Abstract
Gravity plays a fundamental role in plant growth and development, yet little is understood about the early events of gravitropism. To identify genes affected in the signal perception and/or transduction phase of the gravity response, a mutant screen was devised using cold treatment to delay the gravity response of inflorescence stems of Arabidopsis. Inflorescence stems of Arabidopsis show no response to gravistimulation at 4°C for up to 3 h. However, when gravistimulated at 4°C and then returned to vertical at room temperature (RT), stems bend in response to the previous, horizontal gravistimulation (H. Fukaki, H. Fujisawa, M. Tasaka [1996] Plant Physiology 110: 933–943). This indicates that gravity perception, but not the gravitropic response, occurs at 4°C. Recessive mutations were identified at three loci using this cold effect on gravitropism to screen for gravity persistence signal (gps) mutants. All three mutants had an altered response after gravistimulation at 4°C, yet had phenotypically normal responses to stimulations at RT. gps1-1 did not bend in response to the 4°C gravity stimulus upon return to RT. gps2-1 responded to the 4°C stimulus but bent in the opposite direction. gps3-1 over-responded after return to RT, continuing to bend to an angle greater than wild-type plants. At 4°C, starch-containing statoliths sedimented normally in both wild-type and the gps mutants, but auxin transport was abolished at 4°C. These results are consistent with GPS loci affecting an aspect of the gravity signal perception/transduction pathway that occurs after statolith sedimentation, but before auxin transport.
Gravity is a constant stimulus governing the orientation of plant growth. In response to changes in the gravity vector, plants reorient by differential growth. When placed horizontally, shoots and roots of a plant exhibit asymmetric growth resulting in upward or downward curvature, respectively. Gravitropism is only one part of a complex response network that integrates information from developmental and environmental stimuli (Ramussen, 1995). For simplicity, the response pathway has been separated into three sequential steps: gravity perception, signal transduction, and asymmetric growth response (for review, see Sack, 1991). The first step in gravity perception is the sedimentation of amyloplasts in shoots and roots (Sack, 1991; Kiss, 2000; Weise et al., 2000). Later aspects of the gravity response are driven by the asymmetric distribution of auxin that is thought to induce the differential growth that is required for gravitropic curvature (for review, see Muday, 2001). What is much less clear are the mechanisms by which statolith sedimentation initiates the transduction of the gravitropic signal and directs the asymmetric distribution of auxin.
Arabidopsis has emerged as a powerful genetic model and has been useful in the dissection of the molecular mechanisms of the gravity response (Tasaka et al., 1999; Chen et al., 2002). Arabidopsis mutants with gravitropic response defects specific to the inflorescence stem, hypocotyl, or root have been isolated. Several mutants with delayed gravitropic bending have reductions in starch content and are presumed to be defective in stimulus perception (for review, see Kiss, 2000). Other mutants are pleiotropic with dramatic effects on growth (Estelle and Somerville, 1987; Fukaki et al., 1997; Mullen et al., 1998) or are altered in their response to auxin or in the proteins that mediate or regulate auxin transport (for review, see Muday, 2001). Only a handful of mutants have been identified as potential mutants in gravity signal transduction. One such Arabidopsis mutant, arg1, lacks normal root and hypocotyl gravitropism but has normal starch content and hormone response (Sedbrook et al., 1999). The arg1/rhg mutant has a defect in a gene encoding a protein with a DnaJ domain, found in a number of proteins that interact with the cytoskeleton (Fukaki et al., 1997; Sedbrook et al., 1999; Kato et al., 2002). Another, the rcn1 mutant, has a reduced rate of gravitropic bending that is the result of a mutation in a protein phosphatase regulatory subunit that leads to altered regulation of auxin transport (Rashotte et al., 2001).
Several shoot gravity response (sgr) mutants have been isolated that have a reduced gravitropic response of the inflorescence stem of Arabidopsis (Fukaki et al., 1996b; Yamauchi et al., 1997). sgr1/scr and sgr7/shr appear to encode transcriptional factors that are required for development of the statolith-containing shoot endodermis, and both appear to be defective in stimulus perception (Di Laurenzio et al., 1996; Fukaki et al., 1998; Helariutta et al., 2000). The recent cloning of two more of the SGR genes, SGR2 and ZIG/SGR4, suggests that the vacuolar membrane system may have a role in the early events of shoot gravitropism (Kato et al., 2002; Morita et al., 2002). Many of the other sgr mutants appear to be affected in the differential growth response (Fukaki et al., 1996b; Yamauchi et al., 1997). But sgr5, and sgr6 have normal starch accumulation and normal phototropic response, suggesting that these defects are also attributable to a step between gravity perception and differential growth (Yamauchi et al., 1997). As yet, these mutant genes have not been cloned, and no other Arabidopsis mutants have been reported that are likely to be involved in the early events of gravitropic signal transduction.
The gravitropic response of the inflorescence stem of Arabidopsis is rapid, with curvature visible within 30 min of stimulation and a complete reorientation of the inflorescence apex resulting within 2 h (Fukaki et al., 1996a). However, horizontal gravistimulation for up to 3 h at 4°C does not cause any curvature. When the stems that are gravistimulated in the cold are subsequently placed in the vertical position at room temperature (RT), they show a transient bend in response to the previous, gravistimulation at 4°C (Fukaki et al., 1996a). These results indicate that the gravity perception step can occur at 4°C but that part of the response is sensitive to cold. Stems incubated at 4°C horizontally for 30 min and then vertically for up to 60 min still bend in response to the horizontal gravistimulation when returned to RT, suggesting that the signal initiated at 4°C can persist for up to 1 h (Fukaki et al., 1996a).
To identify components of early signal transduction events, we used the cold effect on gravity signal persistence, a phenomenon that is here designated the gravity persistent signal (GPS) response, to select for mutants with an altered gravitropic signal transduction and/or storage mechanism. In this paper, the identification and initial characterization of several gravity persistent signal (gps) mutants at three independent loci (GPS1, GPS2, and GPS3) are described.
RESULTS
Isolation and Genetic Characterization of Arabidopsis Mutants with Altered Response to Gravistimulation at 4°C
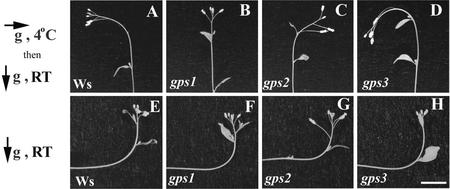
Wild-type inflorescence stems did not bend after 2 h of gravitropic stimulation at 4°C, however, upon return to RT in a vertical orientation, wild-type inflorescences bent in response to the gravity vector at 4°C as shown in Figure 1A. This bending was transient, and wild-type stems began to respond to the new RT gravity vector after 2 h, as shown in Figure 2.
Figure 1.
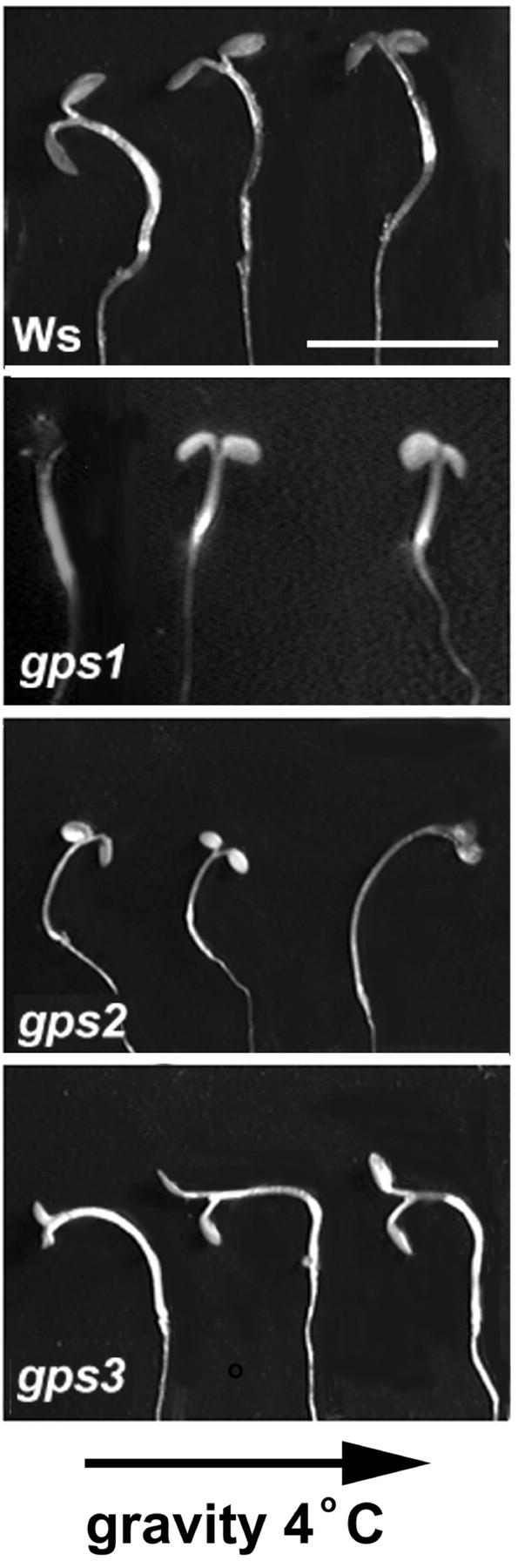
Phenotypes of gps1-1, gps2-1, and gps3-1. Ws is the wild-type Arabidopsis ecotype background from which the mutants were isolated. gps1-1 (no response) represents plants that did not respond to gravistimulation at 4°C and thus remain vertical when returned to RT. gps2-1 (wrong way) plants bent the “wrong way” after the cold stimulus. gps3-1 (over achiever) plants over-responded to the stimulus; they bend in the correct direction but continue to bend past a 90° reorientation. All three gps mutants respond normally to constant gravistimulation at RT (bottom). The arrows indicate the gravity vector in relation to the plants at the temperatures indicated. Scale bar = 1 cm.
Figure 2.
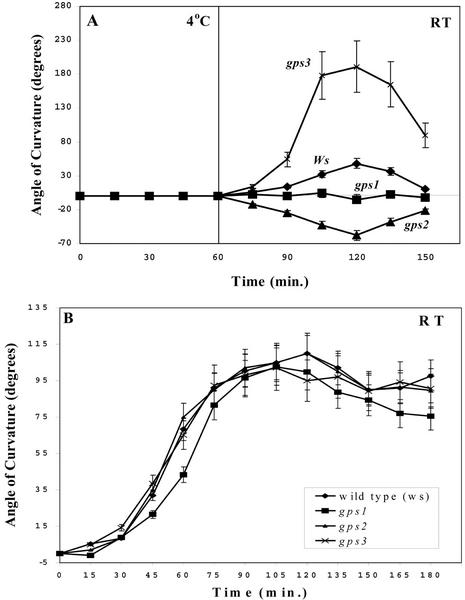
Kinetics of the delayed gravitropic response are similar in gps2-1 and wt. A, Plants were placed horizontally for 1 h at 4°C and then returned to vertical at 21°C and measurements of the resulting angle of curvature were taken at 15-min intervals. B, Plants were placed horizontally in the dark at 21°C, and measurements of the angle of curvature were taken at 15-min intervals. Zero degrees of curvature represents no bending of the inflorescence stem. Each point is the average of a minimum of 10 plants. Bars indicate sd of the mean.
To isolate novel gravitropic mutants, we screened a T-DNA mutagenized seed population for mutant lines whose inflorescence stem showed an altered RT response after gravistimulation at 4°C. Eight mutant lines were isolated that have an altered RT response after a 4°C gravity stimulus from a screen of mutagenized seed lots representing 6,500 independent T-DNA transformants. Figure 1 shows the altered gravitropic response of the inflorescence stems compared with wild-type for three of those lines, gps1-1, gps2-1 and gps3-1, representing three distinct mutant phenotypes designated no response, wrong way, and over achiever, respectively. The first, gps1-1, showed no response to gravistimulation at 4°C when returned to RT (Fig. 1B). gps2-1 showed a reversed gravitropic response to the 4°C gravity stimulus compared with wild-type after return to RT (bent the wrong way, Fig. 1C). The gps3-1 mutant over-responded to the stimulus compared with wild-type after return to RT (Fig. 1D). It continued to bend to a greater angle than wild-type, before the RT gravity stimulation reversed the direction of growth. All three mutants, like wild-type, showed no gravity response during the 2-h stimulation in the cold and exhibited a normal gravity response when the stimulus was presented at RT (Fig. 1, F–H).
The genetic properties of the gps mutants were examined by crossing the mutant lines with wild-type plants and determining the segregation of the mutant traits in F1 and F2 progeny. In the F1 generation, all progeny showed a normal response to gravistimulation at 4°C, indicating that the gps mutations were recessive. In the F2 progeny, the mutations segregated approximately 3:1 wt:mutant phenotype (for gps1-1, 74:23; for gps2-1, 132:41; and for gps3-1, 67:21) confirming the recessive character and demonstrating that the traits in these mutant lines segregated as single Mendelian mutations. To determine the number of complementation groups, the gps lines were crossed to each other, both within and across phenotype classes. Complementation tests revealed three alleles at the gps1 loci (gps1-1, -2, and -3), four alleles at the gps2 loci (gps2-1, -2, -3, and -4) and a single allele at the gps3 loci (gps3-1), and that each locus is distinct.
Growth Characteristics of the gps Mutants
Many gravity mutants are pleiotropic (Chen et al., 1999). To determine whether the gps mutants showed altered growth or phototropism, measurements were made of several parameters in mutant and wild-type plants grown under identical conditions and are summarized in Table I. gps1-1 and gps2-1 appeared normal for all aspects of growth that were examined; gps3-1 showed only a slight decrease in the height of the inflorescence stem but was not significantly different in any other phenotype examined (Table I). Root and hypocotyl lengths in both full light and total darkness were not statistically different from wild-type plants. The phototropic response of inflorescences, as determined by assessment of the angle of curvature after 3 h of unidirectional light, was also normal in all cases. The mutant plants appeared normal except for their ability to respond to gravistimulation at low temperatures upon return to RT.
Table I.
Growth characteristics of the gps mutants
| Wild Type | gps1 | gps2 | gps3 | |
|---|---|---|---|---|
| Adult aerial phenotype | ||||
| Primary inflorescence length (cm) | 10.62 ± 0.55 | 11.51 ± 1.0 | 7.87 ± 0.6 | 6.15 ± 0.7** |
| No. of lateral branches | 2.7 ± 0.3 | 2.9 ± 0.3 | 2.3 ± 0.2 | 2.3 ± 0.2 |
| No. of rosette leaves | 10.2 ± 0.6 | 10.8 ± 0.4 | 9.6 ± 0.4 | 9.8 ± 0.4 |
| Phototropic response | Normal | Normal | Normal | Normal |
| Seedling phenotype | ||||
| Primary root length (mm) | ||||
| Full light | 6.9 ± 0.5 | 7.85 ± 0.6 | 7.01 ± 0.4 | 7.41 ± 0.5 |
| Etiolated | 7.55 ± 0.5 | 7.61 ± 0.7 | 7.83 ± 0.6 | 7.18 ± 0.4 |
| Hypocotyl length (mm) | ||||
| Full light | 4.8 ± 0.3 | 5.05 ± 0.2 | 4.15 ± 0.3 | 4.13 ± 0.2 |
| Etiolated | 10.48 ± 0.8 | 10.68 ± 0.4 | 10.31 ± 0.54 | 10.01 ± 0.5 |
| Phototropic response | Normal | Normal | Normal | Normal |
Data represent the mean of 10 (adult) to 30 (seedling) plants ± se. n, Normal, determined as the response to unidirectional light as compared with wild type. Parameters showing a significant difference from wild type are indicated by a double asterisk. Differences were determined using a one-way ANOVA with a level of 0.05.
Gravitropic Response Kinetics of the gps Mutants
To determine whether the gps mutants showed alterations in the duration or bending rate of the initial gravitropic response, a kinetic analysis was performed. Figure 2 shows a time course for the response of the inflorescence stems of wild-type plants, gps1-1, gps2-1, and gps3-1 after 2 h of gravistimulation in the cold and return to RT. As previously noted, wild-type inflorescences bent in response to the gravity vector at 4°C. gps1-1 either did not recognize the stimulus at 4°C or was incapable of retaining that signal and, therefore, showed no response to 2 h of gravistimulation at 4°C. However, gps1-1 did respond normally to gravistimulation at ambient temperatures, as shown in Figure 2B. In contrast, gps2-1 recognized the 2-h cold gravity stimulus but responded by bending in the wrong direction with similar kinetics to wild-type.
The direction of curvature of gps3-1 was similar to that of WT; however, gps3-1 had an exaggerated response to the gravitropic stimulus. The stem continued to bend past the predicted 90° reorientation, in some cases to as much as 230°. The time required to reach the maximum gravitropic response, before plants reoriented relative to the RT vertical orientation, was equivalent (Fig. 2). Thus, the rate of bending in this mutant is increased compared with WT. The increased rate of bending appeared to be specific to the differential growth phase after the cold gravistimulation. The overall growth rate of the inflorescence of gps3-1 does not appear to be elevated, though, because the inflorescence size is slightly reduced compared with wild-type (Table I).
The defects in these mutants are only in response to gravitropic stimulation in the cold. The gravitropic bending in these mutants was also compared with wild-type after gravity stimulation at RT, as shown in Figure 2B. The direction and rate of gravitropic bending in all three mutants was not significantly different from wild-type. In addition, the response to the RT vertical vector after cold treatment appeared to be wild-type for at least gps2-1 and gps3-1, the mutants that responded, because the shoot apex was restored to a vertical angle in both mutant classes by 2 h after return to RT.
Statolith Sedimentation and Auxin Transport in Response to Cold Treatment
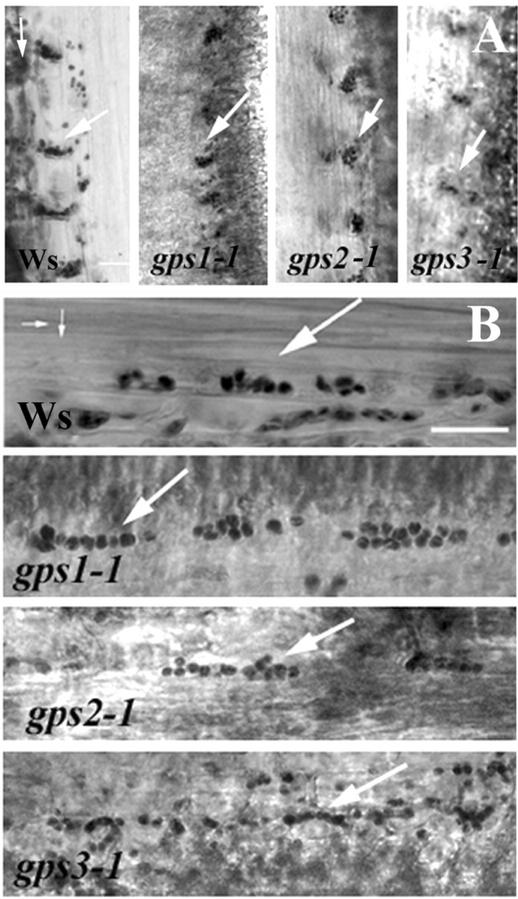
To determine whether the absence of gravitropic bending during gravity stimulation at cold temperatures was attributable to impaired statolith movement, statolith sedimentation in inflorescence stem sections of WT Arabidopsis and the gps mutants was examined in the cold at two consecutive time points. Figure 3 shows sections from stems incubated at 4°C vertically for 30 min (Fig. 3A) and vertically for 30 min and then turned horizontally for 60 min (Fig. 3B). In each case, statoliths in the endodermis sedimented in the direction of the gravity vector, indicating that statolith sedimentation was not prevented by cold temperatures, nor was it affected by the gps mutations.
Figure 3.
Starch in the endodermis of inflorescence stems of both wild-type Arabidopsis and the gps mutants sediments at 4°C. Stems were incubated at 4°C vertically for 30 min (A) or vertically for 30 min then turned horizontally for 60 min (B). The apical 5 cm of the inflorescence stems was removed and fixed at 4°C to maintain their previous orientation. The photographs are positioned to show the final orientation of the plant. Small arrows indicate the direction of the gravity vector, first vertical relative to the inflorescence stems, then horizontal. Longitudinal sections were prepared and stained with I2 for detection of starch. Large arrows indicate amyloplasts within the endodermis of the inflorescence stems. Scale bar = 5 μm.
Chilling temperatures have been shown to slow or stop transport of exogenous auxin from the shoot apex in a variety of plant species (Morris, 1979), suggesting that the lack of a gravitropic response at 4°C may be because of a lack of auxin redistribution in target tissues. To test whether low temperatures affected auxin transport in Arabidopsis inflorescence stems, basipetal auxin transport was first assessed using continuous loading of [3H]indole-3-acetic acid ([3H]IAA) from a buffered solution (Okada et al., 1991). A comparison of auxin transport in plants at 4°C and RT is shown in Table II. When plants were exposed to 4°C before the assay and auxin transport measured at 4°C, almost no auxin movement was detectable. When plants were exposed to 4°C before the assay, but transport was measured at RT, the amount of IAA transported was similar to plants pretreated and tested at RT. As [3H]IAA was supplied continuously throughout the 5-h transport assay, the lack of effect of the cold pretreatment was not surprising and demonstrates that transport recovered during the assay.
Table II.
Effect of cold on [3H]IAA basipetal transport in Arabidopsis inflorescence
| Temperature (°C)
|
IAA Transport
|
||
|---|---|---|---|
| Pretreatment | Transport | pmola | P valueb |
| 25 | 25 | 26.03 + 3.13 | – |
| 4 | 25 | 27.59 + 4.63 | 0.39 |
| 4 | 4 | 1.67 + 0.31 | 5.9 × 10−5 |
The values of IAA transported are the average and se from 13 to 15 plants. The radioactivity transported into the basal 5 mm was determined after 5 h.
The P values were obtained by a one-tailed Student's t test for unequal variance with samples compared to the samples with 25°C pretreatment and transport.
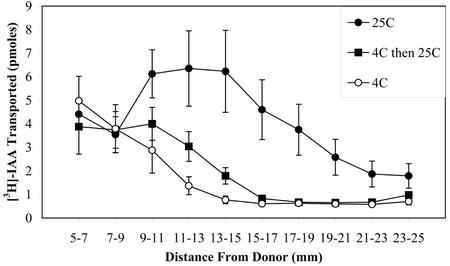
To further test whether the cold pretreatment affected auxin transport when plants were returned to RT, a pulse-chase approach was used. In this assay, plants were incubated with [3H]IAA for only 10 min after return to RT. Plants were then transferred to buffer with nonradioactive IAA, and the transport was determined in multiple segments down the length of the inflorescence, as shown in Figure 4. In this case, it was clear that a cold pretreatment was sufficient to dramatically reduce auxin transport in Arabidopsis inflorescence tissue. These data suggest that the GPS response is caused by an aspect of the gravity signal perception and/or transduction pathway that is after statolith movement but that leads to redistribution or transport of auxin during the gravity response. Initial polar auxin transport experiments with the gps mutants have not been informative, in part because the lateral redistribution of auxin across the inflorescence is of particular interest, and methodology to examine lateral auxin transport is not yet available. A more detailed assessment of auxin transport and redistribution after cold treatment is in progress but is beyond the scope of this paper.
Figure 4.
Effect of cold temperature on auxin transport in Arabidopsis inflorescence. Inflorescence segments (25 mm) were placed such that only their apical end was in contact with a [3H]IAA solution at either 4°C or 25°C for 10 min following either a 4°C or 25°C pretreatment of the intact plants. Segments were then washed of [3H]IAA solution and moved to a solution containing nonradioactive IAA for 90 min. Then each segment was removed from solution and cut into 2-mm pieces, omitting the apical 5 mm that was in contact with the solution, and counted for radioactive IAA. The average and se of 10 segments for each treatment is reported.
Seedling Gravitropic Response of the gps Mutants
Previous studies demonstrated that gravitropic responses of roots, hypocotyls, and shoots could be separated genetically (Tasaka et al., 1999). To determine whether the altered GPS response in mutant inflorescence stems extended to other organs, the roots and hypocotyls of seedlings were examined. When roots were presented with gravistimulation at 4°C, neither wild-type nor the mutants showed perception of that stimulus or a RT response to the 4°C stimulation (data not shown). When wild-type hypocotyls were presented with the cold gravistimulation and returned to vertical at RT, they exhibited a transient gravitropic bending similar to the inflorescence stem, as shown in Figure 5. For the mutants, the hypocotyls of gps1-1 showed no gravitropic bending, those of gps2-1 bent the wrong way at RT in response to the 4°C stimulus, and hypocotyls of gps3-1 showed an exaggerated response but in the predicted direction. Thus, all three gps mutants demonstrated similar mutant phenotypes in both inflorescence shoots and hypocotyls.
Figure 5.
The hypocotyls of the gps mutants show the same gravity (or GPS) response as the inflorescence stem. Arabidopsis Ws wild-type, gps1-1, gps2-1, and gps3-1 seeds were plated onto nutrient agar, allowed to germinate, and grown vertically under full light. Four days after germination, the plates were transferred to 4°C and rotated 90° to provide gravistimulation for 1 h. The seedlings were then returned to vertical at RT and images acquired using infrared LEDs to avoid complications caused by phototropism. The arrow indicates the direction of the gravity vector in relation to the seedlings during the 4°C pretreatment. Scale bar = 1 cm.
DISCUSSION
When Arabidopsis inflorescence stems are gravistimulated at 4°C for several hours, they do not bend in response to gravity stimulation until they are returned to RT (Fukaki et al., 1996a). This behavior suggests that gravity is perceived at 4°C, but the gravitropic response is inhibited by the cold. This “memory” effect in plants was first described in 1958 (Brauner and Hager, 1958). Although clearly different from memory in animals, plants do have a mechanism by which a component of signal transduction is stored, or persists, even when the response is inhibited by cold. This “stored” signal can be interpreted when the inhibition of the response is removed, appearing as if the plants “remember” the previous stimulus. The persistence of the 4°C gravity stimulation upon return of plants to RT has the potential to allow the identification of mutants that fail to store the signal or do not transduce the signal normally.
This screen resulted in the identification of three different classes of mutants that were defective in gravitropic bending in response to a 4°C gravitropic signal. These were designated gps1, -2, and -3. gps1-1 did not recognize a 2-h gravity stimulus at 4°C or was incapable of retaining that signal and, therefore, showed no response to the gravistimulation at 4°C when returned to RT (Fig. 1). gps2-1 recognized the stimulus but responded by bending in the wrong direction. Interestingly, the timing of the response was equivalent to the WT response; however, the curvature occurred in the opposite direction (Fig. 2). The gps3-1 mutant was affected in yet another aspect of signal perception/transduction. The direction of response of gps3-1 was similar to that of WT, but the rate of bending was greater so that the stem reached a greater angle of curvature before the new RT gravity vector began to influence the stem orientation (Fig. 2).
It is unlikely that the gps mutants are altered in the earliest steps of the perception of gravity. Sedimentation of amyloplasts in shoots and roots is an early step in the gravity response. Several mutants that have reduced gravitropic response in roots, hypocotyls, and inflorescence stems do not accumulate starch in amyloplasts, suggesting that amyloplasts act as statoliths in all three organs (for review, see Kiss, 2000). All three gps mutants had a normal gravitropic response in all tissues at RT, accumulate starch in gravity responsive tissues including the inflorescence and hypocotyl endodermis and showed normal sedimentation of amyloplasts in the inflorescent stems at 4°C (Fig. 3). Although we cannot rule out the possibility that gps1-1 is defective in some aspect of stimulus perception, it is likely that steps beyond statolith sedimentation are altered in this mutant. It also appears highly unlikely that gps2-1 and gps3-1 are defective in stimulus perception. Both perceive the gravity stimulus at 4°C but respond to that stimulus in an abnormal manner.
Furthermore, the gps mutants are not likely to be impaired in differential growth. The Cholodny-Went hypothesis suggests that asymmetric growth, whether the result of phototropic or gravitropic stimuli, occurs as a result of asymmetric auxin distribution (for review, see Trewavas, 1992; Chen et al., 1999; Muday, 2001). The gps mutants respond normally to both phototropic and gravitropic stimuli at RT. In addition, auxin transport does not occur at 4°C but was restored when the plants were returned to RT (Fig. 4). These data suggest that the GPS response is controlled by a component or components of signal transduction upstream of auxin transport. Therefore, the GPS genes most likely act on a perception or signal transduction step before auxin transport that is independent of starch accumulation.
The proteins encoded by the GPS genes are likely to function in the transduction of the gravitropic signal. Gravitropic signal transduction may be viewed as a timeline of events leading from stimulus perception to asymmetric auxin transport. Cold temperature blocks this flow of events, and the signal is “stored.” When plants are returned to RT, this block is released, and the plants are again able to respond to the stimulus. The inability of gps1-1 to respond to the cold stimulus indicates that the mutant is defective in some aspect of this timeline, possibly in the mechanism for storing or receiving and transducing the signal. The gps3-1 mutant, however, seems to have an enhanced response to the stimulus, suggesting that the amplitude or duration of some component of the signaling events may be increased.
The bending of gps2-1 plants in the wrong direction is unique and may serve as a catalyst to understanding how the polarity of the gravitropic response might be determined. Polar auxin transport and the cellular events downstream seem to be normal in gps2-1, but the mechanism that establishes the direction of lateral auxin redistribution may be reversed after the cold treatment. There are several examples in nature of inflorescence stems that have different angles of gravity response that are similar to the gps2-1 mutant response. For example, peanut gynophores carry the recently fertilized ovules into the soil for fruit and seed development. This unusual gravitropic behavior of an otherwise normal stem tissue is associated with an accumulation of auxin on the upper side of the organ in contrast to the normal accumulation of auxin on the lower side of plant organs in response to changes in gravity (Moctezuma and Feldman, 1999).
In addition, a number of mutants have been identified that have a trailing or “lazy” phenotype. The lz-2 mutant of tomato exhibits this “lazy” or reverse shoot gravicurvature but only when exposed to red light over several days (Roberts, 1987; Gaiser and Lomax, 1993). The graviresponse of lz-2 is completely normal in the dark or under blue light, and the mutant phenotype has been shown to be regulated by the photoreceptor phytochrome (Gaiser and Lomax, 1993). Like lz-2, gps2-1 exhibits reversed shoot gravicurvature, but only under specific conditions. Unlike lz-2, the reversed response to gravistimulation at 4°C of gps2-1 is independent of light (data not shown), suggesting that the establishment of polarity in the gravitropic response may have components both dependent and independent of phytochrome and may be more analogous to systems like the peanut gynophore. In either case, gps2-1 may prove useful in determining components of signal transduction that establish polarity of auxin transport and, thus, determine the growth direction relative to the gravity vector.
To understand the GPS response, it is important to consider what possible effects cold may have on cellular structures and other potential components of the signal transduction pathway. Cold treatment may modify membrane fluidity, membrane protein activity, and cytoskeletal organization. Membrane fluidity is one of the earliest effects of changes in temperature (Levitt, 1980). Using chemical agents to mimic effects of temperature on membrane fluidity, Orvar et al. (2000) found that membrane rigidification could mimic the effects of cold treatment on cold acclimation. Cold-induced alterations in membrane fluidity could then affect the activity of membrane proteins, such as ion channels and transporters. In fact, Orvar et al. (2000) found that cold-induced rigidification of membranes was required for Ca2+ influx. The molecular defects in two shoot agravitropic mutants have been identified recently and implicate the vacuolar membrane system in gravitropism. ZIG/SGR4 encodes a SNARE involved in vesicle transport to the vacuole (Kato et al., 2002; Morita et al., 2002). SRG2 encodes a possible phospholipase A1 (Kato et al., 2002). It may regulate vacuolar membrane structure or fluidity and thus affect amyloplast distribution, or it may release membrane lipids that act as signaling molecules or contribute to the release of signaling ions such as Ca2+ or H+ stored within the vacuole (Kato et al., 2002; Morita et al., 2002). A rapid increase and oscillation of inositol 1,4,5-trisphosphate (IP3) levels was found in maize (Zea mays) pulvini after a gravitropic stimulus, lending further support to the role of phospholipid signaling in early gravitropic events (Perera et al., 1999). Perera et al. (2001) showed that although cold treatment prevented the gravitropic response, IP3 concentrations still changed in response to the gravity stimulation. The authors proposed that the cold treatment interrupted the events between IP3 signaling and the establishment of an auxin asymmetry (Perera et al., 2001).
As an alternative, cold may also delay gravity response by altering the organization of the cytoskeleton. Work by Mazars et al. (1997) has shown that the cold-induced Ca2+ influx was stimulated by disruption of microtubules and actin microfilaments. Cold-induced actin reorganization may also regulate ion movements across the membrane (Orvar et al., 2000), and cold has been shown to affect stability of the microtubule cytoskeleton in a number of plants (Mizuno, 1992). Finally, the possibility that the cytoskeleton functions in either gravity perception or response (for review, see Baluska and Hasenstein, 1997) suggests that cold might prevent gravitropic response by altering the cytoskeleton. A role for the cytoskeleton in translating the physical force of gravity into altered growth has also been explored (for review, see Sievers et al., 1996; Baluska and Hasenstein, 1997; Muday, 2001). Several groups have observed changes in the organization of the microtubule cytoskeleton in gravity-stimulated maize roots or coleoptiles (Nick et al., 1990; Blancaflor and Hasenstein, 1993; Himmelspach et al., 1999). Reorientation of microtubules induced by gravitropic stimulation seem important to the control of gravitropic bending in coleoptiles (Himmelspach and Nick, 2001), however, in roots, the reorientation appears to be delayed until after the gravity response has been initiated (Blancaflor and Hasenstein, 1995).
Although inhibition studies have not detected a role for the actin cytoskeleton in the gravity response of roots (Blancaflor and Hasenstein, 1997; Staves et al., 1997; Godbole et al., 2000), recent isolation of several mutants has suggested a connection. The arg1 mutant has a defect in a gene encoding a protein with a DnaJ domain, which is used by a number of proteins that interact with the cytoskeleton (Sedbrook et al., 1999). The Yin-Yang mutant isolated from rice (Oryza sativa) coleoptiles shows a more rapid initiation and slower rate of gravitropic bending but continues to bend past the vertical (Wang and Nick, 1998). This phenotype can be mimicked by cytochalasin D, and the actin microfilaments become depolymerized in response to auxin treatment (Wang and Nick, 1998).
In conclusion, to identify genes encoding proteins that function in the signal perception and/or transduction of gravity response, a mutant screen was devised that used cold treatment to delay the gravity response of Arabidopsis inflorescence stems. Recessive mutations were identified at three different loci using this cold effect on gravitropism. All three gps mutants had an altered response after gravistimulation at 4°C, yet were phenotypically normal at RT. The gps mutants represent potentially three independent aspects of signal transduction in the gravitropic response: perception or retention of the gravity signal (gps1-1), determination of the polarity of the response (gps2-1), and the rate of response to the signal (gps3-1). Using the GPS effect we have focused on some components of signal transduction subsequent to statolith sedimentation but before auxin transport. By identifying the genes affected in the gps mutants we may be able to identify components of early signal transduction that link the biophysical signal of statolith movement to the biochemical effects that establish differential auxin transport.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
T-DNA insertion seed stocks (T4 generation) were obtained from the Arabidopsis Biological Resource Center at Ohio State University. Arabidopsis var Wassilewskija (Ws) was the parental strain of the T-DNA insertion mutagenized seed and was used as wild-type for all experiments (Feldmann, 1992). In the experiments involving tropic responses of the inflorescence stems, wild-type and mutant seed were sown onto Premier Pro Mix PGX (Hummert International, Springfield, MO) and grown at 21°C; 60% to 75% relative humidity under long-day conditions (16 h light, 8 h dark; light intensity = 300 μmol m−2 s−1). For experiments involving tropic responses of the hypocotyl and root, seeds were surface sterilized for 30 min in 30% (w/v) bleach and plated onto nutrient agar (1× Murashige and Skoog salts [Invitrogen, Carlsbad, CA], 1× B5 vitamins, 1% [w/v] Suc, 0.05% [w/v] MES, pH 5.8, and 1% [w/v] Phytagar [Sigma]) and grown at 21°C under constant light (50 μmol m−2 s−1).
Mutant Screen
Seed representing 6,500 independent transformants was screened for abnormal response to a gravitropic stimulus given at 4°C for 1 h. The mutant screen involved two stages, analysis of the gravity response after stimulation at 4°C and analysis of the normal (RT) gravity response to eliminate mutants affected in mechanisms downstream of signal transduction (i.e. growth or auxin transport). Plants were grown until the primary inflorescence stem was 8 to 10 cm and then given a horizontal gravistimulation by placing pots, containing 10 to 15 plants, on their side for 1 h at 4°C in total darkness. Plants were then restored to vertical at RT, and their response to gravistimulation at 4°C visually assessed at 5-min intervals for 2 h. Putative mutants showing no response or an altered response to the gravistimulation were saved for the second stage of the screen.
To assess the ability of putative mutants to respond to gravistimulation at RT, plants were presented with a horizontal gravistimulation for 2 h at RT. Plants that showed an altered gravitropic response at 4°C but a normal response at RT were then grown to obtain T5 seeds. The T5 seeds were sown in pots and plants screened as above to determine whether they exhibited the parental phenotype. Putative lines were crossed to each member of their own GPS phenotype group and mutants in other phenotypic groups to test for allelism.
Histology
Arabidopsis plants were grown in pots until the inflorescence stems were 8–10 cm tall. Pots were incubated vertically for 30 min at 4°C, placed on their side to provide gravistimulation at 4°C for 1 h, and returned to vertical for 30 min 4°C. The apical 5 cm was removed at 4°C from plants that were vertical only or vertical then horizontal and immediately placed in prechilled fixative (4% [w/v] paraformaldehyde and 2% [w/v] glutaraldehyde) overnight. Longitudinal sections were cut with paired double edged razor blades, stained with KI2 for detection of starch, and imaged with a RT digital camera (Spot, Diagnostic Products, Los Angeles) connected to an Optiphot microscope (Nikon, Tokyo). Images were manipulated using Adobe Photoshop (Adobe Systems, Mountain View, CA).
Growth Characteristics of the gps Mutants
To determine whether the gps mutants showed altered growth, measurements were made of several parameters in mutant and wild-type plants grown under identical conditions. For assessment of inflorescence stems, seeds were planted to soil and allowed to grow for approximately 32 d until the primary inflorescence stem had reached an approximate height of 8 to 10 cm. Documentation was made using direct measurement of rosette leaf number, inflorescence height, and number of lateral branches produced off the primary inflorescence. Measurements of the inflorescence height and lateral branching were taken 4 d after the primary inflorescence was first detected. A minimum of 10 plants was used for each measurement. For experiments involving growth parameters of hypocotyls and roots, seeds were surface sterilized and plated, 10 to 20 seeds per plate, vertically onto growth medium as described above. Seedlings were grown at 22°C under either constant light or in total darkness for 4 d. Images were captured using DAGE MTI CCD 100 camera and measurements of root and hypocotyl length made using documentation software from Image Pro Plus. Statistical analysis was performed using a one-way ANOVA with an α-value of 0.05. All experiments were repeated at least twice.
Analysis of Tropic Responses
To test the gravitropic response of inflorescence stems, plants were grown as above until the primary inflorescence stem was 8 to 10 cm tall. Plants were gravistimulated by placing the pots on their side. For photographs of the mutant phenotypes, plants were gravistimulated for 2 h at 4°C and returned to vertical at RT for 90 min. To assess the kinetics of the GPS response in the mutants, plants were placed horizontally for 1 h at 4°C then returned to vertical at RT and measurements of the resulting angle of curvature were taken at 15-min intervals. For analysis of the effects of constant gravistimulation at ambient temperatures, plants were placed horizontally in the dark at RT, and measurements of the angle of curvature were taken at 15-min intervals. Photographs were taken after 2 h of gravistimulation. The angle of curvature for both experiments was determined as the difference between the angle of the growing tip at time 0 (immediately after gravistimulation) and each time point thereafter using a protractor on photographs or by direct measurement of the inflorescence stems. For phototropic responses, pots were covered with a black opaque box with an opening on one side and illuminated with approximately 80 μmol m−2s−1 white light through the opening for 3 h at RT.
For experiments involving tropic responses of the hypocotyl and root, seeds were surface sterilized and plated vertically onto growth medium as described above. Seedlings were grown at 22°C under either constant light or in total darkness. For the gravitropic response of the root and hypocotyl, the plates were rotated 90° in darkness, and images were taken, using infrared light emitting diodes (LEDs), every 5 min for 6 and 2 h respectively. For mutant phenotype analysis, plates were transferred to 4°C, rotated 90°, and held for 1 h to provide the seedling with gravistimulation. The plates were then returned to vertical at RT. Images were captured using a DAGE MTI CCD 100 camera equipped with time-lapse video with lighting from infrared LEDs. Image manipulation was done in Adobe Photoshop.
Auxin Transport
Plants were grown in soil as described above until the inflorescence stems were 8 to 10 cm. Auxin transport was measured using continuous delivery of [3H]IAA from a buffered solution under three conditions. Auxin transport was assessed in the cold, at RT and at RT after cold pretreatment using a procedure modified from Okada et al. (1991). Pots containing 10 to 20 plants each were placed at 4°C or RT for a 1 h for pretreatment. After 1 h, a single 2.5-cm segment of the inflorescence stem was cut 5 cm from the base. These segments were then inverted and put into microfuge tubes containing 30 μL of [3H]IAA solution (27 Ci mol−1 from Amersham Biosciences AB, Uppsala) so that the apical end was in contact with the solution (100 nm [3H]IAA and 1.35 μm cold IAA in pH 5.5 MES, 1% [w/v] Suc). Tubes were placed in the dark for 5 h at either 4°C or RT. Basipetal auxin transport was assessed by removing the basal 5 mm of the inflorescence segment, incubating it in 2.5 mL of scintillation fluid for 18 h before scintillation counting for 2 min on a scintillation counter (LS6500 Beckman Coulter, Inc., Fullerton, CA).
The pulse-chase basipetal auxin transport assay was conducted in a similar manner as the above transport with the following modifications. The overall time of transport was shorter, consisting of a 10-min pulse (400 nm [3H]IAA and 1.05 μm cold IAA in pH 5.5 MES, and 1% [w/v] Suc) followed by a 90-min cold IAA chase (1.45 μm cold IAA in pH 5.5 MES, 1% [w/v] Suc). The amount of [3H]IAA used was increased to facilitate detection of this short pulse, although the overall amount of total IAA in each assay is identical. Finally, the inflorescence segment, except for the 5 mm in contact with the IAA solution, was cut into 2 mm segments and each section was separately analyzed for [3H]IAA content.
ACKNOWLEDGMENTS
We thank Dr. Ken Feldmann and the Arabidopsis Biological Resource Center at Ohio State University for generously providing the seed from the T-DNA tagged population. The efforts of Patti McDermott and Reathel Geary at North Carolina State University are also greatly appreciated for their assistance with the mutant screen and initial characterization. Special thanks goes to Darrin Rubino at Ohio University for his help with the statistical analysis of the growth parameters.
Footnotes
This work was supported by the National Aeronautics and Space Administration (grant no. NAGW–4984 awarded to the Specialized Center of Research and Training in Gravitational Biology at North Carolina State University, which supported S.E.W., A.M.R., G.K.M., and D.R.) and by a Research Challenge award (to S.E.W.) and a Program to Aid Career Exploration award (to M.J.S.) from Ohio University.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.010579.
LITERATURE CITED
- Baluska F, Hasenstein K. Root cytoskeleton: its role in perception of and response to gravity. Planta. 1997;203:S69–S78. doi: 10.1007/pl00008117. [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Hasenstein KH. Organization of cortical microtubules in graviresponding maize roots. Planta. 1993;191:231–237. doi: 10.1007/BF00199754. [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Hasenstein KH. Time course and auxin sensitivity of cortical microtubule reorientation in maize roots. Protoplasma. 1995;185:72–82. doi: 10.1007/BF01272755. [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Hasenstein KH. The organization of the actin cytoskeleton in vertical and graviresponding primary roots of maize. Plant Physiol. 1997;113:1447–1455. doi: 10.1104/pp.113.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner L, Hager A. Versuche zur analyse der geotropischen perzeption. Planta. 1958;51:115–147. doi: 10.1007/BF00392282. [DOI] [PubMed] [Google Scholar]
- Chen R, Guan C, Boonsirichai K, Masson PH. Complex physiological and molecular processes underlying root gravitropism. Plant Mol Biol. 2002;49:305–317. [PubMed] [Google Scholar]
- Chen R, Rosen E, Masson PH. Gravitropism in higher plants. Plant Physiol. 1999;120:343–350. doi: 10.1104/pp.120.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Laurenzio L, Wysocka-Diller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, Hahn MG, Feldmann KA. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell. 1996;86:423–433. doi: 10.1016/s0092-8674(00)80115-4. [DOI] [PubMed] [Google Scholar]
- Estelle MA, Somerville CR. Auxin resistant mutants of Arabidopsis with an altered morphology. Mol Gen Genet. 1987;206:200–206. [Google Scholar]
- Feldmann KA. T-DNA insertion mutagenesis in Arabidopsis: seed infection/transformation. In: Koncz D, Chua N-H, Schell J, editors. Methods in Arabidopsis Research. Singapore: World Scientific; 1992. pp. 274–289. [Google Scholar]
- Fukaki H, Fujisawa H, Tasaka M. Gravitropic response of inflorescence stems in Arabidopsis thaliana. Plant Physiol. 1996a;110:933–943. doi: 10.1104/pp.110.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H, Fujisawa H, Tasaka M. SGR1, SGR2, and SGR3: novel genetic loci involved in shoot gravitropism in Arabidopsis thaliana. Plant Physiol. 1996b;110:945–955. doi: 10.1104/pp.110.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H, Fujisawa H, Tasaka M. The RHG gene is involved in root and hypocotyl gravitropism in Arabidopsis thaliana. Plant Cell Physiol. 1997;38:804–810. doi: 10.1093/oxfordjournals.pcp.a029238. [DOI] [PubMed] [Google Scholar]
- Fukaki H, Wysocka-Diller J, Kato T, Fujisawa H, Benfey PN, Tasaka M. Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J. 1998;14:425–430. doi: 10.1046/j.1365-313x.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- Gaiser JC, Lomax TL. The altered gravitropic response of the lazy-2 mutant of tomato is phytochrome-regulated. Plant Physiol. 1993;102:339–344. doi: 10.1104/pp.102.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbole R, Michalke W, Nick P, Hertel R. Cytoskeletal drugs and gravity induced lateral auxin transport in rice coleoptiles. Plant Biol. 2000;1:379–381. [Google Scholar]
- Helariutta Y, Fukaki H, Wuysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser MT, Benfey PN. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell. 2000;101:555–567. doi: 10.1016/s0092-8674(00)80865-x. [DOI] [PubMed] [Google Scholar]
- Himmelspach R, Nick P. Gravitropic microtubule reorientation can be uncoupled from growth. Planta. 2001;212:184–189. doi: 10.1007/s004250000378. [DOI] [PubMed] [Google Scholar]
- Himmelspach R, Wymer CL, Lloyd CW, Nick P. Gravity-induced reorientation of cortical microtubules observed in vivo. Plant J. 1999;18:449–453. doi: 10.1046/j.1365-313x.1999.00467.x. [DOI] [PubMed] [Google Scholar]
- Kato T, Morita MT, Fukaki H, Yamauchi Y, Uehara M, Niihama M, Tasaka M. SGR2, a phospholipase-like protein, and ZIG/SGR4, a SNARE, are involved in the shoot gravitropism of Arabidopsis. Plant Cell. 2002;14:33–46. doi: 10.1105/tpc.010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JZ. Mechanisms of the early phases of plant gravitropism. Crit Rev Plant Sci. 2000;19:551–573. doi: 10.1080/07352680091139295. [DOI] [PubMed] [Google Scholar]
- Levitt J. Responses of Plants to Environmental Stress. New York: Academic Press; 1980. Freezing resistance: types, measurement and changes; pp. 137–141. [Google Scholar]
- Mazars C, Thion L, Thuleau P, Graziana A, Knight MR, Moreau M, Ranjeva R. Organization of cytoskeleton controls changes in cytosolic calcium of cold-shocked Nicotiana plumbaginifolia protoplasts. Cell Calcium. 1997;22:413–420. doi: 10.1016/s0143-4160(97)90025-7. [DOI] [PubMed] [Google Scholar]
- Mizuno K. Induction of cold stability of microtubules in cultured tobacco cells. Plant Physiol. 1992;100:740–748. doi: 10.1104/pp.100.2.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moctezuma E, Feldman LJ. Auxin redistributes upwards in graviresponding gynophores of the peanut plant. Planta. 1999;209:180–186. doi: 10.1007/s004250050620. [DOI] [PubMed] [Google Scholar]
- Morita MT, Kato T, Nagafusa K, Saito C, Ueda T, Nakano A, Tasaka M. Involvement of the vacuoles of the endodermis in the early process of shoot gravitropism in Arabidopsis. Plant Cell. 2002;14:47–56. doi: 10.1105/tpc.010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DA. The effect of temperature on the velocity of exogenous auxin transport in intact chilling-sensitive and chilling-resistant plants. Planta. 1979;146:603–605. doi: 10.1007/BF00388839. [DOI] [PubMed] [Google Scholar]
- Muday GK. Auxins and tropisms. J Plant Growth Regul. 2001;20:226–243. doi: 10.1007/s003440010027. [DOI] [PubMed] [Google Scholar]
- Mullen JL, Turk E, Johnson K, Wolverton C, Ishikawa H, Simmons C, Soll D, Evans ML. Root-growth behavior of the Arabidopsis mutant rgr1: roles of gravitropism and circumutation in the waving/coiling phenomenon. Plant Physiol. 1998;118:1139–1145. doi: 10.1104/pp.118.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick P, Bergfeld R, Schafer E, Schopfer P. Unilateral reorientation of microtubules at the outer epidermal cell wall during photo and gravitropic curvature of maize coleoptiles and sunflower hypocotyles. Planta. 1990;181:162–168. doi: 10.1007/BF02411533. [DOI] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;3:677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvar BL, Sangwan V, Omann F, Dhindsa RS. Early steps in cold sensing by plant cells: the role of actin cytoskeleton and membrane fluidity. Plant J. 2000;23:785–794. doi: 10.1046/j.1365-313x.2000.00845.x. [DOI] [PubMed] [Google Scholar]
- Perera IY, Heilmann I, Boss WF. Transient and sustained increases in inositol 1,4,5-trisphosphate precede the differential growth response in gravistimulated maize pulvini. Proc Natl Acad Sci USA. 1999;96:5838–5843. doi: 10.1073/pnas.96.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera IY, Heilmann I, Chang SC, Boss WF, Kaufman PB. A role for inositol 1,4,5-trisphosphate in gravitropic signaling and the retention of cold-perceived gravistimulation of oat (Avena sativa) shoot pulvini. Plant Physiol. 2001;125:1499–1507. doi: 10.1104/pp.125.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramussen H. Intracellular signaling: from simplicity to complexity. Am Soc Grav Space Biol Bull. 1995;8:7–17. [PubMed] [Google Scholar]
- Rashotte AM, DeLong A, Muday GK. Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response and lateral root growth. Plant Cell. 2001;13:1683–1697. doi: 10.1105/TPC.010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JA. Mutants and gravitropism. In: Thomas H, Grierson D, editors. Developmental Mutants in Higher Plants. Cambridge: Cambridge University Press; 1987. pp. 135–153. [Google Scholar]
- Sack FD. Plant gravity sensing. Int Rev Cytol. 1991;127:193–252. doi: 10.1016/s0074-7696(08)60695-6. [DOI] [PubMed] [Google Scholar]
- Sedbrook JC, Chen R, Masson PH. ARG1 (altered response to gravity) encodes a DnaJ-like protein that potentially interacts with the cytoskeleton. Proc Natl Acad Sci USA. 1999;96:1140–1145. doi: 10.1073/pnas.96.3.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers A, Buchen B, Hodick D. Gravity sensing in tip-growing cells. Trends Plant Sci. 1996;1:273–279. doi: 10.1016/1360-1385(96)10028-5. [DOI] [PubMed] [Google Scholar]
- Staves M, Wayne R, Leopold A. Cytochalasin D does not inhibit gravitropism in roots. Am J Bot. 1997;84:1530–1535. [PubMed] [Google Scholar]
- Tasaka M, Kato T, Fukaki H. The endodermis and shoot gravitropism. Trends Plant Sci. 1999;4:103–107. doi: 10.1016/s1360-1385(99)01376-x. [DOI] [PubMed] [Google Scholar]
- Trewavas AJ. FORUM: What remains of the Cholodny-Went theory? Plant Cell Environ. 1992;15:759–794. [PubMed] [Google Scholar]
- Wang Q, Nick P. The auxin response of actin is altered in the rice mutant Yin-Yang. Protoplasma. 1998;204:22–23. doi: 10.1007/BF01282290. [DOI] [PubMed] [Google Scholar]
- Weise SE, Kuznetsov OA, Hasenstein KH, Kiss JZ. Curvature in Arabidopsis inflorescence stems is limited to the region of amyloplast displacement. Plant Cell Physiol. 2000;41:702–709. doi: 10.1093/pcp/41.6.702. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Fukaki H, Fujisawa H, Tasaka M. Mutation in the SGR4, SGR5, and SGR6 loci of Arabidopsis thaliana alter the shoot gravitropism. Plant Cell Physiol. 1997;38:530–535. doi: 10.1093/oxfordjournals.pcp.a029201. [DOI] [PubMed] [Google Scholar]