Abstract
To investigate the configuration and function of microtubules (MTs) in tip-growing Medicago truncatula root hairs, we used immunocytochemistry or in vivo decoration by a GFP linked to a MT-binding domain. The two approaches gave similar results and allowed the study of MTs during hair development. Cortical MTs (CMTs) are present in all developmental stages. During the transition from bulge to a tip-growing root hair, endoplasmic MTs (EMTs) appear at the tip of the young hair and remain there until growth arrest. EMTs are a specific feature of tip-growing hairs, forming a three-dimensional array throughout the subapical cytoplasmic dense region. During growth arrest, EMTs, together with the subapical cytoplasmic dense region, progressively disappear, whereas CMTs extend further toward the tip. In full-grown root hairs, CMTs, the only remaining population of MTs, converge at the tip and their density decreases over time. Upon treatment of growing hairs with 1 μm oryzalin, EMTs disappear, but CMTs remain present. The subapical cytoplasmic dense region becomes very short, the distance nucleus tip increases, growth slows down, and the nucleus still follows the advancing tip, though at a much larger distance. Taxol has no effect on the cytoarchitecture of growing hairs; the subapical cytoplasmic dense region remains intact, the nucleus keeps its distance from the tip, but growth rate drops to the same extent as in hairs treated with 1 μm oryzalin. The role of EMTs in growing root hairs is discussed.
Root hairs are lateral extensions of epidermal root cells involved in the uptake of water and nutrients, in anchoring the plant in the soil (Peterson and Farquhar, 1996; Gilroy and Jones, 2000), and in the interaction between nitrogen-fixing rhizobacteria and their Fabacean host plants (Mylona et al., 1995; Long, 1996). They emerge as bulges from the outer periclinal cell wall of epidermal cells and elongate almost perpendicularly to the root axis by tip growth (Medicago truncatula: Shaw et al., 2000), which reflects an underlying polarity of the cytoarchitecture (M. truncatula: Sieberer and Emons, 2000).
In growing root hairs, cytoplasm, including the nucleus, is concentrated in the subapical region, whereas the basal part is highly vacuolated (vetch [Vicia sativa]: De Ruijter et al., 1998; M. truncatula: Sieberer and Emons, 2000). The subapical cytoplasmic dense region contains endoplasmic reticulum (ER), mitochondria, plastids, and Golgi bodies (vetch: Miller et al., 2000). At the base of this subapical cytoplasmic dense region is the nucleus, which follows the expanding tip at a certain distance (M. truncatula: Sieberer and Emons, 2000). The extreme apex of the hair looks smooth in the differential interference light microscope, and has been shown with electron microscopy to be filled with vesicles (freeze fixation/freeze substitution: Equisetum hyemale: Emons, 1987; Vicia hirsuta: Ridge, 1988, 1993; Vicia villosa: Sherrier and Van den Bosch, 1994; Arabidopsis: Galway et al., 1997; vetch: Miller et al., 2000).
The subapical cytoplasmic dense region is configured by fine bundles of actin filaments, called FB-actin (Miller et al., 1999). It is thought to function in the transport and/or keeping of Golgi vesicles to the vesicle-rich region in the hair dome. After chemical fixation and fluorescein-phalloidin staining of growing hairs, this vesicle-rich region at the very tip appears to be devoid of filamentous actin when observed with a confocal laser-scanning microscope (CLSM; vetch: Miller et al., 1999). The strongest indication that this typical actin cytoskeleton is involved in tip growth comes from its reaction to Nod factor, a lipochito-oligosaccharide secreted by rhizobacteria. Nod factor enhances this cytoskeleton configuration in all developmental stages of root hairs (De Ruijter et al., 1999), whereupon hair tips swell and new tip growth restarts in those that were arresting growth (De Ruijter et al., 1998). Furthermore, tip growth in vetch root hairs is inhibited by cytochalasin D, an actin-depolymerizing drug (Miller et al., 1999).
Most of the earlier work on microtubules (MTs) in root hairs has dealt with cortical MTs (CMTs) and their role in cellulose microfibril orientation (for review, see Emons and Mulder, 1998; Ketelaar and Emons, 2000). Endoplasmic MTs (EMTs) have been observed only in legume root hairs, and their precise configuration and role remain unclear (Lloyd et al., 1987). Authors have proposed several functions for MTs in root hairs. They may control growth orientation (Arabidopsis: Bibikova et al., 1999), regulate the organization of actin filaments (Hydrocharis: Tominaga et al., 1997), connect the nucleus to the expanding tip (V. hirsuta: Lloyd et al., 1987), or determine the width of the root hair tube (E. hyemale: Emons et al., 1990). Despite these studies, the functions of MTs in tip growth are still less clear than those of actin filaments. Furthermore, no description exists of MTs during all stages of root hair development.
We made use of green fluorescent protein (GFP) technology to visualize MTs in all developmental stages of living root hairs of M. truncatula, a legume well studied for the interaction with rhizobia and mycorrhizal fungi (Cook, 1999). The dynamics of the cytoskeleton in living cells may occlude its clear observation. Compare for instance GFP-talin-labeled actin (Baluska et al., 2000) with fluorescein-phalloidin-stained actin (Miller et al., 1999) in root hairs. Furthermore, we wanted to know whether the same population of MTs is labeled with the GFP microtubule-binding domain (MBD) fusion protein as with immunocytochemistry. The MBD is of animal origin and thus might not label all MTs in root hairs. Therefore, we compared the results obtained with in vivo labeling of MTs by GFP-MBD with results obtained with immunocytochemistry after rapid freeze fixation/freeze substitution (FF/FS), the most reliable fixation method (Emons, 1987), also for light microscopy (Baskin et al., 1996; Vos and Hepler, 1998). With both methods, we made similar observations and it was possible to monitor the configuration of MTs in all stages of root hair development.
Each stage of root hair development had a specific organization of MTs. CMTs were present in all stages of hair development in stage-specific configurations, but EMTs were only present in the subapical region of vigorously growing root hairs. Studies with the MT-inhibiting drugs oryzalin and taxol gave evidence that EMTs are essential in maintaining the specific cytoarchitecture of growing root hairs, including a fixed distance between nucleus and hair tip, as well as to keep the growth rate of these hairs at a high level.
RESULTS
We studied the organization of MTs in M. truncatula root hairs in all developmental stages using CLSM. MTs were visualized by immunocytochemistry after FF/FS, or in vivo by GFP-MBD decoration. With both methods, we find the same configuration of the MT cytoskeleton, but MTs decorated with GFP-MBD appear slightly thicker than MTs labeled with antibodies. Because MTs in living cells are dynamic, they appear thicker in in vivo imaging techniques than in fixed specimens. Root hairs of transformed roots developed normally and grew, with similar speed and pattern, as hairs of nontransformed roots.
Trichoblast (before Bulge Formation)
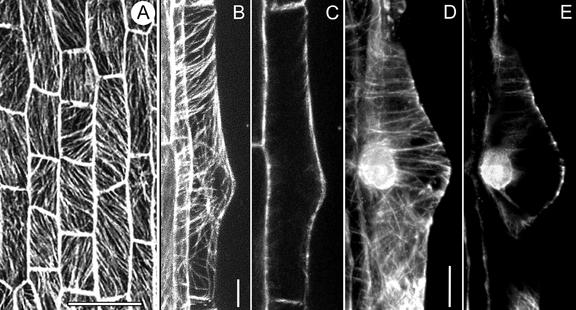
In M. truncatula, every root epidermal cell has the potential to form a bulge, and thus, in this species, all epidermal cells are trichoblasts (Sieberer and Emons, 2000). This is different from Arabidopsis, for instance, where trichoblasts and atrichoblasts are organized in cell files (Dolan et al., 1994). A trichoblast of M. truncatula has one large main vacuole, which fills the cell except for a few cytoplasmic strands traversing the vacuole and a thin layer of peripheral cytoplasm containing all organelles and the nucleus. Within one cell, CMTs are oriented obliquely with different angles and/or transversely to the long axis of the root (Fig. 1A). At the presumptive site of bulge formation, CMTs are transverse to the root axis and parallel to each other. We have not detected EMTs in transvacuolar cytoplasmic strands of trichoblasts before bulge formation; therefore, in this aspect, they are the same as other diffuse growing plant cells (BY-2 tobacco cells: Collings et al., 1998; Granger and Cyr, 2000).
Figure 1.
CMTs in trichoblasts before bulge formation (A) and during bulge formation (B–E), visualized with a CLSM in scanning steps of 1 μm. A through C, GFP-MBD; D and E, Immunocytochemistry. A, CMTs are obliquely or longitudinally oriented to the long axis of the root. Bar = 50 μm. B, Full-stack projection of CMTs. CMTs loop through the tip of the bulge and are transversely or slightly helically oriented to the long axis of the root in the epidermal part. Bar = 10 μm. C, Projection of four median sections. There are no detectable EMTs in this developmental stage. D, Full-stack projection of CMTs; for explanation see B. Bar = 10 μm. E, Note position of the nucleus. For explanation see C.
Bulge
In M. truncatula, root hair development starts with the formation of a bulge in the middle of the outer periclinal wall or slightly toward the root tip. Young bulges have a triangular shape with the large vacuole extending into the bulge. The nucleus is located at the site opposite to the bulge at the inner periclinal wall (Sieberer and Emons, 2000). At the site of the bulge, CMTs are mainly transverse to the long axis of the root (Fig. 1, B and D). Toward both distal ends of the cell, CMTs may be slightly obliquely oriented at this stage (Fig. 1, B and D). CMTs in the bulge are continuous with CMTs in the epidermal part of the trichoblast and pass through the tip of the bulge where they loop through (Fig. 1, B and D). At the tip of the bulge, the distance between CMTs increases when the bulge expands. We did not observe any EMTs between the nucleus and the tip of the bulge (Fig. 1, C and E).
Initiation of Polar Growth
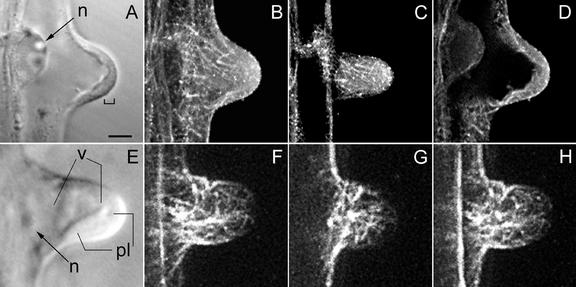
During the transition from bulge to the tip-growing root hair stage, cytoplasm accumulates at the very tip of the bulge and forms a short cytoplasmic dense region there. The nucleus is still in the epidermal part of the cell (Fig. 2A). CMTs are still oriented transversely to the root axis and a few of them still loop through the tip of the developing bulge (Fig. 2, B, C, F, and G). It is interesting that within the cytoplasmic layer at the tip, single EMTs appear (Fig. 2, D and H) and increase in density as the subapical cytoplasmic dense region becomes larger (compare Fig. 2, A with E and D with H). We observed EMTs in living GFP-MBD expressing hairs and in immunolabeled FF/FS hairs of this developmental stage.
Figure 2.
MTs during transition from a bulge into a growing root hair, visualized with a CLSM in scanning steps of 1 μm (B–D and F–H). B through D, Immunocytochemistry; F through H, GFP-MBD. In this developmental stage, polar growth is initiated. A, Corresponding bright-field image to B through D; the bracket indicates a thin layer of cytoplasm at the tip. B, Full-stack projection of MTs. C, Projection of three peripheral sections showing CMTs. A few CMTs still reach the very tip and are net-axially oriented below the tip region. D, Projection of four median sections; EMTs start to appear at this stage of hair development. E, Bright-field image of a hair at a somewhat later stage than shown in A. The cytoplasmic layer in the tip region has increased in length. F, Full-stack projection of MTs. G, Projection of three peripheral sections. Single CMTs still reach the very tip. H, Projection of four median sections showing EMTs. There is a concomitant increase in the length of the subapical cytoplasmic dense region and the density of EMTs. A through D show an earlier stage than E through H. Magnification is the same in all images. Bar = 20 μm. n, Nucleus; v, vacuole; pl, cytoplasmic layer.
Growing Root Hairs
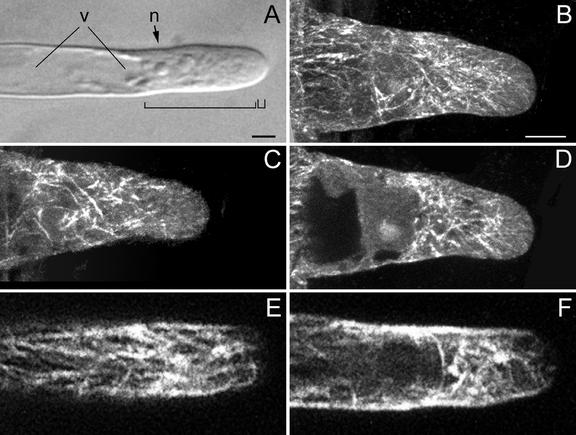
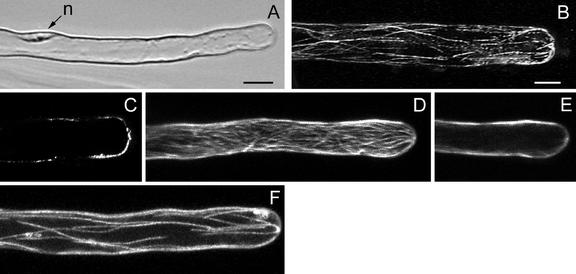
Regularly growing root hairs exclusively elongate by tip growth. A growing root hair of M. truncatula has a characteristic cytoarchitecture (Sieberer and Emons, 2000). It is shown in Figure 3A and it consists of an apical smooth, vesicle-rich region at the very tip, which is followed by a subapical cytoplasmic dense region containing the nucleus. The nucleus follows the expanding tip at a distance of 30 to 40 μm, measured from the hair tip to the middle of the nucleus (see below). During this stage of vigorous tip growth, the central vacuole never enters the subapical cytoplasmic dense region, although extensions of the central vacuole may temporarily penetrate this region.
Figure 3.
MTs in growing root hairs visualized with a CLSM in scanning steps of 1 μm (B–F). B through D, Immunocytochemistry; E and F, GFP-MBD. A, Bright-field image of a living hair; the small bracket indicates the vesicle-rich region and the large bracket indicates the subapical cytoplasmic dense region. n, Nucleus; v, central vacuole. Bar = 10 μm. B, Full-stack projection of MTs. C, Projection of three sections of the cell periphery showing net-axially aligned CMTs. CMTs do not reach the very tip. D, Projection of four median sections showing EMTs. EMTs are abundant close to the nucleus and in the lower part of the subapical cytoplasmic dense region. The density of EMTs in the upper part of the subapical cytoplasmic dense region is low. Only a few EMTs reach the very tip. E, Projection of three sections of the cell periphery showing CMTs. For explanation see C. F, Projection of four median sections; for explanation see D. Magnification in B through F is the same. Bar in B = 10 μm.
CMTs in the base of a young growing root hair are net-axially oriented to the root hair axis and are continuous with CMTs in the subapex, which have the same orientation (Fig. 3, B, C, and E). Later, still during tip growth, CMTs are longitudinal in the lower part of the hair tube and net-axial in the subapex (data not shown). In the CLSM images taken from living root hairs and immunolabeled FF/FS samples, the very tip of a growing root hair is devoid of CMTs (Fig. 3, B, C, and E). In the shank of the root hair, the density of CMTs is higher in growing root hairs than in hairs of other developmental stages.
EMTs are located exclusively in the subapical cytoplasmic dense region between the basal part of the nucleus and the tip of the hair in a densely structured three-dimensional array (Fig. 3, D and F). We observed EMTs around and close to the nucleus in a high density. To determine whether these EMTs originate from the nuclear envelope, attach to it, or just surround it was not part of this study and would require a completely different set of experiments, including, for instance, lifetime imaging during fluorescence resonance energy transfer. EMTs in the lower part of the subapical cytoplasmic dense region occur in a higher density and deviate more from the long axis of the hair than the EMTs close to the root hair tip. Some EMTs are reaching the very tip (Fig. 3, D and F).
Growth-Arresting Root Hairs
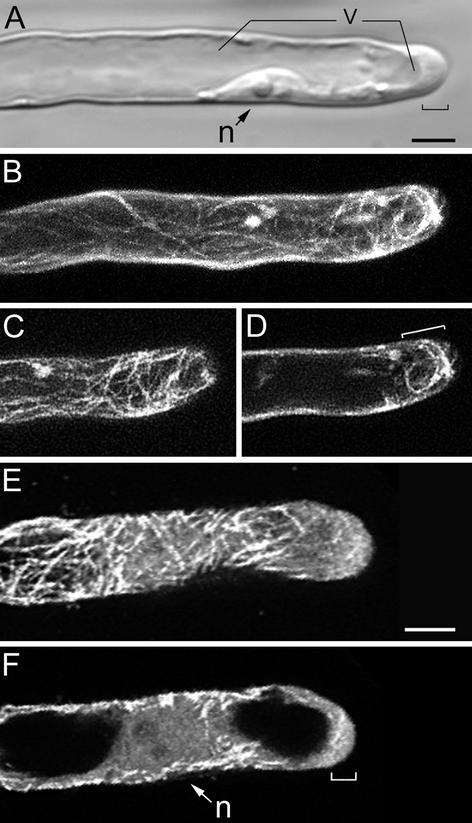
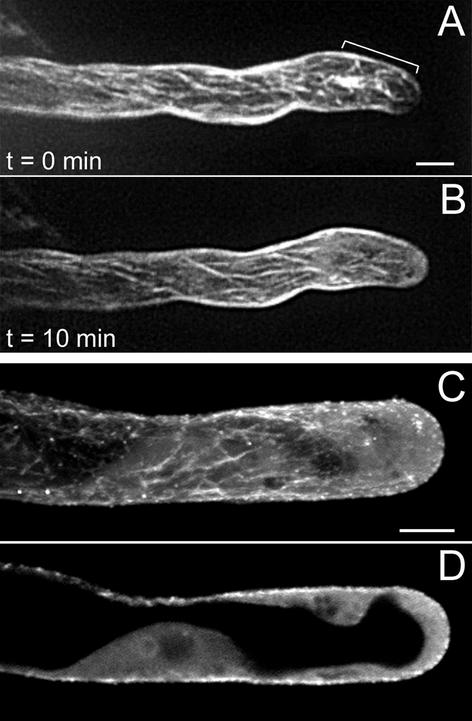
Root hair growth-arrest starts when the main vacuole has permanently passed the nucleus (Sieberer and Emons, 2000). Thin extensions of the vacuole are reaching the very tip; growth rate subsequently drops, and eventually cell elongation stops. During growth arrest, the nucleus does not follow the tip of the hair any longer and the cytoplasmic dense region decreases progressively in length. While the cytoplasmic dense region disappears, the central vacuole expands toward the tip (Fig. 4A). Growth arrest is a gradual process, and root hairs in this developmental stage are still growing to a certain extent before stopping growth (Sieberer and Emons, 2000). In growth-arresting hairs, CMTs (Fig. 4, B, C, and E) are net-axially oriented all along the root hair tube and they are reaching the very tip. As the subapical cytoplasmic dense region gets smaller, the area with EMTs gets shorter, but as long as growth continues, EMTs remain present close to the tip (Fig. 4, D and F). When growth stops, all EMTs have disappeared.
Figure 4.
MTs in growth-arresting hairs visualized with a CLSM in scanning steps of 1 μm (B–F). B through D, GFP-MBD; E and F, immunocytochemistry. At this developmental stage, the subapical cytoplasmic dense region has almost disappeared and the distance tip-nucleus has increased. A, Bright-field image of a living hair; the bracket indicates the remaining subapical cytoplasmic dense region and the smooth (vesicle rich) region at the very tip. Bar = 10 μm (A–D). B, Full-stack projection of MTs. C, Projection of three peripheral sections showing CMTs. CMTs are net-axially oriented. D, Projection of four median sections. Region of EMTs has decreased in length (bracket). E, Projection of three peripheral sections showing CMTs. For explanation see C. Bar = 10 μm. F, Projection of four median sections; for explanation see D. n, Nucleus; v, vacuole.
Full-Grown Root Hairs
A full-grown hair typically has a thin peripheral layer of cytoplasm around the large central vacuole (Fig. 5A), and the nucleus has a random position within the hair (Sieberer and Emons, 2000). CMTs, the only remaining population of MTs in full-grown root hairs (Fig. 5, B–F), are net-axial in the hairs that recently have stopped growth, and mainly longitudinal in the hairs that terminated growth earlier (compare Fig. 5, D with F). CMTs are converging at the very tip of the hair (Fig. 5, B–E). In full-grown root hairs, the density of CMTs decreases over time (Fig. 5F), resulting in living hairs with few detectable MTs left. Full-grown root hairs never have EMTs (Fig. 5, C and E).
Figure 5.
MTs in full-grown root hairs visualized with a CLSM in scanning steps of 1 μm. B and C, Immunocytochemistry; D through F, GFP-MBD. A, Bright-field image of a living hair. Note position of the nucleus (n). Bar = 10 μm. B, Full-stack projection of CMTs. CMTs, the only remaining population of MTs in this developmental stage, are longitudinally oriented; they converge at the very tip. Bar = 10 μm (B–F). C, Projection of two median sections. Full-grown hairs have no EMTs. D, Full-stack projection of CMTs. The hair has stopped growth recently and the density of CMTs, which are net-axially oriented, is similar to previous developmental stages. E, Projection of two median sections; for explanation see C. F, Full-stack projection of CMTs in a hair of a later stage than shown in D. The density of CMTs is lower than in hairs that have just terminated growth.
Effect of Low Concentrations of MT Inhibitors on Growing Root Hairs
To study the function of EMTs, they were depolymerized with oryzalin and stabilized with taxol. Oryzalin is a dinitroaniline herbicide that binds rapidly and reversibly to plant tubulin with high affinity (Hugdahl and Morejohn, 1993) and depolymerizes plant MTs (Morejohn et al., 1987). It binds to tubulin heterodimers in the cytoplasm, and thereby prevents further growth of MTs, leading to depolymerization of MTs, beginning with the more dynamic ones (for review, see Anthony et al., 1999). Thus, the use of low concentrations of oryzalin and the observation of its effect in time may enable us to discriminate between more and less stable MTs. We applied oryzalin in concentrations of 0.25, 0.5, and 1 μm to growing root hairs that had a length of 100 to 150 μm by the time of drug application. None of these concentrations affected the viability of the root hairs or the cytoplasmic streaming. In all these concentrations, oryzalin did not inhibit bulge formation (data not shown). Furthermore, with all these concentrations, EMTs disappeared within 5 to 10 min, whereas CMTs remained present. Figure 6 shows this for 1 μm oryzalin in a GFP-MBD-expressing root hair and an immunolabeled sample. EMTs, which normally are abundant in the subapical cytoplasmic dense region of growing hairs, were completely absent in oryzalin-treated growing root hairs, whereas CMTs in the same hairs were not obviously affected.
Figure 6.
MTs in growing root hairs treated with 1 μm oryzalin. A and B, GFP-MBD; C and D, immunocytochemistry. A, Hair before treatment; a full-stack projection shows abundant MTs in the subapical region (bracket). B, Ten minutes after treatment. The subapical region contains less MTs and the MTs (cortical) in the shank of the hair are unchanged. C, Hair 15 min after treatment; a full-stack projection shows CMTs. D, A projection of four median sections of the hair in C shows that EMTs and the subapical cytoplasmic dense region have completely disappeared, but the vesicle-rich region still is present at the very tip (see also Fig. 7, B and C). Bar in A = 10 μm; bar in C = 10 μm.
Taxol, on the other hand, is a drug that binds to MTs and causes free tubulin in the cytoplasm to assemble into MTs, thus stabilizing MTs (Morejohn, 1991). We found that the concentration of a range tested (0.25, 0.5, and 1 μm) that had a clear effect on growth rate but did not affect cell viability was 1 μm. We used taxol in a concentration that had a similar effect on root hair growth rate as 1 μm oryzalin, which appeared to be 1 μm. However, at 1 μm, taxol root hairs completely recovered their growth rate within 2 to 3 h. Therefore, we refreshed the taxol every 120 min.
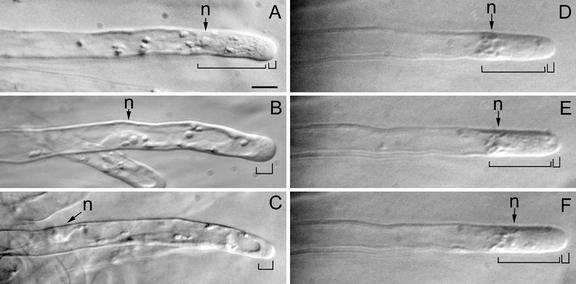
Oryzalin, at a concentration of 1 μm, had striking effects on the cytoarchitecture of growing root hairs (Fig. 7, A–C). Within minutes after application of oryzalin, growing root hairs lost their typical cytoarchitecture. The subapical cytoplasmic dense region disappeared gradually within 5 to 10 min, and the vacuole passed the nucleus and expanded toward the tip of the hair. An oryzalin-treated hair finally had no subapical cytoplasmic dense region, the tip-nucleus distance had increased (see below), and the smooth region containing the Golgi vesicles was still present at the very tip and became even slightly longer over time. The smooth region in an oryzalin-treated hair could even reach a length of 15 to 20 μm instead of the normal length of approximately 3 μm (Sieberer and Emons, 2000). As long as oryzalin was not washed out of the growth medium, the growing root hairs did not reestablish their typical cytoarchitecture. Hairs were observed up to 7 h after drug application.
Figure 7.
The effect of treatment with oryzalin or taxol on the cytoarchitecture of growing root hairs. A, Before treatment. B, Thirty minutes after 1 μm oryzalin. C, Sixty minutes after 1 μm oryzalin. D, Before treatment. E, Thirty minutes after 1 μm taxol. F, Sixty minutes after 1 μm taxol. Bright-field images from living root hairs. Large bracket indicates the subapical cytoplasmic dense region, and the small bracket indicates the smooth (vesicle-rich) region. n, Nucleus. Magnification is the same in all images. Bar = 20 μm.
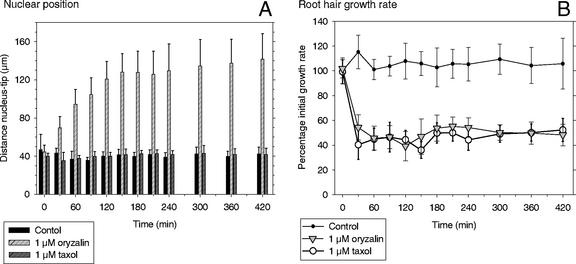
The nucleus of an oryzalin-treated growing root hair lost its typical position of 30 to 40 μm from the tip and moved slowly backward in the root hair tube within the first 90 to 120 min after treatment (Fig. 8A). After that time, the nucleus was again following the expanding root hair tip at a significantly larger distance (Fig. 8A). A nuclear movement at the pace with cell elongation was observed in all hairs, but the distance between the nucleus and the tip was not the same in all hairs. In approximately 70% of the hairs, this distance was 140 ± 30 μm over a period of 4 to 5 h. The other 30%, not represented in Figure 8A, had a smaller (minimally 80 μm) or larger (maximally 250 μm) nucleus-tip distance, but also in these hairs, the nucleus followed the tip. The exact distance is not relevant here, but the fact that the nucleus keeps following the growing root hair tip is.
Figure 8.
Nuclear position (A) and root hair growth rate (B) in growing M. truncatula root hairs after control treatment and treatment with 1 μm oryzalin or 1 μm taxol. In controls and taxol-treated hairs, the nucleus kept a distance of 30 to 40 μm to the tip over time, but it was significantly increased in oryzalin (A). Growth rate in the hairs remained high in controls, but dropped by approximately 60% in oryzalin and taxol (B). Results for each treatment are presented as the means of 10 hairs with their sd. Results are representative for three independent replicates for each treatment.
Taxol at a concentration of 1 μm had no effect on the cytoarchitecture (Fig. 7, D–F) or the position of the nucleus (Fig. 8A). The distance nucleus-tip of 30 to 40 μm remained constant. Observed with bright-field microscopy over 7 h, the subapical cytoplasmic dense region did not show any obvious changes in cytoarchitecture and length compared with controls. Immunocytochemistry showed that 1 μm taxol did not change the configuration of EMTs (data not shown).
Oryzalin, at the above-mentioned concentration of 1 μm, had a significant effect on the growth rate of growing root hairs, but did not inhibit tip growth per se (Fig. 8B). Treated hairs did grow, but with a lower growth rate than untreated growing root hairs. We followed growing root hairs treated with oryzalin over a time span of 7 h. During this period, we did not observe any changes in shape and width of the root hair tube. Furthermore, these hairs always maintained one point of growth (i.e. the expanding tip). Shortly after application of 1 μm oryzalin, most root hairs exhibited a single deviation from their normal growth axis, which is perpendicular to the root. In some root hairs, several deviations from the hairs' growth axis occurred irregularly over time in the presence of oryzalin. However, we never observed a wavy growth pattern of root hairs after MT inhibitor treatment, as described for Arabidopsis (Bibikova et al., 1999). An important difference with that work is that these authors depolymerized all MTs, whereas in our approach, only EMTs were depolymerized and not the CMTs. It is not known whether Arabidopsis has EMTs; they have not been reported.
When oryzalin was washed out after 7 h by replacing the medium with fresh plant growth medium (PGM), 35% to 40% of the growing root hairs fully recovered their cytoarchitecture, but their growth rate reached a maximum 70% of the initial growth rate (data not shown). This recovery took place over a period of 4 to 6 h.
Taxol, at a concentration of 1 μm, had a similar effect on the growth rate of growing root hairs as oryzalin (Fig. 8B). Although the growth rate in taxol-treated growing root hairs dropped to approximately 40% of the initial growth rate, the distance nucleus-tip of 30 to 40 μm remained constant. In 1 μm taxol, root hairs maintained one single point of growth, always kept the same directionality of growth, and always had the same shape as control root hairs.
Oryzalin in concentrations of 10 and 30 μm had the same effect on the cytoarchitecture of growing M. truncatula root hairs as a concentration of 1 μm had (data not shown). Growing root hairs that had a length of 100 to 150 μm by the time of drug application were observed for at least 5 h. Within minutes of treatment, the distance between nucleus and hair tip increased, whereas the subapical cytoplasmic dense region disappeared. The apical vesicle-rich region remained unchanged and tip growth itself proceeded, but growth rate dropped in a similar way as in hairs treated with 1 μm oryzalin. Using immunocytochemistry after a 30-min treatment with 30 μm oryzalin, we could not detect EMTs or intact CMTs in these hairs, whereas they kept on growing for hours.
DISCUSSION
In this paper, we report changes in the spatial configuration of the MT cytoskeleton during development of M. truncatula root hairs and the effect of MT inhibitors on the MT configuration, the cytoarchitecture, the nuclear position, and the growth rate of growing hairs. The results obtained with the two approaches used, cells expressing a GFP-MBD fusion protein (Marc et al., 1998) and immunolabeled fixed whole-mount samples, were similar. Thus, we conclude that the GFP-MBD construct decorates the same set of MTs as does the anti-α-tubulin mouse monoclonal antibody clone DM 1α. We identified two different populations of MTs in root hairs: CMTs, present in all developmental stages, and more dynamic and labile EMTs, unique for growing root hairs. Furthermore, we did not observe any measurable effect on root hair development, cytoarchitecture, growth pattern, and growth rate of GFP-MBD-expressing root hairs.
CMTs during Root Hair Development
All diffuse growing interphase plant cells have CMTs, which are transverse to the direction of elongation of the cell. They become less frequent and obliquely aligned when cell elongation decelerates (for review, see Traas et al., 1985; Cyr, 1994; Wasteneys, 2000). These MTs are involved in cell elongation and appear often, but not always, in the same orientation as nascent cellulose microfibrils (for review, see Sugimoto et al., 2000; Wasteneys, 2000). As expected, the diffuse growing trichoblasts have CMTs transverse to the root axis. The time of bulge formation seems to be the time these cells begin to stop growing. At their upper and lower edges, MTs are becoming helical when bulges first appear. However, at the site of bulge formation, the CMTs are transverse, and after bulge appearance, they are still in this orientation running over the tip of the bulge. During bulge formation, the distance between CMTs increases; they seem to passively part at the bulge tip. In the presence of oryzalin, bulges are being formed normally. In addition, from studies on the cytoskeleton (actin: Miller et al., 1999; actin and MTs: Baluska et al., 2000), ultrastructure (vetch: Miller et al., 2000), and cytoplasmic [Ca2+] gradients in wild-type Arabidopsis and the rhd-2 mutant (Wymer et al., 1997), it was concluded that bulge formation is a distinctly different process than tip growth of the root hair proper.
CMTs have been found in root hairs of all species examined in helical or mostly net-axial orientations (for review, see Ketelaar and Emons, 2000, 2001). The function of CMTs in root hairs is still not clear. Arabidopsis root hairs with depolymerized MTs (Bibikova et al., 1999) or a mutant with disorganized CMTs (Whittington et al., 2001) continue to elongate by tip growth, but in a slightly wavy pattern. Thus, CMTs do not seem to be required for growth per se, but appear to be involved in determining direction of elongation (Bibikova et al., 1999). Because CMTs are often found to be aligned with microfibrils, it is often thought that CMTs are responsible for directing nascent cellulose microfibrils. However, contradictory examples have been reported, and other mechanisms have been proposed (for review, see Emons and Mulder, 2000). The configuration of CMTs we now find in growing root hairs is net-axial in the upper part of growing root hairs and helical in the basal part. A cell wall study has yet to be performed for M. truncatula.
In M. truncatula root hairs, CMTs are absent from the extreme tip in the GFP-MBD plant and after FF/FS immunolabeling. In electron microscopy images of FF/FS root hairs of other species, CMTs were very close to the tip. They have been observed in the area where the Golgi vesicles are located (E. hyemale: Emons, 1989; Arabidopsis: Galway et al., 1997). The absence of CMTs from the very tip could be explained by insufficient labeling of MTs at this place. The specific binding sites of CMTs close to the tip of a growing root hair may not be fully accessible for GFP-MBD or antibodies because CMTs at this site are highly decorated with MT-binding proteins. Therefore, the absence of CMTs in M. truncatula should be confirmed with electron microscopy.
EMTs during Root Hair Development
EMTs are unique for the subapical region in tip-growing root hairs of M. truncatula. From our experiments with low concentrations of oryzalin, it is clear that EMTs are far more sensitive to depolymerization than CMTs and thus are more dynamic than CMTs (Anthony and Hussey, 1999).
Until now, EMTs have only been reported for legume root hairs, and not for any other species. Lloyd et al. (1987) found EMTs in growing root hairs of V. hirsuta that were hypothesized to connect the migrating nucleus to the root hair tip, where they fountain out upon the cortex. We have not seen similar structures in M. truncatula. Lloyd et al. (1987) used chemical fixation and immunolocalization to visualize MTs and, due to the limited resolving power of the fluorescence microscope as compared with a CLSM, the precise configuration of EMTs is still a matter of speculation. In our hands, EMTs appear at the root hair tip when tip growth starts from bulges, they remain present in the tip region during cell elongation, and they disappear upon growth arrest. In growing root hairs, EMTs form a densely structured three-dimensional array close to and in front of the nucleus. They extend throughout the subapical cytoplasmic dense region toward the tip region, appearing less dense there. A few EMTs reach the very tip. What is the function of these EMTs? Are they involved in cell elongation or the determination of growth direction, nuclear positioning, regulation of tube width, or actin filament configuration? We discuss the function of EMTs in cell elongation and in configuring cell architecture, including nuclear position.
Function of EMTs in Configuring the Subapical Cytoplasm, Including the Localization of the Nucleus at a Certain Distance from the Hair Tip
The MT-depolymerizing drug, oryzalin, at a concentration of 1 μm, caused a dramatic change in the cytoarchitecture of the subapex of growing M. truncatula root hairs, whereas taxol, an MT-stabilizing drug, left the cytoarchitecture intact. EMTs, but not CMTs, were depolymerized by 1 μm oryzalin. The most straightforward conclusion from these experiments is that EMTs must contribute directly or indirectly to the cytoarchitecture of growing root hairs. Therefore, we conclude that EMTs are involved in supporting the organization and maintenance of the subapical cytoplasmic dense region. The question then arises: What is the relationship with the actin cytoskeleton? The actin cytoskeleton forms a specific configuration of FB-actin in the subapical cytoplasmic dense region (Miller et al., 1999). An interesting option for the function of EMTs is the one suggested by Tominaga et al. (1997) for Hydocharis morsus-ranae root hairs. From their experiments in which they combined actin and MT drugs, these authors suggest that MTs regulate the organization of actin filaments in the cortex of root hairs. In diverse cell types, functional interactions may exist between the actin and MT cytoskeleton (for review, see Goode et al., 2000) and have been suggested for tip-growing plant cells (for review, see Kropf et al., 1998).
Their role in the positioning of the nucleus at a distance of 30 to 40 μm to the growing root hair tip is related to the role of EMTs in the formation of the subapical cytoarchitecture. In an oryzalin-treated hair, the nucleus first loses its position, but after a period of backward movement, it is again actively following the (now more slowly) growing tip at a larger but again fixed distance. For Arabidopsis root hairs, it has been reported that MT inhibitors such as oryzalin and taxol had no effect on nuclear movement (Chytilova et al., 2000). The question, not only for drug-treated cells, but also for control cells is: What keeps the nucleus following the root hair tip? Our results show that EMTs are necessary for keeping the nucleus at a certain position to the root hair tip, but are not sufficient for nuclear movement.
Function of EMTs in Cell Elongation
Oryzalin, at a concentration at which only EMTs are depolymerized, caused a completely different cytoarchitecture, but had the same effect on growth rate as taxol at a concentration that kept the cells viable. Oryzalin caused the disappearance of the subapical cytoplasmic dense region, but in taxol-treated hairs, the cytoarchitecture of apex and subapex remained unaltered. After treatment with oryzalin, the smooth vesicle-rich region at the very tip remained present and even increased in length. What is the same in both treatments is that the vesicle-rich region at the root hair tip remains present. This is the one and most important prerequisite for tip growth. The fusion of the vesicles with the plasma membrane is the actual cell elongation process, which of course cannot take place if the vesicles are not there. We conclude that exocytotic vesicles are still being produced and delivered to the vesicle-rich region, and they fuse with the plasma membrane in growing root hairs treated with oryzalin or taxol. Tip growth proceeds, though at a low rate. From electron microscopy experiments, we know that the subapical region of legume root hairs contains longitudinal cisternae of the ER, mitochondria, and Golgi bodies (vetch: Miller et al., 2000). Actin labeling has shown the occurrence of subapical net-axial FB-actin (vetch: Miller et al., 1999). Because hairs in which the subapical EMTs are not present anymore (oryzalin) or stabilized (taxol) do still grow, EMTs, possibly together with or in relation to FB-actin, configure the subapical cytoplasm, but the vesicle transport system appears to be actin based. The combined EMTs, FB-actin, and ER cell structure may form a subapical buffer reservoir for exocytotic vesicles in transit to the apical vesicle-rich region.
In the presence of oryzalin and taxol, the growth rate of root hairs drops by 60%. The simplest explanation for this in the case of oryzalin is that the delivery of exocytotic vesicles to the vesicle-rich region is inefficient because there is no buffer of vesicles. Along the same line, this delivery may be inefficient in the presence of taxol because the EMTs are not functioning properly. In addition, there are root hairs of Limnobium stoloniferum (A.M.C. Emons, unpublished data) or H. morsus-ranae (see figures in Tominaga et al., 1997), for instance, that do not have a subapical cytoplasmic dense region or that have an extremely short one. Of course, they do possess a vesicle-rich region. Growth rates of root hairs of species with and without a subapical cytoplasmic dense region have not been compared.
One should have an open mind for other possible explanations. One of the important observations is that although the subapical cytoplasmic dense region disappears in oryzalin-treated hairs, the vesicle-rich region remains present and even enlarges. The fact that upon oryzalin treatment, the vesicle-rich region enlarges, whereas growth rate is tempered, suggests that the drug may interfere with the growth process, exocytosis, itself. In that case, taxol should interfere with exocytosis in a similar fashion.
Relevance of Endoplasmic MTs for Legumes
It is striking that EMTs have only been reported for legume root hairs, although MTs in root hairs of several species have been studied (for review, see Ketelaar and Emons, 2000). Several of these studies were designed to investigate the relationship of CMTs with cellulose microfibrils. Therefore, only the cell cortex was investigated, and EMTs may have been missed.
However, we should consider that legume root hairs might be special. Legumes have developed symbiosis with rhizobia. During the root hair curling around rhizobia, one of the first steps during the infection process, the cytoplasm (cytoskeleton, ER, Golgi bodies, etc.) mediates the curling and the formation of an infection thread through which the bacteria traverse the hair toward the root cortex. One can imagine that for curling and infection thread formation, EMTs are important.
MATERIAL AND METHODS
Plant Culture
Seeds of Medicago truncatula cv Jemalong (Fabaceae) were scarified with 97% (w/v) sulfuric acid for 10 min and were surface sterilized with a mixture of 96% (v/v) ethanol and 30% (v/v) hydrogen peroxide (ethanol:hydrogen peroxide [1:1, v/v]) for 3 min. After rinsing with sterile water, seeds were imbibed for 4 h at 30°C in sterile water and then placed on 1% (w/v) agarose plates. Plates were sealed and stored upside down in the dark for 2 to 4 d at 4°C to synchronize germination. During germination for 6 to 8 h at 24°C, the seeds were protected from light. Seedlings were transferred onto wet filter paper and were grown in vertical orientation for 24 to 48 h under sterile conditions at 24°C with a 16-h day length. The PGM, which was used to humidify the filter paper, contained 1.36 mm CaCl2, 0.97 mm MgSO4, 1.12 mm Na2PO4, 1.36 mm KH2PO4, and 20 μm Fe-citrate, pH 6.5 (Miller et al., 1999).
In a different setup, seedlings were grown at an angle of approximately 25o for 24 to 30 h in microchambers in between a coverslip and a glass slide (Fåhraeus slides: Fåhraeus, 1957; Heidstra et al., 1994) in sterile conditions at 24°C with a 16-h day length. Each Fåhraeus slide contained two seedlings and 3 mL of PGM.
Whole-Mount Immunocytochemistry of MTs
For rapid FF, seedlings were plunged into liquid propane cooled to −180°C with liquid nitrogen and were kept there for at least 20 s. Roots were excised and transferred into cryogenic vials, freeze substituted in water-free methanol containing 0.05% (v/v) glutaraldehyde for 48 h at −90°C, and allowed to warm to room temperature over a 24-h period. Samples were rehydrated in a graded series of methanol in phosphate-buffered saline (PBS; 137 mm NaCl, 2.7 KCl, 1.5 mm KH2PO4, and 8.1 mm Na2HPO4, pH 7.4) containing fixative (0.1% [v/v] glutaraldehyde and 4% [w/v] paraformaldehyde). After rehydration, a partial cell wall digestion was carried out in a saturated suspension of driselase (Fluka, Buchs, Switzerland) and macerocyme R10 (Serva, Heidelberg) in 100 mm MES for 40 min at 35°C and pH 6.15. The specimens were then washed two times for 5 min in PBS and, to block unspecific sites, were incubated for 5 min in PBS containing 0.1% (w/v) acetylated bovine serum albumin (BSAac; Aurion, Wageningen, Netherlands) and 0.05% (v/v) Triton X-100 (BDH Laboratory Supplies, Poole, UK). The samples were incubated for 12 h at 4°C in the monoclonal primary antibody anti-α-tubulin mouse, clone DM 1α (Sigma, St. Louis). The primary antibody was diluted 1:300 (v/v) in PBS containing 0.1% (w/v) BSAac and 0.05% (v/v) Triton X-100. After washing three times for 5 min in PBS, the samples were incubated for 12 h at 4°C in the secondary antibody goat against mouse/IgG/Alexa 488 (Molecular Probes, Eugene, OR). The secondary antibody was diluted 1:300 (v/v) in PBS containing 0.1% (w/v) BSAac and 0.05% (v/v) Triton X-100. Specimens were washed twice for 5 min with PBS and for 5 min with CITIFLUOR PBS solution (Citifluor, London), and were mounted in an antifading medium (PROLONG ANTIFADE; Molecular Probes).
Microscopic Observation
Anti-α-tubulin-labeled MTs were visualized with a CLSM (MCR 600; Bio-Rad, Hertfordshire, UK) with an argon-krypton ion laser attached to an inverted microscope (DIAPHOT300; Nikon Europe B.V., Badhoevedorp, The Netherlands) equipped with a 60× FL 1.4 n.a. oil immersion objective (Nikon). Samples were scanned in subsequent steps of 1 μm. Images were acquired and projected with Confocal Assistant, version 4.02 (Bio-Rad, written by Todd Clark Brelje) and were processed with Scion Image Beta 4.0.2. (Scion Corporation, Frederick, MD) and Adobe Photoshop 5.5 (Adobe Systems, Mountain View, CA).
GFP-MBD Decoration of MTs/Transgenic M. truncatula Plants
The GFP-MBD fusion gene with expression under the control of the 35S promotor was provided in a pUC18 vector by Richard Cyr (Marc et al., 1998). The complete insert from this plasmid was transferred to pCambia1390 (CAMBIA, Canberra, Australian Capitol Territory, Australia) by using the HindIII-EcoRI sites, and was kindly provided by W.J. Theodorus Gadella, Jr. (Wageningen University, Wageningen, The Netherlands).
Plant Transformation and Culture
Transformed roots of M. truncatula cv Jemalong were obtained by using Agrobacterium rhizogenes according to the protocol described by Boisson-Dernier et al. (2001). About 3 to 4 weeks later, plants with transformed roots were put into square 12-cm plastic dishes (Greiner Labortechnik, Kremsmünster, Austria) with one of the four sides containing a round perforation in the middle of about 5 mm in diameter. Each individual plant was put in the perforation in such a way that the root was inside on Fåhraeus medium containing 0.8% (w/v) agar and the stem part was outside the plate. Plates were put vertically in a culture room at 25°C and an 18-h day length.
Microscopic Observations
For observation, the roots growing on agar were submerged in sterile water and were covered with a gas-permeable plastic foil (bioFOLIE 25; Sartorius AG, Vivascience Support Center, Göttingen, Germany) to prevent them from drying. The opened dish was put on the microscope stage of a ZEISS LSM510 (Carl Zeiss SA, Le Pecq, France), and observations were carried out with a 40×/0.8 WPH2 Achroplan or a 63×/0.9 WPH3 Achroplan objective. In general, optical sections were made of whole root hairs with a separating distance of 1 μm between subsequent sections. Image projections were made with ZEISS LSM Image Examiner (Zeiss), and images were processed with Image-Pro plus (Media Cybernetics, Silver Spring, MD).
Light Microscopy
Root hairs were observed with a 20× 0.4 n.a. or a 40× 0.55 n.a. objective (Nikon) on an inverted microscope with Hoffman modulation contrast system (DIAPHOT200; Nikon) equipped with a CCD camera (DXC-95OP; Sony, Tokyo). Image recording and processing and quantitative live measurements were done with a real-time digital contrast and low-light enhancement image processor (ARGUS-20; Hamamatsu Photonics, Hamamatsu City, Japan). To prevent light-induced stress, low light and green filters were used during quantitative live measurements and related image recording.
Drug Studies
Oryzalin (Greyhound Chromatography, Birkenhead, UK) was dissolved in dimethyl sulfoxide (DMSO; Merck, Darmstadt, Germany) as a 10 mm stock solution and was used at 0.25, 0.5, 1, 10, and 30 μm in PGM. Taxol (paclitaxel; Sigma) was dissolved in DMSO as a 10 mm stock solution and was used at 0.25, 0.5, and 1 μm in PGM.
In Fåhraeus slides, drug solutions were applied on the microscope stage with a constant flow of 1 mL min−1, gradually replacing the PGM in the slides. The final volume in each slide was 3 mL. Although oryzalin was applied only once, taxol was refreshed every 120 min in the same way as it was applied the first time (see above). To prevent evaporation of water during observation, the slides were covered in the microscope stage with a large plastic petri dish. Measurements on growth rate and nuclear position were done on growing root hairs of nontransformed M. truncatula cv Jemalong roots. Growing root hairs had a length of 100 to 150 μm when measurements were started. Control roots were treated with PGM only containing the same amount of DMSO as the drug solutions.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owner of all parts of the material. Obtaining any permission will be the responsibility of the requestor.
ACKNOWLEDGMENTS
A.C.J.T. would like to thank Rainer Pepperkok, Jens Rietdorf, Timo Zimmermann, and Andreas Girod of the Advanced Light Microscopy Facility for their hospitality and help during his stay at the EMBL. B.J.S. would like to thank Jan Vos for stimulating discussion and helpful comments on the manuscript.
Footnotes
This work was supported by the European Community Training and Mobility of Researchers Program (grant no. FMRX CT 98 0239 to B.J.S. and F.G.P.L.) and by the European Advanced Light Microscopy Facility of the EMBL (short-term fellowship to A.C.J.T.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.004267.
LITERATURE CITED
- Anthony RG, Hussey PJ. Dinitroaniline herbicide resistance and the microtubule cytoskeleton. Trends Plant Sci. 1999;4:112–116. doi: 10.1016/s1360-1385(99)01378-3. [DOI] [PubMed] [Google Scholar]
- Baluska F, Salaj J, Mathur J, Braun M, Jasper F, Samaj J, Chua NH, Barlow PW, Volkmann D. Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev Biol. 2000;227:618–632. doi: 10.1006/dbio.2000.9908. [DOI] [PubMed] [Google Scholar]
- Baskin TI, Miller DD, Vos JW, Wilson JE, Hepler PK. Cryofixing single cells and multicellular specimens enhances structure and immunocytochemistry for light microscopy. J Microsc. 1996;182:149–161. doi: 10.1046/j.1365-2818.1996.135417.x. [DOI] [PubMed] [Google Scholar]
- Bibikova TN, Blancaflor EB, Gilroy S. Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 1999;17:657–665. doi: 10.1046/j.1365-313x.1999.00415.x. [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier A, Chabaud M, Garcia F, Bécard G, Rosenberg C, Barker DG. Agrobacterium rhizogenes-transformed roots of Medicago truncatula for the study of nitrogen-fixing and endomycorrhizal symbiotic associations. Mol Plant-Microbe Interact. 2001;14:695–700. doi: 10.1094/MPMI.2001.14.6.695. [DOI] [PubMed] [Google Scholar]
- Chytilova E, Macas J, Sliwinska E, Rafelski SM, Lambert GM, Galbraith DW. Nuclear dynamics in Arabidopsis thaliana. Mol Biol Cell. 2000;11:2733–2741. doi: 10.1091/mbc.11.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collings DA, Asada T, Allen NS, Shibaoka H. Plasma membrane-associated actin in bright yellow 2 tobacco cells. Plant Physiol. 1998;118:917–928. doi: 10.1104/pp.118.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. Medicago truncatula: a model in the making! Curr Opin Plant Biol. 1999;2:301–304. doi: 10.1016/s1369-5266(99)80053-3. [DOI] [PubMed] [Google Scholar]
- Cyr RJ. Microtubules in plant morphogenesis: role of the cortical array. Annu Rev Cell Biol. 1994;10:153–180. doi: 10.1146/annurev.cb.10.110194.001101. [DOI] [PubMed] [Google Scholar]
- De Ruijter NCA, Bisseling T, Emons AMC. Rhizobium Nod factors induce an increase in sub-apical fine bundles of actin filaments in Vicia sativa root hairs within minutes. Mol Plant-Microbe Interact. 1999;12:829–832. [Google Scholar]
- De Ruijter NCA, Rook MB, Bisseling T, Emons AMC. Lipochito-oligosaccharides re-initiate root hair tip growth in Vicia sativa with high calcium and spectrin-like antigen at the tip. Plant J. 1998;13:341–350. [Google Scholar]
- Dolan L, Duckett CM, Grierson C, Linstead P, Schneider K, Lawson E, Dean C, Poethig S, Roberts K. Clonal relationships and cell patterning in the root epidermis of Arabidopsis. Development. 1994;120:2465–2474. [Google Scholar]
- Emons AMC. The cytoskeleton and secretory vesicles in root hairs of Equisetum and Limnobium and cytoplasmic streaming in root hairs of Equisetum. Ann Bot. 1987;60:625–632. [Google Scholar]
- Emons AMC. Helicoidal microfibril deposition in a tip-growing cell and microtubule alignment during tip morphogenesis: a dry-cleaving and freeze-substitution study. Can J Bot. 1989;67:2401–2408. [Google Scholar]
- Emons AMC, Mulder BM. The making of the architecture of the plant cell wall: how cells exploit geometry. Proc Natl Acad Sci USA. 1998;95:7215–7219. doi: 10.1073/pnas.95.12.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emons AMC, Mulder BM. How the deposition of cellulose microfibrils builds cell wall architecture. Trends Plant Sci. 2000;5:35–40. doi: 10.1016/s1360-1385(99)01507-1. [DOI] [PubMed] [Google Scholar]
- Emons AMC, Wolters-Arts AMC, Traas JA, Derksen J. The effect of colchicine on microtubules and microfibrils in root hairs. Acta Bot Neerl. 1990;39:19–28. [Google Scholar]
- Fåhraeus G. The infection of white clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957;16:374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- Galway ME, Heckman JW, Jr, Schiefelbein JW. Growth and ultrastructure of Arabidopsis root hairs: The rhd 3 mutation alters vacuole enlargement and tip-growth. Planta. 1997;201:209–218. doi: 10.1007/BF01007706. [DOI] [PubMed] [Google Scholar]
- Goode BL, Drubin DG, Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Curr Opin Cell Biol. 2000;12:63–71. doi: 10.1016/s0955-0674(99)00058-7. [DOI] [PubMed] [Google Scholar]
- Gilroy S, Jones DL. Through form to function: root hair development and nutrient uptake. Trends Plant Sci. 2000;5:56–60. doi: 10.1016/s1360-1385(99)01551-4. [DOI] [PubMed] [Google Scholar]
- Granger CL, Cyr RJ. Microtubule reorganization in tobacco BY-2 cells stably expressing GFP-MBD. Planta. 2000;210:502–509. doi: 10.1007/s004250050037. [DOI] [PubMed] [Google Scholar]
- Heidstra R, Geurts R, Franssen H, Spaink HP, van Kammen A, Bisseling T. Root hair deformation activity of nodulation factors and their fate on Vicia sativa. Plant Physiol. 1994;105:787–797. doi: 10.1104/pp.105.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl JD, Morejohn LC. Rapid and reversible high-affinity binding of the dinitroanaline herbicide oryzalin to tubulin from Zea mays L. Plant Physiol. 1993;102:725–740. doi: 10.1104/pp.102.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketelaar T, Emons AMC. The role of microtubules in root hair growth and cellulose microfibril deposition. In: Ridge RW, Emons AMC, editors. Root Hairs: Cell and Molecular Biology. Tokyo: Springer-Verlag; 2000. pp. 17–28. [Google Scholar]
- Ketelaar T, Emons AMC. The cytoskeleton in plant cell growth: lessons from root hairs. New Phytol. 2001;152:409–418. doi: 10.1046/j.0028-646X.2001.00278.x. [DOI] [PubMed] [Google Scholar]
- Kropf DL, Bisgrove SR, Hable WE. Cytoskeletal control of polar growth in plant cells. Cur Opin Cell Biol. 1998;10:117–122. doi: 10.1016/s0955-0674(98)80094-x. [DOI] [PubMed] [Google Scholar]
- Long SR. Rhizobium symbiosis: Nod factor in perspective. Plant Cell. 1996;8:1855–1898. doi: 10.1105/tpc.8.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CW, Pearce KJ, Rawlins DJ, Ridge RW, Shaw PJ. Endoplasmic microtubules connect the advancing nucleus to the tip of legume root hairs, but F-actin is involved in basipetal migration. Cell Motil Cytoskel. 1987;8:27–36. [Google Scholar]
- Marc J, Granger CL, Brincat J, Fisher DD, Kao T, McCubbin AG, Cyr RJ. A GFP-MAP4 reporter gene for visualizing cortical microtubule rearrangements in living epidermal cells. Plant Cell. 1998;10:1927–1939. doi: 10.1105/tpc.10.11.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DD, De Ruijter NCA, Bisseling T, Emons AMC. The role of actin in root hair morphogenesis: studies with lipochito-oligosaccharide as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J. 1999;17:141–154. [Google Scholar]
- Miller DD, Leferink-ten Klooster HB, Emons AMC. Lipochito-oligosaccharide nodulation factors stimulate cytoplasmic polarity with longitudinal endoplasmic reticulum and vesicles at the tip in vetch root hairs. Mol Plant-Microbe Interact. 2000;13:1385–1390. doi: 10.1094/MPMI.2000.13.12.1385. [DOI] [PubMed] [Google Scholar]
- Morejohn LC. The molecular pharmacology of plant tubulin and microtubules. In: Lloyd CW, editor. The Cytoskeletal Basis of Plant Growth and Form. New York: Academic Press; 1991. pp. 29–43. [Google Scholar]
- Morejohn LC, Bureau TE, Molé-Bajer AS, Fosket DE. Oryzalin, a dinitroaniline herbicide, binds to plant tubulin and inhibits microtubule polymerization in vitro. Planta. 1987;172:252–264. doi: 10.1007/BF00394595. [DOI] [PubMed] [Google Scholar]
- Mylona P, Pawlowski K, Bisseling T. Symbiotic nitrogen fixation. Plant Cell. 1995;7:869–885. doi: 10.1105/tpc.7.7.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LR, Farquhar ML. Root hairs: specialized tubular cells extending root surfaces. Bot Rev. 1996;62:1–35. [Google Scholar]
- Ridge RW. Freeze-substitution improves the ultrastructural preservation of legume root hairs. Bot Mag Tokyo. 1988;101:427–441. [Google Scholar]
- Ridge RW. A model of legume root hair growth and Rhizobium infection. Symbiosis. 1993;14:359–373. [Google Scholar]
- Shaw SL, Dumais J, Long SR. Cell surface expansion in polarly growing root hairs of Medicago truncatula. Plant Physiol. 2000;124:959–969. doi: 10.1104/pp.124.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrier DJ, Van den Bosch KA. Secretion of cell wall polysaccharides in Vicia root hairs. Plant J. 1994;5:185–195. [Google Scholar]
- Sieberer B, Emons AMC. Cytoarchitecture and pattern of cytoplasmic streaming in root hairs of Medicago truncatula during development and deformation by nodulation factors. Protoplasma. 2000;214:118–127. [Google Scholar]
- Sugimoto K, Williamson RE, Wasteneys GO. New techniques enable comparative analysis of microtubule orientation, wall texture, and growth rate in intact roots of Arabidopsis. Plant Physiol. 2000;124:1493–1506. doi: 10.1104/pp.124.4.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Morita K, Sonobe S, Yokota E, Shimmen T. Microtubules regulate the organization of actin filaments at the cortical region in root hair cells of Hydrocharis. Protoplasma. 1997;199:83–92. [Google Scholar]
- Traas JA, Braat P, Emons AMC, Meekes H, Derksen J. Microtubules in root hairs. J Cell Sci. 1985;76:303–320. doi: 10.1242/jcs.76.1.303. [DOI] [PubMed] [Google Scholar]
- Vos JW, Hepler PK. Calmodulin is uniformly distributed during cell division in living stamen hair cells of Tradescantia virginata. Protoplasma. 1998;201:158–171. [Google Scholar]
- Wasteneys GO. The cytoskeleton and growth polarity. Curr Opin Plant Biol. 2000;3:503–511. doi: 10.1016/s1369-5266(00)00120-5. [DOI] [PubMed] [Google Scholar]
- Whittington AT, Vugrek O, Wei KJ, Hasenbein NG, Sugimoto K, Rashbrooke MC, Wasteneys GO. MOR1 is essential for organizing cortical microtubules in plants. Nature. 2001;411:610–613. doi: 10.1038/35079128. [DOI] [PubMed] [Google Scholar]
- Wymer C, Bibikova TN, Gilroy S. Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J. 1997;12:427–439. doi: 10.1046/j.1365-313x.1997.12020427.x. [DOI] [PubMed] [Google Scholar]