Abstract
Wild-type (wt) Arabidopsis plants, the starch-deficient mutant TL46, and the near-starchless mutant TL25 were grown in hydroponics under two levels of nitrate, 0.2 versus 6 mm, and two levels of CO2, 35 versus 100 Pa. Growth (fresh weight and leaf area basis) was highest in wt plants, lower in TL46, and much lower in TL25 plants under a given treatment. It is surprising that the inability to synthesize starch restricted leaf area development under both low N (NL) and high N (NH). For each genotype, the order of greatest growth among the four treatments was high CO2/NH > low CO2/NH, > high CO2/NL, which was similar to low CO2/NL. Under high CO2/NL, wt and TL46 plants retained considerable starch in leaves at the end of the night period, and TL25 accumulated large amounts of soluble sugars, indicative of N-limited restraints on utilization of photosynthates. The lowest ribulose-1,5-bisphosphate carboxylase/oxygenase per leaf area was in plants grown under high CO2/NL. When N supply is limited, the increase in soluble sugars, particularly in the starch mutants, apparently accentuates the feedback and down-regulation of ribulose-1,5-bisphosphate carboxylase/oxygenase, resulting in greater reduction of growth. With an adequate supply of N, growth is limited in the starch mutants due to insufficient carbohydrate reserves during the dark period. A combination of limited N and a limited capacity to synthesize starch, which restrict the capacity to use photosynthate, and high CO2, which increases the potential to produce photosynthate, provides conditions for strong down-regulation of photosynthesis.
In plants grown under elevated CO2 there is often acclimation, reducing the capacity of photosynthesis. A variety of factors have been proposed to contribute to the down-regulation of photosynthesis under prolonged elevated CO2, including limited sink capacity, N limitation, end-product limitation, excess accumulation of starch, a decrease in photosynthetic enzymes such as Rubisco, and accelerated senescence.
Rubisco small subunit (rbcS) transcripts often decrease in elevated CO2, for example, as reported in wheat (Triticum aestivum; Nie et al., 1995), Arabidopsis (Cheng et al., 1998), pea (Pisum sativum; Majeau and Coleman, 1996), and tomato (Lycopersicon esculentum; Van Oosten and Besford, 1995). This correlates with a decrease in Rubisco activity (Van Oosten and Besford, 1995; Majeau and Coleman, 1996) and content (Cheng et al., 1998; Moore et al., 1998). The decrease in Rubisco has been associated with an increase in the levels of soluble sugars in some studies (Van Oosten and Besford, 1995; Majeau and Coleman, 1996; Cheng et al., 1998), implicating sugar-mediated repression of photosynthetic gene expression of which hexokinase is one of the likely sensors in the signaling pathway (Jang and Sheen, 1994; 1997; Pego et al., 2000; Smeekens, 2000). The decrease in gene transcripts is not always correlated with absolute levels of soluble sugars (Nie et al., 1995; Moore et al., 1998), and sugar repression of photosynthesis has been observed to correlate with acid invertase activity, and therefore increased hexoses from Suc cycling (Goldschmidt and Huber, 1992; Moore et al., 1998).
The accumulation of carbohydrates in elevated CO2 may be due to limited sink capacity, and therefore a limitation on the use of photosynthate (Stitt, 1991). Arp (1991) found that photosynthetic capacity under elevated CO2 was decreased, in line with a decline in sink capacity resulting from factors such as low N and restricted root growth. In tomato, removal of sink by the detachment of young leaves resulted in elevated hexose levels and a more substantial decrease in photosynthetic gene transcripts in elevated CO2 (Van Oosten et al., 1994). In a converse manner, when sink capacity is increased relative to the source, down-regulation of photosynthesis was not observed in ryegrass (Lolium perenne; Rogers et al., 1998). Some studies suggest that the observed accumulation of carbohydrates under elevated CO2 may not be due to sink limitation. The decline in photosynthesis has been ascribed to a limitation on triose-P utilization for synthesis of products like starch and Suc (Cure et al., 1991; Ludewig et al., 1998). Photosynthesis may exceed the rate of end-product synthesis, therefore, the recycling of Pi is decreased and can feedback to limit the rate of photosynthesis. A decline in Pi and an increase in phosphoglyceric acid will also induce starch synthesis (Preiss, 1982). In clover (Trifolium subterraneum), when photosynthesis was stimulated by growth in high irradiance, there was no accumulation of starch, in contrast to plants grown in elevated CO2, which had similar photosynthetic rates (Morin et al., 1992). Therefore, in high irradiance, carbon is exported from leaves, indicating there was not a limitation on sink capacity. It is postulated that the decrease in photorespiration in elevated CO2 is causing a decrease in Pi, and ATP synthesis, thereby altering carbon partitioning (Morin et al., 1992).
The accumulation of starch grains has been suggested to disrupt chloroplast structure (Cave et al., 1981) and increase diffusive resistance to CO2 (Nafziger and Koller, 1976; Grub and Mächler, 1990). There is often a more pronounced down-regulation of photosynthesis in starch-accumulating species when sink capacity is limiting (Goldschmidt and Huber, 1992), and in elevated CO2-grown plants, a decline in CO2 assimilation has been seen to correlate with increased leaf starch (Ehret and Jolliffe, 1985). This might occur because starch is metabolized to Glc in the dark period, which may then function in sugar signaling to further decrease photosynthetic genes (Cheng et al., 1998). However, others have found no relationship between starch accumulation and a decrease in photosynthesis (Van Oosten et al., 1994; Moore et al., 1998). In potato (Solanum tuberosum) with antisense inhibition of leaf AGPase, resulting in a reduction in starch content, CO2 assimilation was lower in antisense plants than wild type (wt) when grown in elevated CO2; therefore, in this case, acclimation is not caused by an accumulation of starch (Ludewig et al., 1998).
Another explanation for the decline in photosynthetic capacity in elevated CO2 is accelerated senescence. Earlier leaf senescence in elevated CO2 was responsible for the decreased photosynthetic rates, Rubisco activity, and chlorophyll in tobacco (Nicotiana tabacum; Miller et al., 1997). In agreement, rbcS and other photosynthetic genes declined in elevated CO2, which was also attributed to accelerated senescence (Ludewig and Sonnewald, 2000). There is evidence for interaction between N supply and plant response to growth in elevated CO2 (Stitt and Krapp, 1999). N limitation leads to decreased growth (Paul and Stitt, 1993; Scheible et al., 1997a, 1997b) and an accumulation of starch (Rufty et al., 1988; Paul and Stitt, 1993; Paul and Driscoll, 1997; Scheible et al., 1997a). Under low N, sink strength is decreased and acclimation of photosynthesis to elevated CO2 is usually more marked (Pettersson and McDonald, 1994; Sage, 1994; Bowler and Press, 1996). For example, in tobacco grown under elevated CO2, rbcS was decreased in low N supply, but not when grown with sufficient N (Geiger et al., 1999). The decrease in Rubisco and other photosynthetic proteins in elevated CO2 has also been ascribed to a general decrease in leaf protein due to N limitation, which is accentuated in elevated CO2 (Nakano et al., 1997).
In a previous paper (Sun et al., 1999), we investigated the performance of starch mutants of Arabidopsis (TL46 and TL25) grown in soil under low and high light regimes. Under these conditions, photosynthesis and growth were correlated with the capacity for starch production. In the following study, we examined the effects of elevated CO2 and N availability on wt and starch mutants of Arabidopsis during growth in hydroponics. We aimed at providing insight into the physiological significance of starch and hexose in relation to photosynthate production and utilization under different CO2 and N levels. Our results indicate that there is a complex interplay between N and C that affects the extent of starch accumulation and starch turnover and, in turn, plant growth.
RESULTS
C and N Composition and Growth
Figure 1 shows the results of growth of Arabidopsis wt and leaf starch mutants (TL46, a starch-deficient mutant, and TL25, a near-starchless mutant), under low N (NL) and high N (NH) nutrition and low (CL) and high (CH) CO2, on the C and N composition of shoots and roots. It is apparent that the total C content of the shoots and roots was similar across all treatments (approximately 40% [w/w]), whereas the total N content decreased substantially under Nl nutrition. Under NH nutrition, the N content across all treatments, low versus high CO2, and across the three genotypes was 5% to 6.5% (w/w) of the dry weight, with shoots from wt plants having a slightly lower N content than those from the starch mutants. Thus, on average, the C/N ratio of shoots under the NH treatment was about 6 for the starch mutants and approximately 7 for the wt. With growth under Nl nutrition, the N content of the tissue decreased, resulting in an increase in the C/N ratio of the tissue. In leaf tissue, the largest increase in C/N ratio under Nl nutrition occurred in wt plants compared with the starch mutants. In roots, the increase in the C/N ratio in Nl-grown plants was very similar across the three genotypes; the increase was slightly higher in the low CO2-grown plants.
Figure 1.
C and N content of roots and tops of wt and starch mutants of Arabidopsis grown under different levels of nitrate and CO2. Material from three individual plants was pooled and used for analysis. Plants were grown under different levels of CO2 and N nutrition during the last 16 d of growth as follows: CLNH, growth under 35 Pa CO2, 6 mm nitrate; CLNL, growth under 35 Pa CO2, 0.2 mm nitrate; CHNH, growth under 100 Pa CO2, 6 mm nitrate; and CHNL, growth under 100 Pa CO2, 0.2 mm nitrate.
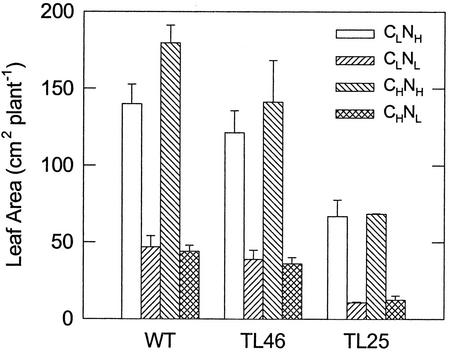
Measurements of fresh weight as an indicator of growth showed a decrease in the ratio of top (aerial)/root fresh weight under Nl across all three genotypes and under low and high CO2 (Fig. 2). This ratio decreased under Nl due to a large decrease in the top growth, whereas the growth of roots was less affected. In wt and TL46 plants, the root growth was very similar across treatments; however, in TL25, the root growth was less, and it was lowest in the Nl plants. In wt plants, the top growth under NH was significantly enhanced under high CO2. The average growth of tops in wt plants under higher N was higher than in TL46, although with the degree of variation, it was not significantly different. However, the growth of tops and roots of TL25 was much lower than wt and TL46 plants under all treatments. The differences in cumulative leaf area per plant (Fig. 3) were very similar to that of top fresh weight (Fig. 2A) across all treatments.
Figure 2.
Growth of shoots and roots of wt and starch mutants of Arabidopsis under different CO2 and N treatments on a fresh weight basis. Analyses were made after 35 d (±2 d) of growth. See Figure 1 for growth conditions.
Figure 3.
Cumulative leaf area of wt and starch mutants of Arabidopsis under different CO2 and N treatments. Analyses were made after 35 d (±2 d) of growth. See Figure 1 for growth conditions.
Leaf Starch and Soluble Carbohydrates
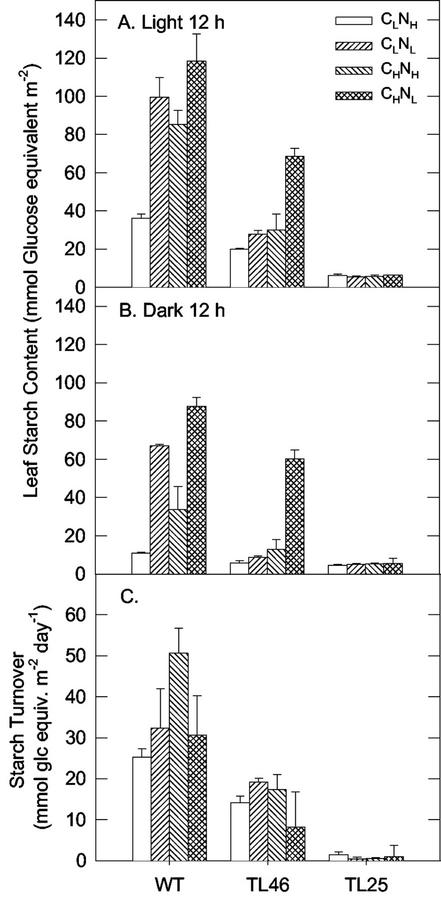
Leaf starch was analyzed at the end of the light (Fig. 4A) and dark periods (Fig. 4B). In wt plants under CLNH, a moderate amount of starch accumulated during the day and it was largely used during the dark period. Under CHNH, there was a substantial increase in starch levels during the day, and although starch turnover rate was twice that observed under CLNH, substantial levels remained at the end of the dark period. In the NL treatments, 2.5- to 3-fold increases in starch levels were observed at the end of the light period compared to CLNH. As there was little difference in starch turnover in NL treatments versus CLNH, the bulk of the starch remained in NL plants at the end of the dark period. TL46 plants showed only moderate increases in starch levels across all treatments, with the apparent exception of CHNL. In CHNL-treated TL46 plants, much of the starch remained at the end of the night period due to substantially reduced turnover. In TL25, starch content was very low under all conditions. Under NH, the cumulative leaf area per plant increased with increasing synthesis and turnover of starch across genotypes and CO2 levels. Under NL, cumulative leaf area was smaller when synthesis and turnover of starch was negligible (in TL25 mutant), whereas there was little or no difference with increasing starch turnover (Fig. 5).
Figure 4.
Leaf starch content at the end of the light period (A, 12 h of light) and the end of the dark period (B, 12 h of dark) and diurnal starch turnover (C) in wt and starch mutants of Arabidopsis under different CO2 and nitrogen treatments. See Figure 1 for growth conditions.
Figure 5.
Correlation of cumulative leaf area with starch turnover in plants grown with Nl and NH in wt and starch mutants of Arabidopsis. See Figure 1 for growth conditions.
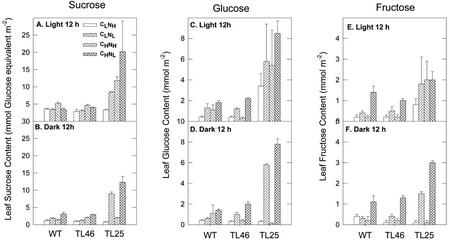
The level of Suc in leaves at the end of the day was very similar in all treatments in wt and TL46 plants (Fig. 6). At the end of the dark period, the level of Suc in wt and TL46 plants was higher in CLNL plants than in CLNH plants, whereas the highest levels of Suc in these genotypes at the end of the dark period was in the CHNL plants. In TL25 plants, the level of Suc in CLNH plants at the end of the day was similar to that in wt and TL46 plants. However, in the other treatments of TL25 plants, there were large increases in Suc levels during the day. Under CHNL and CLNL treatments, there was substantial Suc remaining in the leaves at the end of the dark period, whereas under CHNH treatment, Suc levels decrease to levels similar to that evident in wt and TL46.
Figure 6.
Leaf soluble carbohydrates, Suc, Glc, and Fru at the end of the light period (12 h of light) and the end of the dark period (12 h of dark) in wt and starch mutants of Arabidopsis under different CO2 and N treatments. See Figure 1 for growth conditions.
In wt and TL46 plants, the highest levels of Glc and Fru occurred in CHNL plants, with levels being similar at the end of the light and dark periods. This coincides with the highest level of starch accumulation during the day and retention during the night. At the end of the light period, the levels of Glc and Fru in wt and TL46 were also higher under the CLNL treatment than in the NH treatments. Thus, Nl results in an increase in these soluble sugars.
In TL25 plants, the levels of Glc and Fru at the end of the light period were considerably higher than in wt and TL46 plants across all C-N treatments. At the end of the dark period, high levels of Glc and Fru remained in TL25 in the Nl treatments, with the highest in CHNL, whereas in the NH treatments, the levels of these sugars were low and similar to that in wt and TL46 plants.
Rubisco
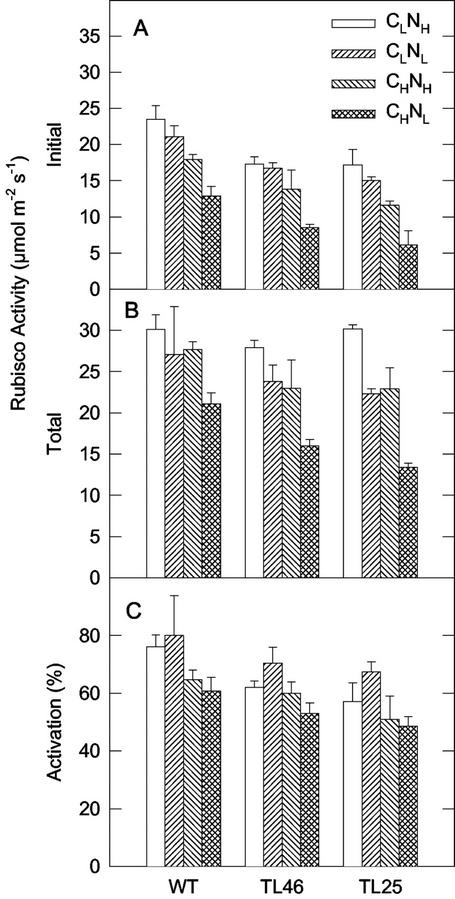
The effects of growth under NH, Nl, CH, and CL on Rubisco activity and content and total soluble protein per unit leaf area were determined (Figs. 7 and 8). For each genotype, the initial extractable activity of Rubisco (which is dependent on the amount of Rubisco and its state of activation) showed a general pattern, with decreasing activity in the following order: CLNH and CLNL being the highest, followed by CHNH, and CHNL. For each C-N treatment, the mutant TL46 and TL25 plants had lower initial extractable activities than the wt.
Figure 7.
Initial and total extractable activity of Rubisco, and percentage of activation, of wt and starch mutants of Arabidopsis under different CO2 and N treatments. Analyses were made after 35 d (±2 d) of growth. See Figure 1 for growth conditions.
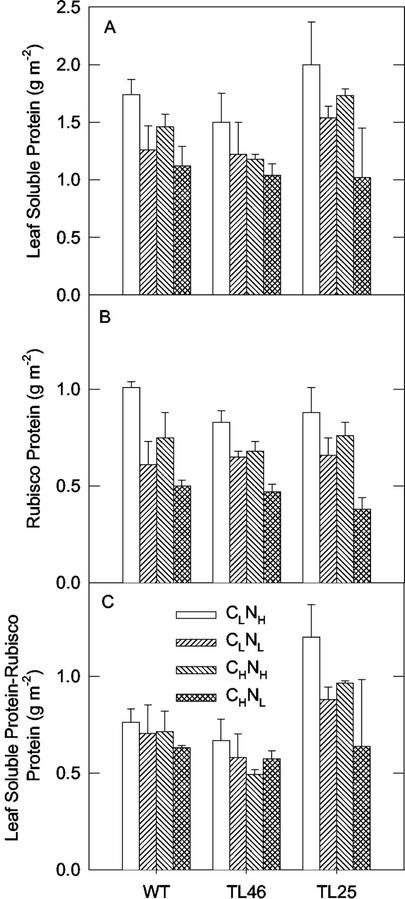
Figure 8.
Rubisco content and total soluble protein on leaf area basis of wt and starch mutants of Arabidopsis under different CO2 and N treatments. See Figure 1 for growth conditions.
Also, for each genotype there was a similar pattern in change in total Rubisco activity (the maximum activity after activation in vitro) and Rubisco protein per unit leaf area (the correlation coefficient across all treatments was R = 0.88**; results not shown). For each genotype, the highest total activity and Rubisco protein was in CLNH, plants; the CLNL and CHNH plants had a similar reduction in total activity and Rubisco protein, whereas the CHNL plants had the largest reduction. Although the initial Rubisco activity was lower in mutants than in wt plants (Fig. 7A), the three genotypes were very similar in total activity (Fig. 7B) and content of Rubisco (Fig. 8A) in response to the CO2 and N treatments, except for CHNL. In the CHNL treatment, it was apparent that the total activity and Rubisco content progressively decreased from wt to TL46 to TL25. The calculated in vivo state of activation of Rubisco (initial/total × 100) indicates the initial state of activation for each genotype was lowest in the high CO2-grown plants. Also, the results showed a pattern of declining state of activation from wt to TL46 to TL25 for a given C-N treatment.
The changes in Rubisco protein content for the C-N treatments and the total soluble protein showed a very similar relationship (Fig. 8, A and B). For each genotype, the CLNH plants had the highest Rubisco and highest soluble protein, and the CHNL plants had the lowest Rubisco and soluble protein. The amount of leaf soluble protein excluding that in Rubisco was calculated (Fig. 8C) to show how the remaining pool of soluble proteins change per leaf area under the various treatments. In wt and TL46, there was little effect of C-N treatments on the remaining total soluble proteins. With the exception of the CHNL treatment, the remaining soluble proteins tended to be higher in TL25 than in wt or TL46.
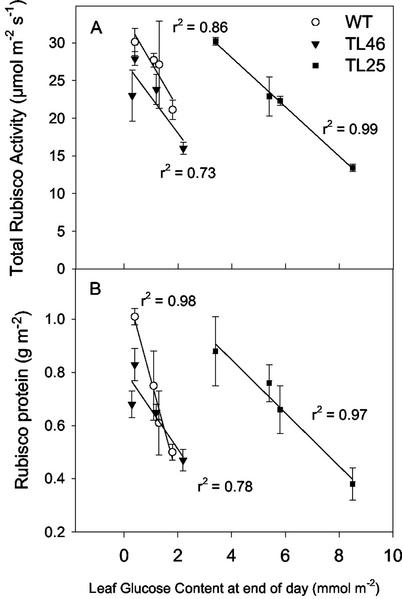
Rubisco total activities as well as Rubisco protein were correlated with leaf Glc concentration (Fig. 9). Rubisco content and activity decreased with increasing levels of Glc in all three genotypes. There was a large shift in the response in TL25, with decreasing Rubisco occurring at higher Glc levels (Fig. 9). Plots of Fru and Suc versus Rubisco showed a less significant correlation (see legend to Fig. 9).
Figure 9.
The relationship between Rubisco total activity (A) and Rubisco protein (B) and leaf Glc concentration. For each genotype, the four data points are values under the two different CO2 and nitrogen regimes. r2 is the correlation of the different Rubisco factors with Glc concentration. r2 values for Rubisco versus Suc and Fru; total activity versus Suc: wt r2 = 0.06, TL46 r2 = 0.33, TL25 r2 = 0.95. Rubisco protein versus Suc: wt r2 = 0.03, TL46 r2 = 0.26, TL25 r2 = 0.85. Total activity versus Fru: wt r2 = 0.93, TL46 r2 = 0.8, Tl25 r2 = 0.66. Rubisco protein versus Fru: wt r2 = 0.55, TL46 r2 = 0.83, TL25 r2 = 0.46.
DISCUSSION
Growth and Source versus Sink Limitation
With growth of wt plants under Nl nutrition, the N content of the tissue decreased, resulting in an increase in the C/N ratio. There was a large reduction in shoot growth (decreased shoot/root ratio) and leaf area under Nl nutrition, indicating N supply was limiting for growth (Paul and Stitt, 1993). With CO2 enrichment under NH in wt plants, there was an enhancement of growth (top fresh weight and leaf area per plant), whereas with CO2 enrichment under Nl, there was no enhancement of growth. Similar results have been found in several other species (Bowler and Press, 1996; Rogers et al., 1996a, 1996b; Ziska et al., 1996; Geiger et al., 1999). Hence, when N is limiting, it has a more dominant role than photosynthetic capacity in affecting plant growth and development.
With the growth of starch mutants under NH nutrition, the C/N ratio of plant tissue was very similar to that of wt plants. Also, as in wt plants, with growth under Nl, the N content of the tissue decreased, resulting in an increase in the C/N ratio. It was evident for both mutants, as in wt, that N was limiting for growth in the Nl treatment from measurements of fresh weights of shoots and roots, and total leaf area per plant. Leaf expansion is decreased when nitrate becomes limiting, possibly through interactions with a nitrate-cytokinin signaling pathway (Forde, 2002).
In comparisons of growth of the three genotypes based on fresh weight and leaf area measurements, it is clear that in each C-N treatment there is a strong reduction in growth of TL25 plants compared with that of wt and TL46. Wt and TL46 plants had similar growth, but in general, growth of TL46 plants was lower. The differences in growth between genotypes may be a function of the photoperiod; with a 12-h dark period, starch reserves are important for growth because when starch mutants were grown under continuous light, differences were not evident (Caspar et al., 1985).
The highest growth occurred in wt plants in the CHNH treatment. TL46 plants also had high growth in the CHNH treatment, although not significantly higher than in CLNH. Although wt plants produced more starch during the day and used more at night (Fig. 4), TL46 plants might partly compensate by increasing partitioning into Suc during the day (Sun et al., 1999). The results suggest in the CHNH treatment, growth is not limiting in wt by supply of photosynthate as there is substantial starch remaining at the end of the dark period. In TL25 plants, growth in the CHNH treatment was only about 40% to 50% of that in wt and TL46 plants, which indicates that the inability of TL25 to make starch as a carbon reserve, for use in the dark is limiting growth. Although TL25 plants accumulate substantial Suc, Glc, and some Fru in the light period, this is not sufficient to compensate for its inability to synthesize starch as a dark period carbon reserve. In a previous study, it was shown in TL25 plants grown under normal CO2 that there is only a partial compensation during photosynthesis with exposure to high levels of 14CO2 for loss of capacity for starch synthesis by increased Suc synthesis in TL25 plants (Sun et al., 1999). Also, some of the sugars produced in the light during photosynthesis in TL25 plants may be lost by respiration in the dark period and thus be unavailable to support growth because an increase in respiration correlates with increased carbohydrate, and therefore respiratory substrates (Wullschleger et al., 1994). An increase in respiration was also found to contribute to decreased growth in other starchless Arabidopsis mutants (Caspar et al., 1985; Schulze and Schulze, 1994).
Wt plants in the CLNH treatment had lower growth than in the CHNH treatment. This may occur by higher Suc production during the day, and increased starch use at night in the CHNH- compared with CLNH-grown plants (55 versus 25 mmol Glc equivalents m−2 used in the dark, respectively; Fig. 4). This suggests the capacity for production of photosynthate is limiting growth in CLNH plants. On the other hand, CHNH plants are producing more starch than they can use at night, indicating some limitation on sink capacity. In TL46 plants, there was no significant difference in growth under CLNH and CHNH conditions; that could be due to limited capacity for production of starch, and a similar degree of starch use in the dark period (Fig. 4). Under CLNH growth, the level of Suc at the end of the light period in TL25 plants was similar to that in wt and TL46 plants, whereas the level of Glc and Fru was higher (Fig. 6). This suggests there is some excess production of Suc in the light, which is not exported, but is converted to Fru and Glc by hydrolysis in leaves.
In wt plants under the CLNL treatment there is a large reduction in growth due to limited N compared with the CLNH treatment. This results in a large accumulation of starch during the light period, although the amount of starch used at night (33 mmol Glc equivalents m−2) is slightly higher than in CLNH-grown plants. The large accumulation of starch during the day in CLNL plants indicates there is limited capacity to use Suc due to N limitation for synthesis of amino acids and development of sinks to use carbohydrate. Therefore, the capacity for photosynthesis and carbohydrate synthesis is greater than sink capacity. In the CLNL treatment, TL46 was slightly more restricted in growth than the wt. In contrast to the wt, TL46 showed only a moderate increase in starch levels under CLNL. However, this level was sufficient to sustain growth nearly to the level of the wt under the limiting N treatment. In the CLNL treatment, the TL46 and TL25 plants had high levels of Glc and Fru at the end of the light and dark periods, whereas in wt, there was less accumulation. This suggests that under limiting N, some of the Suc may be hydrolyzed to Glc and Fru, or starch may be converted to Glc and not exported from the leaf. In the TL25 plants, the growth under CLNL was much lower than in wt and TL46 plants. This indicates that even when N is limiting, the inability to synthesize starch can limit growth.
In the CHNL treatments, the growth of wt and TL46 plants was strongly suppressed, similar to that in CLNL-grown plants, again due to the N limitation. This resulted in a large accumulation of starch during the light, and a substantial retention of starch at the end of the dark period. In wt, there is greater accumulation of starch under conditions of Nl in both CO2 treatments. Leaves have an increased capacity for starch synthesis when N is low, possibly through increased agpS transcript expression, which encodes the regulatory subunit of ADP-Glc pyrophosphorylase (AGPase; Scheible et al., 1997a; Geiger et al., 1999) and allosteric activation of AGPase catalytic activity. In all genotypes, and especially TL25, the levels of Glc and Fru were high at the end of the light and dark period. Therefore, the inability of TL25 plants to produce starch when N is low results in elevated levels of soluble sugars and limited growth, which indicates that N is limiting for synthesis of amino acids and sink development. However, in wt, increased starch production through increased AGPase activity may reduce the accumulation of soluble sugars, thereby limiting feedback effects on photosynthesis. Lack of sink demand contributed to the reduced growth in a starch-deficient Arabidopsis mutant at the rosette stage because relative growth rate increased during flowering when sink strength was increased (Schulze et al., 1994). Therefore, the effect of a lack of sinks in TL25 plants may be exacerbated under limiting N supply. In plants grown under limiting N manipulated to have a decreased source:sink ratio through partial removal of the source leaves in ryegrass (Rogers et al., 1998) or shading in tobacco (Paul and Driscoll, 1997), there was no accumulation of carbohydrates, and down-regulation of photosynthesis was absent. Therefore, sink capacity plays a major role in the acclimation to Nl (Paul and Driscoll, 1997) as well as elevated CO2 (Rogers et al., 1998).
When plants have sufficient N available, the capacity of sinks to use carbohydrates is increased. In NH-grown Arabidopsis, the correlation between cumulative leaf area and production and use of starch across genotypes and CO2 levels indicates the importance of starch reserves in the dark period to plant growth. Unexpectedly, under N limited growth, cumulative leaf area also increased with increased rate of use of starch, even though starch mutants had increased soluble sugars that were not fully used in the dark period. Excess sugars may lead to excess respiration and loss of CO2, storage of hexoses in compartments where they are not available for metabolism (e.g. in vacuoles or the apoplastic space), and sugar signaling that down-regulates the capacity for photosynthesis. An increase in sugars in the apoplastic space could also cause a loss of turgor and limits on cell/leaf expansion.
Feedback Regulation of Rubisco
In each genotype, there was a progressive decrease in the initial extractable Rubisco activity on a leaf area basis under growth conditions from CLNH to CLNL to CHNH to CHNL. Also, for each CO2/N treatment, the initial extractable activity of Rubisco was higher in the wt than in the starch mutants. This suggests feedback regulation of Rubisco due to limitations on sink capacity. The Nl treatments will limit development of sinks due to limiting supply of amino acids. The starch mutants are limited in capacity for synthesis of carbohydrates. Under Nl supply, elevated CO2 often leads to a decrease in Rubisco activity as seen in pea (Riviere-Rolland et al., 1996) and tobacco (Geiger et al., 1999), although this may depend on the level of N supplied (Riviere-Rolland et al., 1996).
What is particularly interesting is that even under Nl, which limits growth, the starch mutants have lower initial extractable activity of Rubisco and lower growth than wt. This suggests that synthesis of starch is important for growth even when N is limiting. One reason for this may be that the starch mutants have increased synthesis of soluble sugars that are not as effectively used for growth (e.g. stored in vacuoles, apoplastic space, or respired) as starch as noted earlier. In an alternate manner, down-regulation of Rubisco in the starch mutants may be mediated by increased soluble sugars, resulting in limited capacity for photosynthesis compared with the wt. There are two ways to account for lower initial extractable activity of Rubisco: control of Rubisco synthesis and control of Rubisco state of activation. The results indicate both contribute to the lower initial extractable activity.
Rubisco total activity and content decreased with increasing Glc levels, and this occurred to a greater degree in TL25 where Glc levels were higher due to limited ability to synthesize starch. Because the decrease in Rubisco protein was much greater than the decrease in other soluble protein, it indicates some selectivity in the down-regulation of Rubisco. A selective decrease in Rubisco relative to other proteins under Nl in elevated CO2 has been reported in spinach (Spinacia oleracea; Evans and Terashima, 1988) and bean (Phaseolus vulgaris; Nakano et al., 1998) where other photosynthetic proteins remained constant. This is also supported by Cheng et al. (1998) who found a decrease in Rubisco transcripts in Arabidopsis with elevated CO2. The results are consistent with the proposed sugar-mediated repression of photosynthetic genes due to increased hexose metabolism (Graham et al., 1994; Jang and Sheen, 1994; Cheng et al., 1998). The down-regulation of Rubisco is more pronounced in Nl, when Glc levels were at their highest. Also, sugar-mediated down-regulation of photosynthesis is particularly effective at Nl supply (Nielsen et al., 1998). Rubisco may be used as an N store and mobilized, as a result of sugar repression, when N becomes limiting (Paul and Stitt, 1993). Because there is an interaction between N and sugar signaling, the increased C:N ratio in Nl (Fig. 1C) may contribute to triggering the sugar-mediated gene repression (Lam et al., 1994; Paul and Driscoll, 1997). The relationship between leaf sugars and Rubisco activity will be influenced not only by compartmentation of sugars in the leaf, but by interaction with other metabolites involved in gene regulation. For example, in antisense potato plants with decreased capacity for starch production, there was an increase in hexoses in elevated CO2. However, this did not result in an inhibition of Rubisco activity or rbcS transcripts, although photosynthesis was decreased. The decreased photosynthetic rate was not due to sugar repression, rather it was limited by end-product synthesis and triose-P use (Ludewig et al., 1998).
When photosynthesis is limited by sink capacity, and if down-regulation of Rubisco synthesis is insufficient to balance the capacity of the source with sink, then further regulation may occur through feedback and decreased state of activation of Rubisco. The state of activation of Rubisco was lower in the starch mutants than wt plants, and the lowest states of activation occurred under CO2 enrichment. Limited capacity for synthesis of starch (starch mutants) or limited capacity to use Suc (under N deficiency) can result in accumulation of organic phosphates, reduction in Pi and synthesis of ATP in the chloroplast, and decreased state of activation of Rubisco, which is dependent on ATP (Sharkey, 1990).
In summary, this study on Arabidopsis indicates that when synthesis of starch is limiting with an adequate supply of N, growth is limited due to insufficient carbohydrate reserves during the dark period (also supported by Sun et al., 1999). When synthesis of starch is restricted under conditions where N supply is limited, the large increase in soluble sugars apparently accentuates the feedback and down-regulation of Rubisco, resulting in greater reduction of growth.
MATERIALS AND METHODS
Plant Growth
Arabidopsis cv Columbia wt, starch-deficient TL46 (10%–40% starch of the wt compared on a w/v basis), and near starchless TL25 (Lin et al., 1988b, 1988a) were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus). TL46 contains a missense mutation of adg2 gene that codes the large subunit structural gene of AGPase (Wang et al., 1997). TL25 contains a mutation of the adg1 gene that codes the small subunit structural gene of AGPase (Lin et al., 1988b).
Plants were grown in controlled environmental growth chambers with a 12-h photoperiod and photosynthetic photon flux density of 300 μmol m−2 s−1 provided by metal halide lamps. Day and night temperatures were 24°C ± 1°C and 18°C ± 1°C, respectively. Relative humidity in the growth chambers was 70%. Plants were cultured hydroponically in modified Hoagland solution (Hoagland and Arnon, 1950). Hydroponic culture was adapted from the previous reports (Sun et al., 1996; Gibeaut et al., 1997). Polyethylene boxes (32L; Rubbermaid, Wooster, OH) were used, and a sheet of Plexiglas was placed on top of the cover. Holes (2.8 cm) were cut through the Plexiglas and the cover to hold 35 to 59 No. 6 rubber stoppers (depending on the plant size). A 1.3-cm hole was drilled in the center of each plug to accept rockwool cylinders (1.5 × 4 cm), which was used for supporting the seedlings with one plant per hole. Seeds were placed on rockwool for germination.
The hydroponic solution consisted of one-quarter-strength Hoagland macronutrients, full-strength Hoagland micronutrients, and various nitrate levels (0.625 mm K2SO4, 0.5 mm MgSO4, 0.25 mm KH2PO4, 3 mm Ca, 20 μm Fe-EDTA, 35 μm 330 Fe [Sequestrene 330; Ciba-Geigy, Greensboro, NC], 46 μm H3BO3, 9 μm MnCl2, 0.76 μm ZnSO4, 0.32 μm CuSO4, 0.12 μm NaMoO4, and various nitrates).
Plants were first established by growth under normal atmospheric levels of CO2 and medium levels of nitrate (3 mm) for 3 weeks (i.e. before the rapid phase of leaf expansion). The plants were then grown under two different levels of nitrate, at 6 mm, designated as NH, and at 0.2 mm, designated as NL (plus another 0.1 mm every week), and two different levels of CO2, 100 Pa (CH) versus 35 Pa (CL) for 16 ± 2 d.
Leaf Area and Fresh Weight
Leaf areas were determined with a leaf area meter (Li-3000; LI-COR, Lincoln, NE). Leaf area and fresh weight measurements were taken at 35 ± 2 d after germination.
Nitrogen Analysis
Three plants from each treatment were dried, pooled, and ground to a powder. The samples were combusted, and C was measured by infrared absorption and N was determined by thermal conductivity (LECO CNS 2000; LECO, St. Joseph, MI).
Starch, Suc, and Hexoses (Glc and Fru) Determination
Leaf starch, Suc, and hexoses were extracted and determined as previously described (Angelov et al., 1993). Leaf discs (2 × 0.33 cm2), acquired using a paper punch, were extracted with 80% (v/v) ethanol several times until the extract was colorless. The ethanol soluble fractions from each sample were pooled, dried at 55°C under vacuum (speed-vac, Savant, Farmingdale, NY), resolubilized in 0.5 mL of distilled water, and frozen (−20°C) until analyzed for sugars. The leaf residue was briefly air dried and was then homogenized in 0.2 mL of 0.5 m KOH. The homogenate was then boiled for 30 min, and the pH was adjusted to approximately 5.5 by the addition of 0.2 mL of 1 m acetic acid. Amyloglucosidase (Sigma, St. Louis), which was used to digest starch, was dissolved in 50 mm MOPS, pH 7.5, centrifuged to remove starch, and desalted to remove sugar. To convert starch to Glc, samples were incubated with amyloglucosidase (10 units in a sample volume of 0.4 mL) at 55°C for 2 h (preliminary tests showed no additional sugars were released beyond 2 h). Free sugars were determined spectrophotometrically in each extract by the coupled enzyme methods as previously described (Angelov et al., 1993; Winder et al., 1998).
Rubisco Enzyme Extraction and Assay
Arabidopsis leaves were collected about 2 h into the light period and were stored in liquid nitrogen until analysis. The leaves were extracted and analyzed the same day as sampled. Protein content was determined using the Bradford procedure with bovine serum albumin as the standard (Bradford, 1976).
Rubisco Activity
Two leaf discs (0.3 cm2) were acquired using a paper punch that was precooled in liquid nitrogen and they were homogenized in 200 μL of solution containing 100 mm Bicine, pH 8.0, 15 mm MgCl2, 0.5 mm EDTA-Na2, 0.01% (v/v) Triton, and 5 mm dithiothreitol. The homogenate was centrifuged in a microcentrifuge (model 235; Fisher Scientific, Pittsburgh) at maximum speed (approximately 12,000g) for 1 min at 4°C. Ten microliters of the supernatant was assayed in a reaction mixture (final volume of 100 μL) containing 70 mm Bicine, pH 8.0, 10 mm MgCl2, 2.5 mm dithiothreitol, 20 mm NaH14CO3 (1 Ci mol−1), and 1 mm RuBP at 25°C. To determine initial activity, the enzyme was added to the above mixture. To determine total activatable activity, the enzyme was incubated in the above mixture for 5 min in the absence of RuBP, and then the reaction was initiated by addition of RuBP. After incubating at 25°C for 1 min, the reaction was stopped by addition of 30% (v/v) acetic acid. The mixture was dried at 55°C and then 100 μL of distilled water was added to dissolve the sample. Ten milliliters of scintillation cocktail was then added and the radioactivity was determined by a liquid scintillation counter (LS7000; Beckman, Fullerton, CA).
Rubisco Protein Determination
The crude extract (see Rubisco activity assay above) was incubated in presence of 20 mm NaHCO3 for 10 min at room temperature. Then 14C-CABP (specific activity 94 dpm pmol−1, made from reaction of 14C-KCN and RuBP; Collatz et al., 1979) was added to the mixture and was incubated for 45 min at room temperature. The proteins were then precipitated in the presence of 20% (w/v) polyethylene glycol 4000 (in 100 mm Bicine, pH 8.0, and 25 mm MgCl2), incubated for 10 min at room temperature, and then centrifuged for 5 min at 15,000g. The pellet was washed once with 20% (w/v) polyethylene glycol 4000 containing 20 mm MgCl2. The pellet was resolved in a solution containing 100 mm Bicine, pH 8.0, and 10 mm MgCl2. Ten milliliters of scintillation cocktail was then added and the radioactivity was determined by a liquid scintillation counter (LS7000; Beckman).
ACKNOWLEDGMENT
We thank Mary Fauci for assistance with nitrogen analysis.
Footnotes
This research was supported by the U.S. Department of Agriculture (grant no. 2001–35318–10126 to T.W.O. and G.E.E.) and by the U.S. Department of Energy (grant no. DE–FG03–96ER20216 to T.W.O.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010058.
LITERATURE CITED
- Angelov MN, Sun J, Byrd GT, Brown RH, Black CC. Novel characteristics of cassava, Manihot esculenta Crantz, a reputed C3-C4intermediate photosynthetic species. Photosynth Res. 1993;38:61–72. doi: 10.1007/BF00015062. [DOI] [PubMed] [Google Scholar]
- Arp WJ. Effects of source-sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ. 1991;14:869–875. [Google Scholar]
- Bowler JM, Press MC. Effects of elevated CO2, nitrogen form and concentration on growth and photosynthesis of a fast- and slow-growing grass. New Phytol. 1996;132:391–401. doi: 10.1111/j.1469-8137.1996.tb01859.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caspar T, Huber SC, Somerville C. Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana(L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol. 1985;79:11–17. doi: 10.1104/pp.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave G, Tolley LC, Strain BR. Effect of carbon dioxide enrichment on chlorophyll content, starch content and starch grain structure in Trifolium subterraneumleaves. Physiol Plant. 1981;51:171–174. [Google Scholar]
- Cheng S-H, Moore BD, Seemann JR. Effects of short- and long-term elevated CO2 on the expression of Ribulose-1,5-bisphosphate carboxylase/oxygenase genes and carbohydrate accumulation in leaves of Arabidopsis thaliana. Plant Physiol. 1998;116:715–723. doi: 10.1104/pp.116.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collatz GJ, Badger M, Smith C, Berry JA. A radioimmune assay for RuP2carboxylase protein. Carnegie Inst Yearbook. 1979;78:171–175. [Google Scholar]
- Cure JD, Rufty TW, Israel DW. Assimilate relations in source and sink leaves during acclimation to a CO2-enriched environment. Physiol Plant. 1991;83:687–695. [Google Scholar]
- Ehret DL, Jolliffe PA. Leaf injury to bean plants grown in carbon dioxide enriched atmospheres. Can J Bot. 1985;63:2015–2020. [Google Scholar]
- Evans JR, Terashima I. Photosynthetic characteristics of spinach leaves grown with different nitrogen treatments. Plant Cell Physiol. 1988;29:157–165. [Google Scholar]
- Forde BG. Local and long-range signaling pathways regulating plant responses to nitrate. Annu Rev Plant Physiol Plant Mol Biol. 2002;53:203–224. doi: 10.1146/annurev.arplant.53.100301.135256. [DOI] [PubMed] [Google Scholar]
- Geiger M, Haake V, Ludewig F, Sonnewald U, Stitt M. The nitrate and ammonium nitrate supply have a major influence on the response of photosynthesis, carbon metabolism, nitrogen metabolism and growth to elevated carbon dioxide in tobacco. Plant Cell Environ. 1999;22:1177–1199. [Google Scholar]
- Gibeaut DM, Hulett J, Cramer GR, Seemann JF. Maximal biomass of Arabidopsis thalianausing a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiol. 1997;115:317–319. doi: 10.1104/pp.115.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt EE, Huber SC. Regulation of photosynthesis by end-product accumulation in leaves of plants storing starch, sucrose, and hexose sugars. Plant Physiol. 1992;99:1443–1448. doi: 10.1104/pp.99.4.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham IA, Denby KJ, Leaver CJ. Carbon catabolite repression regulates glyoxylate cycle gene expression in cucumber. Plant Cell. 1994;6:761–772. doi: 10.1105/tpc.6.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grub A, Mächler F. Photosynthesis and light activation of ribulose 1,5-bisphosphate carboxylase in the presence of starch. J Exp Bot. 1990;41:1293–1301. [Google Scholar]
- Hoagland DR, Arnon DI. The College of Agriculture, ed, California Agricultural Experiment Station Circular 347, Revised January 1950. Berkeley: University of California; 1950. The water-culture method for growing plants without soil. [Google Scholar]
- Jang J-C, Sheen J. Sugar sensing in higher plants. Plant Cell. 1994;6:1665–1679. doi: 10.1105/tpc.6.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J-C, Sheen J. Sugar sensing in higher plants. Trends Plant Sci. 1997;2:208–214. [Google Scholar]
- Lam H-M, Peng SS-Y, Coruzzi GM. Metabolic regulation of the gene encoding glutamine-dependent asparagine synthetase in Arabidopsis thaliana. Plant Physiol. 1994;106:1347–1357. doi: 10.1104/pp.106.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T-P, Caspar T, Somerville CR, Preiss J. Isolation and characterization of a starchless mutant of Arabidopsis thalianalacking ADP glucose pyrophosphorylase activity. Plant Physiol. 1988a;86:1131–1135. doi: 10.1104/pp.86.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T-P, Caspar T, Somerville CR, Preiss J. A starch deficient mutant of Arabidopsis thalianawith low ADP glucose pyrophosphorylase activity lacks one of the two subunits of the enzyme. Plant Physiol. 1988b;88:1175–1181. doi: 10.1104/pp.88.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig F, Sonnewald U. High CO2-mediated down-regulation of photosynthetic gene transcripts is caused by accelerated leaf senescence rather than sugar accumulation. FEBS Lett. 2000;479:19–24. doi: 10.1016/s0014-5793(00)01873-1. [DOI] [PubMed] [Google Scholar]
- Ludewig F, Sonnewald U, Kauder F, Heineke D, Geiger M, Stitt M, Müller-Röber BT, Gillissen B, Kühn C, Frommer WB. The role of transient starch in acclimation to elevated atmospheric CO2. FEBS Lett. 1998;429:147–151. doi: 10.1016/s0014-5793(98)00580-8. [DOI] [PubMed] [Google Scholar]
- Majeau N, Coleman JR. Effect of CO2concentration on carbonic anhydrase and ribulose-1,5-bisphosphate carboxylase/oxygenase expression in pea. Plant Physiol. 1996;112:569–574. doi: 10.1104/pp.112.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A, Tsai C-H, Hemphill D, Endres M, Rodermel S, Spalding M. Elevated CO2effects during leaf ontogeny. Plant Physiol. 1997;115:1195–1200. doi: 10.1104/pp.115.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BD, Cheng S-H, Rice J, Seemann JR. Sucrose cycling, Rubisco expression, and prediction of photosynthetic acclimation to elevated atmospheric CO2. Plant Cell Environ. 1998;21:905–915. [Google Scholar]
- Morin F, André M, Betsche T. Growth kinetics, carbohydrate, and leaf phosphate content of clover (Trifolium subterraneum L.) after transfer to a high CO2atmosphere or to high light and ambient air. Plant Physiol. 1992;99:89–95. doi: 10.1104/pp.99.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafziger ED, Koller HR. Influence of leaf starch concentration on CO2assimilation in soybean. Plant Physiol. 1976;57:560–563. doi: 10.1104/pp.57.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H, Makino A, Mae T. The effect of elevated partial pressure of CO2on the relationship between photosynthetic capacity and N content in rice leaves. Plant Physiol. 1997;115:191–198. doi: 10.1104/pp.115.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H, Makino A, Mae T. The responses of Rubisco protein to long-term exposure to elevated CO2in rice and bean leaves. In: Garab G, editor. Photosynthesis: Mechanisms and Effects. V. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 3391–3394. [Google Scholar]
- Nie G, Hendrix DL, Webber AN, Kimball BA, Long SP. Increased accumulation of carbohydrates and decreased photosynthetic gene transcript levels in wheat grown at an elevated CO2concentration in the field. Plant Physiol. 1995;108:975–983. doi: 10.1104/pp.108.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen TH, Krapp A, Röper-Schwarz U, Stitt M. The sugar-mediated regulation of genes encoding the small subunit of Rubisco and the regulatory subunit of ADP glucose pyrophosphorylase is modified by phosphate and nitrogen. Plant Cell Environ. 1998;21:443–454. [Google Scholar]
- Paul MJ, Driscoll SP. Sugar repression of photosynthesis: the role of carbohydrates in signaling nitrogen deficiency through source:sink imbalance. Plant Cell Environ. 1997;20:110–116. [Google Scholar]
- Paul MJ, Stitt M. Effects of nitrogen and phosphorus deficiencies on levels of carbohydrates, respiratory enzymes and metabolites in seedlings of tobacco and their response to exogenous sucrose. Plant Cell Environ. 1993;16:1047–1057. [Google Scholar]
- Pego JV, Kortstee AJ, Huijser C, Smeekens CM. Photosynthesis, sugars and the regulation of gene expression. J Exp Bot. 2000;51:407–416. doi: 10.1093/jexbot/51.suppl_1.407. [DOI] [PubMed] [Google Scholar]
- Pettersson R, McDonald AJS. Effects of nitrogen supply on the acclimation of photosynthesis to elevated CO2. Photosynth Res. 1994;39:389–400. doi: 10.1007/BF00014593. [DOI] [PubMed] [Google Scholar]
- Preiss J. Biosynthesis of starch and its regulation. In: Loewus FA, Tanner W, editors. Encyclopedia of Plant Physiology. 13A: Intracellular Carbohydrates. Berlin: Springer-Verlag; 1982. pp. 397–417. [Google Scholar]
- Riviere-Rolland H, Contard P, Betsche T. Adaptation of pea to elevated atmospheric CO2: Rubisco, phosphoenolpyruvate carboxylase and chloroplast phosphate translocator at different levels of nitrogen and phosphorus nutrition. Plant Cell Environ. 1996;19:109–117. [Google Scholar]
- Rogers A, Fischer BU, Bryant J, Frehner M, Blum H, Raines CA, Long SP. Acclimation of photosynthesis to elevated CO2 under low-nitrogen nutrition is affected by the capacity for assimilate utilization: perennial ryegrass under free-air CO2enrichment. Plant Physiol. 1998;118:683–689. doi: 10.1104/pp.118.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GS, Milham PJ, Gillings M, Conroy JP. Sink strength may be the key to growth and nitrogen responses in N-deficient wheat at elevated CO2. Aust J Plant Physiol. 1996a;23:253–264. [Google Scholar]
- Rogers GS, Milham PJ, Thibaud M-C, Conroy JP. Interactions between rising CO2concentration and nitrogen supply in cotton: growth and leaf nitrogen concentration. Aust J Plant Physiol. 1996b;23:119–125. [Google Scholar]
- Rufty TW, Huber SC, Volk RJ. Alterations in leaf carbohydrate metabolism in response to nitrogen stress. Plant Physiol. 1988;88:725–730. doi: 10.1104/pp.88.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF. Acclimation of photosynthesis to increasing atmospheric CO2: the gas exchange perspective. Photosynth Res. 1994;39:351–368. doi: 10.1007/BF00014591. [DOI] [PubMed] [Google Scholar]
- Scheible W-R, González-Fontes A, Lauerer M, Müller-Röber BT, Caboche M, Stitt M. Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell. 1997a;9:783–798. doi: 10.1105/tpc.9.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible W-R, Lauerer M, Schulze E-D, Caboche M, Stitt M. Accumulation of nitrate in the shoot acts as a signal to regulate shoot-root allocation in tobacco. Plant J. 1997b;11:671–691. [Google Scholar]
- Schulze W, Schulze E-D. The significance of assimilatory starch for growth in Arabidopsis thalianawild-type and starchless mutants. In: Schulze E-D, Caldwell MM, editors. Ecological Studies 100: Ecophysiology of Photosynthesis. Berlin: Springer-Verlag; 1994. pp. 123–131. [Google Scholar]
- Schulze W, Schulze E-D, Stadler J, Heilmeier H, Stitt M, Mooney HA. Growth and reproduction of Arabidopsis thalianain relation to storage of starch and nitrate in the wild-type and in starch-deficient and nitrate uptake-deficient mutants. Plant Cell Environ. 1994;17:795–809. [Google Scholar]
- Sharkey TD. Feedback limitation of photosynthesis and the physiological role of ribulose bisphosphate carboxylase carbamylation. Bot Mag Tokyo. 1990;2:87–105. [Google Scholar]
- Smeekens S. Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:49–81. doi: 10.1146/annurev.arplant.51.1.49. [DOI] [PubMed] [Google Scholar]
- Stitt M. Rising CO2levels and their potential significance for carbon flow in photosynthetic cells. Plant Cell Environ. 1991;14:741–762. [Google Scholar]
- Stitt M, Krapp A. The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ. 1999;22:583–621. [Google Scholar]
- Sun J, Nishio JN, Vogelman TC. High-light effects on CO2fixation gradients across leaves. Plant Cell Environ. 1996;19:1261–1271. [Google Scholar]
- Sun J, Okita TW, Edwards GE. Modification of carbon partitioning, photosynthetic capacity, and O2sensitivity in Arabidopsis plants with low ADP-glucose pyrophosphorylase activity. Plant Physiol. 1999;119:267–276. doi: 10.1104/pp.119.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oosten J-J, Besford RT. Some relationships between the gas exchange, biochemistry and molecular biology of photosynthesis during leaf development of tomato plants after transfer to different carbon dioxide concentrations. Plant Cell Environ. 1995;18:1253–1266. [Google Scholar]
- Van Oosten J-J, Wilkins D, Besford RT. Regulation of the expression of photosynthetic nuclear genes by CO2 is mimicked by regulation by carbohydrates: a mechanism for the acclimation of photosynthesis to high CO2? Plant Cell Environ. 1994;17:913–923. [Google Scholar]
- Wang S-M, Chu B, Lue W-L, Yu T-S, Eimert K, Chen J. adg2-1 represents a missense mutation in the ADPG pyrophosphorylase large subunit gene of Arabidopsis thaliana. Plant J. 1997;11:1121–1126. doi: 10.1046/j.1365-313x.1997.11051121.x. [DOI] [PubMed] [Google Scholar]
- Winder TL, Sun J, Okita TW, Edwards GE. Evidence for the occurrence of feedback inhibition of photosynthesis in rice. Plant Cell Physiol. 1998;39:813–820. [Google Scholar]
- Wullschleger SD, Ziska LH, Bunce JA. Respiratory responses of higher plants to atmospheric CO2enrichment. Physiol Plant. 1994;90:221–229. [Google Scholar]
- Ziska LH, Weerakoon W, Namuco OS, Pamplona R. The influence of nitrogen on the elevated CO2response in field-grown rice. Aust J Plant Physiol. 1996;23:45–52. [Google Scholar]