Abstract
The light state transition regulates the distribution of absorbed excitation energy between the two photosystems (PSs) of photosynthesis under varying environmental conditions and/or metabolic demands. In cyanobacteria, there is evidence for the redistribution of energy absorbed by both chlorophyll (Chl) and by phycobilin pigments, and proposed mechanisms differ in the relative involvement of the two pigment types. We assayed changes in the distribution of excitation energy with 77K fluorescence emission spectroscopy determined for excitation of Chl and phycobilin pigments, in both wild-type and state transition-impaired mutant strains of Synechococcus sp. PCC 7002 and Synechocystis sp. PCC 6803. Action spectra for the redistribution of both Chl and phycobilin pigments were very similar in both wild-type cyanobacteria. Both state transition-impaired mutants showed no redistribution of phycobilin-absorbed excitation energy, but retained changes in Chl-absorbed excitation. Action spectra for the Chl-absorbed changes in excitation in the two mutants were similar to each other and to those observed in the two wild types. Our data show that the redistribution of excitation energy absorbed by Chl is independent of the redistribution of excitation energy absorbed by phycobilin pigments and that both changes are triggered by the same environmental light conditions. We present a model for the state transition in cyanobacteria based on the x-ray structures of PSII, PSI, and allophycocyanin consistent with these results.
The effective absorption of sunlight by antenna pigments is the critical first step in photosynthesis. All oxygenic photosynthetic organisms share a common core antenna pigment complement of about 40 chlorophyll (Chl) a in PSII and about 100 Chl a in PSI (Rögner et al., 1990). Photosynthetic organisms do not, however, limit their photon capturing ability to this level, but rather use some form of additional peripheral antenna pigments to increase the effective “absorption cross section” of one or both PSs. Higher plants and algae have evolved diverse mechanisms to increase their ability to absorb sunlight. In cyanobacteria, the soluble phycobiliproteins are organized into phycobilisomes (PBSs), which are primarily associated excitonically with PSII in a manner analogous to the family of intrinsic thylakoid membrane Chl a/b-containing light-harvesting complex polypeptides (LHCII), which serve the same function in higher plants (Glazer, 1984; Zilinskas and Greenwald, 1986).
Both cyanobacteria and higher plants can regulate the efficiency of excitation energy transfer to the two PSs. The light state transition appears designed to adjust the relative activities of PSII and PSI in response to a dynamic environment or to changing metabolic demands (Yu et al., 1993). The mechanism in higher plants involves a reversible association of LHCII with PSII and PSI triggered by the redox state of intersystem electron transport carriers and driven by the reversible phosphorylation of LHCII (for review, see Allen, 1992; Wollman, 2001). There is no consensus for the mechanism of the state transition in phycobilisome-containing cyanobacteria (for review, see Van Thor et al., 1998; Mullineaux, 1999).
The state transition in cyanobacteria is triggered in the same way as that in higher plants. State 1 is achieved by oxidation of intersystem electron carriers (usually by “excess” excitation of PSI). Reduction of intersystem electron carriers, most likely plastoquinone (Mullineaux and Allen, 1990), either by “excess” excitation of PSII or by a dark respiratory pathway (Mullineaux and Allen, 1986), triggers the conversion to state 2. State 2 is characterized by a decrease in PSII variable fluorescence, a decrease in the PSII absorbance cross section, and an increase in the PSI absorbance cross section as compared with state 1 (Mullineaux, 1992). It is clear that excitation energy absorbed by the PBS has a lower probability of reaching PSII and a higher probability of reaching PSI in state 2 than in state 1. How this change is effected and the role of Chl a in the state transition mechanism have been controversial points (Salehian and Bruce, 1992; Mullineaux, 1994).
Early proposals for mechanisms for the state transition were based on either the idea of a mobile PBS (Allen and Holmes, 1986), which changes its association with PSII and PSI, or a “spillover” of energy from PSII Chl a to PSI Chl a (Biggins and Bruce, 1989; Bruce et al., 1989; Rouag and Dominy, 1994). Recent work supporting the idea of a mobile PBS comes from fluorescence recovery after photobleaching (FRAP) measurements that clearly but surprisingly indicate that PBSs are much more mobile than PSII (Mullineaux et al., 1997). Mutants of the apcD gene, which codes for the α-subunit of the allophycocyanin (APC) B core subunit (αAP-B), have been shown to be impaired in state transitions and appear to be stuck in state 1 (Zhao et al., 1992). This has been interpreted to support the idea of a mobile PBS mechanism and that the apcD gene product is the site of energy transfer from the PBS to PSI (Ashby and Mullineaux, 1999). The mobile PBS model is not sufficient to fully explain the state transition, however, because changes in the relative contribution of Chl a-absorbed excitation energy to PSII and PSI are also observed. This result is not predicted by the mobile PBS model. The observation of changes in the distribution of Chl a-absorbed excitation has supported the “spillover” model for the state transition, originally proposed by Murata (1969). The spillover model suggests that changes in the rate constant for excitation energy transfer between PSII Chl a and PSI Chl a are responsible for the observed changes in the distribution of both PBS- and Chl a-absorbed energy between the two PSs. This mechanism depends on a strong coupling between the PBS and PSII Chl a and a variable coupling between PSII Chl a and PSI Chl a. The spillover model predicts that the state transition-induced change in the relative contribution of PBS to PSII would have to be equal to or less than the relative change in the contribution of Chl a to PSII. A number of reports show, however, that the relative changes in the distribution of Chl a-absorbed energy are somewhat smaller than the relative changes in the distribution of PBS-absorbed energy (Mullineaux and Holzwarth, 1990; Salehian and Bruce, 1992).
Freeze fracture electron microscopy has shown that large-scale organization changes of ectoplasmic face particles (containing PSII) in the thylakoid membrane accompany the state transition in cyanobacteria (Olive et al., 1986). The ectoplasmic face particles exhibit a nonrandom alignment into long rows in state 1 and a more random distribution in state 2. Linear dichroism studies have also shown that the state transition in cyanobacteria is associated with changes in the orientation of APC and Chl a (Bruce and Biggins, 1985; Brimble and Bruce, 1989; Homer-Dixon et al., 1994).
Clearly, the state transition mechanism in cyanobacteria is more complex than either the simple mobile PBS or spillover mechanism, and somehow involves changes in both PBS and Chl a. A structural model for the state transition in cyanobacteria based primarily on data from electron microscopy suggests that changes in both dimerization of PSII and trimerization of PSI are involved, as well as differential association of the PBS with PSII and PSI (Bald et al., 1996). It appears likely that a number of changes are involved in the state transition mechanism that affect both the relative association of PBS with PSII and PSI and also the probability of excitation energy transfer between PSII and PSI Chl a. Excellent recent work has not simplified the situation. For example, it was reported that a genetically engineered strain of Synechocystis sp. PCC 6803, in which thylakoid membranes were more rigid because of the absence of di- and tri-unsaturated fatty acids, were unable to do state transitions at temperatures below the lipid phase transition temperature (El Bissati et al., 2000). Thus, membrane fluidity plays an important role in cyanobacterial state transitions, supporting the idea of some kind of involvement of mobile PSII and PSI in the mechanism, even though the FRAP data indicate that the PBS are more mobile than PSII (Mullineaux et al., 1997). Reversible changes in the association of isolated PSII and PSI induced by changing detergent concentration have been shown to be associated with state transition-like changes in 77K emission spectra, which again suggest a role for changes in the association of PSII and PSI (Federman et al., 2000).
An insertional inactivation mutant in Synechocystis sp. PCC 6803 (Δsll1926 or rpaC−) was unable to perform state transitions and to grow more slowly than the respective wild type under light-limiting conditions (Emlyn-Jones et al., 1999). The deleted gene product, designated RpaC (regulator of PBS association C), bears no sequence similarity to any known photosynthesis-associated polypeptide, and no recognizable sequence motifs. Interestingly, the mutant lacked the characteristic differences in 77K fluorescence emission spectra indicative of a state transition for excitation of the PBS, but did appear to retain some state transition-like changes when emission spectra were collected for excitation of Chl a (Emlyn-Jones et al., 1999). That work suggested the possibility of differing origins for the fluorescence changes indicative of state transitions in cyanobacteria for excitation of Chl a and PBS.
Are the redistributions of Chl a- and PBS-absorbed excitation energy with PSII and PSI associated with the state transition independent of each other? If the PBS and Chl antenna do act independently, are their light-induced changes in distribution triggered by the same environmental light conditions?
To address these questions, we used two different species of cyanobacteria. The Synechocystis sp. PCC 6803 wild type and rpaC− strain described above were compared with Synechococcus sp. PCC 7002 wild type and a mutant with impaired PBS function, apcD−. The apcD mutant lacks the αAP-B subunit of the APC core, which has been suggested to facilitate the transfer of absorbed excitation energy from the PBS to PSI (Maxson et al., 1989; Ashby and Mullineaux, 1999). The apcD mutant has also been reported to be unable to perform state transitions (Zhao et al., 1992).
Our work confirms the observation that the rpaC− strain of Synechocystis PCC 6803 exhibits state transition-associated Chl fluorescence yield changes upon excitation of Chl but not upon excitation of PBS and shows for the first time, to our knowledge, that this is also the case for the apcD− strain of Synechococcus sp. PCC 7002. In addition, action spectra for the fluorescence yield changes in both species clearly show that the redistribution of both Chl and phycobilin antennae, although separable, are driven by the same environmental light conditions and, thus, share the same triggering mechanism. We also present a new structural model for the light state transition in cyanobacteria that is consistent with independent pathways for the redistribution of PBS- and Chl-absorbed excitation. Our model is based on the x-ray structures of PSII, PSI, and APC and is supported by recent kinetic modeling studies of excitation energy transfer in PSII (Vasil'ev et al., 2001).
RESULTS
Room Temperature Fluorescence
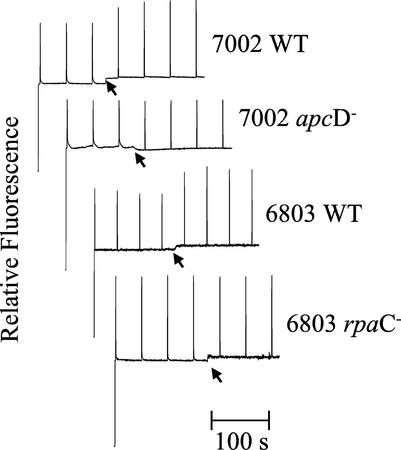
Figure 1 displays the steady-state room temperature variable fluorescence kinetics of Synechococcus sp. PCC 7002 wild type and apcD mutant and Synechocystis sp. PCC 6803 wild type and the rpaC mutant determined with a PAM fluorometer. In both wild-type cyanobacteria, illumination with blue light induces an increase in maximal level of variable fluorescence (Fm) indicative of a transition to state 1. The approximately 20% increases in Fm displayed by both of the wild-type cyanobacteria are absent in the room temperature fluorescence traces of their respective mutants.
Figure 1.
Room temperature pulse-amplitude-modulated (PAM) fluorescence kinetic traces for Synechococcus sp. PCC 7002 wild-type and apcD− cells and Synechocystis sp. PCC 6803 wild-type and rpaC− cells. Cells were dark adapted to state 2 and the arrow indicates the application of blue light in an attempt to drive the cells to state 1. See “Materials and Methods” for details.
77K Fluorescence Emission Spectra
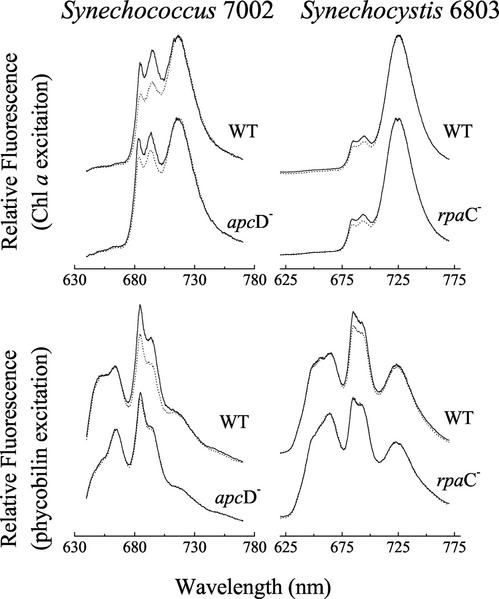
77K fluorescence emission spectra of Synechococcus sp. PCC 7002 wild type and apcD mutant and of Synechocystis sp. PCC 6803 wild type and rpaC mutant are shown in Figure 2. In the wild type of both species, increases in PSII fluorescence emission (685- and 695-nm peaks) relative to PSI fluorescence emission (715-nm peak in Synechococcus and 725-nm peak in Synechocystis) are associated with the transition to state 1 and observed for excitation of both the PBS at 580 nm (lower) and Chl a at 435 nm (upper). In contrast, no significant changes in the shape of the emission spectra are observed in the mutant cells of either species for excitation of the PBS, although a significant increase in the PSII emission relative to the PSI emission is seen in emission spectra of the mutant cells when excited at 435 nm. In both species, the wild-type cells exhibit state transition-like changes in the relative emission yields of PSII relative to PSI for excitation of either PBS or Chl antenna, whereas the mutant cells show changes only for excitation of Chl.
Figure 2.
77K fluorescence emission spectra of Synechococcus sp. PCC 7002 wild-type and apcD− cells and Synechocystis sp. PCC 6803 wild-type and rpaC− cells. Spectra were collected for excitation of Chl a at 435 nm (top) and for excitation of phycobilin pigments at 580 nm (bottom). All cells were pre-illuminated before freezing in liquid nitrogen with either 420 nm of light in an attempt to drive a transition to state 1 (solid lines) or 560 nm of light in an attempt to drive a transition to state 2 (dotted line). See “Materials and Methods” for details.
Action Spectra
To investigate the environmental light conditions that trigger the state transition-associated changes in ratio of PSII emission relative to PSI emission (F695/F715 in Synechococcus; F695/F725 in Synechocystis) observed in the 77K fluorescence emission spectra, we determined action spectra for the ratio as a function of wavelength of the pre-illumination light used to drive the state transition. As described in “Materials and Methods,” cells were pre-illuminated at room temperature at the wavelengths used to construct the action spectra, quickly frozen in liquid nitrogen, and then assayed for 77K fluorescence emission.
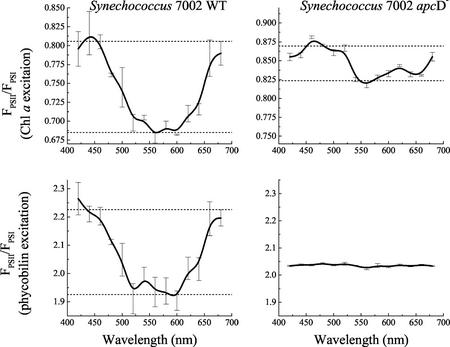
In Figure 3 action spectra for the Synechococcus sp. PCC 7002 wild type and apcD mutant are shown. The F695/F715 ratio is used as an indicator of the “state” attained by any particular pre-illumination wavelength. State 1 is characterized by a high F695/F715 ratio and state 2 is characterized by a low F695/F715 ratio. For each sample, the F695/F715 ratio was determined for excitation of the PBS (580 nm) and Chl a (435 nm). This allowed the construction of two action spectra, one for pre-illumination-induced changes in distribution of light absorbed by the PBS and one for pre-illumination-induced changes in distribution of light absorbed by Chl a. In each action spectrum, the dashed horizontal lines show values of F695/F715 characteristic of state 1 (blue light driven) and state 2 (dark adapted or orange light driven). In wild-type cells, the action spectra for the F695/F715 ratio for excitation of PBS and for excitation of Chl a are very similar to each other. They show that pre-illumination with blue and red light, which is preferentially absorbed by Chl a, drives the cells to state 1 and that pre-illumination with green or orange light, where light is preferentially absorbed by the PBS, drives the cells to state 2 regardless of whether changes in the distribution of PBS- or Chl a-absorbed light are measured. In contrast, the apcD− cells show no pre-illumination-induced changes in F695/F715 when fluorescence emission was assayed with PBS excitation, but do show an action spectrum of smaller magnitude but very similar shape to the spectra of the wild type when assayed with Chl a excitation.
Figure 3.
Action spectra for the light state transition in Synechococcus sp. PCC 7002 wild-type and apcD− cells. The relative amplitude of PSII fluorescence emission divided by PSI emission (FPSII/FPSI) at 77K was plotted versus the pre-illumination wavelength used to drive the state transition at room temperature. Action spectra in the top two panels were generated from analysis of emission spectra excited with light-absorbed by Chl a, whereas the action spectra in the bottom two panels were generated from an analysis of emission spectra from the same samples excited with light absorbed by phycobilin pigments. See “Materials and Methods” for details. For each pre-illumination wavelength, the mean of three independent measures is plotted, and the error bars show the sd.
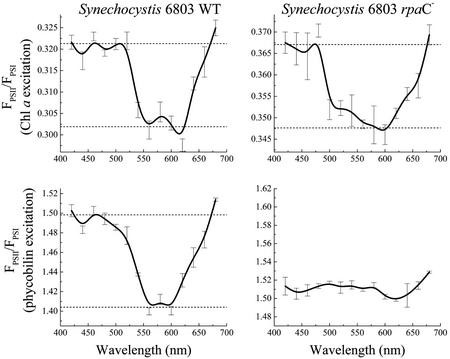
Figure 4 shows action spectra for the F695/F725 ratio as a function of pre-illumination wavelength of the wild-type and rpaC− cells of Synechocystis sp. PCC 6803. This set of action spectra show the same pattern observed in Figure 3 for the action spectra of wild-type and apcD− cells of Synechococcus sp. PCC 7002. All action spectra, with the exception of that from the rpaC− cells under PBS excitation, show clear evidence of a state transition. These action spectra have very similar shapes to each other and to the action spectra in Figure 3 for Synechococcus sp. PCC 7002. The F695/F725 ratio is high with blue and red pre-illumination (Chl a-absorbed light, preferential excitation of PSI) and low with green or orange pre-illumination (PBS-absorbed light, preferential excitation of PSII). In the action spectrum of the rpaC− cells, determined for PBS excitation, the characteristic peaks and troughs indicative of a state transition are absent.
Figure 4.
Action spectra for the light state transition in Synechocystis sp. PCC 6803 wild-type and rpaC− cells. The relative amplitude of PSII fluorescence emission divided by PSI emission (FPSII/FPSI) at 77K was plotted versus the pre-illumination wavelength used to drive the state transition at room temperature. Action spectra in the top two panels were generated from analysis of emission spectra excited with light-absorbed by Chl a, whereas the action spectra in the bottom two panels were generated from an analysis of emission spectra from the same samples excited with light absorbed by phycobilin pigments. See “Materials and Methods” for details. For each pre-illumination wavelength, the mean of three independent measures is plotted, and the error bars show the sd.
Absorbance Cross Sections
To determine whether the pre-illumination-induced changes in 77K fluorescence emission were representative of relative changes in the antenna size of PSII, the PSII absorbance cross sections for excitation of both Chl a and PBS in wild-type and apcD− cells of Synechococcus sp. PCC 7002 were measured at room temperature. PSII absorbance cross sections were determined from Poisson fits to pump probe fluorescence flash saturation curves generated for excitation of Chl a and PBS as described in “Materials and Methods.” Table I shows a summary of the PSII absorbance cross sections of the wild-type and apcD− cells in states 1 and 2 for excitation of both Chl a at 674 nm and the PBS at 630 nm. Wild-type cells show significant decreases in the absorbance cross sections for both PBS and Chl a excitation upon transition from state 1 to state 2. In agreement with the 77K fluorescence emission data, the apcD− cells do not show a significant decrease in the PSII absorbance cross section for PBS excitation upon transition from state 1 to state 2 conditions, but do show a significant decrease in Chl a-sensitized absorbance cross section.
Table I.
Absorbance cross sections (ς II) of PSII in Synechococcus sp. PCC 7002 wild type and apcD mutant
| Cell Type | Excitation | ςII State 2 | ςII State 1 | Change |
|---|---|---|---|---|
| Å2 | % | |||
| Wild type | PBS | 282 ± 12 | 407 ± 32 | 44 |
| Wild type | Chl a | 103 ± 5 | 121 ± 6 | 17 |
| ApcD− | PBS | 441 ± 34 | 475 ± 32 | Not significant |
| ApcD− | Chl a | 134 ± 7 | 163 ± 7 | 22 |
Room temperature PSII absorbance cross sections (ς II) for wild-type and mutant cells in states 1 and 2 for excitation of Chl a at 674 nm and for excitation of the PBS at 630 nm. The percent change column shows the percent increase in ς II on transition from state 2 to state 1. Cross sections were determined from Poisson fits to pump probe Chl a fluorescence flash saturation curves, as described in “Materials and Methods.” Values are mean ± sd, n = 4.
DISCUSSION
Our results confirm previous observations (Zhao et al., 1992; Emlyn-Jones et al., 1999) that both the Synechococcus sp. PCC 7002 apcD− strain and the Synechocystis sp. PCC 6803 rpaC− strain are state transition impaired. Furthermore, our results confirm the interesting observation that the Synechocystis sp. PCC 6803 rpaC− strain retains characteristic changes in 77K fluorescence yield indicative of a state transition when spectra are collected for excitation of Chl a but not for excitation of the PBS. In addition, we show that the Synechococcus sp. PCC 7002 apcD− strain also retains changes in emission spectra indicative of a state transition when spectra are collected for excitation of Chl a. The Synechococcus sp. and Synechocystis sp. mutants are both impaired in PBS-associated proteins and had been characterized initially as lacking state transitions.
It is interesting that both of these state transition mutants appear to be only impaired in state transitions when assayed for changes in the distribution of excitation energy absorbed by the PBS. The observation of clear changes in the distribution of Chl a in both mutants clarifies an apparent contradiction in previous experimental results with two Synechococcus sp. PCC 7002 mutants. A mutant of Synechococcus sp. PCC 7002 lacking PBS (Δapc cpc−) was originally characterized as being state transition competent (Bruce et al., 1989). In that study, low-temperature fluorescence emission spectra characteristic of the state transition were obtained by manipulating the oxidation state of the plastoquinone pool, and it was concluded that the PBS was not essential to the state transition mechanism. A later study with the apcD− strain showed it to be unable to perform state transitions, as assayed by both room temperature fluorescence induction and low-temperature fluorescence emission spectroscopy (Zhao et al., 1992). In contradiction to the work with the PBS-less mutant, the study with the apcD− strain concluded that the αAP-B subunit of the APC core of the PBS was essential for the state transition mechanism. However, fluorescence emission spectra used to assess state transitions in the experiments with the PBS-less mutant were determined for excitation of Chl a (Bruce et al., 1989), and the study with the apcD− strain used excitation of the PBS (Zhao et al., 1992). Rather than contradicting each other, these two studies support our present work and show that changes in the distribution of Chl a-absorbed excitation energy can occur independently of changes in the distribution of PBS-absorbed energy.
The Mechanism of the State Transition, Spillover, and Mobile PBS
The spillover model (Murata, 1969; Dominy and Williams, 1987; Biggins and Bruce, 1989; Bruce et al., 1989) predicts that “excess” energy absorbed by pigments associated with PSII (either the PBS or the Chl a core antenna) “spills over” to PSI in state 2. The mechanism assumes that energy absorbed by the PBS is transferred to the antenna Chl a of PSII, which forms the bridge for excitation energy transfer to PSI. In this way, energy absorbed by either the PBS or the PSII core Chl a antenna will ultimately reach PSI and any spillover-induced changes in the distribution of Chl a-absorbed excitation must be correlated with changes in the distribution of PBS-absorbed excitation. A change in only one rate constant, the rate of energy transfer from the PSII Chl antenna to the PSI Chl antenna, thus changes the distribution of both PBS- and Chl-absorbed excitation. This basic feature of the spillover model is not consistent with our observation that only changes in Chl a-absorbed excitation energy are retained in both of the “state transition-impaired” mutants used in this study.
What are the other options for the mechanism of the state transition? The mobile PBS model (Allen and Holmes, 1986; Kruip et al., 1994; Bald et al., 1996; Mullineaux, 1999) predicts that preferential binding of the PBS to PSII in state 1 and to PSI in state 2 accounts for the changes in excitation distribution. FRAP data indicate that the lateral mobility of the PBS on the surface of the thylakoid membrane is much higher than that of PSII within the membrane (Mullineaux et al., 1997). However, there is also evidence that the redistribution of PBS-absorbed energy requires a fluid thylakoid membrane (El Bissati et al., 2000), suggesting the requirement for mobile PSII and/or PSI. Regardless of the details, the idea of a shift in a dynamic equilibrium between two states, one characterized by preferential transfer from the PBS to PSII and the other by transfer from the PBS to PSI, remains a strong candidate for the explanation of changes in distribution of PBS-absorbed excitation. The major limitation of this mechanism is the failure to explain redistribution of Chl a excitation energy associated with the state transition.
It has been suggested that the state transition-associated changes in fluorescence yield observed at 77K for excitation of Chl a may only exist at low temperature and, thus, may not be indicative of changes in the functional absorbance cross sections of PSII and PSI at room temperature (Mullineaux, 1999). Uncertainty about this point is reinforced by the lack of direct measurements of functional absorbance cross sections accomplished under physiological conditions. However, the room temperature PSII absorbance cross section measurements in our present report show that the functional antenna size of PSII decreases in state 2 for excitation of either the PBS or Chl a antenna pigments. More importantly, our data show that changes in PSII cross section for Chl a excitation are retained in the apcD mutant even though the changes in PSII cross section for PBS excitation are not. Changes in the distribution of Chl a excitation do accompany the state transition in cyanobacteria and must be accounted for.
The preceding point has been addressed by models for the state transition in cyanobacteria, which combine elements of spillover and mobile PBS models (Koblízek et al., 1998; Mullineaux, 1999). Combination models are consistent with the observed redistribution of both PBS- and Chl a-absorbed excitation energy and with the observation that the relative amplitude of the change in distribution of PBS-absorbed excitation energy is often larger than that of the change in Chl a-absorbed excitation energy (Salehian and Bruce, 1992). However, as discussed above for the spillover model, combination models cannot easily explain how changes in Chl a distribution can occur in the absence of changes in PBS distribution.
A Structure-Based Model for the Light State Transition in Cyanobacteria
In light of recent studies on excitation energy transfer within the PSII core complex of cyanobacteria, we believe the spillover model can be modified so that changes in the distribution of Chl a-absorbed excitation energy can be independent of changes in the distribution of PBS-absorbed excitation. The x-ray structure of PSII has revealed a physical “gap” between the antenna Chl a associated with both CP43 and CP47 and the reaction center chromophores associated with D1/D2 (Zouni et al., 2001). Kinetic modeling and simulation of excitation energy transfer based on the x-ray structure has shown that the six reaction center chromophores are not in equilibrium with the antenna Chl a molecules of CP43 or of CP47 (Vasil'ev et al., 2001). The PSII core complex is best thought of as a three-compartment system, the central D1/D2 reaction center chromophores being flanked on one side by antenna Chl a in CP43 and on the other by those in CP47. Excitation energy transfer between the chlorin pigments within any one of the compartments is very fast, and the chromophores within a compartment are effectively in equilibrium within a few picoseconds after excitation. However, energy transfer between the compartments is considerably slower and is also slower than the rate of charge separation in D1/D2 (Vasil'ev et al., 2001).
This “compartmentalization” of excitation energy within PSII makes the transfer of energy from the PBS to PSII and the transfer of energy from PSII to PSI more complex than assumed in the original spillover model. It becomes important which compartments of PSII are involved in energy transfer with the PBS and PSI. For example, energy transferred from the PBS to the chromophores in D1/D2 would have a relatively low probability of reaching Chl a in either CP43 or CP47. In addition, energy transferred from the PBS to CP43 would have a relatively low probability of reaching CP47 and vice versa. Taking this energetic compartmentalization of PSII into account, it is possible to construct a model for the state transition in cyanobacteria that can explain the independent changes in distribution of PBS- and Chl-absorbed energy (Fig. 5).
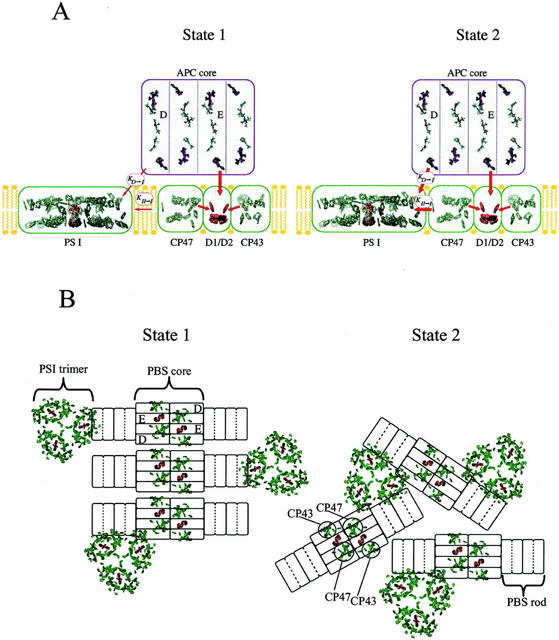
Figure 5.
A model for the organization of chromophores associated with the cyanobacterial thylakoid membrane and for the state transition based on the x-ray structures of PSII, PSI, and APC. A, “Side view” in the plane of the thylakoid membrane showing possible associations between the chromophores in one PSII complex, one PSI complex, and one APC core cylinder in states 1 and 2. Antenna chromophores in PSII and PSI are shown in green and reaction center chromophores are shown in red. Chromophores in PSII are shown divided into three compartments, D1/D2, CP47, and CP43, to reflect the energetic isolation of the two Chl a-containing antenna from the reaction center pigments. Energy transfer from the APC core to PSII occurs from the ApcE subunit to D1/D2. Energy transfer from the APC core to PSI is via the ApcD subunit and is defined by the rate constant KD-I. Energy transfer from PSII to PSI, spillover, is assumed to occur from CP47 to PSI and is defined by the rate constant KII-I. The state transition is proposed to control both KD-I and KII-I. Both rate constants are larger in state 2 than state 1. B, “Top view” down onto the surface of the thylakoid membrane and includes antenna chromophores (in green) and reaction center chromophores (in red) associated with PSII dimers and PSI trimers. APC core cylinders are shown associated with PSII dimers and phycocyanin rods with the core cylinders. In state 2, trimeric PSI is found in close contact with both CP47 of PSII and the ApcD subunits of the core cylinders. In state 1, the PBS-PSII supercomplexes are organized into rows that exclude the PSI trimer from the immediate vicinity of ApcD and CP47. One of the PBS-PSII supercomplexes is shown to remain associated with one of the PSI trimers via ApcD to represent the idea that some complexes may be more involved than others in the state transition. The organization of the PBS-PSII supercomplex and the association of PSI trimers with ApcD and the terminal rod disc in this figure has been adapted from Bald et al. (1996).
The model uses the x-ray structures of PSII (Vasil'ev et al., 2001), PSI (Jordan et al., 2001), and APC (Reuter et al., 1999). A minimal version of the model showing one APC core cylinder, one PSII, and one PSI is displayed in side view in Figure 5A. The APC core cylinder consists of four APC disc-shaped trimers, (αβ)3 (for review, see Sidler, 1994). In this model, two sets of two trimers were assembled face to face in a manner analogous to the assembly of trimers into hexamers proposed for C-phycocyanin in PBS rods (Sauer and Scheer, 1988; Wang et al., 2001). This assembly is similar to the somewhat “looser” association of face-to-face trimers found in the x-ray structure of APC hexamers (lacking linker polypeptides) from the red algae Porphyra yezoensis (Liu et al., 1999). One of the two internal APC core discs is assumed to contain the core membrane linker polypeptide (ApcE) that binds a long wavelength emitting phycocyanobilin chromophore (Gindt et al., 1994) and has a hydrophobic loop domain thought to function in association of the PBS to PSII and/or the thylakoid membrane. ApcE is believed to act as both a physical attachment point and an energetic bridge from the APC core to PSII. One of the external trimeric discs is assumed to contain ApcD, a specialized α-subunit of APC, characterized by long-wavelength absorbance and fluorescence emission maxima, which is believed to be responsible for excitation energy transfer from the APC core to PSI (Maxson et al., 1989; Zhao et al., 1992).
The model shows the core associated with a “three-compartment” version of PSII (showing CP43, D1/D2, and CP47) and PSI. The core cylinder was placed on top of the x-ray structure of PSII and oriented in a manner consistent with electron microscopy of cyanobacterial membranes and PSII core particles as suggested previously by Bald et al. (1996). Arrows show excitation energy transfer from the presumed long-wavelength chromophore in ApcE to chromophores in D1/D2 in PSII. Excitation energy transfer from the APC core to PSI is shown occurring through the presumed long-wavelength chromophore in ApcD. Changes in the distribution of PBS-absorbed excitation energy between PSII and PSI are represented in this model by changes in the association of PSI with the PBS-PSII supercomplex, which affects the value of the rate constant KD-I. In state 2, KD-I is high and the absorbance cross section of PSI would increase at the expense of PSII for PBS-absorbed light. Changes in the distribution of Chl a-absorbed light are accomplished in this model by a spillover mechanism characterized by excitation energy transfer from only one component of PSII (CP47) to PSI. Changes in the rate constant for spillover, KII-I, in this model would predominantly affect the distribution of excitation energy absorbed by the Chl a in CP47 between PSII and PSI. Energy reaching the PSII reaction center from the PBS either directly or via CP43 would have a relatively low probability of visiting CP47 pigments and, thus, would not be “lost” via spillover. The compartmentalization of PSII could effectively serve to separate energy transferred from the PBS to PSII from energy lost from PSII to PSI via spillover. Thus, the two processes are more independent than predicted by the classic spillover mechanism, which assumed all antenna and reaction center chromophores in PSII to be in equilibrium. The choice of CP47 as the PSII component involved in spillover is consistent with state 1 minus state 2 fluorescence emission difference spectra, such as those reported by Salehian and Bruce (1992), which show a dominant peak at 695 nm, characteristic of the long-wavelength Chl a associated with CP47. However, because both CP47 and CP43 are somewhat energetically isolated from D1/D2, either or both could be involved in spillover. It is interesting to note that because of the strong distance dependence for excitation energy transfer, a relatively small change in distance between PSI and the PBS-PSII supercomplex would be sufficient to significantly affect both KD-I and KII-I. The relative magnitude of changes in PBS- and Chl a-absorbed energy with the state transition will depend not only on the relative changes in these two rate constants, but also on the distribution of PBS-absorbed excitation energy between the two terminal emitters of the PBS, ApcE, and ApcD.
Our model is consistent with results obtained from the two mutants used in this study. The model predicts that the observation of changes in PBS distribution requires control of the association of the PBS core with PSI and the presence of ApcD. Control of the association of the PBS with PSI is presumably lacking in the Synechocystis sp. rpaC− strain and ApcD itself is lacking in the Synechococcus sp. apcD− strain. Loss of either would not necessarily affect spillover from CP47 to PSI.
Figure 5B is a top view of the model and shows the association of PSII dimers with two APC core cylinders (from one PBS) and PSI trimers. This view also shows two PBS rods (each consisting of two hexameric phycocyanin discs) associated with the core lying on the surface of the thylakoid membrane. This view of the model is a modified and more detailed version (including chlorin chromophores of PSII and PSI) of a structural model previously presented by Bald et al. (1996) based primarily on electron microscopic data. This figure reflects the likely oligomerization states of PSII (Morschel and Schatz, 1987) and PSI (Kruip et al., 1994) found in cyanobacterial membranes. In addition, Figure 5B is fairly consistent with the relative numbers of PSII, PSI, and PBS usually found in Synechococcus sp. PCC 7002 (Zhao et al., 2001), although the PSII/PSI ratio is higher than that found in Synechocystis sp. PCC 6803. In state 2, PSI trimers are shown in close proximity to both ApcD and CP47, which would facilitate energy transfer from both the presumed long-wavelength ApcD chromophore and CP47 Chl a to PSI Chl a. Although a relatively small displacement of PSI from the PBS-PSII supercomplex would result in large changes in the distribution of both PBS- and Chl a-absorbed excitation, this figure shows a large-scale change in organization consistent with electron microscopic data (Olive et al., 1986). As discussed previously (Bald et al., 1996), the alignment of PSII and PBS in rows in state 1 may exclude PSI from the vicinity of PSII and of ApcD. Therefore, this alignment would serve as an effective means of decreasing both KD-I and KII-I in state 1. In states 1 and 2, the PSI trimer is shown associated with a terminal rod disc, consistent with the idea that cyanobacterial ferredoxin NADP reductase (FNR) serves to “link” PSI to the PBS rod. The cyanobacterial FNR has an N-terminal extension homologous to the rod terminating linker protein CpcD (Schluchter and Bryant, 1992). Although evidence is lacking in cyanobacteria, cross-linking studies have shown FNR to be associated with the stromal-exposed PsaE subunit of PSI in higher plants (Andersen et al., 1992).
State 1 is characterized by a relative decrease in the contribution of PBS-absorbed excitation to PSI, but not a complete loss of all PBS contribution. Although this is fairly easily represented by fine-tuning the two rate constants in Figure 5A, it is more difficult to envisage in Figure 5B if all PSI complexes are excluded from access to the APC core. Thus, the representation of state 1 in Figure 5B includes one PBS-PSII supercomplex that remains associated with a PSI trimer via ApcD. This was included to represent the idea that some complexes may be “more involved” in the state transition than others. Although the state transition may be represented by a small change in the two rate constants characterizing the association of PBS-PSII supercomplexes with PSI as shown in Figure 5A, it could also be represented by a much larger change in these constants for a subset of PBS-PSII supercomplexes involved in the formation of rows as shown in Figure 5B.
It has been suggested previously that changes in the oligomerization state of both PSII and PSI may accompany the state transition (Bald et al., 1996; Mullineaux, 1999). However, because there is no strong direct evidence for changes in oligomerization with the state transition, and they are not required to effect significant changes in energy distribution for either PBS- or Chl a-absorbed excitation energy, we have not included them in this model.
The two APC core cylinders are “antiparallel” and the ApcE occupies an internal disc position in the core. The reaction center chromophores of PSII, shown in red, are seen to lie directly under the ApcE-containing core discs. Although the phycocyanobilin chromophores are not shown in this figure, in our model we have rotated the two cylinders to allow the two presumed ApcE-associated long-wavelength chromophores to come close to the thylakoid membrane. In this orientation, each of the presumed long-wavelength chromophores is located almost directly above each of the two D1/D2s in the PSII dimer. The transition dipoles of the presumed ApcE chromophores are oriented at an angle of approximately 15° to the normal of the thylakoid membrane plane. The transition dipoles of the two pheophytins of the reaction center are also oriented closer to the normal to the thylakoid membrane plane than the majority of nearby Chl a molecules in CP43 or CP47 whose dipoles are mostly aligned parallel to the membrane plane. We have made some initial calculations (data not shown), based on Förster theory and analogous to those described in (Vasil'ev et al., 2001), of the relative efficiency of energy transfer from the presumed ApcE chromophore to the nearest chromophores in D1/D2 and CP43 and CP47. Although specific results are dependent on the exact details of placement of the PBS core onto PSII, it is clear that as long as the presumed ApcE chromophore is somewhere above D1/D2, the pheophytins would carry the largest proportion of energy from the ApcE chromophore to the reaction center. This confirms that it is possible to envisage a “direct connection” between the PBS and PSII reaction center that would be, to some extent, energetically independent of spillover from CP47 to PSI.
Our model for the state transition is consistent with most of the experimental data for state transitions in cyanobacteria. The most notable exception is the FRAP data, which have indicated a higher lateral mobility of the PBS compared with PSII-associated Chl a molecules after photobleaching with a laser flash (Mullineaux et al., 1997). Our model suggests less drastic changes in association of PSII, PSI, and the PBS than the mobile PBS model and cannot easily be reconciled with the FRAP data. However, it seems unlikely that the FRAP technique is completely noninvasive. The apparent mobility of the PBS that has been observed could reflect changes in its association with the thylakoid membrane that arise from local heating generated by the high-energy laser flashes required to bleach the pigments.
Our model does not address the underlying forces responsible for inducing the proposed changes in association between the PBS-PSII supercomplexes and PSI trimers. The state transition is clearly triggered by the redox state of intersystem electron carriers and the action spectra in this work show that changes in both PBS and Chl distribution are triggered by the same environmental light conditions. However, what makes the complexes move? Early reports of reversible phosphorylation of PBS-associated components (Allen et al., 1985) championed a mechanism based on electrostatic interactions and changes in charge density associated with differential phosphorylation. However, state transition-associated reversible phosphorylation has not been supported in the subsequent literature and is unlikely to be involved (for review, see Biggins and Bruce, 1989; Mullineaux, 1999) and strong evidence for an alternative has not been presented. Unfortunately, after more than 30 years of investigation, this aspect of the state transition in cyanobacteria is still a mystery.
CONCLUSIONS
We have shown that regulation of the distribution of Chl a-absorbed excitation energy with the state transition is independent of the regulation of energy absorbed by the PBS in two different cyanobacterial mutants impaired in state transitions. Despite this independence, the regulation of both Chl a and phycobilin pigments are triggered by very similar environmental light conditions. We have constructed a model for the state transition using the x-ray structures of PSII, PSI, and APC that is consistent with our results and most previous work. Our model combines regulation of direct energy transfer from the PBS core to PSI with regulation of the spillover of excitation energy from one of the core antenna complexes of PSII, CP47, to PSI. Key to the mechanism is the idea that a significant proportion of excitation energy from the PBS is transferred directly to the reaction center chromophores of PSII. Because these chromophores are not in energetic equilibrium with CP43 and CP47, changes in spillover from CP47 to PSI will affect the distribution of Chl a-absorbed excitation energy much more than the distribution of PBS-absorbed excitation energy. Our model predicts that relatively small movements of PSI with respect to the PBS/PSII complex would be sufficient to change the distribution of both Chl a- and PBS-absorbed energy. However, the model is also consistent with the previously proposed larger scale changes in the distribution of PBS/PSII complexes in the thylakoid membrane, from ordered rows in state 1 to a more randomized pattern in state 2. Our model is presented as a work in progress. We believe this model currently offers the simplest explanation for our data and for most of the data found in the literature on state transitions in cyanobacteria.
MATERIALS AND METHODS
Cyanobacteria Used and Culture Conditions
Synechococcus sp. PCC 7002 wild-type and apcD− cells were a generous gift from Don Bryant (Pennsylvania State University, University Park).
Synechocystis sp. PCC 6803 wild-type and rpaC− cells were a generous gift from Conrad W. Mullineaux (University College, London).
The wild-type and apcD− cells of Synechococcus sp. PCC 7002 were grown photo-autotrophically in 100-mL batch cultures at 32°C in A+ growth medium, containing vitamin B12, at a light intensity of 50 μmol m−2 s−1. Batch cultures were bubbled with air. All cultures of Synechocystis sp. PCC 6803 were grown under similar conditions with the exception of light intensity and growth medium. In this case, BG11 growth media with 10 mm NaHCO3 and 10 mm TES was used to grow both wild-type and mutant cells photo-autotrophically at a light intensity of 25 μmol m−2 s−1. For all experiments, cells were harvested at mid- to mid-late exponential growth phase.
Room Temperature Fluorescence
A 1-cm optical path fluorescence cuvette was filled (3 mL) with cells at a Chl a concentration of approximately 10 μg Chl mL−1 and was dark adapted to state 2 (Rouag and Dominy, 1994). The dark adaptation drives the transition to state 2 as a result of a dark reduction of the plastoquinone pool (Mullineaux and Allen, 1990). The cuvette was placed in the PAM Chl fluorometer (model PAM 101, H. Walz, Effeltrich, Germany) and variable fluorescence measured for a dark interval followed by exposure to blue light (425-nm interference filter, 10-nm bandpass, 30 μmol m−2 s−1) used to drive the cells to state 1. During the course of the experiment, cells were exposed to saturating 600-ms flashes of white actinic light (150-W tungsten halogen, approximately 10,000 μmol m−2 s−1) spaced 80 s apart, which were used to determine the Fm.
77K Fluorescence Emission Spectra
Samples were assayed for 77K fluorescence emission with a purpose-built fluorescence spectrophotometer previously described by Salehian and Bruce (1992).
Samples for analysis were prepared in the following way. About 100 mL of cells were kept in a stirred flask in their respective growth medium at a Chl a concentration of 10 μg Chl mL−1 and illuminated with room light (approximately 25 μmol m−2 s−1). A small volume of cells (35 μL) was taken from this flask and placed in a Pasteur pipette with a heat-sealed end. The cells in the Pasteur pipette were then immediately exposed to a particular light wavelength, intensity, and duration (details dependent on the experiment), quickly frozen by plunging into liquid nitrogen, and kept in liquid nitrogen until emission spectra were collected.
77K fluorescence emission spectra were collected for every sample for two different excitation wavelengths: 435 nm to excite Chl a and 580 nm to excite the PBS. The intensity of fluorescence emission at 695 nm (F695), arising from the long-wavelength Chl of the PSII core antenna polypeptide CP47 of PSII, was divided by the intensity of emission at 715 nm (F715) in Synechococcus sp. or at 725 nm (F725) in Synechocystis sp., arising from the long-wavelength Chl of PSI. F695/F715 and F695/F725 were used as relative indicators of the distribution of excitation energy between PSII and PSI, and, therefore, the “state” of the cells in Synechococcus sp. and Synechocystis sp., respectively.
Action Spectra for the State Transition-Induced Changes in 77K Fluorescence Emission
To generate an action spectrum for the state transition, 14 samples were illuminated with 14 different wavelengths selected between 420 and 680 nm with a 75-W Xenon Arc lamp dispersed by a 0.25-m monochromator (Sciencetech, London, Canada). Each 35-μL sample was illuminated at room temperature in a Pasteur pipette as described in the previous section for 90 s at one wavelength and then quickly frozen in liquid nitrogen. The bandwidth for all illumination wavelengths was about 6 nm and the intensity of the illumination was 30 μmol m−2 s−1. For each illumination wavelength, three repeats using independent samples of cyanobacteria from each of the four strains were used. As described above, 77K emission spectra were obtained for excitation at both 435 nm (Chl a) and 580 nm (PBS) for each frozen sample and the F695/F715 or F695/F725, depending on species, were calculated for each spectrum. The average ratio for each room temperature illumination wavelength was determined for the three repeats and plotted to give two action spectra (one for 435-nm excitation, the other for 580-nm excitation) for each of the four sample types investigated. The action spectra show the resulting F695/F715 (for Synechococcus sp. PCC 7002 wild type and mutant) or F695/F725 (for Synechocystis sp. PCC 6803 wild type and mutant) as a function of the room temperature illumination wavelength chosen to drive the state transition. A high ratio shows a transition to state 1, and a low ratio shows a transition to state 2.
Absorbance Cross Sections
PSII absorbance cross sections were determined from flash saturation curves generated with a pump probe fluorescence spectrometer as described by Samson and Bruce (1995). Cross sections were determined for excitation of Chl a at 674 nm and for excitation of the PBS at 630 nm. Flash saturation curves were fit with a single-hit Poisson distribution as described by Mauzerall and Greenbaum (1989).
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Footnotes
This work was supported by the Natural and Scientific Engineering Research Council of Canada.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.009845.
LITERATURE CITED
- Allen JF. Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta. 1992;1098:275–335. doi: 10.1016/s0005-2728(09)91014-3. [DOI] [PubMed] [Google Scholar]
- Allen JF, Holmes NG. A general model for regulation of photosynthetic unit function by protein phosphorylation. FEBS Lett. 1986;202:175–181. [Google Scholar]
- Allen JF, Sanders CF, Holmes NG. Correlation of membrane protein phosphorylation with excitation energy distribution on the cyanobacterium Synechocystis 6301. FEBS Lett. 1985;193:271–275. [Google Scholar]
- Andersen B, Scheller HV, Moller BL. The PSI-E subunit of photosystem I binds ferredoxin:NADP+ oxidoreductase. FEBS Lett. 1992;311:169–173. doi: 10.1016/0014-5793(92)81391-x. [DOI] [PubMed] [Google Scholar]
- Ashby MK, Mullineaux CW. The role of ApcD and ApcF in energy transfer from phycobilisomes to PS I and PS II in a cyanobacterium. Photosynth Res. 1999;61:169–179. [Google Scholar]
- Bald D, Kruip J, Rogner M. Supramolecular architecture of cyanobacterial thylakoid membranes: How is the phycobilisome connected with the photosystems? Photosynth Res. 1996;49:118. doi: 10.1007/BF00117661. [DOI] [PubMed] [Google Scholar]
- Biggins J, Bruce D. Regulation of excitation energy transfer in organisms containing phycobilins. Photosynth Res. 1989;20:1–34. doi: 10.1007/BF00028620. [DOI] [PubMed] [Google Scholar]
- Brimble S, Bruce D. Pigment orientation and excitation energy transfer in Porphyridium cruentum and Synechococcus sp. PCC 6301 cross-linked in light state 1 and light state 2 with glutaraldehyde. Biochim Biophys Acta. 1989;973:315–323. [Google Scholar]
- Bruce D, Biggins J. Mechanism of the light-state transition in photosynthesis: V. 77 K linear dichroism of Anacystis nidulans in state 1 and state 2. Biochim Biophys Acta. 1985;810:295–301. [Google Scholar]
- Bruce D, Brimble S, Bryant DA. State transitions in a phycobilisome-less mutant of the cyanobacterium Synechococcus sp. PCC 7002. Biochim Biophys Acta. 1989;974:66–73. doi: 10.1016/s0005-2728(89)80166-5. [DOI] [PubMed] [Google Scholar]
- Dominy PJ, Williams WP. The role of respiratory electron flow in the control of excitation energy distribution in blue-green algae. Biochim Biophys Acta. 1987;892:264–274. [Google Scholar]
- El Bissati K, Delphin E, Murata N, Etienne AL, Kirilovsky D. Photosystem II fluorescence quenching in the cyanobacterium Synechocystis PCC 6803: involvement of two different mechanisms. Biochim Biophys Acta. 2000;1457:229–242. doi: 10.1016/s0005-2728(00)00104-3. [DOI] [PubMed] [Google Scholar]
- Emlyn-Jones D, Ashbey MK, Mullineaux CW. A gene required for the regulation of photosynthetic light harvesting in the cyanobacterium Synechocystis 6803. Mol Microbiol. 1999;33:1050–1058. doi: 10.1046/j.1365-2958.1999.01547.x. [DOI] [PubMed] [Google Scholar]
- Federman S, Malkin R, Scherz A. Excitation energy transfer in aggregates of photosystem I and photosystem II of the cyanobacterium Synechocystis sp. PCC 6803: Can assembly of the pigment-protein complexes control the extent of spillover? Photosynth Res. 2000;64:199–207. doi: 10.1023/A:1006485823403. [DOI] [PubMed] [Google Scholar]
- Gindt YM, Zhou J, Bryant DA, Sauer K. Spectroscopic studies of phycobilisome subcore preparations lacking key core chromophores: assignment of excited state energies to the Lcm, beta 18 and alpha AP-B chromophores. Biochim Biophys Acta. 1994;1186:153–162. doi: 10.1016/0005-2728(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Glazer AN. Phycobilisome: a macromolecular complex optimized for light energy transfer. Biochim Biophys Acta. 1984;768:29–51. [Google Scholar]
- Homer-Dixon J, Gantt E, Bruce D. Pigment orientation changes accompanying the light state transition in Synechococcus sp. PCC 6301. Photosynth Res. 1994;40:35–44. doi: 10.1007/BF00019043. [DOI] [PubMed] [Google Scholar]
- Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauss N. Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature. 2001;411:909–917. doi: 10.1038/35082000. [DOI] [PubMed] [Google Scholar]
- Koblízek M, Komenda J, Masojídek J. State transitions in the cyanobacterium Synechococcus PCC 7942. Mobile antenna or spillover? In: Garab G, editor. Photosynthesis: Mechanisms and Effects. Vol. 1. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 213–216. [Google Scholar]
- Kruip J, Bald D, Boekema EJ, Rogner M. Evidence for the existence of trimeric and monomeric photosystem I complexes in thylakoid membranes from cyanobacteria. Photosynth Res. 1994;40:279–286. doi: 10.1007/BF00034777. [DOI] [PubMed] [Google Scholar]
- Liu JY, Jiang T, Zhang JP, Liang DC. Crystal structure of allophycocyanin from red algae Porphyra yezoensis at 2.2-A resolution. J Biol Chem. 1999;274:16945–16952. doi: 10.1074/jbc.274.24.16945. [DOI] [PubMed] [Google Scholar]
- Mauzerall D, Greenbaum NL. The absolute size of a photosynthetic unit. Biochim Biophys Acta. 1989;974:119–140. [Google Scholar]
- Maxson P, Sauer K, Zhou JH, Bryant DA, Glazer AN. Spectroscopic studies of cyanobacterial phycobilisomes lacking core polypeptides. Biochim Biophys Acta. 1989;977:40–51. doi: 10.1016/s0005-2728(89)80007-6. [DOI] [PubMed] [Google Scholar]
- Morschel E, Schatz GH. Correlation of photosystem 2 complexes with exoplasmatic freeze-fracture particles of thylakoids of the cyanobacterium Synechococcus sp. Planta. 1987;172:145–154. doi: 10.1007/BF00394582. [DOI] [PubMed] [Google Scholar]
- Mullineaux CW. Excitation energy transfer from phycobilisomes to photosystem I in a cyanobacterium. Biochim Biophys Acta. 1992;1100:285–292. doi: 10.1016/j.bbabio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Mullineaux CW. Excitation energy transfer from phycobilisomes to photosystem I in a cyanobacterial mutant lacking photosystem II. Biochim Biophys Acta. 1994;1184:71–77. [Google Scholar]
- Mullineaux CW. The thylakoid membranes of cyanobacteria: structure, dynamics and function. Aust J Plant Physiol. 1999;26:671–677. [Google Scholar]
- Mullineaux CW, Allen JF. The state 2 transition in the cyanobacterium Synechococcus 6301 can be driven by respiratory electron flow into the plastiquinone pool. FEBS Lett. 1986;205:155–160. [Google Scholar]
- Mullineaux CW, Allen JF. State 1-state 2 transitions in the cyanobacterium Synechococcus 6301 are controlled by the redox state of electron carriers between photosystem I and II. Photosynth Res. 1990;23:297–311. doi: 10.1007/BF00034860. [DOI] [PubMed] [Google Scholar]
- Mullineaux CW, Holzwarth AR. A proportion of photosystem II core complexes are decoupled from the phycobilisome in light-state 2 in the cyanobacterium Synechococcus 6301. FEBS Lett. 1990;260:245–248. [Google Scholar]
- Mullineaux CW, Tobin MJ, Jones MR. Mobility of photosynthetic complexes in thylakoid membranes. Nature. 1997;390:421–424. [Google Scholar]
- Murata N. Control of excitation energy transfer in photosynthesis: I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta. 1969;172:242–251. doi: 10.1016/0005-2728(69)90067-x. [DOI] [PubMed] [Google Scholar]
- Olive J, M'Bina J, Vernotte C, Astier C, Wollman FA. Randomization of the EF particles in thylakoid membranes of Synechocystis 6714 upon transition from state 1 to state 2. FEBS. 1986;208:308–312. [Google Scholar]
- Reuter W, Wiegand G, Huber R, Than ME. Structural analysis at 2.2 Å of orthorhombic crystals presents the asymmetry of the allophycocyanin-linker complex, AP.LC7.8, from phycobilisomes of Mastigocladus laminosus. Proc Natl Acad Sci USA. 1999;96:1363–1368. doi: 10.1073/pnas.96.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouag D, Dominy PJ. State adaptations in the cyanobacterium Synechococcus 6301 (PCC): dependence on light intensity or spectral composition. Photosynth Res. 1994;40:107–117. doi: 10.1007/BF00019049. [DOI] [PubMed] [Google Scholar]
- Rögner M, Nixon PJ, Diner BA. Purification and characterization of photosystem I and photosystem II core complexes from wild-type and phycocyanin-deficient strains of the cyanobacterium Synechocystis PCC 6803. J Biol Chem. 1990;265:6189–6196. [PubMed] [Google Scholar]
- Salehian O, Bruce D. Distribution of excitation energy in photosynthesis: quantification of fluorescence yields from intact cyanobacteria. J Luminescence. 1992;51:91–98. [Google Scholar]
- Samson G, Bruce D. Complementary changes in absorbance cross-section of photosystem I and photosystem II due to phosphorylation and magnesium depletion in spinach thylakoids. Biochim Biophys Acta. 1995;1232:21–26. [Google Scholar]
- Sauer K, Scheer H. Excitation transfer in C-phycocyanin. Förster transfer rate and exciton calculations based on new crystal structure data for C-phycocyanins for Agmenellum quadruplicatum and Mastigocladus laminosus. Biochim Biophys Acta. 1988;936:157–170. [Google Scholar]
- Schluchter WM, Bryant DA. Molecular characterization of ferredoxin-NADP+ oxidoreductase in cyanobacteria: cloning and sequence of the petH gene of Synechococcus sp. PCC 7002 and studies on the gene product. Biochemistry. 1992;31:3092–3102. doi: 10.1021/bi00127a009. [DOI] [PubMed] [Google Scholar]
- Sidler WA. Phycobilisomes and phycobiliprotein structures. In: Bryant DA, editor. Molecular Biology of Cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 139–216. [Google Scholar]
- Van Thor JJ, Mullineaux CW, Matthijs HCP, Hellingwerf KJ (1998) Light harvesting and state transitions in cyanobacteria. Bot Acta 430–443
- Vasil'ev S, Orth P, Zouni A, Owens TG, Bruce D. Excited-state dynamics in photosystem II: insights from the x-ray crystal structure. Proc Natl Acad Sci USA. 2001;98:8602–8607. doi: 10.1073/pnas.141239598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XQ, Li LN, Chang WR, Zhang JP, Gui LL, Guo BJ, Liang DC. Structure of C-phycocyanin from Spirulina platensis at 2.2 Å resolution: a novel monoclinic crystal form for phycobiliproteins in phycobilisomes. Acta Crystallogr D Biol Crystallogr. 2001;57:784–792. doi: 10.1107/s0907444901004528. [DOI] [PubMed] [Google Scholar]
- Wollman FA. State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J. 2001;20:3623–3630. doi: 10.1093/emboj/20.14.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Zhoa J, Bryant DA, Golbeck JH. PsaE is required for in vivo cyclic electron flow around photosystem I in the cyanobacterium Synechococcus sp. PCC 7002. Plant Physiol. 1993;103:171–180. doi: 10.1104/pp.103.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Shen G, Bryant DA. Photosystem stoichiometry and state transitions in a mutant of the cyanobacterium Synechococcus sp. PCC 7002 lacking phycocyanin. Biochim Biophys Acta. 2001;1505:248–257. doi: 10.1016/s0005-2728(01)00175-x. [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhao J, Bryant DA. Energy transfer processes in phycobilisomes as deduced from analyses of mutants of Synechococcus sp. PCC 7002. In: Murata N, editor. Research in Photosynthesis. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 25–32. [Google Scholar]
- Zilinskas BA, Greenwald LS. Phycobilisome structure and function. Photosynth Res. 1986;10:7–35. doi: 10.1007/BF00024183. [DOI] [PubMed] [Google Scholar]
- Zouni A, Witt H-T, Kern J, Fromme P, Krauβ N, Saenger W, Orth P. Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution. Nature. 2001;409:739–743. doi: 10.1038/35055589. [DOI] [PubMed] [Google Scholar]