Abstract
A fundamental problem of plant science is to understand the biochemical basis of plant/pathogen interactions. The foliar disease tan spot of wheat (Triticum aestivum), caused by Pyrenophora tritici-repentis, involves Ptr ToxA, a proteinaceous host-selective toxin that causes host cell death. The fungal gene ToxA encodes a 17.2-kD pre-pro-protein that is processed to produce the mature 13.2-kD toxin. Amino acids 140 to 142 of the pre-pro-protein form an arginyl-glycyl-aspartic (RGD) sequence, a motif involved in the binding of some animal proteins and pathogens to transmembrane receptor proteins called integrins. Integrin-like proteins have been identified in plants recently, but their role in plant biology is unclear. Our model for Ptr ToxA action predicts that toxin interacts with a putative host receptor through the RGD motif. Mutant clones of a ToxA cDNA, created by polymerase chain reaction such that the RGD in the pro-toxin was changed to arginyl-alanyl-aspartic or to arginyl-glycyl-glutamic, were expressed in Escherichia coli. Extracts containing mutated forms of toxin failed to cause host cell death, but extracts from E. coli expressing both a wild-type pro-protein cDNA and a control mutation away from RGD were active in cell death development. In competition experiments, 2 mm RGD tripeptide reduced the level of electrolyte leakage from wheat leaves by 63% when co-infiltrated with purified Ptr ToxA (15 μg mL−1) obtained from the fungus, but the control peptide arginyl-glycyl-glutamyl-serine provided no protection. These experiments indicate that the RGD motif of Ptr ToxA is involved with toxin action, possibly by interacting with a putative integrin-like receptor in the host.
One of the fundamental problems of plant biology is to understand the molecular and biochemical basis of plant disease caused by microbes. Some of the best models for understanding fungal pathogenicity and host susceptibility are those systems that involve pathogen-produced metabolites called host-selective toxins (HSTs). In many of these systems, host sensitivity to a toxin has been genetically associated with disease susceptibility. Further, toxin production by the pathogen has been associated with pathogenicity (the ability to cause disease) or with enhanced virulence (disease severity; Scheffer and Livingston, 1984; Walton and Panaccione, 1993). The simplest model for HST action predicts that toxin perception by the host is mediated through a host receptor (Scheffer and Livingston, 1984). Toxin/receptor interactions have been demonstrated for some HSTs, such as victorin, produced by Cochliobolus victorae, causal agent of victoria blight of oats (Avena sativa; Wolpert and Macko, 1989), and with Hmt toxin, the HST produced by Cochliobolus heterostrophus race T, the Southern corn (Zea mays) leaf blight pathogen (Braun et al., 1990). Toxin perception activates biochemical, physiological, and molecular events in the host that are associated with plant disease.
Pyrenophora tritici-repentis (Died.) Dreschs. is the causal agent in the foliar disease tan spot of wheat (Triticum aestivum). Field isolates of the fungus produce Ptr ToxA, a proteinaceous 13.2-kD HST (Ciuffetti et al., 1997) that causes cell death in sensitive wheat (Tuori et al., 1995; Kwon et al., 1996). Sensitivity to the toxin is conditioned by a single dominant gene located on host chromosome 5BL (Faris et al., 1996; Stock et al., 1996). As is typical of HSTs, production of toxin has been associated with pathogenicity (Tomas et al., 1990; Ciuffetti et al., 1997), and host sensitivity to this toxin has been associated with susceptibility (Lamari and Bernier, 1989, 1991) and with disease severity (Friesen et al., 2002). Neither the host gene for toxin sensitivity nor its product have been identified, but conventional views of toxin interactions would predict that the gene encodes a toxin receptor.
Functional studies on the action of Ptr ToxA using an electrolyte leakage assay (Kwon et al., 1996) have demonstrated that active host processes, including de novo mRNA and protein synthesis, are required (Kwon et al., 1998). The requirement of host metabolism and toxin-directed gene expression in cell death qualifies this toxin's action as inducing a form of programmed cell death in the host (Greenberg, 1996).
Ptr ToxA is directly encoded for by the fungal gene ToxA (Ciuffetti et al., 1997). This encodes a 178-amino acid (approximately 17.8-kD) pre-pro-protein, the first 16 to 22 amino acids of which is proposed to make up a signal peptide (Ciuffetti et al., 1997; Zhang, 1997). The remaining 156 to 162 amino acid pro-protein includes a 38- to 44-amino acid pro-peptide, apparently required for proper folding (Cheng, 2000; Tuori et al., 2000), that is cleaved by the fungus before secretion, producing the mature 13.2-kD protein (Zhang, 1997). Analysis of the mature protein sequence revealed the presence of an arginyl-glycyl-aspartic (RGD) tri-peptide, amino acids 140–142, on a predicted loop region of the protein (Zhang et al., 1997). The RGD sequence that has been associated with the binding of extracellular matrix proteins to a class of plasma membrane proteins called integrins (Ruoslahti and Pierschbacher, 1986; d'Souza et al., 1991). In mammalian systems, integrins have been shown to be an important class of receptors involved in transmitting signals both into and out of the cell (Coppolino and Dedhar, 2000; Clark and Brugge, 1995) and in mediating adhesion, migration, and invasion (Hynes, 1992; Schwartz et al., 1995; Critchley et al., 1999). Many mammalian pathogens have exploited the presence of integrins as adhesion sites (Isberg and Tran Van Nhieu, 1994) and as binding sites for toxins secreted by the pathogen. For example exotoxin B of group A Streptococcus (Stockbauer et al., 1999), Actinobacillus actinmycetamcomitans leukotoxin, and Escherichia coli α-hemolysin (Lally et al., 1997) all bind to integrins presented by the host. Only in the last 5 years have integrin-like proteins been identified in plant systems (Faik et al., 1998; Lynch et al., 1998; Laboure et al., 1999; Nagpal and Quatrano, 1999), but little is known about their structure and function. We present evidence here that the RGD tripeptide in Ptr ToxA is required for its function. This suggests that a plant integrin-like protein may act as the receptor that stimulates a programmed cell death response.
RESULTS
cDNA Cloning and Mutagenesis
Primers 1 and 2 (Table I) and first-strand cDNA for ToxA pro-protein made from P. tritici-repentis mRNA were used in a PCR reaction to amplify a 539-bp cDNA. Sequence analysis of the cloned fragment revealed the predicted 486-bp sequence of ToxA that encoded the 162-amino acid wild-type pro-protein for Ptr ToxA proposed by Zhang (1997; Fig. 1). The cDNA was subcloned into pET21c(+) to form pSM1.
Table I.
PCR primers used to create wild type and mutant cDNAs for the Ptr ToxA proprotein
| Primera | Sequenceb | Uses |
|---|---|---|
| 1 | AAGGCTCCATATGCAGGGAAGT | Primed coding strand synthesis of DNA for amino terminus of Ptr ToxA proprotein. |
| TGTATGTCAATCACAATCAAC | Paired with primers 4, 6, and 8 in PCR reactions to create desired mutations. | |
| Paired with primer 2 for final amplification of wild type and mutant cDNAs. | ||
| 2 | GCCGCGCACGAATTCTAATTTTC | Primed noncoding strand synthesis of DNA encoding carboxy terminal (including stop codon) of Ptr ToxA proprotein. |
| TAGCTGCATTCTC | Paired with primers 3, 5, and 7 to create mutations. | |
| Paired with primer 1 for final amplification of cDNAs. | ||
| 3 | CTGGTGCTATAGCCTCGTGGGA | Modified G (96) to A. |
| ACTG | ||
| 4 | CAGTTCCCACGAGGCTATAGCA | |
| CCAG | ||
| 5 | GGATAATACTGTCACTCGGGCG | Modified G (141) to A. |
| GACGTTTATGAG | ||
| 6 | CTCATAAACGTCCGCCCGATGA | |
| CAGTATTATCC | ||
| 7 | GTCACTCGGGGGGAAGTTTATG | Modified D (142) to E. |
| AGC | ||
| 8 | GCTCATAAACTTCCCCCCGAGT | |
| GAC |
Odd-numbered sequences primed coding strand synthesis and even-numbered primed non-coding strand synthesis.
NdeI and EcoRI sites, underlined, were manufactured into primers 1 and 2, respectively. Directed nucleic acid mutations in primers 3 through 8 are indicated with bold italics.
Figure 1.
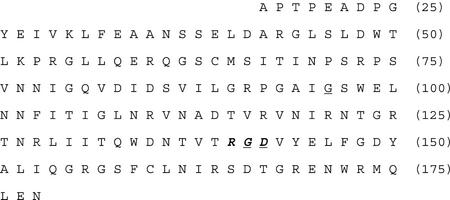
The 162-amino acid wild-type sequence of Ptr ToxA pro-protein proposed by Zhang (1997) and encoded for by pSM1 in this study. This sequence does not include the 16-amino acid signal peptide proposed by Zhang (1997), so the first amino acid shown is the 17th encoded for by ToxA (Ciuffetti et al., 1997). The RGD motif (amino acids 140–142) is in bold italics. Amino acids targeted for mutation are underlined. The G at amino acid 96 was changed to A (pSM2), the G at 141 was changed to A (pSM3), and the D at 142 was changed to E (pSM4). Numbers at the end of each line correspond to the amino acid position of the entire pre-pro-protein (including signal peptide) deduced from ToxA.
pSM1 was used as a template for additional PCR reactions aimed at altering the amino acid sequence of the pro-protein. In accordance, primers 1, 2, 3, and 4 (Table I) were used in double overlap extension reactions to create a cDNA that altered Gly-96 to Ala. As expected, the final PCR fragment that resulted was the same size as the wild-type fragment (data not shown). This fragment was cloned into pET21C(+) to create pSM2. Another mutant cDNA the same size as the wild type was similarly created to alter the RGD motif to RAD. This cDNA, created with primers 1, 2, 5, and 6 (Table I), was cloned to create pSM3. Finally, primers 1, 2, 7, and 8 (Table I) were used in PCR reactions designed to alter the RGD to RGE. This cDNA was cloned to create pSM4. Nucleic acid sequencing revealed that each clone possessed the desired mutation and lacked extraneous, nonspecific mutations (data not shown).
Expression of Wild-Type and Mutant cDNAs
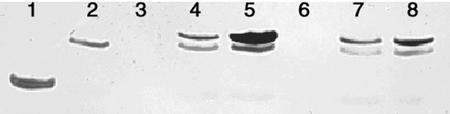
Western blots made from crude cell lysates of cells expressing the toxin exhibited two bands (Fig. 2). One band was at approximately 17 kD and represents the expressed Ptr ToxA pro-protein. The second band, at a slightly lower molecular mass, also appeared in cells containing only the vector without the inserted Ptr ToxA gene. In each case when the Ptr ToxA gene was inserted, the 17-kD band appeared, indicating the expression of the toxin in E. coli. Also shown in lane 1 is purified Ptr ToxA at a concentration of 100 μg mL−1. Comparison of the band intensities indicated that in each case the toxin was expressed at levels of 25 to 100 μg mL−1.
Figure 2.
Western blot of cleared cell lysates from E. coli cells expressing Ptr ToxA pro-protein and mutants. Cleared cell lysates were diluted 1:5 in sample buffer and electrophoresed using a 12:3 gel with the Shägger and von Jagow buffer system. Proteins were electroblotted onto nitrocellulose and probed using anti-Ptr ToxA rabbit antibodies raised in our lab and alkaline phosphatase-linked anti-rabbit antibodies. Lane 1, Purified Ptr ToxA from fungus (0.5 μg); lane 2, vector with no insert; lane 3, empty; lane 4, pro-protein with RGD mutated to RAD; lane 5, pro-protein with RGD mutated to RGE; lane 6, empty; lane 7, pro-protein with G96A mutation; and lane 8, wild-type pro-protein.
Effect of ToxA Mutations on Cell Death Development
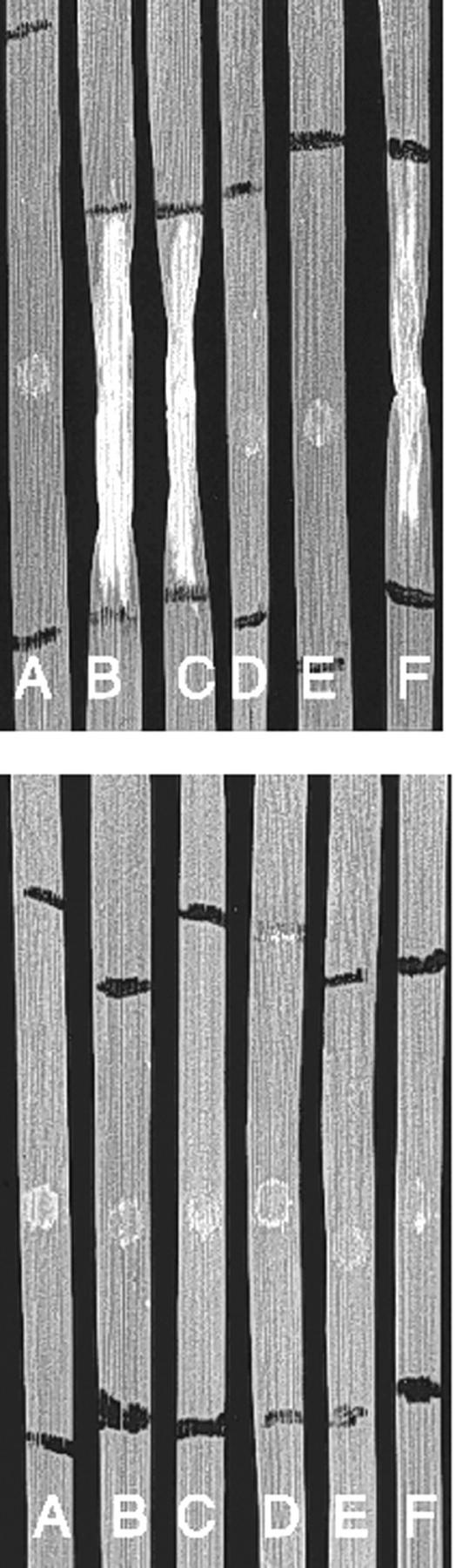
Ptr ToxA obtained from the fungus and from expression of the wild-type ToxA cDNA (pSM1) in E. coli induced cell death in wheat cv ND495 72 h after infiltration. Previous studies indicate that these are physiologically relevant concentrations (Tuori et al., 1995; Kwon et al., 1996). The control G to A mutation at amino acid 96 of Ptr ToxA protein was also active in cell death development. However, E. coli extracts containing Ptr ToxA protein with the mutations from RGD to RAD and RGD to RGE were inactive (Fig. 3, top). None of the preparations induced cell death in the toxin-insensitive wheat cv Erik (Fig. 3, bottom). Full-strength extracts gave identical results (data not shown).
Figure 3.
Effect of expressed Ptr ToxA and mutant proteins on wheat cvs ND495 and Erik. Cleared cell lysates from E. coli expressing the following plasmids were diluted 1:5 with distilled water and then were infiltrated into leaves of wheat cv ND495 (top) or cv Erik (bottom). A, Pet 21C plasmid with no inert; B, plasmid with wild-type pro-protein gene; C, plasmid with Ptr ToxA pro-protein gene containing G96A mutation; D, plasmid with Ptr ToxA pro-protein gene containing G141A mutation; E, plasmid with Ptr ToxA pro-protein gene containing D142E mutation; F, mature Ptr ToxA purified from the fungus (10 μg mL−1). The final concentration of pro-Ptr ToxA in the cell extracts was between 5 and 20 μg mL−1. Black lines indicate the extent of fluid infiltration into the leaves. Round marks near the center of the infiltration area are attributable to damage caused by the syringe during infiltration.
Effect of RGD and RGES Peptides on Toxin-Induced Electrolyte Leakage
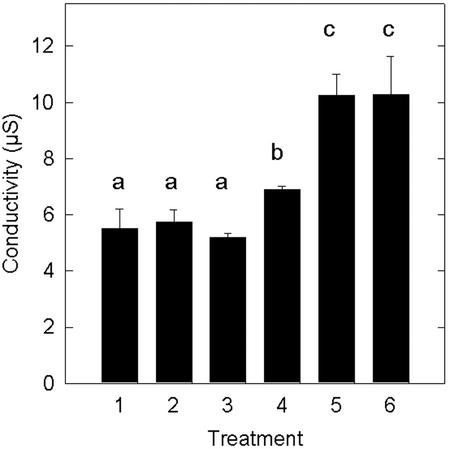
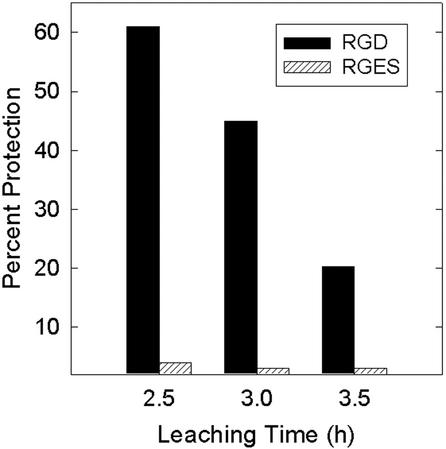
The co-application of RGD tripeptide with purified Ptr ToxA gave a statistically significant reduction in the level of electrolyte leakage relative to the toxin only application (Fig. 4). By comparison, the control RGES tetrapeptide failed to protect wheat leaves when co-infiltrated with toxin. Neither the RGD nor the RGES peptides by themselves induced electrolyte leakage relative to water controls (Fig. 4). In this experiment, RGD provided 63% protection from toxin-induced electrolyte leakage after a 2.5-h leaching time. However, this level of protection was transient. In another experiment, the level of protection was 61% after 2.5 h, but fell to 20% after 3.5 h of leaching (Fig. 5).
Figure 4.
Inhibition of toxin-induced electrolyte leakage by co-application of RGD tripeptide. Wheat leaves were infiltrated with water (1), 2 mm RGD peptide only (2), 2 mm RGES peptide only (3), 15 μg mL−1 Ptr ToxA + RGD peptide (4), Ptr ToxA + RGES peptide (5), or Ptr ToxA only (6). After exposure to experimental solutions for 4 h, leaf sections were placed in distilled water. Conductivity values of the ambient solutions were determined after a 2.5-h incubation. Data are average and sd of two replicates of an experiment that was repeated and produced similar results. Averages with different letters above were significantly different (P = 0.05).
Figure 5.
Transient protection from toxin-induced electrolyte leakage by RGD tripeptide. The experiment was conducted as described in Figure 4. The level of protection from either RGD or RGES was determined by the formula: [1 − (CToxin + Peptide − CPeptide)/(CToxin − CWater)] × 100, where C is the conductivity value at the time point in question.
DISCUSSION
A long-range objective of our research is to understand the molecular basis of cell death development in wheat plants induced by Ptr ToxA. Our current model holds that the toxin sensitivity gene located on chromosome 5BL encodes a receptor. Toxin perception, however mediated, directs de novo gene expression and protein synthesis in the host associated with cell death (Kwon et al., 1998).
Details of a putative toxin receptor are unknown, but our hypothesis is that the receptor interacts with the RGD motif found in Ptr ToxA. Certain extracellular matrix proteins in animals, such as vitronectin and fibronectin, possess the RGD motif. The RGD sequence of these proteins binds to transmembrane proteins called integrins. Integrin binding directs various cellular processes in animals, including apoptosis under some conditions, through a complex signaling cascade that involves calcium fluxes and several protein phosphorylation and dephosphorylation events (Cary et al., 1999; Coppolino and Dedhar, 2000). Along with binding extracellular matrix proteins of cells, some animal integrins have been implicated as binding sites for certain pathogenic bacteria and viruses (Isberg and Tran Van Nhieu, 1994). In plants, RGD-containing peptides have been shown to disrupt cell wall-plasma membrane interactions in plasmolyzed Arabidopsis cells, in onion cells during plasmolysis (Canut et al., 1998), and in pea (Pisum sativum) leaves during plasmolysis (Mellersh and Heath, 2001). RGD peptides have also been shown to disrupt the interaction between a Pro-rich plasma membrane protein and the cell wall in beans (Phaseolus vulgaris; Garcia-Gomez et al., 2000). Interestingly, the Pro-rich proteins identified did not contain the RGD sequence. In Arabidopsis, proteins showing a high degree of similarity to integrins have been identified (Nagpal and Quatrano, 1999). These results suggest that a system of plasma membrane proteins, similar to that found in animals, exists in plants to connect the plasma membrane and the extracellular matrix (Mellersh and Heath, 2001).
We used two independent lines of experimentation to demonstrate that the RGD motif of Ptr ToxA is critical for its effect on sensitive wheat. In the first set of experiments, PCR was used to create mutant clones of ToxA that changed the toxin's RGD sequence protein to RAD and to RGE. Cellular extracts from E. coli producing the mutant form of toxin failed to cause cell death development in wheat (Fig. 1, top). However, extracts from E. coli that had expressed both the wild-type ToxA and a control induced cell death in wheat (Fig. 1, top). By comparison, none of the extracts caused cell death in wheat cv Erik (Fig. 1, bottom), a wheat cultivar long known to be insensitive to toxin (Lamari and Bernier, 1989, 1991; Kwon et al., 1996, 1998). The lack of response in wheat cv Erik indicates that the cell death that developed in leaves B and C of wheat cv ND495 (Fig. 1, top) was in response to toxin rather than to a nonspecific compound in the E. coli extracts.
In preliminary experiments, we observed that significant amounts of Ptr ToxA produced in E. coli is sequestered in inclusion bodies that accumulated in the pellet after centrifugation of the lysate. Various levels of success were achieved in multiple attempts to solubilize and refold toxin in the pellet (Cheng, 2000; Tuori et al., 2000). However, in all cases, cell death developed in a pattern identical to that shown in Figure 3 when these preparations were infiltrated into wheat leaves. Overall, we found that “cleaner” toxin preparations, as determined by western blots (Fig. 2), were obtained with less effort by simply using the supernatant of cells that were lysed.
The second, independent line of experimentation aimed at the RGD motif was more quantitative. Exogenous RGD tripeptide, when co-infiltrated into wheat leaves with Ptr ToxA, reduced the magnitude of toxin-induced electrolyte leakage. The RGD tripeptide gave 63% protection from electrolyte leakage after 2.5 h of leaching in distilled water, relative to infiltration with toxin, RGD, or water alone. By comparison, the control peptide RGES gave no protection when co-infiltrated with toxin, indicating that the protection by RGD was specific (Figs. 4 and 5).
Although exogenous RGD tripeptide gave more than 60% protection after 2.5 h of leaching, the protection was transient and was reduced to only 20% after 3.5 h (Fig. 5). This could be attributable to the diffusion of RGD during course of the experiment or to higher affinity of Ptr ToxA to the putative receptor site. In previous studies that used the electrolyte leakage bioassay, we observed transient protection from toxin action with inhibitors of mRNA synthesis (Kwon et al., 1998).
Several possible interpretations of our results exist. Molar excesses of exogenous RGD tripeptide or larger peptides containing the RGD motif have been used to disrupt integrin-mediated processes in animals (Haas and Plow, 1994). A common interpretation of these experiments is that the exogenous RGD acts as a competitive inhibitor to block the binding of the RGD-containing protein to integrins. By extension, one interpretation of our data could be that the exogenous RGD tripeptide may have blocked the putative interaction of toxin and receptor.
Another possibility is suggested by the work of Mellersh and Heath (2001), who demonstrated that the addition of RGD-containing peptides disrupts the interaction of the plasma membrane with the cell wall, and this resulted in a decrease in cell wall-mediated defense from the host. This was manifested by a decrease in the cell wall-plasma membrane connections known as Hechtian strands. One possible interpretation of our results is that the action of Ptr ToxA requires the cell wall-plasma membrane interaction and that the RGD-containing peptide disrupts this interaction resulting in a loss of toxin perception. Another possibility is that Ptr ToxA, which contains an RGD tripeptide that is necessary for its function, may be affecting the plasma membrane-cell wall interactions in wheat. This could result in the observed necrosis and may reduce the magnitude of the membrane-mediated defense systems of the host. Without the identification of the toxin-binding protein, we cannot distinguish between these possibilities.
MATERIALS AND METHODS
Production of Mycelium and Ptr ToxA from Fungus
Strain 86-124, a race 2 isolate of Pyrenophora tritici-repentis, originally obtained from Dr. Lahkdar Lamari (University of Manitoba, Canada) was used throughout. The fungus was grown on modified Fries medium (Lamari and Bernier, 1989) to produce mycelium and Ptr ToxA. Toxin was purified and quantified according to Zhang et al. (1997).
Wild-Type cDNA Cloning
Total RNA was purified from 4-week-old fungal cultures using the method of de Vries et al. (1988). mRNA was isolated from this preparation by the method of Sambrook et al. (1989), and first-strand cDNA was obtained by reverse transcription as described by Zhang (1997).
PCR was used to produce a wild-type cDNA clone for 156-amino acid pro-protein of Ptr ToxA. The PCR reaction used first-strand cDNA as a template with primers 1 and 2 (Table I). The reaction was in a final volume of 50 μL with 1.25 units of Taq DNA polymerase (Promega, Madison, WI), 1× reaction buffer, and 25 mm MgCl2 supplied by the manufacturer, 10 ng of template DNA, 50 pmol of each primer, and 25 mm each dNTP (Boehringer Mannheim Biochemica, Indianapolis). Denaturation was for 1 min at 95°C. The annealing temperature was 55°C (1 min), followed by a 1-min extension at 72°C. The last of 32 cycles ended with a 7-min extension at 72°C. All reactions were carried out in a thermocycler (Robocycler Gradient 96, Stratagene, La Jolla, CA). The desired PCR product (537 bp) was cloned, and its identity was confirmed by sequencing.
Mutant cDNA Synthesis, Cloning, and Expression
The double overlap extension PCR technique (Horton and Pease, 1991) was used to create three mutant clones. Each mutation was designed to change a specific amino acid in the toxin protein (Fig. 1). One mutant clone derived from PCR (pSM2) changed the nucleotide sequence encoding the RGD to encode RAD (5′-CGG GGG GAC-3′ was changed to 5′-CGG GCG GAC-3′; Table I). The nucleic acid sequence for this region in pSM3 similarly was 5′-CGG GGG GAA-3′, which changed amino acid sequence of toxin from RGD to RGE. pSM4, which resulted in a G to A mutation at amino acid 96, served as a control. This mutation was expected to affect an amino acid on a loop of the folded toxin on the opposite side of the β-sheet from the RGD sequence (S.W. Meinhardt, unpublished data) and therefore not affect activity. PCR primers used to create all clones are summarized in Table I.
Mutant cDNAs were ligated into pCR2.1 (Invitrogen, San Diego). Ligation products were used to transform INVaF'One Shot competent cells to ampicillin resistance according to the manufacturer's instructions (Invitrogen). Clones were sequenced in both directions to confirm the presence of the desired mutation and the absence of nonspecific mutations. Once identified, the appropriate clones were double digested with EcoRI and NdeI and directionally ligated into the pET 21c(+) vector (Novagen, Madison, WI). Ligation products were transformed into Escherichia coli strain BL21(DE3) (Novagen).
Expression was carried out according to the instructions supplied with the cells with a final induction of 20 h at 4 mm isopropylthio-β-galactoside. Cells were collected by centrifugation (5,000g for 10 min), resuspended in 2 mL of extraction buffer (50 mm Tris-HCl and 2 mm EDTA, pH 8.0), and then collected by centrifugation as before. Cells were resuspended in 2 mL of extraction buffer, 1 mL of 5% (w/v) lysozyme was added, and the cell incubated on ice for 30 min. Cells were broken by sonication (Sonifer-450, Branson, Danbury, CT) with a one-eighth-inch tapered microtip. To accomplish this, three 10-s cycles of sonication at 20% output power, each interrupted by a 30-s cooling period, were used. The preparations that resulted were subjected to centrifugation (12,000g) for 15 min. The pellets were discarded, and the supernatants were used in bioassays and western blotting.
Western Blots
E. coli extracts were diluted 1:5 with sample buffer (62.5 mm Tris-HCl, pH 6.8, 10% [v/v] glycerol, 2% [w/v] SDS, 2.5% [v/v] 2-mercaptoethanol, and 0.01% [w/v] bromphenol blue) and heated at 95°C for 5 min. Five microliters was loaded into a 12:3 (T:C) polyacrylamide gel and was subjected to electrophoresis in a Mini Protein II (Bio-Rad, Hercules, CA) using the buffer system of Shägger and Von Jagow (1987). The proteins were electroblotted using the Bio-Rad mini trans-blot onto nitrocellulose membranes as described in the users' manual. Membranes were blocked with a 2.5% (w/v) dry nonfat milk solution in washing buffer (150 mm NaCl, 100 mm Tris-HCl, pH 7.5, and 0.05% [v/v] Tween 20) with gentle shaking overnight. The blot was then probed with the primary antibody, raised against Ptr ToxA in rabbits, in blocking solution (1:2,500 dilution) for 1.5 h. The membranes were washed three times for 10 min with washing buffer and then treated with blocking solution containing goat anti-rabbit antibodies conjugated to alkaline phosphatase (1:5,000 dilution, Pierce, Rockford, IL) for 1 h. The membranes were washed four times for 10 min with washing buffer and rinsed with water once. Bands were visualized according to the instructions in the Bio-Rad alkaline phosphatase conjugate substrate kit.
Plant Materials and Bioassays
The toxin-sensitive hard red spring wheat (Triticum aestivum) cv ND495, was used throughout. Some experiments made use of the toxin-insensitive wheat cv Erik. Plants were grown in a growth chamber for 2 to 3 weeks at 21°C with a 16-h photoperiod.
A bioassay based on cell death development was used for the wild-type or mutant Ptr ToxA protein obtained from expression of ToxA clones in E. coli. The second leaf of intact seedlings was infiltrated with pure Ptr ToxA (10 μg mL−1) purified from the fungus, or wild-type or mutant Ptr ToxA pro-protein expressed in E. coli. Each E. coli extract was diluted 1:5 with distilled water before infiltration into the leaf. The concentration of infiltrated toxin in the E. coli extracts was estimated to be approximately 5 to 20 μg mL−1, as determined by intensities in western blots. All solutions were applied to the second leaf with a disposable 1-mL syringe without a needle, and seven leaves were infiltrated with each solution. Infiltrated seedlings were returned to the growth chamber (21°C), and the presence or absence of cell death was scored 72 h later. This experiment was performed a total of three times with three different sets of E. coli extracts. Each experiment gave identical results. Data shown are representative photographs from one experiment.
A bioassay based on electrolyte leakage was used to determine the effects of exogenous 2 mm RGD and RGES (Sigma-Aldrich, St. Louis) on toxin action (Fig. 4). These experiments were performed precisely as described (Kwon et al., 1998). Each treatment consisted of 10 seedlings divided into two replicates of five seedlings each that were infiltrated with Ptr ToxA (15 μg mL−1) obtained from the fungus, toxin, and peptide together, or water or peptide only as controls. After exposure to the experimental solutions for 4 h, a 2.5-cm leaf section was obtained from the infiltrated region of each leaf. Five leaf sections were combined to form each replicate that was wrapped in cheesecloth. Each replicate was vacuum-infiltrated in 15 mL of distilled water. The conductivity of the ambient solution was determined at the end of the vacuum-infiltration, and the conductivity of the ambient solution was measured at intervals thereafter (Kwon et al., 1996, 1998). Data reported are the average conductivity values of the two replicates. Percent inhibition of electrolyte leakage by inhibitors was determined by the formula
 |
where C is the conductivity value at the time point in question. Each electrolyte leakage bioassay was repeated and similar results were obtained. Data presented are from a representative experiment.
ACKNOWLEDGMENT
We thank James Jordahl for his assistance in the greenhouse.
Footnotes
This work was supported by the U.S. Department of Agriculture/National Research Initiative Competitive Grants Program (grant nos. 96–35303–3436 and 98–35311–6843) and by the North Dakota Agricultural Experiment Station.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.006684.
LITERATURE CITED
- Braun CJ, Siedow JV, Levings CS., III Fungal toxins binding to the URF13 protein in maize mitochondria and Escherichia coli. Plant Cell. 1990;2:153–161. doi: 10.1105/tpc.2.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canut H, Carrasco A, Galaud JP, Cassan C, Bouyssou H, Vita N, Ferrara P, Pont-Lezica R. High affinity RGD-binding sites at the plasma membrane of Arabidopsis thaliana links the cell wall. Plant J. 1998;16:63–71. doi: 10.1046/j.1365-313x.1998.00276.x. [DOI] [PubMed] [Google Scholar]
- Cary LA, Han CD, Guan JL. Integrin-mediated signal transduction pathways. Histol Histopathol. 1999;14:1001–1009. doi: 10.14670/HH-14.1001. [DOI] [PubMed] [Google Scholar]
- Cheng W. Necrosis toxin (Ptr Tox A) from Pyrenophora tritici-repentis: expression in E. coli and toxin-receptor binding assay. MS thesis. Fargo: North Dakota State University; 2000. [Google Scholar]
- Ciuffetti LM, Tuori RP, Gaventa JM. A single gene encodes a selective toxin causal to the development of tan spot of wheat. Plant Cell. 1997;9:135–144. doi: 10.1105/tpc.9.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Coppolino MG, Dedhar S. Bi-directional signal transduction by integrin receptors. Int J Biochem Cell Biol. 2000;32:171–188. doi: 10.1016/s1357-2725(99)00043-6. [DOI] [PubMed] [Google Scholar]
- Critchley DR, Holt MR, Barry ST, Priddle H, Hemmings L, Norman J. Integrin-mediated cell adhesion: the cytoskeletal connection. Biochem Soc Symp. 1999;65:6579–6599. [PubMed] [Google Scholar]
- de Vries S, Hoge H, Bisseling T. Isolation of total and polysomal RNA from plant tissues. In: Gelvin SB, Schilperoot RA, Verma SPS, editors. Plant Molecular Biology Manual. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 1–13. [Google Scholar]
- d'Souza SE, Ginsberg MH, Plow EF. Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. Trends Biochem Sci. 1991;16:246–250. doi: 10.1016/0968-0004(91)90096-e. [DOI] [PubMed] [Google Scholar]
- Faik A, Laboure AM, Gulino D, Mandaron P, Falconet D. A plant surface protein sharing structural properties with animal integrins. Eur J Biochem. 1998;253:552–559. doi: 10.1046/j.1432-1327.1998.2530552.x. [DOI] [PubMed] [Google Scholar]
- Faris JD, Anderson JA, Francl LJ, Jordahl JG. Chromosomal location of a gene conditioning insensitivity in wheat to a necrosis-inducing culture filtrate from Pyrenophora tritici-repentis. Phytopathology. 1996;86:459–463. [Google Scholar]
- Friesen TL, Rasmussen JB, Kwon CY, Ali S, Francl LJ, Meinhardt SW. Reaction of Ptr ToxA-insensitive wheat mutants to Pyrenophora tritici-repentis race 1. Phytopathology. 2002;92:38–42. doi: 10.1094/PHYTO.2002.92.1.38. [DOI] [PubMed] [Google Scholar]
- Garcia-Gomez BI, Campos F, Hernandez M, Covarrubias AA. Two bean cell wall proteins more abundant during water deficit are high in proline and interact with a plasma membrane protein. Plant J. 2000;22:277–288. doi: 10.1046/j.1365-313x.2000.00739.x. [DOI] [PubMed] [Google Scholar]
- Greenberg JT. Programmed cell death: a way of life for plants. Proc Natl Acad Sci USA. 1996;93:12094–12097. doi: 10.1073/pnas.93.22.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas TA, Plow EF. Integrin-ligand interactions: a year in review. Curr Opin Cell Biol. 1994;6:656–662. doi: 10.1016/0955-0674(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Horton R, Pease L. Recombination and mutagenesis of DNA sequences using PCR. In: McPherson M, editor. Directed Mutagenesis: A Practical Approach. Oxford: IRL Press; 1991. pp. 217–247. [Google Scholar]
- Hynes RO. Integrins: versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Isberg RR, Tran Van Nhieu G. Binding and internalization of microorganisms by integrin receptors. Trends Microbiol. 1994;2:10–14. doi: 10.1016/0966-842x(94)90338-7. [DOI] [PubMed] [Google Scholar]
- Kwon CY, Rasmussen JB, Francl LJ, Meinhardt SW. A quantitative bioassay for necrosis toxin from Pyrenophora tritici-repentis based on electrolyte leakage. Phytopathology. 1996;86:1360–1363. [Google Scholar]
- Kwon CY, Rasmussen JB, Meinhardt SW. Activity of Ptr ToxA from Pyrenophora tritici-repentis requires host metabolism. Physiol Mol Plant Pathol. 1998;52:201–212. [Google Scholar]
- Laboure AM, Faik A, Mandaron P, Falconet D. RGD-dependent growth of maize calluses and immunodetection of an integrin-like protein. FEBS Lett. 1999;442:123–128. doi: 10.1016/s0014-5793(98)01634-2. [DOI] [PubMed] [Google Scholar]
- Lally ET, Kieba IR, Sato A, Green CL, Rosenbloom J, Korostoff J, Wang JF, Shenker BJ, Ortlepp S, Robinson MK et al. RTX toxins recognize a β2 integrin on the surface of human target cells. J Biol Chem. 1997;272:30463–30469. doi: 10.1074/jbc.272.48.30463. [DOI] [PubMed] [Google Scholar]
- Lamari L, Bernier CC. Toxin of Pyrenophora tritici-repentis: host-specificity, significance in disease, and inheritance of host reaction. Phytopathology. 1989;79:740–744. [Google Scholar]
- Lamari L, Bernier CC. Genetics of tan necrosis and extensive chlorosis in tan spot of wheat caused by Pyrenophora tritici-repentis. Phytopathology. 1991;81:1092–1095. [Google Scholar]
- Lynch TM, Lintilhac PM, Domozych D. Mechanotransduction molecules in the plant gravisensory response: amyloplast/statolith membranes contain a β1 integrin-like protein. Protoplasma. 1998;201:92–100. doi: 10.1007/BF01280715. [DOI] [PubMed] [Google Scholar]
- Mellersh DG, Heath MC. Plasma membrane-cell wall adhesion is required for expression of plant defense responses during fungal penetration. Plant Cell. 2001;13:413–424. doi: 10.1105/tpc.13.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal P, Quatrano RS. Isolation and characterization of a cDNA clone from Arabidopsis thaliana with partial sequence similarity to integrins. Gene. 1999;230:33–40. doi: 10.1016/s0378-1119(99)00068-2. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Pierschbacher MD. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986;44:517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Scheffer RP, Livingston RS. Host-selective toxins and their role in plant diseases [disease-inducing microorganisms] Science. 1984;223:17–21. doi: 10.1126/science.223.4631.17. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Shägger H, Von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Stock WS, Brule-Babel AL, Penner GA. A gene for resistance to a necrosis-inducing isolate of Pyrenophora tritici-repentis located on 5BL of Triticum aestivum cv Chinese Spring. Genome. 1996;39:598–604. doi: 10.1139/g96-075. [DOI] [PubMed] [Google Scholar]
- Stockbauer KE, Magoun L, Liu M, Burns EH, Gubba S, Renish S, Pan X, Bodary SC, Baker E, Coburn J et al. A neutral variant of the cysteine protease virulence factor of group A Streptococcus with an arginine-glycine aspartic acid (RGD) motif preferentially binds human integrins α5β3 and αIIbβ3. Proc Natl Acad Sci USA. 1999;96:242–247. doi: 10.1073/pnas.96.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas A, Feng GH, Reeck GR, Bockus WE, Leach JE. Purification of a cultivar-specific toxin from Pyrenophora tritici-repentis, causal agent of tan spot of wheat. Mol Plant-Microbe Interact. 1990;3:221–224. [Google Scholar]
- Tuori RP, Wolpert TJ, Ciuffetti LM. Purification and immunological characterization of toxic components from cultures of Pyrenophora tritici-repentis. Mol Plant-Microbe Interact. 1995;8:41–48. doi: 10.1094/mpmi-8-0041. [DOI] [PubMed] [Google Scholar]
- Tuori RP, Wolpert TJ, Ciuffetti LM. Heterologous expression of functional Ptr ToxA. Mol Plant-Microbe Interact. 2000;13:456–464. doi: 10.1094/MPMI.2000.13.4.456. [DOI] [PubMed] [Google Scholar]
- Walton JD, Panaccione DG. Host-selective toxins and disease specificity: perspectives and progress. Annu Rev Phytopathol. 1993;31:275–303. doi: 10.1146/annurev.py.31.090193.001423. [DOI] [PubMed] [Google Scholar]
- Wolpert TJ, Macko V. Specific binding of victorin to a 100-kD protein from oats. Proc Natl Acad Sci USA. 1989;86:4092–4096. doi: 10.1073/pnas.86.11.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H-F. A Pyrenophora tritici-repentis necrosis toxin: protein isolation and characterization and gene cloning and expression. PhD thesis. Fargo: North Dakota State University; 1997. [Google Scholar]
- Zhang H-F, Francl LJ, Jordahl JG, Meinhardt SW. Structural and physical properties of a necrosis-inducing toxin from Pyrenophora tritici-repentis. Phytopathology. 1997;87:154–160. doi: 10.1094/PHYTO.1997.87.2.154. [DOI] [PubMed] [Google Scholar]