Abstract
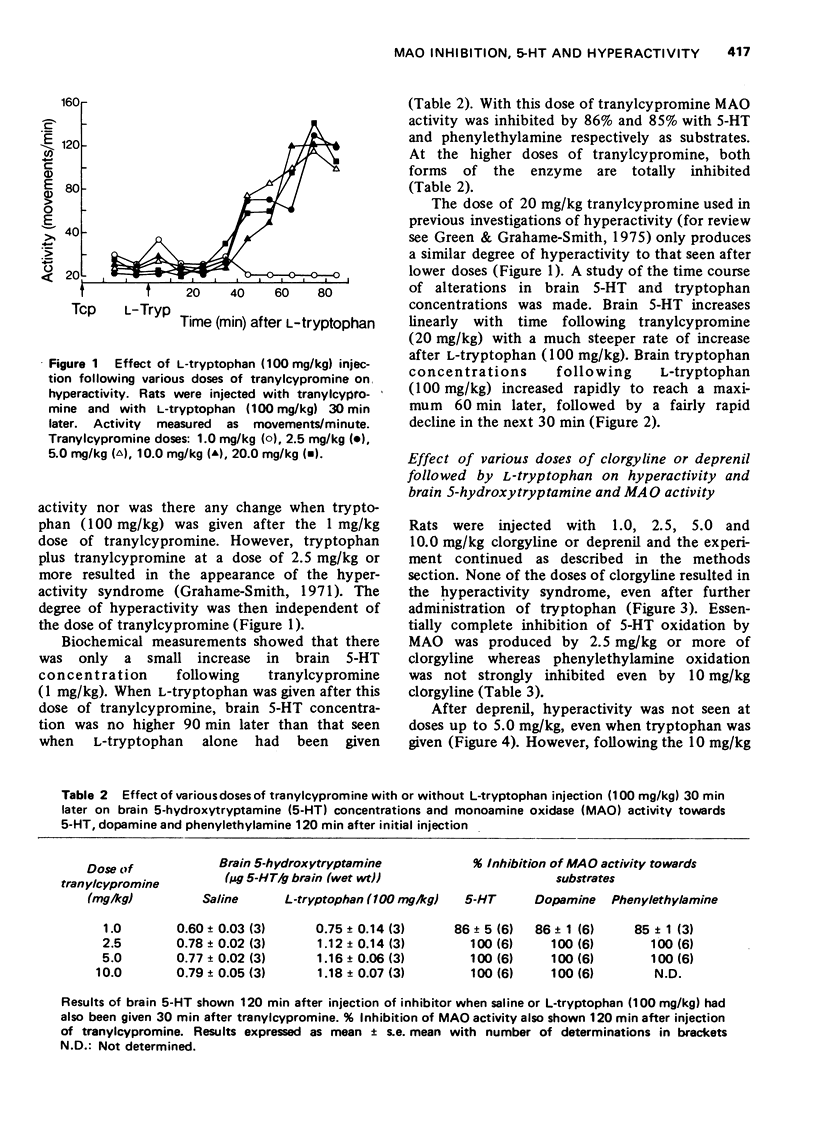
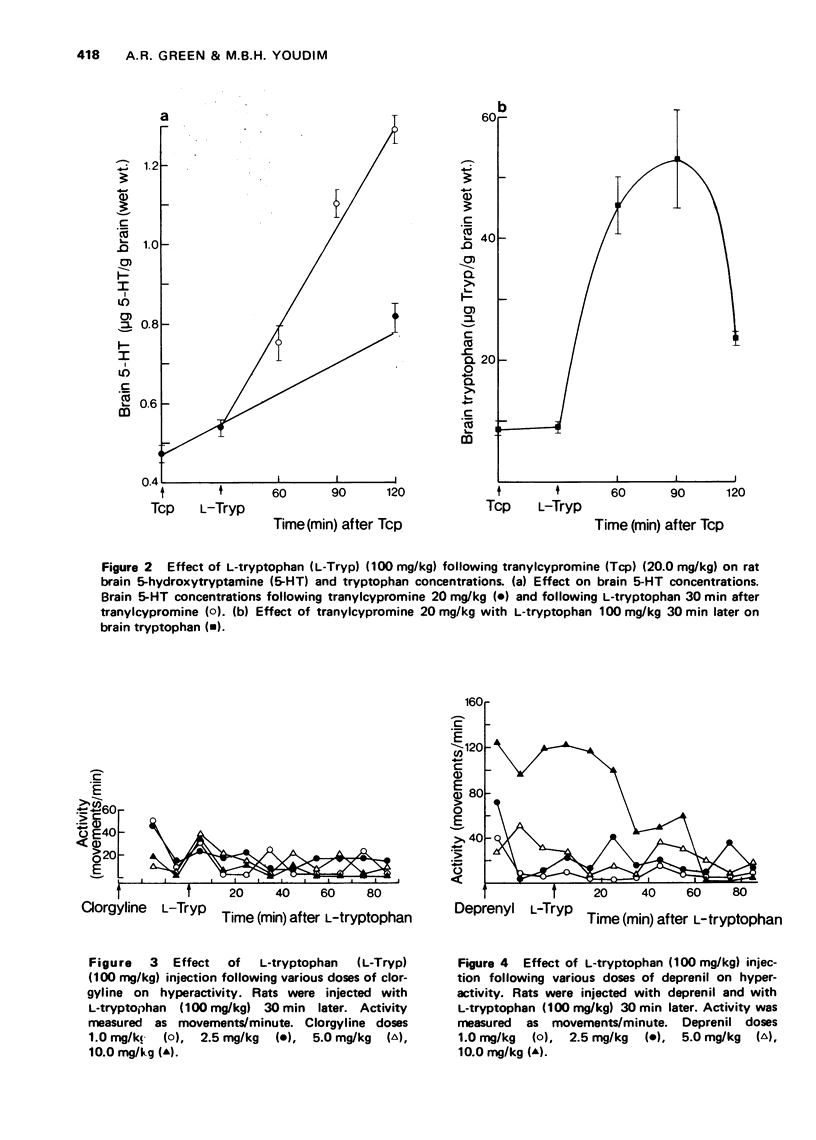
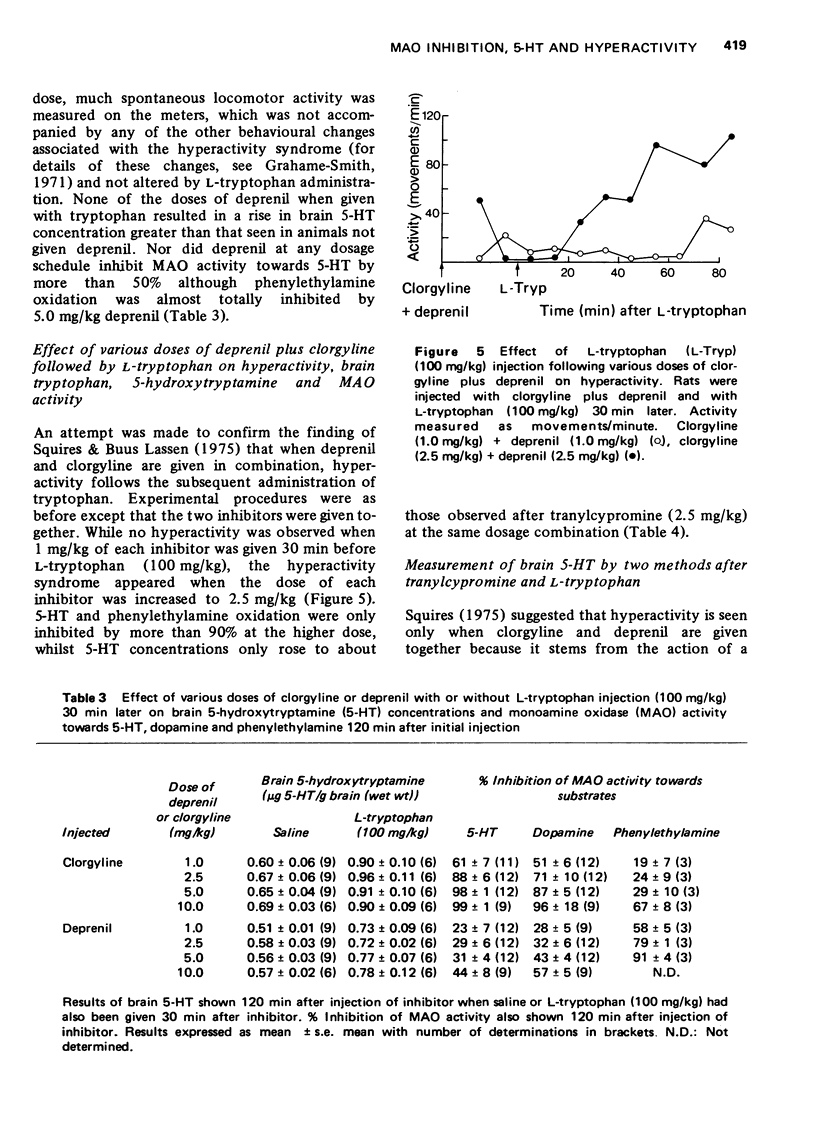
1 The effect of various doses of tranylcypromine on the degree of inhibition of rat brain monoamine oxidase (MAO) using 5-hydroxytryptamine (5-HT), dopamine and phenylethylamine as substrates has been examined 120 min after injection of the inhibitor. The concentration of brain 5-HT was also examined both after tranylcypromine alone and also when L-tryptophan (100 mg/kg) had been given 30 min after the tranylcypromine. 2 All doses of tranylcypromine greater than 2.5 mg/kg totally inhibited MAO oxidation of 5-HT, phenylethylamine and dopamine as measured in vitro and produced a similar rise of brain 5-HT in vivo. When tryptophan was also given, there was a further rise of brain 5-HT, which was comparable after all doses of tranylcypromine above 2.5 mg/kg and the characteristic syndrome of hyperactivity made is appearance. 3 Clorgyline (a "Type A" MAO inhibitor), in doses up to 10 mg/kg, did not totally inhibit MAO activity towards phenylethylamine although it did inhibit 5-HT oxidation by 100%. Deprenil (a "Type B" MAO inhibitor) at doses up to 10 mg/kg did not fully inhibit 5-HT oxidation although phenylethylamine oxidation was inhibited almost completely. Administration of either compound alone did not produce as great an accumulation of brain 5-HT as that seen after tranylcypromine (2.5 mg/kg) and subsequent administration of tryptophan did not cause hyperactivity or the rise of brain 5-HT seen after tranylcypromine (2.5 mg/kg) plus tryptophan. 4 Administration of clorgyline plus deprenil (2.5 mg/kg of each) almost totally inhibited oxidation of both 5-HT and phenylethylamine; subsequent tryptophan administration resulted in a rise of brain 5-HT nearly as great as that seen following tranylcypromine (2.5 mg/kg) plus tryptophan and the animals became hyperactive. 5 No evidence was found pointing to the formation of any other 5-substituted indole in the brain following tranylcypromine plus L-tryptophan administration as suggested by others. 6 It is concluded that while 5-HT may normally be metabolized in the brain by "Tye A" MAO in vivo, when this form is inhibited, 5-HT can still be metabolized by "Type B" enzyme. It is only when both forms are almost totally inhibited that the largest rise of brain 5-HT is seen and subsequent tryptophan administration produces the hyperactivity syndrome.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANG C. C. A SENSITIVE METHOD FOR SPECTROPHOTOFLUOROMETRIC ASSAY OF CATECHOLAMINES. Int J Neuropharmacol. 1964 Dec;3:643–649. doi: 10.1016/0028-3908(64)90089-9. [DOI] [PubMed] [Google Scholar]
- Curzon G., Green A. R. Rapid method for the determination of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in small regions of rat brain. Br J Pharmacol. 1970 Jul;39(3):653–655. doi: 10.1111/j.1476-5381.1970.tb10373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denckla W. D., Dewey H. K. The determination of tryptophan in plasma, liver, and urine. J Lab Clin Med. 1967 Jan;69(1):160–169. [PubMed] [Google Scholar]
- Gascoigne J. E., Williams D., Williams E. D. Proceedings: Histochemical demonstration of an additional form of rat brain MAO. Br J Pharmacol. 1975 Jun;54(2):274P–274P. [PMC free article] [PubMed] [Google Scholar]
- Grahame-Smith D. G. Studies in vivo on the relationship between brain tryptophan, brain 5-HT synthesis and hyperactivity in rats treated with a monoamine oxidase inhibitor and L-tryptophan. J Neurochem. 1971 Jun;18(6):1053–1066. doi: 10.1111/j.1471-4159.1971.tb12034.x. [DOI] [PubMed] [Google Scholar]
- Green A. R., Grahame-Smith D. G. The role of brain dopamine in the hyperactivity syndrome produced by increased 5-hydroxytryptamine synthesis in rats. Neuropharmacology. 1974 Nov;13(10-11):949–959. doi: 10.1016/0028-3908(74)90086-0. [DOI] [PubMed] [Google Scholar]
- Green A. R., Hughes J. P., Tordoff A. F. The concentration of 5-methoxytryptamine in rat brain and its effects on behaviour following its peripheral injection. Neuropharmacology. 1975 Aug;14(8):601–606. doi: 10.1016/0028-3908(75)90127-6. [DOI] [PubMed] [Google Scholar]
- Green A. R., Koslow S. H., Costa E. Identification and quantitation of a new indolealkylamine in rat hypothalamus. Brain Res. 1973 Mar 15;51:371–374. doi: 10.1016/0006-8993(73)90392-2. [DOI] [PubMed] [Google Scholar]
- Green A. R., Sourkes T. L., Young S. N. Liver and brain tryptophan metabolism following hydrocortisone administration to rats and gerbils. Br J Pharmacol. 1975 Feb;53(2):287–292. doi: 10.1111/j.1476-5381.1975.tb07360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. R., Woods H. F., Knott P. G., Curzon G. Letter: Factors influencing effect of hydrocortisone on rat brain tryptophan metabolism. Nature. 1975 May 8;255(5504):170–170. doi: 10.1038/255170a0. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Tipton K. F. A kinetic evaluation of monoamine oxidase activity in rat liver mitochondrial outer membranes. Biochem J. 1974 Jun;139(3):645–652. doi: 10.1042/bj1390645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. P. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968 Jul;17(7):1285–1297. doi: 10.1016/0006-2952(68)90066-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Neff N. H., Tozer T. N. In vivo measurement of brain serotonin turnover. Adv Pharmacol. 1968;6(Pt A):97–109. doi: 10.1016/s1054-3589(08)61160-6. [DOI] [PubMed] [Google Scholar]
- Sandler M., Youdim M. B. Monoamine oxidases: the present status. Int Pharmacopsychiatry. 1974;9(1):27–34. doi: 10.1159/000468113. [DOI] [PubMed] [Google Scholar]
- Sandler M., Youdim M. B. Multiple forms of monoamine oxidase: functional significance. Pharmacol Rev. 1972 Jun;24(2):331–348. [PubMed] [Google Scholar]
- Snyder S. H., Axelrod J., Zweig M. A sensitive and specific fluorescence assay for tissue serotonin. Biochem Pharmacol. 1965 May;14(5):831–835. doi: 10.1016/0006-2952(65)90102-4. [DOI] [PubMed] [Google Scholar]
- Southgate J., Collins G. G. The estimation of monoamine oxidase using 14C-labelled substrates. Biochem Pharmacol. 1969 Sep;18(9):2285–2287. doi: 10.1016/0006-2952(69)90341-4. [DOI] [PubMed] [Google Scholar]
- Squires R. F. Evidence that 5-methoxy-N, N-dimethyl tryptamine is a specific substrate for MAO-A in the rat: implications for the indoleamine dependent behavioural syndrome. J Neurochem. 1975 Jan;24(1):47–50. doi: 10.1111/j.1471-4159.1975.tb07626.x. [DOI] [PubMed] [Google Scholar]
- Squires R. F., Lassen J. B. The inhibition of A and B forms of MAO in the production of a characteristic behabioural syndrome in rats after 1-tryptophan loading. Psychopharmacologia. 1975;41(2):145–151. doi: 10.1007/BF00421072. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Neff N. H. The monoamine oxidases of brain: selective inhibition with drugs and the consequences for the metabolism of the biogenic amines. J Pharmacol Exp Ther. 1974 Jun;189(3):733–740. [PubMed] [Google Scholar]
- Youdim M. B., Collins G. G., Sandler M., Bevan Jones A. B., Pare C. M., Nicholson W. J. Human brain monoamine oxidase: multiple forms and selective inhibitors. Nature. 1972 Mar 31;236(5344):225–228. doi: 10.1038/236225b0. [DOI] [PubMed] [Google Scholar]
- Youdim M. B. Multiple forms of mitochondrial monoamine oxidase. Br Med Bull. 1973 May;29(2):120–122. doi: 10.1093/oxfordjournals.bmb.a070980. [DOI] [PubMed] [Google Scholar]


