Abstract
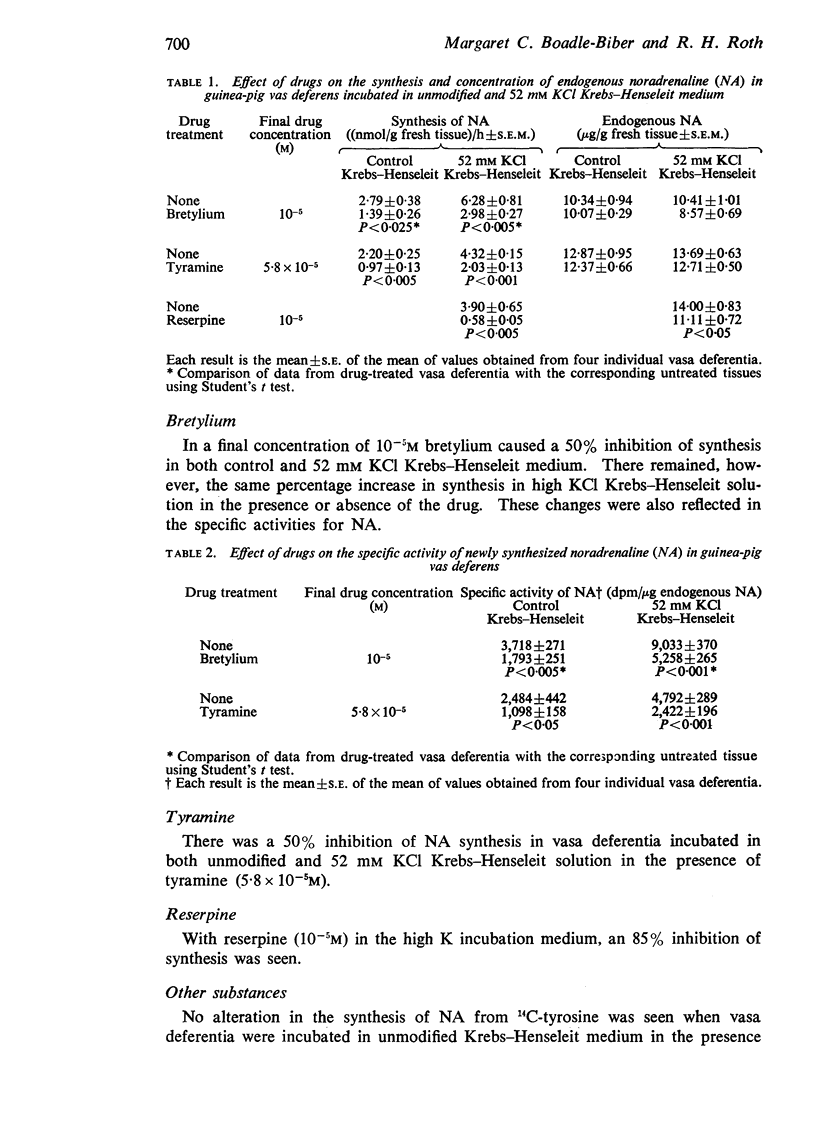
1. Reserpine in vitro (10-5M) caused a profound inhibition (>85%) of the formation of both 14C-catecholamine (14C-CA) and 14C-dihydroxyphenylalanine (14C-DOPA) (in the presence of the amino acid decarboxylase inhibitor brocresine) from 14C-tyrosine in guinea-pig vas deferens. The magnitude of the inhibition was similar for both 14C-CA and 14C-DOPA suggesting that the inhibition occurred primarily at the tyrosine hydroxylase step.
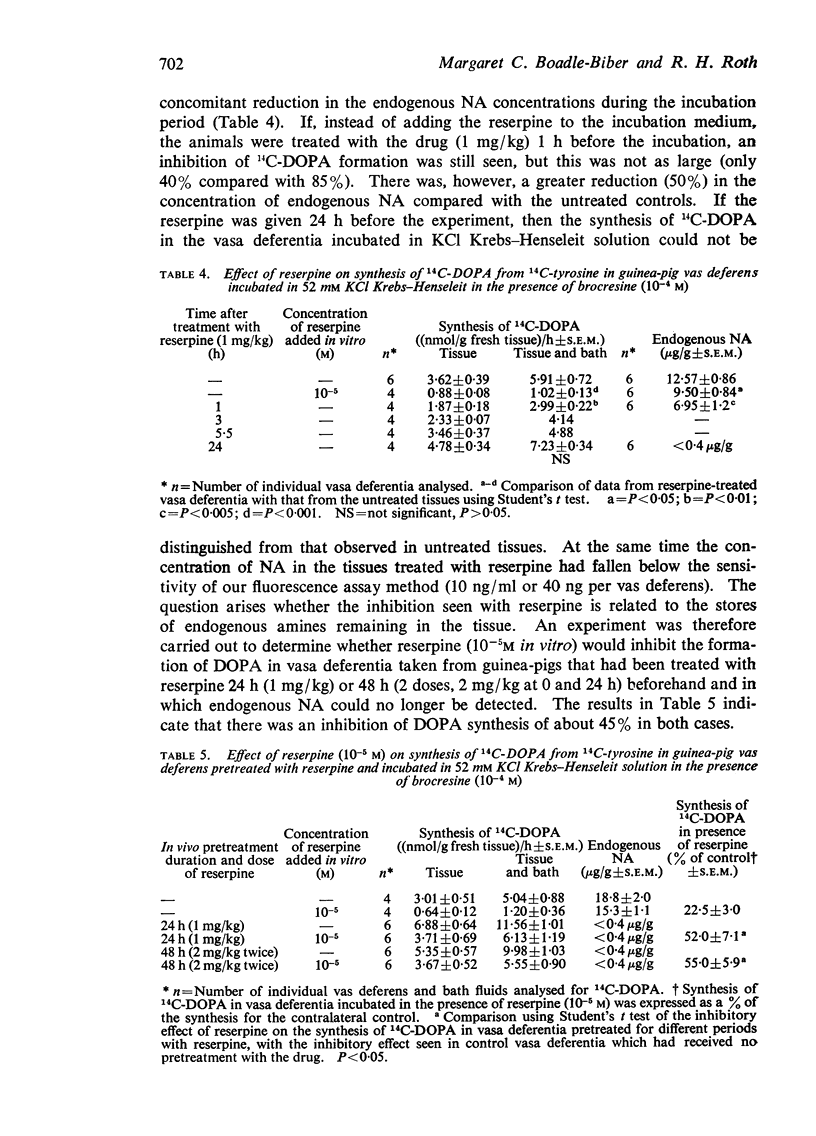
2. One hour after in vivo treatment with reserpine (1 mg/kg) when tissue stores of noradrenaline (NA) were depleted by 50%, there was a significant inhibition of the formation of 14C-DOPA. Twenty-four hours after such treatment, when endogenous NA could no longer be detected, synthesis of 14C-DOPA was indistinguishable from untreated controls. However a 45% inhibition of 14C-DOPA synthesis from 14C-tyrosine could be produced in tissues which had been depleted of NA for 24 h or 48 h by the addition of reserpine, 10-5M, to the incubation medium.
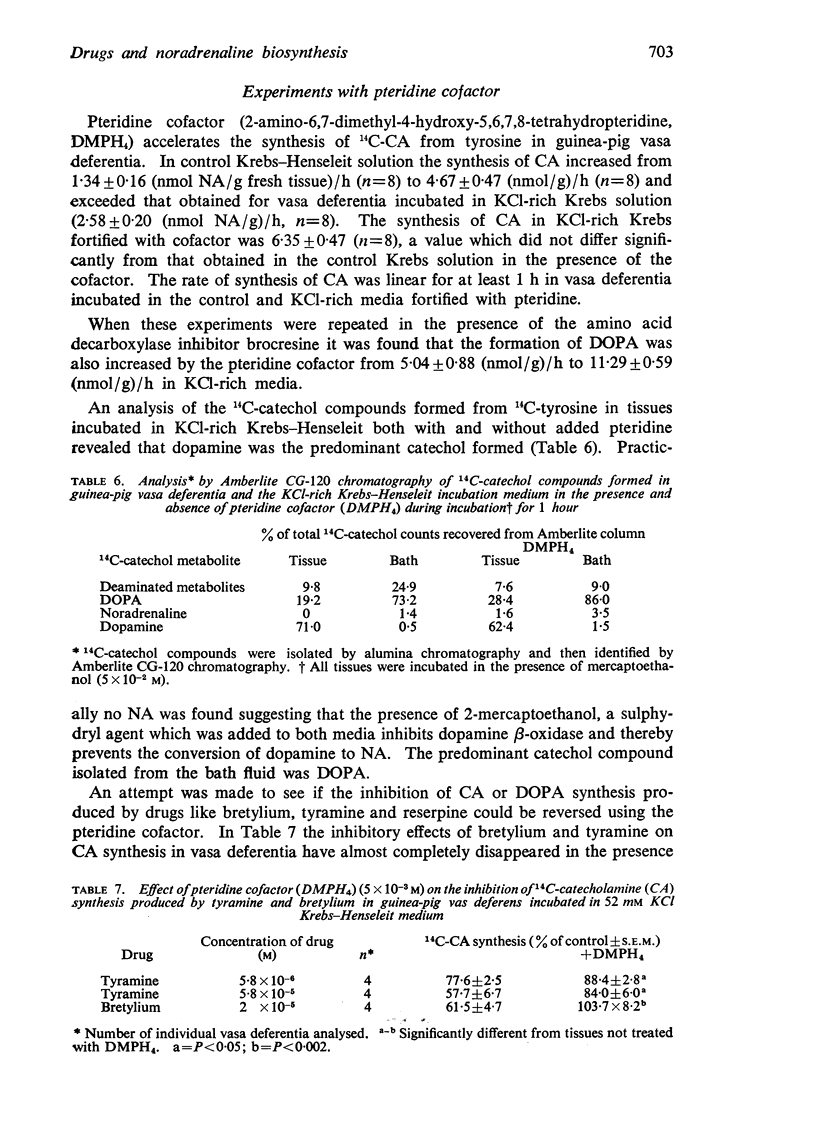
3. Addition of pteridine cofactor, 2-amino-6,7,-dimethyl-4-hydroxy-5,6,7,8-tetrahydropteridine, to the incubation medium in a concentration of 5 × 10-3M enhanced the formation of both 14C-CA and 14C-DOPA from 14C-tyrosine in guinea-pig vas deferens. In 52 mM KCl Krebs-Henseleit medium 14C-CA formation increased from 2·58±0·20 (nmol/g)/h to 6·35±0·47 (nmol/g)/h whilst 14C-DOPA formation increased from 5·04±0·88 (nmol/g)/h to 11·29±0·59 (nmol/g)/h.
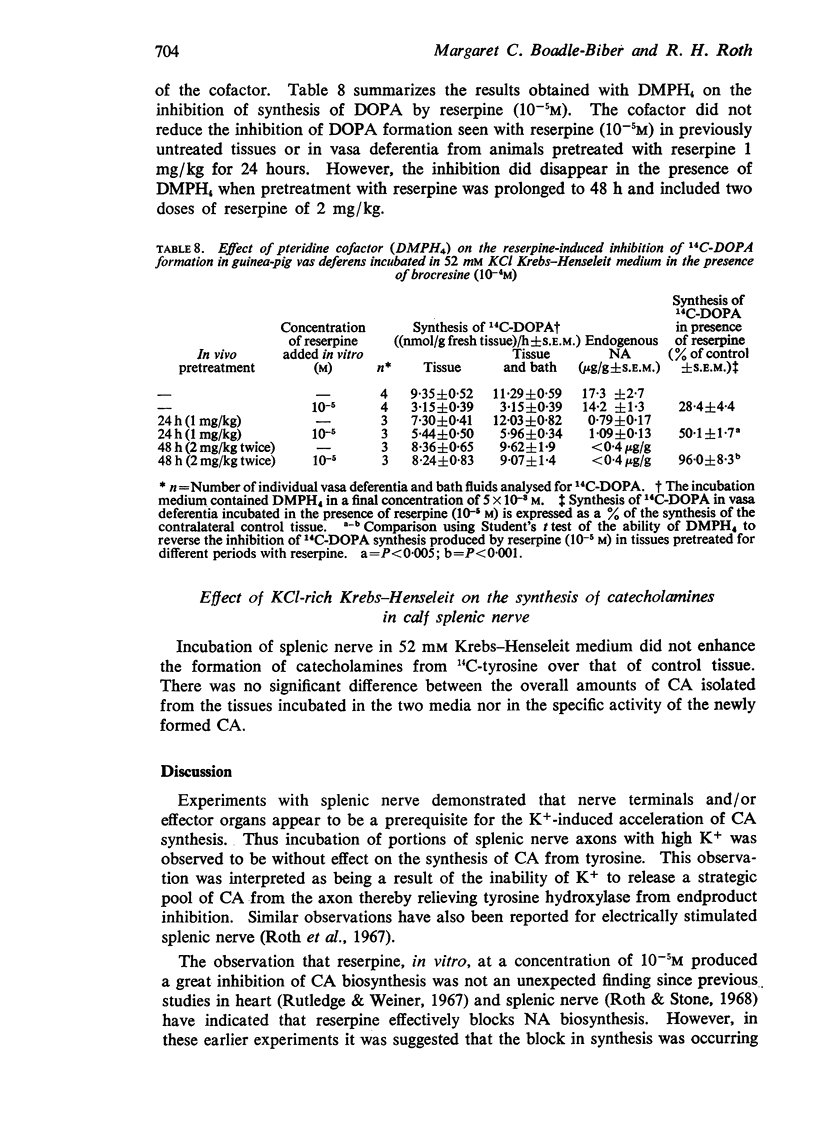
4. Pteridine cofactor (5 × 10-3M) did not reverse the inhibition of 14C-DOPA formation seen with reserpine (10-5M) in previously untreated tissues or in vasa deferentia from animals pretreated with reserpine 1 mg/kg for 24 hours. However, the inhibition did disappear in the presence of pteridine cofactor when treatment with reserpine was prolonged to 48 h and included two doses of reserpine of 2 mg/kg.
5. Tyramine (5·8 × 10-5M) and bretylium (10-5M) in vitro inhibited the formation of 14C-CA and 14C-DOPA from 14C-tyrosine to the same extent in guinea-pig vas deferens again indicating that their major site of action is on tyrosine hydroxylase. The inhibitory effects were reversed by pteridine cofactor.
6. Synthesis of 14C-NA from 14C-tyrosine in calf splenic nerve was not increased by incubating the tissue in 52 mM KCl-Krebs-Henseleit solution.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alousi A., Weiner N. The regulation of norepinephrine synthesis in sympathetic nerves: effect of nerve stimulation, cocaine, and catecholamine-releasing agents. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1491–1496. doi: 10.1073/pnas.56.5.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin L., Livett B. G., Chubb I. W. Increased synthesis and release of noradrenaline and dopamine during nerve stimulation. Life Sci. 1967 Jan 1;6(1):97–104. doi: 10.1016/0024-3205(67)90366-9. [DOI] [PubMed] [Google Scholar]
- Boadle-Biber M. C., Hughes J., Roth R. H. Acceleration of noradrenaline biosynthesis in the guinea-pig vas deferens by potassium. Br J Pharmacol. 1970 Dec;40(4):702–720. doi: 10.1111/j.1476-5381.1970.tb10648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R., Reid J. V., Sjoerdsma A., Udenfriend S. Increased synthesis of norepinephrine in the rat heart on electrical stimulation of the stellate ganglia. Mol Pharmacol. 1966 Nov;2(6):610–613. [PubMed] [Google Scholar]
- Harris J. E., Roth R. H. Potassium-induced acceleration of catecholamine biosynthesis in brain slices. I. A study of the mechanism of action. Mol Pharmacol. 1971 Nov;7(6):593–604. [PubMed] [Google Scholar]
- Ikeda M., Fahien L. A., Udenfriend S. A kinetic study of bovine adrenal tyrosine hydroxylase. J Biol Chem. 1966 Oct 10;241(19):4452–4456. [PubMed] [Google Scholar]
- KIRSHNER N. Uptake of catecholamines by a particulate fraction of the adrenal medulla. J Biol Chem. 1962 Jul;237:2311–2317. [PubMed] [Google Scholar]
- KOPIN I. J., GORDON E. K. Metabolism of norepinephrine-H3 released by tyramine and reserpine. J Pharmacol Exp Ther. 1962 Dec;138:351–359. [PubMed] [Google Scholar]
- McGeer E. G., Gibson S., McGeer P. L. Some characteristics of brain tyrosine hydroxylase. Can J Biochem. 1967 Oct;45(10):1557–1563. doi: 10.1139/o67-185. [DOI] [PubMed] [Google Scholar]
- Roth R. H., Boadle M., Hughes J. Acceleration of the rate limiting step in norepinephrine biosynthesis by potassium. Experientia. 1970 May 15;26(5):494–495. doi: 10.1007/BF01898465. [DOI] [PubMed] [Google Scholar]
- Roth R. H., Stjärne L., von Euler U. S. Acceleration of noradrenaline biosynthesis by nerve stimulation. Life Sci. 1966 Jun;5(12):1071–1075. doi: 10.1016/0024-3205(66)90089-0. [DOI] [PubMed] [Google Scholar]
- Roth R. H., Stjärne L., von Euler U. S. Factors influencing the rate of norepinephrine biosynthesis in nerve tissue. J Pharmacol Exp Ther. 1967 Dec;158(3):373–377. [PubMed] [Google Scholar]
- Roth R. H., Stone E. A. The action of reserpine on noradrenaline biosynthesis in sympathetic nerve tissue. Biochem Pharmacol. 1968 Aug;17(8):1581–1590. doi: 10.1016/0006-2952(68)90218-9. [DOI] [PubMed] [Google Scholar]
- Rutledge C. O., Weiner N. The effect of reserpine upon the synthesis of norepinephrine in the isolated rabbit heart. J Pharmacol Exp Ther. 1967 Aug;157(2):290–302. [PubMed] [Google Scholar]
- Sedvall G. C., Kopin I. J. Acceleration of norepinephrine synthesis in the rat submaxillary gland in vivo during sympathetic nerve stimulation. Life Sci. 1967 Jan 1;6(1):45–51. doi: 10.1016/0024-3205(67)90360-8. [DOI] [PubMed] [Google Scholar]
- Stjärne L., Lishajko F. Localization of different steps in noradrenaline synthesis to different fractions of a bovine splenic nerve homogenate. Biochem Pharmacol. 1967 Sep 9;16(9):1719–1728. doi: 10.1016/0006-2952(67)90247-x. [DOI] [PubMed] [Google Scholar]
- VON EULERU, LISHAJKO F. EFFECT OF ADENINE NUCLEOTIDES ON CATECHOLAMINE RELEASE AND UPTAKE IN ISOLATED ADRENERGIC NERVE GRANULES. Acta Physiol Scand. 1963 Dec;59:454–461. doi: 10.1111/j.1748-1716.1963.tb02761.x. [DOI] [PubMed] [Google Scholar]
- WONG P. W., O'FLYNN M. E., INOUYE T. MICROMETHODS FOR MEASURING PHENYLALANINE AND TYROSINE IN SERUM. Clin Chem. 1964 Dec;10:1098–1104. [PubMed] [Google Scholar]
- Weiner N., Rabadjija M. The effect of nerve stimulation on the synthesis and metabolism of norepinephrine in the isolated guinea-pig hypogastric nerve-vas deferens preparation. J Pharmacol Exp Ther. 1968 Mar;160(1):61–71. [PubMed] [Google Scholar]
- Weiner N. Regulation of norepinephrine biosynthesis. Annu Rev Pharmacol. 1970;10:273–290. doi: 10.1146/annurev.pa.10.040170.001421. [DOI] [PubMed] [Google Scholar]
- Weiner N., Selvaratnam I. The effect of tyramine on the synthesis of norepinephrine. J Pharmacol Exp Ther. 1968 May;161(1):21–33. [PubMed] [Google Scholar]
- von EULER U., LISHAJKO F. Effect of reserpine on the release of catecholamines from isolated nerve and chromaffin cell granules. Acta Physiol Scand. 1961 Jun;52:137–145. doi: 10.1111/j.1748-1716.1961.tb02209.x. [DOI] [PubMed] [Google Scholar]
- von Euler U. S., Lishajko F. Effect of directly and indirectly acting sympathomimetic amines on adrenergic transmitter granules. Acta Physiol Scand. 1968 May-Jun;73(1):78–92. doi: 10.1111/j.1748-1716.1968.tb04085.x. [DOI] [PubMed] [Google Scholar]


