Abstract
The activation sequence-1 (as-1)-like element found in the promoter of some glutathione S-transferase (GST) genes, has been previously described as a salicylic acid (SA)- and auxin-responsive element. In this paper, we tested the hypothesis that the activating effect of SA on the as-1 element is mediated by oxidative species. Supporting this hypothesis, our results show that the antioxidants dimethylthiourea (DMTU) and 3-t-butyl-4-hydroxy-anizole (BHA) inhibit the SA-induced transcription of genes controlled by as-1 elements in tobacco (Nicotiana tabacum) plants [i.e. GNT35 gene coding for a GST and (as-1)4/β-glucuronidase (GUS) reporter transgene]. DMTU and BHA also inhibit SA-activated as-1-binding activity in nuclear extracts. Further support for the hypothesis that the as-1 element is activated by oxidative species comes from our result showing that light potentiates the SA-induced activation of the as-1 element. Furthermore, methyl viologen, a known oxidative stress inducer in plants, also activates the as-1 element. Increasing H2O2 levels by incubation with H2O2 or with the catalase inhibitor 3-amino-1,2,5-triazole does not activate the (as-1)4/GUS gene. On the contrary, 3-amino-1,2,5-triazole inhibits the activating effect of SA on the (as-1)4/GUS gene. These results suggest that oxidative species other than H2O2 mediate the activation of the as-1 element by SA. Our results also suggest that even though the as-1 binding activity is stimulated by oxidative species, this is not sufficient for the transactivation of genes controlled by this element. The complex interplay between SA and reactive oxygen species in the transcriptional activation of defense genes is discussed.
Salicylic acid (SA) is a phenolic hormone that plays a crucial role in stress resistance in plants (Durner et al., 1997; Alvarez, 2000). Cellular levels of SA increase in the onset of pathogen-induced defense reactions, locally in the infected tissues or systemically in noninfected tissues (Malamy et al., 1990). Increased levels of SA are required to activate the transcription of defense genes and to develop an efficient pathogen resistance response (Gaffney et al., 1993; Delaney et al., 1994). It is interesting that accumulation of SA and the activation of defense genes have been also reported to occur after exposure of plants to ozone or UV radiation (Yalpani et al., 1994; Rao and Davis, 1999). Pathogen infection and exposure to ozone or UV radiation are associated with an accumulation of reactive oxygen species (ROS) in plants. The appropriate balance in the cellular levels of SA and ROS seems to be crucial for the efficient activation of defense responses against the above-mentioned environmental stressors (Draper, 1997; Van Camp et al., 1998; Alvarez, 2000; Van Breusegem et al., 2001).
One class of defense genes activated by SA is the glutathione S-transferase (GST) class of genes that code for the GSTs. In plants, GSTs are key enzymes in the metabolism of xenobiotics and secondary products. They catalyze the formation of glutathione conjugates, which are transported into the vacuole for further metabolism (Edwards et al., 2000). In addition, plant GSTs play a role in the binding and transport of hormones and in the reduction of organic hydroperoxides, thus protecting the cells against oxidative stress (Edwards et al., 2000). Therefore, it is not surprising that expression of GST genes is activated under stressful conditions, such as pathogen infection (Alvarez et al., 1998; Maleck et al., 2000; Pontier et al., 2001). It is interesting that the GST genes that are activated by SA are also activated by high concentrations of auxins and methyl jasmonate (Ulmasov et al., 1994; Xiang et al., 1996; Chen and Singh, 1999).
A defined SA-responsive element has been found in the promoter of several SA-inducible GST genes such as GNT35 from tobacco (Nicotiana tabacum) and GST6 from Arabidopsis (Ulmasov et al., 1994; Droog et al., 1995; Chen and Singh, 1999). This element, named activation sequence-1 (as-1), was first described in viral and bacterial promoters (Lam et al., 1989) and is characterized by two TGACG motifs that bind basic/Leu zipper transcription factors of the plant TGA family in vitro and in vivo (Xiang et al., 1997; Johnson et al., 2001a). It is interesting that this promoter element is also responsive to high concentrations of auxins and methyl jasmonate (Ulmasov et al., 1994; Xiang et al., 1996).
One of the intriguing aspects in the mechanism of gene activation via as-1-like promoter elements is that the same element is responsive to several chemically unrelated phytohormones. One possibility is that activation of the as-1 element is mediated by a common oxidative species produced by these hormones (Ulmasov et al., 1994). Several lines of evidence support this idea. First, it has been reported that treatment of plants with SA and auxins increases the levels of oxidative species (Chen et al., 1993; Candeias et al., 1996; Rao et al., 1997; Anderson et al., 1998; Kawano et al., 1998; Joo et al., 2001). Second, the as-1 promoter element has high homology with the AP-1 box (TGACTCAT), a well-known oxidative stress-responsive element in mammals (Karin et al., 1997). TGA factors consistently share homology in their DNA-binding domain with c-jun, a member of the AP-1 transcription complex (Katagiri et al., 1989). Third, AP-1-like sequences found in the promoter of yeast and mammalian GST genes have been defined as elements responsive to oxidative signals (Rushmore and Picket, 1993).
In this paper, we provide new evidence supporting the hypothesis that the as-1 promoter sequence acts as an oxidative stress-responsive element and that its activation by SA is mediated by oxidative species. In light of these results, the role of SA and ROS in the transcriptional activation of defense genes is discussed.
RESULTS
Effect of Antioxidants on the Activation of the as-1 Promoter Element by SA
To determine whether the activation of the as-1 promoter element by SA is mediated by oxidative species, we first evaluated if antioxidants are able to inhibit some SA responses in tobacco plants. We measured three effects induced by SA on the as-1 element: the increased binding of nuclear proteins to the as-1 sequence (Stange et al., 1997), the induction of the GUS reporter gene controlled by four copies of the as-1 element [(as-1)4/GUS transgene; Hidalgo et al., 2001], and the transcriptional activation of the GNT35 endogenous gene, which contains a functional as-1 element in its promoter (Droog et al., 1995). The antioxidants used were dimethylthiourea (DMTU), described mainly as a trap of hydroxyl radicals (Fox, 1984), and butylated hydroxyanisole (BHA), a general radical scavenger (Rehwoldt, 1986).
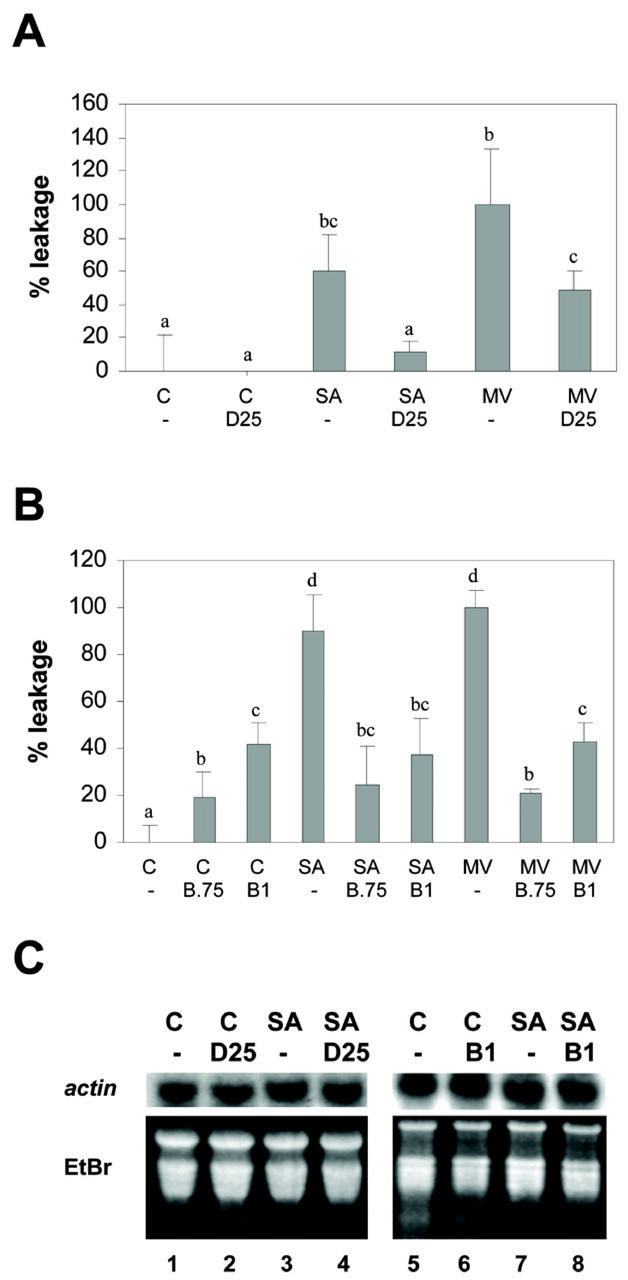
Before evaluating whether DMTU and BHA inhibit the SA effect, we determined their efficiency and specificity at the concentrations used in our study. The efficiency of DMTU and BHA as antioxidants was evaluated by their ability to prevent the oxidative membrane damage produced by methyl viologen (MV) and SA. MV has been reported to generate superoxide radicals, causing cell membrane damage, as evidenced by ion leakage assays (Bowler et al., 1991). The effect of SA as an ROS generator has been also described (Chen et al., 1993; Rao et al., 1997; Anderson et al., 1998; Kawano et al., 1998), and in this paper, we measured its effect on cell membrane damage by using the ion leakage assay. Figure 1A shows that DMTU (25 mm) inhibited MV- and SA-induced membrane damage by 50% and 80%, respectively. Larger DMTU concentrations were unable to further inhibit the effect of MV or SA (data not shown). On the other hand, BHA (0.75 and 1 mm) completely inhibited the oxidative damage produced by MV and SA (Fig. 1B). Figure 1, A and B, also shows that differences in the effect of SA (1 mm) and MV (50 μm) are not statistically significant.
Figure 1.
Effect of DMTU and BHA on oxidative membrane damage induced by MV and SA, and on transcription of a constitutive gene. A and B, Oxidative membrane damage was assayed by ion leakage from tobacco leaf discs (see “Materials and Methods”) after the following treatments. A, Preincubation for 30 min with water (-) or 25 mm DMTU (D25), followed by incubation for 5 h in the absence (C) or in the presence of 1 mm SA or 50 μm MV. B, Preincubation for 30 min with 0.1% (v/v) methanol (-), 0.75 mm BHA (B 0.75), or 1 mm BHA (B1), followed by incubation for 5 h in the absence (C) or in the presence of 50 μm MV or 1 mm SA. To calculate the percentage of leakage, the mean conductivity value of the control treatment with water (Fig. 1A) or 0.1% (v/v) methanol (Fig. 1B) was subtracted from the conductivity value of each sample, and then the percentage was calculated considering as 100% the treatment with 50 μm MV. Contribution of the treatment solution alone to the conductance of each sample was previously subtracted. Data presented are the mean ± sd of three independent experiments. Different letters indicate significantly different values (P < 0.05; ANOVA). C, Actin mRNA levels were detected by northern hybridization, using total RNA (20 μg per lane) isolated from samples subjected to the follow- ing treatments: Leaf discs obtained from wild-type tobacco plants were pretreated for 30 min with water (lanes 1 and 3), 25 mm DMTU (D25, lanes 2 and 4), 0.1% (v/v) methanol (lanes 5 and 7), and 1 mm BHA (B 1, lanes 6 and 8), and they were then treated for 2.5 h in the absence (C) or in the presence of 1 mm SA. A specific 32P-labeled actin gene fragment was used as a probe. Staining the gels with ethidium bromide (EtBr) controlled equal RNA loading.
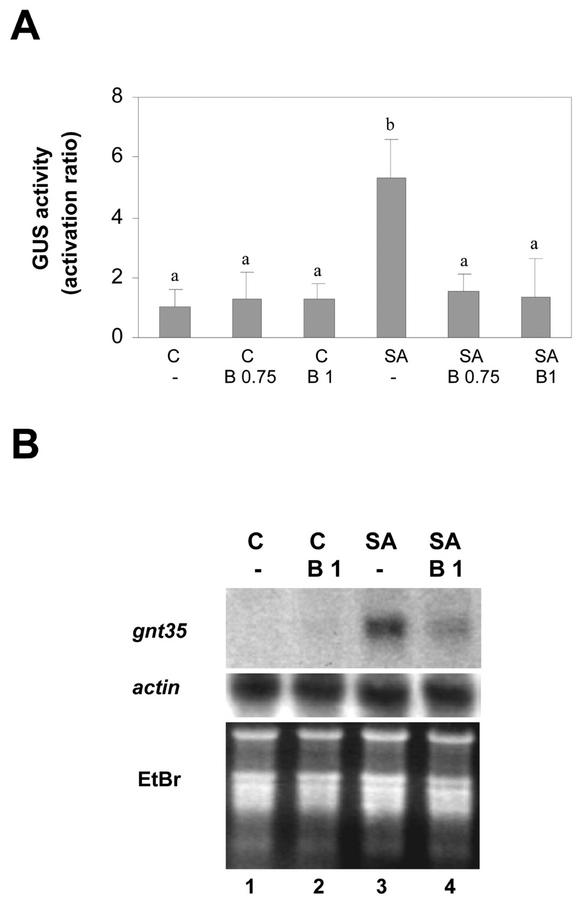
To test the specificity of DMTU and BHA, we measured mRNA levels of the constitutive actin gene in the presence of these antioxidants. Total RNA was isolated from tobacco leaf discs pretreated with the antioxidants for 30 min, followed by a treatment in the presence or absence of 1 mm SA for 2.5 h. The levels of actin mRNA were detected by northern-blot analysis. As shown in Figure 1C, SA (1 mm), DMTU (25 mm), and BHA (1 mm) treatments did not affect the levels of actin mRNA, suggesting that these compounds do not exert nonspecific effects on transcription. In the same experiment, treatment with SA stimulated GNT35 gene expression, whereas BHA treatment inhibits this effect, as shown later in Figure 3B.
Figure 3.
Effect of BHA on the SA-activated expression of genes controlled by the as-1 promoter element. Leaf discs obtained from tobacco plants transformed with the (as-1)4/GUS gene were pretreated for 30 min with 0.1% (v/v) methanol (-), 0.75 mm BHA (B 0.75), or 1 mm BHA (B1), and were then treated for 5 h (A) or for 2.5 h (B) in the absence (C) or in the presence of 1 mm SA. A, GUS activity measured in protein extracts obtained from samples subjected to the indicated treatments. Values are mean ± sd of three independent experiments. Different letters indicate significantly different values (P < 0.05; ANOVA). B, GNT35 mRNA levels detected by northern hybridization using total RNA (20 μg per lane) isolated from samples subjected to the indicated treatments. A specific 32P-labeled GNT35 gene fragment was used as a probe. Staining the gels with EtBr controlled equal RNA loading.
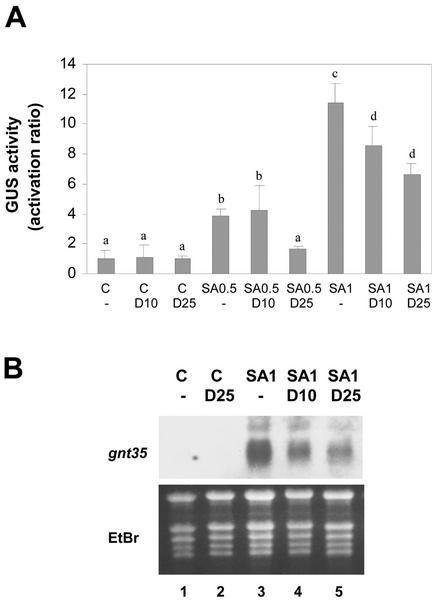
Once the controls for efficiency and specificity were completed, we evaluated the effects of DMTU and BHA on the SA-activated expression of (as-1)4/GUS and GNT35 genes. For this purpose, tobacco leaf discs were pretreated for 30 min with DMTU (10 or 25 mm) or BHA (0.75 or 1 mm), and then treatment proceeded in the presence or absence of SA (0.5 or 1 mm) for the indicated periods (Figs. 2 and 3). Expression of the (as-1)4/GUS gene was detected by assaying GUS activity, whereas expression of the GNT35 gene was detected by northern blot. DMTU partially inhibited the SA-activated expression of the (as-1)4/GUS gene (Fig. 2A) and the GNT35 gene (Fig. 2B). Twenty-five millimolar DMTU inhibited, by 76% and 46%, the GUS expression activated by 0.5 and 1 mm SA, respectively. Ten millimolar DMTU, on the other hand, inhibited by 28% the GUS expression induced by 1 mm SA.
Figure 2.
Effect of DMTU on the SA-activated expression of genes controlled by the as-1 promoter element. Leaf discs obtained from tobacco plants transformed with the (as-1)4/GUS gene were pretreated for 30 min with water (-), 10 mm DMTU (D 10), or 25 mm DMTU (D 25), and were then treated for 5 h (A) or for 2.5 h (B) in the absence (C) or in the presence of 0.5 mm SA (SA 0.5) or 1 mm SA (SA 1). A, GUS activity measured in protein extracts obtained from samples subjected to the indicated treatments. Values are mean ± sd of three to six independent experiments. Different letters indicate significantly different values (P < 0.05; ANOVA). B, GNT35 mRNA levels detected by northern hybridization using total RNA (20 μg per lane) isolated from samples subjected to the indicated treatments. A specific 32P-labeled GNT35 gene fragment was used as a probe. Staining the gels with EtBr controlled equal RNA loading.
BHA, a general radical scavenger, was more efficient than DMTU in inhibiting the SA-activated expression of the (as-1)4/GUS (Fig. 3A) and GNT35 gene (Fig. 3B). GUS expression activated by 1 mm SA was reduced 95% and 98% by 0.75 and 1 mm BHA, respectively (Fig. 3A). In addition, the accumulation of the GNT35 mRNA induced by 1 mm SA was also strongly reduced in the presence of 1 mm BHA (Fig. 3B).
Differences in the magnitude of GUS activation ratios after SA treatment, such as those found in our study (compare Figs. 2 and 3), are usually detected when we used greenhouse plants grown at different periods of the year. These differences in SA effectiveness could be due to differences in the cellular redox balance determined by seasonal changes in light intensity and ozone concentration (Bowler et al., 1991; Rao and Davis, 1999). Similar GUS activation ratio values were consistently obtained in greenhouse plants from the same batch.
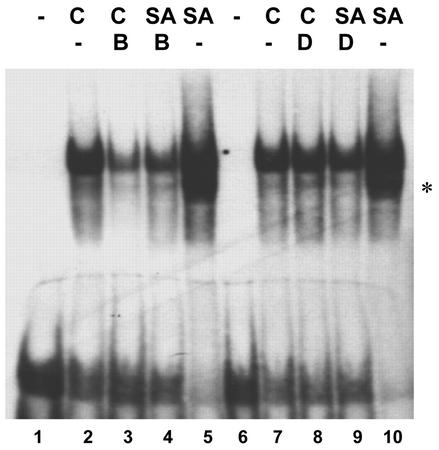
The effect of antioxidants on the SA-activated binding of nuclear factors to the as-1 sequence was evaluated in nuclear extracts obtained from leaf samples pretreated for 30 min with DMTU (25 mm) or BHA (1 mm), and then incubated in the absence or presence of 1 mm SA for 1 h (Fig. 4). The as-1-binding activity was detected by gel mobility shift assay using a 36-bp DNA fragment containing one copy of the as-1 sequence as a probe. As shown in Figure 4, the SA-activated binding of nuclear proteins to the as-1 sequence was completely inhibited by 1 mm BHA and 25 mm DMTU. Furthermore, BHA alone inhibited the basal as-1-binding activity (Fig. 4), but had no effect on the basal GUS activity (Fig. 3A). A possible explanation for this difference is that plants used in the experiment in Figure 4 had a higher oxidative status than those used in the experiment in Figure 3. This different plant oxidative status could explain the high level of basal as-1-binding activity (Fig. 4), susceptible to be inhibited by BHA, compared with the low level of basal GUS and GNT35 genes expression (Fig. 3, A and B). This result is also consistent with the hypothesis that the as-1-binding activity is more sensitive to oxidative species than the transcriptional activity. Results from experiments made with MV support this last possibility (see Figs. 7 and 8 and “Discussion”). Specificity of the binding to the as-1 sequence was demonstrated by competition experiments (see Fig. 8B).
Figure 4.
Effect of DMTU and BHA on the SA-activated binding of nuclear factors to the as-1 sequence. Gel mobility shift assays were carried out with nuclear extracts obtained from tobacco plants leaves pretreated for 30 min with 0.1% (v/v) methanol (-, lanes 2, 5, 7, and 10), 1 mm BHA (B, lanes 3 and 4), or 25 mm DMTU (D, lanes 8 and 9), and were then treated for 1 h in the absence (C) or in the presence of 1 mm SA. A 32P-labeled DNA fragment containing one copy of the as-1 sequence was used as probe. Lanes 1 and 6 show control reactions without nuclear extract. An asterisk indicates that this second band, although it specifically binds as-1 (see Fig. 8), did not appear in all experiments.
Figure 7.
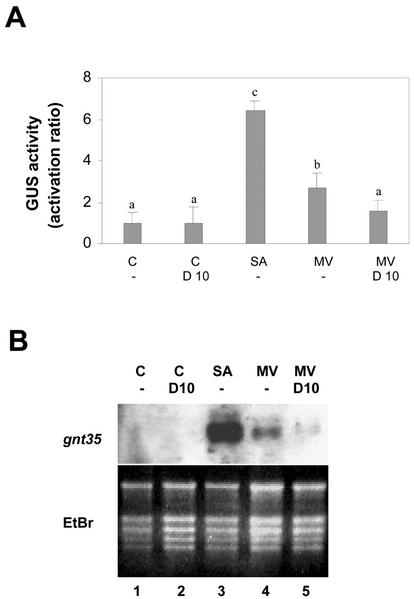
Effect of MV on transcription of genes controlled by the as-1 promoter element. Leaf discs obtained from tobacco plants transformed with the (as-1)4/GUS gene were pretreated for 30 min with water (-) or 10 mm DMTU (D 10), and were then treated for 5 h (A) or for 2.5 h (B) in the absence (C) or in the presence of 1 mm SA, or 50 μm MV. A, GUS activity measured in protein extracts obtained from samples subjected to the indicated treatments. Values are mean ± sd of three to six independent experiments. Different letters indicate significantly different values (P < 0.05; ANOVA). B, GNT35 mRNA levels detected by northern hybridization using total RNA (20 μg per lane) isolated from samples subjected to the indicated treatments. A specific 32P-labeled GNT35 gene fragment was used as a probe. Staining the gels with EtBr controlled equal RNA loading.
Figure 8.
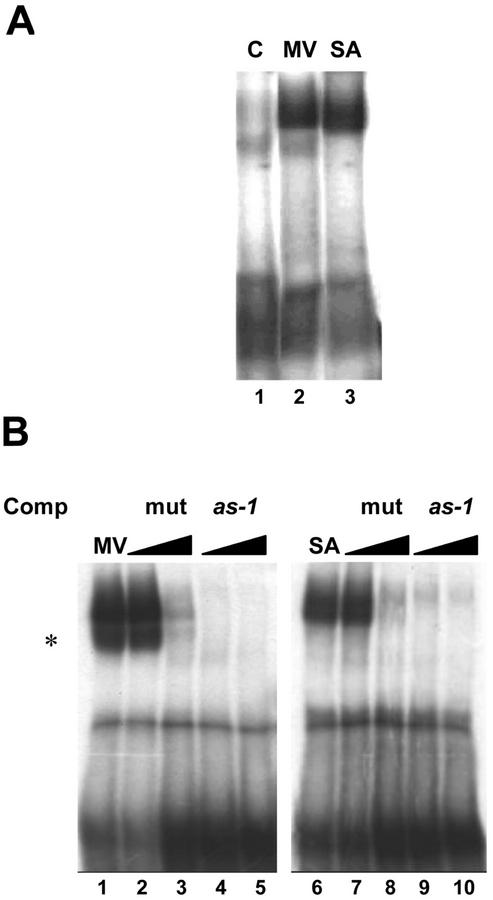
Effect of MV on the binding of nuclear factors to the as-1 sequence. Gel mobility shift assays were carried out with nuclear extracts from leaves of tobacco plants treated with water (C), 50 μm MV, or 1 mm SA. A 32P-labeled DNA fragment containing one copy of the as-1 sequence was used as a probe. Competition experiments were performed with 50× (B, lanes 4 and 9) and 150× molar excess (B, lanes 5 and 10) of the as-1 sequence, or 50× (B, lanes 2 and 7) and 150× molar excess (B, lanes 3 and 8) of a mutated as-1 sequence. An asterisk indicates that this second band, although it specifically binds as-1, did not appear in all experiments.
In summary, our results indicate that the ability of SA to activate the as-1 element is inhibited by antioxidants, with BHA being more efficient than DMTU. The higher efficiency of BHA (Fig. 1, A and B) may be related to the broader spectrum activity of BHA as a radical scavenger as compared with DMTU (Fox, 1984; Rehwoldt, 1986).
Effect of Light on the Activation of the as-1 Element by SA
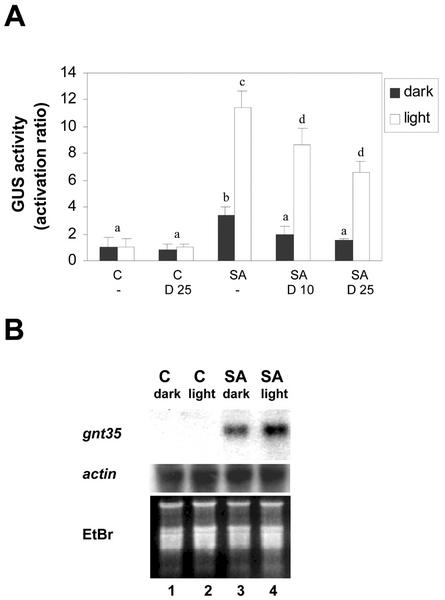
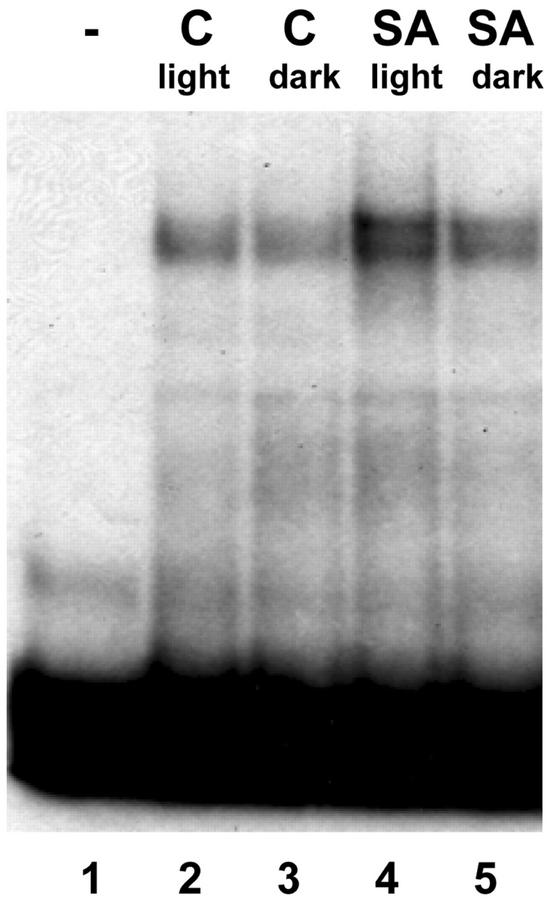
Unpublished results from our group indicate that the activation of the (as-1)4/GUS gene by SA is stronger in the presence of light than in darkness. It is interesting that it has been described that slight increments in light intensity lead to the accumulation of oxidized ascorbate and glutathione in tobacco plants (Willekens et al., 1997). The accumulation of these species is thought to be due to an increased production of ROS generated by the electron transport chain in the chloroplast (Willekens et al., 1997). If ROS levels are higher in the presence of light, we expected that the effect of SA on nuclear protein binding activity to the as-1 element and expression of GNT35 and (as-1)4/GUS genes would also be potentiated by light. As shown in Figure 5A, the SA-activated expression of the (as-1)4/GUS gene was 4.3 times higher in the presence of light than in the dark, and was significantly inhibited by DMTU in both cases. In a similar manner, the SA-activated expression of the GNT35 gene was also higher in the presence of light (Fig. 5B). The effect of SA on binding of nuclear proteins to the as-1 sequence was also stimulated by light (Fig. 6). In sum, results shown in Figures 5 and 6 indicate that the activation of the as-1 element by SA is potentiated by light. These findings are consistent with our hypothesis that oxidative species mediate the effect of SA on gene activation.
Figure 5.
Effect of light on the SA-activated transcription of genes controlled by the as-1 promoter element. A, Leaf discs obtained from tobacco plants transformed with the (as-1)4/GUS gene were pretreated for 30 min with water (-), 10 mm DMTU (D 10), or 25 mm DMTU (D 25), and were then treated for 5 h in the absence (C) or in the presence of 1 mm SA. Treatments were performed in the presence of light (90 μmol m−2 s−1, white bars) or in the dark (black bars). GUS activity was measured in protein extracts obtained from these samples. Values are mean ± sd of three to six independent experiments. Different letters indicate significantly different values (P < 0.05; ANOVA). B, Leaf discs obtained from tobacco plants transformed with the (as-1)4/GUS gene were treated for 2.5 h with water as a control (C) or with 1 mm SA in the presence of light (90 μmol m−2 s−1) or in the dark. GNT35 mRNA levels were detected by northern hybridization using total RNA (20 μg per lane) isolated from these samples. Specific 32P-labeled GNT35 and actin gene fragments were used as probes. Staining the gels with EtBr controlled equal loading.
Figure 6.
Effect of light on the SA-activated binding of nuclear factors to the as-1 sequence. Gel mobility shift assays were carried out with nuclear extracts from leaves of tobacco plants treated with water as a control (C) or with 1 mm SA for 1 h in the presence of light (90 μmol m−2 s−1) or in the dark. A 32P-labeled DNA fragment containing one copy of the as-1 sequence was used as a probe. Lane 1, A control reaction without nuclear extract.
Effect of MV, H2O2, and the Catalase Inhibitor 3-Amino-1,2,5-Triazole (3AT) on the Activity of the as-1 Element
To provide further evidence for the idea that the as-1 element is responsive to oxidative signals, the activity of the as-1 promoter element was evaluated after treating plants with MV, H2O2, and 3AT, compounds known to alter the cellular concentration of oxidative species. MV increases superoxide radical production preferentially in the chloroplast (Bowler et al., 1991), H2O2 is a diffusible ROS (Van Breusegem et al., 2001), and 3AT is a specific inhibitor of plant and animal catalases (Chen et al., 1993), increasing intracellular H2O2 levels.
To evaluate the effect of MV on the expression of (as-1)4/GUS and GNT35 genes, leaf samples were treated with MV (50 μm) for the indicated periods of time (Fig. 7). Treatment with SA (1 mm) was used as a positive control, and pretreatment with DMTU (10 mm) was used to evaluate the participation of ROS. Results shown in Figure 7, A and B, indicate that MV induced the expression of (as-1)4/GUS and GNT35 genes, albeit to a lesser extent than SA. GUS gene expression activated by MV was significantly higher than that of controls and was partially reduced by 10 mm DMTU (Fig. 7A). The level of GNT35 mRNA was also increased by the treatment with MV, an effect that was counteracted by 10 mm DMTU (Fig. 7B).
To evaluate the effects of MV on as-1 binding, we compared the binding activities in MV- and SA-treated plants. For this purpose, we obtained leaf nuclear extracts from plants treated for 1 h with water, 1 mm SA, and 50 μm MV. The as-1-binding activity of these extracts was assayed. Fifty micromolar MV increased the as-1-binding activity as efficiently as 1 mm SA (Fig. 8A). Nuclear protein binding to the as-1 sequence activated by MV and SA can be competed with a 50× molar excess of the nonradioactive as-1 sequence, but not with the same excess of a mutated as-1 sequence (Fig. 8B), thus indicating the specificity of the binding. It is interesting to note that although MV was as efficient as SA in producing oxidative damage (Fig. 1) and in increasing as-1-binding activity (Fig. 8), it was much less efficient than SA in activating transcription of genes controlled by the as-1 promoter element (Fig. 7).
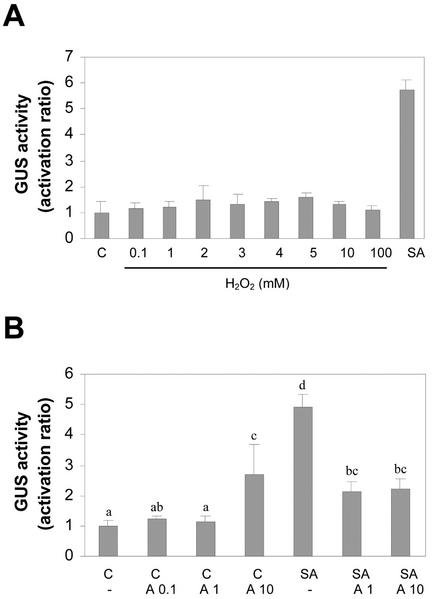
To evaluate the effect of increasing extracellular concentration of H2O2 on the expression of the (as-1)4/GUS gene, we treated leaf samples for 5 h with the indicated concentrations of H2O2, or with SA (1 mm) as a positive control. As shown in Figure 9A, H2O2 was not able to activate transcription of the (as-1)4/GUS gene. The lack of effect by H2O2 was not related to H2O2 degradation in the incubation solution, because H2O2 levels were not altered after 5 h of treatment (data not shown).
Figure 9.
Effect of H2O2 and the catalase inhibitor 3AT on the expression of the (as-1)4/GUS gene. A, Leaf discs obtained from tobacco plants transformed with the (as-1)4/GUS gene were treated for 5 h with 1 mm SA or with the indicated concentrations of H2O2. Treatments with H2O2 were done in the dark to avoid H2O2 decomposition. GUS activity was measured in protein extracts obtained from these samples. Values are mean ± sd of three independent experiments. B, Leaf discs obtained from tobacco plants transformed with the (as-1)4/GUS gene were pretreated for 30 min with water (-), 0.1 mm 3AT (A 0.1), 1 mm 3AT (A 1), or 10 mm 3AT (A 10), and were then treated for 5 h in the absence (C) or in the presence of 1 mm SA. GUS activity was measured in protein extracts obtained from these samples. Values are mean ± sd of three independent experiments. Different letters indicate significantly different values (P < 0.05; ANOVA).
The effect of inhibiting plant cellular catalases by treatment with 3AT on the expression of the (as-1)4/GUS gene was also evaluated. Inhibition of catalases leads to an increased intracellular level of H2O2 (Chen et al., 1993). SA inhibits catalases, generating the accumulation of H2O2 and also a SA radical species. On the other hand, 3AT inactivates catalases irreversibly without generating free radicals (Durner and Klessig, 1996; Kvaratskhelia et al., 1997; Anderson et al., 1998). Therefore, the use of 3AT in the presence of SA allows us to evaluate not only the effect of increasing H2O2 concentration, but also the possible effect of preventing the accumulation of the SA-free radical (Anderson et al., 1998).
Results shown in Figure 9B indicate that treatment of leaf discs for 5 h with 0.1 and 1 mm 3AT did not activate the (as-1)4/GUS gene, whereas a similar treatment with 10 mm 3AT activated it slightly. On the other hand, 3AT (1 and 10 mm) significantly reduced the effect of SA on (as-1)4/GUS gene activation (Fig. 9B).
Therefore, the present results do not support a role for H2O2 in the SA signaling pathway. Far from potentiating the SA effects on (as-1)4/GUS gene activation, inhibition of catalases activity by 3AT inhibited the activating effect of SA.
Taken together, the results shown in Figures 7 through 9 suggest that oxidative species different from H2O2 can be important for the activation of the as-1 promoter element by SA.
DISCUSSION
Oxidative Species as Signals in the SA-Mediated Activation of the as-1 Promoter Element
The findings of this study support the hypothesis that SA activates the as-1 sequence through oxidative species. According to our results, the oxidative species involved in the SA effect seem to be different from H2O2. Our results strengthen the idea that the as-1 sequence acts as an oxidative stress responsive element.
The idea that oxidative species act as intermediate signals of SA in the transcriptional regulation of defense genes has been extensively discussed in the literature (Chen et al., 1993; Bi et al., 1995; Neuenschwander et al., 1995; Durner and Klessig, 1996; Anderson et al., 1998; Chamnongpol et al., 1998). However, the discussion has been mainly focused on the role of H2O2 in the late transcriptional activation of pathogenesis-related (PR) genes by SA. Current evidence indicates that increased levels of H2O2 produced after SA treatment may play a role in potentiating SA-induced cell death in the local defense reaction (Alvarez, 2000). However, high levels of H2O2 do not seem to be directly involved in the systemic activation of PR genes by SA (Bi et al., 1995; Neuenschwander et al., 1995). Several reports present evidence supporting the idea that H2O2 mediates activation of PR genes by SA. In fact, it was reported that SA inhibits catalases, the major H2O2-degrading enzymes in plants (Conrath et al., 1995; Durner and Klessig, 1996). This, together with evidence of increased levels of H2O2 in tobacco leaves by SA treatment (1 mm, 3–24 h), and induction of PR-1a gene by H2O2 (1–5 mm, 48 h) and by the catalase inhibitor 3AT (1–4 mm, 48 h), lead Klessig and coworkers to postulate H2O2 as a second messenger for SA-mediated activation of PR genes (Chen et al., 1993). Thereafter, several reports using different strategies indicated that SA is required to have an effect of H2O2 on PR-1a expression, which supports the idea that H2O2 plays a role upstream rather than downstream of SA (Bi et al., 1995; Neuenschwander et al., 1995; Chamnogpol et al., 1998).
One of the main difficulties in the study of the SA-mediated signaling pathway leading to the activation of late genes, such as the PR genes, is the prolonged periods of incubation of plant tissues or cells with SA (over 24 h) required to activate PR transcription (Qin et al., 1994). The conclusions obtained from experiments using prolonged incubations with ROS-scavenging or ROS-generating compounds must be carefully considered because under these conditions, ROS not only influence PR expression, but also SA biosynthesis (León et al., 1995; Neuenschwander et al., 1995).
In this paper, we explored the role of ROS downstream of SA in the immediate early transcriptional activation of genes controlled by the as-1 element. In our experiments, the effects of ROS-scavenging or ROS-generating compounds were evaluated using incubation periods of 1 to 5 h. Even though we cannot be sure that under our conditions, H2O2 treatments did not affect SA biosynthesis, published information and our results suggest that SA concentrations were not increased. León et al. (1995) reported increased SA levels after a 6 h treatment of tobacco leaves with 150 mm H2O2. Treatment of tobacco leaves with H2O2 (0.1–100 mm) for 5 h did not consistently activate the expression of the (as-1)4/GUS reporter gene, which is responsive to SA levels (Hidalgo et al., 2001).
An important question arising from this work is: Which are the oxidative species involved in the activation of the as-1 sequence? Results of treatment with H2O2 and 3AT shown in this paper suggest that H2O2 is not this signal. Although H2O2 has been reported to activate genes containing as-1-like promoter elements, like plant GST genes (Levine et al., 1994; Chen and Singh, 1999) or the NOS gene (Dai and An, 1995), there is no convincing evidence that this activation involves the participation of as-1 promoter elements (Dai and An, 1995; Xiang et al., 1996). The report presented by Chen and Singh (1999) found that H2O2 activates the as-1-like element from the GST6 promoter after long incubation periods (18 h). Taking into account previous reports (León et al., 1995), it can be expected that SA biosynthesis occurred during the 18-h incubation. On the other hand, Xiang et al. (1996) showed activation of the as-1-like element from the NOS promoter by H2O2 in tobacco cell suspension culture (incubation for 2 h), but they were unable to reproduce this effect in whole seedlings. More conclusively, Dai and An (1995) reported that the H2O2-responsive element in the NOS gene promoter is located downstream the as-1-like element. Thus, it is unlikely that H2O2 functions as a signal in the SA activation of early genes.
On another hand, our results support the idea that oxidative species generated by SA, probably through its interaction with catalases, can be important for SA-activated expression of genes controlled by the as-1 element. Previous studies on the inhibitory effect of SA on plant and animal catalases (Durner and Klessig, 1996) and plant ascorbate peroxidase (Kvaratskhelia et al., 1997) indicate that the SA free radical can be produced in this process. Furthermore, the inhibitory effect of 3AT on lipid peroxidation produced by SA has been suggested as an evidence that 3AT prevents the generation of the SA-free radical (Anderson et al., 1998). In this context, we can speculate that our finding that 3AT inhibits the effect of SA on the expression of the (as-1)4/GUS gene may suggest the involvement of SA free radical and lipoperoxides. Furthermore, the fact that BHA acts as a better inhibitor of SA than DMTU also suggests that SA-free radicals or lipoperoxides could play a role in this pathway because BHA is a broader ROS scavenger than DMTU and it efficiently breaks the chain reactions that generate lipoperoxides (Rehwoldt, 1986).
Another interesting possibility is that activation of the as-1 element is regulated by the general oxidative status of the cell or of certain subcellular compartments, rather than by a specific ROS. If this is the case, it is possible that SA and MV, but not H2O2, are able to generate the appropriate oxidative status required to activate this pathway. Concerning the subcellular compartmentalization, it has been reported that ROS can be generated in mitochondria, chloroplasts, peroxisomes, microsomes, and apoplast (Grant and Loake, 2000). More specific experiments will be required to clarify whether or not particular subcellular compartments are involved in ROS generation after SA increases.
Mechanism of Activation of the as-1 Promoter Element by ROS and SA
A second question that emerges from our results is how oxidative signals can activate the as-1 promoter element. Several research groups are contributing to the unraveling of the mechanisms by which SA and other signals activate gene transcription via as-1-like promoter elements (Qin et al., 1994; Jupin and Chua, 1996; Stange et al., 1997; Pascuzzi et al., 1998; Niggeweg et al., 2000; Hidalgo et al., 2001; Johnson et al., 2001b, Pontier et al., 2001). Recent reports using dominant-negative mutants of TGA transcription factors indicate that the as-1-binding activity detected in nuclear extracts is mainly due to members of this protein family (Niggeweg et al., 2000; Pontier et al., 2001). Furthermore, in TGA activity-depleted plants, activation of the GNT35 gene by SA, 2,4-dichlorophenoxyacetic acid, and methyl jasmonate is suppressed, indicating that TGA factors are required for activation of this gene by these chemical stressors (Pontier et al., 2001). The consistent in vivo binding of one of the TGA factors to the as-1 element present in the GNT35 promoter was recently reported (Johnson et al., 2001a). Current evidence indicate that the as-1-binding activity of TGA factors (Jupin and Chua, 1996; Stange et al., 1997; Johnson et al., 2001a, 2001b) and the trans-activation capacity of these factors (Pascuzzi et al., 1998; Johnson et al., 2001b) can be regulated by SA or auxins through mechanisms involving protein-protein dissociation and protein phosphorylation. In agreement with these findings, we have recently reported participation of a nuclear protein kinase CK2 in the SA-activated binding of nuclear proteins to the as-1 sequence (Hidalgo et al., 2001). The possible relationship between protein phosphorylation by CK2 and oxidative signals in this system is an interesting issue to explore. Evidence of activation of CK2 by conditions associated to oxidative stress in mammalian cells support this idea (Gerber et al., 2000).
Results shown in this paper indicate that transcription factor binding to the as-1 sequence is more susceptible to ROS-scavenging or ROS-generating compounds than transcription of genes controlled by this sequence. In fact, MV was as efficient as SA in promoting the binding of proteins to the as-1 sequence, but was much less efficient than SA in the transcriptional activation of GNT35 or (as-1)4/GUS. Furthermore, antioxidants inhibit the SA-activated binding to the as-1 sequence more efficiently than the SA-activated transcription of GNT35 or (as-1)4/GUS. Therefore, it can be postulated that SA promotes the binding of transcription factors to the as-1 sequence and increases the trans-activation capacity of the factors by different mechanisms. Our results are consistent with the idea that the participation of ROS is more important in the transcription factor binding than in the trans-activation process.
It is interesting that several mammalian transcription factors have been reported to be regulated by ROS. For example, it is well known that ROS activate genes related to immune and inflammatory responses through the activation of nuclear factor-κB and AP-1 transcription factors (Gabbita et al., 2000). Thioredoxin-mediated redox modification of Cys residues involved in DNA-binding activity of both factors seems to be an important regulatory mechanism (Matthews et al., 1992; Hirota et al., 1997). Based on the homology between c-jun and TGA factors, and their respective target sequences (Katagiri et al., 1989), we are currently evaluating possible redox modification of TGA factors induced by SA or auxins.
Role of SA in Activation of Antioxidant Defenses
Experimental evidence accumulated up to now has led researchers to postulate a dual role for SA in the protection of plants against pathogen-induced oxidative stress. Although high levels of SA potentiate programmed cell death in infected tissues, low levels of SA activate antioxidant defenses required to maintain the cellular redox state in systemic tissues (Van Camp et al., 1998; Grant and Loake, 2000). In infected tissues, potentiation of programmed cell death (Draper, 1997; Van Camp et al., 1998; Grant and Loake, 2000) can be explained by a dual effect: a direct effect of SA by increasing levels of ROS, and an indirect effect of SA by inhibiting ROS-detoxifying enzymes such as catalases (Rao et al., 1997; Grant and Loake, 2000). In systemic tissues, during systemic acquired resistance, a moderate accumulation of SA may play a role in the activation of ROS-detoxifying genes such as GSTs. It is interesting to note that according to the experimental data shown in this work, the most probable mechanism by which SA activates these genes is also by increasing ROS levels. In this case, ROS could activate binding of nuclear factors to oxidative stress-responsive elements such as as-1 found in the promoter of GSTs. This dual effect of SA on the cellular redox balance can also be important in the establishment of defense reactions against ozone exposure (Rao and Davis, 1999).
MATERIALS AND METHODS
Plant Material
Wild-type tobacco (Nicotiana tabacum) plants used in this study were cv Xanthi nc. Transgenic tobacco plants were tobacco cv Xanthi nc. transformed with the (as-1)4/GUS reporter gene, which contains a tetramer of the as-1 sequence fused to the 35S cauliflower mosaic virus minimal promoter (−46 to +8) and to the GUS coding sequence. The sequence of the tetramer of as-1 is agctt(CTGACGTAAGGGATGACGCAC)2tctaga(CTGACGTAAGGGATGACGCAC)2-tcga (as-1 motifs in bold). Details for construction of the (as-1)4/GUS gene, cloning in a binary vector, and obtainment and selection of the transgenic tobacco clones were previously reported (Hidalgo et al., 2001). All plants were grown in a greenhouse (at 15°C–25°C with 16 h of light at 30 μmol m−2 s−1) until they had eight to 15 expanded leaves.
Treatment of Plants with SA, MV, H2O2, and Antioxidants
The expression of GUS and GNT35 genes was assayed in transgenic tobacco plants harboring the (as-1)4/GUS gene. On the other hand, the as-1-binding activity was measured in wild-type tobacco plants. For these assays, leaf discs of 1.2 cm in diameter were subjected to chemical treatments with SA (Sigma, St. Louis, or Riedel-deHaën Laborchemkallen GmbH & Co., Seelze, Germany), MV (Sigma), or H2O2 (Merck, Whitehouse Station, NJ). The discs were placed, top side up, in 15 mL of the corresponding solution and they were vacuum-infiltrated for 3 min. Stock solutions of SA, MV, and H2O2 were freshly prepared in water. The exact concentration of the H2O2 in the stock solution was determined spectrophotometrically before being used (ε240 nm = 39.6 m−1 cm−1). Control samples were incubated in water. Incubations were carried out in a growth chamber under constant temperature (22°C–25°C). To test the effects of antioxidants, leaf discs were incubated for 30 min in the dark in a solution containing DMTU (Sigma) or BHA (Sigma). SA or MV was subsequently added and the incubation was continued in the presence of white light (90 μmol m−2 s−1) for the period indicated in each case. Stock solutions of DMTU and BHA were freshly prepared in water and 100% (v/v) methanol, respectively. Control samples for DMTU or BHA treatment were pretreated under the same conditions with water or with 0.1% (v/v) methanol, respectively. Immediately after treatments, leaf discs were frozen in liquid nitrogen and stored at −70°C.
Measurements of Ion Leakage
Oxidative membrane damage produced by MV and SA was assayed by measuring ion leakage from tobacco leaf discs punched out from adult soil-grown plants with at least 10 fully expanded leaves (Bowler et al., 1991). For each treatment, three discs (1.2 cm of diameter) were half cut and floated, top side up, on 15 mL of the corresponding antioxidant solution (BHA or DMTU) or the control solution (0.1% [v/v] methanol or water, respectively). The samples were vacuum-infiltrated for 3 min and were preincubated for 30 min in the dark at room temperature. MV (50 μm) or SA (1 mm) was then added and the samples were incubated in the presence of white light (90 μmol m−2 s−1) for 5 h in a growth chamber at 25°C. Control samples were treated under the same conditions, without addition of MV or SA. Thereafter, the samples were protected from light and the incubation was continued for 16 h in the growth chamber. Leaf discs were carefully removed and the conductivity of the bathing solution was measured with a conductivity meter. To calculate the percentage of leakage, the mean conductivity value of the control treatment with water or 0.1% (v/v) methanol was subtracted from the conductivity value of each sample, and then the percentage was calculated considering as 100% the treatment with 50 μm MV. Contribution of the treatment solution alone to the conductance of each sample was previously subtracted.
GUS Activity Assay
GUS activity was assayed in protein extracts by a fluorescence method using 4-methylumbelliferyl glucuronide as substrate (Jefferson, 1987). 4-Methylumbelliferone (MU), the fluorescent product, was quantified using a fluorometer (TKO 100; Hoefer Scientific Instruments). Standard solutions of MU in 0.2 m Na2CO3 were used for calibration purposes. To prepare protein extracts, the frozen tissue was ground in liquid nitrogen, extracted with buffer (50 mm sodium phosphate, pH 7.0, 1 mm EDTA, 0.1% [v/v] Triton X-100, and 10 mm 2-mercaptoethanol), and centrifuged for 10 min at 4°C in a microcentrifuge. Protein concentration was determined according to the Bio-Rad protocol provided with the protein assay kit. GUS activity was calculated as picomoles MU per minute per milligram of protein and was then expressed as activation ratio (ratio between treatment and control activities).
RNA Extraction and Northern Analysis
Total RNA was extracted from frozen leaf samples essentially as described by Logemann et al. (1987). Samples containing 20 μg of RNA were separated on formaldehyde-agarose gels. Staining the gels with EtBr controlled for equal loading. After RNA transfer onto nylon membranes (Hybond N; Amersham Biosciences, Piscataway, NJ), filters were hybridized with the 32P-labeled probe (30–50 × 106 cpm) in a buffer containing 6× SSC, 5× Denhardt's' solution, 50% (w/v) formamide, 0.5% (w/v) SDS, 1 mm EDTA, and 150 μg mL−1 salmon sperm carrier DNA (Figs. 2 and 7), or in a ultrahybrid solution from Ambion (Austin, TX; Figs. 1, 3, and 5). The filters were then washed twice in 2× SSC and 0.1% (w/v) SDS for 10 min at 55°C (Figs. 2 and 7) or 42°C (Figs. 1, 3, and 5), and twice in 0.2× SSC and 0.1% (w/v) SDS for 5 min at 55°C (Figs. 2 and 7) or 42°C (Figs. 1, 3, and 5). A 410-bp DNA fragment obtained by PCR using previously described synthetic primers (Xiang et al., 1996) was used as a probe for tobacco GNT35 gene. In turn, a 130-bp DNA fragment obtained by PCR using the primers 5-GCTATGTATGTCGCCATTCAAGC and 5-CATCATATTCTGCCTTTGC(A/G)ATCC was used as a probe for the tobacco actin gene. The amplified fragments were sequenced and labeled by random priming using [α-32P]dCTP (Megaprime DNA labeling system; Amersham).
Nuclear Extracts and Gel Mobility Shift Assays
Nuclear protein extracts were prepared as described by Hidalgo et al. (2001). The final yield was 5 to 10 μg of nuclear protein per gram of fresh leaf tissue weight. DNA-protein binding assays were carried out with nuclear protein extracts (2–10 μg of protein) incubated with the 32P-labeled probe (25,000 cpm, 0.06–0.8 pmol) in 15 μL of binding buffer (50 mm HEPES, pH 7.9, 100 mm KCl, 2 mm MgCl2, 10 mm dithiothreitol, 3.75% [v/v] glycerol, 10 mm NaF, 8 mm Na2MoO4, and 15 ng poly(dG)·poly(dC); Amersham Biosciences) for 10 to 15 min at room temperature. For competition experiments, the indicated molar excess of the nonradioactive probe was included in the binding assay 10 min before the labeled probe was added. DNA-protein complexes were separated from the unbound probe by electrophoresis in a 6% (w/v) polyacrylamide gel (6.072% T and 1.186% C) in Tris borate-EDTA buffer. After electrophoresis, gels were dried and subjected to autoradiography at −70°C for 14 to 16 h. To obtain the DNA probes, the following oligonucleotides were used: 5′-CTGCAGACTGACGTAAGGGATGACGCACAACTCGAG-3′ for the as-1 sequence (protein-binding motifs are indicated in bold), and 5′-CTGCAGACCGACGATAGGGACGACGACCAACTCGAG-3′ for the mutated as-1 sequence (mutated nucleotides are underlined). The complementary strands were synthesized using the primer 5′-CTCGAGT-3′, dNTPs, and Klenow DNA polymerase following standard protocols (Ausubel, 1997). (α-32P)dCTP was included in this reaction for labeling the probe.
Statistical Analysis of Data
Differences were evaluated by analysis of variance (ANOVA) for repeated measurements with Duncan adjustments, using the statistical program SPSS 7.5 (SPSS, Chicago). A P value of 0.05 was considered significant.
ACKNOWLEDGMENTS
We thank Dr. Marcela Bitran for improving the manuscript and Alejandra San Martín for the statistical analysis of data.
Footnotes
This work was supported by Fondecyt-Conicyt, Chile (grant nos. 8980005 and 2980065).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.009886.
LITERATURE CITED
- Alvarez ME. Salicylic acid in the machinery of hypersensitive cell death an disease resistance. Plant Mol Biol. 2000;44:429–442. doi: 10.1023/a:1026561029533. [DOI] [PubMed] [Google Scholar]
- Alvarez ME, Pennell RI, Meijer P-J, Ishikawa A, Dixon RA, Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;92:773–784. doi: 10.1016/s0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- Anderson MD, Chen Z, Klessig DF. Possible involvement of lipid peroxidation in salicylic acid-mediated induction of PR-1 gene expression. Phytochemistry. 1998;47:555–566. [Google Scholar]
- Ausubel FM. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1997. [Google Scholar]
- Bi YM, Kenton P, Mur L, Darby R, Draper J. Hydrogen peroxide does not function downstream of salicylic acid in the induction of PR protein expression. Plant J. 1995;8:235–245. doi: 10.1046/j.1365-313x.1995.08020235.x. [DOI] [PubMed] [Google Scholar]
- Bowler C, Slooten L, Vandenbranden S, De Rycke R, Botterrman J, Sybesma C, Van Montagu M, Inzé D. Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. EMBO J. 1991;10:1723–1732. doi: 10.1002/j.1460-2075.1991.tb07696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candeias LP, Folkes LK, Wardman P. Enhancement of peroxidase-induced lipid peroxidation by indole-3-acetic acid and effect of antioxidants. Redox Rep. 1996;2:141–147. doi: 10.1080/13510002.1996.11747041. [DOI] [PubMed] [Google Scholar]
- Chamnongpol S, Willekens H, Moeder W, Langebartels C, Sandermann H, Jr, Van Montagu M, Inzé D, Van Camp W. Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc Natl Acad Sci USA. 1998;95:5818–5823. doi: 10.1073/pnas.95.10.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Singh KB. The auxin, hydrogen peroxide and salicylic acid induced expression of the Arabidopsis GST6 promoter is mediated in part by an ocs element. Plant J. 1999;19:667–677. doi: 10.1046/j.1365-313x.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- Chen Z, Silva H, Klessig DF. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science. 1993;262:1883–1886. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- Conrath U, Chen Z, Ricigliano JR, Klessig DF. Two inducers of plant defense responses, 2, 6-dichloroisonicotinec acid and salicylic acid, inhibit catalase activity in tobacco. Proc Natl Acad Sci USA. 1995;92:7143–7147. doi: 10.1073/pnas.92.16.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, An G. Induction of nopaline synthase promoter activity by H2O2 has no direct correlation with salicylic acid. Plant Physiol. 1995;109:1191–1197. doi: 10.1104/pp.109.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E et al. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–1250. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- Draper J. Salicylate, superoxide synthesis and cell suicide in plant defense. Trends Plant Sci. 1997;2:162–165. [Google Scholar]
- Droog F, Spek A, van der Kooy A, de Ruyter A, Hoge H, Libbenga K, Hooykaas P, van der Zaal B. Promoter analysis of the auxin-regulated tobacco glutathione S-transferase genes Nt103-1 and Nt103-35. Plant Mol Biol. 1995;29:413–429. doi: 10.1007/BF00020974. [DOI] [PubMed] [Google Scholar]
- Durner J, Klessig DF. Salicylic acid is a modulator of tobacco and mammalian catalases. J Biol Chem. 1996;271:28492–28501. doi: 10.1074/jbc.271.45.28492. [DOI] [PubMed] [Google Scholar]
- Durner J, Shah J, Klessig DF. Salicylic acid and disease resistance in plants. Trends Plant Sci. 1997;2:266–274. [Google Scholar]
- Edwards R, Dixon DP, Walbot V. Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci. 2000;5:193–198. doi: 10.1016/s1360-1385(00)01601-0. [DOI] [PubMed] [Google Scholar]
- Fox RB. Prevention of granulocyte-mediated oxidant lung injury in rats by a hydroxyl radical scavenger, dimethylthiourea. J Clin Invest. 1984;74:1456–1464. doi: 10.1172/JCI111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbita SP, Robinson KA, Stewart CA, Floyd RA, Hensley K. Redox regulatory mechanisms of cellular signal transduction. Arch Biochem Biophys. 2000;376:1–13. doi: 10.1006/abbi.1999.1685. [DOI] [PubMed] [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- Gerber DA, Souquere-Besse S, Puvion F, Dubois MF, Bensaude O, Cochet C. Heat-induced relocalization of protein kinase CK2: implication of CK2 in the context of cellular stress. J Biol Chem. 2000;275:23919–23926. doi: 10.1074/jbc.M002697200. [DOI] [PubMed] [Google Scholar]
- Grant JJ, Loake GJ. Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol. 2000;124:21–29. doi: 10.1104/pp.124.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo P, Garretón V, Berríos CG, Ojeda H, Jordana X, Holuigue L. A nuclear casein kinase 2 activity is involved in early events of transcriptional activation induced by salicylic acid in tobacco. Plant Physiol. 2001;125:396–405. doi: 10.1104/pp.125.1.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Matsui M, Iwata S, Nishiyama A, Mori K, Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc Natl Acad Sci. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5:387–405. [Google Scholar]
- Johnson C, Boden E, Desai M, Pascuzzi P, Arias J. In vivo target promoter-binding activities of a xenobiotic stress-activated TGA factor. Plant J. 2001a;28:237–243. doi: 10.1046/j.1365-313x.2001.01147.x. [DOI] [PubMed] [Google Scholar]
- Johnson C, Glover G, Arias J. Regulation of DNA binding and trans-activation by a xenobiotic stress-activated plant transcription factor. J Biol Chem. 2001b;276:172–178. doi: 10.1074/jbc.M005143200. [DOI] [PubMed] [Google Scholar]
- Joo JH, Bae YS, Lee JS. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001;126:1055–1060. doi: 10.1104/pp.126.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupin I, Chua N-H. Activation of the CaMV as-1 cis-element by salicylic acid: differential DNA-binding of a factor related to TGA1a. EMBO J. 1996;15:5679–5689. [PMC free article] [PubMed] [Google Scholar]
- Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Katagiri F, Lam E, Chua N-H. Two tobacco DNA-binding proteins with homology to the nuclear factor CREB. Nature. 1989;340:727–729. doi: 10.1038/340727a0. [DOI] [PubMed] [Google Scholar]
- Kawano T, Sahashi N, Takahashi K, Uozumi N, Muto S. Salicylic acid induces extracellular superoxide generation followed by an increase in cytosolic calcium ion in tobacco suspension culture: the earliest events in salicylic acid signal transduction. Plant Cell Physiol. 1998;39:721–730. [Google Scholar]
- Kvaratskhelia M, George SJ, Thorneley RN. Salicylic acid is a reducing substrate and not an effective inhibitor of ascorbate peroxidase. J Biol Chem. 1997;272:20998–21001. doi: 10.1074/jbc.272.34.20998. [DOI] [PubMed] [Google Scholar]
- Lam E, Benfey PN, Gilmartin PM, Fang R-X, Chua N-H. Site-specific mutations alter in vitro factor binding and change promoter expression pattern in transgenic plants. Proc Natl Acad Sci USA. 1989;86:7890–7894. doi: 10.1073/pnas.86.20.7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León J, Lawton MA, Raskin I. Hydrogen peroxide stimulates salicylic acid biosynthesis in tobacco. Plant Physiol. 1995;108:1673–1678. doi: 10.1104/pp.108.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987;163:16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Malamy J, Carr JP, Klessig DF, Raskin I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Maleck K, Levine A, Eulgem T, Morgan A, Schmid J, Lawton KA, Dangl JL, Dietrich RA. The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat Genet. 2000;26:403–410. doi: 10.1038/82521. [DOI] [PubMed] [Google Scholar]
- Matthews JR, Wakasugi N, Virelizier J-L, Yodoi J, Hay RT. Thioredoxin regulates the DNA binding activity of NF-κB by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuenschwander U, Vernooij B, Friedrich L, Uknes S, Kessmann H, Ryals J. Is hydrogen peroxide a second messenger of salicylic acid in systemic acquired resistance? Plant J. 1995;8:227–233. [Google Scholar]
- Niggeweg R, Thurow C, Kegler C, Gatz C. Tobacco transcription factor TGA2.2 is the main component of as-1 binding factor ASF-1 and is involved in salicylic acid- and auxin-inducible expression of as-1 containing target promoters. J Biol Chem. 2000;275:19897–19905. doi: 10.1074/jbc.M909267199. [DOI] [PubMed] [Google Scholar]
- Pascuzzi P, Hamilton D, Bodily K, Arias J. Auxin-induced stress potentiates trans-activation by a conserved plant basic/leucine-zipper factor. J Biol Chem. 1998;273:26631–26637. doi: 10.1074/jbc.273.41.26631. [DOI] [PubMed] [Google Scholar]
- Pontier D, Miao ZH, Lam E. Trans-dominant suppression of plant TGA factors reveals their negative and positive roles in plant defense responses. Plant J. 2001;27:529–538. doi: 10.1046/j.1365-313x.2001.01086.x. [DOI] [PubMed] [Google Scholar]
- Qin X-F, Holuigue L, Horvath DM, Chua N-H. Immediate early transcription activation by salicylic acid via the cauliflower mosaic virus as-1 element. Plant Cell. 1994;6:863–874. doi: 10.1105/tpc.6.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Davis KR. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J. 1999;17:603–614. doi: 10.1046/j.1365-313x.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- Rao MV, Paliyath G, Ormrod DP, Murr DP, Watkins CB. Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes. Plant Physiol. 1997;115:137–149. doi: 10.1104/pp.115.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehwoldt R. Tracking the use of antioxidants through industry surveys. Food Chem Toxicol. 1986;24:1039–1041. doi: 10.1016/0278-6915(86)90286-3. [DOI] [PubMed] [Google Scholar]
- Rushmore TH, Picket CB. Glutathione S-transferases, structure, regulation, and therapeutic implications. J Biol Chem. 1993;268:11475–11478. [PubMed] [Google Scholar]
- Stange C, Ramírez I, Gómez I, Jordana X, Holuigue L. Phosphorylation of nuclear proteins directs binding to salicylic acid-responsive elements. Plant J. 1997;11:1315–1324. doi: 10.1046/j.1365-313x.1997.11061315.x. [DOI] [PubMed] [Google Scholar]
- Ulmasov Y, Hagen G, Guilfoyle T. The ocs element in the soybean GH2/4 promoter is activated by both active and inactive auxin and salicylic acid analogues. Plant Mol Biol. 1994;26:1055–1064. doi: 10.1007/BF00040688. [DOI] [PubMed] [Google Scholar]
- Van Breusegem F, Vranová E, Dat JF, Inzé D. The role of active oxygen species in plant signal transduction. Plant Sci. 2001;161:405–414. [Google Scholar]
- Van Camp W, Van Montagu M, Inzé D. H2O2 and NO: redox signals in disease resistance. Trends Plant Sci. 1998;3:330–334. [Google Scholar]
- Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inzé D, Van Camp W. Catalase is a sink for H2O2 and is indispensable for stress defense in C3 plants. EMBO J. 1997;16:4806–4816. doi: 10.1093/emboj/16.16.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C, Miao ZH, Lam E. Coordinated activation of as-1 type elements and a tobacco glutathione S-transferase gene by auxins, salicylic acid, methyl-jasmonate and hydrogen peroxide. Plant Mol Biol. 1996;32:415–426. doi: 10.1007/BF00019093. [DOI] [PubMed] [Google Scholar]
- Xiang C, Miao ZH, Lam E. DNA-binding properties, genomic organization and expression pattern of TGA6, a new member of the TGA family of bZIP transcription factors in Arabidopsis thaliana. Plant Mol Biol. 1997;34:403–415. doi: 10.1023/a:1005873500238. [DOI] [PubMed] [Google Scholar]
- Yalpani N, Enyedi AJ, León J, Raskin I. Ultraviolet light and ozone stimulate accumulation of salicylic acid, pathogenesis-related proteins and virus resistance in tobacco. Planta. 1994;193:372–376. [Google Scholar]