Abstract
A voltage-independent cation (VIC) channel has been identified in the plasma membrane of wheat (Triticum aestivum) root cells (P.J. White [1999] Trends Plant Sci 4: 245–246). Several physiological functions have been proposed for this channel, including roles in cation nutrition, osmotic adjustment, and charge compensation. Here, we observe that Ca2+ permeates this VIC channel when assayed in artificial, planar lipid bilayers, and, using an energy barrier model to describe cation fluxes, predict that it catalyzes Ca2+ influx under physiological ionic conditions. Thus, this channel could participate in Ca2+ signaling or cytosolic Ca2+ homeostasis. The pharmacology of 45Ca2+ influx to excised wheat roots and inward cation currents through the VIC channel are similar: Both are insensitive to 20 μm verapamil or 1 mm tetraethylammonium, but inhibited by 0.5 mm Ba2+ or 0.5 mm Gd3+. The weak voltage dependency of the VIC channel (and its lack of modulation by physiological effectors) suggest that it will provide perpetual Ca2+ influx to root cells. Thus, it may effect cytosolic Ca2+ homeostasis by contributing to the basal Ca2+ influx required to balance Ca2+ efflux from the cytoplasm through ATP- and proton-coupled Ca2+ transporters under steady-state conditions.
Cation channels in the plasma membrane of root cells perform a variety of functions (White, 1997). The physiological roles of inward-rectifying K+ channels that facilitate nutritional K+ uptake (Hirsch et al., 1998), outward-rectifying K+ channels that provide charge compensation during membrane depolarization and mediate K+ efflux to the xylem (Gaymard et al., 1998), and Ca2+ channels involved in cell signaling (White, 2000) have all been substantiated. In contrast, the role(s) of voltage-independent cation (VIC) channels in the plasma membrane of root cells is unknown (White, 1999a; Demidchik et al., 2002).
VICs have been characterized after the incorporation of plasma membrane vesicles from both rye (Secale cereale; White and Tester, 1992) and wheat (Triticum aestivum; Davenport and Tester, 2000) roots into artificial, planar lipid bilayers (PLBs) and by patch clamping root cell protoplasts (White and Lemtiri-Chlieh, 1995; Roberts and Tester, 1997; Tyerman et al., 1997; Buschmann et al., 2000; Maathuis and Sanders, 2001; Demidchik and Tester, 2002). When studied in PLBs, VIC channels are open 60% to 80% of the time at voltages more positive than about −120 mV, but their probability of being open (Po) may decrease at more negative voltages (White and Tester, 1992; Davenport and Tester, 2000). They are permeable to a range of monovalent cations with the selectivity sequence NH4+ > Rb+ ≥ K+ > Cs+ > Na+ > Li+ > tetraethylammonium (TEA+; White and Tester, 1992; White, 1996; Roberts and Tester, 1997; Tyerman et al., 1997; Davenport and Tester, 2000; Demidchik and Tester, 2002). This catholic permeability identifies VIC channels as nonselective cation channels (Davenport and Tester, 2000; Demidchik et al., 2002). Inward currents through VIC channels are insensitive to TEA+, verapamil, and nifedipine, but partially inhibited by Ca2+, Ba2+, Gd3+, and, in some plant species, quinine (White and Tester, 1992; White and Lemtiri-Chlieh, 1995; Roberts and Tester, 1997; Tyerman et al., 1997; Davenport and Tester, 2000; Demidchik and Tester, 2002). Based on their permeability to monovalent cations, it has been speculated that VIC channels could contribute to low-affinity NH4+ uptake (White, 1996), that cation fluxes through VIC channels could allow rapid osmotic adjustment independent of the membrane potential (White, 1997; Tyerman and Skerrett, 1999), and that VIC channels could provide compensatory cation fluxes for the many electrogenic transport processes occurring across the plasma membrane of root cells (White, 1997; Amtmann and Sanders, 1999; Tyerman and Skerrett, 1999). It has also been argued that VIC channels mediate most of the Na+ (Amtmann and Sanders, 1999; Tyerman and Skerrett, 1999; White, 1999a) and Cs+ (White and Broadley, 2000; Broadley et al., 2001) influx to root cells.
Several observations indicate that VIC channels are also permeable to Ca2+. First, VIC channels from the plasma membrane of wheat roots have an appreciable Ca2+ conductance when studied after incorporation into PLB and assayed in bi-ionic (cytoplasmic:extracellular) 100 mm NaCl:50 mm CaCl2 or 100 mm KCl:50 mm CaCl2. This yielded permeability ratios for Ca2+ versus Na+ (PCa:PNa) of 0.21 (Davenport and Tester, 2000) and for Ca2+ versus K+ (PCa:PK) of 0.04 (Davenport, 1998) calculated using the equation of Fatt and Ginsborg (1958). Second, the addition of Ca2+ to monovalent cation solutions bathing the extracellular side of the VIC channel results in a positive shift in the zero current (reversal) potential (Erev; Davenport, 1998). Third, VIC channels from the plasma membrane of rye roots have a measurable Ca2+ conductance (6.05 ± 0.74 pS, n = 5) when incorporated into PLB and assayed in the presence of 100 mm CaCl2 (P.J. White, unpublished data). In this paper, Ca2+ currents through VIC channels from the plasma membrane of wheat roots assayed in PLB have been quantified using an energy barrier model for cation permeation (Hille, 2001). The predictions of this model have been used to investigate possible roles for these channels in cytosolic Ca2+ ([Ca2+]cyt) homeostasis or [Ca2+]cyt signaling.
RESULTS
Proposed 3B2S Model for Cation Permeation
The permeation of monovalent cations through the VIC channel in the plasma membrane of rye roots has been modeled using a free-energy profile composed of three energy barriers and two ion-binding sites (energy wells). This 3B2S model assumed single-file permeation through a pore, which could be occupied at most by two cations that repelled each other, and allowed for surface potential effects in the vestibules to the pore (White and Ridout, 1995; White, 1996). This model was chosen because the channel exhibited: (a) rectification in unitary conductance when identical solutions bathed both sides of the channel, (b) non-Michaelian relationships between unitary conductance and cation concentration, and (c) apparent relative permeabilities for cations that changed with concentration. Similar phenomena have been observed for the VIC channel in the plasma membrane of wheat roots (Davenport, 1998; Davenport and Tester, 2000), suggesting that cation permeation through the pore of the wheat VIC channel might also be fitted using a 3B2S model. In addition to estimating the free-energy profiles for Na+ and K+ permeation through the VIC channel in the plasma membrane of wheat roots, the free-energy profile for Ca2+ permeation was also estimated (Table I; Fig. 1).
Table I.
Estimated parameters for the permeation of K+, Na+, and Ca2+ through the pore of the VIC channel in the plasma membrane of wheat roots
| Parameter | K+ | Na+ | Ca2+ |
|---|---|---|---|
| G1 | 6.97 ± 0.080 | 5.95 ± 0.138 | 9.18 ± 0.315 |
| G2 | 3.11 ± 0.323 | 7.49 ± 0.110 | 6.48 ± 0.990 |
| G3 | 5.00* | 5.00* | 8.92 ± 0.228 |
| U1 | −5.39 ± 0.140 | −5.50 ± 0.136 | −6.69 ± 0.302 |
| U2 | −3.76 ± 0.776 | −3.25 ± 0.159 | −5.00* |
| A | 5.00* | ||
| D1 | 0.07 ± 0.021 | ||
| D2 | 0.31 ± 0.087 | ||
| D3 | 0.61 ± 0.049 | ||
| D4 | 0.66* | ||
| D5 | 0.70* | ||
| Rstrans | 16.27 ± 0.390 | ||
| Rscis | 11.59 ± 0.550 |
Permeation was modeled as single-file movement through a free-energy profile with three energy barriers and two ion-binding sites that could be occupied simultaneously. Parameters were estimated from unitary current versus voltage relationships collected across a wide variety of ionic conditions. The total no. of observations was 624 and the weighted residual sum of squares was 200.51. ses of the parameters are indicated. Reliable estimates of the ses of values marked with asterisks are not available. These parameters could not be estimated with any precision and were fixed to their optimal values. The parameters G1, U1, G2, U2, and G3 define the free energies at positions D1, D2, D3, D4, and D5, respectively. They are expressed in (dimensionless) multiples of thermal energy (RT). The solution reference state is 55.5 m. The postscript refers to their position relative to the trans- (cytoplasmic) compartment. The parameter A defines the magnitude of ionic interactions, which raise the free energy profile by A(z1z2)/d, where z1 and z2 are the valencies of the interacting cations and d is the electrical distance from the occupied well. The parameters Rstrans and Rscis (expressed in Å) correspond to the radii of circles containing one electron charge in the trans- and cis-vestibules of the pore, respectively.
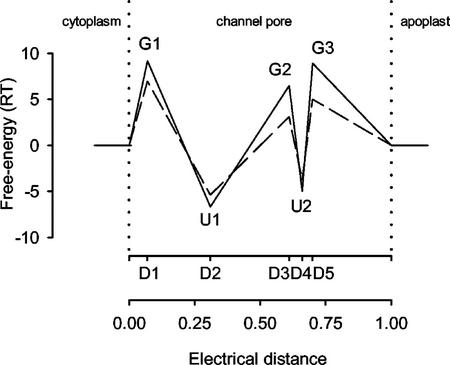
Figure 1.
Schematic representation of the free-energy profiles for K+ (− − −) and Ca2+ (——) permeation through the pore of an unoccupied VIC channel in the plasma membrane of a wheat root cell in the absence of an electric field. The positions of free-energy maxima (G1–G3) and minima (U1 and U2) are indicated (D1–D5). The solution reference state is 55.5 m.
The proposed 3B2S model for the VIC channel in the plasma membrane of wheat roots indicated that free-energy peaks and wells for cation permeation were situated asymmetrically within the pore (Table I; Fig. 1). The free-energy profiles for Na+ and K+ permeation were similar to each other and differed from that for Ca2+. The free-energy peaks (G1–G3) were generally lower for Na+ and K+ than for Ca2+, and the free-energy wells (U1 and U2) were higher for Na+ and K+ than for Ca2+. The proposed 3B2S model also indicated considerable surface charge in the vestibules of the pores and significant interactions between cations within the pore.
In general, the placement of free-energy peaks and wells in the pore of the wheat VIC channel was similar to that proposed for the rye VIC channel (White, 1997), except that the energy peak adjacent to the extracellular medium (D5) was situated further into the pore than its rye counterpart. However, the proposed free-energy peaks and wells for Na+ and K+ permeation of the wheat VIC channel were universally higher than those proposed for the rye VIC channel, and considerable surface charge was indicated in the vestibules to the pore of the wheat VIC channel, but not the rye VIC channel. These differences may have arisen because the wheat VIC channel was assayed in a negatively charged bilayer, whereas the rye VIC channel was assayed in an uncharged phosphatidylethanolamine (PE) bilayer. Consistent with this notion, the apparent Km for the relationship between unitary conductance and Na+ activity for the wheat VIC channel was much lower in negatively charged bilayers composed of PE/phosphatidyl-Ser/phosphatidylcholine than in those composed solely of PE (Davenport, 1998). It is likely that a mixed-lipid, negatively charged bilayer will resemble the plasma membrane of a root cell more closely than an uncharged PE bilayer.
Monovalent Cations Permeate the VIC Channel
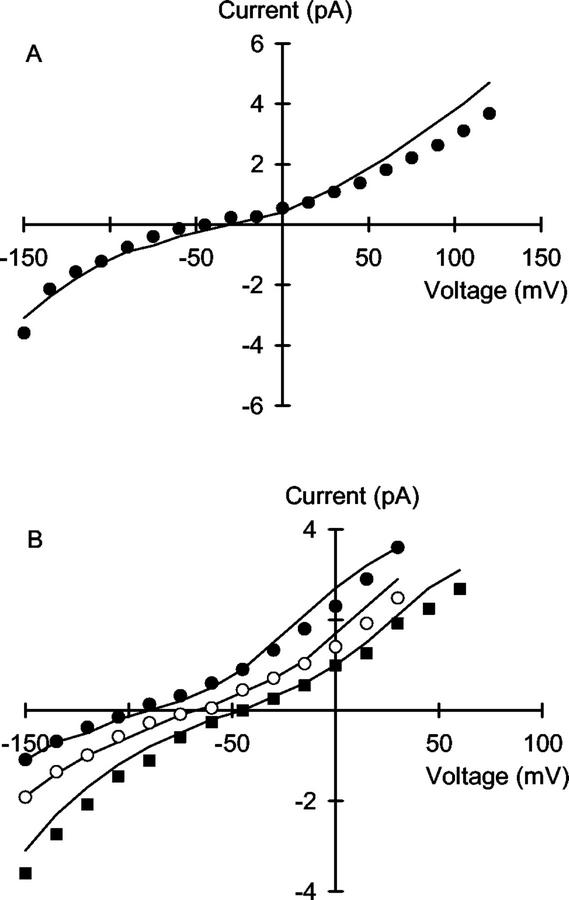
The VIC channel in the plasma membrane of wheat roots was permeable to both Na+ and K+. The 3B2S model proposed for this channel accurately described the rectification of unitary current when Na+ was the sole charge carrier (Fig. 2). It also fitted the magnitude of the unitary conductance, and the low apparent Km inherent in the relationship between unitary conductance and Na+ activity when identical NaCl solutions bathed both sides of the channel (Fig. 3). It also accurately described the rectifying unitary current versus voltage (I/V) relationships obtained under bi-ionic (cis:trans) KCl:NaCl (Fig. 4A). However, under the reciprocal ionic conditions (bi-ionic cis:trans NaCl:KCl), the I/V relationships predicted by the proposed 3B2S model deviated slightly from the experimental data (Fig. 4B). This was most apparent at low-NaCl concentrations, when the rectification of the unitary current was underestimated. The reasons for this are unknown. Apparent permeability ratios for PNa:PK of 0.60, 0.89, and 0.78 were calculated from Erev obtained under bi-ionic equimolar (cis:trans) 100 mm NaCl:KCl (Erev = −12.9 mV), 10 mm KCl:NaCl (Erev = 3.0 mV), and 100 mm KCl:NaCl (Erev = 6.4 mV) using the Fatt and Ginsborg (1958) transformation of the Goldman-Hodgkin-Katz equation. However, because the apparent PNa:PK changes with concentration, it is inappropriate to describe cation permeation of the channel in terms of the Goldman-Hodgkin-Katz equation (Hille, 2001) and permeability ratios are presented here for comparative purposes only.
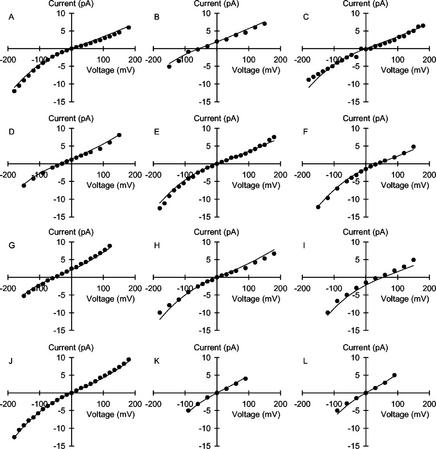
Figure 2.
Unitary current versus voltage relationships for the VIC channel in the plasma membrane of wheat roots. Solutions contained (cis:trans): A, 1:1; B, 1:10; C, 5:5; D, 10:50; E, 10:10; F, 50:10; G, 10:100; H, 50:50; I, 100:10; J, 100:100; K, 250:250; and L, 500:500 mm NaCl. The curves are derived from a 3B2S model for cation permeation using the parameters given in Table I.
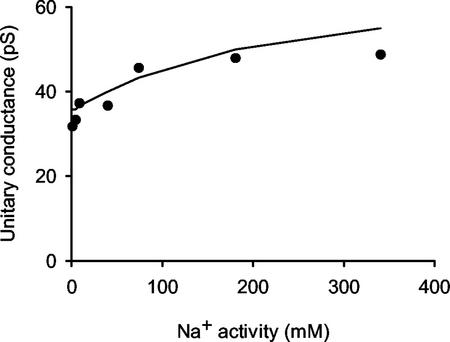
Figure 3.
The relationship between the unitary chord conductance (determined between −60 mV and +60 mV) of the VIC channel in the plasma membrane of wheat roots and Na+ activity. The channel was incorporated in a PLB separating identical NaCl solutions. The curve was derived from a 3B2S model for cation permeation using the parameters given in Table I.
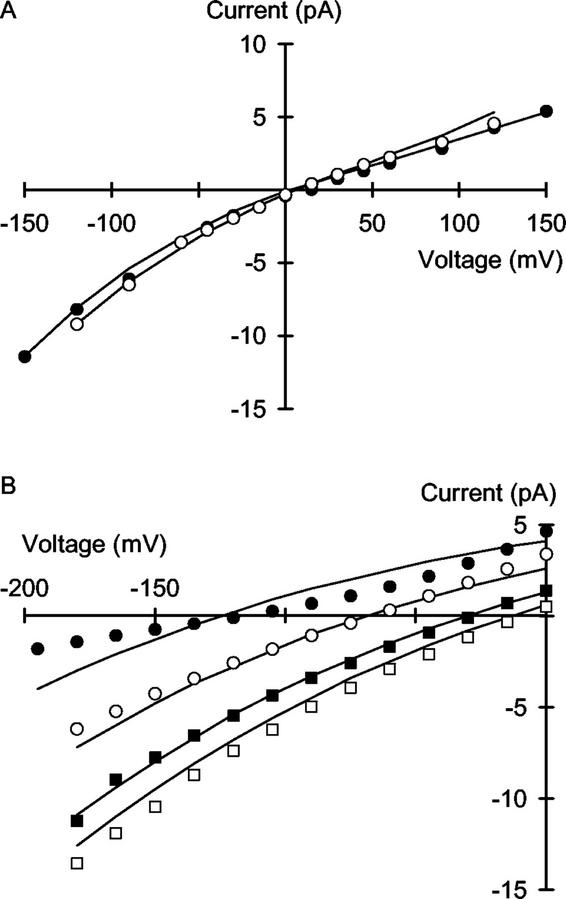
Figure 4.
Unitary current versus voltage relationships for the VIC channel in the plasma membrane of wheat roots assayed in the presence of (cis:trans): A, 10 mm KCl:10 mm NaCl (●) or 100 mm KCl:100 mm NaCl (○); and B, 1 mm NaCl:100 mm KCl (●), 10 mm NaCl:100 mm KCl (○), 50 mm NaCl:100 mm KCl (▪), and 100 mm NaCl:100 mm KCl (□). Curves were derived from a 3B2S model for cation permeation using the parameters given in Table I.
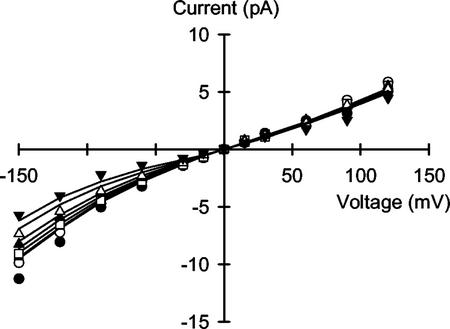
The VIC Channel Is Permeable to Ca2+
The VIC channel from the plasma membrane of wheat roots displayed an appreciable Ca2+ conductance in bi-ionic (cis:trans) CaCl2:NaCl or CaCl2:KCl (Fig. 5), and the proposed 3B2S model fitted the complex I/V relationships observed under these conditions. From the Erev obtained in the presence of 50 mm CaCl2:100 mm NaCl (−30.0 mV), an apparent permeability ratio for PCa:PNa of 0.42 can be calculated using the equation of Fatt and Ginsborg (1958). Similarly, apparent permeability ratios for PCa:PK of 1.35, 0.45, and 0.18 can be calculated from I/V curves obtained in the presence of 0.5 mm CaCl2:100 mm KCl (Erev = −90.0 mV), 5 mm CaCl2:100 mm KCl (Erev = −67.5 mV), and 50 mm CaCl2:100 mm KCl (Erev = −48.0 mV), respectively.
Figure 5.
Unitary current versus voltage relationships for the VIC channel in the plasma membrane of wheat roots assayed in the presence of (cis:trans): A, 50 mm CaCl2:100 mm NaCl; B, 0.5 mm CaCl2:100 mm KCl (●), 5 mm CaCl2:100 mm KCl (o), or 50 mm CaCl2: 100 mm KCl (▪). Curves were derived from a 3B2S model for cation permeation using the parameters given in Table I.
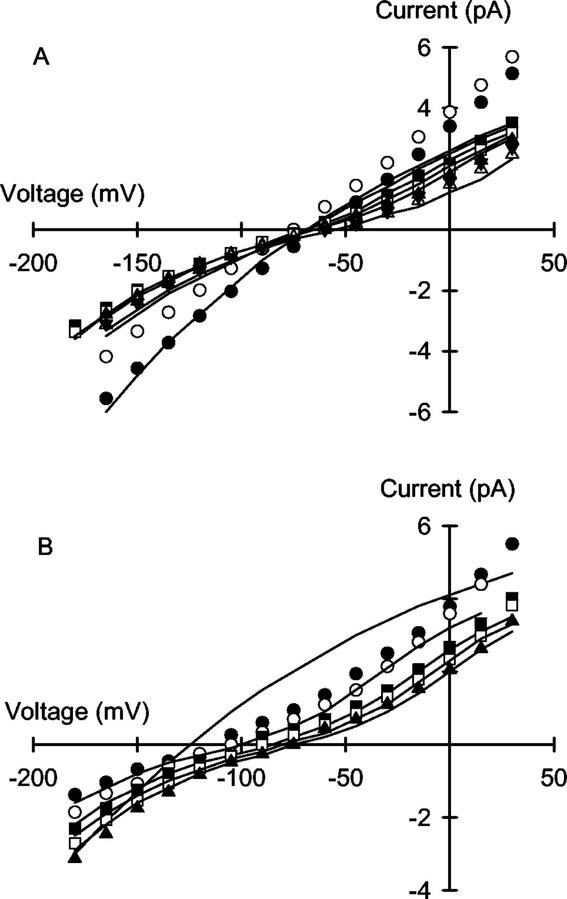
The addition of Ca2+ to a solution of monovalent cations bathing the extracellular (cis) side of the VIC channel reduced both negative and positive unitary currents and shifted the Erev to more positive voltages (Figs. 6 and 7). This was most apparent when the solution bathing the extracellular side of the VIC channel contained low concentrations of monovalent cations. When assays were performed with 100 mm NaCl on both sides of the channel, the addition of Ca2+ to the solution on the cis side of the channel reduced the negative unitary current more than the positive unitary current and barely shifted the Erev (Fig. 6). When assays were performed in the presence of (cis:trans) 10 mm NaCl:100 mm KCl the addition of Ca2+ to the solution on the cis side of the channel reduced both negative and positive unitary currents and shifted the Erev to slightly more positive voltages (Fig. 7A). When assays were performed in the presence of (cis:trans) 1 mm NaCl:100 mm KCl, the addition of Ca2+ to the solution on the cis side of the channel altered the I/V relationship for the channel substantially, increasing the unitary current at extreme negative voltages, decreasing it at extreme positive voltages and shifting the Erev to much more positive voltages (Fig. 7B).
Figure 6.
The effect of varying extracellular (cis) Ca2+ concentration on the unitary current versus voltage relationships for the VIC channel in the plasma membrane of wheat roots. Assay solutions contained 100 mm NaCl plus 0 (●), 0.1 (○), 0.5 (▪), 1 (□), 2 (▴), 5 (▵), or 10 (▾) mm CaCl2 (cis) and 100 mm NaCl (trans). Curves were derived from a 3B2S model for cation permeation using the parameters given in Table I.
Figure 7.
The effect of varying extracellular (cis) Ca2+ concentration on the unitary current versus voltage relationships for the VIC channel in the plasma membrane of wheat roots assayed in the presence of: A, 10 NaCl:100 KCl; or B, 1 NaCl:100 KCl. Calcium chloride was added to the solution in the cis chamber to give final concentrations in: A, 0 (●), 0.1 (○), 0.5 (▪), 1 (□), 2 (▴), 5 (▵), and 10 (▾) mm; and B, 0 (●), 0.1 (○), 0.5 (▪), 1 (□), and 2 (▴) mm. Curves were derived from a 3B2S model for cation permeation using the parameters given in Table I.
The proposed 3B2S model fitted the experimental data obtained in solutions containing 100 mm NaCl. However, when the channel was assayed in (cis:trans) 10 mm NaCl:100 mm KCl, the proposed 3B2S model fitted the decrease in unitary current at positive voltages, but underestimated the current at positive voltages in the absence of Ca2+ in the cis solution. Similarly, when the channel was assayed in (cis:trans) 1 mm NaCl:100 mm KCl, the proposed 3B2S model fitted the experimental data obtained in the presence of Ca2+ in the cis solution, but the shape of the I/V relationship obtained in the absence of Ca2+ was not described well by the proposed 3B2S model. The latter observation might imply contamination of experimental solutions by micromolar Ca2+. A Ca2+ activity of about 20 μm would generate an appropriate I/V relationship.
Physiological Evidence That VIC Channels Mediate Ca2+ Influx to Wheat Root Cells
Inward cation currents through VIC channels in the plasma membrane of wheat root cells are partially inhibited by Ba2+ and Gd3+, but insensitive to verapamil, TEA+, and quinine (Tyerman et al., 1997; White, 1999a; Davenport and Tester, 2000). Here, a similar pharmacology was obtained for Ca2+ influx to excised wheat roots (Table II). This is consistent with the hypothesis that VIC channels contribute to Ca2+ influx to wheat root cells under steady-state conditions. Curiously, the presence of quinine in the extracellular medium stimulated Ca2+ influx to excised wheat roots. The reasons for this are unknown.
Table II.
The pharmacology of Ca2+ influx to excised wheat roots
| Pharmaceutical | Ca2+ Influx |
|---|---|
| μmol g−1 fresh wt h−1 | |
| None | 0.81 ± 0.057 |
| Verapamil (20 mm) | 0.77 ± 0.047 |
| Tetraethylammonium (1 mm) | 0.81 ± 0.079 |
| Barium (0.5 mm) | 0.35 ± 0.024 |
| Gadolinium (0.5 mm) | 0.11 ± 0.012 |
| Quinine (0.5 mm) | 1.92 ± 0.198 |
Data are expressed as mean ± se from eight experiments.
DISCUSSION
The VIC Channel Mediates Ca2+ Influx under Physiological Ionic Conditions
The appreciable Ca2+ conductance when assayed in bi-ionic (cis:trans) CaCl2:KCl or CaCl2:NaCl (Fig. 4) and the positive shift in Erev when Ca2+ was added to monovalent cation solutions in the cis chamber (Figs. 6 and 7) indicate that the VIC channel in the plasma membrane of wheat roots is permeable to Ca2+. A unitary chord conductance (calculated between −30 and + 30 mV) of 5.94 pS is predicted by the proposed 3B2S model for this channel when assayed in 100 mm CaCl2. This value is similar to that measured for a VIC channel in the plasma membrane of rye roots under comparable conditions (6.05 ± 0.74 pS, n = 5; P.J. White, unpublished data).
Under physiological conditions, there is a considerable electrochemical gradient for Ca2+ influx to root cells (White, 2000). Thus, the VIC channel in the plasma membrane of wheat roots will facilitate Ca2+ influx to the cytoplasm and, therefore, may have a role in [Ca2+]cyt homeostasis and/or [Ca2+]cyt signaling. The role of the VIC channel in [Ca2+]cyt dynamics can be addressed by investigating the Ca2+ influx through the channel under physiological conditions. This can be estimated using the proposed 3B2S model for this channel. Because the proposed 3B2S model was found to err only under a few experimental conditions (at positive voltages and/or in the absence of extracellular Ca2+), the model can be used to predict I/V relationships under physiological conditions, when the membrane potential is substantially negative and millimolar Ca2+ is present in the extracellular solution.
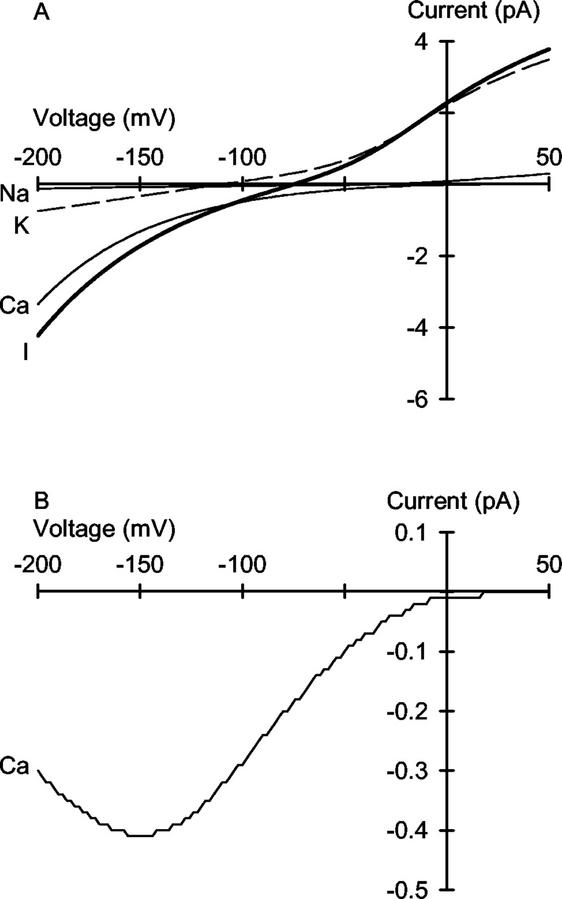
Under physiologically relevant ionic conditions, with 0.904 mm K+, 0.930 mm Na+, and 0.696 mm Ca2+ activities in the extracellular solution and 71 mm K+, 3.5 mm Na+, and 100 nm Ca2+ activities in the cytoplasmic solution (Marschner, 1995), the predicted unitary current through the VIC channel is dominated by Ca2+ influx at negative voltages (Fig. 8A). This is greater than either the predicted K+ or Na+ influx through the channel under these ionic conditions. This conclusion underlies the complex changes in I/V curves obtained when Ca2+ is added to the 1 mm NaCl on the cis side of the channel when 100 mm KCl is present on the trans side (Fig. 7B). Thus, it is clear that Ca2+ permeates the VIC channel and that, if open, VIC channels will facilitate Ca2+ influx under physiological conditions.
Figure 8.
A, The predicted voltage-dependence of the unitary current (——) and net unitary Ca2+ (——), K+ (− − −), and Na+ (——) currents through the VIC channel in the plasma membrane of wheat roots under ionic conditions approximating those present physiologically (Marschner, 1995). Curves were derived from a 3B2S model for cation permeation using the parameters given in Table I. The extracellular solution contained ionic activities of 0.904 mm K+, 0.930 mm Na+, and 0.696 mm Ca2+, and the cytoplasmic solution contained ionic activities of 72 mm K+, 3.5 mm Na+, and 100 nm Ca2+. B, The predicted voltage dependence of the Ca2+ current though a single VIC channel under physiological conditions. The curve was calculated as the product of the unitary conductance, shown in A, and the open probability of the VIC channel [Po = 0.75/(1 + exp(−139 − V)/(RT/0.82F)); Davenport and Tester, 2000].
The Po of the VIC channel in the plasma membrane of wheat roots has been fitted with a modified Boltzmann equation with a gating charge of 0.82, a maximal Po of 0.75, and half-maximal Po occurring at −139 mV (Davenport and Tester, 2000). This equation can be combined with the 3B2S model for cation permeation to estimate Ca2+ influx to a root cell through the VIC channel under steady-state conditions (Fig. 8B). It can be observed that the channel will mediate significant Ca2+ influx at all physiological membrane potentials.
It is not yet known whether the VIC channel in the plasma membrane of wheat root cells is controlled by physiological effectors. Activities of ion channels are frequently modulated by the binding of ligands or by covalent modification, but the VIC channel in the plasma membrane of wheat roots does not appear to respond dramatically to any cytosolic ligand or phosphatase inhibitor. Changing [Ca2+]cyt in the physiological range had no effect on the kinetics of the VIC channel from wheat roots, and the channel was unaffected by 0.5 mm spermine or 4 mm Mg2+ on the cytoplasmic side (Davenport and Tester, 2000). Decreasing the pH of the solution on the cytoplasmic side of the channel from 7.4 to 5.5 decreased the inward Na+ current by about 25% (Davenport and Tester, 2000). Similar observations have been made for the VIC channel in the plasma membrane of rye roots (White, 1997, 1999b). Neither cAMP nor cGMP at 100 μm strongly affected the wheat root VIC channel (Davenport and Tester, 2000). Neither 2 nm deltamethrin (an inhibitor of type 2B protein phosphatases) nor 100 nm okadaic acid (an inhibitor of type 2A protein phosphatases) applied extracellularly greatly affected the VIC-mediated, instantaneous Na+ current in protoplasts from wheat roots (Buschmann et al., 2000). Furthermore, the high Po of the VIC channels observed in rye and wheat root plasma membrane preparations (White and Tester, 1992; Davenport and Tester, 2000), and the ubiquity of VIC-mediated currents in protoplasts from plant tissues (Demidchik et al., 2002), suggests that this type of channel is constitutively active in the plasma membrane of plant cells.
In conclusion, the weak voltage dependency of the VIC channel in the plasma membrane of wheat roots (and its lack of modulation by physiological effectors) suggests that it will be open and provide a weakly voltage-dependent inward Ca2+ current under physiological conditions.
Ca2+ Influx through the VIC Channel May Have a Role in [Ca2+]cyt Homeostasis
Changes in [Ca2+]cyt initiate the physiological responses to many environmental challenges and developmental stimuli (Trewavas, 2000; White, 2000). To facilitate these signals, plant cells maintain a low, and relatively constant, [Ca2+]cyt under steady-state conditions. This is affected primarily by balancing cytoplasmic Ca2+ influx and Ca2+ efflux. The activity of Ca2+-ATPases and H+/Ca2+ antiporters located on the plasma membrane, tonoplast, and endoplasmic reticulum determines the rate of Ca2+ efflux from the cytoplasm (Sanders et al., 1999). Because the cell can ill afford to down-regulate Ca2+ efflux activities, because high [Ca2+]cyt are extremely toxic, these enzymes are probably working constantly. Therefore, to prevent [Ca2+]cyt depletion, it is likely that a controlled Ca2+ influx to the cytoplasm counterbalances this perpetual Ca2+ efflux. The plasma membrane VIC channels, by providing a weakly voltage-dependent Ca2+ influx under most physiological conditions, could perform this function.
That such a system operates across the plasma membrane of root cells is evidenced by the large unidirectional Ca2+ fluxes across this membrane, which are far greater than either the net Ca2+ flux across it or the unidirectional Ca2+ fluxes across intracellular membranes (White et al., 1992). Furthermore, the I/V relationship for Ca2+ currents through the wheat VIC channel appears to be the mirror image of that for Ca2+ currents through the plasma membrane Ca2+-ATPase, which dominates Ca2+ efflux across this membrane (Felle et al., 1992). Thus, it is possible that the physiological role of VIC channels is to allow the Ca2+ influx to the cell required to balance the Ca2+ efflux driven by the plasma membrane Ca2+-ATPase, and thereby effect [Ca2+]cyt homeostasis under steady-state conditions. This function is not trivial because the provision of [Ca2+]cyt homeostasis is necessary not only for intracellular signaling through changes in [Ca2+]cyt but also for cell survival.
The Curse of Ca2+ Signaling
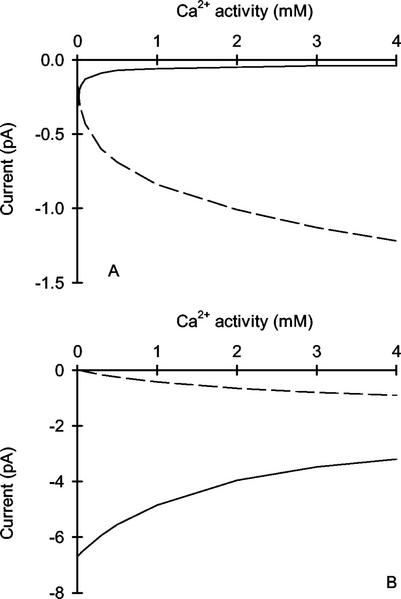
It has been suggested that VIC channels contribute significantly to the uptake of toxic monovalent cations, such as Na+ and Cs+, by plant root cells (White, 1999a; White and Broadley, 2000). This argument follows from the omnipresence of VIC channels in the plasma membrane of root cells, their lack of selectivity, and their high Po. The Na+ influx to a root cell through a VIC channel in the plasma membrane of wheat roots can be estimated using the 3B2S model described here (Fig. 9). Under nonsaline conditions, the model predicts that little Na+ enters cells through a VIC channel (Figs. 8A and 9A). However, under saline conditions, the model predicts that Na+ influx dominates the cation fluxes through this channel (Fig. 9B). Furthermore, the model predicts that increasing extracellular Ca2+ to millimolar activities can reduce Na+ influx only partially. This is consistent with the effects of increasing extracellular Ca2+ on 22Na+ influx to wheat roots (Davenport et al., 1997), and earlier arguments that VIC channels are responsible for the bulk of Na+ influx to root cells (Tyerman and Skerrett, 1999; White, 1999a). Thus, one consequence of effecting [Ca2+]cyt homeostasis through a nonselective VIC channel is its potential for poisoning a cell. This is the curse of Ca2+ signaling in plant cells.
Figure 9.
The predicted dependence of the net unitary Na+ (——) and Ca2+ (− − −) currents through the VIC channel in the plasma membrane of wheat roots on extracellular Ca2+ activity under nonsaline (A) and saline (B) ionic conditions. Curves were derived from a 3B2S model for cation permeation using the parameters given in Table I. A membrane potential of −120 mV was assumed. The extracellular activities were 0.904 mm K+, 0.696 mm Ca2+, and 0.930 mm (nonsaline) or 72 mm (saline) Na+. The cytoplasmic activities were 72 mm K+, 100 nm Ca2+, and 3.5 mm (nonsaline) or 7.2 mm (saline) Na+.
MATERIALS AND METHODS
Plasma Membrane Isolation and Incorporation into PLBs
Wheat (Triticum aestivum cv Hunter) seedlings were grown hydroponically over a 0.5 mm CaSO4 solution in growth cabinet on a 16-h-light (25°C)/8-h-dark (15°C) cycle. Photon irradiance was 200 μmol m−2 s−1. Roots were harvested on the 7th d after germination and plasma membrane vesicles were obtained by aqueous polymer two-phase partitioning of a root microsomal membrane fraction (Davenport and Tester, 2000).
Plasma membrane vesicles were incorporated into PLBs generated from a dispersion of 8 mm PE, 6 mm phosphatidyl-Ser, 3 mm phosphatidylcholine, and 15 mm cholesterol in n-decane, as described by Davenport and Tester (2000). The bilayer (300 μm in diameter) separated volumes of 500 μL in the cis compartment and 1.5 mL in the trans compartment. Plasma membrane vesicles were added to the solution in the cis compartment and, after incorporation, the VIC channel became orientated with its extracellular surface facing the cis solution (White and Tester, 1992). Ionic conditions were established either by the addition of stock solutions or by perfusing compartments with an appropriate solution. All solutions were filtered and adjusted to pH 5.5 with HCl, unless otherwise indicated. Activities of ions in solutions were calculated using GEOCHEM-PC version 2.1 (Parker et al., 1995).
Single-Channel Recordings
Experiments were performed at room temperature. Current recordings were obtained under voltage clamp conditions using a List EPC-7 amplifier (List Electronics, Darmstadt, Germany) connected to the bilayer chambers via 3 m KCl/1% (w/v) agar salt bridges. Voltages were referenced to the cis chamber, which was grounded. This accords with the physiological convention (i.e. cytosol with respect to the external medium). Movement of cations from the extracellular to the cytoplasmic side of the channel is indicated by a negative current. Data were recorded unfiltered on digital audiotape (DTC-75ES, Sony, Tokyo) and/or filtered at 100 Hz with an eight-pole low pass Bessel filter (Kemo, Beckenham, UK) and recorded with pCLAMP 6.03 software (Axon Instruments, Foster City, CA). The pCLAMP files were sampled at 1 kHz for analysis, and Gaussian distributions of current amplitude were determined using the Simplex least-squares method provided by pSTAT (pCLAMP6) software.
Some of the experimental data presented in Figures 2 and 4 were published by Davenport and Tester (2000), who also showed examples of single-channel recordings of the VIC channel in the plasma membrane of wheat roots. The remaining experimental data can be found in Davenport (1998). In addition to the experimental data presented in the figures, unitary I/V relationships obtained in solutions containing (cis:trans) 100 μm NaCl:100 mm KCl were also input for regressions. Because no attempt was made to standardize data from different experiments, it should be noted that VIC channel activities from different membrane preparations could differ slightly in their unitary conductances (Davenport, 1998).
It is evident from the genome sequencing projects that families of genes, with many individual members, encode similar cation channels that may have specific but subtle functional differences. The data presented in this paper were obtained from single-channel electrical recordings of many individual VIC channels. Because these electrical recordings were remarkably consistent between individual VIC channels, this suggests that the model presented here describes the permeation of cations through the dominant VIC channel in the plasma membrane of wheat root cells.
Modeling Cation Permeation
Estimates of energy profiles for permeant cations and structural characteristics of the wheat VIC channel were obtained using a version of the FORTRAN computer program AJUSTE (Alvarez et al., 1992) modified for the presence of both monovalent and divalent cations (White and Ridout, 1999). The model chosen (a 3B2S model) had energy profiles consisting of three energy barriers and two ion-binding sites (energy wells), and allowed for single-file permeation, double-cation occupancy, ion-ion repulsion, and surface potential effects. The energies of the unoccupied channel at zero voltage (expressed as RT) were defined by three peaks (G1–G3) and two wells (U1 and U2) with the postscript referring to their position relative to the trans (cytoplasmic) compartment. The distances D1 to D5 refer to the position of successive peaks and wells in the electrical field relative to the trans (cytoplasmic) compartment.
The effects of ion-ion interactions (electrostatic and/or allosteric) were simulated by the addition of an energy factor to the peaks and wells adjacent to an occupied well. This was calculated as A(z1z2)/d, where A is the ionic-repulsion energy parameter, z1 and z2 are the valencies of the interacting cations, and d is the electrical distance from the occupied well. Two parameters (Rscis and Rstrans) were included in the model to describe surface charge effects. These parameters correspond to the radii of circles (expressed in Ångstroms) containing one electron charge in the cis and trans vestibules of the pore, respectively.
Rate constants were formulated by the standard Eyring rate theory expression equal to the product of a pre-exponential term, kT/h (where k/h is Boltzmann's constant divided by Planck's constant), and an exponential function of the energy difference, exp(ΔG/(RT)). A similar expression was used for the bimolecular rate constants describing the entry of ions from the internal or external solutions. The reference energy state of our model corresponds to 55.5 m solution. To compare our energy values with models that use a 1 m reference state, 4.02 RT units must be added to our values.
Parameters describing the free-energy profiles for K+, Na+, and Ca2+ permeation were fitted to the complete data set of 624 datapoints obtained in 42 different solution combinations in which these cations were present. Fitting began with a symmetrical model, with electrical distances of 0.00, 0.25, 0.50, 0.75, and 1.00 for D1 through D5, respectively. Starting parameters for the free-energy profile for K+ and Na+ permeation were based on those of the corresponding VIC channel in the plasma membrane of rye (Secale cereale) roots (White, 1997). Starting parameters for the well depths for Ca2+ were based on the concentration giving half-maximal conductance and those for barrier heights were based on the maximal Ca2+ conductance. Three contrasting sets of initial parameter estimates were pursued and the one with the lowest residual sum of squares (RSS) is presented here. In total, over 120 regressions were run in the course of obtaining the final parameters describing the free-energy profiles for K+, Na+, and Ca2+ permeation. Several parameters could not be estimated with any precision and were, therefore, fixed to their optimal values.
Calcium Influx to Excised Roots
Caryopses of wheat were surface sterilized using NaOCl (1% [w/v] active chlorine), rinsed in distilled water for 8 h, and germinated at 17°C in the dark. After germination, seedlings were grown hydroponically for a further 7 d in an aerated solution containing 0.5 mm CaCl2 in a constant environment room with a relative humidity of 70% and a temperature of 25°C. Seedlings were illuminated by a bank of TLDW/84 lights (Philips, Eindhoven, The Netherlands) providing a photosynthetically active photon flux of 75 μmol m−2 s−1 at plant height. Shoots were removed immediately before experimentation, and roots were placed in an aerated solution containing 0.5 mm CaCl2, in which Ca was isotopically labeled with approximately 3 MBq l−1 45Ca (NEN Life Science Products, Zavantem, Belgium). Calcium (45Ca) influx to excised roots was assayed over 20 min in the absence or presence of 20 μm verapamil, 1 mm TEACl, 0.5 mm BaCl2, 0.5 mm GdCl3, or 0.5 mm quinine. After 20 min, roots were transferred to a solution lacking 45Ca but containing 0.5 mm CaCl2 and 1 mm LaCl3 for 5 min to remove 45Ca from the cell wall. Roots were blotted on filter paper, and their fresh weights and 45Ca contents were determined. Tissue 45Ca content was determined by liquid scintillation counting. To estimate 45Ca content, 100 μL of a solution containing 3.68 mg mL−1 CaCl2 was added to the root tissue before it was frozen at −20°C for 30 min. Each sample was thawed and 10 mL of Ecoscint A (National Diagnostics, Hull, UK) was added. Radioactivity was determined using an LS 6000TA scintillation counter (Beckman Instruments, Fullerton, CA). In each of eight replicated experiments, roots from four seedlings were exposed to an assay treatment.
ACKNOWLEDGMENTS
We thank Mark Tester (Plant Sciences, Cambridge, UK) and Martin R. Broadley (Horticulture Research International) for their comments on the original manuscript, Martin S. Ridout (Institute of Mathematics and Statistics, Kent, UK) for his statistical expertise, and Helen C. Bowen and John Hammond (Horticulture Research International) for technical assistance.
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council (to P.J.W.) and by a UK Commonwealth Scholarship (to R.J.D.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.005769.
LITERATURE CITED
- Alvarez O, Villarroel A, Eisenman G. Calculation of ion currents from energy profiles and energy profiles from ion currents in multibarrier, multisite, multioccupancy channel models. Methods Enzymol. 1992;207:816–854. doi: 10.1016/0076-6879(92)07058-v. [DOI] [PubMed] [Google Scholar]
- Amtmann A, Sanders D. Mechanisms of Na+ uptake by plant cells. Adv Bot Res. 1999;29:75–112. [Google Scholar]
- Broadley MR, Escobar-Gutiérrez AJ, Bowen HC, Willey NJ, White PJ. Influx and accumulation of Cs+ by the akt1 mutant of Arabidopsis thaliana (L.) Heynh. lacking a dominant K+ transport system. J Exp Bot. 2001;52:839–844. doi: 10.1093/jexbot/52.357.839. [DOI] [PubMed] [Google Scholar]
- Buschmann PH, Vaidyanathan R, Gassmann W, Schroeder JI. Enhancement of Na+ uptake currents, time-dependent inward-rectifying K+ channel currents, and K+ channel transcripts by K+ starvation in wheat root cells. Plant Physiol. 2000;122:1387–1397. doi: 10.1104/pp.122.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport RJ. Mechanisms of toxic sodium influx in wheat. PhD thesis. UK: Cambridge University; 1998. [Google Scholar]
- Davenport RJ, Reid RJ, Smith FA. Sodium-calcium interactions in two wheat species differing in salinity tolerance. Physiol Plant. 1997;99:323–327. [Google Scholar]
- Davenport RJ, Tester M. A weakly voltage-dependent, nonselective cation channel mediates toxic sodium influx in wheat. Plant Physiol. 2000;122:823–834. doi: 10.1104/pp.122.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Davenport RJ, Tester M. Nonselective cation channels in plants. Annu Rev Plant Biol. 2002;53:67–107. doi: 10.1146/annurev.arplant.53.091901.161540. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Tester M. Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiol. 2002;128:379–387. doi: 10.1104/pp.010524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatt P, Ginsborg BL. The ionic requirements for the production of action potentials in crustacean muscle fibers. J Physiol. 1958;142:516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle HH, Tretyn A, Wagner G. The role of the plasma-membrane Ca2+-ATPase in Ca2+ homeostasis in Sinapis alba root hairs. Planta. 1992;188:306–313. doi: 10.1007/BF00192796. [DOI] [PubMed] [Google Scholar]
- Gaymard F, Pilot G, Lacombe B, Boucherez D, Bruneau D, Michaux-Ferrière N, Thibaud J-B, Sentenac H. Identification and disruption of a plant Shaker-like outward channel involved in K+ release into the xylem. Cell. 1998;94:647–655. doi: 10.1016/s0092-8674(00)81606-2. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Ed 3. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- Hirsch RE, Lewis BD, Spalding EP, Sussman MR. A role for the AKT1 potassium channel in plant nutrition. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D. Sodium uptake in Arabidopsis roots is regulated by cyclic nucleotides. Plant Physiol. 2001;127:1617–1625. [PMC free article] [PubMed] [Google Scholar]
- Marschner H. Mineral Nutrition of Higher Plants. Ed 2. London: Academic Press; 1995. [Google Scholar]
- Parker DR, Norvell WA, Chaney RL. GEOCHEM-PC: a chemical speciation program for IBM and compatible computers. In: Loeppert RH, Schwab AP, Goldberg S, editors. Chemical Equilibrium and Reaction Models, Special Publication 42. Madison, WI: Soil Science Society of America; 1995. pp. 253–269. [Google Scholar]
- Roberts SK, Tester M. A patch-clamp study of Na+ transport in maize roots. J Exp Bot. 1997;48:431–440. doi: 10.1093/jxb/48.Special_Issue.431. [DOI] [PubMed] [Google Scholar]
- Sanders D, Brownlee C, Harper JF. Communicating with calcium. Plant Cell. 1999;11:691–706. doi: 10.1105/tpc.11.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A. Signal perception and transduction. In: Buchanan BB, Gruissem W, Jones RL, editors. Biochemistry and Molecular Biology of Plants. Rockville, MD: American Society of Plant Physiologists; 2000. pp. 930–987. [Google Scholar]
- Tyerman SD, Skerrett IM. Root ion channels and salinity. Sci Hortic. 1999;78:175–235. [Google Scholar]
- Tyerman SD, Skerrett M, Garill A, Findlay GP, Leigh R. Pathways for the permeation of Na+ and Cl− into protoplasts derived from the cortex of wheat roots. J Exp Bot. 1997;48:459–480. doi: 10.1093/jxb/48.Special_Issue.459. [DOI] [PubMed] [Google Scholar]
- White PJ. The permeation of ammonium through a voltage-independent K+ channel in the plasma membrane of rye roots. J Membr Biol. 1996;152:89–99. doi: 10.1007/s002329900088. [DOI] [PubMed] [Google Scholar]
- White PJ. Cation channels in the plasma membrane of rye roots. J Exp Bot. 1997;48:499–514. doi: 10.1093/jxb/48.Special_Issue.499. [DOI] [PubMed] [Google Scholar]
- White PJ. The molecular mechanism of sodium influx to root cells. Trends Plant Sci. 1999a;4:245–246. doi: 10.1016/s1360-1385(99)01435-1. [DOI] [PubMed] [Google Scholar]
- White PJ. The mechanism of sodium influx into root cells. In: Hagin J, Johnston AE, Glasscock J, editors. Proceedings of the Dahlia Greidinger International Symposium on Nutrient Management under Salinity and Water Stress. Haifa, Israel: Technion; 1999b. pp. 11–16. [Google Scholar]
- White PJ. Calcium channels in higher plants. Biochim Biophys Acta. 2000;1465:171–189. doi: 10.1016/s0005-2736(00)00137-1. [DOI] [PubMed] [Google Scholar]
- White PJ, Banfield J, Diaz M. Unidirectional Ca2+ fluxes in roots of rye (Secale cereale L.). A comparison of excised roots with roots of intact plants. J Exp Bot. 1992;43:1061–1074. [Google Scholar]
- White PJ, Broadley MR. Mechanisms of caesium uptake by plants. New Phytol. 2000;147:241–256. [Google Scholar]
- White PJ, Lemtiri-Chlieh F. Potassium currents across the plasma membrane of protoplasts derived from rye roots: a patch-clamp study. J Exp Bot. 1995;46:497–511. [Google Scholar]
- White PJ, Ridout M. The K+ channel in the plasma membrane of rye roots has a multiple ion residency pore. J Membr Biol. 1995;143:37–49. doi: 10.1007/BF00232522. [DOI] [PubMed] [Google Scholar]
- White PJ, Ridout MS. An energy-barrier model for the permeation of monovalent and divalent cations through the maxi cation channel in the plasma membrane of rye roots. J Membr Biol. 1999;168:63–75. doi: 10.1007/s002329900498. [DOI] [PubMed] [Google Scholar]
- White PJ, Tester MA. Potassium channels from the plasma membrane of rye roots characterized following incorporation into planar lipid bilayers. Planta. 1992;186:188–202. doi: 10.1007/BF00196248. [DOI] [PubMed] [Google Scholar]