Abstract
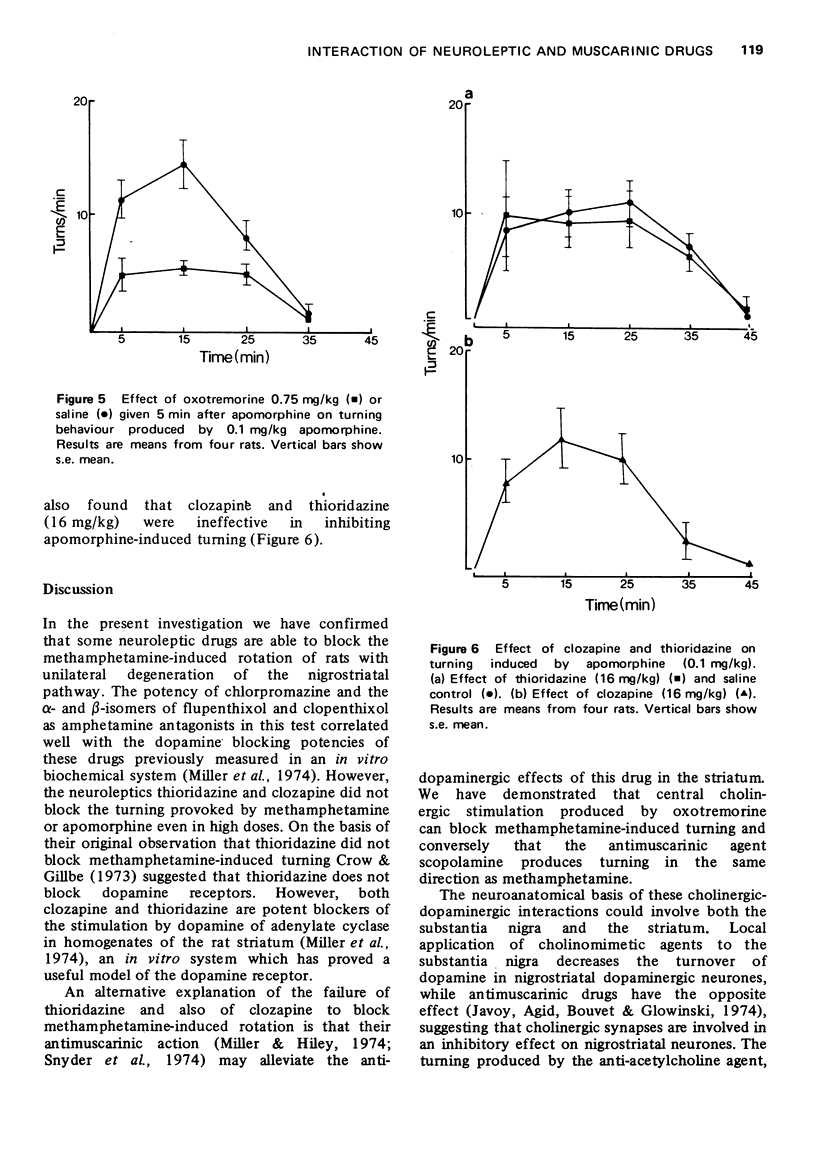
1. The effect of muscarinic and neuroleptic agents on the turning behaviour induced by methamphetamine and apomorphine in rats with unilateral lesions of the substantia nigra induced by 6-hydroxydopamine has been examined. 2. Turning towards the side of the lesion induced by (+)-methamphetamine (5 mg/kg) was inhibited by alpha-flupenthixol (1 mg/kg) and alpha-clopenthixol (8 mg/kg) but not by high doses of their beta-isomers. 3. Turning was inhibited by chlorpromazine (4 mg/kg) and pimozide (0.2 mg/kg). Thioridazine and clozapine (16 mg/kg) were ineffective. Turning in the same direction produced by scopolamine (10 mg/kg) was also inhibited by alpha-flupenthixol (1 mg/kg) and pimozide (0.25 mg/kg). 4. Turning produced by methamphetamine (5 mg/kg) was inhibited by oxotremorine (0.75 mg/kg) even in the presence of methylatropine (5 mg/kg). 5. Turning away from the side of the lesion induced by apomorphine (0.1 mg/kg) was inhibited by oxotremorine (0.75 mg/kg) but not by thioridazine or clozapine (16 mg/kg). 6. These results are discussed with regard to the mode of action of neuroleptic drugs in producing anti-psychotic effects and drug-induced Parkinsonism.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andén N. E., Butcher S. G., Corrodi H., Fuxe K., Ungerstedt U. Receptor activity and turnover of dopamine and noradrenaline after neuroleptics. Eur J Pharmacol. 1970;11(3):303–314. doi: 10.1016/0014-2999(70)90006-3. [DOI] [PubMed] [Google Scholar]
- Bürki H. R., Ruch W., Asper H., Baggiolini M., Stille G. Pharmakologische und neurochemische Wirkungen von Clozapin: neue Gesichtspunkte in der medikamentösen Behandlung der Schizophrenie. Schweiz Med Wochenschr. 1973 Dec 1;103(48):1716–1724. [PubMed] [Google Scholar]
- COLE J. O., CLYDE D. J. Extrapyramidal side effects and clinical response to the phenothiazines. Rev Can Biol. 1961 Jun;20:565–574. [PubMed] [Google Scholar]
- Clement-Cormier Y. C., Kebabian J. W., Petzold G. L., Greengard P. Dopamine-sensitive adenylate cyclase in mammalian brain: a possible site of action of antipsychotic drugs. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1113–1117. doi: 10.1073/pnas.71.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B., Naylor R. J., Olley J. E. Catalepsy and circling behaviour after intracerebral injections of neuroleptic, cholinergic and anticholinergic agents into the caudate-putamen, globus pallidus and substantia nigra of rat brain. Neuropharmacology. 1972 Sep;11(5):645–663. doi: 10.1016/0028-3908(72)90073-1. [DOI] [PubMed] [Google Scholar]
- Crow T. J., Gillbe C. Dopamine antagonism and antischizophrenic potency of neuroleptic drugs. Nat New Biol. 1973 Sep 5;245(140):27–28. doi: 10.1038/newbio245027a0. [DOI] [PubMed] [Google Scholar]
- Horn A. S., Cuello A. C., Miller R. J. Dopamine in the mesolimbic system of the rat brain: endogenous levels and the effects of drugs on the uptake mechanism and stimulation of adenylate cyclase activity. J Neurochem. 1974 Feb;22(2):265–270. doi: 10.1111/j.1471-4159.1974.tb11589.x. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine in the basal ganglia. Its role and therapeutic implications (including the clinical use of L-DOPA). Br Med Bull. 1973 May;29(2):172–178. doi: 10.1093/oxfordjournals.bmb.a070990. [DOI] [PubMed] [Google Scholar]
- Javoy F., Agid Y., Bouvet D., Glowinski J. Changes in neostriatal DA metabolism after carbachol or atropine microinjections into the substantia nigra. Brain Res. 1974 Mar 22;68(2):253–260. doi: 10.1016/0006-8993(74)90394-1. [DOI] [PubMed] [Google Scholar]
- Miller R. J., Hiley C. R. Anti-muscarinic properties of neuroleptics and drug-induced Parkinsonism. Nature. 1974 Apr 12;248(449):596–597. doi: 10.1038/248596a0. [DOI] [PubMed] [Google Scholar]
- Sethy V. H., Van Woert M. H. Regulation of striatal acetylcholine concentration by dopamine receptors. Nature. 1974 Oct 11;251(5475):529–530. doi: 10.1038/251529a0. [DOI] [PubMed] [Google Scholar]
- Shintomi K., Yamamura M. Letter: Effects of antiparkinsonian drugs on neuroleptic-induced extrapyramidal signs in monkeys. J Pharm Pharmacol. 1973 Aug;25(8):666–667. doi: 10.1111/j.2042-7158.1973.tb10661.x. [DOI] [PubMed] [Google Scholar]
- Snyder S., Greenberg D., Yamamura H. I. Antischizophrenic drugs and brain cholinergic receptors. Affinity for muscarinic sites predicts extrapyramidal effects. Arch Gen Psychiatry. 1974 Jul;31(1):58–61. doi: 10.1001/archpsyc.1974.01760130040006. [DOI] [PubMed] [Google Scholar]
- Stadler H., Lloyd K. G., Gadea-Ciria M., Bartholini G. Enhanced striatal acetylcholine release by chlorpromazine and its reversal by apomorphine. Brain Res. 1973 Jun 15;55(2):476–480. doi: 10.1016/0006-8993(73)90317-x. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U., Arbuthnott G. W. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970 Dec 18;24(3):485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]


