Abstract
The role of growth temperature and growth irradiance on the regulation of the stoichiometry and function of the photosynthetic apparatus was examined in the cyanobacterium Plectonema boryanum UTEX 485 by comparing mid-log phase cultures grown at either 29°C/150 μmol m−2 s−1, 29°C/750 μmol m−2 s−1, 15°C/150 μmol m−2 s−1, or 15°C/10 μmol m−2 s−1. Cultures grown at 29°C/750 μmol m−2 s−1 were structurally and functionally similar to those grown at 15°C/150 μmol m−2 s−1, whereas cultures grown at 29°C/150 μmol m−2 s−1 were structurally and functionally similar to those grown at 15°C/10 μmol m−2 s−1. The stoichiometry of specific components of the photosynthetic apparatus, such as the ratio of photosystem (PS) I to PSII, phycobilisome size and the relative abundance of the cytochrome b6/f complex, the plastoquinone pool size, and the NAD(P)H dehydrogenase complex were regulated by both growth temperature and growth irradiance in a similar manner. This indicates that temperature and irradiance may share a common sensing/signaling pathway to regulate the stoichiometry and function of the photosynthetic apparatus in P. boryanum. In contrast, the accumulation of neither the D1 polypeptide of PSII, the large subunit of Rubisco, nor the CF1 α-subunit appeared to be regulated by the same mechanism. Measurements of P700 photooxidation in vivo in the presence and absence of inhibitors of photosynthetic electron transport coupled with immunoblots of the NAD(P)H dehydrogenase complex in cells grown at either 29°C/750 μmol m−2 s−1 or 15°C/150 μmol m−2 s−1 are consistent with an increased flow of respiratory electrons into the photosynthetic intersystem electron transport chain maintaining P700 in a reduced state relative to cells grown at either 29°C/150 μmol m−2 s−1 or 15°C/10 μmol m−2 s−1. These results are discussed in terms of acclimation to excitation pressure imposed by either low growth temperature or high growth irradiance.
Cyanobacteria are a large and diverse group of prokaryotes performing oxygenic photosynthesis in a manner similar to green algae and plants. The cyanobacterial photosynthetic apparatus consists of five multiprotein complexes. PSII, cytochrome b6f, PSI, and ATP synthase are common to both cyanobacteria and plants. However, the fifth complex, a light-harvesting antenna of PSII, is functionally but not structurally homologous (Gantt, 1994). In cyanobacteria, light harvesting is mediated by phycobilisomes (PBSs), complex protein structures located on the cytoplasmic surface of the thylakoid membranes. The major components of the PBS are the biliproteins, allophycocyanin (AP), phycocyanin (PC), phycoerythrin, and phycoerythrocyanin, with covalently attached bilin chromophores. Different colorless linker polypeptides are specifically associated with each type of phycobiliprotein and function to stabilize the PBS and optimize their absorbance and energy transfer characteristics. The PBSs are composed of two structural domains: an AP core that is in direct contact with the thylakoid membrane and generally six rods of stacked PC and, in some strains, phycoerythrin or phycoerythrocyanin hexamers radiating from the core (Sidler, 1994).
Another important distinction between cyanobacteria and chloroplasts is that in cyanobacteria, both respiratory and photosynthetic electron transport chains function within thylakoid membranes, where they share electron transport components such as the plastoquinone (PQ) pool and cytochrome b6f complex (Scherer, 1990; Schmetterer, 1994; Cooley et al., 2000; Cooley and Vermaas, 2001). Thus, electron fluxes in the intersystem chain may be affected by the electron supply from PSII, NAD(P)H dehydrogenase (Ndh)-, and succinate dehydrogenase-mediated electron transport pathway from respiratory donors and cyclic electron pathway around PSI as well as the electron consumption by cytochrome oxidase (Cooley et al., 2000; Cooley and Vermaas, 2001).
The composition of cyanobacterial photosynthetic apparatus is regulated in response to environmental factors such as light, temperature, and nutrient availability. Growth of cyanobacteria at high irradiance induces changes in the abundance of light-harvesting antennae (Raps et al., 1985; de Lorimier et al., 1992; Reuter and Muller, 1993; Garnier et al., 1994; Samson et al., 1994; Nomsawai et al., 1999) as well as changes in the stoichiometry between PSI and PSII (Murakami and Fujita, 1991; Fujita et al., 1994). In general, the mechanisms of regulation of light harvesting in response to high-light intensity include a decrease in cellular content of chlorophyll (Chl) a as well as a reduction in the number of PBSs and/or size of PBSs by a decrease of the peripheral biliprotein complexes (Raps et al., 1985; de Lorimier et al., 1992; Reuter and Muller, 1993; Garnier et al., 1994; Samson et al., 1994; Nomsawai et al., 1999). Moreover, some species may vary the composition of their PBS by induction of new polypeptides associated with PBS or modifications of the PBS components (Reuter and Muller, 1993; Garnier et al., 1994; Samson et al., 1994; Nomsawai et al., 1999). The PSI to PSII ratio becomes higher under low irradiance and lower at high-light intensity, and PSI seems to be the variable component of the photosynthetic apparatus (Murakami and Fujita, 1991; Fujita et al., 1994). Moreover, the activity or the amount of cytochrome c oxidase in the respiratory system is adjusted concomitantly with the level of PSI (Adhikary et al., 1990; Murakami et al., 1997). Both terminal components of the electron transport system in cyanobacteria appear to be regulated in response to modulation of the redox state of the intersystem PQ pool and/or the cytochrome b6f complex. Alterations in the redox state of these intersystem electron transport components may be induced by changes in either light quality, irradiance, CO2 availability, or Na+ stress (Murakami and Fujita, 1993; Fujita et al., 1994; Grossman et al., 1994; Murakami et al., 1997). Recently, Grossman et al. (2001) have shown that the responses to both high light and nutrient stress in Synechococcus sp. PCC 7942 is regulated by a two-component sensory system. NblR is the response regulator that appears to control PBS degradation in response to high light and nutrient stress. NblS is the sensor His kinase that regulates the phosphorylation on nblR (Grossman et al., 2001). Furthermore, the sensor for chromatic adaptation in cyanobacteria also is a two-component sensor His kinase similar to that of plant phytochromes (Kehoe and Grossman, 1996).
Recently, it has been suggested that low temperatures specifically induce damage to the PSI reaction center in the cyanobacterium Synechocystis sp. PCC 6803 (Zak and Pakrasi, 2000). Growth of Synechocystis sp. PCC 6803 at low temperatures causes a destabilization of the PSI complex that, in turn, leads to a degradation of the PSI core proteins, PsaA and PsaB. In contrast, the content and activity of PSII do not exhibit significant changes under these conditions. The stability of the PSI reaction center seems to be dependent on the presence of the extrinsic thylakoid protein BtpA (Zak and Pakrasi, 2000).
We have reported previously that the filamentous cyanobacterium Plectonema boryanum UTEX 485 grown at low temperature/moderate irradiance (15°C/150 μmol m−2 s−1) mimicked the cells grown at moderate temperature/high-light intensity (29°C/750 μmol m−2 s−1) with respect to pigmentation and photosynthetic characteristics (Miskiewicz et al., 2000). Cells grown under these conditions exhibited reduced cellular contents of Chl a and concomitantly higher levels of myxoxanthophyll, lower apparent quantum yields of oxygen evolution, and enhanced resistance to photoinhibition under visible (Miskiewicz et al., 2000) as well as UV light (Ivanov et al., 2000a). However, decreasing growth irradiance from 150 to 10 μmol m−2 s−1 at 15°C resulted in low temperature-grown cells that were photosynthetically indistinguishable from cells grown under control conditions of 29°C and 150 μmol m−2 s−1 (Miskiewicz et al., 2000). These results indicate that photosynthetic acclimation of P. boryanum is the result of the combined effects of growth temperature and light, rather than because of either low temperature or high light per se. A similar phenomenon was reported for the green algae Chlorella vulgaris and Dunaliella salina (Huner et al., 1998). In the present study, we test the hypothesis that the stoichiometry of photosynthetic components in P. boryanum is not regulated in response to absolute growth temperature or irradiance, but rather, the interaction of both environmental factors. The roles of excitation pressure and redox sensing are discussed.
RESULTS
Relative Abundance of Photosynthetic Components
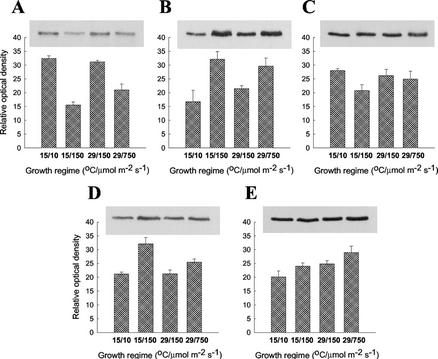
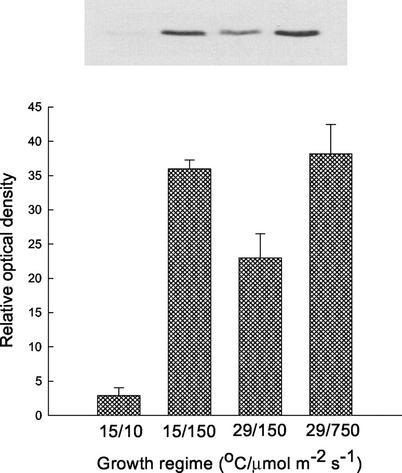
The abundance of polypeptides involved in electron transport, ATP synthesis, and CO2 assimilation in P. boryanum cells exposed to varying growth regimes was examined by immunoblot analysis of the PSII reaction center D1 polypeptide, cytochrome f, the PSI reaction center PsaB polypeptide, the large subunit of Rubisco, and the α-subunit of the CF1 ATP synthase (Fig. 1). In addition, we determined the total PQ and Chl a contents by HPLC (Table I). Cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 exhibited a 2-fold lower content of PsaB polypeptide but a 50% higher cytochrome f content on a Chl basis than cells grown at either 15°C/10 μmol m−2 s−1 or 29°C/150 μmol m−2 s−1 (Fig. 1, A and B). These changes were accompanied by a 2- to 3-fold increase in the PQ to Chl a ratio (Table I) in cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 compared with those grown at either 15°C/10 μmol m−2 s−1 or 29°C/150 μmol m−2 s−1. The changes in the PQ to Chl a ratio appeared to be because of a 2.2- to 4.5-fold decrease in Chl a accompanied by only a concomitant 30% decrease in PQ content (Table I). In contrast, the relative abundance of the D1 polypeptide and Rubisco large subunit exhibited minimal changes irrespective of growth temperature or irradiance (Fig. 1, C and E). The content of ATP synthase was unaffected by growth regime, except that it was approximately 50% higher only in cells grown at 15°C/150 μmol m−2 s−1 compared with cells grown at any other conditions (Fig. 1D).
Figure 1.
Immunoblot and densitometric analysis of polypeptides involved in photosynthetic electron transport and CO2 assimilation in P. boryanum cells grown under various conditions of temperature (°C)/irradiance (μmol m−2 s−1): A, PsaB polypeptide of the PSI reaction center; B, cytochrome f; C, D1 polypeptide of the PSII reaction center; D, α-subunit of the CF1 ATP synthase; and E, Rubisco large subunit. Representative immunoblots and mean values ± se of densitometric data from five independent experiments are shown.
Table I.
Total and functional PQ pool size in P. boryanum cells grown under various conditions of temperature/irradiance
| Growth Regime | PQ/Chl a | PQ | Chl a | A−DCMU/A+DCMU |
|---|---|---|---|---|
| °C/μmol m−2 s−1 | mmol mol−1 | mg g dry wt−1 | ||
| 15/10 | 21.5 ± 1.5 | 0.118 ± 0.008 | 17.73 ± 1.43 | 4.38 ± 0.19 |
| 15/150 | 73.9 ± 2.1 | 0.091 ± 0.003 | 3.98 ± 0.54 | 1.11 ± 0.03 |
| 29/150 | 29.5 ± 2.4 | 0.130 ± 0.010 | 14.26 ± 1.96 | 6.45 ± 0.37 |
| 29/750 | 51.4 ± 2.4 | 0.102 ± 0.005 | 6.38 ± 0.56 | 1.33 ± 0.08 |
The PQ was extracted from the cells with diethylether in hexane and its content estimated by HPLC as described in “Materials and Methods.” The apparent PQ pool size was determined from the ratio of areas over the Chl fluorescence induction curves from Chl fluorescence at open PSII centers (Fo) to maximum fluorescence at closed PSII centers (Fm) in the absence (A−DCMU) and presence (A+DCMU) of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU). Prior to the measurements, samples were dark adapted at the growth temperature in the absence or presence of 20 μm DCMU. The data represent mean values ± se from five independent experiments.
PBS Composition and Structure
To examine the structure of light-harvesting antennae in P. boryanum grown under different conditions of temperature and irradiance, purified PBSs were obtained as a single deep-blue band in Suc gradient centrifugation and analyzed by fluorescence emission spectroscopy, absorption spectroscopy, and SDS-PAGE. Isolated PBSs exhibited single fluorescence emission peaks at 679 nm at room temperature and at 688 nm at 77K upon excitation at 580 nm (data not shown). This fluorescence originates from the terminal energy emitters of PBSs and reflects an efficient transfer of excitation energy between phycobiliproteins within the PBS and the structural integrity of isolated complexes (Glazer, 1988; Sidler, 1994). Absorption spectra of isolated PBS from P. boryanum cells grown under various conditions of temperature and irradiance exhibited a maximum at 625 nm with a shoulder at 652 nm corresponding to PC and AP, respectively (data not shown). The ratios of A625 to A652 indicated that the PC content relative to AP was reduced in P. boryanum grown at either 15°C/150 μmol m−2 s−1 (A625/A652 = 1.36) or 29°C/750 μmol m−2 s−1 (1.56) compared with cells grown at either 15°C/10 μmol m−2 s−1 (1.96) or 29°C/150 μmol m−2 s−1 (1.73).
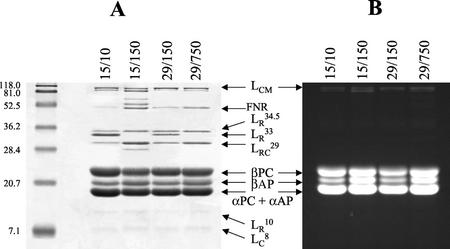
The polypeptide components of isolated PBSs were detected by Coomassie blue staining of the gels (Fig. 2A), whereas phycobilin-containing proteins were also detected by zinc-enhanced fluorescence (Fig. 2B). PBS from cells grown under control conditions (29°C/150 μmol m−2 s−1) contained chromophore-bearing proteins, the α- and β-subunits of AP and PC, and the core-membrane linker (LCM; 94–98 kD), as well as colorless polypeptides identified as linker polypeptides (8-kD core linker [LC8], 10-kD rod linker [LR10], 29-kD rod-core linker [LRC29], 33-kD rod-rod linker [LR33], and 34.5-kD rod-rod linker [LR34.5]) and possibly ferredoxin:NADP+ reductase (47 kD; Glazer, 1988; Sidler, 1994). The PC subunits of the PBS rods are held together by specific linker polypeptides: LRC29 attaches the first PC subunit to the AP core, whereas LR34.5 and LR33 attach the second and third PC subunit to the AP core, respectively (Sidler, 1994). Because of this order, the length of PBS rods may be determined from the abundance of these different linker polypeptides. The electrophoretic analysis of isolated PBS indicated that considerable alterations in PBS structure occurred in P. boryanum with changes in growth conditions (Fig. 2). In cells grown at either 15°C/10 μmol m−2 s−1 or 29°C/150 μmol m−2 s−1, LR33 was present in substantial amounts with respect to the core components. However, in cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1, LR33 was not detected, and its absence correlated with the lower amounts of PC relative to AP in agreement with the spectroscopic analyses. Furthermore, the apparently higher β-AP content in cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 may indicate an alteration in the composition of the PBS core (Sidler, 1994). We note that additional bands of 27, 52, and 65 kD were observed in PBS isolated from cells grown at 15°C/150 μmol m−2 s−1 (Fig. 2A). These bands may represent minor degradation products of the PBS components, contaminants and/or new polypeptides associated with the PBS fraction. The appearance of new polypeptides of unknown function that copurify with PBS was previously observed in Spirulina maxima (Garnier et al., 1994) and Spirulina platensis (Nomsawai et al., 1999) grown under high light. The densitometric analysis of the gels showed that the ratio of LCM to AP did not change significantly in cells grown under various conditions of temperature and irradiance. The number of PBS per Chl a did not seem to vary in P. boryanum with changes in growth regime as indicated by the similar levels of LCM polypeptide detected by ZnSO4 staining of total protein gels on a Chl a basis (data not shown).
Figure 2.
SDS-PAGE of PBS isolated from P. boryanum cells grown under various conditions of temperature (°C)/irradiance (μmol m−2 s−1): A, Coomassie blue staining; and B, ZnSO4 staining. Twenty micrograms of protein was loaded per lane. Molecular mass standards (kD) are indicated on the left. Identities of resolved polypeptides are indicated on the right.
Energy Transfer and Redox State of PSII
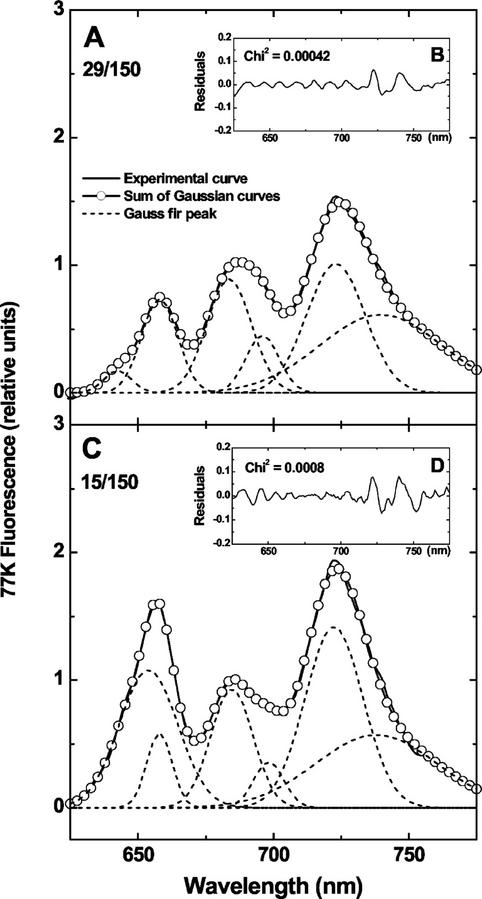
To determine whether structural modifications in the photosynthetic apparatus of P. boryanum were accompanied by functional alterations, energy distribution between PBS and PSII was examined by fluorescence emission spectroscopy at 77K. Figure 3 shows the fluorescence emission spectra of P. boryanum cells grown at 29°C/150 μmol m−2 s−1 and 15°C/150 μmol m−2 s−1 obtained by excitation of PC at 580 nm and resolved into six components. The emission spectrum for cells grown at 15°C/10 μmol m−2 s−1 was similar to that of cells grown at 29°C/150 μmol m−2 s−1 and the emission spectrum of cells grown at 29°C/750 μmol m−2 s−1 was similar to that of cells grown at 15°C/150 μmol m−2 s−1 (Miskiewicz et al., 2000). The bands around 645 and 658 nm are associated with PBS components; the bands around 685, 697, and 743 nm are associated with the terminal energy emitters of PBS and PSII; and the band around 723 nm is associated with PSI (Salehian and Bruce, 1992). P. boryanum grown at either 15°C/150 μmol m−2 s−1 (Fig. 3) or 29°C/750 μmol m−2 s−1 (Miskiewicz et al., 2000) exhibited a higher fluorescence emission from PBS components relative to that of PSII than cells grown at either 15°C/10 μmol m−2 s−1 (Miskiewicz et al., 2000) or 29°C/150 μmol m−2 s−1 (Fig. 3). This indicated that light energy absorbed by PBS was transferred to PSII with a lower efficiency in cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 than in cells grown at either 15°C/10 μmol m−2 s−1 or 29°C/150 μmol m−2 s−1.
Figure 3.
Low temperature (77K) fluorescence emission spectra and their decomposition in Gaussian sub-bands of P. boryanum cells grown under various conditions of temperature (°C)/irradiance (μmol m−2 s−1). The excitation wavelength was 580 nm. The experimental curves (—), representing an average of three scans, were normalized to the emission band around 685 nm. Both the sum of the Gaussian bands (–ο–) and the individual sub-bands (− − − −) are shown. The differences between the sum of the components and the emission curves are shown as residuals in the figure inserts.
The redox state of the PSII acceptor side was assessed by monitoring Chl a fluorescence. Growth of P. boryanum at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 resulted in a 2-fold reduction of the maximal photochemical efficiency measured as Fv/Fm compared with cells grown at either 15°C/10 μmol m−2 s−1 or 29°C/150 μmol m−2 s−1 (Table II). However, cells acclimated to different growth regimes exhibited only minimal differences in qP (Table II), indicating that the proportion of open PSII centers remained relatively constant at between 75% and 85% in P. boryanum regardless of growth conditions. This trend is not observed during acclimation of winter rye (Secale cereale; Gray et al., 1997) or green algae, C. vulgaris, and D. salina to either high light or low temperature (Maxwell et al., 1995a, 1995b). However, only about 56% of the PSII reaction centers remained open after P. boryanum cells grown at 29°C/150 μmol m−2 s−1 were shifted to 15°C (Table II). This effect of low temperature stress on qP is consistent with that observed for plants and green algae (Maxwell et al., 1995a, 1995b; Gray et al., 1997).
Table II.
Effects of growth temperature and irradiance on the maximal photochemical efficiency of PSII (Fv/Fm) and photochemical Chl fluorescence quenching (qP) of P. boryanum grown under various conditions of temperature/irradiance
| Growth Conditions | Fv/Fm | qP |
|---|---|---|
| ° C/μ mol m−2 s−1 | ||
| 15/10 | 0.518 ± 0.016 | 0.760 ± 0.051 |
| 15/150 | 0.280 ± 0.028 | 0.827 ± 0.018 |
| 29/150 | 0.509 ± 0.015 | 0.864 ± 0.020 |
| 29/150 → 15°C | 0.424 ± 0.010 | 0.559 ± 0.005 |
| 29/750 | 0.255 ± 0.023 | 0.743 ± 0.047 |
The sample 29/150 → 15°C was grown at 29°C/150 μmol m−2 s−1 but measured at 15°C to illustrate the effect of measuring temperature on the photosynthetic parameters. The data represent mean values ± se from six to nine independent experiments.
The kinetics of the fluorescence rise from Fo to Fm is related to the rate of reduction of the PQ pool and has been used to determine the size of the functional PQ pool (Krause and Weis, 1991). This is expressed as a ratio of complementary area over the fluorescence rise for control samples to the complementary area over the fluorescence rise for samples treated with DCMU. DCMU blocks the oxidation of the primary electron-accepting quinone of PSII (QA) by the secondary electron-accepting plastoquinone of PSII (QB); therefore, the fluorescence rise in DCMU-treated cells is much faster than in the absence of the inhibitor. A larger complementary area above the fluorescence rise in the absence of DCMU would indicate a relatively larger functional PQ pool. P. boryanum grown at either 29°C/150 μmol m−2 s−1 or 15°C/10 μmol m−2 s−1 exhibited a 4- to 5-fold larger functional PQ pool in comparison with cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 (Table I).
Redox State of P700 and Intersystem Electron Transport
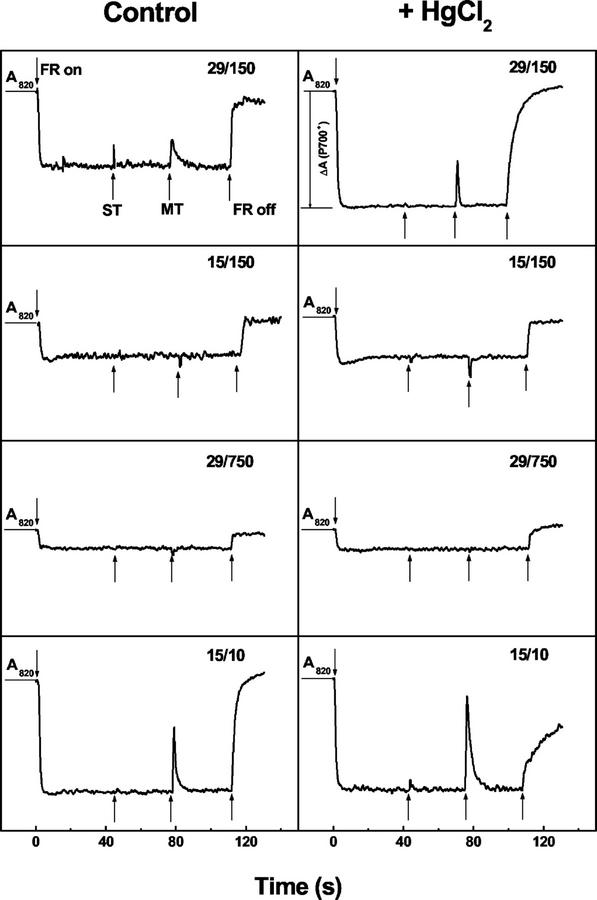
The functional activity of PSI as well as the electron flux through the intersystem electron transport chain can be assessed in vivo by measurements of the redox state of P700 (Schreiber et al., 1988; Mi et al., 1992a, 1992b; Yu et al., 1993; Herbert et al., 1995; Schubert et al., 1995). Figure 4 shows typical traces illustrating the oxidation and reduction of P700 in P. boryanum grown under different conditions of temperature and irradiance. Illumination of the sample with FR light resulted in a change in A820 (ΔA820/A820), which reflects the oxidation of P700 to P700+ (Schreiber et al., 1988). Application of ST or MT flashes of white AL caused a rapid, transient reduction of P700+. Because DCMU inhibited the transient reduction of P700+ induced by the ST and MT flashes (data not shown) with minimal effect on the overall P700+ signal (ΔA820/A820), the source of electrons for the ST- and MT-induced reduction of P700+ is PSII, which does not appear to contribute significantly to ΔA820/A820 (Table III). When the FR light was turned off, the kinetics of the subsequent P700+ reduction in the dark is presumed to occur as a consequence of cyclic electron transport around PSI (Mi et al., 1992b) as well as respiratory electron transport.
Figure 4.
The redox state of P700 in P. boryanum cells grown under various conditions of temperature (°C)/irradiance (μmol m−2 s−1) in the absence (control) and presence of 100 μm HgCl2. The measurements were performed at growth temperatures of either 15°C or 29°C. After reaching a steady-state level of P700+ in the presence of far-red (FR) background light, single-turnover (ST) and multiple-turnover (MT) flashes of actinic light (AL) were applied as described in “Materials and Methods.” Representative traces of three to five independent experiments are shown.
Table III.
The redox state of P700 (Δ A820/A820 × 10−3) and the rate of dark reduction of P700+ (t1/2) in P. boryanum cells grown under various conditions of temperature (oC)/irradiance (μ mol m−2 s−1) in the absence (control) and presence of electron transport inhibitors
| Growth Regime | Control
|
+DCMU
|
+HgCl2
|
+DBMIB
|
||||
|---|---|---|---|---|---|---|---|---|
| Δ A820/A820 | t1/2 | Δ A820/A820 | t1/2 | Δ A820/A820 | t1/2 | Δ A820/A820 | t1/2 | |
| s | s | s | s | |||||
| 15/10 | 5.22 ± 0.35 | 1.53 ± 0.06 | 5.80 ± 0.16 | 1.50 ± 0.05 | 5.28 ± 0.11 | 9.74 ± 0.84 | 6.19 ± 0.07 | 1.91 ± 0.12 |
| 15/150 | 1.15 ± 0.08 | 0.62 ± 0.04 | 1.01 ± 0.21 | 0.71 ± 0.04 | 1.70 ± 0.23 | 2.07 ± 0.43 | 1.88 ± 0.11 | 0.88 ± 0.05 |
| 29/150 | 2.19 ± 0.22 | 1.51 ± 0.21 | 2.05 ± 0.08 | 1.59 ± 0.04 | 2.92 ± 0.31 | 6.88 ± 0.93 | 3.46 ± 0.21 | 2.49 ± 0.41 |
| 29/750 | 0.77 ± 0.08 | 0.66 ± 0.04 | 0.82 ± 0.07 | 0.74 ± 0.05 | 1.21 ± 0.11 | 1.99 ± 0.44 | 1.38 ± 0.01 | 0.85 ± 0.02 |
The data represent mean values ± se from four to eight independent experiments.
Under FR light, ΔA820/A820 was at least 2- to 5-fold lower in cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 than in cells grown at either 15°C/10 μmol m−2 s−1 or 29°C/150 μmol m−2 s−1 (Fig. 4; Table III). A transient reduction of P700+ was induced by ST and MT pulses of white AL in cells grown at either 15°C/10 μmol m−2 s−1 or 29°C/150 μmol m−2 s−1. However, transient reductions of P700+ by ST and MT flashes were not detectable in cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 (Fig. 4). Furthermore, the t1/2 for P700+ reduction in the dark after FR light was turned off was 2.5-fold faster in cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 than in cells grown at either 15°C/10 μmol m−2 s−1 or 29°C/150 μmol m−2 s−1 (Table III), indicating an increased capacity for cyclic electron transport around PSI and respiratory electron transport in these cells (Mi et al., 1992b). The apparent lower values for ΔA820/A820 for cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 (Table III) are consistent with the lower PsaB content observed by immunoblot analysis (Fig. 1A).
To assess whether the FR light used was sufficient to oxidize all of the P700, we examined the effect of supplementing FR light with white AL (data not shown). When cells grown at either 15°C/10 μmol m−2 s−1 or 29°C/150 μmol m−2 s−1 were illuminated with FR light, P700 became oxidized and the addition of AL caused the reduction of P700+ to P700. This indicates that the FR light was of sufficient intensity to oxidize all of the P700 pool. In contrast, oxidation of P700 in cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 was not complete with FR light alone because further oxidation of P700 was observed upon the addition of AL. Regardless, the ΔA820/A820 in these cells was still at least 2-fold lower than in cells grown at either 15°C/10 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 (data not shown).
Effects of Electron Transport Inhibitors on P700 Oxidation/Reduction
In cyanobacteria, the photosynthetic electron transport chain shares redox components with respiratory electron flow (Scherer, 1990; Schmetterer, 1994; Cooley and Vermaas, 2001). Thus, donation of electrons from PSII and Ndh- and succinate dehydrogenase-mediated electron flow from cytosolic respiratory donors and the cyclic electron pathway around PSI as well as consumption of electrons by cytochrome oxidase may all contribute, to varying extents, to the intersystem electron transport and affect PSI photochemistry (Mi et al., 1992a, 1992b; Yu et al., 1993; Herbert et al., 1995; Schubert et al., 1995; Cooley et al., 2000; Cooley and Vermaas, 2001). To assess the contribution of different electron transfer pathways to PSI photochemistry in P. boryanum grown under different conditions of temperature and irradiance, P700 oxidation/reduction transients were measured in the presence of the following inhibitors of electron flow: DCMU, to inhibit the electron donation from PSII; 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (DBMIB), to inhibit entry of electrons from all sources to the cytochrome b6f complex (Trebst, 1980; Yu et al., 1993); and HgCl2, to inhibit Ndh-mediated electron flow from the cytosolic sources (Mi et al., 1992a, 1992b; Fig. 4; Table III). DCMU and DBMIB were tested at various concentrations to determine the minimum concentration required to inhibit photosynthetic electron transport and O2 evolution (Miskiewicz et al., 2000). Similarly, we found that concentrations of HgCl2 less than or equal to 100 μm did not inhibit PSII activity (data not shown). Under these conditions, DCMU had minimal effects on either the ratio of ΔA820/A820 or the rate of reduction of P700+ in the dark in P. boryanum regardless of growth conditions (Table III). This indicated that the excitation of PSII by FR light had a negligible effect on P700 photooxidation.
DBMIB, the inhibitor of the oxidation of the PQ pool by cytochrome b6f complex (Trebst, 1980), induced an increase in the level of P700 photooxidation by approximately 20% in cells grown at 15°C/10 μmol m−2 s−1, 60% in cells grown at 15°C/150 μmol m−2 s−1, and 29°C/150 μmol m−2 s−1 and 80% in cells grown at 29°C/750 μmol m−2 s−1 compared with untreated cells (Table III). However, the ΔA820/A820 remained lower in cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 than in cells grown at either 15°C/10 μmol m−2 s−1 or 29°C/150 μmol m−2 s−1. Moreover, DBMIB-treated cells also exhibited a slower rate of P700+ reduction in the dark than untreated cells (Table III).
HgCl2 at a concentration of 100 μm is considered to block cytosolic electron donation to the intersystem chain in the cyanobacteria Synechocystis sp. PCC 6803 via a dehydrogenase complex (Mi et al., 1992b) and Synechococcus sp. PCC 7002 (Mi et al., 1992a). In P. boryanum grown under control conditions (29°C/150 μmol m−2 s−1), HgCl2 treatment resulted in a 30% increase in the level of P700+ as well as a 4.5-fold decrease in the rate of P700+ dark reduction (Fig. 4; Table III). HgCl2-treated cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 exhibited a 50% increase in the extent of P700 photooxidation, as well as a 3-fold decrease in the rate of P700+ reduction in the dark (Fig. 4; Table III). In contrast, HgCl2 did not induce any changes in the level of P700 photooxidation in cells grown at 15°C/10 μmol m−2 s−1. However, the rate of P700+ reduction in these cells was 6-fold slower in the presence of the inhibitor (Table III). Moreover, HgCl2 also increased the partial reduction of P700+ in response to ST and MT flashes in cells grown at either 15°C/10 μmol m−2 s−1 or 29°C/150 μmol m−2 s−1 (Fig. 4), indicating that HgCl2 at a concentration of 100 μm affected neither PSII nor intersystem electron transport. However, regardless of the inhibitor used, the ΔA820/A820 was always less in cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 than in those grown at either 15°C/10 μmol m−2 s−1 or 29°C/150 μmol m−2 s−1 (Table III).
The changes in P700 oxidation/reduction in the presence of 100 μm HgCl2 in P. boryanum indicate a possible rapid donation of electrons to P700+ mediated by a cytosolic donor such as the Ndh-dehydrogenase complex as suggested by Mi et al. (1992a, 1992b). To assess this possibility, the abundance of the Ndh complex in P. boryanum cells exposed to varying growth regimes was examined by immunoblot analysis of the NdhH subunit (Fig. 5). Cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 exhibited an approximately 60% increase in the level of the NdhH subunit on a Chl basis compared with cells grown at control conditions (29°C/150 μmol m−2 s−1). However, this polypeptide was barely detected in cells grown at 15°C/10 μmol m−2 s−1 (Fig. 5).
Figure 5.
Immunoblot and densitometric analysis of the NdhH subunit of Ndh in P. boryanum cells grown under various conditions of temperature (°C)/irradiance (μmol m−2 s−1). Representative immunoblot and mean values ± se of densitometric data from four independent experiments are shown.
DISCUSSION
It was reported previously that in cyanobacteria, changes in light-harvesting antennae (Raps et al., 1985; de Lorimier et al., 1992; Reuter and Muller, 1993; Garnier et al., 1994; Samson et al., 1994; Nomsawai et al., 1999) and PSI abundance (Zak and Pakrasi, 2000) solely reflect responses to either high growth irradiance or low growth temperature, respectively. However, we suggest that the structural and functional alterations in the photosynthetic apparatus of P. boryanum are not induced by either growth temperature or irradiance per se, but rather reflect a common response to excess excitation for the following reasons. Growth of P. boryanum at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 resulted in a comparable decrease of the PBS size, increase in the relative levels of the PQ pool and cytochrome b6f complex, and reduction in PSI abundance compared with cells grown at either 15°C/10 μmol m−2 s−1 or 29°C/150 μmol m−2 s−1. Moreover, cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 exhibited a comparable decrease in the efficiency of energy transfer from PBS to PSII, decrease in photochemical efficiency, a decrease in the photooxidation level of P700, and an increase in the rate of dark reduction of P700+. These results cannot be explained as a simple growth irradiance response because cells grown at 15°C/150 μmol m−2 s−1 appear to mimic high light-grown cells (29°C/750 μmol m−2 s−1), even though they were grown under moderate irradiance. These results also cannot be explained as a simple growth temperature effect because decreasing growth irradiance from 150 to 10 μmol m−2 s−1 at 15°C results in low temperature-grown cells that are comparable with control cells grown at 29°C/150 μmol m−2 s−1. Thus, we conclude that the stoichiometry of certain specific components of the photosynthetic apparatus, as well as PBS structure, are regulated by both growth temperature and growth irradiance in a similar manner in P. boryanum. This suggests that temperature and light may share a common sensing/signaling mechanism to control the relative abundance of PSI, the cytochrome b6/f complex, the PQ pool, and PBS structure. However, our data clearly indicate that the accumulation of either the Rubisco LS, D1 polypeptide of PSII or the α-subunit of CF1 do not appear to be regulated by this mechanism. Thus, not all components of the photosynthetic apparatus respond to this proposed common sensing/signaling mechanism in P. boryanum.
These results are consistent with those reported for the photosynthetic acclimation of P. boryanum as well as green algae C. vulgaris and D. salina induced by growth under high excitation pressure conditions created either by high light or by low temperature (Huner et al., 1998; Miskiewicz et al., 2000; Wilson and Huner, 2000). Exposure of photosynthetic organisms to such conditions results in an imbalance in energy budget. Under high-light conditions (29°C/750 μmol m−2 s−1), the rate of light absorption through photochemistry exceeds the rate of energy utilization through metabolism and/or the rate of non-radiative dissipation of excess light. A comparable energy imbalance is created by growth at low temperature and moderate irradiance (15°C/150 μmol m−2 s−1) because low temperature preferentially reduces the rate of energy utilization versus photochemistry. This energy imbalance is sensed as a change in the redox poise of intersystem electron transport (Huner et al., 1998). The putative redox sensor for this acclimation process appears to be localized downstream of the cytochrome b6f complex in P. boryanum (Miskiewicz et al., 2000), whereas in green algae, it appears to reside between PSII and the cytochrome b6f complex, indicating that the most likely candidate is the PQ pool (Escoubas et al., 1995; Wilson and Huner, 2000). Thus, we suggest that the modifications in the structure and function of the photosynthetic apparatus in P. boryanum reflect responses to changes in the redox state of the intersystem electron transport chain rather than to temperature or light per se. This conclusion is in agreement with the recent reports of Fujita and coworkers indicating that the redox state of the components of electron transport system is involved in the regulation of the PSI to PSII ratio in cyanobacteria (Murakami and Fujita, 1993; Fujita et al., 1994; Murakami et al., 1997).
Lower levels of P700+ observed in P. boryanum grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 compared with cells grown at either 15°C/10 μmol m−2 s−1 or 29°C/150 μmol m−2 s−1 could reflect lower absolute amounts of PSI and/or enhanced Ndh-mediated electron transfer from cytosolic sources to P700+ (Mi et al., 1992a, 1992b). Because the photooxidation level of P700 was lower in these cells regardless of the treatment used (application of AL, electron transfer inhibitor treatments), it is more likely that this is a consequence of the lower abundance of PSI as shown by immunoblot analysis. Thus, in addition to adjusting light-harvesting efficiency, P. boryanum appears to adjust the stoichiometry of PSII and PSI in favor of PSII when grown under potentially high-excitation pressure (15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1), which allows these cells to keep PSII reaction centers relatively oxidized and consequently prevent photodamage. This is in contrast to the green alga C. vulgaris (Wilson and Huner, 2000) and winter rye (Gray et al., 1998), which exhibited no significant changes in PS stoichiometry when grown under high excitation pressure. Thus, we demonstrate for the first time, to our knowledge, that the stoichiometry of the photosynthetic apparatus of the cyanobacterium P. boryanum, together with the PBS structure and function, is regulated by excess excitation rather than light or temperature per se. Moreover, it appears that in P. boryanum alternative electron transport pathways such as Ndh-mediated electron supply from cytosolic sources to PSI and cyclic electron transport around PSI may play an important role in the maintenance of energy budget and protection of PSII from excess excitation. These pathways were previously shown to be essential for the adjustment of cyanobacteria to high light (Herbert et al., 1995), salt (Tanaka et al., 1997), and iron (Ivanov et al., 2000b) stress.
P. boryanum maintained a comparable capacity to keep about 80% of the QA pool oxidized during growth at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1. Thus, the excitation pressure (1 − qP) estimated for cells grown and acclimated at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 was similar to that observed for cells grown and acclimated at either 15°C/10 μmol m−2 s−1 or 29°C/150 μmol m−2 s−1 and equal to a 1 − qP value of about 0.20 (Table II). This is in sharp contrast to the results observed for overwintering cereals as well as C. vulgaris (Maxwell et al., 1995a; Gray et al., 1997; Wilson and Huner, 2000). However, when P. boryanum grown at 29°C/150 μmol m−2 s−1 were cold stressed by shifting these cells to 15°C/150 μmol m−2 s−1, only 56% of the QA pool remained oxidized, indicating a doubling of the excitation pressure to a value of 0.44 (Table II). Although qP, and hence 1 − qP, may be good estimates of PSII closure in plants and green algae, Campbell et al. (1998) suggest these fluorescence parameters are not good indicators of the relative redox state of PSII reaction centers in Synechococcus sp. PCC 7942 because of the more complex interactions between photosynthetic and respiratory electron transport in cyanobacteria. However, we maintain that qP, and hence 1 − qP, are reasonable indicators of PSII closure in P. boryanum exposed to low temperature stress (Table II). Cells exposed to low temperature stress induced by a sudden shift from 29°C to 15°C at constant irradiance exhibit a 2-fold higher value of 1 − qP than the non-stressed cells. The fact that P. boryanum cells grown and acclimated at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 exhibited similar values of qP as those grown at either 15°C/10 μmol m−2 s−1 or 29°C/150 μmol m−2 s−1 illustrates the greater capacity of this cyanobacterium to acclimate to environmental extremes and to maintain an energy balance than either winter cereals or green algae (Maxwell et al., 1995a; Gray et al., 1997; Wilson and Huner, 2000). We suggest that this may, in part, reflect the more complex interactions between photosynthetic and respiratory electron transport in cyanobacteria than in eukaryotic species. However, caution must be used in the interpretation of such qP data obtained from cyanobacteria because of possible bilin contributions to the Chl fluorescence associated with PSII.
Cyanobacteria are characterized by their dynamic capacity to alter the structure and composition of their photosynthetic apparatus in response to various environmental stimuli such as light quality, irradiance, nutrient stress, and temperature (Fujita et al., 1994; Grossman et al., 1994; Nishida and Murata, 1996; Nomsawai et al., 1999). The responses to both high light and nutrient stress in Synechococcus sp. PCC 7942 is regulated by a two-component sensory system (Grossman et al., 2001). It has been suggested that photoautotrophs may respond to an imbalance in energy budget induced by environmental stresses such as high light and low temperature by reducing light absorption and/or enhancing the capacity of electron sinks (Huner et al., 1998). Growth of P. boryanum at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 appears to induce a reduction in PBS size as well as a decrease in efficiency of energy transfer from PBS to PSII, which would minimize light-harvesting capacity compared with cells grown at either 15°C/10 μmol m−2 s−1 or 29°C/150 μmol m−2 s−1. These results are consistent with a lower photochemical efficiency and lower rates of photosynthesis exhibited by cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 (Miskiewicz et al., 2000). In addition, P. boryanum is unable to adjust growth rates in response to irradiance regardless of the growth temperature (Miskiewicz et al., 2000).
PBSs are considered to be primary light-harvesting antenna for PSII in cyanobacteria and red algae (Sidler, 1994). We assume that PBSs remain primarily attached to PSII regardless of their growth condition. The enhancement of the oxidation of P700 with the addition of white AL to the FR light in cells grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1 might be interpreted to indicate enhanced energy transfer of PBS to PSI rather than to PSII in cells grown under high-excitation pressure. However, with no evidence that PBSs become primarily attached to PSI in high-excitation pressure cells, we consider the following the most likely explanation. PBS remain attached to PSII in cells grown at high-excitation pressure cells and the requirement for white AL in addition to the FR light to oxidize all the P700 in these cells is because of an increased flux of electrons from the cytosol, which would tend to keep P700 in the reduced state.
The lack of P700+ reduction after ST and MT flashes in FR background as well as the effects of inhibitors of electron transport on the P700 photooxidation under FR light in cells grown under high-excitation pressure indicates significant alterations in electron flux in the intersystem electron transport chain. The measurements of P700+ reduction by ST and MT flashes indicate that PSII alone cannot account for the reduction of P700+ in cells grown at high-excitation pressure in contrast to cells grown at low-excitation pressure. Thus, we suggest that the inability to detect P700+ by ST and MT flashes in cells grown at high-excitation pressure may be a consequence of the increased flow of electrons from respiratory sources to the intersystem electron transport chain with a concomitant limitation on the acceptor side of PSI keeping P700 in the reduced state. Furthermore, a more highly reduced PQ pool may also account for the fact that cells grown at high-excitation pressure may exhibit a smaller functional PQ pool size (Table II). This conclusion is supported by the following: (a) Cells grown at high-excitation pressure exhibit lower photosynthetic capacity on a dry weight basis than cells grown at low excitation pressure (Miskiewicz et al., 2000). Furthermore, P. boryanum is unable to increase its exponential growth rate in response to increased irradiance when grown at either 29°C or 5°C (Miskiewicz et al., 2000); (b) Immunoblot analysis of the NdhH subunit showed an increase in the abundance of the Ndh complex in cells grown at high-excitation pressure compared with cells grown at low-excitation pressure; (c) Cells grown at high-excitation pressure exhibit higher rates of respiration than cells grown at low-excitation pressure (Miskiewicz et al., 2000); (d) The presence of either DBMIB or HgCl2 caused a proportionally greater increase in the P700 oxidation in cells grown at high-excitation pressure than those grown at low-excitation pressure; and (e) the rate of dark reduction of P700+ increased when P. boryanum was grown at either 15°C/150 μmol m−2 s−1 or 29°C/750 μmol m−2 s−1, indicating the combined effects of an increased respiratory electron transport as well as possibly higher rates of PSI cyclic electron transport (Mi et al., 1992b). The increased rates of dark respiration and PSI cyclic electron flow appear to be associated with higher ratios of PQ to Chl a and increased levels of the cytochrome b6f complex on a Chl basis. Further studies are needed to assess the contribution of respiratory electron acceptors to the photosynthetic intersystem electron fluxes.
MATERIALS AND METHODS
Culture Conditions
Plectonema boryanum strain UTEX 485 was grown axenically in batch cultures in BG-11 medium (Rippka et al., 1979) buffered with 10 mm HEPES, pH 8.0, at either control temperature of 29°C or low temperature of 15°C under ambient CO2 conditions. Continuous illumination of the cultures was provided by fluorescent lamps (CW-40, Sylvania, Danvers, MA) at 10 or 150 μmol m−2 s−1 or by a 300-W halogen lamp (General Electric, Fairfield, CT) at 750 μmol m−2 s−1. The irradiance was measured at the center of the culture tubes using a quantum sensor attached to a radiometer (model LI-189, LI-COR, Lincoln, NE). The Chl a concentration was determined in 80% (v/v) acetone according to the equation of Arnon (1949). Cells in midexponential growth phase were used for all experiments.
SDS-PAGE and Immunoblot Analysis
Total cellular proteins were extracted from frozen cell pellets as described by Clarke et al. (1993). Protein samples containing equal amounts of Chl a (2.5 μg) were separated on 15% (w/v) SDS-PAGE in the presence of 6 m urea, transferred to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA) and immunodecorated with specific polyclonal antibodies raised against the D1 polypeptide of the PSII reaction center, cytochrome f, the PsaB polypeptide of the PSI reaction center, the α-subunit of ATP synthase, the large subunit of Rubisco, and the NdhH subunit of Ndh. Immunodetection was performed using horseradish peroxidase-conjugated secondary antibodies (Sigma, St. Louis) and enhanced chemiluminescence according to the manufacturer (Amersham-Pharmacia Biotech, Uppsala). Density scanning of x-ray films from each replicate immunoblot was performed with a Scan Jet 4200C Desktop scanner (Hewlett-Packard, Palo Alto, CA) and Scion Image densitometry software (Scion Corporation 1998, Fredrick, MD).
Analysis of PQ Content
The total PQ content was determined as described by Giacometti et al. (1996). Quinones were extracted with dietheylether:hexane (1:3 [v/v]) from cells collected on glass fiber filters (GF/C, Whatman, Clifton, NJ). The extracts were dried under nitrogen and dissolved in 100% (v/v) methanol. HPLC was performed using a Beckman System Gold apparatus (Beckman Instruments, San Ramon, CA) fitted with a CSC-Spherisorb ODS-1 reverse-phase column (5-μm particle size, 250- × 4.6-mm i.d.), an Upchurch Perisorb A guard column (Chromatographic Specialties, Concord, ON, Canada), and a diode array detector. Samples were eluted isocratically with a solvent system of methanol:ethylacetate (68:32 [v/v]) at a flow rate of 1 mL min−1, and absorbance was monitored at 255 nm. The retention time and response factor for PQ was determined by injection of known amounts of decylplastoquinone standard (Sigma).
Isolation and Analysis of PBS
PBS were isolated by Suc gradient centrifugation according to the method of Glazer (1988). The structural integrity of isolated complexes was assayed by fluorescence spectroscopy at room temperature and 77K using a PTI fluorometer (model LS-1, Photon Technology International, Monmouth Junction, NJ). The isolated PBSs were analyzed by absorption spectroscopy and SDS-PAGE. Absorption spectra were recorded with a Beckman DU640 spectrophotometer (Beckman Instruments). The protein concentration was determined using the Bio-Rad DC system according to the manufacturer's instruction. Samples containing 20 μg of protein were precipitated with ice-cold 10% (w/v) trichloroacetic acid and kept at 0°C for 15 min. Precipitated proteins were resuspended in Laemmli buffer (100 mm Tris-HCl [pH 6.8] containing 4% [w/v] SDS, 0.1% [w/v] bromphenol blue, 10% [v/v] glycerol, and 5% [v/v] 2-mercaptoethanol), boiled for 2 min, and separated on 15% (w/v) SDS-PAGE. The chromophore-containing proteins were visualized on the gels, before Coomassie blue staining, by treatment with 100 mm ZnSO4 and illumination on a UV transilluminator (Raps, 1990).
Low-Temperature (77K) Fluorescence Spectra
77K fluorescence emission spectra of whole cells were measured with an excitation wavelength of 580 nm using a PTI Fluorometer (model LS-1, Photon Technology International). The cells were harvested and resuspended in fresh BG-11 medium to a Chl a concentration of 5 μg mL−1, dark adapted for 30 min, and quickly frozen in liquid nitrogen before measurement. The spectra were analyzed in terms of Gaussian bands by a nonlinear least-squares algorithm that minimizes the chi square function using a Microcal Origin Version 6.0 software package (Microcal Software Inc., Northampton, MA).
Chl a Fluorescence Measurements
Chl fluorescence of dark-adapted P. boryanum cell suspensions at the corresponding growth temperatures of either 15°C or 29°C was measured with a PAM-101 Chl fluorescence measuring system (Heinz Walz GmbH, Effeltrich, Germany) equipped with an emitter-detector cuvette assembly unit ED-101US/D as described by Schreiber (1994). Fo was excited by a nonactinic modulated measuring beam (650 nm, 0.12 μmol m−2 s−1) at 1.6 kHz in the dark and 100 kHz in the light. Fm was induced by saturating light pulses (800 ms, 2,800 μmol m−2 s−1) provided by a Schott lamp (KL1500, Schott Glaswerke, Mainz, Germany) and controlled from a Walz PAM 103 Trigger Control Unit. The AL corresponded to the growth irradiance. The qP parameter was evaluated under steady-state photosynthesis and calculated according to Schreiber (1994). The functional size of the PQ pool was estimated as the ratio of the complementary area over the fluorescence rise from Fo to Fm in the absence and presence of 20 μm DCMU (Krause and Weis, 1991).
Measurements of the Redox Level of P700
The redox state of P700 in P. boryanum samples prepared according to Herbert et al. (1995) was estimated as a change in absorbance around 820 nm (ΔA820/A820) at the growth temperature of either 15°C or 29°C using a PAM-101 Chl fluorescence measuring system (Heinz Walz GmbH) equipped with ED-800T emit-detector and PAM-102 units according to Schreiber et al. (1988). The oxidation of P700 was induced by illumination with FR light (λmax 715 nm, 10 W m−2, Schott filter 715) or white AL, equivalent to growth irradiance, provided by the FL-101 light source. After reaching a steady-state level of P700+ in the presence of FR background, ST (half-peak width of 14 μs), and MT (50 ms), pulses of AL were applied with an XMT-103 and XST-103 power/control units, respectively, via a multibranched fiber optic system connected to the emitter-detector unit and the cuvette. The P700 transients were measured in the absence and presence of the following inhibitors of electron transport: 10 μm DCMU, 12 μm DBMIB, and 100 μm HgCl2. Freshly prepared stock solutions of DCMU and DBMIB in 95% (v/v) ethanol were added to the cell suspension to a final solvent concentration of 0.5% (v/v).
ACKNOWLEDGMENTS
We thank Dr. Parag R. Chitnis (Iowa State University, Ames), Dr. Eva-Marie Aro (University of Turku, Finland), Dr. John E. Thompson (University of Waterloo, Canada), Dr. Adrian K. Clarke (Göteborg University, Sweden), and Dr. Dominique Rumeau (Commissariat à l'Energie Atomique, Paris) for their generous gifts of antibodies against PsaB protein, D1 protein, cytochrome f, the α-subunit of ATP synthase, and the NdhH subunit of Ndh, respectively.
Footnotes
This work was supported by the Natural Sciences and Engineering Research Council of Canada (grant to N.P.A.H.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.008631.
LITERATURE CITED
- Adhikary SP, Murakami A, Ohki K, Fujita Y. Photoregulation of respiratory activity in the cyanophyte Synechocystis PCC 6714: the possibility of the simultaneous regulation of the amount of PSI complex and the activity of respiratory terminal oxidase in thylakoids. Plant Cell Physiol. 1990;31:527–532. [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D, Hurry V, Clarke AK, Gustafsson P, Öquist G. Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol Mol Biol Rev. 1998;62:667–683. doi: 10.1128/mmbr.62.3.667-683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AK, Soitamo A, Gustafsson P, Öquist G. Rapid exchange between two distinct forms of cyanobacterial photosystem II reaction-center protein D1 in response to photoinhibition. Proc Natl Acad Sci USA. 1993;90:9973–9977. doi: 10.1073/pnas.90.21.9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley JW, Hewitt CA, Vermaas WFJ. Succinate:quinol oxidoreductases in the cyanobacterium Synechocystis sp. strain PCC 6803: presence and function in metabolism and electron transport. J Bacteriol. 2000;182:714–722. doi: 10.1128/jb.182.3.714-722.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley JW, Vermaas WFJ. Succinate dehydrogenase and other respiratory pathways in thylakoid membranes of Synechocystis sp. strain PCC 6803: capacity comparisons and physiological function. J Bacteriol. 2001;183:4251–4258. doi: 10.1128/JB.183.14.4251-4258.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorimier RM, Smith RL, Stevens SE. Regulation of phycobilisome structure and gene expression by light intensity. Plant Physiol. 1992;98:1003–1010. doi: 10.1104/pp.98.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoubas J-M, Lomas M, LaRoche J, Falkowski PG. Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool. Proc Natl Acad Sci USA. 1995;92:10237–10241. doi: 10.1073/pnas.92.22.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Murakami A, Aizawa K, Ohki K. Short-term and long-term adaptation of the photosynthetic apparatus: homeostatic properties of thylakoids. In: Bryant DA, editor. The Molecular Biology of Cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 677–692. [Google Scholar]
- Gantt E. Supramolecular membrane organization. In: Bryant DA, editor. The Molecular Biology of Cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 119–138. [Google Scholar]
- Garnier F, Dubacq J-P, Thomas J-C. Evidence for a transient association of new proteins with the Spirulina maxima phycobilisome in relation to light intensity. Plant Physiol. 1994;106:747–754. doi: 10.1104/pp.106.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. Phycobilisomes. Methods Enzymol. 1988;167:304–312. [Google Scholar]
- Giacometti GM, Barbato R, Chiaramonte S, Friso G, Rigoni F. Effects of ultraviolet-B radiation on photosystem II of the cyanobacterium Synechocystis sp. PCC 6083. Eur J Biochem. 1996;242:799–806. doi: 10.1111/j.1432-1033.1996.0799r.x. [DOI] [PubMed] [Google Scholar]
- Gray GR, Chauvin L-P, Sarhan F, Huner NPA. Cold acclimation and freezing tolerance: a complex interaction of light and temperature. Plant Physiol. 1997;114:467–474. doi: 10.1104/pp.114.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GR, Ivanov AG, Krol M, Huner NPA. Adjustment of thylakoid plastoquinone content and photosystem I electron donor pool size in response to growth temperature and growth irradiance in winter rye (Secale cereale L.) Photosynth Res. 1998;56:209–221. [Google Scholar]
- Grossman AR, Bhaya D, He Q. Tracking the light environment by cyanobacteria and the dynamic nature of light harvesting. J Biol Chem. 2001;276:11449–11452. doi: 10.1074/jbc.R100003200. [DOI] [PubMed] [Google Scholar]
- Grossman AR, Schaefer MR, Chiang GG, Collier JL. The responses of cyanobacteria to environmental conditions: light and nutrients. In: Bryant DA, editor. The Molecular Biology of Cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 641–675. [Google Scholar]
- Herbert SK, Martin RE, Fork DC. Light adaptation of cyclic electron transport through photosystem I in the cyanobacterium Synechococcus sp. PCC 7942. Photosynth Res. 1995;46:277–285. doi: 10.1007/BF00020441. [DOI] [PubMed] [Google Scholar]
- Huner NPA, Öquist G, Sarhan F. Energy balance and acclimation to light and cold. Trend Plant Sci. 1998;3:224–230. [Google Scholar]
- Ivanov AG, Miskiewicz E, Clarke AK, Greenberg BM, Huner NPA. Protection of photosystem II against UV-A and UV-B radiation in the cyanobacterium Plectonema boryanum: the role of growth temperature and irradiance. Photochem Photobiol. 2000a;72:772–779. doi: 10.1562/0031-8655(2000)072<0772:popiau>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ivanov AG, Park Y-I, Miskiewicz E, Raven JA, Huner NPA, Öquist G. Iron stress restricts photosynthetic intersystem electron transport in Synechococcus sp. PCC 7942. FEBS Lett. 2000b;485:173–177. doi: 10.1016/s0014-5793(00)02211-0. [DOI] [PubMed] [Google Scholar]
- Kehoe D, Grossman AR. Sensor of chromatic adaptation is similar to the phytochrome and ethylene receptor. Science. 1996;273:1409–1412. doi: 10.1126/science.273.5280.1409. [DOI] [PubMed] [Google Scholar]
- Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:313–345. [Google Scholar]
- Maxwell DP, Falk S, Huner NPA. Photosystem II excitation pressure and development of resistance to photoinhibition: I. Light-harvesting complex II abundance and zeaxanthin content in Chlorella vulgaris. Plant Physiol. 1995b;107:687–694. doi: 10.1104/pp.107.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DP, Laudenbach DE, Huner NPA. Redox regulation of light-harvesting complex II and cab mRNA abundance in Dunaliella salina. Plant Physiol. 1995a;109:787–795. doi: 10.1104/pp.109.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Endo T, Schreiber U, Asada K. Donation of electrons from cytosolic components to the intersystem chain in the cyanobacterium Synechococcus sp. PCC 7002 as determined by the reduction of P700+ Plant Cell Physiol. 1992a;33:1099–1105. [Google Scholar]
- Mi H, Endo T, Schreiber U, Ogawa T, Asada K. Electron donation from cyclic and respiratory flows to the photosynthetic intersystem chain is mediated by pyridine nucleotide dehydrogenase in the cyanobacterium Synechocystis PCC 6803. Plant Cell Physiol. 1992b;33:1233–1237. [Google Scholar]
- Miskiewicz E, Ivanov AG, Williams JP, Khan MU, Falk S, Huner NPA. Photosynthetic acclimation of the filamentous cyanobacterium, Plectonema boryanum UTEX 485, to temperature and light. Plant Cell Physiol. 2000;41:767–775. doi: 10.1093/pcp/41.6.767. [DOI] [PubMed] [Google Scholar]
- Murakami A, Fujita Y. Regulation of photosystem stoichiometry in the photosynthetic system of the cyanophyte Synechocystis PCC 6714 in response to light-intensity. Plant Cell Physiol. 1991;32:223–230. [Google Scholar]
- Murakami A, Fujita Y. Regulation of stoichiometry between PSI and PSII in response to light regime for photosynthesis observed with Synechocystis PCC 6714: relationship between redox state of cyt b6-f complex and regulation of PSI formation. Plant Cell Physiol. 1993;34:1175–1180. [Google Scholar]
- Murakami A, Kim S-J, Fujita Y. Changes in photosystem stoichiometry in response to conditions for cell growth observed with the cyanophyte Synechocystis PCC 6714. Plant Cell Physiol. 1997;38:392–397. doi: 10.1093/oxfordjournals.pcp.a029181. [DOI] [PubMed] [Google Scholar]
- Nishida I, Murata N. Chilling sensitivity in plants and cyanobacteria: the crucial contribution of membrane lipids. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:541–568. doi: 10.1146/annurev.arplant.47.1.541. [DOI] [PubMed] [Google Scholar]
- Nomsawai P, Tandeau de Marsac N, Thomas JC, Tanticharoen M, Cheevadhanarak S. Light regulation of phycobilisome structure and gene expression in Spirulina platensis C1 (Arthrospira sp. PCC 9438) Plant Cell Physiol. 1999;40:1194–1202. [Google Scholar]
- Raps S. Differentiation between phycobiliprotein and colorless linker polypeptides by fluorescence in the presence of ZnSO4. Plant Physiol. 1990;92:358–362. doi: 10.1104/pp.92.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raps S, Kycia JH, Ledbetter MC, Siegelman HW. Light intensity adaptation and phycobilisome composition of Microcystis aeruginosa. Plant Physiol. 1985;79:983–987. doi: 10.1104/pp.79.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter W, Muller C. Adaptation of the photosynthetic apparatus of cyanobacteria to light and CO2. J Photochem Photobiol B Biol. 1993;21:3–27. [Google Scholar]
- Rippka D, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- Salehian O, Bruce D. Distribution of excitation energy in photosynthesis: quantification of fluorescence yields from intact cyanobacteria. J Lumines. 1992;51:91–98. [Google Scholar]
- Samson G, Herbert SK, Fork DC, Laudenbach DE. Acclimation of the photosynthetic apparatus to growth irradiance in a mutant strain of Synechococcus lacking iron superoxide dismutase. Plant Physiol. 1994;105:287–294. doi: 10.1104/pp.105.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S. Do photosynthetic and respiratory electron transport chains share redox proteins? Trends Biochem Sci. 1990;15:458–462. doi: 10.1016/0968-0004(90)90296-n. [DOI] [PubMed] [Google Scholar]
- Schmetterer G. Cyanobacterial respiration. In: Bryant DA, editor. The Molecular Biology of Cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 409–435. [Google Scholar]
- Schreiber U. New emitter-detector-cuvette assembly for measuring modulated chlorophyll fluorescence of highly diluted suspensions in conjunction with the standard PAM fluorometer. Z Naturforsch. 1994;49c:646–656. [Google Scholar]
- Schreiber U, Klughammer C, Neubauer C. Measuring P700 absorbance changes around 830 nm with a new type of pulse modulated system. Z Naturforsch. 1988;43c:686–698. [Google Scholar]
- Schubert H, Matthijs HCP, Mur LR. In vivo assay of P700 redox changes in the cyanobacterium Fremyella diplosiphon and the role of cytochrome c oxidase in regulation of photosynthetic electron transfer. Photosynthetica. 1995;31:517–527. [Google Scholar]
- Sidler WA. Phycobilisome and phycobiliprotein structures. In: Bryant DA, editor. The Molecular Biology of Cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 139–216. [Google Scholar]
- Tanaka Y, Katada S, Ishikawa H, Ogawa T, Takabe T. Electron flow from NAD(P) H dehydrogenase to photosystem I is required for adaptation to salt shock in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 1997;38:1311–1318. [Google Scholar]
- Trebst A. Inhibitors of electron flow: tools for the functional and structural localization of carriers and energy conservation sites. Methods Enzymol. 1980;69:675–715. [Google Scholar]
- Wilson KE, Huner NPA. The role of growth rates, redox-state of the plastoquinone pool and the trans-thylakoid ΔpH in photoacclimation of Chlorella vulgaris to growth irradiance and temperature. Planta. 2000;212:93–102. doi: 10.1007/s004250000368. [DOI] [PubMed] [Google Scholar]
- Yu L, Zhao, Mohlenhoff U, Bryant DA, Golbeck JH. PsaE is required for in vivo cyclic electron flow around photosystem I in the cyanobacterium Synechococcus sp. PCC 7002. Plant Physiol. 1993;103:171–180. doi: 10.1104/pp.103.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak E, Pakrasi HB. The BtpA protein stabilizes the reaction center proteins of photosystem I in the cyanobacterium Synechocystis sp. PCC 6803 at low temperature. Plant Physiol. 2000;123:215–222. doi: 10.1104/pp.123.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]