Abstract
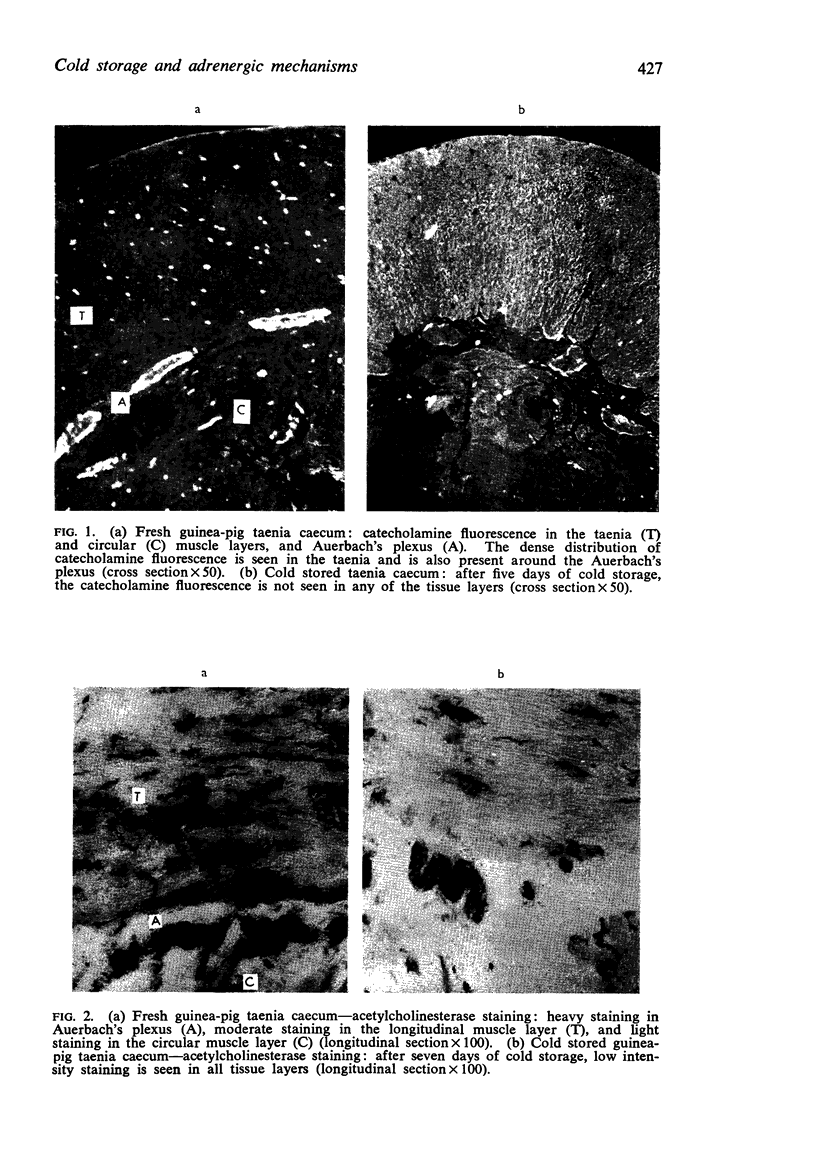
1. In the guinea-pig taenia caecum, fluorescent adrenergic fibres terminate in both muscle layers. The density of these fibres is greater in the taenia than in the underlying circular muscle layer. The myenteric plexus and individual ganglion cells are also densely innervated by intensely fluorescent adrenergic nerve fibres.
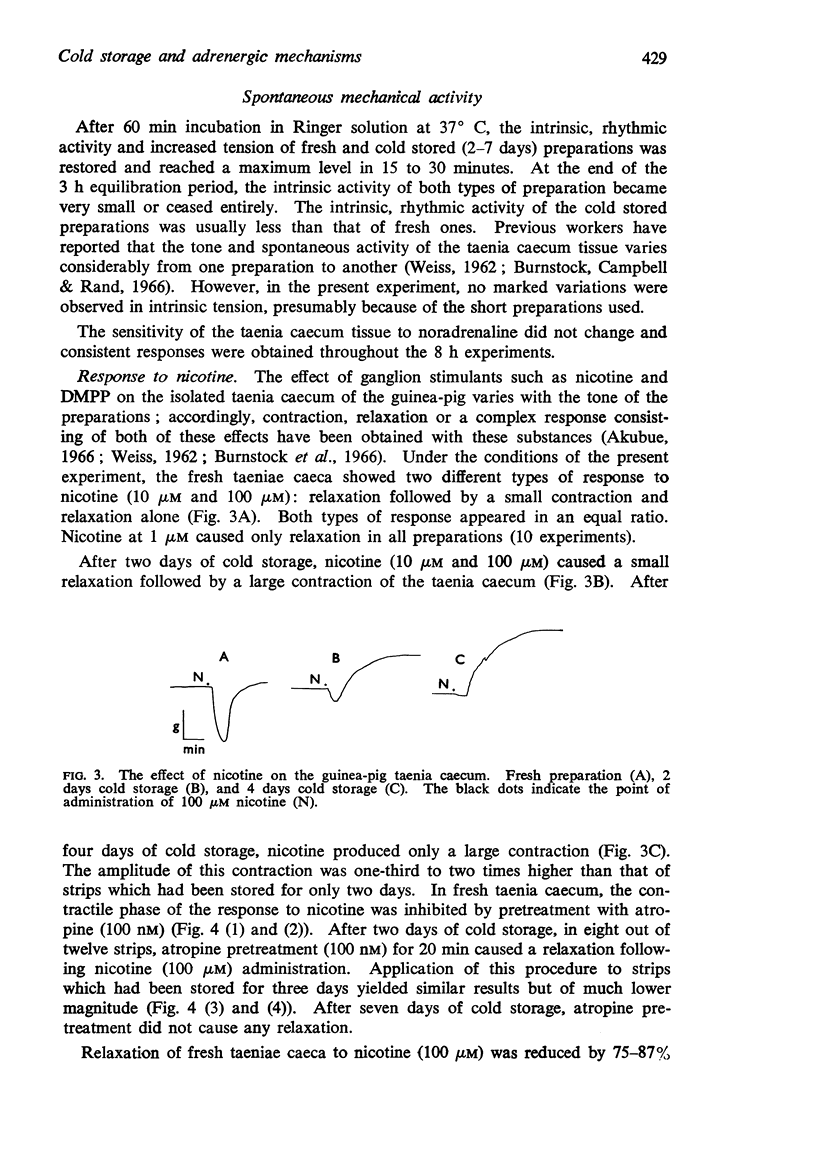
2. After three days of cold storage, the specific fluorescence disappeared from all tissue layers of the taenia caecum and smooth muscle fibres. In contrast, cholinesterase active substances were still demonstrable in all tissue layers even after seven days of cold storage but the density of these substances was decreased.
3. Cold storage (3-7 days) decreased the tissue noradrenaline content and did not modify the cholinesterase enzyme activity (4 days).
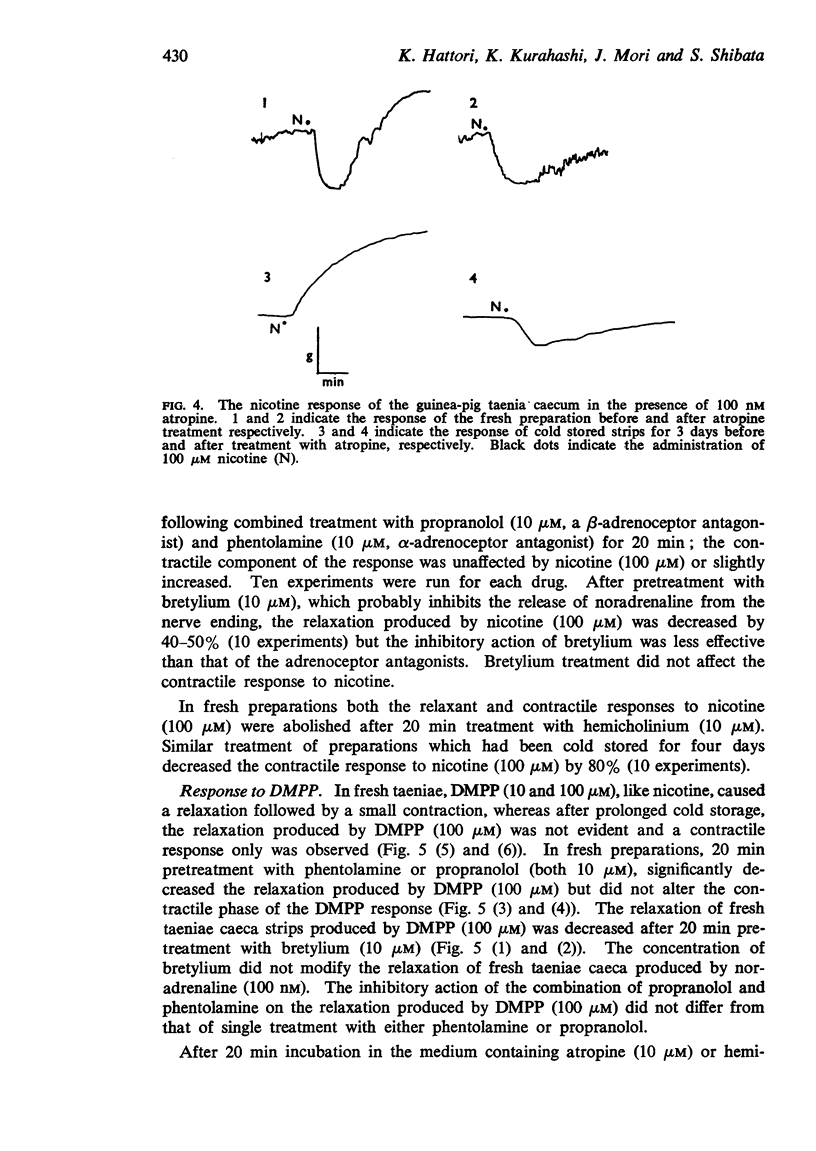
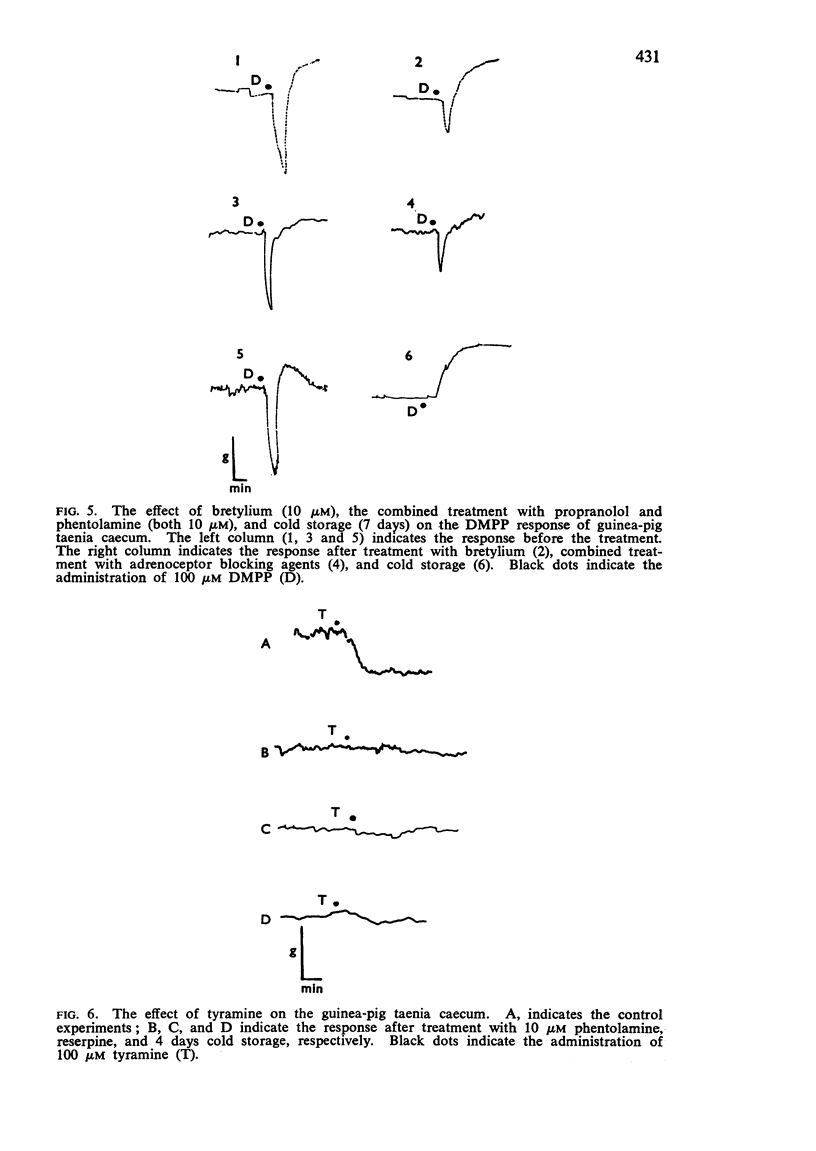
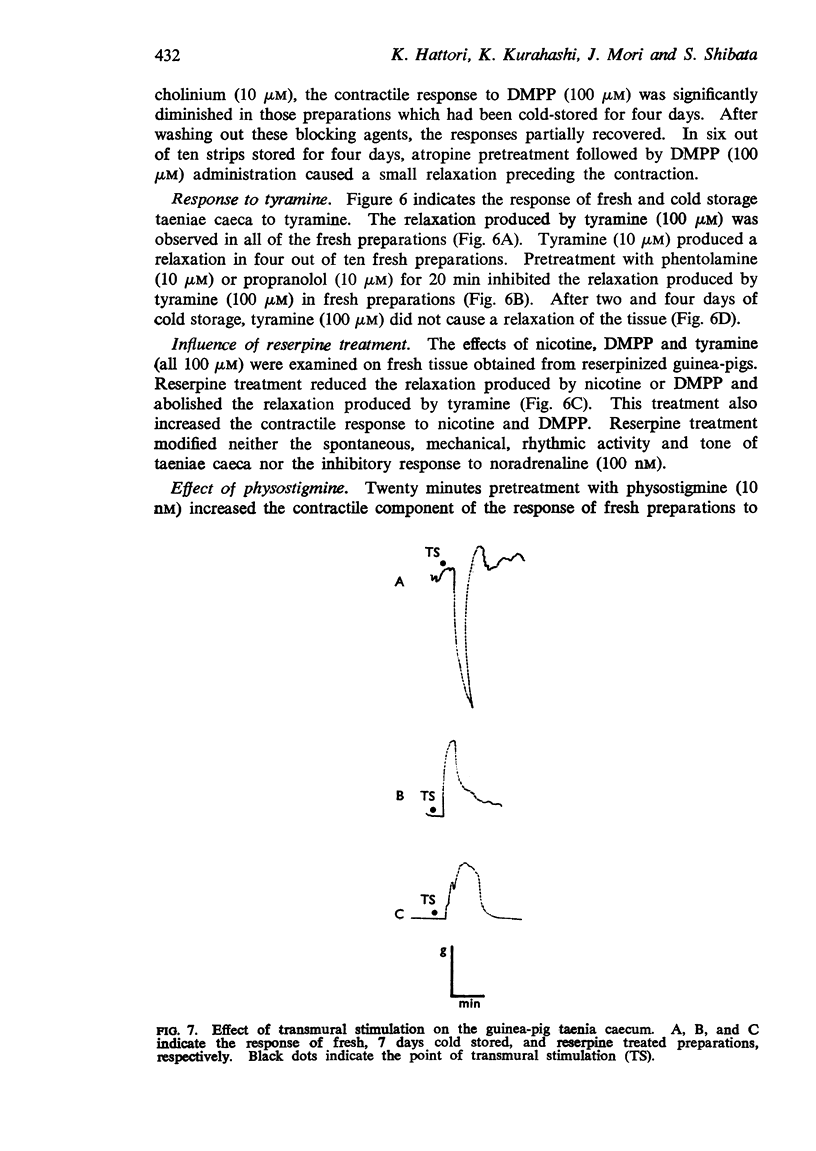
4. In cold stored strips, the inhibitory response to nicotine, 1,1-dimethyl-4-phenylpiperazinium iodide (DMPP) or electrical transmural stimulation was abolished and enhancement of the contractile response occurred. Cold storage also inhibited the inhibitory action of tyramine. Similar results were observed after reserpine treatment.
5. In fresh taenia, the relaxation produced by nicotine, DMPP and electrical transmural stimulation was inhibited by adrenoceptor blocking agents and bretylium. In cold storage preparations, contraction produced by these stimuli was blocked by parasympathetic blocking agents and potentiated by anti-cholinesterase. These results indicate that the inhibitory response to these stimulants is mediated by stimulation of the adrenergic nerve system more than by non-adrenergic nerves; the excitatory effect is probably due to stimulation of cholinergic nerves.
6. These results suggest that the adrenergic mechanisms of the taenia caecum are more labile in cold storage than the cholinergic mechanisms. Thus, the inhibitory action of cold storage on the relaxation produced by nicotine, DMPP, and transmural stimulation is probably explained by selective physical degeneration of the adrenergic nerve terminal. Also, enhancement of the contractile response to these stimulants in cold stored preparations is explained by the lack of adrenergic inhibitory mechanisms.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aberg G., Eränkö O. Localization of noradrenaline and acetylcholinesterase in the taenia of the guinea-pig caecum. Acta Physiol Scand. 1967 Apr;69(4):383–384. doi: 10.1111/j.1748-1716.1967.tb03535.x. [DOI] [PubMed] [Google Scholar]
- Akubue P. I. The site of action of drugs on the isolated taenia caeci from the guinea-pig. Br J Pharmacol Chemother. 1966 Aug;27(2):347–365. doi: 10.1111/j.1476-5381.1966.tb01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTLER A., CARLSSON A., ROSENGREN E. A method for the fluorimetric determination of adrenaline and noradrenaline in tissues. Acta Physiol Scand. 1958 Dec 15;44(3-4):273–292. doi: 10.1111/j.1748-1716.1958.tb01627.x. [DOI] [PubMed] [Google Scholar]
- BERTLER A., CARLSSON A., ROSENGREN E. A method for the fluorimetric determination of adrenaline and noradrenaline in tissues. Acta Physiol Scand. 1958 Dec 15;44(3-4):273–292. doi: 10.1111/j.1748-1716.1958.tb01627.x. [DOI] [PubMed] [Google Scholar]
- BURN J. H., GIBBONS W. R. THE SYMPATHETIC POSTGANGLIONIC FIBRE AND THE BLOCK BY BRETYLIUM; THE BLOCK PREVENTED BY HEXAMETHONIUM AND IMITATED BY MECAMYLAMINE. Br J Pharmacol Chemother. 1964 Jun;22:549–557. doi: 10.1111/j.1476-5381.1964.tb01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., CAMPBELL G., BENNETT M., HOLMAN M. E. INNERVATION OF THE GUINEA-PIG TAENIA COLI: ARE THERE INTRINSIC INHIBITORY NERVES WHICH ARE DISTINCT FROM SYMPATHETIC NERVES? Int J Neuropharmacol. 1964 May;3:163–166. doi: 10.1016/0028-3908(64)90003-6. [DOI] [PubMed] [Google Scholar]
- Bianchi C., Beani L., Frigo G. M., Crema A. Further evidence for the presence of non-adrenergic inhibitory structures in the guinea-pig colon. Eur J Pharmacol. 1968 Aug;4(1):51–61. doi: 10.1016/0014-2999(68)90009-5. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Rand M. J. The inhibitory innervation of the taenia of the guinea-pig caecum. J Physiol. 1966 Feb;182(3):504–526. doi: 10.1113/jphysiol.1966.sp007834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Satchell D., Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970 Dec;40(4):668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORRODI H., HILLARP N. A., JONSSON G. FLUORESCENCE METHODS FOR THE HISTOCHEMICAL DEMONSTRATION OF MONOAMINES. 3. SODIUM BOROHYDRIDE REDUCTION OF THE FLUORESCENT COMPOUNDS AS A SPECIFICITY TEST. J Histochem Cytochem. 1964 Aug;12:582–586. doi: 10.1177/12.8.582. [DOI] [PubMed] [Google Scholar]
- Day M. D., Warren P. R. A pharmacological analysis of the responses to transmural stimulation in isolated intestinal preparations. Br J Pharmacol Chemother. 1968 Feb;32(2):227–240. doi: 10.1111/j.1476-5381.1968.tb00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE J. S., MACKENNA B. R. The inhibitory action of nicotine on the rabbit colon. J Physiol. 1960 Jul;152:191–205. doi: 10.1113/jphysiol.1960.sp006481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G., Costa M. Adrenergic innervation of the intestinal smooth musculature. Experientia. 1969 Apr 15;25(4):395–396. doi: 10.1007/BF01899944. [DOI] [PubMed] [Google Scholar]
- HOLMAN M. E., HUGHES J. R. INHIBITION OF INTESTINAL SMOOTH MUSCLE. Aust J Exp Biol Med Sci. 1965 Jun;43:277–290. doi: 10.1038/icb.1965.27. [DOI] [PubMed] [Google Scholar]
- INNES I. R., KOSTERLITZ H. W., ROBINSON J. A. The effects of lowering the bath temperature on the responses of the isolated guinea-pig ileum. J Physiol. 1957 Aug 6;137(3):396–409. doi: 10.1113/jphysiol.1957.sp005821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARRETT R. J. Action of nicotine on the rabbit muscular organ (ileo-colic sphincter). Br J Pharmacol Chemother. 1962 Apr;18:397–404. doi: 10.1111/j.1476-5381.1962.tb01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobowitz D. Histochemical studies of the autonomic innervation of the gut. J Pharmacol Exp Ther. 1965 Sep;149(3):358–364. [PubMed] [Google Scholar]
- Norberg K. A. Transmitter histochemistry of the sympathetic adrenergic nervous system. Brain Res. 1967 Jun;5(2):125–170. doi: 10.1016/0006-8993(67)90084-4. [DOI] [PubMed] [Google Scholar]
- Shibata S. Effect of prolonged cold storage on the contractile response of strips of rabbit aorta to various agents. Circ Res. 1969 Feb;24(2):179–187. doi: 10.1161/01.res.24.2.179. [DOI] [PubMed] [Google Scholar]
- Shibata S., Hattori K., Sakurai I., Mori J., Fujiwara M. Adrenergic innervation and cocaine-induced potentiation of adrenergic responses of aortic strips from young and old rabbits. J Pharmacol Exp Ther. 1971 Jun;177(3):621–632. [PubMed] [Google Scholar]
- Shibata S., Hattori K., Timmerman D. Effect of cold storage on the response of guinea-pig taenia coli to certain catecholamines and other agents. Eur J Pharmacol. 1970;11(3):321–331. doi: 10.1016/0014-2999(70)90008-7. [DOI] [PubMed] [Google Scholar]
- Su C., Bevan J. A. The release of H3-norepinephrine in arterial strips studied by the technique of superfusion and transmural stimulation. J Pharmacol Exp Ther. 1970 Mar;172(1):62–68. [PubMed] [Google Scholar]