Abstract
1. The responses to non-adrenergic, non-cholinergic nerve stimulation have been compared with those to exogenously applied ATP on seventeen different tissues from a number of vertebrate classes.
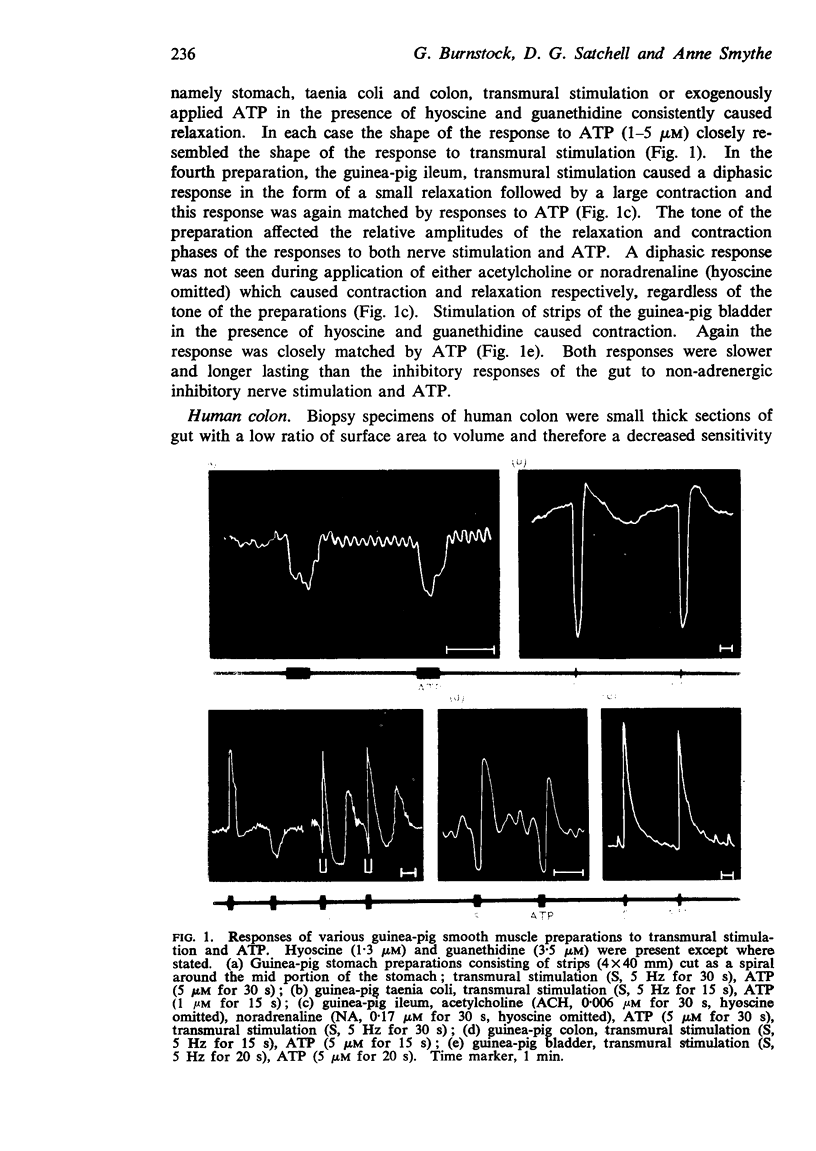
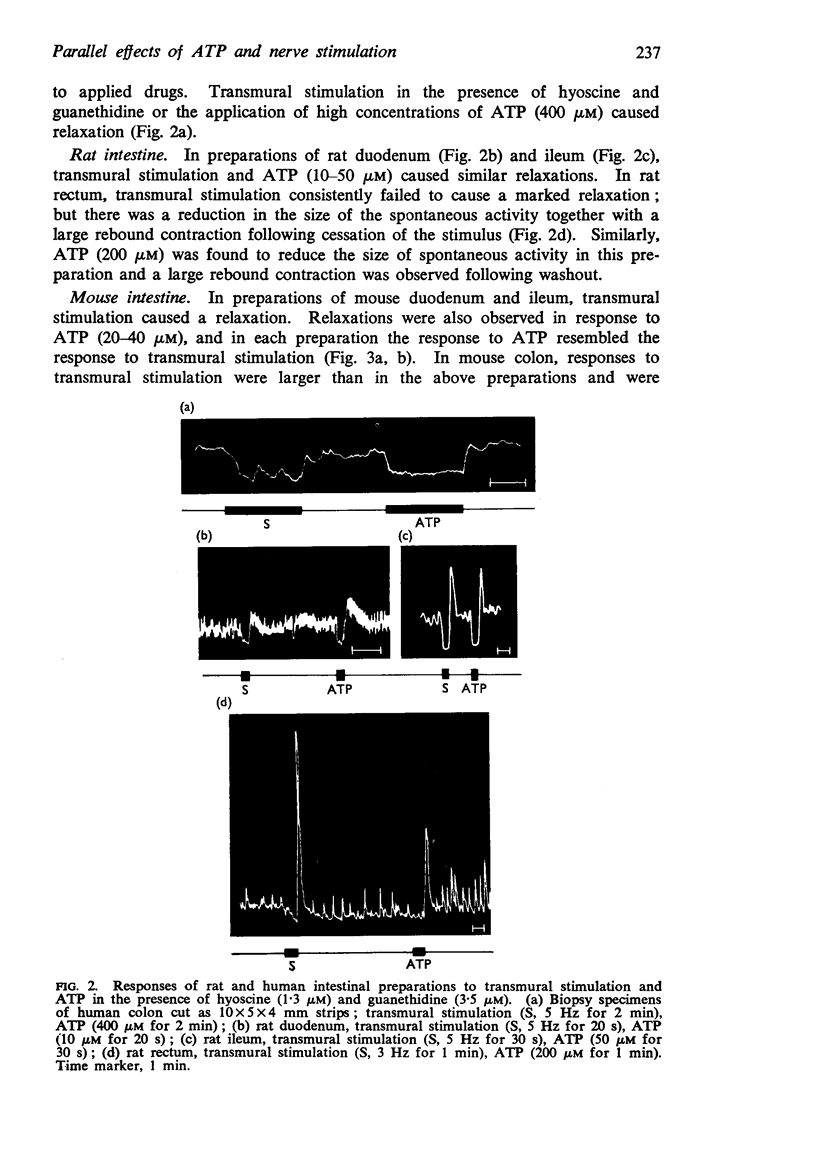
2. Stimulation of all the mammalian gut preparations studied (with the exception of the guinea-pig ileum) after blockade of the effects of adrenergic and cholinergic nerve stimulation by guanethidine (3·5 μM) and hyoscine (1·3 μM) caused inhibition; exogenously applied ATP mimicked this inhibitory response.
3. Stimulation of the guinea-pig ileum in the presence of hyoscine and guanethidine, usually caused a diphasic response, relaxation followed by contraction; exogenously applied ATP mimicked this response, in contrast to acetylcholine and noradrenaline which caused excitation and relaxation respectively.
4. Stimulation of preparations of lower vertebrate gut and guinea-pig bladder in the presence of hyoscine and guanethidine caused contraction; exogenously applied ATP mimicked this contractile response.
5. In each preparation the time course of the response to ATP was similar or identical to the response to non-adrenergic, non-cholinergic nerve stimulation.
6. The results are consistent with the hypothesis that a purine nucleotide may be the transmitter substance released from non-adrenergic, non-cholinergic nerves supplying smooth muscle preparations from a number of vertebrate classes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N., Freeman M. A. Atropine-resistant longitudinal muscle spasms due to excitation of non-cholinergic neurones in Auerbach's plexus. J Physiol. 1968 Dec;199(3):705–727. doi: 10.1113/jphysiol.1968.sp008674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambache N., Verney J., Zar M. A. Evidence for the release of two atropine-resistant spasmogens from Auerbach's plexus. J Physiol. 1970 May;207(3):761–782. doi: 10.1113/jphysiol.1970.sp009093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambache N., Zar M. A. Non-cholinergic transmission by post-ganglionic motor neurones in the mammalian bladder. J Physiol. 1970 Oct;210(3):761–783. doi: 10.1113/jphysiol.1970.sp009240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlet A. L., Hassan T. Contraction of chicken rectum to nerve stimulation after blockade of sympathetic and parasympathetic transmission. Q J Exp Physiol Cogn Med Sci. 1971 Jul;56(3):178–183. doi: 10.1113/expphysiol.1971.sp002117. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Satchell D., Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970 Dec;40(4):668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Dumsday B., Smythe A. Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol. 1972 Mar;44(3):451–461. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Evolution of the autonomic innervation of visceral and cardiovascular systems in vertebrates. Pharmacol Rev. 1969 Dec;21(4):247–324. [PubMed] [Google Scholar]
- Burnstock G. Neural nomenclature. Nature. 1971 Jan 22;229(5282):282–283. doi: 10.1038/229282d0. [DOI] [PubMed] [Google Scholar]
- Campbell G. Autonomic innervation of the lung musculature of a toad (Bufo marinus). Comp Gen Pharmacol. 1971 Sep;2(7):281–286. doi: 10.1016/0010-4035(71)90052-8. [DOI] [PubMed] [Google Scholar]
- Carter R. H. Resistance to tetrodotoxin in toad sympathetic nerves. J Pharm Pharmacol. 1969 Jun;21(6):394–395. doi: 10.1111/j.2042-7158.1969.tb08274.x. [DOI] [PubMed] [Google Scholar]
- Chesher G. B. Acetylcholine in extracts and perfusates of urinary bladder. J Pharm Pharmacol. 1967 Jul;19(7):445–455. doi: 10.1111/j.2042-7158.1967.tb08108.x. [DOI] [PubMed] [Google Scholar]
- Dumsday B. Atropine-resistance of the urinary bladder innervation. J Pharm Pharmacol. 1971 Mar;23(3):222–225. doi: 10.1111/j.2042-7158.1971.tb08649.x. [DOI] [PubMed] [Google Scholar]
- Everett S. D. Pharmacological responses of the isolated innervated intestine and rectal caecum of the chick. Br J Pharmacol Chemother. 1968 Jun;33(2):342–356. doi: 10.1111/j.1476-5381.1968.tb00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B. An electrophysiological study of the innervation of the smooth muscle of the colon. J Physiol. 1969 Dec;205(3):549–562. doi: 10.1113/jphysiol.1969.sp008982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B. Secondary excitation of intestinal smooth muscle. Br J Pharmacol. 1971 Feb;41(2):213–226. doi: 10.1111/j.1476-5381.1971.tb08023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg M. M. Analysis of the inhibitory innervation of the isolated gerbil colon. Arch Int Pharmacodyn Ther. 1968 Oct;175(2):347–364. [PubMed] [Google Scholar]
- HOLMAN M. E., HUGHES J. R. INHIBITION OF INTESTINAL SMOOTH MUSCLE. Aust J Exp Biol Med Sci. 1965 Jun;43:277–290. doi: 10.1038/icb.1965.27. [DOI] [PubMed] [Google Scholar]
- Hughes J., Vane J. R. Relaxations of the isolated portal vein of the rabbit induced by nicotine and electrical stimulation. Br J Pharmacol. 1970 Jul;39(3):476–489. doi: 10.1111/j.1476-5381.1970.tb10356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterlitz H. W., Lydon R. J. Spontaneous electrical activity and nerve-mediated inhibition in the innervated longitudinal muscle strip of the guinea-pig ileum. J Physiol. 1969 Feb;200(2):126P–128P. [PubMed] [Google Scholar]
- Kuriyama H., Osa T., Toida N. Nervous factors influencing the membrane activity of intestinal smooth muscle. J Physiol. 1967 Jul;191(2):257–270. doi: 10.1113/jphysiol.1967.sp008249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNRO A. F. Effect of autonomic drugs on the responses of isolated preparations from the guinea-pig intestine to electrical stimulation. J Physiol. 1953 Apr 28;120(1-2):41–52. doi: 10.1113/jphysiol.1953.sp004871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P. M., McLean J. R., Burnstock G. Ultrastructural identification of non-adrenergic inhibitory nerve fibers. J Pharmacol Exp Ther. 1971 Nov;179(2):149–160. [PubMed] [Google Scholar]
- Schnizer W., Hoang N. D., Brecht K. Transmitter in der Froschlunge. Pflugers Arch. 1968;304(3):271–283. doi: 10.1007/BF00592130. [DOI] [PubMed] [Google Scholar]
- Wood M. J., Burnstock G. Innervation of the lungs of the toad (Bufo marinus). I. Physiology and pharmacology. Comp Biochem Physiol. 1967 Sep;22(3):755–766. doi: 10.1016/0010-406x(67)90768-2. [DOI] [PubMed] [Google Scholar]



