Abstract
The influence of external phosphorus (P) on carbon (C) allocation and metabolism as well as processes related to P metabolism was studied in monoxenic arbuscular mycorrhiza cultures of carrot (Daucus carota). Fungal hyphae of Glomus intraradices proliferated from the solid minimal medium containing the colonized roots into C-free liquid minimal medium with different P treatments. The fungus formed around three times higher biomass in P-free liquid medium than in medium with 2.5 mm inorganic P (high-P). Mycelium in the second experiment was harvested at an earlier growth stage to study metabolic processes when the mycelium was actively growing. P treatment influenced the root P content and [13C]glucose administered to the roots 7 d before harvest gave a negative correlation between root P content and 13C enrichment in arbuscular mycorrhiza fungal storage lipids in the extraradical hyphae. Eighteen percent of the enriched 13C in extraradical hyphae was recovered in the fatty acid 16:1ω5 from neutral lipids. Polyphosphate accumulated in hyphae even in P-free medium. No influence of P treatment on fungal acid phosphatase activity was observed, whereas the proportion of alkaline-phosphatase-active hyphae was highest in high-P medium. We demonstrated the presence of a motile tubular vacuolar system in G. intraradices. This system was rarely seen in hyphae subjected to the highest P treatment. We concluded that the direct responses of the extraradical hyphae to the P concentration in the medium are limited. The effects found in hyphae seemed instead to be related to increased availability of P to the host root.
Arbuscular mycorrhizal (AM) association is the only way for fungi in the order Glomales to proliferate and reproduce (Bécard and Fortin, 1988; Smith and Read, 1997; Bago et al., 2000). It is well known that C is transferred from colonized plants to AM fungi (Ho and Trappe, 1973), whereas the plants in many cases receive most of their P through hyphal uptake and fungal transfer to the host root (Pearson and Jakobsen, 1993). Colonization by AM fungi increases the C sink strength of roots (Douds et al., 1988). The fungal C demand upon root colonization can constitute a significant cost to the host plant, as indicated by reduced growth at high P levels (Peng et al., 1993). This also implies that there is an important connection between external P supply and the regulation of C allocation to the fungal partner in the symbiosis.
C metabolism of the AM fungus Glomus intraradices has been studied using 13C-NMR in monoxenic cultures (Pfeffer et al., 1999). Although no hexose uptake occurs in the extraradical mycelium, intraradical AM fungal mycelium takes up C supplied as hexoses to the root. Triacylglycerols are synthesized from this C in the fungus and transported to the extraradical mycelium. These triacylglycerols are substantial sinks for C in the AM fungal mycelium (Bago et al., 2000; Olsson and Johansen, 2000).
Formation of AM is important for P acquisition in most plants. The adverse effect of high soil P levels on AM formation is well documented (Mosse, 1973; Menge et al., 1978; Jasper et al., 1979; Abbott et al., 1984; Bååth and Spokes, 1989), and is mainly caused by P concentrations being higher in the roots (Sanders, 1975). It has, however, also been shown that high P levels inhibit AM fungi more directly by reducing spore germination and hyphal growth from the germinated spores (Miranda and Harris, 1994; Nagahashi et al., 1996). P-regulated gene expression in fungi includes phosphatases (Kaffman et al., 1994), P transporters (Versaw, 1995), and proteins related to polyphosphate metabolism (Ogawa et al., 2000). A high-affinity P transporter is expressed in the extraradical mycelium of the AM fungus Glomus versiforme (Harrison and Van Buuren, 1995). In G. intraradices, a similar P transporter is regulated by P availability in the external medium and possibly also by the P status of the host root (Maldonado-Mendoza et al., 2001). The P-related processes of polyphosphate accumulation and intracellular phosphatase activities are mainly localized to the vacuolar compartment (Gianinazzi and Gianinazzi-Pearson, 1978; Smith and Read, 1997). The vacuolar compartment in fungi may consist of separate spherical vacuoles, but there is increasing evidence for functioning vacuoles in fungi having the shape of dynamic tubular networks (Cole et al., 1998). This has recently also been shown in the AM fungus Gigaspora margarita (Uetake et al., 2002).
We studied the influence of external P availability on C allocation and metabolism as well as P metabolism in a monoxenic system with carrot (Daucus carota) root-organ cultures in symbiosis with the AM fungus G. intraradices. This system has proved suitable for study of growth strategies of the fungus (Bago et al., 1998) and is now well established as a model system for metabolism and transport processes in the AM symbiosis (Bago et al., 2000; Fortin et al., 2002). We used a two-compartment petri dish system where the root grew in a solid medium in one compartment and extraradical AM fungal mycelium proliferated over a barrier into a second compartment containing liquid medium subjected to different P treatments. The method is described in more detail by Maldonado-Mendoza et al. (2001).
RESULTS
Growth of Mycelium and Roots and Fungal Fatty Acid Composition
The high-P treatment (containing 2.5 mm inorganic P) reduced the growth of extraradical mycelium in the liquid medium in experiment 1, and fungal biomass was 3-fold higher in the P-free medium (Table I). Mycelium of both treatments sporulated, but finely branched absorption hyphae were rarely seen. Mycelium covered 100% of the area of the liquid medium compartment. The high-P treatment had a similar effect on the total amounts of phospholipid fatty acids (PLFAs) and neutral lipid fatty acids (NLFAs) of the mycelium (Table I). The fatty acid composition of the mycelium was influenced by the high-P treatment in that a higher proportion of unsaturated fatty acids was found in mycelium growing in P-free medium (Table I). The fatty acid 16:1ω5 dominated the neutral lipid fraction. All saturated NLFAs occurred at a higher proportion after the high-P treatment than the P-free treatment (Table II).
Table I.
Influence of P treatment on AM fungal growth
| Treatment | Biomass of Mycelium | Mycelium Fatty Acid Content
|
Proportion Unsaturated
|
||
|---|---|---|---|---|---|
| PLFAs | NLFAs | PLFAs | NLFAs | ||
| mg dry wt | nmol | % | |||
| P-free | 3.56 ± 0.36 | 6.85 ± 0.76 | 2400 ± 327 | 45.3 ± 3.3 | 81.1 ± 0.2 |
| High-P | 1.23 ± 0.23 | 3.52 ± 0.47 | 782 ± 345 | 34.4 ± 2.1 | 76.4 ± 1.0 |
| P value | 0.002 | 0.001 | 0.015 | 0.03 | 0.004 |
Experiment 1. Growth and fatty acid content of the G. intraradices mycelium in P-free medium and in 2.5 mm KH2PO4 (high-P). Total amounts of PLFAs and NLFAs extracted from mycelia in liquid medium (see Table II) are given together with the proportion of unsaturated PLFAs and NLFAs (mean ± se, n = 4). The effect of P treatment was tested with Student's t test.
Table II.
Fatty acid composition of AM fungal mycelium
| Lipid Fraction | Treatment | Unsaturated Fatty Acids
|
Saturated Fatty Acids
|
|||||
|---|---|---|---|---|---|---|---|---|
| 16:1ω5 | 18:2ω6,9 | 18:1ω9 | 18:1ω7 | 16:0 | 18:0 | 20:0 | ||
| % | ||||||||
| PLFAs | P-free | 23.2 | 1.8 | 6.2 | 15.1 | 31.0 | 11.4 | 11.2 |
| High-P | 14.7 | 0.0 | 6.2 | 13.8 | 40.0 | 18.1 | 7.1 | |
| NLFAs | P-free | 77.6 | 0.4 | 1.1 | 2.1 | 17.7 | 0.7 | 0.5 |
| High-P | 74.8 | 0.3 | 0.9 | 1.7 | 19.8 | 1.4 | 1.1 | |
Experiment 1. Mean fatty acid composition (n = 4) of PLFAs and NLFAs in mycelium of G. intraradices in P-free medium and with 2.5 mm KH2PO4 (high-P). The nomenclature for fatty acids follows that of Tunlid and White (1992).
Between 15% and 80% of the area of the liquid medium compartment was covered by mycelium at the time of harvest in experiment 2. Mycelia all sporulated, and finely branched hyphae occurred frequently. The addition of P increased the biomass of the roots and influenced the root P concentration in experiment 2 (Table III) in a fashion that could be expected from the amounts of orthophosphate in the liquid medium. The P treatment did not influence the N concentration of the roots. There was no significant difference in mycelium biomass between the P-free, low-P (containing 25 μm inorganic P), or high-P treatment in experiment 2 (ranging from 0.89 to 1.13 mg dry weight), and the results were similar for total amounts of PLFAs and NLFAs. A higher mycelium dry weight was found in the organic P (org-P) medium (containing 2.5 mm organic P as sodium-phytate). This was presumably attributable to a precipitate (probably containing sodium-phytate) on the hyphae, and the total amounts of PLFAs and NLFAs were not influenced by this treatment.
Table III.
Influence of P treatment on root biomass and nutrient content
| Treatment | Root P Concentration | Total Root P | Root N Content | Root Dry Wt |
|---|---|---|---|---|
| mg g−1 | μg | mg g−1 | mg | |
| P-free | 1.5 ± 0.12 a | 62 ± 4.5 a | 12.3 ± 1.4 | 41.5 ± 1.2 a |
| Low-P | 1.7 ± 0.16 ab | 80 ± 8.7 ab | 13.5 ± 1.7 | 47.7 ± 2.1 ab |
| Org-P | 1.9 ± 0.06 bc | 91 ± 8.2 bc | 15.2 ± 1.0 | 47.8 ± 4.1 ab |
| High-P | 2.0 ± 0.05 c | 104 ± 8.0 c | 13.1 ± 0.6 | 51.6 ± 3.4 b |
| P value | 0.020 | 0.012 | 0.45 | 0.16 |
Experiment 2. The effect of P treatment was tested with one-way ANOVA (mean values ± se, n = 4), and different letters indicate significantly different values (P < 0.05, Fischer's lsd).
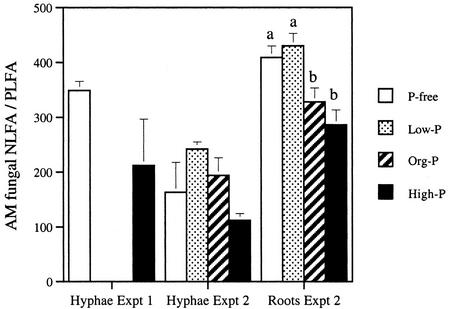
Although in the extraradical hyphae, the total NLFA to PLFA ratio (indicating the relative amount of C allocated to storage lipids) was lower after the high-P treatment than the other kinds of treatment, this difference was not statistically significant (Fig. 1). The relative amount of C allocated to storage lipids of the AM fungus inside the roots was estimated from the amount of the AM fungal fatty acid 16:1ω5. The ratio between NLFA 16:1ω5 and PLFA 16:1ω5 was significantly lower after the high-P treatment than the low-P and P-free treatment, indicating less allocation to storage lipids at high P.
Figure 1.
The relative allocation of C to storage lipids is indicated by the ratio of NLFAs to PLFAs (average ± se, n = 4; for hyphae in experiment 2, n = 3). For hyphae, the ratio was calculated using all detected fatty acids (see Table II), whereas the AM fungal signature fatty acid 16:1ω5 (Olsson et al., 1997) was used for the intraradical mycelium because of a background from other fatty acids in the root. The P treatment significantly influenced the NLFA to PLFA ratio in the root (P = 0.004; one-way ANOVA) but not in hyphae. Different letters indicate significantly different values (P < 0.05, Fischer's lsd).
Changes in the Liquid Medium during Experiment 2
Mycelia proliferated into the liquid medium after all kinds of P treatment. Most roots that crossed the barrier between the compartments were cut, but at the time of harvest, some roots had entered the liquid medium in most of the replicates of each P treatment. The orthophosphate in the low-P medium was almost depleted by roots and hyphae after the experimental period, whereas no depletion could be observed in the high-P treatment (Table IV). Only about 60% to 70% of the originally added liquid remained after the experimental period because of evaporation, meaning that the orthophosphate concentration in the remaining liquid medium would have increased. This was also the reason for an increase in orthophosphate concentration in the control dishes incubated without cultures (Table IV). The org-P medium contained 88 μm orthophosphate in addition to the 2.5 mm sodium-phytate at the commencement of the experiment. This orthophosphate had to a large extent been depleted by the end of the experiment. In all kinds of P treatment, the growth of the AM fungal mycelia raised the pH compared with that of the control dishes (Table IV). At the end of the experiment, the pH of the P-free and low-P treatments was higher than that of the high-P treatment and the org-P treatment.
Table IV.
Orthophosphate concentration and pH in liquid medium with G. intraradices
| PO4− concentration
|
pH
|
||||
|---|---|---|---|---|---|
| Treatment | Initial | Final | Control | Final | Control |
| μm | |||||
| P-free | 0.06 | 0.36 | 0.06 | 7.4 ± 0.18 a | 5.0 |
| Low-P | 23 | 1.0 | 29 | 7.6 ± 0.14 a | 5.5 |
| Org-P | 88 | 16 | 114 | 6.6 ± 0.22 b | 4.7 |
| High-P | 2.5*103 | 2.6*103 | 2.9*103 | 6.8 ± 0.03 b | 5.5 |
| ANOVA | P = 0.002 | ||||
Experiment 2. Final values were measured at the end of the experimental period (mean ± se, n = 4). Initial values were measured in media stored at −20°C. Liquid medium incubated in petri dishes without cultures was used as control (n = 2). The pH of the medium was set at 5.5 before incubation, but because the organic P was added after autoclaving, the pH could not be adjusted after this addition. The effect of P treatment on pH was tested with one-way ANOVA and different letters indicate significantly different values (P < 0.05, Fischer's lsd).
13C Enrichment in Hyphae and Roots
13C enrichment in the extraradical mycelium was low and variable in experiment 1 (0.021% ± 0.015% for P-free and 0.030% ± 0.027% for high-P treatment, averages ± se) after a 4-d chase period, and there was no significant difference between various P treatments. The results were similar for 13C enrichment in NLFA 16:1ω5. 13C enrichment in PLFA 16:1ω5 (0.27% ± 0.17% for P-free and 0.58% ± 0.30% for high-P treatment) was higher than both total 13C enrichment (P = 0.04, paired t test, n = 8) and 13C enrichment in NLFA 16:1ω5 (P = 0.04, paired t test, n = 8).
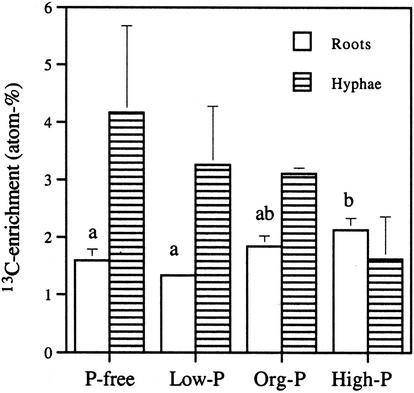
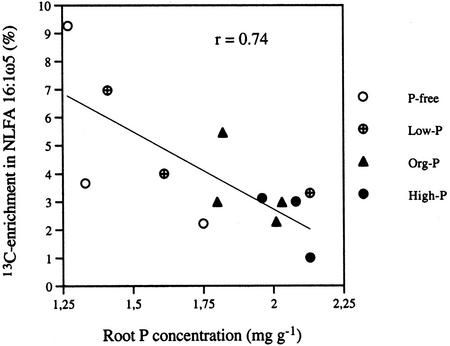
High initial orthophosphate content of the liquid medium gave high Glc uptake in the root in experiment 2, as shown by high 13C enrichment, whereas the opposite trend was found for the extraradical mycelium, although this was not statistically significant (Fig. 2). The P content of the roots correlated negatively with 13C enrichment in NLFA 16:1ω5 (P < 0.01) in extraradical hyphae, indicating that neutral lipids accumulate in particular in mycelia growing with root organs with limited P availability (Fig. 3). The 13C enrichment in hyphae in the liquid medium was higher than in roots (Table V). The 13C enrichment could be determined in the AM fungal fatty acid 16:1ω5 and in fatty acid 18:2ω6,9 which is a common plant fatty acid. Fatty acid 16:0 is common both in plants and AM fungi, whereas in roots, PLFA 16:0 mainly reflects plant origin; NLFA 16:0 in roots normally also represents AM fungal lipids to a large extent. The 13C enrichment in fatty acids indicated higher C accumulation in the fungus than in the root (Table V).
Figure 2.
Experiment 2. 13C enrichment expressed as excess atomic percent in roots and hyphae (average ± se, n = 4 for roots, n = 3 for hyphae). The background attributable to the natural abundance of 13C (1.14%) has been subtracted. P treatment influenced 13C enrichment significantly in roots (P = 0.038; one-way ANOVA) but not in hyphae. Different letters indicate significantly different values (P < 0.05, Fischer's lsd).
Figure 3.
Experiment 2. The relationship between root P concentration and 13C enrichment in NLFA 16:1ω5 in hyphae in liquid medium after different kinds of P treatment (P < 0.01). The background attributable to the natural abundance of 13C (1.14%) has been subtracted.
Table V.
13C enrichment in signature fatty acids and total root and hyphal C
|
13C Enrichment
|
|||||||
|---|---|---|---|---|---|---|---|
| PLFA-C
|
NLFA-C
|
Total C | |||||
| 16:1ω5 | 16:0 | 18:2ω6,9 | 16:1ω5 | 16:0 | 18:2ω6,9 | ||
| excess atom % 13C | |||||||
| Roots (n = 16) | nd | 3.0 ± 0.20 | 2.7 ± 0.22 | 1.7 ± 0.18 | 3.7 ± 0.38 | 1.3 ± 0.10 | 1.7 ± 0.11 |
| Mycelium (n = 12) | 4.2 ± 0.58 | 4.5 ± 0.64 | nd | 3.9 ± 0.61 | 5.3 ± 0.72 | nd | 3.0 ± 0.50 |
| P value | 0.031 | 0.006 | 0.051 | 0.029 | |||
Experiment 2. Values from NLFA-C and PLFA-C in roots and hyphae together with the total 13C enrichment in roots and hyphae are given (mean ± se). The background attributable to natural abundance of 13C (1.14%) has been subtracted. Differences between roots and hyphae were tested with the paired t test. In some cases, it was not possible to determine the 13C enrichment (nd).
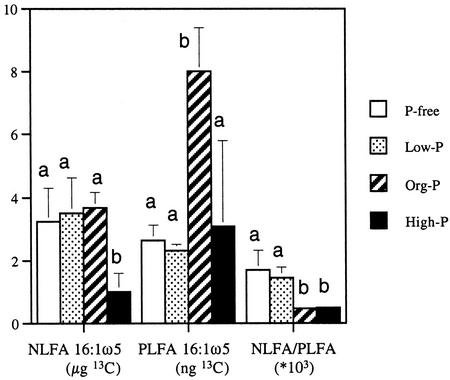
The total amount of 13C transported to the mycelium in the liquid medium and incorporated into NLFA 16:1ω5 was lower after the high-P treatment than for the other kinds of P treatment (Fig. 4). Mycelia growing in org-P medium exhibited the highest incorporation in PLFA 16:1ω5. In relation to incorporation into PLFA 16:1ω5, mycelia growing in P-free and low-P media allocated more C to neutral lipids than fungi growing in high-P and org-P media. In total, 18% (average for all treatments) of the excess 13C in extraradical mycelium was recovered in NLFA 16:1ω5. The corresponding figure for the roots was 7.1% of the excess 13C in NLFA 16:1ω5.
Figure 4.
Experiment 2. Amount of enriched 13C in NLFA and PLFA 16:1ω5 in hyphae of G. intraradices in the liquid medium calculated after the background attributable to natural abundance of 13C (1.14%) had been subtracted (average ± se, n = 3). P treatment influenced the [13C]NLFA to [13C]PLFA ratio significantly (P = 0.016; one-way ANOVA). There was also an indication of effects on NLFA 16:1ω5-13C (P = 0.068) and PLFA 16:1ω5-13C (P = 0.055). Different letters indicate significantly different values (P < 0.05, Fischer's lsd).
Metabolic Processes Related to Fungal P Uptake
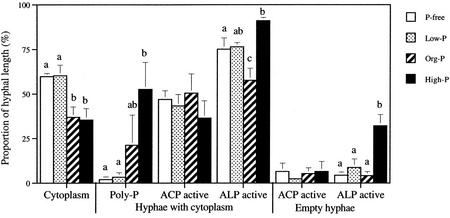
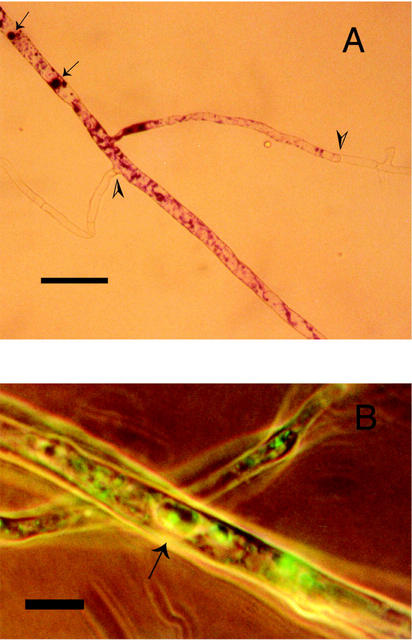
The proportion of hyphal length with an intact cytoplasm (Fig. 5) was reduced by 40% in solutions subjected to high-P and org-P treatment compared with P-free and low-P treatment (Fig. 5). Polyphosphate accumulated in the high-P-treated hyphae, as indicated by the proportion of hyphae containing cytoplasm with metachromasy (see Fig. 6A). One of the replicates of the org-P treatment also showed high polyphosphate accumulation, but no significant difference was found compared with the other treatments. Polyphosphate was not detected in hyphae without cytoplasm. Cross-walls were formed in empty hyphae and polyphosphate was detected on the cytoplasmic side of the cross-walls (Fig. 6A). Low levels of polyphosphate were detected after the P-free and low-P treatment.
Figure 5.
Microscopically investigated processes in the hyphae of G. intraradices. The proportion of hyphal length with polyphosphate (Poly-P) and hyphae with acid phosphatase (ACP) and alkaline phosphatase (ALP) activity was determined both in hyphae with cytoplasm and in empty hyphae (average ± se, n = 4). No polyphosphate was detected in empty hyphae. The P treatment significantly influenced the proportion of hyphae containing cytoplasm (P = 0.007; one-way ANOVA), hyphae containing cytoplasm with polyphosphate (P = 0.03) and ALP-active cytoplasmic (P = 0.004) and ALP-active empty hyphae (P = 0.002). Different letters indicate significantly different values (P < 0.05, Fischer's lsd).
Figure 6.
A, Hyphae after low-P treatment with metachromasy, indicating the localization of polyphosphate (arrows). Metachromatic hyphae with cytoplasm separated from empty hyphae by cross-walls (arrow heads) can be seen. B, Localization of alkaline phosphatase activity by enzyme-labeled fluorescence substrate (ELF) staining (arrow) of a sample from the low-P treatment. Transmitted long-wavelength light was combined with UV light. Scale bar in A = 25 μm and in B = 9 μm.
The proportion of acid-phosphatase-active length of hyphae was not significantly influenced by the P treatment for hyphae with or without cytoplasm. Both the acid phosphatase activity and the alkaline phosphatase activity was, in many cases, associated with the vacuoles (Fig. 6B). The proportion of alkaline-phosphatase-active length of hyphae containing cytoplasm was, however, increased in the high-P treatment and decreased in the org-P treatment compared with other kinds of P treatment. The high-P treatment also increased the proportion of alkaline-phosphatase-active hyphal length in hyphae without cytoplasm compared with other kinds of P treatment. In the empty hyphae, the alkaline phosphatase activity was associated with cell walls.
Phosphatase activity in the liquid medium was below the usual detection limit of 0.01 unit mL−1 in both experiments 1 and 2. Phosphatase activity was also assayed after decreasing the detection limit to 0.001 unit mL−1 in experiment 2. Still, only occasional acid phosphatase activity and no alkaline phosphatase activity was found. The highest value recorded was 0.0023 unit acid phosphatase activity mL−1 in one of the org-P-treated replicates. This could, however, be attributable to phosphatases released by the few roots observed in the liquid medium.
The Tubular Vacuolar System in G. intraradices
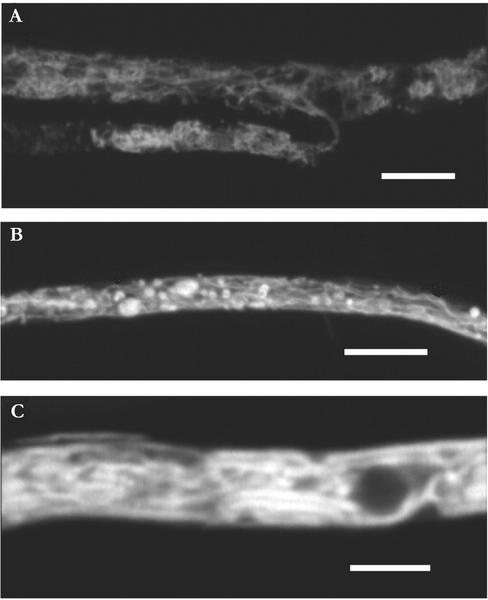
The medium originally containing 35 μm P was almost depleted of P at the time of investigation of the vacuolar structures and contained only 1.1 ± 0.38 μm P. No depletion was detected in the medium initially containing 350 μm P. Tubular vacuoles were seen only in live hyphae with cytoplasmic streaming. The cytoplasmic streaming was bidirectional. In the healthiest of cells, in the 35 μm P medium, the tubular vacuoles often formed a finely branched network (Fig. 7A) and there were few rounded vacuoles. In the medium originally containing 350 μm P, there were more empty hyphae. In living hyphae, cytoplasmic streaming was slower, and in this medium, the hyphae mostly contained rounded vacuoles, sometimes interconnected with tubular vacuoles (such as shown for hyphae in 35 μm P medium; Fig. 7B). Both tubules and rounded vacuoles varied in diameter (Fig. 7B). In P-free medium, the shape and organization of the vacuoles were intermediate between that of the vacuoles in the other two media, although they resembled more closely that of the hyphae in the medium containing 35 μm P.
Figure 7.
Tubular vacuole system of G. intraradices when growing in medium originally containing 35 μm P. A and C, Digital images of a single focus plane. B, Maximum intensity projection of multiple scan (20 images). Scale bar in A = 8 μm, in B = 10 μm, and in C = 4 μm.
In hyphae after all treatments, tubular vacuoles seemed fragile. It was easiest to make observations very soon after transfer of the hyphae to the slide, and after approximately 30 min, the tubular network tended to coalesce (Fig. 7C), increased numbers of rounded vacuolar structures appeared, and streaming was reduced.
DISCUSSION
External P Influences C Metabolism of the AM Fungus
In experiment 1, we found a negative feedback of high P availability on mycelium growth of the AM fungus. Local P sources that can only be accessed by AM fungal hyphae have been shown to reduce root colonization by the fungus (Boddington and Dodd, 1998) and with this possibly also the development of extraradical mycelium, because root colonization and the amount of extraradical mycelium are related (Olsson et al., 1997). A good P status of plants usually inhibits colonization by AM fungi (Sanders, 1975) and prevents the accumulation of AM fungal neutral lipids (Peng et al., 1993; Olsson et al., 1997). In the present study, root colonization was already established when we applied the P treatment. AM fungal hyphae, and later also some roots, reached the liquid medium. The reduced hyphal growth in medium with a high P content indicates that the root may reduce the C flow to the fungus under improved P conditions. Considering the proposed C metabolism in the symbiosis, this would result in reduced lipid transport (mainly triacylglycerols) from intraradical mycelium to the extraradical mycelium (Bago et al., 2000).
The hyphae in experiment 1 had stopped expanding because the whole area of the liquid compartment was covered at the time of harvest. The proportion of unsaturated fatty acids in G. intraradices was higher in neutral lipids than in phospholipids, which is in contrast to many other fungi (Lösel, 1988). The lower proportion of unsaturated fatty acids after the high-P treatment could be a response to a nutritional factor such as the C supply from the host. Plasticity in fatty acid synthesis attributable to nutritional factors is evident in filamentous fungi (Lösel, 1988). Earlier studies have shown that P application influences the lipid metabolism of AM fungi. Graham et al. (1997) found little effect of P application on AM colonization in citrus, but the content of the AM fungal fatty acid 16:1ω5 (from all types of lipids) in roots was reduced at high P. Using the same fatty acid as a signature, Olsson et al. (1997) showed that high-P level in the colonized root particularly reduced the AM fungal storage lipids. Similar effects were shown in this study by a reduced NLFA to PLFA ratio at high P, and coherent with this was the reduced 13C enrichment in NLFA 16:1ω5 at high P (Fig. 3).
In experiment 2, we harvested mycelium while it still was expanding, and a considerable C flow to the mycelium in the liquid medium occurred. The flow of 13C reflects the total flow of C, because there was no C source in the liquid medium. There is normally a net movement of lipids from intraradical mycelium to extraradical in AM fungi, although significant bidirectional translocation occurs (Bago et al., 2002). The higher 13C enrichment in AM fungal signature fatty acids in the extraradical mycelium than in the root compartment (Table V) indicates that the extraradical mycelium had a higher growth rate. It is proposed that triacylglycerols are the major C compounds translocated from the intraradical mycelium to the extraradical mycelium (Bago et al., 2000). We found that 18% of the excess 13C in extraradical mycelium was present in NLFA 16:1ω5, a dominant constituent of the AM fungal triacylglycerols. Calculations based on the assumption that at least 75% of NLFA is 16:1ω5 (see Table II) and that NLFAs constitute about 75% of triacylglycerols (see Olsson and Johansen, 2000) give an estimate that 32% of 13C enrichment in the extraradical mycelium is found in the triacylglycerols. This result supports the suggestion that these lipids are important for AM fungal C metabolism.
P Translocation in the Fungus
The mycelium of G. intraradices grew very well in the P-free medium, which indicates a good allocation ability of P in the mycelium because P could only have been taken up on the root side of the cultures. Polyphosphate with an approximate average chain length of 17 accumulates in the vacuoles of G. intraradices (Rasmussen et al., 2000) and may be important in reducing osmotic stress at high internal P concentrations but is probably also important for the translocation of P (Smith and Read, 1997). Incubation of extracted intraradical mycelium of G. margarita in Glc increased the efflux of P and at the same time decreased the polyphosphate content in the hyphae, indicating a role for polyphosphate in the exchange of C and P between the symbionts (Solaiman and Saito, 2001). Up to 17% of the P in the extraradical mycelium of G. margarita may be stored as poly-phosphate (Solaiman et al., 1999) and 37% in Brewer's yeast (Saccharomyces cerevisiae; Ogawa et al., 2000). The detection of polyphosphate in mycelium growing in P-free medium supported a role for polyphosphate in the transport of P taken up in other parts of the mycelium. This means that bidirectional transport of P, possibly as polyphosphate, may occur in AM fungal hyphae. Ezawa et al. (2001) did not detect polyphosphate in the extraradical hyphae of Glomus coronatum growing in P-free sand. However, they used a slightly different staining method not including the precipitation of polyphosphates with ethanol.
The presence of a motile tubular vacuole system has been demonstrated in a wide range of fungi (Ashford, 1998), and the system is found in the mycelium of Pisolithus sp. when growing in ectomycorrhizal symbiosis (Allaway and Ashford, 2001). The first AM fungus in which this kind of system was demonstrated was G. margarita (Uetake et al., 2002). In G. intraradices, individual spherical vacuoles have been described (Bago et al., 1998). The tubular components of the vacuolar system are, however, not retained in conventional microscopy preparation methods (Cole et al., 1998), and here, we show the presence of a mobile tubular vacuolar system in G. intraradices. Tubular vacuole systems may be important in polyphosphate storage and transport in fungi (Ashford and Allaway, 2002).
Fungi typically respond to P deficiency by increasing the production of acid phosphatases (Kaffman et al., 1994) and, at least in some cases, also by increased production of alkaline phosphatases (Grotelueschen et al., 1994). The induction of phosphatase activity in fungal mycelium because of P starvation can easily be detected by both the methods used in this study, as shown for Aspergillus fumigatus (Van Aarle et al., 2001). However, we observed no such effect in G. intraradices when growing in P-free medium. This is consistent with high-P translocation ability within the mycelium, with the result that external P availability did not limit the growth of mycelium. Instead, the results indicate that C provided by the root limits mycelium growth at high-P levels. In an earlier study, however, Maldonado-Mendoza et al. (2001) observed a response of the mycelium of G. intraradices in a similar system that could indicate P limitation. A high-affinity P transporter gene was expressed in a medium similar to our low-P medium but was repressed at high P and in a medium without P.
Another explanation of the lack of induction of phosphatase activity in the AM fungus could be that acquisition of external organic P is not the major role of AM fungal phosphatases, although at least some easily mineralized organic P compounds can be used by AM fungi (Joner et al., 2000; Koide and Kabir, 2000). In our study, there seemed to be no special response to the applied organic P (sodium-phytate). Instead, the effect of this treatment seemed to be more related to the orthophosphate that was present in this P source because, in most cases, the response to this treatment seemed to be intermediate to responses to the low-P and the high-P treatment. The proportion of alkaline-phosphatase-active hyphae was highest after the high-P treatment, which indicates a role of this enzyme either in polyphosphate metabolism or in the degradation of P compounds in dying hyphae.
CONCLUSIONS
We propose that the response of AM fungi to high external P availability depends, to a large extent, on the influence on the P status of the colonized root. Although the hyphae experienced extreme P levels (no P or P concentration 70 times more than the initial root medium), the influence on the hyphal processes seemed rather to be an effect of the subsequent regulation of the C flow in the symbiosis. This was evident because the variation within each treatment was large, and this variation was explained by variation in root P content (Fig. 3). For an AM fungal mycelium, the sink region for P is normally the intraradical mycelium, because large quantities of P are transferred to the host plant. Here, we showed that extraradical mycelium deprived of a P source may just as well act as a P sink.
MATERIALS AND METHODS
Monoxenic AM Cultures
The AM fungus Glomus intraradices Schenck & Smith was grown monoxenically in mycorrhizal association with root-organ cultures of carrot (Daucus carota). The cultures are clones of carrot roots (line DC1) that were initially transformed using the bacterium Agrobacterium rhizogenes as transformation system with the T-DNA of the root-inducing plasmid transferred to the plant genome (Bécard and Fortin, 1988). AM-colonized cultures were maintained at a constant temperature of 24°C on petri dishes with 0.3% (w/v) Phytagel (Sigma-Aldrich, St. Louis) as the gelling agent and with a minimal nutrient medium (Bécard and Fortin, 1988) containing 10 g Suc L−1 as the C source and 35 μm P (as 4.8 mg KH2PO4 L−1).
Experimental Setups
Plugs of solid medium containing carrot roots and mycelium and spores of G. intraradices were transferred at the start of each experiment from 3- to 4-month-old cultures to the experimental two-compartment petri dishes (Maldonado-Mendoza et al., 2001). One plug was transferred to each new dish and inserted into a hole in the solid minimal nutrient medium (about 20 mL) on the root side. The cultures were sealed with Parafilm “M” (American National Can, Chicago). The second compartment of the petri dishes was at this time still empty. At the start of the experimental treatment, the empty compartment was filled with liquid minimal medium lacking Suc and with the 35 μm P replaced by a specific amount of P for each treatment (see details below). Mycelia from experiment 1 were harvested when they had covered the liquid medium compartment and stopped expanding, whereas those in experiments 2 and 3 were harvested while still under expansion.
Experiment 1
Liquid medium was added 21 d after the root side of two-compartment dishes had been inoculated. Roots passing over the barrier between the two compartments were removed at this time. Two P treatments were applied to the liquid medium, one with no P (P-free treatment) and one with 2.5 mm P as KH2PO4 (high-P treatment). The root side was supplied with 10 mg of [13C]d-Glc (U-13C6, 99% [w/w] 13C, Cambridge Isotope Laboratories, Andover, MA) in four replicate dishes 106 d after adding the liquid medium. One dish with P-free treatment was not labeled and was kept as a control to measure the natural abundance of 13C in roots and AM fungal mycelium. The systems were harvested 4 d after labeling, and the systems were at that time 131 d old. Mycelium from the liquid medium was collected and freeze-dried, the dry weight was determined, and the dry mycelium was stored at −20°C until determination of 13C enrichment and lipid composition.
Experiment 2
Liquid medium was added to the second compartment 54 d after inoculation in the root compartment. Four treatments were applied to the liquid medium: no P added (P-free), 25 μm P as KH2PO4 (low-P), 2.5 mm P as KH2PO4 (high-P), and 2.5 mm P as inositol hexaphosphoric acid (Na salt, from corn, Sigma-Aldrich; org-P). Nutrient solutions incubated in sterile petri dishes without cultures were used as controls. One replicate from each treatment was labeled on each of 4 subsequent d. This was done to be able to harvest over 4 d and still have the same period of labeling for all four replicates. The root side was supplied with [13C]d-Glc (10 mg Glc dish−1) 28 to 31 d after adding the liquid medium (roots close to the barrier to the hyphal side were removed twice during this period). The systems were harvested 7 d after labeling, and were at that time 89 to 92 d old. The mycelium from each dish was collected and transferred to liquid medium containing the same treatment, and subsamples of mycelium were taken for microscopical assessment of acid- and alkaline-phosphatase-active hyphal length on the same day. Another subsample of mycelium was fixed in 96% (v/v) ethanol for microscopical determination of polyphosphate accumulation. The remaining mycelium was stored at −20°C until required for freeze-drying, for determination of 13C enrichment, and for lipid extraction and analysis. The liquid growth medium was collected for determination of pH, orthophosphate content, and external phosphatase activity on the same day. The solid medium of the root compartment was dissolved in 250 mL of 10 mm sodium citrate by mixing on a magnetic stirrer for 1 h at low speed. The roots were collected, freeze-dried, weighed, and stored at −20°C for nutrient analysis, for determination of 13C enrichment, and for lipid analysis.
Experiment 3
The liquid medium was added 75 d after inoculation and included three kinds of P treatment (P-free, 35 μm P, and 350 μm P as KH2PO4). The 350 μm P was chosen to obtain a treatment where the P was not depleted at the time of harvest (see Table III), but avoiding a reduced proportion of hyphae with cytoplasm such as caused by the high P in experiment 2 (see Fig. 5). Mycelia were harvested 30 d after adding the liquid medium, and the amount of orthophosphate remaining in the liquid medium was measured. The formation and morphology of the vacuolar system of extraradical hyphae were studied in three replicates each of the 35 μm P and 350 μm P treatments and 1 replicate of the P-free treatment.
Lipid Analysis
Mycelium samples were milled with iron balls (7 mm diameter) in 50-mL Teflon tubes, and roots were ball-milled in iron beakers. The lipids from mycelium and mycorrhizal roots were then extracted by vortex mixing (1 min) in a one-phase mixture of citrate buffer, methanol, and chloroform (0.8:2:1, v/v, pH 4.0; Bligh and Dyer, 1959). The lipids were fractionated into neutral lipids, glycolipids, and phospholipids on prepacked silica columns (100 mg of sorbent mass, Varian Medical Systems, Palo Alto, CA) by eluting with 1.5 mL of chloroform, 6 mL of acetone, and 1.5 mL of methanol, respectively. The fatty acid residues in neutral lipids and phospholipids were transformed into free fatty acid methyl esters and analyzed by gas chromatography using a 50-m HP5 capillary fused silica column (Hewlett Packard, Palo Alto, CA) with H2 as carrier gas (Frostegård et al., 1993). The fatty acids were identified from their retention times in relation to that of the internal standard (fatty acid methyl ester 19:0). These were compared with those identified earlier by gas chromatography-mass spectrometry.
Determination of 13C Enrichment in Solid Samples and Fatty Acids
Freeze-dried mycelium (approximately 20 μg) or ball-milled root material (approximately 100 μg) was enclosed in tin capsules, and 13C atomic percent was determined on an isotope ratio mass spectrometer (20–20 Stable Isotope Analyser, PDZ Europa Scientific Instruments, Crewe, UK) interfaced to a combustion module (ANCA-NT). Fatty acid methyl esters (prepared as described above) were analyzed on the isotope ratio mass spectrometer, interfaced to a Hewlett Packard gas chromatograph to determine the 13C atomic percent in NLFAs and PLFAs. The gas chromatograph was equipped with a 30-m HP5MS capillary column (Hewlett Packard) with He as carrier gas. The 13C enrichment (excess atomic percent 13C) was calculated by subtracting the natural abundance of 13C (1.14%).
Microscopical Investigation of Hyphal Polyphosphate Accumulation and Phosphatase Activity
Subsamples of the extraradical hyphae were placed in 96% (v/v) ethanol at 5°C to precipitate polyphosphate. After 5 d, the samples were washed for 10 min in water, and then stained for 5 min in 0.05% (w/v) toluidine blue O in 25 mm sodium acetate buffer at pH 4.4 (Ashford et al., 1975). Improvement in the penetration of the stain was obtained by spreading the extraradical hyphae out in the staining solution and shaking this suspension continuously. Extraradical hyphae were then washed for 1 min in 1% (v/v) HCl to remove excess staining of compounds other than polyphosphate. The extraradical hyphae were mounted in water on a microscope slide, and a coverslip was sealed immediately with nail varnish. The polyphosphate accumulation was assessed within 2 h of mounting at 500× magnification. For each sample, approximately 200 hyphal intersections were assessed to determine polyphosphate accumulation, which were classified as containing polyphosphate or not containing polyphosphate. The proportion (%) of hyphal length with polyphosphate was determined for hyphae with and without cytoplasm (Fig. 6A).
Extraradical mycelium was subjected to histochemical assessment of acid phosphatase and alkaline phosphatase activity. Collected samples were incubated with a fluorogenic phosphatase substrate (ELF; Molecular Probes, Leiden, The Netherlands), buffered at either pH 4.8 or 8 (Van Aarle et al., 2001). The substrate-buffer solution was filtered through a 0.22-μm filter (Millex-GV, Millipore, Bedford, MA) before use to remove any aggregates of the substrate that may have formed during storage. The samples were mounted on microscope slides with the ELF mounting medium. For each sample, approximately 200 hyphal intersections were assessed at 300× magnification for ELF precipitation, which were classified as active or nonactive (see Fig. 6B). The proportion (%) of acid- and alkaline-phosphatase-active hyphal length was determined for hyphae with cytoplasm and empty hyphae.
Micrographs were recorded on Ultra Gold film (Eastman Kodak, Rochester, NY) with a microscope camera (Zeiss, Welwyn Garden City, UK), using either transmitted long-wavelength light alone or transmitted long-wavelength light combined with UV light.
External Phosphatase Activity
To measure acid phosphatase and alkaline phosphatase activity of the liquid medium, a modified procedure based on that of Tabatabai and Bremner (1969) was used. Phosphatase activities were determined spectrophotometrically using p-nitrophenyl phosphate as substrate (Sigma-Aldrich). Samples of 100 μL were incubated with 100 μL of p-nitrophenyl phosphate solution (4 mg p-nitrophenyl phosphate mL−1 in water) and 100 μL of buffer solution. Tris-HCl buffer (50 mm Tris, pH 9.0) was used for the alkaline phosphatase activity determination and citrate buffer (90 mm citrate and 10 mm chloride, pH 4.8) for the acid phosphatase activity determination. Adding 1 mL of 0.1 m NaOH after 3 h stopped the reaction, and A420 was measured. One unit of phosphatase activity, under the specified conditions, was defined as the amount of enzyme activity that had liberated 1 μmol of p-nitrophenol in 1 h. Liquid media from each kind of treatment, from control dishes without cultures, were used as controls for background A420.
P and N Content in Medium and Roots
The content of orthophosphate (PO4-P) in the liquid medium was analyzed spectrophotometrically using the molybdate blue method of Murphy and Riley (1962). The P content of nutrient solutions stored at −20°C from the start of the experiment, was measured to assess actual starting levels of orthophosphate. One milliliter of sample (diluted if necessary to contain less than 870 μg PO4-P L−1) was mixed with 200 μL of reaction mixture (H2SO4, ammonium molybdate, ascorbic acid, and potassium antimony tartrate), and A882 was measured after 1.5 h of incubation.
Total P and N concentrations in roots were determined after Kjeldahl combustion. Freeze-dried root samples (20 mg) were heated for 1 h at 337°C in H2SO4 with a catalytic mix of K2SO4 and CuSO4. Ammonia and orthophosphate were analyzed with a flow injection analysis system.
Confocal Visualization of the Fungal Vacuolar System
Extraradical mycelia were stained with Oregon green 488 carboxylic acid diacetate (20 μm). The stain solution was prepared with 10 mm MES buffer at pH 5.5. Ten milliliters of the staining solution was added to the petri dish after removing the liquid medium and incubated in the dark at 20°C. After 8 h of incubation, the staining solution was removed, and the mycelium was washed three times with equivalent amounts of buffer. Part of the extraradical mycelium was carefully removed from the dish and mounted in MES buffer. Samples were immediately observed with a confocal laser scanning microscope (TCS/NT, Leica, Wetzlar, Germany). An Ar/Kr laser (488-nm excitation wavelength) was used for illumination and a 63× water immersion (numerical aperture 1.20, free working distance 220 μm) was used. Confocal images (resolution 512 × 512 pixels) were captured at a green wavelength through a long-pass filter LP 495. The digital single image (Fig. 7, A and C) and the reconstructed projection (Fig. 7B) were processed using Corel Photo-Paint (Corel Corporation, Ottawa).
ACKNOWLEDGMENTS
We thank Sabine Ravnskov of the Risø National Laboratory (Denmark) for the inoculum of monoxenic AM cultures and for valuable advice. W.G.A. and A.E.A. thank Prof. B. Söderström and Lund University for facilities while on study leave.
Footnotes
This work was supported by The Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning and by the Carl Trygger Foundation.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.009639.
LITERATURE CITED
- Abbott LK, Robson AD, De Boer G. The effect of phosphorus on the formation of hyphae in soil by the vesicular-arbuscular mycorrhizal fungus, Glomus fasciculatum. New Phytol. 1984;97:437–446. [Google Scholar]
- Allaway WG, Ashford AE. Motile tubular vacuoles in extramatrical mycelium and sheath hyphae of ectomycorrhizal systems. Protoplasma. 2001;215:218–225. doi: 10.1007/BF01280316. [DOI] [PubMed] [Google Scholar]
- Ashford AE. Dynamic pleiomorphic vacuole systems: Are they endosomes and transport components in fungal hyphae? Adv Bot Res. 1998;28:119–159. [Google Scholar]
- Ashford AE, Allaway WG. The role of the motile tubular vacuole system in mycorrhizal fungi. Plant Soil. 2002;244:177–187. [Google Scholar]
- Ashford AE, Ling-Lee M, Chilvers GA. Polyphosphate in eucalypt mycorrhizas: a cytochemical demonstration. New Phytol. 1975;74:447–453. [Google Scholar]
- Bååth E, Spokes J. The effect of added nitrogen and phosphorus on mycorrhizal growth response and infection in Allium schoenoprasum. Can J Bot. 1989;67:3227–3232. [Google Scholar]
- Bago B, Azcon-Aguilar C, Goulet A, Piché Y. Branched absorbing structures (BAS): a feature of the extraradical mycelium of symbiotic arbuscular mycorrhizal fungi. New Phytol. 1998;139:375–388. [Google Scholar]
- Bago B, Pfeffer PE, Shachar-Hill Y. Carbon metabolism and transport in arbuscular mycorrhizas. Plant Physiol. 2000;124:949–957. doi: 10.1104/pp.124.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bago B, Zipfel W, Williams RM, Jun J, Arreola R, Lammers PJ, Pfeffer PE, Shachar-Hill Y. Translocation and utilization of fungal storage lipid in the arbuscular mycorrhizal symbiosis. Plant Physiol. 2002;128:108–124. [PMC free article] [PubMed] [Google Scholar]
- Bécard G, Fortin JA. Early events of vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol. 1988;108:211–218. doi: 10.1111/j.1469-8137.1988.tb03698.x. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Boddington CL, Dodd JC. A comparison of the development and metabolic activity of mycorrhizas formed by arbuscular mycorrhizal fungi from different genera on two tropical forage legumes. Mycorrhiza. 1998;8:149–157. [Google Scholar]
- Cole L, Orlovich DA, Ashford AE. Structure, function and mobility of vacuoles in filamentous fungi. Fungal Genet Biol. 1998;24:86–100. doi: 10.1006/fgbi.1998.1051. [DOI] [PubMed] [Google Scholar]
- Douds DD, Jr, Johnson CR, Koch KE. Carbon cost of the fungal symbiont relative to net leaf P accumulation in a split-root VA mycorrhizal symbiosis. Plant Physiol. 1988;86:491–496. doi: 10.1104/pp.86.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezawa T, Smith SE, Smith FA. Differentiation of polyphosphate metabolism between the extra- and intraradical hyphae of arbuscular mycorrhizal fungi. New Phytol. 2001;149:555–563. doi: 10.1046/j.1469-8137.2001.00040.x. [DOI] [PubMed] [Google Scholar]
- Fortin JA, Bécard G, Declerck S, Dalpé Y, St-Arnaud M, Coughlan AP, Piché Y. Arbuscular mycorrhiza on root-organ cultures. Can J Bot. 2002;80:1–20. [Google Scholar]
- Frostegård Å, Tunlid A, Bååth E. Phospholipid fatty acid composition, biomass and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl Environ Microbiol. 1993;59:3605–3617. doi: 10.1128/aem.59.11.3605-3617.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianinazzi S, Gianinazzi-Pearson V. Enzymatic studies on the metabolism of vesicular-arbuscular mycorrhiza: III. Ultrastructural localization of acid and alkaline phosphatase in onion roots infected by Glomus mosseae (Nicol. & Gerd.) New Phytol. 1978;82:127–132. [Google Scholar]
- Graham JH, Duncan LW, Eissenstat DM. Carbohydrate allocation patterns in citrus genotypes as affected by phosphorus nutrition, mycorrhizal colonization and mycorrhizal dependency. New Phytol. 1997;135:335–343. [Google Scholar]
- Grotelueschen J, Peleg Y, Glass NL, Metzenberg RL. Cloning and characterization of the pho-2+ gene encoding a repressible alkaline phosphatase in Neurospora crassa. Gene. 1994;113:129–133. doi: 10.1016/0378-1119(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Harrison MJ, Van Buuren ML. A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature. 1995;378:626–629. doi: 10.1038/378626a0. [DOI] [PubMed] [Google Scholar]
- Ho I, Trappe JM. Translocation of 14C from Festuca plants to their endomycorrhizal fungi. Nature. 1973;224:30–31. doi: 10.1038/newbio244030a0. [DOI] [PubMed] [Google Scholar]
- Jasper DA, Robson AD, Abbott LK. Phosphorus and formation of vesicular-arbuscular mycorrhizas. Soil Biol Biochem. 1979;11:501–505. [Google Scholar]
- Joner EJ, Ravnskov S, Jakobsen I. Arbuscular mycorrhizal phosphate transport under monoxenic conditions using radio-labelled inorganic and organic phosphate. Biotechnol Lett. 2000;22:1705–1708. [Google Scholar]
- Kaffman A, Herskowitz I, Tijan R, O'Shea EK. Phosphorylation of the transcription factor PHO4 by a cyclin-CDK complex, PHO80-PHO85. Science. 1994;263:1153–1156. doi: 10.1126/science.8108735. [DOI] [PubMed] [Google Scholar]
- Koide RT, Kabir Z. Extraradical hyphae of the mycorrhizal fungus Glomus intraradices can hydrolyse organic phosphate. New Phytol. 2000;148:511–517. doi: 10.1046/j.1469-8137.2000.00776.x. [DOI] [PubMed] [Google Scholar]
- Lösel DM. Fungal lipids. In: Ratledge C, Wilkinson SG, editors. Microbial Lipids. Vol. 1. London: Academic Press; 1988. [Google Scholar]
- Maldonado-Mendoza IE, Dewbre GR, Harrison MJ. A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus Glomus intraradices is regulated in response to phosphate in the environment. Mol Plant-Microbe Interact. 2001;14:1140–1148. doi: 10.1094/MPMI.2001.14.10.1140. [DOI] [PubMed] [Google Scholar]
- Menge JA, Steirle D, Bagyaraj DJ, Johnson ELV, Leonard RT. Phosphorus concentrations in plants responsible for inhibition of mycorrhizal infection. New Phytol. 1978;80:575–578. [Google Scholar]
- Miranda JCC, Harris PJ. Effects of soil phosphorus on spore germination and hyphal growth of arbuscular mycorrhizal fungi. New Phytol. 1994;128:103–108. doi: 10.1111/j.1469-8137.1994.tb03992.x. [DOI] [PubMed] [Google Scholar]
- Mosse B. Plant growth responses to vesicular-arbuscular mycorrhiza: IV. In soil given additional phosphate. New Phytol. 1973;72:127–136. [Google Scholar]
- Murphy J, Riley JP. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta. 1962;27:31–36. [Google Scholar]
- Nagahashi G, Douds DD, Jr, Abney GD. Phosphorus amendment inhibit hyphal branching of the VAM fungus Gigaspora margarita directly and indirectly through its effect on root exudation. Mycorrhiza. 1996;6:403–408. [Google Scholar]
- Ogawa N, DeRisi J, Brown PO. New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol Biol Cell. 2000;11:4309–4321. doi: 10.1091/mbc.11.12.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson PA, Bååth E, Jakobsen I. Phosphorus effects on the mycelium and storage structures of an arbuscular mycorrhizal fungus as studied in the soil and roots by analysis of fatty acid signatures. Appl Environ Microbiol. 1997;63:3531–3538. doi: 10.1128/aem.63.9.3531-3538.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson PA, Johansen A. Lipid and fatty acid composition of hyphae and spores of arbuscular mycorrhizal fungi at different growth stages. Mycol Res. 2000;104:429–434. [Google Scholar]
- Pearson JN, Jakobsen I. The relative contribution of hyphae and roots to phosphorus uptake by arbuscular mycorrhizal plants, measured by dual labelling with 32P and 33P. New Phytol. 1993;124:489–494. [Google Scholar]
- Peng S, Eissenstat DM, Graham JH, Williams K, Hodge NC. Growth depression in mycorrhizal citrus at high-phosphorus supply: analysis of carbon cost. Plant Physiol. 1993;101:1063–1071. doi: 10.1104/pp.101.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer PE, Douds DD, Jr, Bécard G, Shachar-Hill Y. Carbon uptake and the metabolism and transport of lipids in an arbuscular mycorrhiza. Plant Physiol. 1999;120:587–598. doi: 10.1104/pp.120.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen N, Lloyd DC, Ratcliffe RG, Hansen PE, Jakobsen I. 31P NMR for the study of P metabolism and translocation in arbuscular mycorrhizal fungi. Plant Soil. 2000;226:245–253. [Google Scholar]
- Sanders FE. The effect of foliar-applied phosphate on the mycorrhizal infections of onion roots. In: Sanders FE, Mosse B, Tinker PB, editors. Endomycorrhizas. London: Academic Press; 1975. pp. 261–276. [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal Symbiosis. Ed 2. San Diego: Academic Press; 1997. [Google Scholar]
- Solaiman MZ, Ezawa T, Kojima T, Saito M. Polyphosphates in intraradical and extraradical hyphae of an arbuscular mycorrhizal fungus, Gigaspora margarita. Appl Environ Microbiol. 1999;65:5604–5606. doi: 10.1128/aem.65.12.5604-5606.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaiman MZ, Saito M. Phosphate efflux from intraradical hyphae of Gigaspora margarita in vitro and its implication for phosphorus translocation. New Phytol. 2001;151:525–533. [Google Scholar]
- Tabatabai MA, Bremner JM. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem. 1969;1:301–307. [Google Scholar]
- Tunlid A, White DC. Biochemical analysis of biomass, community structure, nutritional status, and metabolic activity of microbial communities in soil. Soil Biochemistry. Vol. 7. New York: Marcel Dekker; 1992. pp. 229–262. [Google Scholar]
- Uetake Y, Kojima T, Ezawa T, Saito M. Extensive tubular vacuole system in an arbuscular mycorrhizal fungus, Gigaspora margarita. New Phytol. 2002;154:761–768. doi: 10.1046/j.1469-8137.2002.00425.x. [DOI] [PubMed] [Google Scholar]
- Van Aarle IM, Olsson PA, Söderström B. Microscopic detection of phosphatase activity of saprophytic and arbuscular mycorrhizal fungi using a fluorogenic substrate. Mycologia. 2001;93:17–24. [Google Scholar]
- Versaw WK. A phosphate-repressible, high affinity phosphate permease is encoded by the pho-5+ gene of Neurospora crassa. Gene. 1995;153:135–139. doi: 10.1016/0378-1119(94)00814-9. [DOI] [PubMed] [Google Scholar]