Abstract
Abscisic acid (ABA) regulates developmental processes and abiotic stress responses in plants. We recently characterized a new Arabidopsis mutant, abh1, which shows ABA-hypersensitive regulation of seed germination, stomatal closing, and cytosolic calcium increases in guard cells (V. Hugouvieux, J.M. Kwak, J.I. Schroeder [2001] Cell 106: 477–487). ABH1 encodes the large subunit of a dimeric Arabidopsis mRNA cap-binding complex and in expression profiling experiments was shown to affect mRNA levels of a subset of genes. Here, we show that the dimeric ABH1 and AtCBP20 subunits are ubiquitously expressed. Whole-plant growth phenotypes of abh1 are described and properties of ABH1 in guard cells are further analyzed. Complemented abh1 lines expressing a green fluorescent protein-ABH1 fusion protein demonstrate that ABH1 mainly localizes in guard cell nuclei. Stomatal apertures were smaller in abh1 compared with wild type (WT) when plants were grown at 40% humidity, and similar at 95% humidity. Correlated with stomatal apertures from plants grown at 40% humidity, slow anion channel currents were enhanced and inward potassium channel currents were decreased in abh1 guard cells compared with WT. Gas exchange measurements showed similar primary humidity responses in abh1 and WT, which together with results from abh1/abi1-1 double-mutant analyses suggest that abh1 shows enhanced sensitivity to endogenous ABA. Double-mutant analyses of the ABA-hypersensitive signaling mutants, era1-2 and abh1, showed complex genetic interactions, suggesting that ABH1 and ERA1 do not modulate the same negative regulator in ABA signaling. Mutations in the RNA-binding protein sad1 showed hypersensitive ABA-induced stomatal closing, whereas hyl1 did not affect this response. These data provide evidence for the model that the mRNA-processing proteins ABH1 and SAD1 function as negative regulators in guard cell ABA signaling.
The plant hormone abscisic acid (ABA) controls several physiologically important stress and developmental responses throughout the life cycle of plants. During seed development, ABA triggers the acquisition of nutritive reserves, desiccation tolerance, maturation, and dormancy (Marcotte et al., 1992; Koornneef et al., 1998; Finkelstein et al., 2002). Later, during vegetative growth, ABA is the internal signal that enables plant adaptive responses to adverse environmental conditions such as drought, salt, and cold stresses (Marcotte et al., 1992; Koornneef et al., 1998; Leung and Giraudat, 1998).
In response to drought, ABA is synthesized and induces closure of stomatal pores, located on the leaf surface, to limit water loss by transpiration. Stomatal pores are surrounded by pairs of guard cells whose turgor regulates stomatal pore apertures. ABA induces stomatal closure via efflux of K+ and anions from guard cells and removal of organic osmolytes (MacRobbie, 1998; Schroeder et al., 2001). Ion channel-mediated efflux of anions and K+ and stomatal closure are controlled by ABA-induced cytosolic calcium ([Ca2+]cyt) increases (Schroeder and Hagiwara, 1989; McAinsh et al., 1990; MacRobbie, 1998).
An increasing number of genetic mutations that contribute to ABA signaling in guard cells have been characterized recently. These genes include two type 2C protein phosphatases, abi1-1 and abi2-1 (Leung et al., 1994, 1997; Meyer et al., 1994), a farnesyl transferase β-subunit, ERA1 (Cutler et al., 1996; Pei et al., 1998), an ABA-activated protein kinase mutant (aapk; Li et al., 2000), a GTP-binding protein α-subunit, GPA1 (Wang et al., 2001), dominant mutations in a GTPase protein, Atrac1-1 (Lemichez et al., 2001), and an mRNA cap-binding protein, ABH1 (Hugouvieux et al., 2001). Genes that affect ABA responses at the transcriptional level in seed germination and development have also been identified encoding three transcriptional regulators, ABI3 (Giraudat et al., 1992), ABI4 (Finkelstein et al., 1998), and ABI5 (Finkelstein and Lynch, 2000). Two RNA-binding proteins, a double-stranded RNA-binding protein, HYL1 (Lu and Fedoroff, 2000), and a protein similar to an Sm-like snRNP protein, SAD1 (Xiong et al., 2001a) were recently described to affect ABA regulation of seed germination. The sad1 mutation also caused enhanced ABA-induced expression of marker genes, but is drought hypersensitive (Xiong et al., 2001a). The fry1 mutant shows a superinduction of ABA- and stress-responsive genes and revealed the involvement of an inositol polyphosphate 1-phosphatase in ABA signaling (Xiong et al., 2001b).
We recently characterized the abh1 mutation (Hugouvieux et al., 2001) that points to a link between mRNA metabolism and ABA signaling. ABH1 encodes a homolog to yeast (Saccharomyces cerevisiae) and human (Homo sapiens) CBP80 genes and functions as the large subunit of an Arabidopsis mRNA cap-binding complex (CBC). No other ABH1 homologs are present in the Arabidopsis genome. Disruption of ABH1 results in ABA-hypersensitive regulation of seed germination, ABA-hypersensitive stomatal closing, reduced wilting during drought, and, interestingly, ABA-hypersensitive [Ca2+]cyt increases in guard cells, demonstrating amplification of early ABA signaling mechanisms (Hugouvieux et al., 2001). In yeast and mammals, CBC is involved in mRNA metabolism (Lewis and Izaurralde, 1997). DNA microarray experiments comparing the level of expression of genes in wild type (WT) and abh1 showed that a limited number of genes in abh1 are down-regulated, some of which may correspond to negative regulators of ABA signaling in guard cells. The recent isolation of three recessive ABA-hypersensitive mutants, abh1, hyl1, and sad1, which all encode RNA-associated proteins, gives rise to a new model by which RNA processing modulates and/or participates in ABA signal transduction.
To better understand ABH1 functions in plants, in the present work we characterize the pattern of ABH1 gene expression and associated whole-plant growth phenotypes of abh1. We also analyze ABH1 protein localization in guard cells and genetic interactions between ABH1 and the early ABA signaling components abi1-1 and ERA1 during stomatal regulation. In addition, we further characterize stomatal responses of abh1, sad1, and hyl1 which show differential effects on ABA-induced stomatal closing in hyl1 compared with abh1 and sad1. Responses in sad1 strengthen the hypothesis that RNA processing contributes to ABA signal transduction in guard cells.
RESULTS
ABH1 Expression Is Ubiquitous
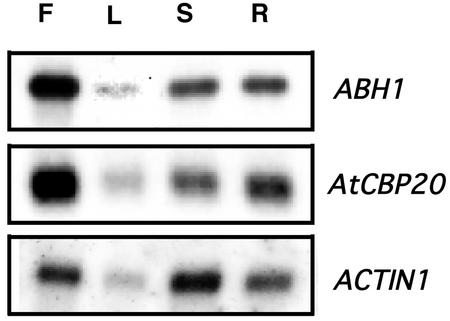
ABH1 was shown to be expressed in guard cells (Hugouvieux et al., 2001). Northern-blot analyses were performed on poly(A+) RNA extracted from WT roots, leaves, flowers, and stems to analyze ABH1 gene expression in various organs. As shown in Figure 1, ABH1 transcript was present in all tissues analyzed, with high expression in flowers. The AtCBP20 gene encodes the small subunit of the CBC and is required for in vitro binding of ABH1 to the 7-methyl guanosine cap structure of mRNA (Hugouvieux et al., 2001). AtCBP20 showed the same expression pattern as ABH1 (Fig. 1).
Figure 1.
Expression level of the CBC subunits, ABH1 and AtCBP20, is ubiquitous. Northern-blot analyses were performed on approximately 2 μg of poly(A+) RNA (extracted from flowers [F], stems [S], leaves [L], and roots [R]) in WT Arabidopsis. ABH1 or AtCBP20 cDNAs were used as probes. Actin1 probe was used as a loading control.
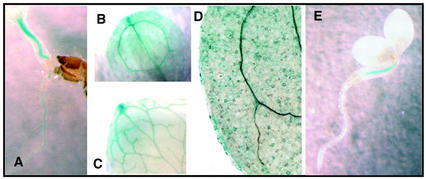
WT transgenic seedlings expressing the β-glucuronidase (GUS) reporter gene under the control of the ABH1 promoter (genomic sequence containing 1.2 kb from the 5′ end of the ABH1 start site; Hugouvieux et al., 2001) were used to further analyze in which tissues the ABH1 promoter was active. As expected, GUS activity was detected in roots and hypocotyls (Fig. 2A), cotyledons (Fig. 2B), and leaves (Fig. 2C). GUS activity was not detected in root or apical meristems (data not shown). The ABH1 promoter was highly active in vascular tissues (hypocotyls, roots, cotyledons, and leaves; Fig. 2, A–C). ABH1 expression was also observed in the vascular tissues of petals (data not shown). In 2-d-old seedlings, GUS activity was often first visually detectable in the hypocotyl (Fig. 2E); however, this restricted expression pattern disappeared in 8-d-old seedlings, suggesting a developmental control of ABH1 expression. The ABH1 promoter was active in guard cells (Fig. 2D; Hugouvieux et al., 2001). The same patterns of expression were observed in three independent homozygous lines.
Figure 2.
Analysis of GUS activity in WT plants transformed with the GUS reporter gene under the control of the ABH1 promoter. A through E, GUS activity in the hypocotyl and root of an 8-d old seedling (A), a cotyledon of an 8-d old seedling (B), a mature leaf (C), guard cells (D), and the hypocotyl of 2-d-old seedling (E).
ABA Treatment Does Not Change ABH1 Transcript Level
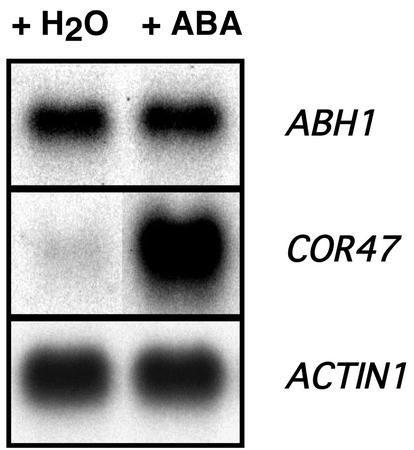
ABH1 is a negative regulator of ABA signaling (Hugouvieux et al., 2001). We further investigated whether ABA treatment induces changes in the ABH1 transcript level. As shown in Figure 3, treatment of WT leaves with 100 μm ABA for 4 h did not change ABH1 transcript levels (Fig. 3; n = 2 experiments). In control experiments, the transcript level of ABA-inducible genes was increased in WT plants, including COR47 (Fig. 3), RAB18, ABI1, and ABI2 (data not shown). Treatments with lower concentrations of ABA (0.1, 1, and 10 μm) were also performed and again showed no ABA regulation of ABH1 at the transcript level. The lack of ABA regulation of ABH1 mRNA levels was also observed after drought treatment (data not shown). As positive controls, under drought conditions, induction of the drought-induced gene, COR47, was observed (data not shown).
Figure 3.
ABH1 expression level is not regulated by ABA. WT leaves from five individual plants were sprayed with 100 μm ABA or water in parallel control experiments. Four hours later, poly(A+) RNA was extracted and 2 μg was used in northern-blot analyses using ABH1 cDNA and the COR47 genomic fragment as a probe. Actin1 probe was used as a loading control. Similar results were obtained in two replicates, and in the WT ecotype Wassilewskija (data not shown).
Growth and Developmental Phenotypes of abh1
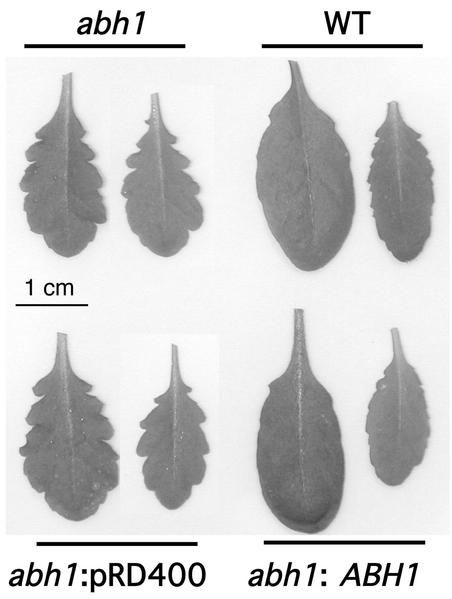
Considering the expression pattern of the ABH1 gene in vegetative tissues (Figs. 1 and 2), several morphological and growth characteristics of abh1 and WT plants were analyzed. abh1 plants showed a slightly serrated leaf phenotype (Fig. 4, top left) that is complemented in abh1 plants expressing the WT copy of the ABH1 gene (Fig. 4, lower right). Interestingly, this leaf phenotype was also observed in homozygous abh1/era1-2 and abh1/abi1-1 double mutants, which were indistinguishable from the abh1 mutant in leaf morphology (data not shown).
Figure 4.
abh1 plants show a serrated leaf phenotype that is complemented by the ABH1 gene. Two rosette leaves of each plant are shown after 7 weeks of growth. abh1 lines complemented with the ABH1 promoter and gene (abh1:ABH1) were generated as described (Hugouvieux et al., 2001). abh1 lines transformed with the vector control pRD400 only (abh1:pDR400) were used as controls.
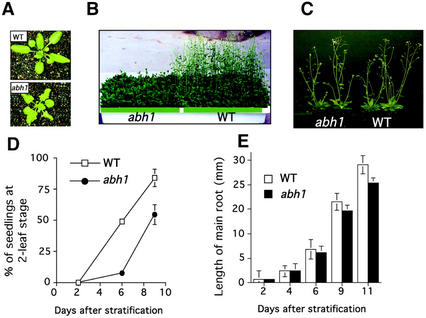
After exposure of seeds to 4°C for 4 d, 6-month-old abh1 and WT seeds germinated at the same rate in the absence of exogenous ABA (Hugouvieux et al., 2001). Under these conditions, abh1 plants were smaller than WT after 3 weeks of growth (Fig. 5A) and showed a delay in bolting and flowering time after 6 weeks (Fig. 5B). However, abh1 and WT appeared very similar at maturity (Fig. 5C). An approximately 5- to 10-d delay was observed in flowering of abh1 plants compared with WT. This delay varied among plants showing slightly different genetic penetrance among individual plants. The same number of leaves (between eight and 10) was observed in abh1 and WT plants when they started bolting, suggesting that abh1 growth is slowed compared with WT and that abh1 is not a late-flowering mutant that shows an increased leaf number when flowering. To further investigate whether abh1 growth was slower compared with WT, we followed the shoot and the main root's development in abh1 and WT seedlings grown in petri dishes. As shown in Figure 5D, the appearance and development of the first two leaves were delayed in abh1 compared with WT after 6 and 9 d of growth. Root length was slightly decreased in abh1 compared with WT after 11 d of growth, although root length was similar after 2 d in both WT and abh1 (Fig. 5E). These data suggested that abh1 growth is delayed compared with WT. Morphological characteristics of abh1 and WT plants were further analyzed and compared at maturity. As summarized in Table I, the only significant difference observed between abh1 and WT was in the length of the main stem, which was decreased in abh1 by approximately 15% to 20%. No significant differences were observed in the number of seeds per plant, the number of petals per flower, or the number of secondary and lateral branches. These data showed that the abh1 mutation does not significantly affect important developmental processes during growth and further reaffirms the relative dearth of pleiotropic effects of the abh1 mutation.
Figure 5.
abh1 growth is slower compared with WT. A, abh1 and WT rosettes are shown after 3 weeks of growth. For the same age, abh1 rosettes are smaller and show a smaller number of leaves. B, WT and abh1 plants are shown after 6 weeks of growth. The delay in flowering is about 5 to 10 d in abh1. C, When abh1 and WT growth was synchronized, WT and abh1 plants showed similar whole-plant phenotypes (see also Table I). D, Development of the first two leaves in abh1 and WT seedlings grown in petri dishes. Data represent the mean of five experiments ± se (n = 30 seedlings per line for each experiment). E, Root elongation after 2, 6, 9, and 11 d in abh1 and WT. The figure shows a representative experiment ± sd (n = 60). D and E, Error bars are smaller than symbols when not visible. The slower growth in abh1 was complemented by the ABH1 gene (data not shown). A, B, D, and E, WT and abh1 plants were grown in parallel in a growth chamber after stratification for 4 d at 4°C from seeds between 3 to 6 months old.
Table I.
Morphological characteristics of abh1 and WT
| Property | WT | abh1 |
|---|---|---|
| Length of the main stem (cm)a | Exp 1: 34.03 ± 1.18 Exp 2: 26.65 ± 0.79 | 29.5 ± 1.49e 21.07 ± 0.7e |
| No. of lateral branchesa | Exp 1: 2.6 ± 0.22 Exp 2: 1.8 ± 0.29 | 2.9 ± 0.18 2 ± 0.39 |
| No. of secondary stemsa | Exp 1: 4.28 ± 0.42 Exp 2: 4.85 ± 0.4 | 4.14 ± 0.34 4.42 ± 0.429 |
| Length of the siliquesb | 1.45 ± 0.017 | 1.41 ± 0.01 |
| No. of seeds/siliquesb | 40.75 ± 1.84 | 41.87 ± 2.16 |
| Weight of seeds/plants (mg)c | 68 ± 0.007 | 70 ± 0.006 |
| No. of petals per flowerd | 4.009 ± 0.09 | 4.049 ± 0.21 |
More than 20 abh1 and WT plants were grown in parallel in each experiment (Exp) after their growth was synchronized (see “Materials and Methods”). Plants used for measurements were selected randomly. Measurements were performed after 9 weeks, except for the petals, which were counted when flowers had opened (6–8 weeks). ses of the mean are shown.
n = 7 and 10 in Exp 1 and 2, respectively.
n = 30.
n = 10.
n = 100.
Significant difference from WT.
ABH1 Is Mainly Localized in the Nucleus
We showed that ABH1, together with AtCBP20, binds the 7-methyl guanosine cap structure of mRNA in vitro, and that its disruption leads to abnormal transcript accumulation of a limited number of genes in planta (Hugouvieux et al., 2001), which suggests that ABH1 has a role in mRNA processing. To obtain further insights into ABH1 function in guard cells, we analyzed where the protein was localized.
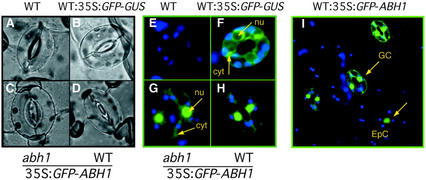
To investigate the subcellular localization of the ABH1 protein, ABH1 and green fluorescent protein (GFP) coding sequences were fused in frame to produce both N- and C-terminal fusions. abh1 mutant plants expressing the N-terminal fusion protein, GFP-ABH1, showed complementation of the abh1 mutant based on seed germination assays with ABA and on suppression of the serrated leaf phenotype (data not shown), illustrating that GFP-ABH1 was functional. The C-terminal fusion protein, ABH1-GFP, showed no complementation of the abh1 mutant based on ABA-dependent seed germination analyses and on the persistence of the serrated leaf phenotype (data not shown). These data suggest that the C-terminal domain of ABH1 is important for ABH1 function. Thus, further analyses were performed on the functional GFP-ABH1 lines. The subcellular localization of GFP-ABH1 was analyzed in guard cells and epidermal cells (Fig. 6, A–I). Untransformed WT plants, as expected, showed no GFP fluorescence (Fig. 6, A and E). The GFP-ABH1 fusion protein was mainly found in nuclei in both WT and abh1 plants and a slight GFP activity was detected in the cytosol (Fig. 6, G–I). The same pattern of expression was observed using the C-terminal fusion (data not shown). In control experiments, in which WT plants expressed the GFP protein fused to the GUS protein, the GFP-GUS fusion protein was detected in the cytosol (Fig. 6F). The nuclear localization of GFP-ABH1 and complementation of abh1 by this fusion protein support the proposed function of ABH1 as a subunit of a nuclear RNA CBC.
Figure 6.
GFP-ABH1 fusion protein is localized mainly in nuclei in WT and abh1. A through D, Bright-field images of guard cells used to study GFP fluorescence in E through H. Blue and green colors show chloroplast (emission 488 nm) and GFP (emission 522 nm) fluorescence, respectively. F, WT control transformed with GFP fused to the GUS protein (pCAMBIA1303; GenBank accession no. AF23299) shows that GFP-GUS is not localized in nuclei (nu), but rather in the cytosol (cyt). G, abh1-complemented lines with 35S:GFP-ABH1 construct show GFP fluorescence mainly in nuclei. H and I, Epidermal strips from WT transformed with the 35S:GFP-ABH1 construct show GFP fluorescence mainly in the nuclei in guard cells (GC) and epidermal cells (EpC). The same pattern of expression was observed in three independent lines.
Anion and Potassium Channel Activity in abh1
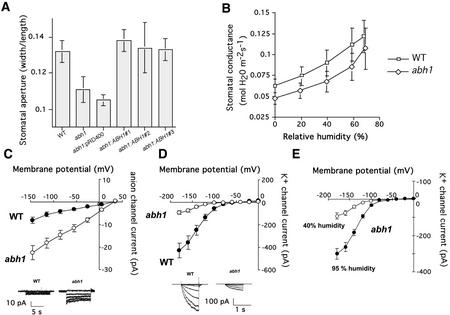
To better understand the function of ABH1 in guard cell signaling, we further investigated guard cell phenotypes in abh1 plants. When stomatal apertures were measured in leaves harvested directly from plants grown under low humidity (40%), stomatal apertures of abh1 were smaller than those of WT plants (Fig. 7A). WT stomatal apertures were restored in three homozygous abh1 lines transformed with the ABH1 gene (Fig. 7A). In contrast, for plants exposed to 95% humidity (overnight treatment), stomatal apertures in abh1 and WT were similar (abh1 stomatal aperture width/length, 0.17 ± 0.04; WT stomatal aperture width/length, 0.16 ± 0.02; n = 3 experiments ± sd). The smaller stomatal apertures observed in abh1 plants exposed to 40% humidity possibly resulted either from hypersensitivity to endogenous ABA or because abh1 showed an altered response to low humidity.
Figure 7.
Stomatal aperture and guard cell anion and K+ in channel activity are modified in abh1. A, Stomatal apertures in abh1 are smaller than in WT in plants grown at 40% humidity. Leaves were directly harvested from plants grown in a 40% humidity growth chamber and stomatal apertures were measured without any pre-incubation in opening solution. Three independent abh1 lines complemented with the ABH1 locus (abh1:ABH1 nos. 1, 2, and 3) show stomatal apertures similar to WT. abh1 control plants transformed with vector only (abh1:pRD400) show abh1 stomatal apertures. The data represent the mean ± sd of three independent experiments. B, Relative changes in leaf gas exchange in response to different humidity levels are similar in abh1 and WT. Stomatal conductance of WT and abh1 at several humidities was measured in intact leaves using a Li-6400 infrared gas analyzer (LI-COR, Inc., Lincoln, NE). Stomata were acclimated first to a high humidity (≥80% RH), then the humidity was sequentially dropped and new steady-state conductances determined at each humidity level. New steady states were achieved within 5 to 15 min after the humidity step. Data represent the mean ± se of three independent experiments. C and D, Whole-cell current voltage relationships recorded in WT and abh1 guard cells isolated from 40% humidity-grown plants showed a constitutive activation of slow anion channel currents and a decreased inward-rectifying K+ (K+in) channel activity in abh1 compared with WT (WT, n = 26 [C]; n = 13 [D]) and (abh1: n = 35 [C]; n = 14 [D]). Inserts show examples of ion current recordings. E, Whole-cell current voltage relationships recorded in abh1 guard cells isolated from either 40% humidity-grown plants (n = 14 cells) or after 72 h of high humidity (95%) treatment (n = 17 cells). Anion and K+ currents were recorded from 4- to 6-week-old plants as described in “Materials and Methods.”
To distinguish between these two possibilities, we investigated the humidity response of abh1 plants by comparing stomatal conductances of WT and abh1 at various humidities using an infrared gas analyzer (Fig. 7B). Gas exchange in abh1 plants at 40% humidity was slightly reduced compared with WT (Fig. 7B), consistent with stomatal aperture measurements (Fig. 7A). Stomata of abh1 closed upon introduction of lower humidity, as did WT stomata (Fig. 7B). The rapid response in gas exchange experiments to step changes in humidity was previously shown to be independent of ABA signaling in Arabidopsis (Assmann et al., 2000). Indistinguishable gas exchange responses to step changes in humidity in abh1 and WT (Fig. 7B) suggest that abh1 does not directly modulate the rapid primary humidity response. We also observed that stomatal apertures of abi1-1 were generally larger than those of WT plants (Landsberg erecta [Ler] background) when no ABA was added, including plants grown at 40% humidity (described later), and abi1-1 does not affect the stomatal response to humidity (Assmann et al., 2000). These findings are consistent with the hypothesis that reduced stomatal apertures in abh1 at low humidity are because of signaling mediated by endogenous ABA.
ABA induces cytosolic Ca2+ increases, which in turn activate slow anion channels and inhibit K+in channels in guard cells (Schroeder and Hagiwara, 1989; McAinsh et al., 1990). The activities of guard cell slow anion channels and K+in channels were investigated in abh1 and WT grown at 40% humidity, which causes reduced stomatal apertures in abh1 (Fig. 7A). Patch clamp experiments performed on guard cell protoplasts from plants grown under low humidity showed that in abh1 guard cells, anion currents were consistently larger than those in the WT guard cells (Fig. 7C; P < 0.001). Furthermore, anion currents (n = 6) showed reduction to WT magnitudes in a complemented line (P = 0.15; data not shown). Control experiments were performed on the ABA-hypersensitive mutant, era1-2. In era1-2, no constitutive activation of guard cell anion channels was observed in the absence of exogenous ABA (n = 8, data not shown), confirming previous findings (Pei et al., 1998). These data show a difference in slow anion channel regulation in the absence of exogenous ABA in abh1 (Fig. 7C) and era1, indicating different functions of these two negative regulators of ABA signaling.
K+in channel currents were substantially smaller in abh1 guard cells than in WT guard cells from plants grown at 40% humidity without addition of exogenous ABA (Fig. 7D; P < 0.001). K+in currents (n = 6) showed recovery of WT magnitudes in a complemented line (P = 0.74). Furthermore, when plants were exposed to high (95%) humidity for 3 d, K+in channel current magnitudes in abh1 guard cells were significantly larger (n = 17) than those in abh1 guard cells from 40% humidity-grown plants (n = 14) (P < 0.001 at −180 mV; Fig. 7E). K+in channel currents in abh1 guard cells grown at 95% showed recovery, but remained slightly smaller than the current magnitudes of WT grown at 40% humidity (Fig. 7, D and E). Anion channel and K+in channel activities recorded in abh1 guard cells isolated from plants grown at 40% humidity (Fig. 7, C and D) correlated with the reduced stomatal apertures and reduced gas exchange found in abh1 leaves (Fig. 7A).
ABA Induction of Stomatal Closure in abh1/abi1-1 and abh1/era1-2 Double Mutants
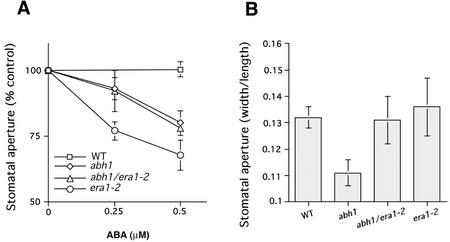
To further investigate genetic interactions of abh1 with previously characterized early ABA signaling mutants, stomatal responses in homozygous abh1/era1-2 and abh1/abi1-1 double mutants were analyzed. In the abh1/era1-2 double mutant, stomatal closure in response to ABA was similar to the response in abh1 alone when plants were treated overnight at high humidity (Fig. 8A). However, stomatal apertures of plants grown at 40% humidity were similar in abh1/era1-2 and era1-2 and similar to WT (Fig. 8B).
Figure 8.
Stomatal aperture phenotypes in the presence and absence of ABA in the abh1/era1-2 double mutant. A, ABA-induced stomatal closure in the abh1/era1-2 double mutant is similar to abh1. Plants were kept overnight in high (95%) humidity and then leaves were pre-incubated for 2 h in opening solution, under light. ABA was then added and stomatal apertures measured after 2 more hours. Stomatal aperture is expressed relative to the mean of stomatal aperture measured with no ABA for each line. Stomatal aperture ratio (width/length) with no ABA were 0.17 ± 0.016, 0.175 ± 0.015, 0.23 ± 0.029, and 0.21 ± 0.016 for WT, abh1, era1-2, and abh1/era1-2, respectively. B, Stomatal apertures in abh1/era1-2 plants grown at 40% humidity show similar opening to era1-2 and WT. Leaves were directly harvested from plants grown in a 40% humidity growth chamber about 6 h after onset of the 16-h day/night period and stomatal apertures were measured without any pre-incubation in stomatal opening solution. Data in A and B represent the mean ± se of three independent experiments with a minimum of 20 stomatal apertures measured per experiment and condition.
The complex interaction of abh1 and era1-2 was also observed for non-guard cell phenotypes. For example, the increased number of petals described in era1-2 (Ziegelhoffer et al., 2000) compared with WT was also observed in the abh1/era1-2 double mutant (data not shown), whereas the abh1 serrated leaf phenotype (Fig. 4A) was unchanged in abh1/era1-2 compared with abh1. However, the delay in growth of abh1/era1-2 was increased compared with both abh1 and era1-2, suggesting additive affects in this response (data not shown). The ability to produce siliques and seeds was strongly reduced in abh1/era1-2 (data not shown). All these data stress the complexity by which abh1 and era1-2 interact in planta, suggesting that they may target distinct processes in the ABA signaling network that have differential relative importance in different tissues and depending on conditions. Furthermore, era1 is known to have pleiotropic phenotypes because it is the only farnesyl transferase β-subunit gene in Arabidopsis; therefore, many era1 phenotypes would be expected not to show an interaction with abh1. ERA1-associated mechanisms that are considered not to be linked to ABA signaling include the Wiggum flower development phenotype and farnesylation of the AP1 transcription factor (Yalovsky et al., 2000; Ziegelhoffer et al., 2000).
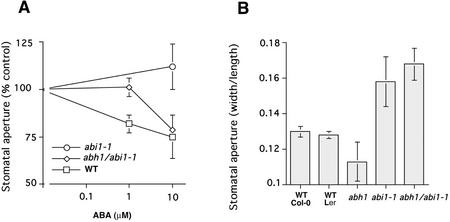
Stomatal apertures in response to ABA were also investigated in the abh1/abi1-1 double mutant. As shown in Figure 9A, 1 μm ABA did not cause significant stomatal closure in the abh1/abi1-1 double mutant, but induced stomatal closing in the WT. At 10 μm ABA, however, stomata closed in the abh1/abi1-1 double mutants, whereas they remained widely opened in abi1-1. These data showed that ABA-induced stomatal closing in the abh1/abi1-1 double mutant is more ABA sensitive than in the abi1-1 background. These data correlate with an intermediate ABA sensitivity of the abh1/abi1-1 double mutant in seed germination that lies between the abh1 and abi1-1 sensitivities (Hugouvieux et al., 2001).
Figure 9.
Stomatal aperture phenotypes in the presence or absence of ABA in the abh1/abi1-1 double mutant. A, ABA-induced stomatal closure in abh1/abi1-1 double mutant shows an intermediate response relative to abi1-1 and WT. Leaves were pre-incubated for 2 h in stomatal opening solution, under light, and then ABA was added and stomatal apertures measured after 2 more h. Stomatal aperture is expressed relative to the mean of stomatal apertures measured with no ABA for each line. Stomatal aperture ratio (width/length) with no ABA were 0.187 ± 0.016, 0.194 ± 0.011, and 0.157 ± 0.015 for WT, abh1/abi1-1, and abi1-1, respectively. B, Stomatal apertures in abh1/abi1-1 plants grown at 40% humidity are as wide as abi1-1. Leaves were directly harvested from plants grown in a 40% humidity growth chamber and stomatal apertures were measured without any pre-incubation in stomatal opening solution. A and B, Data represent the mean ± sd of three independent experiments.
However, because abh1 and abi1-1 mutants are in the Columbia and Ler backgrounds, respectively, we have also isolated a control line that carries the abi1-1 mutation in an ERECTA WT background, from F2 seeds resulting from abh1/abi1-1 crosses. The control line and its progeny showed a strong insensitivity to 10 μm ABA (stomatal aperture after ABA treatment was 93% ± 7% of the maximal stomatal aperture measured with no ABA). Transgenic expression of abi1-1 in tobacco (Nicotiana benthamiano) confers strong insensitivity to ABA (Armstrong et al., 1995), confirming that the dominant abi1-1 mutant protein functions in many backgrounds. The abh1 mutation partially suppresses this dominant abi1-1 phenotype.
Stomatal apertures of abi1-1 plants, measured directly from plants grown at 40% humidity, were generally larger than those of WT plants (Ler background; Fig. 9B). Under the same conditions, stomatal apertures in the abh1/abi1-1 double mutant were similar to abi1-1 (Fig. 9B). These findings are also consistent with the hypothesis that reduced stomatal apertures in abh1 at low humidity are because of a response to low levels of endogenous ABA as the abi1-1 mutation causes ABA insensitivity to low ABA concentrations in the abi1-1/abh1 double mutant (Fig. 9A).
ABA-Hypersensitive Stomatal Closure in sad1
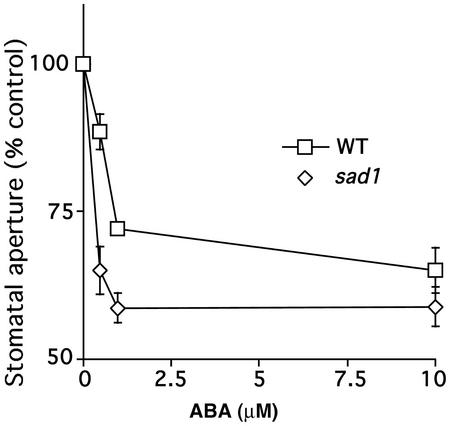
Mutations in three RNA-binding proteins have been described recently that show ABA hypersensitivity (Lu and Fedoroff, 2000; Hugouvieux et al., 2001; Xiong et al., 2001a). In addition to the mRNA cap binding protein ABH1 (Hugouvieux et al., 2001), these genes include a double-stranded RNA-binding protein, HYL1 (Lu and Fedoroff, 2000) and a protein with similarity to an Sm-like snRNP protein, SAD1 (Xiong et al., 2001a). We investigated whether the sad1 and hyl1 mutations affect stomatal movements. ABA-induced stomatal closure in the sad1 mutant showed a reproducible ABA hypersensitivity compared with the WT ecotype C24 (Fig. 10). In contrast, in the hyl1 mutant, ABA-induced stomatal closure was similar to the WT ecotype Nossen in two independent lines of investigation (N. Fedoroff, personal communication; n = 3 independent experiments; data not shown).
Figure 10.
ABA-hypersensitive stomatal closing in the sad1 mutant. Plants were kept overnight in high (95%) humidity and then leaves were pre-incubated for 2 h in opening solution, under light. ABA was then added and stomatal apertures measured after 2 more h in sad1 and WT C24 ecotype. Stomatal apertures are expressed relative to the mean stomatal apertures measured without ABA addition for each line. Stomatal aperture ratios (width/length) without ABA addition were 0.158 ± 0.01 and 0.145 ± 0.006 for WT and sad1, respectively. Data represent the mean ± se of three independent experiments. Error bars are smaller than symbols when not visible.
DISCUSSION
In a previous report, we described the isolation and characterization of a new recessive ABA-hypersensitive Arabidopsis mutant, abh1. abh1 shows ABA hypersensitivity in seed germination, stomatal closure, and ABA-induced guard cell calcium increases (Hugouvieux et al., 2001). ABH1 was shown to function in vitro as a subunit of a dimeric mRNA CBC and ABH1 gene expression is necessary for the correct expression level of a subset of genes in the Arabidopsis genome (Hugouvieux et al., 2001). The isolation of abh1 points to a link between mRNA metabolism and correct ABA signaling.
To better understand ABH1 function at the whole-plant level and in guard cells, we further investigated ABH1 gene expression and regulation, ABH1 protein localization, and we further characterized abh1 mutant phenotypes, sad1 and hyl1 phenotypes in guard cells, and genetic interactions between ABH1 and two well-described early ABA signaling components, ERA1 and abi1-1.
ABH1 Expression Pattern and Nuclear Localization
The ABH1 gene is expressed in all tissues tested (Figs. 1 and 2). However, our data further show that the ABH1 expression level varies between tissues and also appears to depend on the developmental stage. Further research will be needed to determine whether different expression levels affect ABH1 functions in these tissues or at these developmental stages. It is intriguing to note that the abh1 mutation has no visible impact on flower morphology even though ABH1 mRNA levels are high (Fig. 1). Thus, it is possible that some ABH1 functions may be conditional and/or that this expression pattern is related to the abh1 phenotype in seeds.
ABH1 expression does not appear to be affected by external ABA application (Fig. 3), nor by drought stress. Similar results were observed in the case of the SAD1 gene (Xiong et al., 2001a). In humans, CBC activity is increased in response to growth factors (Wilson et al., 1999). It has been proposed that this increased CBC activity may be because of posttranslational modification of the ABH1 homolog, CBP80; for example, by phosphorylation (Wilson and Cerione, 2000), although direct evidence has not been yet reported. The CBC is also known to interact with several proteins from the spliceosome complex and translation machinery (Fortes et al., 2000; Ishigaki et al., 2001; McKendrick et al., 2001). The x-ray crystal structure of a human CBC was recently determined and suggested that CBP80 could behave as a platform protein for nuclear cap-related RNA-processing proteins (Mazza et al., 2001). Thus, it is tempting to speculate that in response to ABA, ABH1 activity may be regulated by posttranslational modifications and/or interaction with new regulators. Note that sad1 does not interact in yeast two-hybrid experiments with either ABH1 or AtCBP20 (Xiong et al., 2001a). However, a larger complex that includes these proteins cannot be excluded.
Using protein database motif searches, no clear nuclear localization signal can be detected in the ABH1 protein sequence, in contrast to the human and yeast homologs (data not shown). The abh1 mutant was complemented by an N-terminal GFP-ABH1 fusion protein, which was predominantly localized to the nucleus (Fig. 6). In contrast, a C-terminal ABH1-GFP fusion did not complement abh1. The ABH1 protein has a C-terminal extension compared with yeast and animal CBP80 that could be related to specific unknown properties of ABH1 (Hugouvieux et al., 2001).
The nuclear localization correlates with the function of ABH1 as a subunit of a nuclear CBC as described in yeast and mammals (Lewis and Izaurralde, 1997). We also observed a slight GFP fluorescence in the cytosol (Fig. 6). Although the steady-state level of CBC is mainly nuclear, it is well established that CBC moves into the cytosol during mRNA export in yeast, insects, and vertebrates and is later recycled back into the nucleus (Goerlich et al., 1996; Visa et al., 1996; Shent et al., 2000).
Whole-Plant and Stomatal Responses of abh1
Although ABH1 is expressed in several plant tissues, abh1 plants showed only a slightly slowed growth, a serrated leaf phenotype, and a slightly smaller stem length at maturity compared with WT (Figs. 4 and 5; Table I). No other clearly visible phenotypes were detected at the morphological level. Similarly, the yeast mutant disrupted in CBP80 shows a growth delay compared with WT, depending on the carbon source used (Uemura and Jigami, 1992).
In the present study, we characterized further abh1 phenotypes in guard cells. abh1 stomatal pore apertures were significantly reduced compared with WT when plants were grown in a growth chamber at 40% humidity (Fig. 7A). Reduced stomatal apertures correlated with both a constitutive activation of slow anion channels and a reduction in K+ in channel activity in abh1 guard cells (Fig. 7, B, C, and E). The stomatal phenotype of abh1 plants grown at 40% humidity correlates with ion channel activities, even though ion channel activities were measured after protoplastation (see “Materials and Methods”). These data suggest that the modulation of ion channel activities in abh1 revealed here may be because of longer term regulatory mechanisms such as posttranslational modification and/or potential alteration of expression levels of ABA transducers (Hugouvieux et al., 2001).
Slow anion channels are activated by cytosolic Ca2+ elevations and ABA (Schroeder and Hagiwara, 1989; Grabov et al., 1997; Pei et al., 1997; Allen et al., 1999) and K+ in channels are down-regulated by cytosolic Ca2+ elevations and by ABA (Schroeder and Hagiwara, 1989; Blatt and Armstrong, 1993). ABA content in abh1 and WT plants are similar in sufficiently watered plants, and ABA content increased to the same extent in both WT and abh1 after desiccation (Hugouvieux et al., 2001). The primary responses of abh1 and WT stomates to rapid humidity changes were similar (Fig. 7B). Therefore, we propose that reduced stomatal apertures observed in abh1 at 40% humidity are because of a response to endogenous ABA. This model is supported by the finding that abh1/abi1-1 double mutants do not show reduced stomatal apertures at 40% humidity (Fig. 9B) and that abi1-1 does not function in humidity signaling (Assmann et al., 2000).
The abh1/era1-2 double mutant shows an additive ABA-hypersensitive phenotype in seed germination assays (Hugouvieux et al., 2001). Interestingly, ABA-induced stomatal closure in the abh1/era1-2 double mutant does not show additive effects of the two mutants (Fig. 8). Under the imposed conditions, the abh1/era1-2 double mutant showed an ABA hypersensitivity similar to abh1. However, when stomatal apertures were measured in plants grown at 40% humidity, stomatal apertures of the double mutant were similar to those in era1-2 stomatal apertures (Fig. 8B). These data suggest complex interactions of the abh1 and era1-2 mutants, indicating that they affect different components or branches in ABA signaling that change their relative contribution to ABA signal transduction depending on conditions. In the abh1/abi1-1 double mutant, however, ABA sensitivity was decreased compared with the abi1-1 mutant (Fig. 9B), which correlated with seed germination assays in response to ABA (Hugouvieux et al., 2001), suggesting a less complex interaction of abi1-1 and ABH1, which may be explained by the dominant nature of the abi1-1 mutant.
Distinct ABA Responses in Guard Cells of sad1 and abh1 Compared with hyl1
The Sm-like snRNP protein, SAD1, was suggested to function in mRNA processing in ABA signaling and homeostasis (Xiong et al., 2001a). The independent isolation of abh1 and sad1 by two different screens strengthens the recent hypothesis that mRNA-processing proteins function as negative regulators in ABA signaling. Furthermore, the recessive double-stranded RNA-binding protein mutant hyl1 also shows ABA hypersensitivity (Lu and Fedoroff, 2000). In the present study, we analyzed stomatal responses of sad1 and hyl1. Although sad1 showed ABA hypersensitivity in stomatal movement responses (Fig. 10), hyl1 did not (data not shown; N. Fedoroff, personal communication). These data suggest that hyl1 may modulate ABA signaling via a mechanism independent of abh1 and sad1. These results are consistent with the finding that HYL1 binds double-stranded RNA, which appears to be mechanistically different from the proposed SAD1 function and ABH1 cap-binding activity. Furthermore, the hyl1 mutation also modulates the sensitivity to auxin and cytokinin (Lu and Fedoroff, 2000), showing a clear difference from abh1 and sad1 (Hugouvieux et al., 2001; Xiong et al., 2001a).
Although both sad1 and abh1 show hypersensitivity to exogenous ABA (Fig. 10; Hugouvieux et al., 2001), there are distinct differences in the two mutants. The sad1 mutation results in reduced ABA levels in drought-stressed plants because of a feedback mechanism from ABA signaling to ABA biosynthesis (Xiong et al., 2001a). Consistent with these findings, detached sad1 rosettes showed an enhanced transpiration rate compared with WT (Xiong et al., 2001a). In contrast, the abh1 mutation does not affect endogenous ABA levels and abh1 causes reduced transpiration (Hugouvieux et al., 2001) and reduced stomatal apertures. Despite these differences, it is conceivable that ABH1 and SAD1 participate in a complex RNA-processing network that modulates ABA signaling, in such a manner that the two mutants have clearly distinguishable effects but some similarities in their phenotypes, which include ABA hypersensitivity in seed germination (Hugouvieux et al., 2001; Xiong et al., 2001) and in stomatal closing (Fig. 10).
CONCLUSIONS
In conclusion, we show that ABH1 is widely expressed in plants and that ABH1 shows preferentially nuclear localization. The negative regulators of ABA signaling, ABH1 and the farnesyl transferase β-subunit ERA1, show complex genetic interactions and differential effects indicating that these two genes act at different locations in the ABA signal network. Findings that both abh1 and sad1 show similar sensitivity to exogenous ABA, whereas the hyl1 mutant did not affect stomatal apertures in response to ABA, further strengthen the hypothesis that RNA processing modulates early ABA signaling.
MATERIALS AND METHODS
Plant Growth and Culture Conditions
abh1, other Arabidopsis mutants, and the corresponding WT ecotypes were grown side by side in growth chambers: 40% humidity, 16-h-light/8-h-dark cycle, temperature 20°C, and photon fluency rate of 75 μmol m−2 s−1. Unless otherwise stated, WT plants are from the Columbia ecotype. When required, the growth of abh1 and WT plants was synchronized by sowing abh1 plants 1 week earlier than WT. To test the sensitivity of seeds to ABA, in the case of abh1 lines complemented with the N-terminal GFP-ABH1 fusion, seeds were plated on minimal medium (0.25× Murashige and Skoog medium) containing 0.3 μm ABA. After 4 d at 4°C, seeds were transferred to 28°C and continuous light and germination was scored after 5 more d. Seeds used for comparative studies were from plants grown and harvested in parallel. For seedling growth assays, 6-month-old abh1 and WT seeds were germinated on Murashige and Skoog plates. Germination was scored after 2 d to make sure that all the seedlings were at the two-cotyledon stages and the root length was estimated using a micrometer under a dissecting microscope. Seeds that showed a delay in germination were removed from the plates in both WT and abh1. After 2, 6, 9, and 11 d of growth, root length was measured and the development of the shoot was studied by analyzing the appearance and development of new leaves under the microscope.
GFP-ABH1 Fusion Constructs and Plant Transformation
To generate N- and C-terminal fusion proteins between ABH1 and GFP proteins, the full-length ABH1 cDNA was amplified using Pfu DNA polymerase (Stratagene, La Jolla, CA). Primers used for PCR included specific restriction sites at both ends, to allow the cloning of ABH1 and GFP in frame in the vector GFP-JFH1 (kindly provided by Dr. Jeff Harper, The Scripps Research Institute, La Jolla, CA). PCR-amplified constructs were confirmed by sequencing (Retrogen, San Diego). Agrobacterium tumefaciens strain C58 was used to generate transgenic Arabidopsis plants using the floral dip technique (Clough and Bent, 1998).
Confocal Microscopy
The subcellular localization of ABH1 fused to GFP was assessed by scanning confocal laser microscopy in epidermal strips prepared as described (Allen et al., 1999). Epidermal strips were mounted between two cover slips on an Axiovert 35M microscope (Zeiss, Jena, Germany) coupled to an MCr-1000 scanning laser confocal system (Bio-Rad Laboratories, Hercules, CA). Argon laser light (488-nm wavelength, 30% power) was used to excite GFP and emission light was measured at 522 nm. Transmission images were collected in parallel. Autofluorescence was monitored at 488 nm.
GUS Staining
GUS activity was assayed on either seedlings grown on Murashige and Skoog plates or soil, after 24 h of incubation in a solution containing 2 mm 5-bromo-4-chloro-3-indolyl-β-d-glucuronide, 0.1 m Na2HPO4 (pH 7.2), 0.1% (w/v) K+ ferrocyanide, 0.1% (w/v) ferricyanide, and 0.1% (v/v) Triton. Experiments were performed on three independent WT:ABH1-GUS lines. WT control plants showed no GUS activity (data not shown).
RNA-Blot Analyses
Total RNA was extracted from flowers, leaves, stems, and roots of 5- to 6-week-old WT plants using Trizol reagent (Life Technologies/Gibco-BRL, Rockville, MD). Poly(A+) RNA was further purified using the μMACS mRNA Isolation Kit (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. To examine whether ABA regulates ABH1 gene expression, total RNA and poly(A+) RNA were extracted from rosette leaves of WT plants sprayed with 100 μm ABA, 4 h before extraction. Several complementary experiments were carried out using 1, 10, and 100 μm ABA for different application times. Total and poly(A+) RNA were separated in a 1.2% (w/v) denaturing agarose gel, and then transferred onto a Hybond N+ membrane (Amersham, Buckinghamshire, UK). The blots were hybridized with 32P-labeled ABH1 cDNA and/or a Cor47 genomic fragment amplified by PCR using the forward and reverse primers, 5′GAT CGA AAT GGT TGA TAA GAG ATC3′ and 5′CAC ACT CTC CGA CAC TGG TAC C3′, respectively.
Stomatal Movement Analyses
Stomatal aperture measurements were performed as described (Pei et al., 1997; Allen et al., 2000). Stomata from 5- to 6-week-old plants were opened by exposing excised leaves for 2 h to white light (intensity: 125 μmol m−2 s−1), floating in a stomatal opening solution containing 5 mm KCl, 10 μm CaCl2, and 10 mm MES (pH 6.15), in the growth chamber at 20°C. Stomatal apertures were measured 2 h after ABA was added (Pei et al., 1997). When experiments were performed with abh1, era1-2, sad1, and abh1/era1-2 double mutants, plants were subjected to an overnight high (95%) humidity treatment before incubation of the leaves in stomatal opening solution. Control experiments were performed in parallel with no ABA added. Leaves were then blended for 30 s and epidermal peels collected as described (Allen et al., 1999). Stomatal apertures were measured (pore width/length) by focusing on the focal plane of guard cells in epidermal strips (Ichida et al., 1997).
Stomatal Conductance
Stomatal responses to humidity were determined by measuring transpiration rates using a Li-6400 infrared gas analyzer (LI-COR, Inc.). Chamber temperature and carbon dioxide concentration were maintained at 23°C and 400 μL L−1, respectively. Constant illumination of 500 μmol quanta m−2s−1 photosynthetically active radiation was provided by a red and blue LED light source.
Electrophysiology
Anion and K+ currents were recorded from guard cell protoplasts of 4- to 6-week-old plants (Ichida et al., 1997; Pei et al., 1997) grown either at 40% humidity or after 72 h of high-humidity treatment (95%). The solutions used in patch clamp experiments were composed of 150 mm CsCl, 2 mm MgCl2, 6.7 mm EGTA, 3.35 mm CaCl2, and 10 mm HEPES-Tris, pH 7.1, in the pipette medium and of 30 mm CsCl, 2 mm MgCl2, 1 mm CaCl2, and 10 mm MES-Tris, pH 5.6, in the bath medium, for anion channel activity measurement (Pei et al., 1997). For K+ current measurements, the pipette solution was composed of 30 mm KCl, 70 mm-Glu, 2 mm MgCl2, 6.7 mm EGTA, 3.35 mm CaCl2, 5 mm ATP, and 10 mm HEPES-Tris, pH 7.1. The bath solution contained 30 mm KCl, 40 mm CaCl2, 2 mm MgCl2, and 10 mm MES-Tris, pH 5.5 (Pei et al., 1997). For all solutions, osmolarity was adjusted to 485 mmol kg−1 for bath solutions and 500 mmol kg−1 for pipette solutions by addition of d-sorbitol.
ACKNOWLEDGMENTS
We thank Gethyn Allen for advice on confocal microscopy and members of the laboratory for discussions, and David Waner, Christine Salomon, and Jorieth Jose for assistance. We thank Drs. Nina Fedoroff and Jian-Kang Zhu for providing hyl1 and sad1 mutants and for discussion.
Footnotes
This research was supported by the National Institutes of Health (grant no. R01GM60396–01), by the Torrey Mesa Research Institute/University of California Biostar (grant), in part by the National Science Foundation (grant no. MCB 0077791 to J.I.S.), and by the Human Frontier Science Program Organization (fellowship to J.M.K.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.009480.
LITERATURE CITED
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF et al. Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science. 2000;289:2338–2342. doi: 10.1126/science.289.5488.2338. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Kuchitsu K, Chu SP, Murata Y, Schroeder JI. Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell. 1999;11:1785–1798. doi: 10.1105/tpc.11.9.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong F, Leung J, Grabov A, Brearley J, Giraudat J, Blatt MR. Sensitivity to abscisic acid of guard-cell K+ channels is suppressed by abi1-1, a mutant Arabidopsis gene encoding a putative protein phosphatase. Proc Natl Acad Sci USA. 1995;92:9520–9524. doi: 10.1073/pnas.92.21.9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM, Snyder JA, Lee Y-RJ. ABA-deficient (aba1) and ABA-insensitive (abi1-1, abi2-1) mutants of Arabidopsis have a wild-type stomatal response to humidity. Plant Cell Environ. 2000;23:387–395. [Google Scholar]
- Blatt MR, Armstrong F. Potassium channels of stomatal guard cells: abscisic acid-evoked control of the outward rectifier mediated by cytoplasmic pH. Planta. 1993;191:330–341. [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P. A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science. 1996;273:1239–1241. doi: 10.1126/science.273.5279.1239. [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD. Abscisic acid signaling in seeds and seedlings. Plant Cell Suppl. 2002;14:S15–45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Lynch TJ. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell. 2000;12:599–609. doi: 10.1105/tpc.12.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM. The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell. 1998;10:1043–1054. doi: 10.1105/tpc.10.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortes P, Inada T, Preiss T, Hentze MW, Mattaj IW, Sachs AB. The yeast nuclear cap binding complex can interact with translation factor elF4G and mediate translation initiation. Mol Cell. 2000;6:191–196. [PubMed] [Google Scholar]
- Giraudat J, Hauge BM, Valon C, Smalle J, Parcy F, Goodman HM. Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell. 1992;4:1251–1261. doi: 10.1105/tpc.4.10.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D, Kraft R, Kostka S, Vogel F, Hartmann E, Laskey RA, Mattaj IW, Izaurralde E. Importin provides a link between nuclear protein import and UsnRNA export. Cell. 1996;87:21–32. doi: 10.1016/s0092-8674(00)81319-7. [DOI] [PubMed] [Google Scholar]
- Grabov A, Leung J, Giraudat J, Blatt MR. Alteration of anion channel kinetics in wild-type and abi1-1 transgenic Nicotiana benthamiana guard cells by abscisic acid. Plant J. 1997;12:203–213. doi: 10.1046/j.1365-313x.1997.12010203.x. [DOI] [PubMed] [Google Scholar]
- Hugouvieux V, Kwak JM, Schroeder JI. An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell. 2001;106:477–487. doi: 10.1016/s0092-8674(01)00460-3. [DOI] [PubMed] [Google Scholar]
- Ichida AM, Pei Z-M, Baizabal-Aguirre VM, Turner KJ, Schroeder JI. Expression of a Cs+-resistant guard cell K+ channel confers CS+-resistant, light-induced stomatal opening in transgenic Arabidopsis. Plant Cell. 1997;9:1843–1857. doi: 10.1105/tpc.9.10.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigaki Y, Li X, Serin G, Maquat LE. Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell. 2001;106:607–617. doi: 10.1016/s0092-8674(01)00475-5. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Leon-Kloosterziel KM, Schwartz SH, Zeevaart JAD. The genetic and molecular dissection of abscisic acid biosynthesis and signal transduction in Arabidopsis. Plant Physiol Biochem. 1998;36:83–89. [Google Scholar]
- Lemichez E, Wu Y, Sanchez JP, Mettouchi A, Mathur J, Chua NH. Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev. 2001;15:1808–1816. doi: 10.1101/gad.900401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Bouvier-Durand M, Morris PC, Guerrier D, Chefdor F, Giraudat J. Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science. 1994;264:1448–1452. doi: 10.1126/science.7910981. [DOI] [PubMed] [Google Scholar]
- Leung J, Giraudat J. Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:199–222. doi: 10.1146/annurev.arplant.49.1.199. [DOI] [PubMed] [Google Scholar]
- Leung J, Merlot S, Giraudat J. The Arabidopsis abscisic acid-insensitive2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell. 1997;9:759–771. doi: 10.1105/tpc.9.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Izaurralde E. The role of the cap structure in RNA processing and nuclear export. Euro J Biochem. 1997;247:461–469. doi: 10.1111/j.1432-1033.1997.00461.x. [DOI] [PubMed] [Google Scholar]
- Li J, Wang X-Q, Watson MB, Assmann SM. Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science. 2000;287:300–303. doi: 10.1126/science.287.5451.300. [DOI] [PubMed] [Google Scholar]
- Lu C, Fedoroff N. A mutation in the Arabidopsis HYL1 gene encoding a dsRNA binding protein affects responses to abscisic acid, auxin, and cytokinin. Plant Cell. 2000;12:2351–2366. doi: 10.1105/tpc.12.12.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie EAC. Signal transduction and ion channels in guard cells. Philos Trans R Soc London. 1998;353:1475–1488. doi: 10.1098/rstb.1998.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte WR, Guiltinan MJ, Quatrano RS. ABA-regulated gene expression: cis-acting sequences and trans-acting factors. Biochem Soc Trans. 1992;20:93–97. doi: 10.1042/bst0200093. [DOI] [PubMed] [Google Scholar]
- Mazza C, Ohno M, Segref A, Mattaj IW, Cusack S. Crystal structure of the human nuclear cap binding complex. Mol Cell. 2001;8:383–396. doi: 10.1016/s1097-2765(01)00299-4. [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM. Abscisic acid-induced elevation of guard cell cytosolic calcium precedes stomatal closure. Nature. 1990;343:186–188. [Google Scholar]
- McKendrick L, Thompson E, Ferreira J, Morley SJ, Lewis JD. Interaction of eukaryotic translation initiation factor 4G with the nuclear cap-binding complex provides a link between nuclear and cytoplasmic functions of the m7 guanosine cap. Mol Cell Biol. 2001;21:3632–3641. doi: 10.1128/MCB.21.11.3632-3641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Leube MP, Grill E. A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science. 1994;264:1452–1455. doi: 10.1126/science.8197457. [DOI] [PubMed] [Google Scholar]
- Pei Z-M, Ghassemian M, Kwak CM, McCourt P, Schroeder JI. Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science. 1998;282:287–290. doi: 10.1126/science.282.5387.287. [DOI] [PubMed] [Google Scholar]
- Pei Z-M, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell. 1997;9:409–423. doi: 10.1105/tpc.9.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:627–658. doi: 10.1146/annurev.arplant.52.1.627. [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S. Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature. 1989;338:427–430. [Google Scholar]
- Shent EC, Stage-Zimmermann T, Chui P, Silver PA. The yeast mRNA-binding protein Npl3p interacts with the cap-binding complex. J Biol Chem. 2000;275:23718–23724. doi: 10.1074/jbc.M002312200. [DOI] [PubMed] [Google Scholar]
- Uemura H, Jigami Y. GCR3 encodes an acidic protein that is required for expression of glycolytic genes in Saccharomyces cerevisiae. J Bacteriol. 1992;174:5526–5532. doi: 10.1128/jb.174.17.5526-5532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visa N, Izaurralde E, Ferreira J, Daneholt B, Mattaj IW. A nuclear cap-binding complex binds Balbiani ring pre-mRNA cotranscriptionally and accompanies the ribonucleoprotein particle during nuclear export. J Cell Biol. 1996;133:5–14. doi: 10.1083/jcb.133.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XQ, Ullah H, Jones AM, Assmann SM. G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science. 2001;292:2070–2072. doi: 10.1126/science.1059046. [DOI] [PubMed] [Google Scholar]
- Wilson KF, Cerione RA. Signal transduction and post-transcriptional gene expression. Biol Chem. 2000;381:357–365. doi: 10.1515/BC.2000.048. [DOI] [PubMed] [Google Scholar]
- Wilson KF, Fortes P, Singh US, Ohno M, Mattaj IW, Cerione RA. The nuclear cap-binding complex is a novel target of growth factor receptor-coupled signal transduction. J Biol Chem. 1999;274:4166–4173. doi: 10.1074/jbc.274.7.4166. [DOI] [PubMed] [Google Scholar]
- Xiong L, Gong Z, Rock CD, Subramanian S, Guo Y, Xu W, Galbraith D, Zhu JK. Modulation of abscisic acid signal transduction and biosynthesis by an Sm-like protein in Arabidopsis. Dev Cell. 2001a;1:771–781. doi: 10.1016/s1534-5807(01)00087-9. [DOI] [PubMed] [Google Scholar]
- Xiong L, Lee B-h, Ishitani M, Lee H, Zhang C, Zhu J-K. FIERY1 encoding an inositol polyphosphate 1-phosphatase is a negative regulator of abscisic acid and stress signaling in Arabidopsis. Genes Dev. 2001b;15:1971–1984. doi: 10.1101/gad.891901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky S, Rodriguez-Concepcion M, Bracha K, Toledo-Ortiz G, Gruissem W. Prenylation of the floral transcription factor APETALA1 modulates its function. Plant Cell. 2000;12:1257–1266. doi: 10.1105/tpc.12.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegelhoffer EC, Medrano LJ, Meyerowitz EM. Cloning of the Arabidopsis WIGGUM gene identifies a role for farnesylation in meristem development. Proc Natl Acad Sci USA. 2000;97:7633–7638. doi: 10.1073/pnas.130189397. [DOI] [PMC free article] [PubMed] [Google Scholar]