Abstract
In commercial crops, maize (Zea mays) plants are typically grown at a larger distance between rows (70 cm) than within the same row (16–23 cm). This rectangular arrangement creates a heterogeneous environment in which the plants receive higher red light (R) to far-red light (FR) ratios from the interrow spaces. In field crops, the hybrid Dekalb 696 (DK696) showed an increased proportion of leaves toward interrow spaces, whereas the experimental hybrid 980 (Exp980) retained random leaf orientation. Mirrors reflecting FR were placed close to isolated plants to simulate the presence of neighbors in the field. In addition, localized FR was applied to target leaves in a growth chamber. During their expansion, the leaves of DK696 turned away from the low R to FR ratio signals, whereas Exp980 leaves remained unaffected. On the contrary, tillering was reduced and plant height was increased by low R to FR ratios in Exp980 but not in DK696. Isolated plants preconditioned with low R/FR-simulating neighbors in a North-South row showed reduced mutual shading among leaves when the plants were actually grouped in North-South rows. These observations contradict the current view that phytochrome-mediated responses to low R/FR are a relic from wild conditions, detrimental for crop yield.
Plants are able to modify their foliage architecture in response to the environment. In maize (Zea mays), for instance, leaf orientation can switch from a random distribution in nearly isolated plants (i.e. 3 plants m−2) to a distich distribution where the leaves are placed perpendicular to rows, when the plants are grown at commercial crop densities (i.e. 9–10 plants m−2; Girardin, 1992; Stewart and Dwyer, 1993; Girardin and Tollenaar, 1994; Maddonni et al., 2001a). Thus, maize canopies may adopt a nonisotropic structure with a preferential across-row orientation of the leaves. Both field measurements and computer simulations indicate that maize canopies with leaves perpendicular to the rows may present increased light interception (about 10% higher) and grain yield (about 10% higher) than similar canopies with randomly oriented leaves (Toler et al., 1999; Maddonni et al., 2001b). Therefore, it is of importance to understand the mechanisms that drive leaf reorientation.
Typically, phototropic (Firn, 1994) and diagravitropic (Ritter and Koller, 1994) responses are dominated by the blue region of the spectrum. These effects are mediated at least partially by phototropins (Briggs et al., 2001). However, reorientation of leaves has been observed at early ontogenic stages of maize crops (Girardin, 1992; Maddonni et al., 2001a), a condition where blue light gradients depend on solar angle rather than the presence of neighbors. In contrast, low red light (R) to far-red light (FR) ratios, caused by FR reflected on green leaves, provide an early signal of the presence of neighbors, which is anticipated to mutual shading and competition for light (Ballaré et al., 1987). The extent of reduction in R to FR ratio correlates with the proximity of the surrounding vegetation (Smith et al., 1990; Sattin et al., 1994). The R/FR signals of spaced canopies promote stem growth in dicotyledonous weeds (Ballaré et al., 1987, 1990) and reduce tillering in grasses (Casal et al., 1986, 1987). Thus, it is tempting to speculate that leaf orientation responds to the R to FR ratio, minimizing mutual shading among plants.
The R to FR ratio signals are perceived by phytochromes, a family of photoreceptors with two photo-interconvertible forms, Pr and Pfr (Smith, 2000). High R to FR ratios increase the proportion of phytochromes in the biologically active Pfr form. Phytochromes modulate growth and development throughout plant development, including seed germination, seedling de-etiolation, shoot morphology, and flowering (Ballaré and Casal, 2000; Casal, 2002).
Phytochrome-mediated changes in the orientation of whole shoots and individual leaves enhance light capture. The shoots of Cucumis sativus colonize empty spaces beneath dense canopies by modifying node spatial distribution toward sites with high R/FR, a reaction missing in the lh mutant deficient in phytochrome B (Ballaré et al., 1995). The tillers of Lolium multiflorum adopt a more erectophile position in response to low R to FR ratios, placing leaf lamina at higher, better illuminated strata of the canopy (Casal et al., 1990). Low R to FR ratios also reduce solar tracking by sunflower (Helianthus annuus) leaves (Casal and Sadras, 1987). The shoots of the parasite plant Cusucuta planiflora move toward low R to FR ratios, searching for host plants that provide a source of photo-assimilates (Orr et al., 1996). Phytochrome also controls tillering and leaf growth in forage grasses (Deregibus et al., 1983, 1985; Casal et al., 1987), wheat (Triticum aestivum; Kasperbauer and Karlen, 1986; Casal, 1988), barley (Hordeum vulgare; Skinner and Simmons, 1993), and sorghum (Sorghum bicolor; Childs et al., 1997), and flowering time in sorghum (Childs et al., 1997), rice (Oryza sativa; Izawa et al., 2000), and barley (Hanumappa et al., 1999). However, information on photomorphogenic responses in maize is limited to seedling stages, where shoot stature is increased by low R to FR ratio (Smith, 1980; Kasperbauer and Karlen, 1994). When maize plants are grown in the field, tillering only takes place at low plant densities (<3.5 plants m−2), suggesting that small reductions in R to FR ratio could severely reduce tillering in this species (Tetio-Kagho and Gardner, 1988).
It is tempting to speculate that leaf orientation in maize responds to the R to FR ratio and is part of a strategy to minimize mutual shading among plants. The aim of this work was to investigate this hypothesis.
RESULTS
R to FR Ratios Provide a Row Orientation Signal
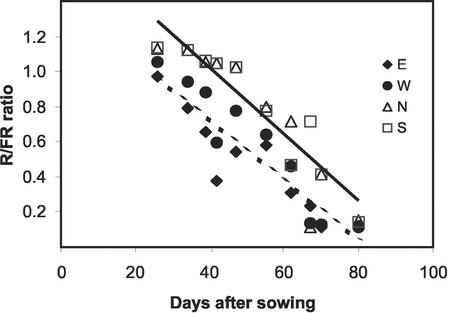
We investigated the horizontal pattern of the R to FR ratio within a maize canopy with east-west-oriented rows sown at a distance of 70 cm between them. We placed the remote probe of the R/FR sensor 8 cm apart from target plants in the direction of the four cardinal points, at the base of the stem, and facing upwards. The R to FR ratio was higher when the sensor was placed toward the north or the south compared with the east or the west (Fig. 1). In field-grown maize, heterogeneity in R/FR environments is evidenced from early ontogenic stages to flowering. This heterogeneity may serve as a signal for leaf reorientation.
Figure 1.
The rectangular arrangement of commercial maize crops creates a heterogeneous horizontal pattern of R to FR ratios. Time course of the R to FR ratio measured at midday with the sensor placed 8 cm toward the east, west, north, or south of target plants grown in east-west-oriented rows with an interrow distance of 70 cm and a density of 8 plants m−2. Data are the means of three measurements.
Azimuthal Orientation of Leaves Is Modified by R to FR Ratio Signals
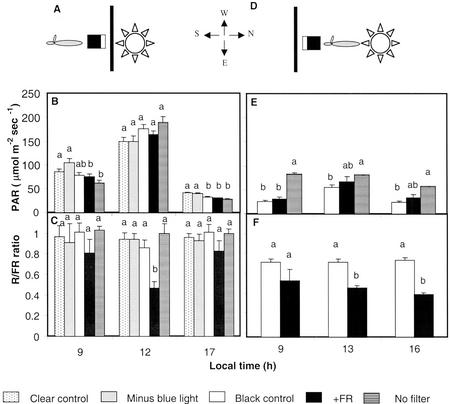
To investigate the effect of R to FR ratio signals on leaf reorientation, we used a filter sandwich selectively reflecting FR placed toward the north side of plants of the hybrid Dekalb 696 (DK696), which were grown isolated in the field (Fig. 2A). As controls, we used a black filter (both black and FR-reflecting filters did not transmit light) and a clear filter. We also included an orange filter (99% reduction of the light intensity between 400–500 nm) to investigate whether any alteration of blue light caused by the black and FR-reflecting filters was having an effect. The filters had negligible effects on the PAR received by the plants (Fig. 2B) because due to the distance between the filter and the plants and the solar angle, the filters did not shade the plants. The most obvious effect was the reduced R to FR ratio caused by the FR-reflecting filter (Fig. 2C). The lowest R to FR ratio (approximately 0.5) occurred at midday and was within the range of R to FR ratios observed in the field at the nine-leaf stage of plants when the sensor was placed facing plants on the same row (0.5, 0.32, and 0.34 for plant densities of 3, 9, or 12 plants m−2, respectively).
Figure 2.
Light environment created by selective filters placed toward the north (A–C) or the south (D–F) of isolated maize plants grown in the field. A and D, Schematic representation of the position of the filters. The photosynthetically active radiation (PAR) and R/FR sensors were placed close to the plants (<1 cm), with their sensing surface (white rectangles) facing toward the filters. B and E, PAR. C and F, R to FR ratio. Data are means and se of four measurements. Different letters above bars indicate significant differences (P < 0.05) among filters for each local time.
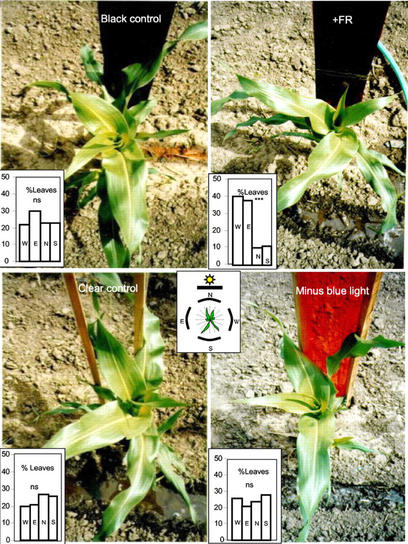
We measured the pattern of leaf orientation on the horizontal plane at the seven-leaf stage. Leaf orientation of plants cultivated toward the south of black, clear, or minus blue light filters showed a random distribution toward the four cardinal axes (Fig. 3). However, plants cultivated toward the south of the FR-reflecting filter presented a distichous phyllotaxy. Their leaves were orientated mainly toward the East-West direction, i.e. parallel to the FR-reflecting filter. Thus, maize leaves are able to turn away from the reflected FR signal, simulating a nearby neighbor.
Figure 3.
Leaf orientation responds to FR-simulating neighbors. Isolated plants of maize were grown in the field with a selective mirror placed 15 cm toward the north. The proportion of leaves was calculated for groups of 10 plants in each filter class. ***, Leaf distribution significantly (P < 0.001) different from random frequency (chi square test).
Cultivar-Specific Responses to Neighbors and R/FR Signals
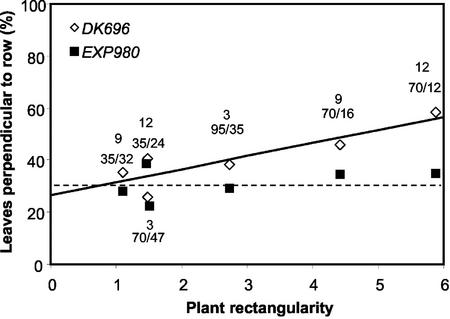
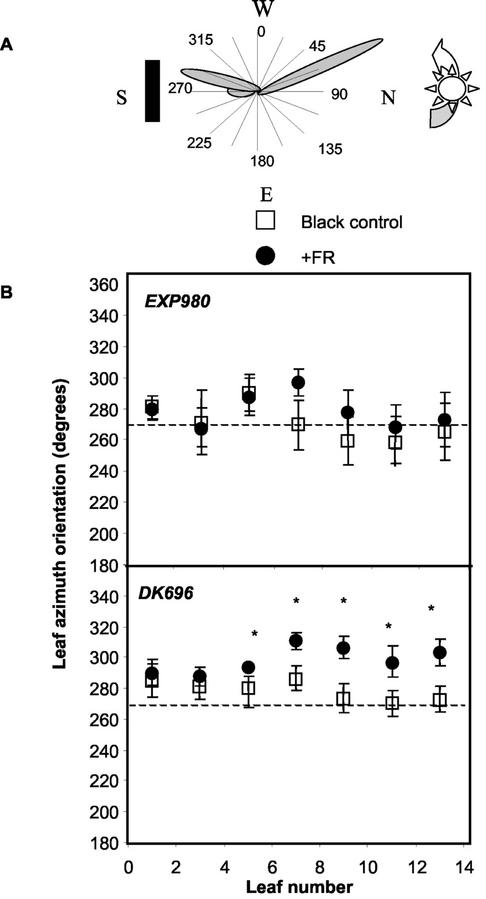
We also compared the azimuthal orientation of leaves in canopies of two hybrids cultivated under different plant distances within the row and between adjacent rows. These plant arrangements resulted in a series of values of plant rectangularity (i.e. the ratio between the longest and the shortest distance between plants; Willey and Heath, 1969). Plant rectangularity close to 1 indicates a regular spatial arrangement, and values larger than 1 represent a rectangular planting geometry. The hybrids differed in the response of leaf orientation to plant spatial distribution (Fig. 4). In Exp980, the proportion of leaves perpendicular to the rows was relatively constant (about 30%) at different plant rectangularities. In DK696, the proportion of leaves perpendicular to row increased significantly with the increment of plant rectangularity (y = 26.5 + 5x; R2 = 0.77, P < 0.05).
Figure 4.
Proportion of leaves of a canopy orientated perpendicular to the row in maize hybrids DK696 and experimental hybrid 980 (Exp980) as affected by plant rectangularity (distance between rows/distance within rows). Leaf orientation was measured at tasseling in 15 plants of each condition. A leaf was classified as perpendicular to the row when its azimuthal angle with the row line (0°–180°) was comprised within either the 67° 30′ to 112° 30′ or 247°30′ to 292° 30′ intervals (Girardin and Tollenaar, 1994). Plant population density (plants m−2) and the ratio between the longest and the shortest distance between two plants are indicated next to the symbols. Regression lines were fitted to each hybrid data. DK696 data are redrawn after Maddonni et al. (2001a).
To investigate whether the differential behavior of the hybrids correlated with differential responses to R/FR signals, we tested leaf orientation responses of DK696 and Exp980 to reflected FR. In this experiment, the FR-reflecting and black filters were placed at the south side of the plants (15 cm apart) and the first leaf pointed toward the filters after transplant (Fig. 2D). Both filters caused a similar reduction in diffuse light incident from the south (Fig. 2E). The FR-reflecting filter reduced the R to FR ratio received by the plants from the south from approximately 0.7 to 0.5 throughout the photoperiod (Fig. 2F). Azimuthal leaf orientation was measured after tasseling. Odd-numbered leaves of DK696 grown beside a FR-reflecting filter departed more from the initial south orientation than those of plants cultivated beside the black control (Fig. 5, A and B). The orientation of leaves inserted above the height of the filters (from the 15th leaf onwards) was not affected. Even numbered leaves, which were conditioned by transplanting to grow toward the north, did not change their orientation in response to FR arriving from the south (data not shown). In Exp980 leaves, orientation was not affected by FR reflection. These results indicate that the FR signal causes leaf reorientation in a hybrid-specific manner. Our results also suggest that leaf reorientation is probably a local reaction to FR because only leaves growing close to the stimulus turn away from the light signal.
Figure 5.
Differential effects of unilateral FR-simulating neighbors on leaf orientation of DK696 and Exp980 plants grown isolated in the field. A, Schematic representation of the experimental setting. B, Azimuthal orientation of odd-numbered leaves measured at tasseling. The dashed line indicates the angle of the first leaf fixed at transplant. Data are means and se of 10 to 15 plants. *, P < 0.05.
Maize Leaf Reorientation Is a Local Reaction to FR
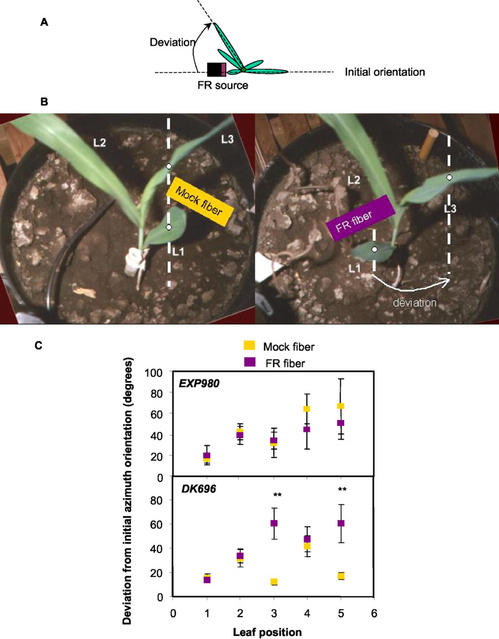
Next, we conducted experiments in a growth chamber to study if leaf reorientation is a local or systemic reaction to FR. The third and fifth leaves were locally FR irradiated during their unfolding periods (Fig. 6, A and B). In DK696, the FR treatment increased the deviation of irradiated leaves from the predicted position based on the orientation of the first leaf (which pointed toward the FR or mock source, Fig. 6). In contrast, the leaves of EXP980 were not significantly affected by FR. The leaf reorientation capacity observed in plants of DK696 was a local reaction to FR because the position of the second and forth (nonirradiated) leaves was not affected (Fig. 6C).
Figure 6.
Localized effects of FR on leaf reorientation. A, Schematic representation of the experimental setting. The first leaf was placed in the direction of the optic fibers providing FR but the probe was adjusted to the height of the third and fifth leaves during their expansion. B, Representative control (left) and FR-treated (right) plants of DK696. Note that in the control, leaf 1 (L1) and leaf 3 (L3) are on the same orthostic, whereas the FR treatment deviated L3 from the vertical plane containing L1. C, Deviation from the azimuthal orientation of L1 in EXP980 and DK696. Data are means and se of six (Exp980) or 12 (DK696) plants. **, P < 0.01.
Leaf Reorientation Capacity Minimizes Interference among Plants
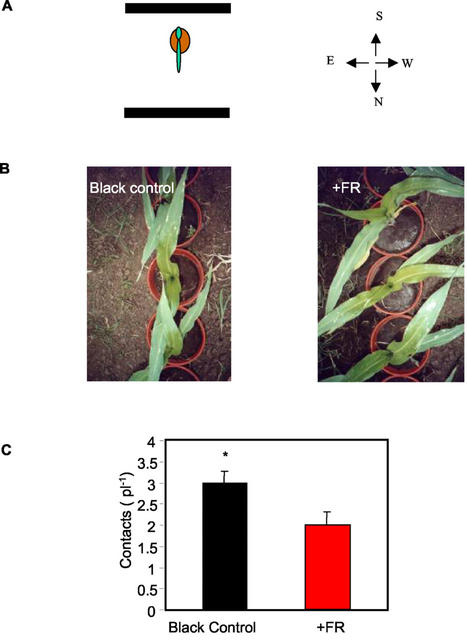
We investigated if the capacity of DK696 to modify leaf orientation alters interference among plants. Isolated plants of DK696 were grown in the field with their first two leaves bearing East-West orientation. Either black or FR-reflecting filters were placed toward the south and the north (Fig. 7A) of the plants. The plants cultivated with black control filters received a similar R to FR ratio (approximately 0.90 ± 0.04) from the four cardinal points. The FR-reflecting filters simulated a north-south-oriented row with a lower R to FR coming from the north and south (about 0.29 ± 0.03) than from the east and west (about 0.71 ± 0.05). After 20 d of preconditioning with or without simulated neighbors (FR-reflecting filters), all the plants were effectively grouped in north-south-oriented rows (with a distance of 0.22 m between plants, which is typical of commercial crops) without altering the orientation that their leaves had achieved (Fig. 7B). The interference of the foliage between adjacent plants within the row was significantly lower when the plants had been preconditioned with stimulated low R/FR signals (Fig. 7C).
Figure 7.
Preconditioning with FR-reflecting filters simulating north-south-oriented rows reduces mutual plant shading when the plants are grouped in north-south-oriented rows. A, Plants of DK696 were grown in pots between black or FR-reflecting filters placed toward the north and toward the south in the field. The first leaf pointed toward the south. B, At the five-leaf stage, plants were grouped in north-south-oriented rows (23 cm between plants). C, Number of contacts among leaves of neighbor plants. Data are means and se of 27 plant pairs.
Tiller Production and Plant Stature Responses to FR
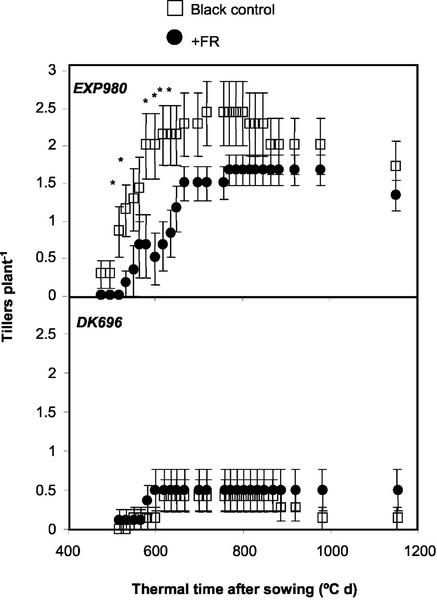
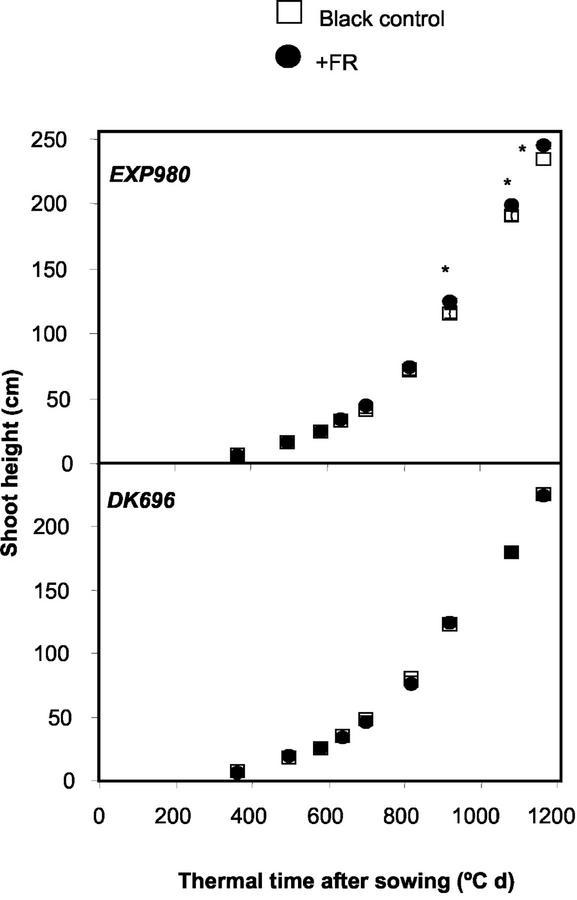
Tiller number and plant stature were also investigated in isolated plants cultivated with black or FR-reflecting filters placed toward the south (Fig. 2, D–F). The hybrid Exp980, i.e. the genotype showing no leaf orientation response, showed reduced tillering (Fig. 8) and slightly enhanced stature (Fig. 9). The hybrid DK696, which exhibited leaf orientation responses, did not show tiller and height responses to FR.
Figure 8.
Differential effects of unilateral FR-simulating neighbors on tiller number of DK696 and Exp980 plants grown isolated in the field. Tiller number is plotted against thermal time (base temperature 8°C) after sowing (Ritchie and NeSmith, 1991). Data are means and se of 10 to 15 plants. *, P < 0.05.
Figure 9.
Differential effects of unilateral FR simulating neighbors on shoot height of DK696 and Exp980 plants grown isolated in the field. Height is plotted against thermal time (base temperature 8°C) after sowing (Ritchie and NeSmith, 1991). Data are means and se of 10 to 15 plants. *, P < 0.05.
DISCUSSION
Maize leaves skip neighbor plants by orientating their growth away from low R/FR signals. Under low R to FR ratios, eudicots tend to place their leaves in a more erect position in a movement articulated at the base of the petioles (Satter and Whetherell, 1968), and forage grasses place the entire shoot in a more erect position (Casal et al., 1990). We did not observe obvious changes in the angle of maize leaves on a vertical plane in response to R/FR signals simulating neighbor plants. In contrast, maize leaves showed a distinctive displacement of the lamina on the horizontal plane.
The shade avoidance syndrome (Smith, 2000) involves a series of changes in plant architecture in response to the low R to FR ratio of vegetation canopies, which improve the exposure of the foliage to photosynthetic light. Phytochrome-mediated changes include enhanced axis growth, reduced branching, organ reorientation, and accelerated flowering. The relative importance of these responses depends on the species. In caulescent eudicots, the most obvious response is the enhanced stem growth (Smith, 1982); in rosette eudicots, the most obvious responses are a combination of enhanced petiole growth and erectophile position of leaves (Satter and Whetherell, 1968). In forage grasses (Deregibus et al., 1983, 1985; Casal et al., 1987) and winter cereals (Kasperbauer and Karlen, 1986; Casal et al., 1986; Casal, 1988, 1990; Skinner and Simmons, 1993), reduced tillering is the most dramatic response to low R to FR ratio. In maize, plant stature (Fig. 9) and tillering (Fig. 8) responded to R to FR ratio but the largest effects were those associated with a redirection of the leaves toward gaps with high R to FR ratio (Figs. 3, 5, and 6).
The gaps within the canopy created areas of higher irradiance and R to FR ratio. Maize leaves moved away from areas of low R to FR ratio via localized reaction. When expanding leaves of plants grown under white light were irradiated unilaterally with supplementary FR, these leaves moved away from the FR-treated site (Fig. 6). The leaves normally appearing with an orientation opposite to the FR-treated site were unaffected by FR (Fig. 6).
Changes in R to FR ratio are perceived by phytochromes. Five phytochromes (A–E) are present in Arabidopsis, where phytochromes B, D, and E mediate responses to the R to FR ratio (Reed et al., 1993; Yanovsky et al., 1995; Devlin et al., 1998, 1999). Only three phytochromes (A–C) are present in monocots (Mathews and Sharrock, 1997), but these genes are duplicated in maize (Christensen and Quail, 1989). Phytochrome B is involved in the control of plant stature, tillering, and flowering time in sorghum (Childs et al., 1997), and the control of flowering time in barley (Hanumappa et al., 1999). Phytochrome A has weaker effects in light-grown grasses seedlings (Casal et al., 1996; Shlumukov et al., 2001; Takano et al., 2001). Thus, the phytochrome B pair is a likely candidate to mediate R/FR perception, inducing changes in maize leaf orientation. Although blue light plays a key role in phototropism and solar tracking (Firn, 1994; Ritter and Koller, 1994), we did not observe changes in leaf orientation in response to altered blue light (Fig. 3). The effect of our blue light filters was negligible during sunny days (Fig. 2), when direct light reached the plants without filter interference, but these filters reduced diffuse blue light. This signal was likely too weak to alter leaf orientation but did modify tillering in one of the genotypes (Exp980 maximum tillers plant−1: control = 1.90 ± 0.1; minus blue light filter = 1.36 ± 0.23).
A number of observations have provided support to the idea that the photomorphogenic responses induced by the low R to FR ratio of crop canopies are detrimental to yield. Increasing the R to FR ratio by supplementing the lower strata of the canopy with R during flowering and early pod set reduces the abscission of reproductive structures in soybean (Glycine max) plants (Heindl and Brun, 1983). Expressing low levels of oat (Avena sativa) phytochrome A increases leaf/stem dry weight ratio of tobacco (Nicotiana tabacum; Robson et al., 1996). Expressing Arabidopsis phytochrome B in potato (Solanum tuberosum) increases maximum photosynthesis rates, at least in isolated plants (Thiele et al., 1999). In sunflower plants grown at low densities, lowering the R to FR ratios reaching the stem reduces grain yield (Libenson et al., 2002). Thus, the current consensus is that photomorphogenic responses were selected under wild conditions and impose a limitation to crop yield that should be eliminated. The observations presented here do not fit this model. The ability of maize leaves to skip areas of low R to FR ratio caused by FR reflected on neighbor plant leaves reduced mutual shading (Fig. 7). The impact of redirection of leaf growth to gaps of high R to FR ratios is predicted to have a larger beneficial effect on light interception in crops sown at a larger distance between rows than between plants within the same row, i.e. rectangular patterns. Field measurements and computer simulations using virtual plant models that explicitly describe the three-dimensional plant geometry (e.g. Andrieu et al., 1995) indicate that under rectangular planting patterns, maize canopies with leaves perpendicular to rows may present higher efficiency of light attenuation than similar canopies where plants have a random leaf orientation (Maddonni et al., 2001b). Thus, the leaf position response is particularly effective under agricultural conditions. A definite judgment of the impact of the R/FR-controlled leaf orientation will require the quantification of grain yield in field experiments with near-isogenic lines. It is not possible to elucidate with current information whether this photomorphogenic response was either actually selected under the pressure of agricultural crops or already present in wild ancestors. It is clear, however, that the leaf position response described here cannot be regarded as a relict providing competitive advantage under wild conditions and a burden under agricultural systems.
The observation that at least some responses to R/FR can be beneficial rather than detrimental to crop performance poses a new paradigm. Although enhanced phytochrome levels in transgenic plants can increase yield, the overexpression strategy is likely to eliminate detrimental as well as beneficial effects of plant responses to R to FR ratio, thus precluding an optimum combination. It is noteworthy that the maize cultivar showing stronger leaf orientation response is the one exhibiting weaker tillering and shoot height responses. This indicates that response-specific genes operating downstream phytochromes provide a way to selectively eliminate those responses with negative impact on yield, while retaining those ones with positive contribution. Identifying these genes is a challenge that can be approached with developing tools of functional genomics.
MATERIALS AND METHODS
Plant Material
Maize (Zea mays) plants of the hybrid cv DK696 and of Exp980 were used in the experiments described here. These hybrids have similar duration of the cycle because of maximum leaf development (approximately 900°C d) and total leaf number (21 leaves per plant). All experiments were conducted without water and nutrient limitations.
Field Experiments with Selective Filters
Field experiments with selective filters were conducted at the experimental unit of the Department of Plant Production (University of Buenos Aires; 34° 25′S, 58° 25′W), on a silty clay loam soil (Vertic Argiudoll, Soil Taxonomy, U.S. Department of Agriculture). Experiments were sown in early December (1998 and 1999) and early January (2001). In the 1998 experiment, seeds were sown directly in the field. Thus, the first leaf of seedlings had a random orientation. In 1999 and 2001 experiments, tested hybrids were seeded in pots (two seeds per pot) and after seedling emergence, they were thinned by hand to one plant per pot. At the two-leaf stage, plants were transplanted to the field with the first leaf pointing to the south. In all experiments, filters were placed close to plants at the three-leaf stage.
Filters had a dimension of 1.4 × 0.3 m. The FR-reflecting filter consisted of a wooden board covered with an aluminum filter, a blue acrylic filter (Paolini 2031, 2.4 mm, La Casa del Acetato, Buenos Aires), and a red acetate filter. The black control was made of a wooden board covered with an opaque black plastic. The blue light-absorbing filter was made of a wooden frame surrounding an orange acetate filter (La Casa del Acetato). The clear control had a similar structure but a clear acetate filter. The filters were characterized with a handheld spectroradiometer (FieldSpec, Analytical Spectral Devices, Inc., Boulder, CO). The transmission of the clear control filter was above 90% in the visible far-red range. The minus blue light filter showed similar transmission above 600 nm, but only 1% and 35% transmission in the 400- to 500-nm and 500- to 600-nm range, respectively. The reflectance (relative to a white reference panel with 100% reflection) was 3% and 2% in the 600- to 700-nm range and 4% and 7% in the 700- to 800-nm range for the black control and the FR-reflecting filter, respectively. The filters were placed either toward the north or toward the south of the plants. In each case, the filter to plant distance was 15 cm.
Effects of Plant Spatial Arrangement in the Field
The experiment was sown in Salto (34° 33′S, 60° 33′W; Province of Buenos Aires) in early November (1998). The soil was a silty clay loam (Typic Argiudoll, Soil Taxonomy, U.S. Department of Agriculture). Plant arrangement was varied by combining three plant densities (3, 9, and 12 plants m−2) and two row spacings (0.35 and 0.70 m).
Localized Irradiation in Growth Chambers
Single plants were grown in 4.3-L pots filled with silty clay loam soil, placed in a growth chamber. Photoperiod was 13 h, PAR was 40 μmol s−1m−2 (provided by 40-W Gro-lux fluorescent tubes, Sylvania, Buenos Aires), the R to FR ratio was >3, and temperature was 21°C. In one-half of the plants, the third and fifth leaf received supplementary FR (0.9 μmol s−1 m−2) during the photoperiod. The FR treatments started when the visible portion of the third and fifth leaf was less than 1 cm long and continued until full expansion. FR was provided by a halogen lamp (placed outside the growth chamber) in combination with an optic fiber (3-mm diameter), a blue acrylic filter (Paolini 2031, 2.4 mm), and a red acetate filter. The tip of the optic fiber was daily adjusted to a position 2 mm above the ligule of the previous leaf.
Light Measurements
The R to FR ratio was measured with a two-channel radiometer bearing a cosine-corrected remote probe with narrow band filters centered at 660 and 730 nm (SKR 110, Skye Instruments, Powys, UK). The PAR (400–700 nm) was measured with a 1-m line quantum-sensor (model LI-191SA, LI-COR, Lincoln, NE).
Plant Measurements
Leaf azimuth was recorded using an azimuthal plastic board circle placed below each leaf along the stem. A slot was cut along the 0° to 180° line of the board to center the stem of each plant in the middle of the azimuthal circle. The circle was divided in 16 sectors (22° 30′ per sector). For each blade, the sector where the projection of the middle part of the midrib occurred was recorded. Measurements were not based on leaf tip position because a twisting and/or shift of the distal part of the blade sometimes occurred (Girardin, 1992).
Plant height (from soil surface to the upper leaf) and tiller production (tillers per plant) were recorded daily or three times a week.
Footnotes
This work was supported by Dekalb Argentina S.A., Agencia Nacional de Promoción Cientifica y Tecnológica (grant no. PICT 08–06608 to M.E.O.), by Secretaria de Ciencia, Tecnologia e Innovación Productiva (grant no. A98B03 to M.E.O. and B.A.), by the University of Buenos Aires (grant no. JG20 to G.A.M.), and by the Fundación Antorchas (grant to G.A.M.). G.A.M., M.E.O., and J.J.C. are members of Consejo Nacional de Investigaciones Científicas y Técnicas, the Research Council of Argentina.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.009738.
LITERATURE CITED
- Andrieu B, Ivanov N, Boissard P. Simulation of light interception from a maize canopy model constructed by stereo plotting. Agric For Meteorol. 1995;75:103–119. [Google Scholar]
- Ballaré CL, Casal JJ. Light signals perceived by crop and weed plants. Field Crop Res. 2000;67:149–160. [Google Scholar]
- Ballaré CL, Sanchez RA, Scopel AL, Casal JJ, Ghersa CM. Early detection of neighboring plants by phytochrome perception of spectral changes in reflected sunlight. Plant Cell Environ. 1987;10:551–557. [Google Scholar]
- Ballaré CL, Scopel AL, Roush ML, Radosevich SSR. How plant find light in patchy canopies. A comparison between wild-type and phytochrome-B-deficient mutant plants of cucumber. Funct Ecol. 1995;9:859–868. [Google Scholar]
- Ballaré CL, Scopel AL, Sanchez RA. Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science. 1990;247:329–332. doi: 10.1126/science.247.4940.329. [DOI] [PubMed] [Google Scholar]
- Briggs WR, Beck CF, Cashmore AR, Christied JM, Hughes J, Jarillo JA, Kagawa T, Kanegae H, Liscum E, Nagatani A et al. The phototropin family of photoreceptors. Plant Cell. 2001;13:993–997. doi: 10.1105/tpc.13.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ. Light quality effects on the appearance of tillers of different orders in wheat. Ann Appl Biol. 1988;112:167–173. [Google Scholar]
- Casal JJ. Environmental cues affecting development. Current Opin Plant Biol. 2002;5:37–42. doi: 10.1016/s1369-5266(01)00218-7. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Clough RC, Vierstra RD. High-irradiance responses induced by far-red light in grass seedlings of the wild type or overexpressing phytochrome A. Planta. 1996;200:132–137. [Google Scholar]
- Casal JJ, Sadras VO. Effects of end-of-day red/far red ratio on growth and orientation of sunflower leaves. Bot Gaz. 1987;148:463–467. [Google Scholar]
- Casal JJ, Sanchez RA, Deregibus VA. Effects of plant density on tillering: the relationship with R/FR and the proportion of radiation intercepted per plant. Environ Exp Bot. 1986;26:365–371. [Google Scholar]
- Casal JJ, Sanchez RA, Deregibus VA. Tillering responses of Lollium multiflorum plants to changes of red/far red ratio typical of sparse canopies. J Exp Bot. 1987;38:1432–1439. [Google Scholar]
- Casal JJ, Sanchez RA, Gibson D. The significance of changes in the red/far red ratio, associated with the neighbour plants or twilight, for tillering in Lolium multiflorum Lam. New Phytol. 1990;116:565–572. [Google Scholar]
- Childs KL, Miller FR, Cordonnier-Pratt MM, Pratt LH, Morgan PW, Mullet JE. The sorghum photoperiod sensitivity gene, Ma3, encodes a phytochrome B. Plant Physiol. 1997;113:611–619. doi: 10.1104/pp.113.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AH, Quail PH. Structure and expression of a phytochrome-encoding gene. Gene. 1989;85:381–390. doi: 10.1016/0378-1119(89)90431-9. [DOI] [PubMed] [Google Scholar]
- Deregibus VA, Sánchez RA, Casal JJ. Light quality effects on tiller production in Lolium spp. Plant Physiol. 1983;72:900–902. doi: 10.1104/pp.72.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deregibus VA, Sánchez RA, Casal JJ, Trlica MJ. Tillering responses to enrichment of red light beneath the canopy in a humid natural grassland. J Appl Ecol. 1985;22:199–206. [Google Scholar]
- Devlin PF, Patel SR, Whitelam GC. Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell. 1998;10:1479–1488. doi: 10.1105/tpc.10.9.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Robson PRH, Patel SR, Goosey L, Sharrock RA, Whitelam GC. Phytochrome D acts in the shade-avoidance syndrome in Arabidopsis by controlling elongation growth and flowering time. Plant Physiol. 1999;119:909–916. doi: 10.1104/pp.119.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firn RD. Phototropism. In: Kendrick RE, Kronrnberg GHM, editors. Photomorphogenesis in Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 659–681. [Google Scholar]
- Girardin P. Leaf azimuth in maize canopies. Eur J Agron. 1992;1:91–97. [Google Scholar]
- Girardin P, Tollenaar M. Effects of intraspecific interference on maize leaf azimuth. Crop Sci. 1994;34:151–155. [Google Scholar]
- Hanumappa M, Pratt LH, Cordonnier-Pratt MM, Deitzer GF. A photoperiod-insensitive barley line contains a light-labile phytochrome B. Plant Physiol. 1999;119:1033–1039. doi: 10.1104/pp.119.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindl JC, Brun WA. Light and shade effects on abscission and 14C-photoassimilate partition among reproductive structures in soybean. Plant Physiol. 1983;73:434–439. doi: 10.1104/pp.73.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa T, Oikawa T, Tokutomi S, Okuno K, Shimamoto K. Phytochromes confer the photoperiodic control of flowering in rice (a short-day plant) Plant J. 2000;22:391–399. doi: 10.1046/j.1365-313x.2000.00753.x. [DOI] [PubMed] [Google Scholar]
- Kasperbauer MJ, Karlen DL. Light-mediated bioregulation of tillering and photosynthate partitioning in wheat. Physiol Plant. 1986;66:159–163. [Google Scholar]
- Kasperbauer MJ, Karlen DL. Plant spacing and reflected far-red light effects on phytochrome-regulated photosynthate allocation in corn seedlings. Crop Sci. 1994;34:1564–1569. [Google Scholar]
- Libenson S, Rodriguez V, López Pereira M, Sánchez RA, Casal JJ. Low red to far-red ratios reaching the stem reduce grain yield in sunflower. Crop Sci. 2002;42:1180–1185. [Google Scholar]
- Maddonni GA, Chelle M, Drouet J-L, Andrieu B. Light interception of contrasting azimuth canopies under square and rectangular plant spatial distributions: simulations and crop measurements. Field Crop Res. 2001b;70:1–13. [Google Scholar]
- Maddonni GA, Otegui ME, Cirilo AG. Plant population density, row spacing and hybrid effects on maize architecture and light attenuation. Field Crop Res. 2001a;71:183–193. [Google Scholar]
- Mathews S, Sharrock RA. Phytochrome gene diversity. Plant Cell Environ. 1997;20:666–671. [Google Scholar]
- Orr GL, Haidar MA, Orr DA. Smallseed dodder (Cuscuta planiflora) phototropism toward far-red when in white light. Weed Sci. 1996;43:88–94. [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis. Dev Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JT, NeSmith DS. Temperature and crop development. In: Hanks J, Ritchie JT, editors. Modeling Plant and Soil Systems, Agronomy Series 31. Madison, WI: ASA, CSSA, SSSA Press; 1991. pp. 5–29. [Google Scholar]
- Ritter S, Koller D. Light-driven movements of the trifoliate leaves of bean (Phaseolus vulgaris L.). Activity of blue light and red light. J Exp Bot. 1994;45:335–341. [Google Scholar]
- Robson PRH, McCormac AC, Irvine AS, Smith H. Genetic engineering of harvest index in tobacco through overexpression of a phytochrome gene. Nat Biotechnol. 1996;14:995–998. doi: 10.1038/nbt0896-995. [DOI] [PubMed] [Google Scholar]
- Sattin M, Zuin MC, Sartorato I. Light quality beneath field-grown maize, soybean and wheat canopies: red:far red variations. Physiol Plant. 1994;91:322–328. [Google Scholar]
- Satter RL, Whetherell DF. Photomorphogenesis in Sinningia speciosa, cv. Queen Victoria: II. Stem elongation: interaction of a phytochrome controlled process and a red-requiring, energy dependent reaction. Plant Physiol. 1968;43:961–967. doi: 10.1104/pp.43.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlumukov LR, Barro F, Barcelo P, Lazzeri P, Smith H. Establishment of far-red high irradiance responses in wheat through transgenic expression of an oat phytochrome A gene. Plant Cell Environ. 2001;24:703–712. [Google Scholar]
- Skinner RH, Simmons SR. Modulation of leaf elongation, tiller appearance and tiller senescence in spring barley by far-red light. Plant Cell Environ. 1993;16:555–562. [Google Scholar]
- Smith H. Evidence that Pfr is not the active form of phytochrome in light-grown maize. Nature. 1980;293:163–165. [Google Scholar]
- Smith H. Light quality, photoperception and plant strategy. Annu Rev Plant Physiol. 1982;33:481–518. [Google Scholar]
- Smith H. Phytochromes and light signal perception by plants: an emerging synthesis. Nature. 2000;407:585–591. doi: 10.1038/35036500. [DOI] [PubMed] [Google Scholar]
- Smith H, Casal JJ, Jackson GM. Reflection signals and perception by phytochrome of the proximity of neighboring vegetation. Plant Cell Environ. 1990;13:73–78. [Google Scholar]
- Stewart DW, Dwyer LM. Mathematical characterization of maize canopies. Agric For Meteorol. 1993;66:247–265. [Google Scholar]
- Takano M, Kanegae H, Shinomura T, Miyao A, Hirochika H, Furuya M. Isolation and characterization of rice phytochrome A mutants. Plant Cell. 2001;13:521–534. doi: 10.1105/tpc.13.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetio-Kagho F, Gardner FP. Responses of maize to plant population density: I. Canopy development, light relationships, and vegetative growth. Agron J. 1988;80:930–935. [Google Scholar]
- Thiele A, Herol M, Lenk I, Quail PH, Gatz C. Heterologous expression of Arabidopsis phytochrome B in transgenic potato influences photosynthesis performance and tuber development. Plant Physiol. 1999;120:73–81. doi: 10.1104/pp.120.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toler JE, Murdock EC, Stapleton GS, Wallace SU. Corn leaf orientation effects on light interception, intraspecific competition, and grain yields. J Prod Agric. 1999;12:396–399. [Google Scholar]
- Willey RW, Heath SB. The quantitative relationships between plant population and crop yield. In: Brady NC, editor. Advances in agronomy. R Halls Press, Ithaca, NY: Cornell University; 1969. pp. 281–321. [Google Scholar]
- Yanovsky MJ, Casal JJ, Whitelam GC. Phytochrome A, phytochrome B and HY4 are involved in hypocotyl-growth responses to natural radiation in Arabidopsis. Weak de-etiolation of the phyA mutant under dense canopies. Plant Cell Environ. 1995;18:788–794. [Google Scholar]