Abstract
The temporal dynamics of N remobilization was studied in walnut (Juglans nigra × regia) trees growing in sand culture. Trees were fed with labeled N (15N) during 1999 and unlabeled N in 2000. Total N and 15N contents in different tree compartments were measured during 80 d after bud burst and were used to estimate N remobilization for spring growth. The seasonal (and occasionally diurnal) dynamics of the concentration and 15N enrichment of the major amino acids in xylem sap were determined concurrently. Sap flow velocity was also measured for sample trees. A new approach coupling amino acid concentrations to sap flow velocity for quantifying N remobilization was tested. A decrease of the labeled N contents of medium roots, tap roots, and trunk was observed concurrently to the increase in the labeled N content of new shoots. Remobilized N represented from previous year storage 54% of N recovered in new shoots. Arginine, citruline, γ-amino butyric acid, glutamic acid, and aspartic acid always represented around 80% of total amino acid and amide N in xylem sap and exhibited specific seasonal trends and significant diurnal trends. N translocation was mainly insured by arginine during the first 15 d after bud burst, and then by glutamic acid and citruline. The pattern of N remobilization estimated by the new approach was consistent with that measured by the classical labeling technique. Implications for quantifying N remobilization for large, field-growing trees are discussed.
Because N is often the most limiting factor for plant growth in terrestrial ecosystems (Cole, 1981; Vitousek and Howarth, 1991), plant N economy is crucial for plant productivity and survival (Chapin et al., 1990). In contrast to annuals, perennial herbaceous and woody species can remobilize N stored during the previous years during growth in the spring (Millard, 1996; Bausenwein et al., 2001). N storage and remobilization enable perennial plants to be partially independent of external N availability for their growth (Nambiar and Fife, 1991; Millard and Proe, 1993; Millard, 1996), because remobilization of stored N supports the growth of new shoots before, or concurrently with, root uptake (Domenach and Kurdali, 1989; Millard and Proe, 1991; Neilsen et al., 1997; Millard et al., 2001). N storage occurs principally in autumn in perennial tissues such as roots and stems (Millard, 1996) in the form of bark and wood storage proteins and amino acids (Wetzel et al., 1989; Sagisaka, 1993; Stepien et al., 1994). In general, leaf growth is the strongest sink for N remobilization during spring growth, and remobilized N can reach nearly up to 90% of total N used for leaf growth (Millard, 1996; Neilsen et al., 1997).
The best approach currently available to quantify N storage and remobilization relies on labeling techniques using 15N enrichment (Millard and Neilsen, 1989) or depletion (Deng et al., 1989). Besides its cost, the applicability of this method for field grown trees has been questioned because the spatial and temporal stability of N enrichment in the rooting zone is difficult to monitor and control. Thus, no reliable, nondestructive method is presently available to directly measure N remobilization in field-growing trees.
Coupling sap flow velocity and nitrogenous compounds translocated in the xylem sap has been suggested as a means to estimate nutrient fluxes to new shoots during spring growth in field-growing trees (Millard et al., 1998). For instance, coupling sap flow velocity and mineral concentrations in xylem sap was found to provide adequate estimates of the amount of nutrients recovered in new shoots of adult spruce tree for Mg but not for Ca, P, and K (Dambrine et al., 1995). However, such an approach cannot distinguish between nutrients taken up by roots as opposed to those remobilized. Several authors have recently demonstrated that spring growth is characterized by an increase in the concentration of one or a few amino acids in xylem sap after bud break in trees. For instance, peaks of Asn, Asp, and Gln were observed after bud burst in Malus spp. (Malaguti et al., 2001), whereas peaks of citruline (Cit) were observed in Alnus spp. (Lewis, 1986), Betula spp. (Millard et al., 1998), and Juglans spp. (Prima-Putra and Botton, 1998). Using 15N labeling, the increase in the amount of Cit translocated in the xylem sap after bud break was shown to be attributable to remobilization in Betula pendula by Millard et al. (1998), who concluded that coupling the concentration of specific amino acids in xylem sap with sap velocity was a promising, nondestructive approach to measure N remobilization.
The objectives of this study were (a) to quantify the importance of N remobilization versus N uptake for new shoot growth in walnut (Juglans nigra × regia), (b) to identify specific N forms translocated in xylem sap during N remobilization, and (c) to test whether coupling the concentrations of specific amino acids in xylem sap with sap velocity could quantify N remobilization. Sand-growing walnut trees were fed with a labeled (15N) nutrient solution during year 1 and fed with unlabeled N during year 2. Xylem sap velocity and the concentrations and 15N signals of amino acids in xylem sap were surveyed during a 3-month period after bud burst in the second year. The dynamics of N remobilization estimated from this new approach were then compared with that obtained by the destructive 15N-budget technique. Implications for measuring N remobilization in large, field-growing trees are discussed.
RESULTS
Seasonal Dynamics of New Shoot Growth and N Remobilization by the 15N-Labeling Technique
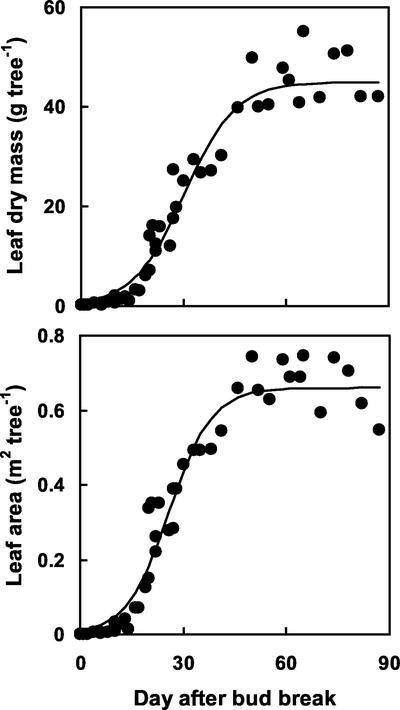
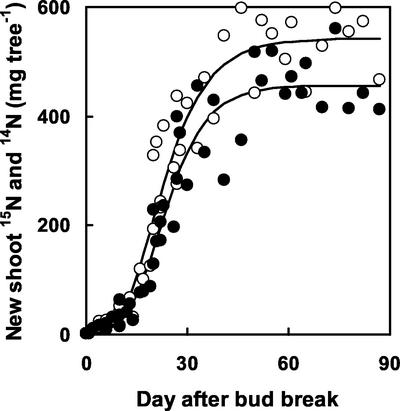
Leaf biomass and area increased exponentially during the first 20 d after bud-break and reached a maximal value of 44 g and 0.66 m2 per tree around 50 d after bud break, respectively (Fig. 1). Unlabeled and labeled N (15N) in new shoots (mainly represented by the leaves that contained around 82% of both the labeled and unlabeled N in new shoots) exhibited similar temporal dynamics (Fig. 2). N remobilization finished around 50 d after bud break and reached 520 mg N tree−1. At this time, the 14N pool in new shoots reached 440 mg tree−1. Remobilized N thus represented 54% of N recovered in new shoots.
Figure 1.
The pattern of leaf growth during 2000. Each point represents one tree at the date of harvest. Data were fitted with logistic curves: y = 0.0 + 44.9/(1 + EXP[−0.13(x − 30.1)]), n = 48, r2 = 0.96 and y = 0.0 + 0.66/(1 + EXP[−0.16(x − 25.9]), n = 48, r2 = 0.96 for leaf dry mass and leaf area, respectively.
Figure 2.
The recovery of labeled (white symbols) and unlabeled (black symbols) N with time after bud break. The 15N and 14N dataset were fitted with logistic curves: y = 0.0 + 500.5/(1 + EXP[−0.16 (x − 24.3)]), n = 48, r2 = 0.94, and y = 0.0 + 403.2/(1 + EXP[−0.19 (x − 24.3)]), n = 48, r2 = 0.96, respectively.
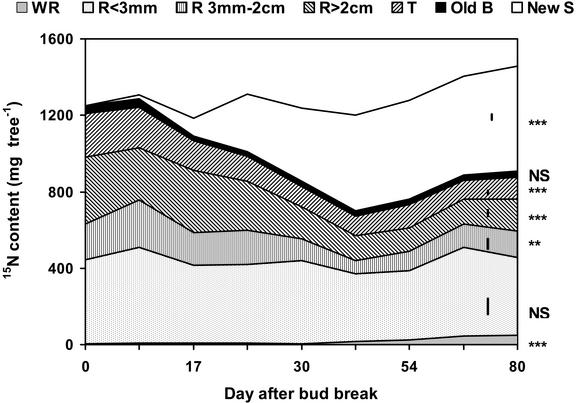
Analysis of the temporal dynamics of the 15N contents in the different tree compartments allowed a determination of which compartments were used for N storage during the winter (Fig. 3). Before bud break, labeled N was mainly located in the root system (78% of total labeled N) and to a lesser extent in old, aboveground parts (trunk and old branches). During spring growth, the 15N pools in fine roots (R ≤ 3 mm) and old branches did not change significantly over time. In contrast, the 15N pools in tap roots (R > 2 cm), medium roots (3 mm < R ≤ 2 cm), and trunk significantly decreased with time (P < 0.001), concurrently to the increase in the 15N pool in new shoots.
Figure 3.
Changes in the content of labeled N in different tree compartments (WR, new growing white roots; R <3 mm, fine roots; R 3 mm–2 cm, medium roots; R > 2 cm, tap roots; T, trunk; Old B, old branches; New S, new shoots including leaf lamina, petioles, and new branches). Values are expressed as milligrams of labeled N per tree and are means of four replicates. Bars are maximal ses for each tree compartment. Significant effects of time were observed for WR (P < 0.0001), medium roots (P = 0.002), tap roots (P < 0.0001), T (P < 0.0001), and New S (P < 0.0001).
N Translocation in Xylem Sap. Key Amino Acids and Diurnal and Seasonal Variations
N was translocated in walnut xylem sap mainly as amino acids (Table I). Five major amino acids were identified: Arg, Cit, γ-amino butyric acid (Gaba), Gln, and Asp, which represent 85% and 79% of total amino acid N in xylem sap 20 and 70 d after bud burst, respectively (Table I). Pro and Thr also represented significant amounts of N in xylem sap. Total amino acid N concentration observed around 20 d after bud break (39 μg N g−1 xylem sap) was 6-fold higher than that observed 70 d after bud break. This trend was observed for all amino acids, although the decrease was less marked for Gaba and Asp than for the other amino acids (Table I).
Table I.
Comparison of the recovery of N in amino acids in the xylem sap of walnut trees harvested 20 and 70 d after bud burst (BB)
| Amino Acid | Amino Acid Concentration (μg N g−1 xylem sap)
|
|
|---|---|---|
| 20 d after BB | 70 d after BB | |
| Ala | 0.44 (0.12) | 0.14 (0.02) |
| Gly | 0.20 (0.06) | 0.07 (0.01) |
| Val | 0.39 (0.15) | 0.12 (0.04) |
| Leu | 0.10 (0.02) | 0.05 (0.01) |
| Ile | 0.15 (0.06) | 0.05 (0.01) |
| Gaba | 6.33 (3.04) | 2.66 (0.42) |
| Pro | 1.24 (0.29) | 0.21 (0.07) |
| Ser | 0.51 (0.10) | 0.16 (0.04) |
| Thr | 1.20 (0.38) | 0.46 (0.14) |
| Phe | 0.09 (0.03) | 0.02 (0.005) |
| Asp | 2.79 (1.62) | 0.54 (0.16) |
| Glu | 0.28 (0.04) | 0.07 (0.02) |
| Cit | 13.56 (6.31) | 0.78 (0.35) |
| Asn | 0.30 (0.05) | 0.06 (0.02) |
| Gln | 4.56 (1.65) | 0.61 (0.27) |
| Arg | 6.95 (5.53) | 0.52 (0.18) |
| Total | 39.1 (9.82) | 6.51 (1.37) |
Means are given for three and four trees for the two dates, respectively. Values between brackets are ses.
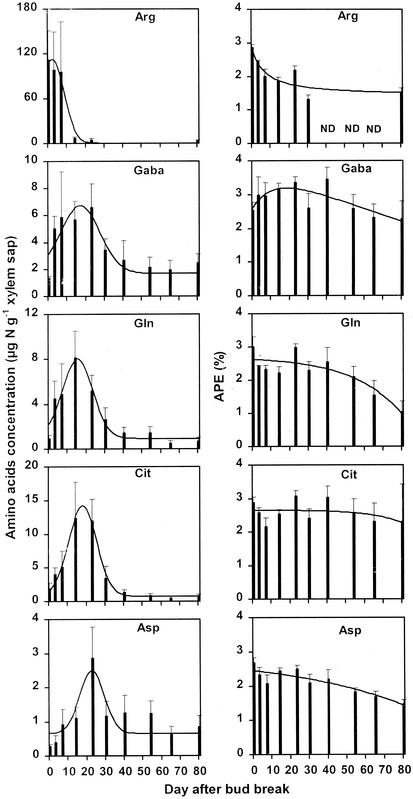
Arg appeared in xylem sap in the very first days after bud break at very high concentration (up to 110 μg N g−1 xylem sap) compared with the other amino acids (always less than 14 μg N g−1 xylem sap; Fig. 4). The concentration of this amino acid remained high only during the first 10 d after bud burst, and after 25 d, it was around 5 μg N g−1 xylem sap. At each sampling date, there was also a large variation in the concentration of Arg recovered, which was attributable to the natural variation between replicate samples, the precision of the analysis of standards being better than 1 μg N g−1 xylem sap. Uncertainty in the determination of first bud burst occurrence for each tree could partly explain this, because an error of a few days could lead to significant differences in the concentration of this amino acid (Fig. 4). Gln, Cit, and Asp exhibited maximum concentration values around 15, 17, and 22 d after bud break, respectively, whereas low values (around 1 μg N g−1 xylem sap) were observed just after bud burst and after d 40. In contrast, Gaba-N concentration exhibited a less pronounced peak through time, with relatively high values (>2 μg N g−1 xylem sap) observed just after bud burst and after d 40 compared with maximum values (6.7 μg N g−1 xylem sap). During the first 40 d after bud burst, the APE of each amino acid remained quite stable and thereafter declined for Gln and Asp (Fig. 4).
Figure 4.
Temporal courses of the concentration and atom % excess (APE) of the five major amino acids present in xylem sap of walnut. Values are means of four trees sampled during 4-d periods. Bars are ses. Lines are the fitted gaussians, the form of which is: y = a + b/[(2*Π)0.5 * s] * exp{[−(x − m)2/2s2). R2 values are 0.96, 0.67, 0.90, 0.97, and 0.58 for Arg, Gaba, Gln, Cit, and Asp, respectively. For concentration, note the differences of scale between amino acids.
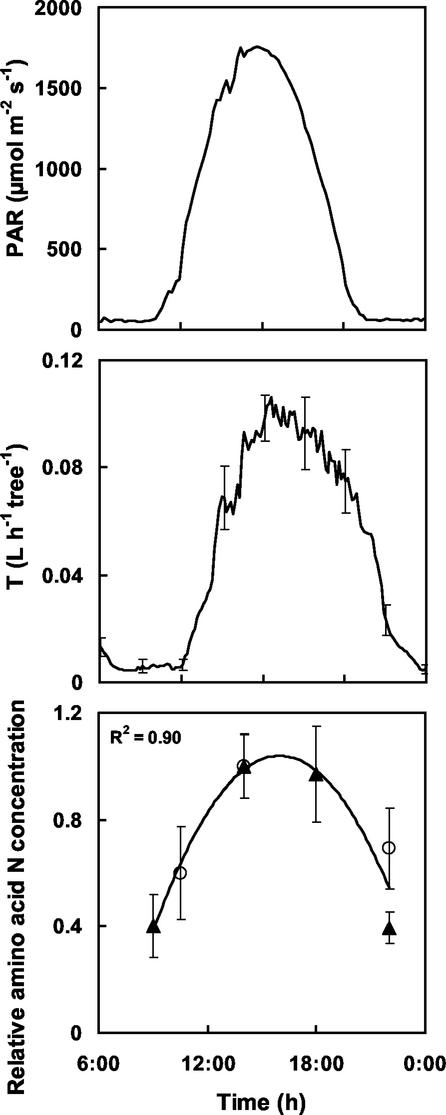
The diurnal courses of incident radiation (PAR) and mean sap flow velocity measured on a sample day (16 June 2000) are shown in Figure 5. Transpiration rate increased with a slight delay compared with PAR, from 0.01 dm3 h−1 tree−1 in the early morning to maximal values around 0.11 dm3 h−1 tree−1 at noon. During the diurnal period, amino acid-N concentration in xylem sap increased concurrently with the transpiration rate (Fig. 5), and similar diurnal dynamics were observed for the five major amino acids but with slightly different amplitude (data not shown). Thus, a correction factor was used for each amino acid to compute N flux in xylem sap.
Figure 5.
Diurnal courses of incident photosynthetically active radiation (PAR) measured on June 16 (top), transpiration rate (T) measured on June 16 (middle), and the relative concentration of total N-amino acids in xylem sap measured on May 27 (▴) and June 16 (○; bottom). T values are means of sap flow measurements on four trees. Concentrations are means for four trees. Relative concentration refers to the ratio of actual to maximum diurnal values. Bars are ses and are presented only every 3 h for sake of legibility. The fitted polynomial is y = −0.009x2 + 0.29x − 1.49.
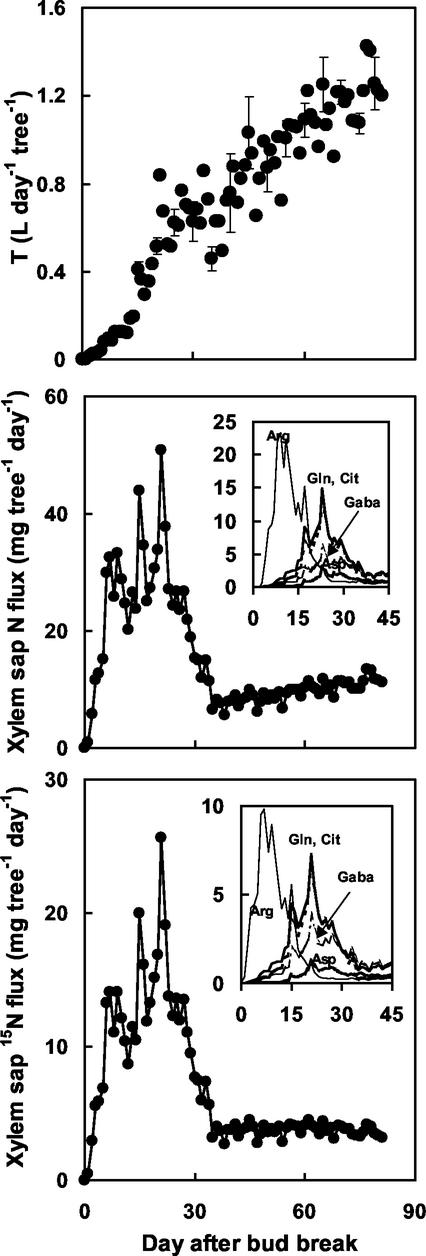
Coupling of the temporal trends in amino acid concentrations and APE with the temporal course of transpiration rate per tree was used to compute daily values for total and labeled N translocated in xylem sap (Fig. 6). Both fluxes exhibited similar temporal courses, with maximal values from d 6 to 28 after bud burst and a strong decrease from d 29 to 35. N fluxes then remained slow and stable. Translocation of both total and labeled N was mainly insured by Arg during the first 15 d after bud burst and by Gln and Cit from d 15 to 30 (Fig. 6).
Figure 6.
Temporal courses of measured daily transpiration rate per tree, T (top), computed total N flux in xylem sap (middle), and computed 15N flux in xylem sap (bottom). Insets, The fluxes computed for individual amino acids. Bars for T are ses.
Comparison of N Remobilization by the Two Methods
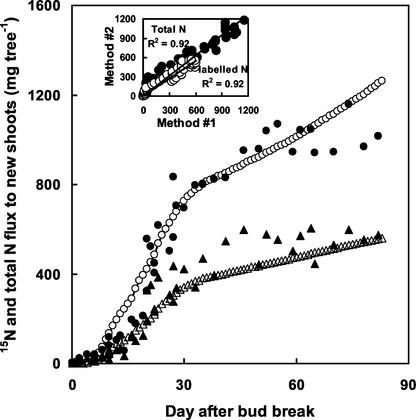
The temporal pattern of total and labeled N fluxes to new shoots estimated from coupling amino acid concentrations and sap flow velocity was in agreement with the patterns of total and 15N recovery measured by the 15N labeling technique (Fig. 7). However, the total N flux to new shoots estimated by the new method was higher than the recovery of labeled N in shoots from d 10 to 20. In addition, after d 50, the 15N and total N pools in the new shoots observed by the recovery of labeled N remained stable, whereas positive fluxes to new shoots were observed in the xylem.
Figure 7.
Temporal dynamics of the measured total N content in new shoots (●) and the total N flux in xylem sap computed by the new method (○) and of the measured 15N content in new shoots (▴) and the 15N flux in xylem sap computed by the new method (▵). Insets, The linear regression between values measured by 15N technique (method 1) and by new approach (method 2).
DISCUSSION
Importance of N Remobilization for Spring Growth in Walnut
Spring remobilization of N stored during the previous year played an important role for new shoot growth in walnut. When shoot growth was completed, N remobilization had provided around 54% of total N to new shoots. This result is consistent with the values of 60% estimated for larger walnut trees grown in the orchard by Weinbaum and Van Kessel (1998), although higher values of 75% have also been reported for field-growing walnut trees by Deng et al. (1989). Similar percentages of N derived from remobilization for new growth were found in young pear (Pyrus communis) trees (Taglivani et al., 1997), in kiwifruit (Actinidia deliciosa) in orchard conditions (Ledgard and Smith, 1992), and in B. pendula, 40% (Millard et al., 1998). Higher fractions were reported for citrus trees growing in sand culture (Legaz et al., 1995), apple (Malus domestica) trees (Neilsen et al., 1997), and peach (Prunus persica) trees (Munoz et al., 1993). It should be noted that we estimated the importance of remobilization of N stored during the previous year only. However, our trees already had reserve organs (tap root and trunk mainly) in early 1999, and N stored during a given year can be used 2 years after, as observed in walnut (Weinbaum and Van Kessel, 1998). Thus, the percentage of total N derived from remobilization for new shoot growth in walnut was probably higher than 54%. This also explains why the APE of amino acids in xylem sap was lower than that of fertilizer applied the previous year.
During growth in the spring, N uptake and remobilization were concurrent. In contrast, N remobilization was found to provide more N to new shoots during the early growing season, and N uptake then became predominant in sycamore (Acer pseudoplatanus), B. pendula, and Sorbus aucuparia (Millard and Proe, 1991; Millard et al., 1998, 2001). This shows that new growth is less decoupled to soil N availability during the early growing season in walnut than the other species.
Different tree organs can be used as storage organs for N during the winter. For instance, old leaves can store N (partly as Rubisco) in evergreen trees, and recycling of N from old needles provides much of N for new growth (Millard and Proe, 1992; Legaz et al., 1995). In deciduous trees, woody roots and old stems are generally major storage organs (Munoz et al., 1993; Millard et al., 1998) where bark- and wood-storage proteins are accumulated (Sauter and Neumann, 1994; Stepien et al., 1994). In the present study, the increase in the labeled N content (i.e. derived from remobilized N) in new shoots was concurrent with a decrease in the labeled N recovered in the trunk and in coarse and medium roots (>3 mm). The pool of labeled N in the fine roots did not change significantly with time, demonstrating that they were not a site used for N storage during the winter, as was already observed for B. pendula (Millard et al., 1998).
Characteristics of N-Translocation in the Xylem
Throughout the experiment, N was translocated in the xylem sap predominantly as Arg, Cit, Gaba, Gln, and Asp, whereas mineral N was found to be negligible. Using data obtained in summer on 60 tree species, Barnes (1963) found that Gln, Cit, Arg, and Asp were the main amino acids in xylem sap of 16, 13, 7, and 7 species, respectively. In particular, Cit was the main amino acid in J. nigra. Cit (and to a lesser extent Gln) was similarly the major amino acid in J. regia (Prima-Putra and Botton, 1998). In contrast to such point measurements, comprehensive studies on the seasonal course of amino acid concentrations in xylem sap are less frequent. Cit and Gln are the major amino acids in xylem sap during spring growth in B. pendula (Millard et al., 1998), whereas Gln remains the major amino acid in xylem sap during the whole vegetative period in poplar (Populus spp.; Sauter and Vancleve, 1992; Schneider et al., 1994) and willow (Salix spp.; Sauter, 1981). Asn, Asp, and Gln were found to be translocated in xylem sap during spring growth in apple trees (Malaguti et al., 2001). In our study, five amino acids accounted for the majority of N translocation in xylem sap. To our knowledge, such a case has not been reported previously. Furthermore, there was a strong seasonal variation in amino acid concentrations, which differed significantly between individual amino acids. In particular, Arg exhibited high concentrations very early after bud burst, whereas Gln, Cit, and Asp exhibited maximum concentration values at later dates. Gaba concentrations also peaked during N remobilization, although the subsequent decrease in concentration was not as pronounced as for Arg, Gln, and Cit. There is evidence from herbaceous plants that Gaba has a role as a temporary N storage compound, as well as being synthesized in response to stress (Bown and Shelp, 1997; Shelp et al., 1999). It is possible, therefore, that the presence of Gaba was due, in part, to a stress response by the trees to being handled.
Our results could explain why point measurements (for example taken around d 23 in our study) would identify Cit as the major amino acid in walnut. The observed differences in temporal variations among individual amino acids contrast with previous results on apple (Tromp and Ovaa, 1969), where the three major amino acids exhibited similar seasonal variations in spring. To our knowledge, only the results of Sauter and Vancleve (1992) showed that the concentration peak for one relatively minor amino acid (Asn) occurred 1 month later than the peaks for the three other amino acids (including Gln, the major one). The present study is the first, to our knowledge, to demonstrate a temporal succession of the main amino acids involved in N remobilization. Our results show the importance of the time step used when measuring temporal trends in amino acid concentrations in xylem saps and the importance of documenting accurately processes occurring very early (i.e. during the 1st week after bud burst) for understanding N remobilization in some species.
Toward a New Method for Assessing N Remobilization?
The amount of N remobilization, estimated by the approach of coupling sap flow velocity and the concentration of N in the main amino acids translocated in xylem sap, was in agreement with that measured by the recovery of labeled in the new shoots. However, during early growth (between 10 and 20 d after bud break), the new method overestimated total N and to a lesser extent remobilized N to new shoots. Also, the new method calculated significant total N and remobilized N fluxes to new shoots after leaf growth was completed (after 60 d), whereas the total and labeled N contents of new shoots remained constant. The new method estimated gross N fluxes, whereas the 15N labeling technique estimated net fluxes. Given the importance of retranslocation of N in the phloem from shoots to roots (Marschner et al., 1997) and the rapid turnover of leaf labile N pools (Dewar et al., 1998; Frak et al., 2001), the two methods should be consistent when new shoots are the strongest N sinks and when N retranslocation in the phloem is negligible compared with the flux in the xylem (i.e. before completion of new shoot growth).
Both approaches provided similar amounts of N remobilized at the end of growth in the spring (i.e. around 60 d after bud break). The data suggest, therefore, that measuring amino acid fluxes in the xylem could be used for quantification of N remobilization by large, field-growing trees. However, there are some points that would need addressing before the method could be applied with confidence.
First, the method is based on the hypothesis that one or several amino acids are specific of remobilization. This would have to be verified for a given species by a labeling technique (Millard et al., 1998). In our experiment, stored N was labeled during 1999 by using a nutrient solution with 15N enriched at 4.98%. However, the enrichment of the five amino acids involved in N remobilization in 2000 was lower (between 3.5 and 2 APE). A first hypothesis is that these amino acids were not specific of N remobilization and that a significant proportion of the flux of each amino acid came from root uptake. A second hypothesis is that the amino acids were specific to N remobilization, but that remobilization of N taken up in 1999 (hence labeled N) and in 1998 (hence unlabeled N) occurred concurrently. The latter hypothesis is supported by the fact that Weinbaum and Van Kessel (1998) demonstrated that N assimilated in a given year can be remobilized 2 years later in walnut. In this case, changes in APE during the 80 d after bud burst could be attributable to changes in the relative importance of remobilization from labeled N taken up in 1999 and unlabeled N taken up in 1998.
Second, the diurnal variations in amino acid concentrations should be quantified and taken into account when necessary. The quantification of N fluxes was found to be sensitive to the diurnal dynamics of amino acids in the present study. Such diurnal variations make difficult the use of a unique value of amino acid concentration measured at a given time to estimate N fluxes without correcting factors. Very few studies have quantified diurnal changes in amino acid concentrations. Weak diurnal variations were observed in total amino acid concentration in Vitis rotundifolia (Andersen and Brodbeck, 1989), Vitis hybrid sp., and pear (Pyrus communis; Andersen et al., 1995), whereas marked variations were observed for four amino acids (Asn, Asp, Gln, and Glu) in the xylem sap of peach (Andersen et al., 1995). The presence of any diurnal pattern of amino acid concentrations in the xylem would, therefore, need to be evaluated.
Finally, given the importance of the N translocation observed in xylem sap immediately after bud burst, sap flow velocity needs to be measured accurately, when leaf area is small and shoot transpiration rates are slow. In walnut, the most important variations in N concentration in xylem sap were observed during the first 20 d after bud break. However we could not measure sap velocity properly with the heat balance system before leaf area was higher than 0.1 m2 (transpiration was measured by weighing before then). Improvements of the techniques able to measure a slow sap flow (such as the heat pulse technique) are thus important for the application of the new method.
However, despite the methodological points discussed above, coupling amino acid concentrations and sap flow velocity already appears to be a promising tool for studying N remobilization in large, field-growing trees.
MATERIALS AND METHODS
Plant Material and Experimental Design
Forty eight, 1-year-old walnut (Juglans nigra × regia) trees were planted in March 1999 in 35-dm3 pots filled with fine sand. The apical bud of each plant was removed to stimulate auxiliary buds and branch development. Plants were watered weekly and kept frost-free until bud break. In late April, the trees were transferred outdoors and randomized in four blocks. Each tree received 500 cm3 of nutrient solution (8 mol N m−3) three times per week. N was applied as 15NH415NO3 enriched with 15N to 4.98 APE. Other nutrients were supplied as described by Millard and Proe (1991). Depending on evaporative demand, the trees were watered automatically during the other days. In early November 1999, the trees were transferred under a shelter and kept moist and frost-free during winter. In February 2000, each plant was carefully removed from its pot, and its root system was washed to remove any remaining sand. The trees were then transplanted in a new pot with fresh sand and kept sheltered until April. The nutrient solution supplied in 2000 had the same composition as in 1999 but contained a natural abundance of 15N. In spring, the number of open buds was surveyed daily and the day of the first open bud was designed as start of bud burst.
Tree Organ Dry Masses and 15N Content
Trees were harvested at 12 dates (April 21; May 3, 12, 18, 23, and 29; June 5, 13, 19, and 30; and July 13 and 21). At each date, one plant from each block was carefully removed from its pot. The root system was washed and sorted into four categories: R ≤ 3 mm, 3 mm < R ≤ 2 cm, R > 2 cm and newly grown white roots. Tree aboveground parts were separated into old branches, trunk, and new shoots of the year, the latter including new stems, leaf laminas, and leaf petioles. The total fresh leaf area of each tree was measured with an area meter (LI-3100, LI-COR, Lincoln, NE). All samples were frozen with liquid N, stored at −80°C, and then freeze-dried. Their dry mass was measured, and the samples were milled before 15N analyses. A Tracer Mat continuous flow mass spectrometer (Finningan MAT, Hemel Hempstead, UK) was used to determine the 15N abundance and total N concentration of each sample.
Xylem Sap Collection and Amino Acid Analysis
Xylem sap was collected on branches cut at 9 h on each sampled tree. Only the biggest branches (around 20 cm long) were used (two–three branches according to the tree). Around 10 cm of bark from the apical part of branch was removed to avoid any contamination with phloem sap. Branches were placed in a vacuum extraction system allowing simultaneous extractions from the different branches by applying 0.1 MPa suction. Sap samples were collected in glass tubes and placed on ice. Sap samples were pooled together when several branches were used from the same tree. Lack of contamination of xylem by phloem sap, as indicated by the presence of ATP (Schneider et al., 1996), was checked on subsamples using the Luciferine-luciferase reagent kit (ATP bioluminescence assay kit CLS II, Roche Diagnostics, Mannheim, Germany).
Xylem sap samples were stored at −80°C. Particulate material was removed by centrifugation in an MSE Micro-Centaur centrifuge for 5 min at 5,800g. Samples (20 mg) were then diluted with 0.5 cm3 demineralized water and a 100-mm3 aliquot of the dilute sap along with an internal standard of nor-Val (25 mm3 containing 0.18 μg) were added to a 1-CWV clear glass crimp top tapered vial (Chromacol Ltd., Welwyn Garden City, UK) and freeze dried. The derivatization reagent (100 mm3), consisting of N-(tert-butyldimethylsilyl)-N-methyltrifluoroacetamide containing 1% (w/v) tert-butyldimethylsilyl chloride (Sigma-Aldrich, Gillingham, UK) in acetonitrile (1:4, v/v), was added to the dried material and left at room temperature for 10 min. The solution was then heated at 70°C for 35 min to convert the free amino acids to their tert-butyldimethylsilyl derivatives. The analyses of the derivatives were carried out using gas chromatography/mass spectrometry (GC/MS) in the single-ion recording mode. The instrumentation used was a Trace 2000 gas chromatograph, fitted with an AS 2000 autosampler and interfaced to a Finnigan Trace quadrupole mass spectrometer (ThermoFinnigan, Hemel Hempstead, UK).
Arg was determined as the N-heptafluorobutryl n-butyl ester (MacKenzie and Tenaschuk, 1979). Xylem sap samples (10 mg) containing nor-Val (50 mm3 containing 0.36 μg) as internal standard were freeze-dried and esterified by heating at 100°C for 30 min in 120 nm−3 reagent (acetyl chloride:n-butanol, 1:10, v/v). After evaporating excess reagent, the residue was acylated for 10 min at 150°C using 120 nm−3 heptafluorobutyric anhydride. After cooling, the excess reagent was evaporated, the residue was dissolved in 100 mm−3 ethyl acetate, and the solution was analyzed using GC/MS. The derivatives were analyzed by GC/MS.
The enrichment of 15N in individual amino acids was calculated from the ratio of the ion monitored at natural abundance and enriched amino acids (Campbell, 1974). Amino acid concentrations were calculated using response factors obtained from the analysis of solutions containing known weights of amino acids. Quality control was assured by analyzing standard solutions of amino acids.
Diurnal Course of Amino Acid Concentrations
The diurnal dynamics of the concentration of amino acids in xylem sap was surveyed at two dates (May 27 and June 16). On each date, xylem sap was extracted from branches of four additional walnut trees at four or three times during the diurnal period. The samples were frozen in liquid N, stored in −80°C, and analyzed for five major amino acids by GC/MS.
Measurements of Sap Velocity
Two methods were used to estimate tree transpiration: weighing and heat balance. The plant plus pot system was weighed daily on 12 trees to measure transpiration from bud burst until the day of harvest. The sand surface was covered to restrict evaporation. Water and nutrient solution inputs were controlled manually and added water was weighed. The heat balance method (Valancogne and Nasr, 1989) was concurrently applied on four of these trees. A sap flow meter was installed on the trunk of each tree. Thermocouples were connected to a data logger scanning and averaging data every 15 s and every 10 min, respectively. When leaf area was less than 0.1 m2 per tree, the heat balance technique could not provide accurate transpiration measurements and so transpiration rates were measured by the weighing method. Concurrent measurements of daily transpiration by both methods were close (y = 0.96x, n = 60, r2 = 0.82).
During the whole study period, the leaf area dynamics were measured on the 12 trees by surveying the size of each leaflet and using an allometric relationship (leaflet area = 0.705 × length × width, n = 50, r2 = 0.98).
Quantification of N Remobilization
N remobilization was quantified by two independent methods. In the first one, the amount of N taken up and stored in 1999 and remobilized in 2000 was measured by the recovery of 15N pool in the new shoot compartment (Millard and Neilsen, 1989). Because N remobilized during spring growth is translocated in xylem sap as free amino acids, the second method used amino acid concentration and 15N enrichment coupled with sap flow velocity measurements. We took the diurnal and seasonal variations in amino acid concentration and 15N enrichment into account. The variations in relative concentration (i.e. ratio of actual to maximum concentrations) measured on May 27 and June 16 were fitted for each main amino acid present in xylem sap (Gaba, Asp, Cit, Gln, and Arg) with a second order polynomial. In addition, the seasonal variation in concentration measured at 9 am was fitted for each amino acid with gaussian curves. The fluxes of total and labeled N to new shoots were computed as follows:
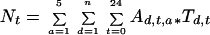 |
1 |
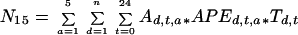 |
2 |
where Nt and N15 are total N and 15N translocated in xylem sap over a period of n days after bud burst (mg N tree−1 and mg 15N tree−1), respectively, Ad,t,a is the N concentration in amino acid a at a given time t of date d (micrograms per gram of xylem sap), Td,t is the measured sap flow velocity at a time t of date d (kilograms per tree per hour), and APEd,t,a is the 15N to total N ratio computed from the APE for amino acid a at a given time t of date d. No diurnal course of APE was actually observed for the amino acids studied and a unique APE value was thus used along a given diurnal period for each amino acid.
Data Fitting and Statistical Analyses
Data for leaf growth (dry mass and area) and temporal increase in new shoot 15N and 14N were fitted with logistic curves by using the Genstat 5 standard curve fitting procedure. The same procedure was used to fit the seasonal patterns of amino acid N concentrations with gaussian curves. The diurnal dynamics of amino acid N concentration in xylem sap was fitted with polynomial function.
Effects of date of harvest on labeled N pools in different tree compartments and in the whole tree, and on amino acid N concentration and N enrichment were tested with a one way analysis of variance.
ACKNOWLEDGMENTS
We thank Stéphane Ploquin and Patrice Chaleil for tree management; Thierry Améglio and Christian Bodet (Physiologie Intégrée de l'Arbre Fruitier et Forestier [PIAF], Institut National de la Recherche Agronomique, Clermont Ferrand) for help during sap flow measurements; Marc Vandame, Brigitte Saint-Joanis, Jean-Pierre Richard, and Arlette Cissoire (PIAF, Institut National de la Recherche Agronomique, Clermont Ferrand) for help during tree sampling and analysis; and Alan Hepburn (Analytical Unit, Macaulay Institute, Aberdeen) for GC/MS analyses.
Footnotes
This work was supported as part of the Twinning Agreement between Institut National de Recherche Agronomique (INRA) and the Macaulay Land Use Research Institute. The Macaulay Institute receives grant-in-aid funding from the Scottish Executive Environment, Agriculture, and Rural Affair Department. E.F., X.L.R., P.M., and R.W. were supported by the Alliance program (no. 99–120). The PhD grant of E.F. was funded by INRA and the Auvergne region.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.002139.
LITERATURE CITED
- Andersen PC, Brodbeck BV. Diurnal and temporal changes in the chemical profile of xylem exudate from Vitis rotundifolia. Physiol Plant. 1989;75:63–70. [Google Scholar]
- Andersen PC, Brodbeck BV, Mizell RF. Diurnal-variations in tension, osmolarity, and the composition of nitrogen and carbon assimilates in xylem fluid of Prunus persica, Vitis Hybrid, and Pyrus communis. J Am Soc Hortic Sci. 1995;120:600–606. [Google Scholar]
- Barnes RL. Organic nitrogen compounds in tree xylem sap. For Sci. 1963;9:98–102. [Google Scholar]
- Bausenwein U, Millard P, Thornton B, Raven JA. Seasonal nitrogen and remobilization in the forb Rumex acetosa. Funct Ecol. 2001;15:370–377. [Google Scholar]
- Bown AW, Shelp BJ. The metabolism and functions of aminobutyric acid. Plant Physiol. 1997;115:1–5. doi: 10.1104/pp.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IM. Incorporation and dilution values: their calculation in mass spectrally assayed stable isotope labelling experiments. Bioorg Chem. 1974;3:386–397. [Google Scholar]
- Chapin FS, Schulze ED, Mooney HA. The ecology and economics of storage in plants. Annu Rev Ecol Syst. 1990;21:423–447. [Google Scholar]
- Cole DW. Nitrogen uptake and translocation by forest ecosystems. Ecol Bull. 1981;33:219–232. [Google Scholar]
- Dambrine E, Martin F, Carisey N, Granier A, Hallgren JE, Bishop K. Xylem sap composition: a tool for investigating mineral uptake and cycling in adult spruce. Plant Soil. 1995;169:233–241. [Google Scholar]
- Deng X, Weinbaum SA, DeJong TM, Muraoka TT. Utilization of nitrogen from storage and current-year uptake in walnut spurs during the spring flush of growth. Physiol Plant. 1989;75:492–498. [Google Scholar]
- Dewar RC, Medlyn BE, McMurtrie RE. A mechanistic analysis of light and carbon use efficiencies. Plant Cell Environ. 1998;21:573–588. [Google Scholar]
- Domenach AM, Kurdali F. Influence of nitrogen reserves on leaf formation in Alnus glutinosa and its consequences in estimating nitrogen-fixation. Can J Bot. 1989;67:865–871. [Google Scholar]
- Frak E, Le Roux X, Millard P, Dreyer E, Jaouen G, Saint-Joanis B, Wendler R. Changes in total leaf nitrogen and partitioning of leaf nitrogen drive photosynthetic acclimation to light in fully developed walnut leaves. Plant Cell Environ. 2001;24:1279–1288. [Google Scholar]
- Ledgard SF, Smith GS. Fate of 15N-labelled nitrogen fertilizer applied to kiwifruit (Actinidia deliciosa) vines. Plant Soil. 1992;147:59–68. [Google Scholar]
- Legaz F, Serna MD, Primo-Millo E. Mobilisation of the reserves N in citrus. Plant Soil. 1995;173:205–210. [Google Scholar]
- Lewis OAM. Plant and Nitrogen: Studies in Biology. Baltimore: Arnold E; 1986. [Google Scholar]
- MacKenzie SL, Tenaschuk D. Quantitative formation of N (O,S)-heptafluorobutyryl amino acids for gas chromatographic analysis: II. Acylation J Chromatogr. 1979;173:53–63. [Google Scholar]
- Malaguti D, Millard P, Wendler R, Hepburn A, Tagliavini M. Translocation of amino acids in the xylem of apple (Malus domestica Borkh.) trees in spring as a consequence of both N remobilization and root uptake. J Exp Bot. 2001;52:1665–1671. [PubMed] [Google Scholar]
- Marschner H, Kirkby EA, Engels C. Importance of cycling and recycling of mineral nutrients within plants for growth and development. Bot Acta. 1997;110:265–273. [Google Scholar]
- Millard P. Ecophysiology of the internal cycling of nitrogen for tree growth. Z Pflanzen Boden. 1996;159:1–10. [Google Scholar]
- Millard P, Hester A, Wendler R, Baillie G. Interspecific defoliation responses of trees depend on sites of winter nitrogen storage. Funct Ecol. 2001;15:535–543. [Google Scholar]
- Millard P, Neilsen GH. The influence of nitrogen supply on the uptake and remobilization of stored N for the seasonal growth of apple trees. Ann Bot. 1989;63:301–309. [Google Scholar]
- Millard P, Proe MF. Leaf demography and the seasonal internal cycling of nitrogen in sycamore (Acer pseudoplatanus L.) seedlings in relations to nitrogen supply. New Phytol. 1991;117:587–596. [Google Scholar]
- Millard P, Proe MF. Storage and internal cycling of nitrogen in relation to seasonal growth of Sitka spruce. Tree Physiol. 1992;10:33–43. doi: 10.1093/treephys/10.1.33. [DOI] [PubMed] [Google Scholar]
- Millard P, Proe MF. Nitrogen uptake, partitioning and internal cycling in Picea sitchensis (Bong) Carr as influenced by nitrogen supply. New Phytol. 1993;125:113–119. doi: 10.1111/j.1469-8137.1993.tb03869.x. [DOI] [PubMed] [Google Scholar]
- Millard P, Wendler R, Hepburn A, Smith A. Variations in the amino acid composition of xylem sap of Betula pendula Roth. trees due to remobilization of stored N in the spring. Plant Cell Environ. 1998;21:715–722. [Google Scholar]
- Munoz N, Guerri J, Legaz F, Primo-Millo E. Seasonal uptake of 15N-nitarate and distribution of absorbed nitrogen in peach trees. Plant Soil. 1993;150:263–269. [Google Scholar]
- Nambiar EKS, Fife DN. Nutrient retranslocation in temperate conifers. Tree Physiol. 1991;9:185–207. doi: 10.1093/treephys/9.1-2.185. [DOI] [PubMed] [Google Scholar]
- Neilsen D, Millard P, Neilsen GH, Hogue EJ. Sources of N for leaf growth in a high-density apple (Malus domestica) orchard irrigated with ammonium nitrate solution. Tree Physiol. 1997;17:733–739. doi: 10.1093/treephys/17.11.733. [DOI] [PubMed] [Google Scholar]
- Prima-Putra D, Botton B. Organic and inorganic compounds of xylem exudates from five woody plants at the stage of bud breaking. J Plant Physiol. 1998;153:670–676. [Google Scholar]
- Sagisaka S. Amino-acid metabolism in nongrowing environment in higher plants. Amino Acids. 1993;4:141–155. doi: 10.1007/BF00805810. [DOI] [PubMed] [Google Scholar]
- Sauter JJ. Seasonal variation of amino acids and amides in the xylem sap of Salix. Z Pflanzen Boden. 1981;101:399–411. [Google Scholar]
- Sauter JJ, Neumann U. The accumulation of storage materials in ray cells of poplar wood (Populus × Canadensis robusta): effect of ringing and defoliation. J Plant Physiol. 1994;143:21–26. [Google Scholar]
- Sauter JJ, Vancleve B. Seasonal variation of amino-acids in the xylem sap of Populus × canadensis and its relation to protein body mobilization. Trees. 1992;7:26–32. [Google Scholar]
- Schneider A, Gessler A, Weber P, von Sengbusch D, Hanemann U, Rennenberg H. Soluble N compounds in trees exposed to high loads of N: a comparison of spruce (Picea abies) and beech (Fagus sylvatica) grown under field conditions. New Phytol. 1996;134:103–114. [Google Scholar]
- Schneider A, Kreuzwieser J, Schupp R, Sauter JJ, Rennenberg H. Thiol and amino-acid-composition of the xylem sap of poplar trees (Populus × Canadensis robusta) Can J Bot. 1994;72:347–351. [Google Scholar]
- Shelp BJ, Bown AW, McLean MD. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 1999;4:446–452. doi: 10.1016/s1360-1385(99)01486-7. [DOI] [PubMed] [Google Scholar]
- Stepien V, Sauter JJ, Martin F. Vegetative storage proteins in woody plants. Plant Physiol Biochem. 1994;32:185–192. [Google Scholar]
- Tagliavini M, Quartieri M, Millard P. Remobilised nitrogen and root uptake of nitrate for spring leaf growth, flowers and developing fruits of pear (Pyrus communis L.) trees. Plant Soil. 1997;195:137–142. [Google Scholar]
- Tromp J, Ovaa JC. The effect of nitrogen application on the seasonal variations in the amino acid composition of xylem sap of apple. Z Pflanzen Boden. 1969;60:232–241. [Google Scholar]
- Valancogne C, Nasr Z. A heat-balance method for measuring the sap flow in small trees. Agronomie. 1989;9:609–617. [Google Scholar]
- Vitousek PM, Howarth RW. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry. 1991;13:87–115. [Google Scholar]
- Weinbaum SA, Van Kessel C. Quantitative estimate of uptake and internal cycling of 14N-labelled fertilizer in mature walnut trees. Tree Physiol. 1998;18:795–801. doi: 10.1093/treephys/18.12.795. [DOI] [PubMed] [Google Scholar]
- Wetzel S, Demmers C, Greenwood JS. Seasonally fluctuating bark proteins are a potential form of nitrogen storage in three temperate hardwoods. Planta. 1989;178:275–281. doi: 10.1007/BF00391854. [DOI] [PubMed] [Google Scholar]