Abstract
In nature, plants encounter a combination of environmental conditions that may include stresses such as drought or heat shock. Although drought and heat shock have been extensively studied, little is known about how their combination affect plants. We used cDNA arrays, coupled with physiological measurements, to study the effect of drought and heat shock on tobacco (Nicotiana tabacum) plants. A combination of drought and heat shock resulted in the closure of stomata, suppression of photosynthesis, enhancement of respiration, and increased leaf temperature. Some transcripts induced during drought, e.g. those encoding dehydrin, catalase, and glycolate oxidase, and some transcripts induced during heat shock, e.g. thioredoxin peroxidase, and ascorbate peroxidase, were suppressed during a combination of drought and heat shock. In contrast, the expression of other transcripts, including alternative oxidase, glutathione peroxidase, phenylalanine ammonia lyase, pathogenesis-related proteins, a WRKY transcription factor, and an ethylene response transcriptional co-activator, was specifically induced during a combination of drought and heat shock. Photosynthetic genes were suppressed, whereas transcripts encoding some glycolysis and pentose phosphate pathway enzymes were induced, suggesting the utilization of sugars through these pathways during stress. Our results demonstrate that the response of plants to a combination of drought and heat shock, similar to the conditions in many natural environments, is different from the response of plants to each of these stresses applied individually, as typically tested in the laboratory. This response was also different from the response of plants to other stresses such as cold, salt, or pathogen attack. Therefore, improving stress tolerance of plants and crops may require a reevaluation, taking into account the effect of multiple stresses on plant metabolism and defense.
Under optimal conditions, cellular homeostasis is achieved by the coordinated action of many biochemical pathways. However, different pathways may have different molecular and biophysical properties, making them different in their dependence upon external conditions. Thus, during events of suboptimal conditions (stress), different pathways can be affected differently, and their coupling, which makes cellular homeostasis possible, is disrupted. This process is usually accompanied by the formation of reactive oxygen intermediates (ROIs) because of an increased flow of electrons from the disrupted pathways to the reduction of oxygen (Halliwell and Gutteridge, 1989; Noctor and Foyer, 1998; Asada, 1999; Dat et al., 2000; Mittler, 2002). One example for this process is the effect of heat shock on mitochondrial electron transfer. It was shown that during heat shock, membrane-bound complexes at the inner mitochondrial membrane are uncoupled or disrupted. Electrons from NADH produced by the soluble, and less temperature-sensitive, Krebs cycle enzymes are then channeled to the reduction of O2 to ROI by different components of the uncoupled electron transport chain (Davidson and Schiestl, 2001).
To counter the effects of stress, plants undergo a process of stress acclimation. This process may require changes in the flow of metabolites through different pathways, the suppression of pathways that may be involved in the production of ROI during stress, and the induction of various defense genes such as heat shock proteins (HSPs) and ROI-scavenging enzymes (Vierling, 1991; Dat et al., 2000; Mittler, 2002).
The complexity of signaling events associated with the sensing of stress and the activation of defense and acclimation pathways is believed to involve ROI, calcium, calcium-regulated proteins, mitogen-activated protein kinase cascades, and cross talk between different transcription factors (Liu et al., 1998; Xiong et al., 1999; Bowler and Fluhr, 2000; Knight and Knight, 2001; Kovtun et al., 2000; Chen et al., 2002). Interestingly, different stress conditions such as drought and cold can result in the activation of similar stress response pathways (Seki et al., 2001; Chen et al., 2002). Thus, a high degree of overlap may exist between gene clusters activated by different stresses. This overlap may explain the well-documented phenomena of “cross tolerance,” in which a particular stress can induce in plants resistance to a subsequent stress that is different from the initial one (Bowler and Fluhr, 2000).
Although the study of abiotic stress response has advanced considerably in recent years, analyzing the effect of a single stress on plants can be very different from the conditions encountered by plants in the field in which a number of different stresses may occur simultaneously (Merquiol et al., 2001; Mittler et al., 2001). These can alter plant metabolism in a novel manner that may be different from that caused by each of the different stresses applied individually, and may require a new type of response that would not have been induced by each of the individual stresses.
To characterize some of the mechanisms involved in the response of plants to a combination of stresses, applied simultaneously, we studied the effect of drought and heat shock on tobacco (Nicotiana tabacum) plants. A combination of drought and heat shock can represent the conditions encountered by many plants and crops growing within arid and semiarid environments (Mittler et al., 2001); therefore, its understanding may be critical for the development of new strategies and tools to enhance stress tolerance via genetic manipulations.
RESULTS
Physiological Characterization of Drought Stress, Heat Shock, and a Combination of Drought Stress and Heat Shock in Tobacco
To mimic the conditions encountered by plants during extended periods of drought, accompanied by brief exposures to heat shock (typically occurring between midday to late afternoon; Merquiol et al., 2001), we subjected tobacco plants to drought stress until they reached a relative water content (RWC) of 65% to 70%. Plants were then exposed to a heat shock treatment and sampled. As controls, we used well-watered plants (control), drought-stressed plants that were not subjected to heat shock (drought), and well-watered plants that were subjected to heat shock (heat shock). All plants were analyzed and sampled at the same time (after the heat shock treatment). Recovery tests indicated that plants subjected to a combination of drought stress and heat shock could recover within a few days upon watering and changing of temperature to 23°C (not shown). The conditions used in our study, therefore, were not lethal to plants.
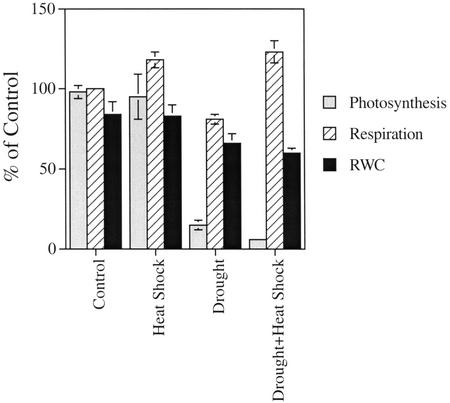
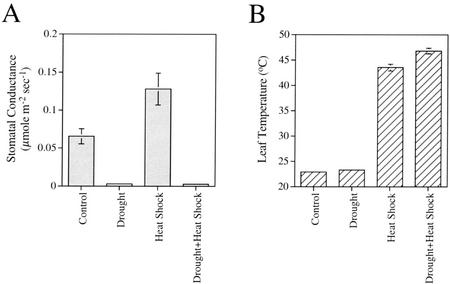
As shown in Figure 1, drought stress resulted in the suppression of respiration and photosynthesis. In contrast, heat shock resulted in the enhancement of respiration, but did not significantly alter photosynthesis. Interestingly, the combination of drought stress and heat shock resulted in the suppression of photosynthesis, similar to drought stress, but the enhancement of respiration to levels that were comparable with those measured in plants after heat shock. Measurements of stomatal conductance, shown in Figure 2A, indicated that heat shock is accompanied by opening of stomata, probably to enable the cooling of leaves via an enhanced transpiration stream. In contrast, stomata remained closed after drought or a combination of drought and heat shock, suggesting that plants subjected to a combination of drought and heat shock may be unable to cool their leaves by enhanced transpiration. Measurements of leaf temperature, shown in Figure 2B, revealed that the leaf temperature of plants subjected to a combination of drought and heat shock was higher by 2°C to 3°C compared with that of plants subjected to heat shock without drought. In addition, measurements of leaf transpiration confirmed that during heat shock transpiration is enhanced, whereas during a combination of drought and heat shock, transpiration is almost completely abolished (not shown). The results presented in Figures 1 and 2 suggest that a combination of drought and heat shock affects plants differently from drought or heat shock applied individually. The differences included changes in photosynthesis, respiration, stomatal conductance, and leaf temperature.
Figure 1.
Measurements of photosynthesis and respiration in plants subjected to heat shock, drought stress, and a combination of heat shock and drought stress. Plants were subjected to stresses as described in “Materials and Methods,” and photosynthetic activity and dark respiration were measured with an LI-6400 apparatus (LI-COR, Lincoln, NE). Photosynthetic activity is shown to be suppressed after drought stress or a combination of drought and heat shock, whereas respiration is enhanced after heat shock and a combination of drought and heat shock. A combination of drought and heat shock, therefore, is different from drought or heat shock by having a high rate of respiration and a low rate of photosynthetic activity. Results are presented as mean and sd of five individual measurements.
Figure 2.
Stomatal conductance (A) and leaf temperature (B) of plants subjected to heat shock, drought stress, and a combination of heat shock and drought stress. Measurements were performed as described in “Materials and Methods.” The temperature of leaves subjected to a combination of drought and heat shock is shown to be higher than that of plants subjected to heat shock in the absence of drought. This difference may result from the inability of plants, subjected to the stress combination, to cool their leaves by transpiration because their stomata are closed.
Molecular Characterization of Gene Expression during Drought Stress, Heat Shock, and a Combination of Drought Stress and Heat Shock in Tobacco
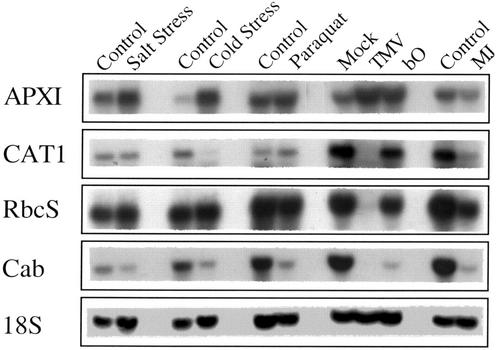
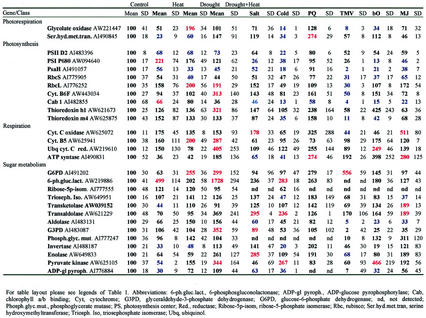
To examine the effect of drought and heat shock on gene expression in tobacco, we designed and used cDNA arrays composed of 170 cDNA clones encoding different defense and metabolic genes. These were spotted in duplicates on nylon filters and used to assay changes in the steady state level of their corresponding transcripts during drought, heat shock, and a combination of drought and heat shock. Identical filters were hybridized with radiolabeled cDNAs obtained from total RNA isolated from plants subjected to the different stresses. The overall pattern of gene expression detected by the filter arrays was different among control, drought stress, heat shock, and a combination of drought stress and heat shock (not shown). A summary of the changes in gene expression calculated as percent of control and averaged over five different experiments, each analyzed individually, is shown in Tables I through III. To compare the changes in expression during heat shock, drought stress, and a combination of drought stress and heat shock with other stresses, we subjected plants to salt stress, cold stress, PQ application, TMV infection, treatment with MJ, or to the expression of bO (Mittler et al., 1995). A summary of the changes in gene expression during these stresses is also shown in Tables I through III. As described previously, TMV infection and bO expression result in the activation of the hypersensitive response and the enhanced generation of ROI (Mittler et al., 1998). Because each of these additional stresses requires a different treatment, e.g. spraying with Tween 20 for PQ, mock infection for TMV, or growth in liquid culture for salt stress, an adequate control was designed for each treatment. These were critical because, as shown in Figures 3 and 4, and as described previously (Mittler and Zilinskas, 1992), different control treatments alter the expression of key genes such as APX and catalase. To confirm that these results, obtained with the cDNA arrays, adequately represent changes in steady-state transcript levels, we tested the expression of nine different cDNAs by RNA blots. These, shown in Figures 3 and 4, were found to be in good agreement with the results presented in Tables I through III (the results shown in Figs. 3 and 4 are from one experiment, whereas the results shown in Tables I through III are the average and sd of five different experiments, for the drought and heat experiments [n = 5], and the average of two different experiments each repeated twice for the other stresses [n = 4], including the experiments shown in Figs. 3 and 4). Because the leaf temperature of plants subjected to drought and heat shock was higher than that of plants subjected to heat shock in the absence of drought (Fig. 2), we performed additional experiments of heat shock at a higher temperature (i.e. 46°C); however, we did not find a significant difference between the induction of HSPs in tobacco plants subjected to heat shock at 46°C or heat shock at 44°C (not shown).
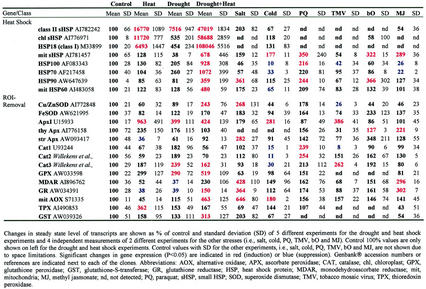
Table I.
Changes in the steady-state level of transcripts encoding heat shock proteins and ROI removal enzymes
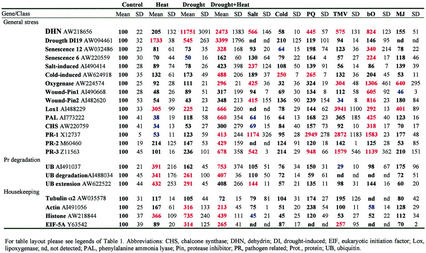
Table III.
Changes in the steady-state level of transcripts encoding general stress, ubiquitin, and “housekeeping” proteins
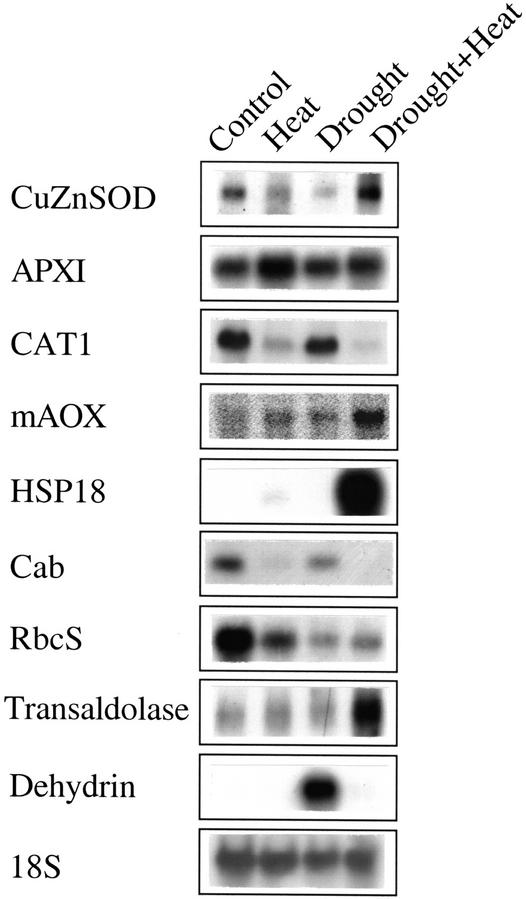
Figure 3.
Changes in the steady-state level of transcripts encoding stress response and metabolic proteins and enzymes during a combination of drought and heat shock. RNA gel blots were used to assay the steady-state level of selected transcripts during a combination of drought and heat shock. Many of the transcripts shown in this figure have a distinct expression pattern during a combination of drought and heat shock. RNA isolation, blots, and analysis are described in “Materials and Methods.”
Figure 4.
Changes in the steady-state level of transcripts encoding stress response and metabolic proteins and enzymes after different environmental stresses. RNA gel blots were used to assay the steady-state level of selected transcripts during different stresses. RNA isolation, blots, and analysis are described in “Materials and Methods.”
Table I summarizes results obtained for cDNAs encoding different HSPs, and different ROI removal enzymes. As shown in Table I, a number of HSPs were induced during a combination of drought and heat shock. These included cytosolic HSP90, HSP70, and HSP100, and sHSPs (cytosolic, mitochondrial, and chloroplastic). Overall, the induction of HSPs was higher in drought and heat shock compared with heat shock or drought. Analyzing the changes in ROI removal enzymes revealed interesting differences among the different stresses. During heat shock, cytosolic APX and thioredoxin peroxidase appeared to be dominant. In contrast, during drought stress, CAT and GPX appeared to be specifically induced. During a combination of drought and heat shock, however, AOX, GPX, glutathione reductase, CuZn-SOD, and glutathione-S-transferase were induced. Thus, the panel of transcripts encoding ROI-detoxifying enzymes induced during each of the different stresses appeared to be different, and ROI detoxification may occur via different routes during the different stresses. The induction of cytosolic APX during heat shock was in agreement with previous reports on the presence of a heat shock factor-binding sequence at the promoter of ApxI (Mittler and Zilinskas, 1992; Storozhenko et al., 1998).
Changes in the steady-state transcript level of different metabolic genes are shown in Table II. As shown in Table II, many of the photosynthetic genes were suppressed during stress. Exceptions were transcripts encoding a PSI reaction center protein, the large subunit of Rubisco, and a subunit of cytochrome B6F. Because cyclic electron flow involves PSI and cytochrome B6F, it is possible that during stress some energy dissipation is obtained via this pathway. Glycolate oxidase, a key enzyme of the photorespiratory pathway induced during drought, was suppressed during a combination of drought and heat shock. In contrast to the suppression of photosynthetic genes, some transcripts encoding enzymes of the pentose phosphate pathway and glycolysis were induced during a combination of drought and heat shock. These included Glc-6-phosphate dehydrogenase and pyruvate kinase. The induction of these transcripts may suggest that during a combination of drought and heat shock, the flow of sugars through these pathways is enhanced, possibly for the production of reducing energy, such as NAD(P) H, in the absence of photosynthesis. In contrast to the suppression of transcripts involved in photosynthesis during a combination of drought and heat shock, transcripts encoding different components of the mitochondrial respiration pathway were not suppressed during a combination of drought stress and heat shock (Table II).
Table II.
Changes in the steady-state level of transcripts encoding metabolic enzymes and proteins
Table III summarizes changes in the expression pattern of different stress response genes. In contrast to drought or heat shock, a combination of drought and heat shock resulted in the induction of a number of different stress response transcripts. These included transcripts encoding PR proteins and PAL. In contrast to PR proteins that were not induced to the same extent as during TMV infection, PAL was induced to levels that were similar to or even higher than those found during pathogen infection. DHN, highly induced during drought stress, was only moderately induced during a combination of drought and heat shock (Table III; see also Fig. 3). In contrast, the induction of a different drought-induced protein (DI-19) was augmented by the combination of drought and heat shock. However, unlike DHN, this transcript was also induced during heat shock. The induction of transcripts encoding different components of the UB protein degradation pathway was also elevated during a combination of drought and heat shock. The induction of the different stress and pathogen response transcripts during a combination of drought and heat shock suggests that this combination may have activated a signal transduction pathway that is also activated during wounding or pathogen infection. This activation might have resulted from the combined synthesis of different plant hormones such as abscisic acid, ethylene, and MJ. The expression of lipoxygenase, involved in jasmonic acid synthesis, was elevated during drought and heat shock.
Expression of Stress Response Transcripts with Homology to Transcripts Isolated from the Desert Plant Retama raetam during a Combination of Drought Stress and Heat Shock in Tobacco
We recently cloned, by a subtraction cDNA cloning method, a number of stress response cDNAs induced in the desert plant R. raetam in response to a combination of different naturally occurring stresses, of which drought and heat shock appear to be the most prominent (Pnueli et al., 2002). To test whether homologs of these transcripts are also involved in the response of laboratory-grown plants to a combination of stresses, we studied their expression in tobacco plants subjected to drought, heat shock, and a combination of drought and heat shock.
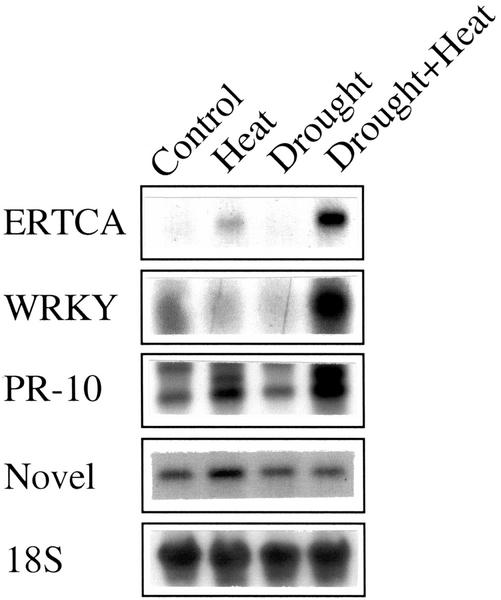
As shown in Figure 5, the expression of two transcripts with a high degree of homology to transcripts induced in the desert plant, i.e. those encoding a WRKY transcription factor, and an ethylene response transcriptional co-activator (ERTCA), was specifically induced during a combination of drought and heat shock in tobacco. The specific induction of these transcription factor homologs during a combination of drought and heat shock may suggest that this combination is accompanied by the activation of a unique genetic program different from the programs activated in plants during drought or heat shock. The expression of another transcript, i.e. a homolog PR-10, induced in the desert plant (Pnueli et al., 2002), was also induced during a combination of drought and heat shock. However, this transcript was also induced during heat shock in the absence of drought. In contrast, a homolog of a novel transcript corresponding to the Arabidopsis gene AC007508.2, induced in the desert plant (Pnueli et al., 2002), was not specifically induced during a combination of drought and heat shock in tobacco (Fig. 5).
Figure 5.
Expression of transcripts with homology to stress response cDNAs isolated from the desert plant R. raetam. RNA gel blots were used to study the expression of different transcripts that hybridized to cDNAs isolated from the desert plant R. raetam subjected to a combination of drought and heat shock in its natural environment. Hybridizations were performed at a high stringency (60°C) using full-length R. raetam cDNA clones as described in “Materials and Methods.”
DISCUSSION
We performed an initial characterization of the response of tobacco plants to a combination of drought stress and heat shock. Our results strongly suggest that the effect of this combination on plants is very different from that of drought or heat shock applied individually. Because in the field or in nature plants are often subjected to a combination of stresses such as drought and heat shock, studying the response of plants to a combination of different stresses may be critical to our understanding of stress tolerance in plants. Thus, stress combinations such as drought and cold, heat shock and high light, or drought and heat shock should be studied before a successful manipulation of plant metabolism can be achieved, to artificially enhance stress tolerance. Future studies using full-scale genome arrays conducted on Arabidopsis plants subjected to similar stress combinations may reveal key regulators of gene clusters activated during a combination of stresses. The identification of two transcripts encoding homologs of proteins involved in the transcriptional regulation of gene expression, i.e. WRKY and ERTCA, specifically induced during a combination of drought and heat shock (Fig. 5), supports the presence of key regulators involved in this response. The finding that a combination of drought and heat shock results in the activation of wound and pathogen response pathways, not activated by each of these stresses applied individually, can also be viewed as an evidence for the induction of a unique genetic program upon stress combination. Our results, therefore, may provide an entry point and a reference to future analysis of gene expression during a combination of stresses. In addition, our results can suggest possible targets for the enhancement of stress tolerance in crops by genetic engineering. Thus, it may be possible to enhance the tolerance of plants to multiple stresses by manipulating the expression of different enzymes of the pentose phosphate pathway, AOX, GPX, and/or homologs of the transcription factors identified by our study (i.e. WRKY and ERTCA).
A number of new findings were uncovered by our analysis. For example, a role for mitochondrial AOX and GPX in the protection of cells from ROI-related damage during a combination of stresses can be suggested. In addition, the finding that the expression of DHN is suppressed during a combination of drought and heat shock may suggest that during this combination, HSPs can replace the stabilizing function of DHN, and it is no longer required for drought-related cellular protection. The source of NAD(P) H used for the removal of ROI during stress is mostly unknown. Our results suggest that the reduction of NAD(P)+ to NAD(P) H during stress, in the absence of photosynthesis, may occur via the pentose phosphate pathway. This suggestion is supported by a number of studies in animal cells and yeast (Saccharomyces cerevisiae), linking the pentose phosphate pathway to the removal of ROI during normal metabolism and stress (Pandolfi et al., 1995; Juhnke et al., 1996), and by our recent findings that plants with suppressed expression of APX and CAT have enhanced expression of transcripts encoding enzymes of the pentose phosphate pathway (Rizhsky et al., 2002). The expression of transcripts encoding enzymes of the pentose phosphate pathway was also elevated during other stresses such as PQ and salt (Table II).
Drought stress and heat shock may affect plant metabolism in a different manner when applied individually. However, it is not entirely clear how they affect plant metabolism when occurring simultaneously. Our analysis suggests that the mitochondria may be critical during a combination of drought and heat shock. During this combination photosynthesis is suppressed, whereas respiration is enhanced (Fig. 1). In addition, the expression of photosynthetic genes is suppressed, whereas the expression of genes involved in respiration is unchanged or induced (Table II). Moreover, the expression of mitochondrial AOX, implicated in the defense of plants from mitochondria-generated ROI during stress (Maxwell et al., 1999), is specifically elevated during a combination of drought and heat shock (Table I; Fig. 3). However, the exact role of the mitochondria, aside from energy supply in the absence of photosynthesis, is unknown.
The response of plants to a combination of drought and heat shock is composed of suppression of photosynthesis, enhancement of respiration, induction of a large number of defense genes, including genes induced during pathogen defense, and changes in genes involved in sugar metabolism. The overall balance between the expression of transcripts encoding different ROI removal enzymes and HSPs is also altered during a combination of drought and heat shock. These changes strongly suggest that the combination of drought and heat shock results in the activation of a unique genetic program that is different from that activated during drought or heat shock. Comparing the expression pattern of the different transcripts shown in Tables I through III between the combination of drought and heat shock and other stresses, such as cold, salt, PQ, or pathogen attack, suggests that the response of plants to the stress combination is also different from the response of plants to these stresses.
Drought and heat shock combination resulted in the induction of at least one senescence-associated transcript (SAG12; Table III). An overlap in the activation of at least 28 different transcription factors was recently reported between senescence and environmental stresses such as cold, salt, and pathogen attack (Chen et al., 2002). Therefore, it is possible that some overlap may also exist between senescence and a combination of drought and heat shock. Interestingly, the study of Chen et al. (2002), although very comprehensive, could not assign a function to a specific WRKY protein, identified as the Arabidopsis homolog of NtWRKY4, also a homolog of the R. raetam WRKY used for the hybridizations shown in Figure 5. From our results (Pnueli et al., 2002; Fig. 5), it is possible that this WRKY is involved in the response of plants to a combination of stresses such as drought and heat shock, or drought and cold stress.
MATERIALS AND METHODS
Growth Conditions and Physiological Measurements
Growth of tobacco (Nicotiana tabacum cv Xanthi-nc NN) plants and experiments were conducted under controlled environmental conditions at 23°C or 44°C. Plants were individually potted in equal amounts of Pro-Mix, and watered with 0.5× Hoagland solution. Continuous illumination was provided by cool-white fluorescent lamps (150 μmol m−2 s−11). Photosynthetic activity, dark respiration, leaf temperature, and stomatal conductance were measured with a LI-COR LI-6400 apparatus using the following measuring cell (6 cm2) parameters: 23°C or 44°C, 150 μmol photons m−2 s−1, and an air flow of 300 μL s−1, as previously described (Mittler et al., 2001). RWC was determined as described by Mittler and Zilinskas (1994).
Stress Treatments
Heat shock was applied by raising the temperature in the growth chamber to 37°C for 1 h, followed by another increase to 44°C for 6 h. Drought stress was imposed by withdrawing water from plants until they reached a RWC of 65% to 70% (typically 6–7 d). A combination of drought and heat shock was performed by subjecting drought-stressed plants (RWC of 65%–70%) to the heat hock treatment. All plants, i.e. drought-stressed plants, well-watered plants subjected to heat shock, drought- and heat-shocked plants, and control well-watered plants kept at 23°C were sampled at the same time for analysis. Cold stress was imposed by changing the temperature in the growth chamber to 4°C for 48 h. Control plants were kept at 23°C. Mock TMV infection plants expressing the bO gene and treatment of plants with MJ were performed as described previously (Mittler et al., 1998). PQ treatment was performed as described by Mittler and Zilinskas (1992). Salt stress was induced by subjecting 7-d-old tobacco seedlings, grown in culture in a medium containing 0.5× Hoagland, to 250 mm NaCl for 3 d. Control seedlings were grown in the same culture media without NaCl. For all stresses, control and stressed tissue were sampled at the same time.
RNA Isolation and RNA Gel Blots
Total RNA was isolated as previously described (Mittler et al., 1998) and subjected to RNA gel-blot analysis (Mittler and Zilinskas, 1992). A probe for 18S rRNA was used to ensure equal loading of RNA. Hybridization conditions were as follows: 0.25 m Na2HPO4, 1 mm EDTA, 7% (w/v) SDS, and 1% (w/v) casein (pH 7.4) at 60°C to 65°C, overnight, and washes were at 1× SSC and 0.1× SSC in the presence of 0.1% (w/v) SDS.
Filter Array Hybridization
Clones for the production of filter arrays were ordered from the tomato (Lycopersicon esculentum) expressed sequence tag library at Clemson University (SC), or obtained from the laboratories of Drs. Dirk Inzé (University of Gent, Belgium), Barbara A. Zilinskas (Rutgers University, NJ), Pierre Goloubinoff (Hebrew University, Jerusalem, Israel), and Gadi Schuster (Technion, Haifa, Israel). Filter cDNA arrays were prepared from the clones by spotting PCR products in duplicates on nylon membranes at the Hadassah Medical School DNA Facility of the Hebrew University. Filters were hybridized with radiolabeled cDNAs prepared from total RNA isolated from the different plants using oligo-dT and Superscript reverse transcriptase (Life Technologies/Gibco-BRL, Cleveland) as suggested by the manufacturer. Hybridization conditions were as follows: 57°C, 5× SSC, 5× Denhart, 0.5% (w/v) SDS, and 100 μg mL−1 salmon sperm DNA, overnight. Washing conditions were as follows: 57°C, 2× SSC, and 0.1% (w/v) SDS for 20 min, followed by 0.2× SSC and 0.1% (w/v) SDS, 57°C, for 20 min. After hybridization and washes, the signals were assayed with a phosphor imager (BAS1000, Fuji Photo Film, Tokyo) and analyzed with TINA software (Raytest, Pittsburgh). A number of control “housekeeping” genes, animal-specific genes (as negative controls), and empty spots (for background) were also spotted on the membrane. These were used to normalize the intensity of signals between the different filters and calculate the changes in gene expression presented in Tables I through III. When pertinent, the expression level of specific genes was verified by RNA blots.
ACKNOWLEDGMENTS
We thank Drs. Dirk Inzé, Barbara A. Zilinskas, Pierre Goloubinoff, and Gadi Schuster for gift of cDNA clones. We also thank Dr. Daniel Goldenberg for his help with preparing filter arrays.
Footnotes
This work was supported by the Israeli Academy of Science, by the Hebrew University Minerva Arid Ecosystem Research Center, by The Biotechnology Council (Iowa State University), and by the fund for the promotion of research at Technion.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.006858.
LITERATURE CITED
- Asada K. The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Bowler C, Fluhr R. The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci. 2000;5:241–246. doi: 10.1016/s1360-1385(00)01628-9. [DOI] [PubMed] [Google Scholar]
- Chen W, Provart NJ, Glazebrook J, Katagiri F, Chang HS, Eulgem T, Mauch F, Luan S, Zou G, Whitham SA et al. Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell. 2002;14:559–574. doi: 10.1105/tpc.010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dat J, Vandenabeele S, Vranova E, Van Montagu M, Inzé D, Van Breusegem F. Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci. 2000;57:779–795. doi: 10.1007/s000180050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JF, Schiestl RH. Mitochondrial respiratory electron carriers are involved in oxidative stress during heat stress in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:8483–8489. doi: 10.1128/MCB.21.24.8483-8489.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford: Clarendon; 1989. [Google Scholar]
- Juhnke H, Krems B, Kotter P, Entian KD. Mutants that show increased sensitivity to hydrogen peroxide reveal an important role for the pentose phosphate pathway in protection of yeast against oxidative stress. Mol Gen Genet. 1996;252:456–464. doi: 10.1007/BF02173011. [DOI] [PubMed] [Google Scholar]
- Knight H, Knight MR. Abiotic stress signalling pathways: specificity and cross-talk. Trends Plant Sci. 2001;6:262–267. doi: 10.1016/s1360-1385(01)01946-x. [DOI] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA. 2000;97:2940–2945. doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merquiol E, Pnueli L, Cohen M, Simovitch M, Goloubinoff P, Kaplan A, Mittler R (2001) Seasonal and diurnal variations in gene expression in the desert legume Retama raetam. Plant Cell Environ (in press)
- Mittler R. Oxidative stress, antioxidants, and stress tolerance. Trends Plant Sci. 2002;9:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mittler R, Feng X, Cohen M. Post-transcriptional suppression of cytosolic ascorbate peroxidase expression during pathogen-induced programmed cell death in tobacco. Plant Cell. 1998;10:461–474. doi: 10.1105/tpc.10.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Merquiol E, Hallak-Herr E, Rachmilevitch S, Kaplan A, Cohen M. Living under a “dormant” canopy: a molecular acclimation mechanism of the desert plant Retama raetam. Plant J. 2001;25:407–416. doi: 10.1046/j.1365-313x.2001.00975.x. [DOI] [PubMed] [Google Scholar]
- Mittler R, Shulaev V, Lam E. Coordinated activation of programmed cell death and defense mechanisms in transgenic tobacco plants expressing a bacterial proton pump. Plant Cell. 1995;7:29–42. doi: 10.1105/tpc.7.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Zilinskas B. Molecular cloning and characterization of a gene encoding pea cytosolic ascorbate peroxidase. J Biol Chem. 1992;267:21802–21807. [PubMed] [Google Scholar]
- Mittler R, Zilinskas B. Regulation of pea cytosolic ascorbate peroxidase and other antioxidant enzymes during the progression of drought stress and following recovery from drought. Plant J. 1994;5:397–406. doi: 10.1111/j.1365-313x.1994.00397.x. [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer C. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Pandolfi PP, Sonati F, Rivi R, Mason P, Grosveld F, Luzzatto L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 1995;14:5209–5215. doi: 10.1002/j.1460-2075.1995.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pnueli L, Hallak-Herr E, Rozenberg M, Cohen M, Goloubinoff P, Kaplan A, Mittler R. Mechanisms of dormancy and drought tolerance in the desert legume Retama raetam. Plant J. 2002;31:319–330. doi: 10.1046/j.1365-313x.2002.01364.x. [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Hallak-Herr E, Van Breusegem F, Rachmilevitch S, Rodermel S, Inzé D, Mittler R (2002) Double antisense plants with suppressed expression of ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants with suppressed expression of ascorbate peroxidase or catalase. Plant J (in press) [DOI] [PubMed]
- Seki M, Narusaka M, Abe H, Kasuga M, Yamaguchi-Shinozaki K, Carninci P, Hayashizaki Y, Shinozaki K. Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell. 2001;13:61–72. doi: 10.1105/tpc.13.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storozhenko S, De Pauw P, Van Montagu M, Inzé D, Kushnir S. The heat-shock element is a functional component of the Arabidopsis APX1 gene promoter. Plant Physiol. 1998;118:1005–1014. doi: 10.1104/pp.118.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling E. The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:579–620. [Google Scholar]
- Willekens H, Villarroel R, Van Montagu M, Inzé D, Van Camp W. Molecular identification of catalases from Nicotiana plumbaginifolia (L.) FEBS Lett. 1994;352:79–83. doi: 10.1016/0014-5793(94)00923-6. [DOI] [PubMed] [Google Scholar]
- Xiong L, Ishitani M, Zhu JK. Interaction of osmotic stress, temperature, and abscisic acid in the regulation of gene expression in Arabidopsis. Plant Physiol. 1999;119:205–212. doi: 10.1104/pp.119.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]