Abstract
Dependence of monoterpenoid emission and fractional composition on stomatal conductance (GV) was studied in Mediterranean conifer Pinus pinea, which primarily emits limonene and trans-β-ocimene but also large fractions of oxygenated monoterpenoids linalool and 1,8-cineole. Strong decreases in GV attributable to diurnal water stress were accompanied by a significant reduction in total monoterpenoid emission rate in midday. However, various monoterpenoids responded differently to the reduction in GV, with the emission rates of limonene and trans-β-ocimene being unaffected but those of linalool and 1,8-cineole closely following diurnal variability in GV. A dynamic emission model indicated that stomatal sensitivity of emissions was associated with monoterpenoid Henry's law constant (H, gas/liquid phase partition coefficient). Monoterpenoids with a large H such as trans-β-ocimene sustain higher intercellular partial pressure for a certain liquid phase concentration, and stomatal closure is balanced by a nearly immediate increase in monoterpene diffusion gradient from intercellular air-space to ambient air. The partial pressure rises also in compounds with a low H, but more than 1,000-fold higher liquid phase concentrations of linalool and 1,8-cineole are necessary to increase intercellular partial pressure high enough to balance stomatal closure. The system response is accordingly slower, and the emission rates may be transiently suppressed by low GV. Simulations further suggested that linalool and 1,8-cineole synthesis rates also decreased with decreasing GV, possibly as the result of selective inhibition of various monoterpene synthases by stomata. We conclude that physicochemical characteristics of volatiles not only affect total emission but also alter the fractional composition of emitted monoterpenoids.
Monoterpenoids emitted by plants constitute a major source of volatile organic compounds (VOC) in the atmosphere (Guenther et al., 1994). Because they play an important part in atmospheric chemistry, in particular in ozone-forming reactions (Guenther et al., 1994; Simpson, 1995), considerable effort has been put toward measuring and prediction of monoterpenoid emission rates from the foliage of emitting species (Guenther et al., 1994).
All plant monoterpenoids are synthesized in plastids (Chappell, 1995; Lichtenthaler, 1999; Davis and Croteau, 2000). In many species, the rates of monoterpenoid synthesis are dependent on both light and temperature similarly to carbon assimilation rates (Schuh et al., 1997; Shao et al., 2001), and there is conclusive evidence that a part of the emitted compounds originates from a small pool of immediately assimilated carbon. However, the current monoterpenoid emission models mostly use a simple two-parameter empirical temperature equation to describe the monoterpene efflux from the foliage (Tingey et al., 1980, 1991; Guenther et al., 1993) assuming that monoterpenoids are emitted primarily from the storage pools such that their emission rates are uncoupled from the synthesis rates. In species without specific storage pools like the Mediterranean sclerophyll holm oak (Quercus ilex), it has been demonstrated that light may also control monoterpenoid emission (Staudt and Seufert, 1995; Ciccioli et al., 1997; Staudt and Bertin, 1998), and light effects on emission are generally described by a hyperbolic equation derived from foliar isoprene emission measurements (Guenther et al., 1993). Evidence has also accumulated to indicate that monoterpenoid efflux rates may even be light sensitive in species with storage pools such as conifers from the genera Pinus (Janson, 1993; Staudt et al., 1997; Kesselmeier and Staudt, 1999; Shao et al., 2001) and Picea (Schürmann, 1993; Schürmann et al., 1993; Steinbrecher and Ziegler, 1997). Light-dependent emissions in these species may be explained by a slower emission from the storage pools in resin ducts compared with the emission from chloroplastic monoterpenoid pools in the mesophyll cells (Schürmann et al., 1993). Such differences in the turnover rates of various pools may result from large diffusion resistances between the outside air and the resin ducts, which are lined by a layer of epithelial cells and an additional layer of thick-walled sclerenchyma cells (Steinbrecher and Ziegler, 1997). Despite of the appealing simplicity of the emission algorithms employing either only temperature or temperature and light as drivers, these models frequently provide relatively poor fits to the experimental observations (e.g. Juuti et al., 1990; Staudt et al., 1997; Llusiá and Peñuelas, 2000; Sabillón and Cremades, 2001) with explained variances generally not exceeding 50% to 70%. A relatively low percentage of explained variance suggests that monoterpenoid emission is not purely a physical phenomenon driven by temperature and also that important foliar characteristics and monoterpenoid physicochemical parameters may affect the emission rates.
Although the experimental work has demonstrated that both isoprene (Fall and Monson, 1992) and monoterpenoids (Schürmann, 1993; Loreto et al., 1996b) are emitted through the stomata (for review, see Kesselmeier and Staudt, 1999), a key assumption of all current empirical emission models is that stomata do not control the isoprenoid efflux. This apparently contrasts with previous observations that there is a strong correlation between foliar monoterpene emission and transpiration rates (Steinbrecher, 1989; Kesselmeier et al., 1996, 1997). Moreover, positive relations between leaf conductance to water vapor (GV) and monoterpenoid emission rates have frequently been observed (Steinbrecher, 1989; Schuh et al., 1997). The correlation between monoterpenoid emission and transpiration rates (E) may, of course, result from simultaneous positive effects of temperature on monoterpene efflux rates and on water vapor pressure deficit between leaf and atmosphere (ΔP; E = ΔPGV). Positive effects of light on both GV and monoterpenoid synthesis rate (e.g. Schuh et al., 1997) may similarly provide an explanation for the scaling of emission rates with GV.
So far, the lack of significant stomatal control over the emission rates has been shown only for isoprene (Monson and Fall, 1989; Fall and Monson, 1992) and for α-pinene (Loreto et al., 1996b) and has been generalized to all volatile compounds (Sharkey, 1991; Kesselmeier and Staudt, 1999). Missing stomatal control has been explained by low- and non-saturated foliar gas phase concentrations of isoprenoids, which readily increase in response to a stomatal closure and thereby balance the decrease in conductance by an enhanced diffusion gradient from the intercellular air space to outside air (Sharkey, 1991; Fall and Monson, 1992; Kesselmeier and Staudt, 1999). In fact, if the monoterpene synthesis rate remains constant after a decrease in GV, no sustained stomatal limitation of the emission rates is possible. An alternative explanation would be the emission of isoprenoids through the cuticle. However, calculations demonstrate that cuticle may account for only up to 10% to 20% of total monoterpenoids emitted (Schürmann, 1993). Thus, cuticular emission alone could not sustain the observed monoterpenoid emission rates in the absence of the emission through stomata.
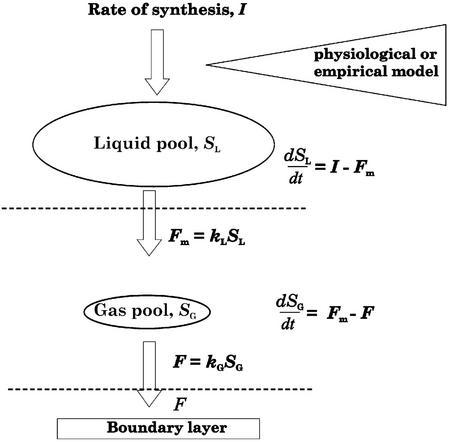
To gain mechanistic insight into varying stomatal controls over the emission of specific compounds, we developed a dynamic model describing the dependence of the VOC emission rate on GV and the compound physicochemical characteristics. Mass-balance approach is employed to simulate the dynamics of leaf gas and liquid pools (Fig. 1). The model explains the compound-specific emission responses to GV by different half-times of the monoterpene internal gas and liquid pools. The compounds that partition primarily to liquid phase such as short-chain alcohols, aldehydes, and carboxylic acids require larger increases in the liquid pool size for a certain rise in partial pressure than the compounds that partition primarily to the gas phase. Thus, provided that the compound intercellular partial pressure (Pi) changes more slowly than stomatal aperture, GV may affect VOC emission in non-steady-state conditions.
Figure 1.
Outline of the dynamic model of foliar monoterpene emission. The leaf internal monoterpene content is divided between liquid and gas pools, and a mass balance approach is used to determine the dynamics of the pools. The rate of monoterpenoid synthesis, I, may be computed by either a process-based model or an empirical model (Eqs. 15–17). The diffusion flux density from the site of synthesis to outer surface of cell walls, Fm, is given by Equation 6, and the diffusion flux density through the stomata, F, by Equation 5.
Apart from the potential stomatal limitations attributable to the slow rise in Pi, increases in the liquid phase VOC concentrations may also directly affect the compound synthesis rates, thereby leading to curbed rates of emission in the steady-state conditions. It is know that accumulation of certain end products and intermediates may lead to differences in the activity profiles of various plant monoterpene synthases (Davis and Croteau, 2000). We use the model developed to discriminate between stomatal and biochemical controls on plant VOC emission in the Mediterranean evergreen conifer Pinus pinea. This species has a distinct emission pattern emitting large amounts of oxygenated monoterpenoids linalool and 1,8-cineole that may potentially be strongly controlled by stomata in a non-steady-state situation. The light-dependent emissions in this species are an order of magnitude larger than the emissions in the dark (Staudt et al., 1997), suggesting that de novo synthesis rather than the storage in resin ducts is the primary source of emitted monoterpenes. Empirical models based on leaf temperature and incident irradiance alone have provided especially poor fits to the diurnal dynamics of oxygenated compounds in P. pinea (Staudt et al., 1997; Sabillón and Cremades, 2001). We further demonstrate that the fractional composition of emitted monoterpenoids changes during the day as the result of selective constraints on the synthesis and emission of oxygenated monoterpenoids. Because various monoterpenoids differ largely with respect to the gas phase rate coefficients for reaction with ozone and hydroxyl radicals (Fehsenfeld et al., 1992; Guenther et al., 1994), a mechanistic prediction of changes in monoterpenoid composition provides an important basis to determine diurnal changes in atmospheric reactivity.
RESULTS
Simulated Responses of Plant Volatile Emission to Changes in Stomatal Conductance (GV). Gas Phase Dynamics
Provided that the volatile synthesis rate is unaffected by changes in GV, stomatal closure leads to an increase in internal leaf volatile concentration. In a steady-state situation, the rise in the internal concentration exactly balances the decrease in GV, and the same flux as before the changes in GV is maintained at a lower stomatal aperture. This means that when the volatile build-up does not affect its synthesis rate, stomata may affect the VOC emission only in a non-steady-state situation, and the turnover rate of leaf gas and liquid phases apparently determines the time required to reach the steady state.
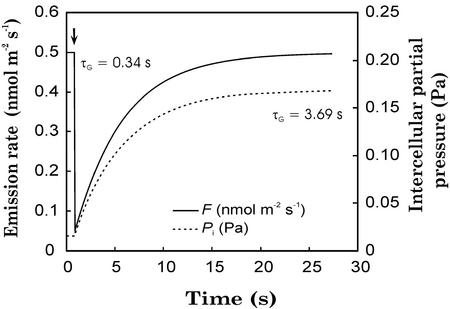
We first use the model version with a gas pool only (Fig. 1; dSG/dt = Fm − F, where Fm is the VOC flux from the site of synthesis to the outer surface of cell walls and F is the flux through stomata) to demonstrate that the size of the gas phase pool is far too small to explain stomatal limitations on VOC emission. Taking GV to water vapor equal to 30 mmol m−2 s−1—a low value that corresponds to highest daily GV values in water-stressed foliage of P. pinea—the rate constant (Eq. 10) of the gas pool of trans-β-ocimene, kG, is 2.05 s−1, and the half-time of the gas pool, τG (Eq. 11), will be 0.34 s (Fig. 2). When GV is low, e.g. 1.5 mmol m−2 s−1, a value corresponding to a closed-stomata situation at night, kG = 0.19 s−1, and τG increases to 3.69 s. Figure 2 illustrates changes in trans-β-ocimene emission rate (Fig. 1; Eq. 10) in response to a hypothetical instant decrease in GV from 30 to 1.5 mmol m−2 s−1, with a trans-β-ocimene input rate from chloroplasts to substomatal cavities of 0.5 nmol m−2 s−1. A new steady state is reached in approximately 15 s. After that, the flux is maintained, because the higher monoterpene Pi compensates for the stomatal closure. Given that the binary diffusion coefficients of monoterpenoids in air vary less than 10% among monoterpenoids (Table I), the system behaves very similarly for other monoterpenoids. Additional simulations demonstrated that the half-times of the gas phase pool, τG, are less than 5 s for all monoterpenoids emitted by P. pinea, even for very low finite gas phase conductances. These values are much lower compared with the time constants of stomatal closure and opening that measure in minutes (e.g. Tinoco-Ojanguren and Pearcy, 1993). Thus, we conclude that the gas phase pool is fast and that changes in gas phase dynamics cannot lead to stomatal limitations of monoterpene emission.
Figure 2.
Gas phase dynamics in P. pinea. Modeled (Fig. 1; Eqs. 8–10) effects of an instant stomatal closure on trans-β-ocimene emission rate (F) and intercellular partial pressure (Pi) at 25°C. Stomatal conductance to water vapor was changed from 30 to 1.5 mmol m−2 s−1 at time 1 s (denoted by arrow). A value of 0.5 nmol m−2 s−1 was used for the initial emission rate. τG is the half-time of the gas pool (Eq. 11). Physicochemical characteristics of trans-β-ocimene are given in Table I, and leaf structural data used for model parameterization are given in an electronic supplement (which can be viewed at www.plantphysiol.org).
Table I.
Physicochemical properties of selected monoterpenoids at 25°C
| Property | Linalool | 1,8-Cineole | Trans-β-Ocimene | d-Limonene |
|---|---|---|---|---|
| Diffusion coefficient in air (DA, m2 s−1) | 5.17 × 10−6 | 5.46 × 10−6 | 5.46 × 10−6 | 5.64 × 10−6 |
| Diffusion coefficient in water (DW, m2 s−1) | 6.26 × 10−10 | 6.64 × 10−10 | 6.52 × 10−10 | 6.82 × 10−10 |
| Saturated vapor pressure (PV, Pa) | 21.3 | 253 | 197 | 253 |
| Water solubility (δ, mol m−3) | 10.2 | 19.1 | 0.0797 | 0.0886 |
| Henry's law constant (H; Pa m3 mol−1) | 2.078 | 13.27 | 3330 | 2850 |
| Log[octanol-water partition coefficient (Ko/w, mol mol−1)] | 2.97 | 2.61 | 4.45 | 4.485 |
| Gas-phase internal conductance (Gias, m s−1) | 1.59 × 10−3 | 1.67 × 10−3 | 1.68 × 10−3 | 1.73 × 10−3 |
| Liquid-phase internal conductance (GL, m s−1) | 5.88 × 10−4 | 3.46 × 10−4 | 1.54 × 10−3 | 1.61 × 10−3 |
Supplemental data of derivation of and references to the physicochemical monoterpene characteristics and internal diffusion conductances are provided at www.plantphysiol.org. Averages were calculated whenever multiple estimates were available.
Simulated Liquid Phase Dynamics
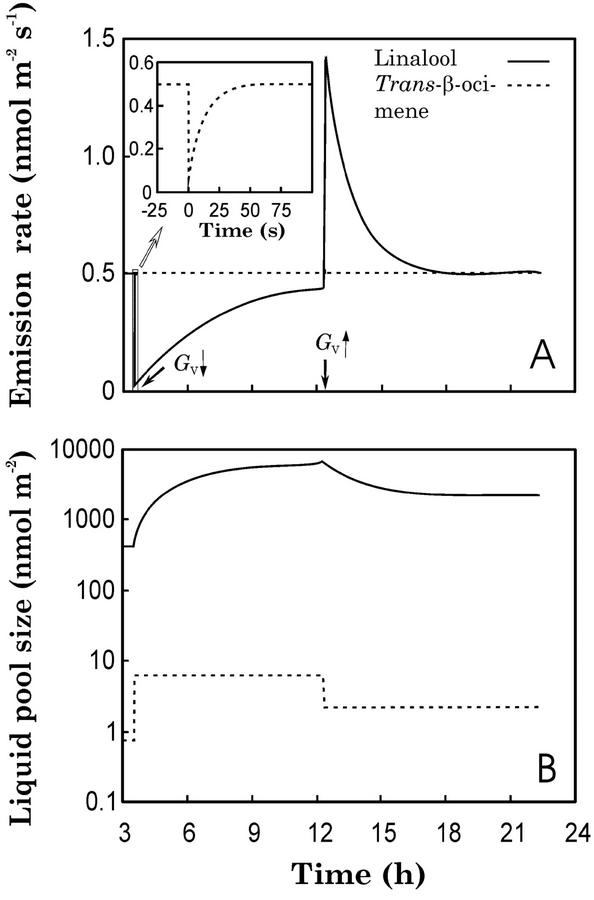
Provided that the gas phase is in the steady state, we now investigate the possibility that compound-to-compound differences in liquid phase dynamics lead to different stomatal sensitivities of VOC emissions. Figure 3 demonstrates the response of the system (Eqs. 12–14) to an instant decrease in GV from 30 to 1.5 mmol m−2 s−1 and a subsequent rise to 5 mmol m−2 s−1 in two monoterpenoids of contrasting Henry's law constant (H, the equilibrium air-water partition coefficient; Eq. 7). Trans-β-ocimene, an olefin, has a H value of 3,330 Pa m3 mol−1, and linalool, a terpene alcohol, has a H of 2.078 Pa m3 mol−1 (see Table I for the physicochemical characteristics of monoterpenoids). For trans-β-ocimene, the half-time of the liquid pool (τL) is 1.03 s before the decrease in GV, and the corresponding value is 551 s for linalool. After simulated stomatal closure, τL increases to 8.5 s in trans-β-ocimene and to 10,120 s in linalool. Thus, the Pi increases rapidly in response to the simulated stomatal closure for trans-β-ocimene, and the rise in the partial pressure balances the stomatal closure in approximately 30 s (Fig. 3A). In contrast, more than 10 h are needed to reach a steady-state situation for linalool (Fig. 3A). This striking difference in compound behavior results from the circumstance that a certain liquid phase concentration supports more than 1,000-fold higher Pi of trans-β-ocimene relative to linalool (Fig. 3B). As a consequence, slow increases in the liquid phase linalool concentration slow down the system response to changes in stomatal aperture.
Figure 3.
Performance of the dynamic model of monoterpene emission for P. pinea (Fig. 1; Eqs. 12–14) with linalool that has a Henry's law constant (H; Eq. 7) of 2.078 Pa m3 mol−1 and with trans-β-ocimene (H = 3,330 Pa m3 mol−1; Table I) at 25°C. The liquid-phase pools were allowed to reach a steady state at a GV of 30 mmol m−2 s−1, and GV was decreased to 1.5 mmol m−2 s−1 at time 3.5 h (denoted by an arrow). The conductance was kept at this value until 12.5 h, and then GV was risen to 5 mmol m−2 s−1. Inset in A shows the initial changes after the decrease in GV in trans-β-ocimene in a higher resolution.
The emission rate dynamics also behaves differently after a moderate increase in stomatal openness. Again, a new steady state is reached in seconds for trans-β-ocimene. However, linalool exhibits a large overshoot of the emission, because the liquid phase pool exceeds severalfold the steady-state linalool pool corresponding to new conditions (Fig. 3A). Such bursts of emission after a change in stomatal openness have been experimentally observed for methanol (Nemecek-Marshall et al., 1995) and acetaldehyde (Holzinger et al., 2000), and they demonstrate the decay of the extensive VOC pool accumulated during periods of low GV.
These simulations demonstrate that the emission rate is essentially always in a steady state in compounds with a high H, but potentially large effects of GV on the emission dynamics are expected for compounds with low values of H. Thus, we predict strong stomatal effects on emission of monoterpenes that preferably partition to aqueous phase (linalool and 1,8-cineole) and no stomatal effects for compounds primarily partitioning to gas phase (pinenes, ocimenes, and limonene; Table I).
Experimental Observations. Seasonal Changes in Monoterpenoid Emission
Emission of all monoterpenoids was strongly light dependent and was lower more than an order of magnitude at night than during the day at a common leaf temperature (Fig. 4). The highest total emission rates with daily maxima (Fmax) of 4 to 9 nmol m−2 s−1 were observed during the July 31 to August 6, 1994 campaign and were followed by the emission rates during June 1 to 14, 1993 (Fmax = 3–8 nmol m−2 s−1), May 5 to 28, 1994 (Fmax = 1–3 nmol m−2 s−1), and October 23 to 27, 1994 (Fmax = 0.5–0.6 nmol m−2 s−1). Periods of low monoterpenoid emission rates in May and October 1994 were accompanied by lower temperatures and reduced emission factors (Fs). Fs is the emission rate at defined standard temperature and light conditions (Eq. 15) and represents the overall foliar capacity to produce monoterpenoids. Periods of high monoterpenoid emission rates in June 1993 and August 1994 were accompanied by high emission factors, high temperatures (Fig. 4A), and foliar water stress. Maximum daily GV values of approximately 45 (June 1993) and 25 mmol m−2 s−1 (August 1994) were observed in early morning, and GV decreased thereafter gradually during the day with moderate recovery in afternoon (Fig. 4B). In contrast, maximum values of GV of up to 120 mmol m−2 s−1 were observed in midday in non-stressed leaves (May and October 1994; data not shown; for further details on seasonal variability in GV in P. pinea, see Manes et al., 1997).
Figure 4.
Examples of diurnal variability (August 3) in incident quantum flux density (Q, dots) and leaf temperature (TL, lines; A), foliar net assimilation rates and GV to water vapor (B), measured (dots) and predicted (lines) emission rates of trans-β-ocimene (C), limonene (D), 1,8-cineole (E), and linalool (F) in P. pinea. Monoterpenoid emission rates were simulated by Guenther et al. (1993) algorithm (Eqs. 15–17), assuming no stomatal effects on the diffusion flux density through the stomata and monoterpene synthesis rate and computing the emission factor, FS (Eq. 15), from the measurements between 9 am and 12 pm.
The most important monoterpenoids emitted were cyclic monoterpene limonene and acyclic monoterpenoids linalool, trans-β-ocimene, and myrcene (Table II). In addition, ether 1,8-cineole and cyclic monoterpenes α- and β-pinene were emitted in large quantities during certain periods. The fraction of monoterpenoids emitted as trans-β-ocimene was large in water-stressed leaves in June 1993 and August 1994 but was low in May and October 1994 when there was no significant foliar water stress.
Table II.
Daily average (±se) fractions of total emission of majora monoterpenoids emitted in P. pinea
| Monoterpenoid | Campaign
|
|||
|---|---|---|---|---|
| May 5–28, 1994 | June 1–14, 1993 | July 3–August 6, 1994 | October 23–27, 1994 | |
| Limonene | 0.413 ± 0.027 | 0.486 ± 0.035 | 0.067 ± 0.014 | 0.400 ± 0.067 |
| Linalool | 0.124 ± 0.013 | 0.180 ± 0.024 | 0.225 ± 0.029 | 0.248 ± 0.053 |
| Trans-β-ocimene | 0.051 ± 0.006 | 0.208 ± 0.023 | 0.397 ± 0.049 | 0.0114 ± 0.0021 |
| 1,8-Cineole | 0.0325 ± 0.0029 | 0.0315 ± 0.0042 | 0.0397 ± 0.0037 | 0.167 ± 0.027 |
| Myrcene | 0.174 ± 0.023 | 0.0355 ± 0.0018 | 0.0149 ± 0.0017 | 0.0559 ± 0.0055 |
| α-Pinene | 0.161 ± 0.012 | 0.0377 ± 0.0030 | 0.0200 ± 0.0038 | 0.0524 ± 0.0048 |
| β-Pinene | 0.0259 ± 0.0033 | 0.0286 ± 0.0042 | 0.0052 ± 0.0043 | 0.0100 ± 0.0025 |
Altogether, 19 monoterpenoids have been detected in the foliar emission patterns of P. pinea (Kesselmeier et al., 1997).
Diurnal Variability in Emission Rates of Various Monoterpenoids
In non-water limited leaves in May and October 1994, the empirical Guenther et al. (1993) model (Eqs. 15–17) that does not include stomatal effects on emission provided good fits to the emission rates of all monoterpenoids (data not shown). In water-stressed leaves in June 1993 (data not shown) and in August 1994, the model gave an excellent description of the emission of monoterpenoids with a low aqueous solubility and a large value of H (Table I) such as trans-β-ocimene (Fig. 4C) and limonene (Fig. 4D). However, the model strongly overestimated the emission of compounds with higher water solubility and a low H such as 1,8-cineole (Fig. 4E) and linalool (Fig. 4F). Diurnal variability in linalool and 1,8-cineole emission rates was similar to GV (compare Fig. 4B with E and F) with the emission rates being highest in early morning and decreasing during the day with a modest rise of the emissions in the afternoon. Because of the strong decrease of more than an order of magnitude of linalool and 1,8-cineole efflux rates, the total emission rate of all monoterpenoids (Fsum) also decreased in midday (Fig. 5). Nevertheless, because the decline in linalool and 1,8-cineole emission rates was somewhat compensated for by increases in the emission of other monoterpenoids, Fsum decreased during the day only by 10% to 25% (Fig. 5).
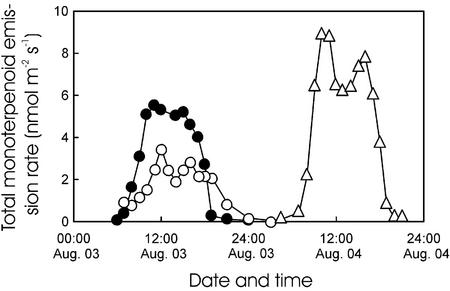
Figure 5.
Sample plot of the diurnal variability in total monoterpenoid emission rates on August 3 in two trees of P. pinea (●, tree 1; ○, tree 2), and on August 4 (▵, tree 1). The samples were analyzed gas-chromatographically by three different laboratories (●, JCT; ○, GRECA; and ▵, ENSCT; for details, see “Materials and Methods”).
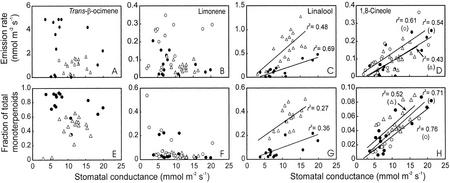
Emission rates of monoterpenoids with a low H value—linalool and 1,8-cineole—were closely related to GV (Fig. 6, C and D). No correlations between the emission rates and GV was observed for monoterpenoids with a high H value such as trans-β-ocimene and limonene (Fig. 6, A and B).
Figure 6.
Correlations of the GV with the emission rates (A–D) and the fractions of total emitted monoterpenoids (E–H) of trans-β-ocimene (A and E), limonene (B and F), linalool (C and G), and 1,8-cineole (D and H) in P. pinea. The GV to specific monoterpenoid is given as GVDA/DV (Eq. 1), where DV is the binary diffusion coefficient for water vapor in air and DA is the diffusion coefficient of specific monoterpenoid in air (Table I). The emission rates were measured on August 3 and 4 in two trees, and all data sampled during these days are given (for an example of daily time-courses, see Fig. 4; for the explanation of symbols, see Fig. 5). Because of problems in resolution of linalool and trans-β-ocimene for tree 2 on August 3 (○) only data for tree 1 are provided for these monoterpenoids. All linear regressions drawn are significant at least at P < 0.05.
Modification of the Composition of Emitted Volatiles
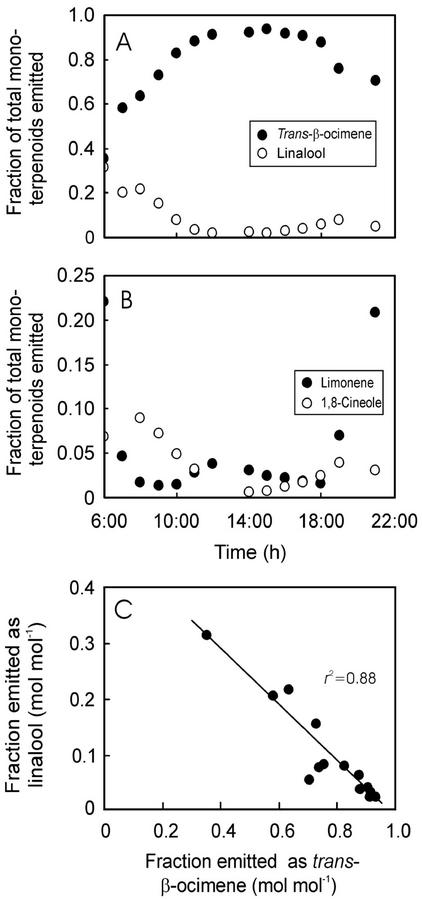
Similarly to the emission rates, the fractions of total monoterpenoids emitted as linalool and 1,8-cineole were strongly related to GV (Fig. 6, G and H) but not the fractions of other monoterpenoids (Fig. 6, E and F). These correlations resulted from parallel diurnal changes in the composition of emitted monoterpenoids (Fig. 7, A and B) and GV (Fig. 4B). In general, the fractions of linalool and 1,8-cineole emitted were the lowest in midday corresponding to lowest emission rates (compare Fig. 4, E and F, with Fig. 7, A and B), whereas the emission rates of trans-β-ocimene and limonene did not exhibit midday minima (Fig. 7, A and B). Because of large increases in trans-β-ocimene emission, the fraction of acyclic monoterpenoids (sum of the fractions of linalool, trans-β-ocimene, and myrcene) were largest in midday (data not shown). Very similar diurnal changes in emission dynamics and fractional composition were observed during all other days. These contrasting variation patterns resulted in a strong negative relationship between the fraction of trans-β-ocimene and the fraction of monoterpenoids emitted as linalool (Fig. 7C). There were no other correlations between the fractional emissions of compounds with large and small H values.
Figure 7.
Sample plots of diurnal variability in the fractions of total monoterpenoids emitted as trans-β-ocimene and linalool (A) and limonene and 1,8-cineole (B), and the correlation between the fractions emitted as linalool and trans-β-ocimene (C) in P. pinea. The data were fitted by a linear regression (P < 0.001). The same data set as in Figure 4.
Stomatal or Biochemical Constraints on Monoterpene Emission?
According to the dynamic monoterpene emission model (Fig. 1; Eqs. 12–14), the midday decline in linalool and 1,8-cineole emission rates may potentially result from changes in GV, because both of these compounds have a low H, and the Pi values of these volatiles respond slowly to modifications in GV. Alternatively, increases in monoterpenoid partial pressures after stomatal closure may suppress the synthesis rates and thereby reduce the emission. To discriminate between these two possible mechanisms, we constructed three contrasting scenarios to simulate the diurnal emission dynamics on the example of linalool (Fig. 8) and trans-β-ocimene (Fig. 9).
Figure 8.
Comparison of measured (Fig. 4F, dots) and modeled (Eqs. 12–14, lines) daily time courses of linalool emission from the needles of P. pinea. In scenario 1 (A–C), GV was assumed to be invariable during the light period and to increase in the morning and decrease symmetrically in the evening with the rate constant determined from the data (solid line in A), whereas the synthesis rate (I) was modeled according to Equations 15 to 17 (dashed line in A). In scenario 2 (D–F), GV was varied in accordance with the measurements in the field (solid line in D), whereas I was as in scenario 1. In scenario 3 (G–I), GV followed the data, and the linalool synthesis rate was assumed to be proportional to actual GV, i.e. the rate of synthesis is equal to GV/GV,maxI, where I is the rate of synthesis predicted in A and D and GV,max is the maximal GV observed during the day. Solid lines in simulated linalool emission rate (B, E, and H) and liquid pool size (C, F, and I) plots are predicted for a temperature-dependent H (inset in F). The punctuated lines in E, H, F, and I demonstrate the hypothetical system dynamics for a constant H of 2.078 Pa m3 mol−1. In simulations, the initial liquid phase pool size at 0 h was taken equal to that at 24 h. The emission factor (FS) was determined from the measurements between 9 and 12 h.
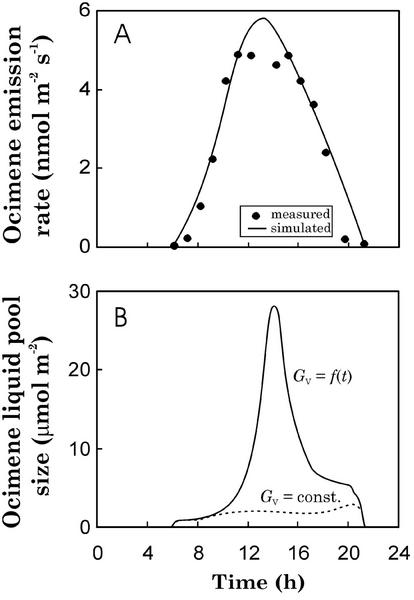
Figure 9.
Measured (Fig. 4C, dots) and simulated (Eqs. 12–14, lines) diurnal variability in trans-β-ocimene emission rates (A) and liquid pool sizes (B) in P. pinea. The synthesis of trans-β-ocimene was predicted as in Figure 8A, and the GV was either as in Figure 8A (dotted line in B) or changed according to data (solid line in A and B). The estimates of trans-β-ocimene emission rate did not differ for these scenarios, and thus, only a single line is provided in A.
In the first scenario (Figs. 8, A–C, and 9), we assumed no diurnal decline in GV (Fig. 8A) while keeping the synthesis at a maximal potential rate determined by actual leaf temperature and incident irradiance (Fig. 4, C and F; Eqs. 15–17). In the second scenario (Figs. 8, D–F, and 9), GV tracked the actual measurements while the synthesis rates were not modified by stomata. In the third scenario (Fig. 8, G–I), the diurnal variation in GV followed the data, and the synthesis rate was set proportional to GV.
Scenario 1 (Figs. 8, A–C, and 9) demonstrates that without GV limitations, both linalool and trans-β-ocimene emission rates closely follow the rates of their synthesis. The second scenario (Figs. 8, D–F, and 9) suggests that stomata may significantly modify the emission rates of linalool. As the stomata close in midday, the half-time of the liquid pool (τL) increases from 502 to 3,040 s, indicating that the liquid phase linalool pool (Fig. 8F) rises more slowly than the changes in GV occur, leading to a midday depression in the emission rates. Yet after a moderate increase in stomatal openness, the suppression is followed by a burst of linalool emission that temporarily exceeds the synthesis rate (Fig. 3). No effect of GV is observed for trans-β-ocimene because the increase in gas phase pool of this monoterpene immediately balances changes in GV (Fig. 9).
However, scenario 2 overestimated the emission rates at low values of GV, hinting at simultaneous changes in linalool synthesis rates with GV. The hypothesis of declining synthesis rates was further strengthened by excellent fits to the data when the linalool synthesis rate was set proportional to GV (Fig. 8, G–I). Thus, these simulations suggested that both stomatal and biochemical constraints modified the linalool emission from the leaves.
The latter model is supported by good fits to diurnal time courses of monoterpenoid Pi (Fig. 10), which determines the gradient for monoterpenoid diffusion from intercellular air space to the outside air. There was a moderate midday overestimation of Pi for trans-β-ocimene (Fig. 10B) that resulted from the higher predicted emission rates during this period (Fig. 9A). This may indicate that the trans-β-ocimene synthesis rate was also somewhat down-regulated compared with the potential rate predicted by the empirical model (Eqs. 15–17). However, given that the needle inclination angles were vertical but the light sensor was arranged horizontally, this may also be associated with a midday overestimation of incident quantum flux densities on needle surface (Staudt et al., 1997).
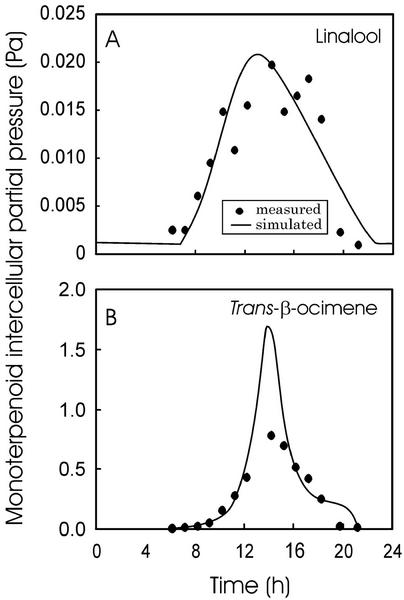
Figure 10.
Linalool (A) and trans-β-ocimene (B) Pi values during August 3 (emission data in Fig. 4, C and F). The partial pressure was determined according to Equation 2 (measured) or from Equation 8 using the simulations in Figures 8, G to I, and 9 (GV changed to track the measurements). Pi is the intercellular partial pressure in equilibrium with cell wall monoterpene concentrations. The total emission data are depicted in Figure 4, C and F.
The evidence that the Pi values of linalool (Fig. 10A) and 1,8-cineole were constant in midday and did not balance the decreases in GV that would have been expected for a constant synthesis rate (Fig. 8, D–F) further suggests that stomatal closure, accompanied by monoterpene build-up in the liquid phase, may potentially shift the chemical equilibrium between the production and interconversion of specific monoterpenoids or lead to changes in gene expression and/or changes in activity profiles of multiple terpene synthases.
In these simulations, the H of linalool was expected to vary from 1.46 to 5.32 Pa m3 mol−1 for a diurnal variability in temperature from 20.3°C to 38.4°C (compare Fig. 4A with Fig. 8F, inset). For trans-β-ocimene, H was predicted to change from 2,520 to 6,620 Pa m3 mol−1 for the same temperature range. Given that the exact temperature relationships of H are not available for these compounds, we also considered the hypothetical situation with an invariable H in scenarios 2 and 3 (Fig. 8F, inset) to determine the potential temperature effect on emission dynamics attributable to varying H (supplemental data can be viewed at www.plantphysiol.org). These simulations indicate that a smaller increase in H is compatible with a more efficient stomatal control of linalool emission in midday but also with a greater linalool burst in response to an increase in stomatal openness (Fig. 8E). A similar modification of the emission dynamics is also observed for the scenario 3 (Fig. 8H), but the effect is damped because of a lower liquid pool of linalool than that in the scenario 2 (Fig. 8, D–F).
Implicit in these calculations was the assumption that the production of oxygenated and nonoxygenated compounds can be described by the same light and temperature algorithms (Eqs. 15–17), allowing the estimation of the basal emission factor, FS (Eq. 15) from emission measurements between 9 and 12 am for all compounds. Given that there may be a burst of emission of monoterpenoids with a low H after increases in stomatal opening (Fig. 3A), the high morning rates of linalool and 1,8-cineole emission (Fig. 4, E and F) may also partly rely on the decay of the liquid phase monoterpenoid pool retained over or accumulated during the night. However, such an mechanism would imply that there is a moderate linalool and 1,8-cineole emission rate in the darkness, which either decreases during the night as the liquid phase pool present after stomatal closure is depleted or increases as the monoterpenoids accumulate in the leaf (Fig. 4A). Given that the emission rates of oxygenated monoterpenoids were essentially zero during the night (Fig. 4), we consider this mechanism unlikely.
DISCUSSION
Diurnal Variability in Monoterpenoid Emission Rates
The current study demonstrated a strong midday decline in the emission rates of oxygenated monoterpenoids linalool and 1,8-cineole in water-stressed needles (Fig. 4, E and F) but no apparent decrease in the efflux rates of other compounds (Fig. 4, C and D). A strong decrease in the emission of linalool, 1,8-cineole, and total monoterpenoids (Fig. 5) clearly indicates that the emission algorithm driven by light and temperature only (Eqs. 15–17) is an oversimplification and does not allow reproduction of the diurnal emission courses of monoterpenoids in P. pinea.
The decline in the emission rates of oxygenated monoterpenoids was accompanied by decreases in GV and decreases in net carbon assimilation rate (Fig. 4B). Because a certain fraction of emitted plant monoterpenoids is always synthesized in chloroplasts (Chappell, 1995) and the synthesis of isoprenoids may rely on a small pool of photosynthetic intermediates (Loreto et al., 1996a; Zimmer et al., 2000), monoterpenoid emission rates often scale positively with carbon assimilation rates (Kesselmeier et al., 1996; Loreto et al., 1996b). Thus, the diurnal decline in monoterpenoid emission rate may have resulted from daily decreases in foliar net carbon assimilation rates. However, this suggestion does not explain away the insensitivity of limonene and trans-β-ocimene to net photosynthesis rates and to GV. Furthermore, recent evidence indicates that the rates of monoterpenoid synthesis may be more closely controlled by the rate of photosynthetic electron transport, i.e. the availability of NADPH and ATP in chloroplasts (Niinemets et al., 1999, 2002a, 2002b). Photosynthetic electron transport is relatively insensitive to GV because photorespiration may effectively substitute for carbon assimilation as an electron sink in water-stressed leaves with closed stomata, thereby avoiding down-regulation of photosynthetic electron transport rates (Kozaki and Takeba, 1996). The control of potential monoterpenoid synthesis rates by photosynthetic electron transport and the close to immediate rise in monoterpene partial pressure after a decrease in stomatal openness is likely the mechanistic explanation of the lack of midday decline in the efflux of the compounds with a high value of H such as limonene and trans-β-ocimene as demonstrated by our simulations (Figs. 2, 3, and 9). Thus, the results of these simulations, quantitatively describe the hypothesis of Sharkey (1991) and Fall and Monson (1992). However, this mechanism cannot account for the stomatal sensitivity of VOC emission observed for linalool and 1,8-cineole in our study.
Explanation of the Stomatal Sensitivity of Oxygenated Compounds
The circumstance that stomata cannot limit the emission over the long term does not mean that they cannot control the emission at all. In fact, slow turnover of leaf liquid phase in compounds that preferably partition to aqueous phase may result in a limited rise of gas phase volatile concentrations and strong temporal stomatal limitations as simulated in Figure 3.
In the current study, there were strong positive correlations between GV and linalool and 1,8-cineole emission rates (Fig. 6, C and D). According to simulations (Figs. 8–10), the slow rise in the monoterpenoid Pi (Fig. 8, D–F) partly explained the midday decline in linalool and 1,8-cineole emission rates. However, for a constant rate of synthesis, the Pi values would have risen to a value supporting high flux rates in the afternoon, indicating that the sustained stomatal limitation is not possible over the entire day for these compounds (Fig. 8E). Thus, our model analyses provide indirect evidence that the synthesis rates of these two monoterpenoid also declined (Fig. 8, G–I) with decreasing GV. More than 1,000-fold higher linalool and 1,8-cineole liquid phase concentrations than limonene and trans-β-ocimene concentrations would have been necessary to overcome the stomatal limitations (compare Fig. 8F with 9B). Although the reductive equivalents and carbon were apparently available to maintain the synthesis at a constant level, high chloroplastic concentrations of linalool and 1,8-cineole apparently inhibited further enzymatic conversion of monoterpenoid precursors to these compounds.
Apart from our study, there is currently conclusive experimental evidence of a strong stomatal sensitivity of methanol emission (Nemecek-Marshall et al., 1995). Given the H values of 0.0132 Pa m3 mol−1 for acetic acid, 5.23 Pa m3 mol−1 for acetaldehyde, and 0.461 Pa m3 mol−1 for methanol (Staudinger and Roberts, 1996), effective stomatal control over the rates of emission of these compounds is expected (Fig. 3). Kesselmeier et al. (1997) observed a large midday depression paralleling changes in GV in the efflux rates of acetic and formic acids and of the respective aldehydes from the foliage of P. pinea. Possibly because of larger stomatal sensitivity, the isoprene emission algorithm of Guenther et al. (1993), which does not consider stomatal effects on emission, predicted emission rates with much greater uncertainty for organic acids (average error 40%) and aldehydes (65%) than the emissions of total monoterpenoids (28%; Kesselmeier et al., 1997), where the contribution of oxygenated compounds was relatively low.
Overall, the stomatal effects are apparently more important than has been acknowledged so far. For example, in deserts, the emission rates are low during drought periods (Winer and Karlik, 2001), but there is often a burst of smell just before a rain. Such a phenomenon can be explained within our modeling framework by stomatal opening attributable to increasing humidity as the rain is approaching. Because in a closed-stomata situation, there is an extensive gas and liquid phase pool within the leaves, a burst of emission is expected for fragrant compounds such as linalool, 1,8-cineole, camphor, thymol, and other oxygenated monoterpene derivatives that may be emitted in trace quantities in steady-state situations (Fig. 3). Such burst of emission have been observed previously for alcohols (Nemecek-Marshall et al., 1995) and aldehydes (Holzinger et al., 2000) but could not be explained by current steady-state emission models.
Diurnal Changes in Monoterpenoid Composition
Composition of monoterpenoids emitted may change during the day (Staudt et al., 1997, 2000) and during the season (Llusiá and Peñuelas, 2000; Staudt et al., 2000). Both seasonal (Table II) and diurnal changes (Fig. 7) in monoterpenoid composition were observed in our study. In particular, the fraction of oxygenated monoterpenoids linalool and 1,8-cineole had a pronounced midday minimum, whereas the fractions of trans-β-ocimene and limonene were generally highest in midday. Because the gas phase rate coefficients (Meylan and Howard, 1993) for reactions with ozone (KO3) and hydroxyl radicals (KOH) differ for various monoterpenoids, the changes observed in monoterpenoid fractional composition have a direct bearing on atmospheric chemistry.
The fraction of acyclic monoterpenoids (sum of the fractions of linalool, myrcene, and trans-β-ocimene) emitted increased during the day, but a reverse correlation was observed between the fractions of linalool and trans-β-ocimene (Fig. 7C). The decrease in emission of linalool during the day was accordingly compensated by increases in the emission of more volatile trans-β-ocimene and myrcene. This may indicate that the reactions leading to linalool and trans-β-ocimene are tightly linked and that their synthesis is regulated in a coordinated manner.
Overall, single monoterpenoid synthases catalyze the multistep reactions leading from the common monoterpene precursor geranyl-pyrophosphate (GPP) to monoterpenoids (Croteau, 1987; Gershenzon and Croteau, 1993; Steinbrecher and Ziegler, 1997). Given that the first step, isomerization of GPP to linalylpyrophosphate, is common for both the synthesis of trans-β-ocimene and linalool, binding of the GPP to a respective terpene synthase may control the product formation. Thus, selective inhibition of linalool synthase activity, e.g. as the result of accumulation of linalool because of stomatal closure, may favor trans-β-ocimene synthesis. So far, the empirical data to support such a substrate level inhibition are scarce, and discrimination between various hypotheses of regulation of stoichiometry of emitted compounds warrants further detailed experimental study. Nevertheless, our study suggests that synthesis of certain monoterpenoids may be selectively inhibited by stomatal closure, leading to a compensatory synthesis of other compounds. In conditions of low volatilization rates of linalool, the whole-reaction pathway may shift toward synthesis of trans-β-ocimene, thereby explaining the observed negative relationship between these compounds (Fig. 7C).
CONCLUSIONS
Analyses of daily time courses of VOC emissions by dynamic models allow gaining fundamental insight into diurnal variabilities in synthesis and emission rates of various volatiles. According to our simulations, the gas pool is very fast for all compounds, and the assumption of a steady state in the gas phase is justified for analyses of the emission responses to stomatal openness. However, the turnover rates of leaf liquid pools differ dramatically among various compounds (Fig. 3). Because stomatal resistance is always finite, stomata may exert a control over VOC efflux from the foliage for minutes to days depending on the H values of specific compounds. Our study provides experimental evidence and a theoretical explanation of strong stomatal sensitivity of emission of oxygenated volatiles from the leaves of plants. Because many important emitting plant species grow in habitats where water stress regularly occurs and because there are also characteristic diurnal variation patterns in GV in non-stressed conditions, our results have major implications for the application and modification of current emission models. Although the simple emission algorithms may provide reasonable fits for the daily average emission rates, accurate simulation of diurnal courses of emission and monoterpenoid composition is extremely relevant for the prediction of atmospheric reactivity. We conclude that stomatal effects on emission of compounds with a low H such as alcohols, aldehydes, carboxylic acids, and oxygenated monoterpenoids are significant under realistic values of GV and must be accounted for in the further models of plant VOC fluxes. As our study demonstrates, a model including the liquid to gas phase monoterpenoid partitioning may provide a valuable tool to differentiate between stomatal and biochemical controls on monoterpenoid emission.
MATERIALS AND METHODS
Dynamic Model of Monoterpene Emission
Steady-State Monoterpene Emission Rates
Because monoterpenes are mainly emitted through the stomata, we relate terpene flux (F, nmol m−2 s−1) from the leaves to stomatal conductance (Tingey et al., 1991) by an equation analogous to that previously employed for CO2 diffusion into the leaf (Farquhar and Sharkey, 1982; Field et al., 1989):
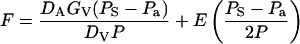 |
1 |
where DA (m2 s−1) is the air phase diffusion coefficient for specific monoterpene (Table I) and DV that for water vapor (m2 s−1), GV is stomatal conductance for water vapor (mmol m−2 s−1), PS is the monoterpene partial pressure in substomatal cavities, Pa is the monoterpene partial pressure in the ambient air (Pa), P is the total air pressure, and E is the leaf transpiration rate (mmol m−2 s−1). The first part of the equation describes the control of F by stomata, and the second part of the flux results from mass flow attributable to net water efflux through the stomata. From Equation 2, the steady-state PS is given by:
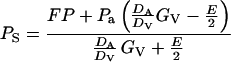 |
2 |
The transpiration correction to the entire flux is generally small. For example, for a typical non-stressed actively transpiring leaf with a GV of 200 mmol m−2 s−1, and moderate water vapor pressure deficit of 1.7 kPa, E equals to 3.4 mmol m−2 s−1, and the flux attributable to mass flow is 120 times less than the flux attributable to diffusion through the stomata. In a situation with closed stomata, the mass flow correction may be larger because of the rise of water vapor pressure deficit and E as the result of an increase in leaf temperature. However, even for a high vapor pressure deficit of 5 kPa and low GV of 5 mmol m−2 s−1, the mass flow correction is less than 2.5%. Thus, for simplicity, we neglect the contribution of mass flow. Further considering that no terpene build-up generally occurs in the boundary layer as well as in the ambient air, Pa is practically zero under natural conditions. Thus, Equation 2 simplifies to:
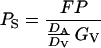 |
3 |
In addition to stomatal conductance, the gas phase monoterpene flux is also limited by the compound diffusion from the outer surface of cell walls to substomatal cavities. This part of the diffusion pathway is determined by the intercellular gas phase conductance, Gias. For the two conductances in series, the total gas phase diffusion conductance is given as:
 |
4 |
The internal conductance, Gias, essentially measures the average path-length from outer surface of cell walls to substomatal cavities (supplemental data can be viewed at www.plantphysiol.org) and differs between various monoterpenoids because of differing binary diffusion coefficients in air (Table I). The flux from the outer surface of the cell walls to the ambient air is further given as:
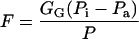 |
5 |
where Pi is the steady-state intercellular partial pressure of the volatile.
Analogously to CO2 diffusion (Laisk and Oja, 1998), we express the monoterpene efflux from the site of synthesis in chloroplasts to substomatal cavities, Fm, as:
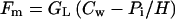 |
6 |
where GL is the liquid phase diffusion conductance (meters per second) for specific volatile from the site of synthesis to the outer surface of cell walls, Cw is the liquid phase monoterpene concentration in the site of synthesis (mol m−3). H, the Henry's law constant (Pa m3 mol−1; Table I), is the equilibrium air-water partition coefficient, which for dilute aqueous solutions is defined as (Staudinger and Roberts, 1996):
 |
7 |
where Ca (mol m−3) is the liquid phase monoterpene concentration at a monoterpene partial pressure of Pi. For environmental applications, aqueous solutions with less than 0.001 to 0.01 mol fraction of solute are considered dilute (Staudinger and Roberts, 1996). Use of H values is justified for all of the 19 monoterpenoid species emitted by Pinus pinea (Staudt et al., 1997), because the aqueous solubility of these compounds is typically in the range of 10−6 to 10−7 mol fraction at 25°C, and for the most soluble monoterpenoid emitted—1,8-cineole—the solubility is 3.44 × 10−4 mol fraction at 25°C (Table I; for the solubility data, see Staudinger and Roberts, 1996).
The internal liquid phase diffusion conductance, GL, is a composite conductance consisting of several conductances in series. This conductance is determined by the monoterpenoid liquid phase diffusion coefficient (Table I), permeabilities of plant membranes, and leaf anatomical characteristics. Supplemental data of calculation of the internal conductances of the diffusion pathway can be viewed at www.plantphysiol.org, and the diffusion conductances used in current simulations are provided in Table I. The values of GL vary because of varying liquid phase diffusion coefficients, but also because of differing membrane permeabilities of monoterpenoids. As the sensitivity analyses demonstrate (Ü. Niinemets and M. Reichstein, unpublished data), the dynamics of the VOC emission rates are not very sensitive to large changes in internal leaf conductance.
Dynamic Model of Monoterpene Diffusion through the Stomata
Given that the rate of monoterpene synthesis, I, is unaffected by modifications in gas phase conductance (GG, Eq. 4), I is equal to the diffusion flux density through the stomata and mesophyll in a steady-state situation, i.e. F = Fm = I (Eqs. 5 and 6). This means that any change in GG is balanced by an appropriate change in Pi such that F is equal before and after stomatal closure and that there could be no stomatal control on F in the steady state. Stomata may accordingly curtail F only in a non-steady-state situation, and the vital question to solve is how fast the leaf reaches a new steady state after a change in GG.
To simplify the analysis, we consider gas (SG, nmol m−2) and liquid (SL, nmol m−2) pools for each monoterpenoid, and use the mass balance approach to describe the dynamics of the pools (Fig. 1). The size of the gas pool is determined as:
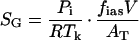 |
8 |
where R is the gas constant (8.314 J mol−1 K−1), Tk is leaf temperature (K), V (m3) is leaf volume, AT total leaf surface area, and fias the fraction of gas volume in total leaf volume. Thus, fiasV/AT gives the intercellular leaf volume per leaf surface area. The liquid pool size is given as:
 |
9 |
where fw is the liquid fraction of total leaf volume. All leaf structural data needed for model parameterization for P. pinea are provided in the electronic supplement, which can be viewed at www.plantphysiol.org.
Dynamics of the Gas and Liquid Phase Pools
Combining Equations 5 and 8, assuming that Pa is negligible, and revealing F leads to a first order kinetics of the gas pool:
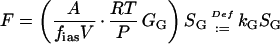 |
10 |
where kG (s−1) is the rate constant of the gas phase, and the half-time of the gas pool, τG, is:
 |
11 |
The following analysis may be significantly simplified if we could consider SG as essentially in a steady state, i.e. if the values of τG are very small relative to the time constants of stomatal closure and opening. According to the “Results” (Fig. 2), the gas phase pool half-time is on the order of seconds. Given that the half-time of stomatal movements is on the order of minutes (Tinoco-Ojanguren and Pearcy, 1993), the gas phase is effectively in a steady state. Thus, Fm = F, allowing the substitution of Pi from Equation 5 into Equation 6, giving:
 |
12 |
Replacing Cw = SLA/(fwV) from Equation 9 in Equation 12, the governing differential equation becomes:
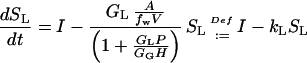 |
13 |
The product kLSL is the flux into the gas pool and, because the gas pool is in a steady state, also the emission flux. Thus, the efflux from the liquid pool obeys a first order kinetics, where the rate constant kL depends on the GL and GG and on the H. The analytical solution of this differential equation is:
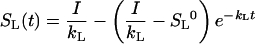 |
14 |
with SL0 being the pool size at t = 0. The analytical solution is applicable for simulations with a constant kL and I. In all other cases, numerical solutions were employed.
Field Measurements
Study Site, Foliar Monoterpenoid Emission, and CO2 and H2O Exchange Measurements
The research was conducted at Castelporziano (Rome, 41°45′N, 12°26′E) mixed evergreen forest in frames of the Biogenic Emissions in the Mediterranean Area (BEMA) project (see Seufert et al., 1997). Enders et al. (1997) provide a detailed description of the stand, which is dominated by P. pinea (50%–60% coverage) and Quercus ilex (10%–20% coverage). Some of the results of the Castelporziano field campaigns have been published previously (Staudt et al., 1997). The current study analyses data from intensive field campaigns conducted by the Environment Institute, Joint Research Centre of the European Commission, Italy (JRC) (Bertin et al., 1997; Staudt et al., 1997) during June 1 to 14, 1993; May 5 to 28, 1994; July 31 to August 6, 1994; and October 23 to 27, 1994.
The techniques for foliar photosynthesis and transpiration and terpenoid emission measurements have been reported in full detail in Bertin et al. (1997) and in Staudt et al. (1997). The cylindrical gas exchange chambers (volume of either 0.02 or 0.05 m3) consisted of a Plexiglas frame supporting a 50-μm-thick, transparent Teflon foil (Nowofol Kunststoffprodukte, Siegsdorf, Germany). The chambers were installed on proximal branch positions in the upper crown at a height of 9 m in the forest of 8 to 12 m total height. The cuvettes enclosed 0.1 to 0.4 m2 of total needle surface area, and multiple trees were sampled simultaneously. Depending on chamber size, the flow rate of charcoal-filtered ambient air (CO2 concentrations around 350 μmol mol−1) was maintained at 0.03 to 0.06 m3 min−1 with a thermal mass flow controller (MKS Instruments, Andover, MA) to yield a mean air residence time of 0.5 to 2 min. Chamber inlet and outlet CO2 concentrations were determined with an infrared CO2 analyzer (BINOS 100, Fisher-Rosemount, Hasselroth, Germany) operated in an absolute mode, and water vapor concentrations were measured with a set of dew point mirrors (MTS-2, H. Walz, Effeltrich, Germany). Foliar gas exchange parameters were computed according to von Caemmerer and Farquhar (1981). Needles enclosed in the cuvette were harvested at the end of the measurement campaign, and the projected surface area was measured with an optical planimeter (Delta-T, Cambridge, UK). An experimentally determined total to projected needle area ratio of 2.25 was used to convert the projected areas to total needle area (Staudt et al., 1997), and foliage photosynthesis rates, GV values, and monoterpenoid emission rates were expressed on the total area basis.
Several labs participated in monoterpenoid sampling from the chamber air and latter monoterpenoid determination. In the current study, the data provided by the JRC, by the Universite Joseph Fourier, Groupe de Recherche sur l'Environnement et la Chimie Appliquée, Grenoble, France (GRECA), and by the Institut National Polytechnique, Ecole Nationale Supérieure de Chimie de Toulouse, France (ENSCT), were used. A comparison with blind monoterpene mixtures between these three and seven other laboratories participating in the BEMA project revealed that the analytical monoterpene sampling and analysis methods allowed efficient detection of most monoterpenes emitted by plants in all laboratories (Larsen et al., 1997). Despite qualitatively similar results, there were occasionally relatively large differences in absolute amounts of various monoterpenoids determined (Larsen et al., 1997). Therefore, we used only complete daily time courses sampled and analyzed by the same group.
Bertin et al. (1997; see also Larsen et al., 1997) gives an overview of the monoterpenoid determination methods employed by JRC, GRECA, and ENSCT. We provide here the outline of the analytical methods for all of these groups, and we provide details of the JRC group protocols, because our conclusions primarily rely on these data. In all cases, monoterpenoids were trapped with Tenax TA adsorbent resin (20–35 mesh, surface area of 35 m2 g−1; Alltech Associates, Deerfield, IL). Tenax TA was selected because it has been demonstrated to completely adsorb all plant monoterpenoids and to release them by thermal desorption without decomposition (Ciccioli et al., 1992). Either glass (JRC) or stainless steel tubes (GRECA) were used for trapping, and the gas-chromatographic analysis with flame ionization detector was independent of trapping and was conducted later in the laboratory (JRC and GRECA). On-line gas-chromatographic analysis including an automated adsorption-desorption device was alternatively employed (ENSCT). To avoid adsorbent trap breakthrough, the air flow rate through the sampling tube was controlled at 0.15 to 0.2 dm3 min−1, and 3 to 6 L of air was sampled. Thus, each sample was a weighted average of a 15- to 40-min time period. Given also the 5- to 10-min bypass periods before and after sampling, one or two samples per hour were obtained. Gas chromatographic analysis (gas-chromatograph CP9001, Chrompack, Varian Medical Systems, Palo Alto, CA) of samples by the JRC group included precooling at −100°C for 3 min, desorption at 200°C (TCT/PTI CP4001, Chrompack) for 10 min, and injection at 200°C for 1 min. After injection, the 25-m column (0.32-mm capillary column coated with 1.2-μm Chrompack CP-Sil 8 CB) was maintained at 65°C for 4 min, followed by 2.5°C min−1 to 80°C, 2.0°C min−1 to 100°C, and 20°C min−1 to 240°C. Purified monoterpene standards (Aldrich Chemie, Steinheim, Germany) were used for monoterpenoid identification and calibration (Bertin et al., 1997). Overall, the detection limit was less than 1 pmol m−2 s−1 for all monoterpenoids.
Empirical Fitting of Diurnal Courses of Monoterpenoid Emission
Because the monoterpene emission rates depend both on incident quantum flux density and temperature in P. pinea (Staudt et al., 1997; Sabillón and Cremades, 2001), we employed an empirical emission algorithm of Guenther et al. (1993), which has been demonstrated to successfully simulate isoprene emission in a broad variety of species (Guenther et al., 1993) as well as monoterpenoid emission rates in Q. ilex (Bertin et al., 1997; Ciccioli et al., 1997). In P. pinea, the application of the isoprene emission algorithm is supported by close to zero monoterpene emission rates at night, indicating that the efflux from the storage pools contributes negligibly to the total emission rate.
According to the model, the emission rate of a specific monoterpenoid, F (nmol m−2 s−1), is given as:
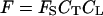 |
15 |
where CT is the temperature correction factor, CL is the light correction factor, and FS is the basal emission rate measured in standard conditions (emission factor). As a rule, FS is estimated at a leaf temperature (TL) of 30°C and incident quantum flux density (Q) of 1,000 μmol m−2 s−1. Implicit in Equation 15 is that stomata exert no control over monoterpenoid emission. We use the equation of Tingey et al. (1980) for CT:
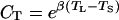 |
16 |
where TS is the leaf temperature in standard conditions (30°C), and β an empirical parameter determining the shape of the F versus TL response curve. Although the values of β may vary depending on the physicochemical properties of specific monoterpenoids as well as temperature characteristics of various monoterpenoid synthases, an estimate of β = 0.09°C−1 appears applicable for a wide range of species and monoterpenoids (Guenther et al., 1993) and was used in the current study. The original model (Guenther et al., 1993) includes a more complex five-parameter temperature function to describe the decrease of emission rates in supra-optimal temperatures. However, we favor Equation 16 in our model exercise, because no appreciable decrease in emission rates was observed even under the highest temperatures of 35°C to 40°C during the measurements in conditions of high soil water availability. The light correction factor is calculated as:
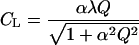 |
17 |
where α and λ are empirical parameters describing the shape of the F versus Q response curve. We used values of α = 0.0027 and λ = 1.066, which were originally determined for isoprene-emitting species (Guenther et al., 1993) and later demonstrated to provide good fits to monoterpenoids emitted by Q. ilex (Ciccioli et al., 1997). Having determined CT and CL, the emission factor (FS, Eq. 15), was computed as an average for the entire measurement campaign using the morning measurements (9 am–12 pm).
Supplementary Material
ACKNOWLEDGMENTS
We thank Profs. Agu Laisk (University of Tartu, Estonia) and Thomas D. Sharkey (University of Wisconsin, Madison) for their invaluable comments on the study.
Footnotes
This work was supported by the European Commission (BEMA, DG XII/D-1, and VOCAMOD; contract no. ENV4–CT97–0424), by the Estonian Science Foundation (grant nos. 3525 and 4584), and by the German Federal Minister of Research and Technology (grant nos. BEO 51–0339476A and EST 001–98).
The online version of this article contains Web-only data. The supplemental material is available at www.plantphysiol.org.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.009670.
LITERATURE CITED
- Bertin N, Staudt M, Hansen U, Seufert G, Ciccioli P, Foster P, Fugit JL, Torres L. Diurnal and seasonal course of monoterpene emissions from Quercus ilex (L.) under natural conditions: applications of light and temperature algorithms. Atmos Environ. 1997;31:135–144. [Google Scholar]
- Chappell J. Biochemistry and molecular biology of the isoprenoid biosynthetic pathways in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:521–547. [Google Scholar]
- Ciccioli P, Cecinato A, Brancaleoni E, Frattoni M, Liberti A. Use of carbon adsorption traps combined with high resolution gas chromatography-mass spectrometry for the analysis of polar and non-polar C4-C14 hydrocarbons involved in photochemical smog pollution. J High Resolut Chromatogr. 1992;15:75–84. [Google Scholar]
- Ciccioli P, Fabozzi C, Brancaleoni E, Cecinato A, Frattoni M, Loreto F, Kesselmeier J, Schäfer L, Bode K, Torres L et al. Use of the isoprene algorithm for predicting the monoterpene emission from the Mediterranean holm oak Quercus ilex L.: performance and limits of this approach. J Geophys Res. 1997;102:23319–23328. [Google Scholar]
- Croteau R. Biosynthesis and catabolism of monoterpenoids. Chem Rev. 1987;87:929–954. [Google Scholar]
- Davis EM, Croteau R. Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes. In: Leeper FJ, Vederas JC, editors. Topics in Current Biochemistry. 209. Biosynthesis: Aromatic Polyketides, Isoprenoids, Alkaloids. Berlin: Springer-Verlag; 2000. pp. 53–95. [Google Scholar]
- Enders G, Kotzias D, Seufert G. General methods used during the Castelporziano campaigns. Atmos Environ. 1997;31:27–34. [Google Scholar]
- Fall R, Monson RK. Isoprene emission rate and intercellular isoprene concentration as influenced by stomatal distribution and conductance. Plant Physiol. 1992;100:987–992. doi: 10.1104/pp.100.2.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. Annu Rev Plant Physiol. 1982;33:317–345. [Google Scholar]
- Fehsenfeld F, Calvert J, Fall R, Goldan P, Guenther AB, Hewitt CN, Lamb B, Liu S, Trainer M, Westberg H et al. Emissions of volatile organic compounds from vegetation and the implications for atmospheric chemistry. Global Biogeochem Cycles. 1992;6:389–430. [Google Scholar]
- Field CB, Ball JT, Berry JA. Photosynthesis: principles and field techniques. In: Pearcy RW, Ehleringer JR, Mooney HA, Rundel PW, editors. Plant Physiological Ecology. Field Methods and Instrumentation. London: Chapman and Hall; 1989. pp. 209–253. [Google Scholar]
- Gershenzon J, Croteau RB. Terpenoid biosynthesis: the basic pathway and formation of monoterpenes, sesquiterpenes, and diterpenes. In: Moore TS, editor. Lipid Metabolism in Plants. Boca Raton, FL: CRC Press; 1993. pp. 339–388. [Google Scholar]
- Guenther AB, Zimmerman PR, Harley PC, Monson RK, Fall R. Isoprene and monoterpene emission rate variability: model evaluations and sensitivity analyses. J Geophys Res. 1993;98:12609–12617. [Google Scholar]
- Guenther A, Zimmerman PR, Wildermuth M. Natural volatile organic compound emission rates for U.S. woodland landscapes. Atmos Environ. 1994;28:1197–1210. [Google Scholar]
- Holzinger R, Sandoval-Soto L, Rottenberger S, Crutzen PJ, Kesselmeier J. Emissions of volatile organic compounds from Quercus ilex L. measured by proton transfer reaction mass spectrometry under different environmental conditions. J Geophys Res. 2000;105:20573–20579. [Google Scholar]
- Janson RW. Monoterpene emissions from Scots pine and Norwegian spruce. J Geophys Res. 1993;98:2839–2850. [Google Scholar]
- Juuti S, Arey J, Atkinson R. Monoterpene emission rate measurements from a Monterey pine. J Geophys Res. 1990;95:7515–7519. [Google Scholar]
- Kesselmeier J, Bode K, Hofmann U, Müller H, Schäfer L, Wolf A, Ciccioli P, Brancaleoni E, Cecinato A, Frattoni M et al. Emission of short chained organic acids, aldehydes and monoterpenes from Quercus ilex L. and Pinus pinea L. in relation to physiological activities, carbon budget and emission algorithms. Atmos Environ. 1997;31:119–133. [Google Scholar]
- Kesselmeier J, Schäfer L, Ciccioli P, Brancaleoni E, Cecinato A, Frattoni M, Foster P, Jacob V, Denis J, Fugit JL et al. Emission of monoterpenes and isoprene from a Mediterranean oak species Quercus ilex L. measured within the BEMA (biogenic emissions in the Mediterranean area) project. Atmos Environ. 1996;30:1841–1850. [Google Scholar]
- Kesselmeier J, Staudt M. Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J Atmos Chem. 1999;33:23–88. [Google Scholar]
- Kozaki A, Takeba G. Photorespiration protects C3 plants from photooxidation. Nature. 1996;384:557–560. [Google Scholar]
- Laisk A, Oja V. Techniques in Plant Sciences. 1. Dynamics of Leaf Photosynthesis: Rapid-Response Measurements and Their Interpretations. Canberra, Australia: CSIRO Publishing; 1998. [Google Scholar]
- Larsen B, Bomboi-Mingarro T, Brancaleoni E, Calogirou A, Cecinato A, Coeur C, Chatzianestis I, Duane M, Frattoni M, Fugit J-L et al. Sampling and analysis of terpenes in air: an interlaboratory comparison. Atmos Environ. 1997;31:35–49. [Google Scholar]
- Lichtenthaler HK. The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:47–65. doi: 10.1146/annurev.arplant.50.1.47. [DOI] [PubMed] [Google Scholar]
- Llusiá J, Peñuelas J. Seasonal patterns of terpene content and emission from seven Mediterranean woody species in field conditions. Am J Bot. 2000;87:133–140. [PubMed] [Google Scholar]
- Loreto F, Ciccioli P, Cecinato A, Brancaleoni E, Frattoni M, Fabozzi C, Tricoli D. Evidence of the photosynthetic origin of monoterpenes emitted by Quercus ilex L. leaves by 13C labeling. Plant Physiol. 1996a;110:1317–1322. doi: 10.1104/pp.110.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Ciccioli P, Cecinato A, Brancaleoni E, Frattoni M, Tricoli D. Influence of environmental factors and air composition on the emission of α-pinene from Quercus ilex leaves. Plant Physiol. 1996b;110:267–275. doi: 10.1104/pp.110.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes F, Seufert G, Vitale M. Ecophysiological studies of Mediterranean plant species at the Castelporziano estate. Atmos Environ. 1997;31:51–60. [Google Scholar]
- Meylan WM, Howard PH. Computer estimation of the atmospheric gas-phase reaction rate of organic compounds with hydroxyl radicals and ozone. Chemosphere. 1993;26:2293–2299. [Google Scholar]
- Monson RK, Fall R. Isoprene emission from aspen leaves: influence of environment and relation to photosynthesis and photorespiration. Plant Physiol. 1989;90:267–274. doi: 10.1104/pp.90.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemecek-Marshall M, MacDonald RC, Franzen JJ, Wojciechowski CL, Fall R. Methanol emission from leaves: enzymatic detection of gas-phase methanol and relation of methanol fluxes to stomatal conductance and leaf development. Plant Physiol. 1995;108:1359–1368. doi: 10.1104/pp.108.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Hauff K, Bertin N, Tenhunen JD, Steinbrecher R, Seufert G. Monoterpene emissions in relation to foliar photosynthetic and structural variables in Mediterranean evergreen Quercus species. New Phytol. 2002a;153:243–256. [Google Scholar]
- Niinemets Ü, Seufert G, Steinbrecher R, Tenhunen JD. A model coupling foliar monoterpene emissions to leaf photosynthetic characteristics in Mediterranean evergreen Quercus species. New Phytol. 2002b;153:257–276. [Google Scholar]
- Niinemets Ü, Tenhunen JD, Harley PC, Steinbrecher R. A model of isoprene emission based on energetic requirements for isoprene synthesis and leaf photosynthetic properties for Liquidambar and Quercus. Plant Cell Environ. 1999;22:1319–1336. [Google Scholar]
- Sabillón D, Cremades LV. Diurnal and seasonal variation of monoterpene emission rates for typical Mediterranean species (Pinus pinea and Quercus ilex) from field measurements: relationship with temperature and PAR. Atmos Environ. 2001;35:4419–4431. [Google Scholar]
- Schuh G, Heiden AC, Hoffmann T, Kahl J, Rockel P, Rudolph J, Wildt J. Emissions of volatile organic compounds from sunflower and beech: dependence on temperature and light intensity. J Atmos Chem. 1997;27:291–318. [Google Scholar]
- Schürmann W. Emission von Monoterpenen aus Nadeln von Picea abies (L.) Karst. sowie deren Verhalten in der Atmosphäre. Dr. Rer. Nat. thesis. München, Germany: Fakultät für Chemie, Biologie und Geowissenchaften der Technischen Universität; 1993. [Google Scholar]
- Schürmann W, Ziegler H, Kotzias D, Schönwitz R, Steinbrecher R. Emission of biosynthesized monoterpenes from needles of Norway spruce. Naturwissenschaften. 1993;80:276–278. [Google Scholar]
- Seufert G, Bartzis J, Bombol T, Ciccioli P, Cieslik S, Dlugi R, Foster P, Hewitt CN, Kesselmeier J, Kotzias D et al. An overview of the Castelporziano experiments. Atmos Environ. 1997;31:5–17. [Google Scholar]
- Shao M, Czapiewski KV, Heiden AC, Kobel K, Komenda M, Koppmann R, Wildt J. Volatile organic compound emissions from Scots pine: mechanisms and description by algorithms. J Geophys Res. 2001;106:20483–20491. [Google Scholar]
- Sharkey TD. Stomatal control of trace gas emissions. In: Sharkey TD, Holland EA, Mooney HA, editors. Physiological Ecology. A Series of Monographs, Texts, and Treatises: Trace Gas Emissions by Plants. San Diego: Academic Press; 1991. pp. 335–339. [Google Scholar]
- Simpson D. Biogenic emissions in Europe: 2. Implications for ozone control strategies. J Geophys Res. 1995;100:22891–22906. [Google Scholar]
- Staudinger J, Roberts PV. A critical review of Henry's law constants for environmental applications. Crit Rev Environ Sci Technol. 1996;26:205–297. [Google Scholar]
- Staudt M, Bertin N. Light and temperature dependence of the emission of cyclic and acyclic monoterpenes from holm oak (Quercus ilex L.) leaves. Plant Cell Environ. 1998;21:385–395. [Google Scholar]
- Staudt M, Bertin N, Frenzel B, Seufert G. Seasonal variation in amount and composition of monoterpenes emitted by young Pinus pinea trees: implications for emission modeling. J Atmos Chem. 2000;35:77–99. [Google Scholar]
- Staudt M, Bertin N, Hansen U, Seufert G, Ciccioli P, Foster P, Frenzel B, Fugit J-L. Seasonal and diurnal patterns of monoterpene emissions from Pinus pinea (L.) under field conditions. Atmos Environ. 1997;31:145–156. [Google Scholar]
- Staudt M, Seufert G. Light-dependent emission of monoterpenes by holm oak (Quercus ilex L.) Naturwissenschaften. 1995;82:89–92. [Google Scholar]
- Steinbrecher R. Gehalt und Emission von Monoterpenen in oberirdischen Organen von Picea abies (L.) Karst. Dr. rer. Nat. thesis. Germany: Institut für Botanik und Mikrobiologie, Lehrstuhl für Botanik der Technischen Universität München; 1989. [Google Scholar]
- Steinbrecher R, Ziegler H. Monoterpene production by trees. In: Rennenberg H, Eschrich W, Ziegler H, editors. Trees: Contributions to Modern Tree Physiology. Leiden, The Netherlands: Backhuys Publishers; 1997. pp. 119–138. [Google Scholar]
- Tingey DT, Manning M, Grothaus LC, Burns WF. Influence of light and temperature on monoterpene emission rates from slash pine. Plant Physiol. 1980;65:797–801. doi: 10.1104/pp.65.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingey DT, Turner DP, Weber JA. Factors controlling the emissions of monoterpenes and other volatile organic compounds. In: Sharkey TD, Holland EA, Mooney HA, editors. Physiological Ecology. A Series of Monographs, Texts, and Treatises: Trace Gas Emissions by Plants. San Diego: Academic Press; 1991. pp. 93–119. [Google Scholar]
- Tinoco-Ojanguren C, Pearcy RW. Stomatal dynamics and its importance to carbon gain in two rainforest Piper species: II. Stomatal versus biochemical limitations during photosynthetic induction. Oecologia. 1993;94:395–402. doi: 10.1007/BF00317115. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Winer AM, Karlik J. Development and validation of databases for modeling biogenic hydrocarbon emissions in California's air sheds. Contract no. 97–320. Los Angeles: California Air Resources Board, California Environmental Protection Agency; 2001. [Google Scholar]
- Zimmer W, Brüggemann N, Emeis S, Giersch C, Lehning A, Steinbrecher R, Schnitzler J-P. Process-based modelling of isoprene emission by oak leaves. Plant Cell Environ. 2000;23:585–595. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.