Abstract
Starch biosynthesis during pollen maturation is not well understood in terms of genes/proteins and intracellular controls that regulate it in developing pollen. We have studied two specific developmental stages: “early,” characterized by the lack of starch, before or during pollen mitosis I; and “late,” an actively starch-filling post-pollen mitosis I phase in S-type cytoplasmic male-sterile (S-CMS) and two related male-fertile genotypes. The male-fertile starch-positive, but not the CMS starch-deficient, genotypes showed changes in the expression patterns of a large number of genes during this metabolic transition. In addition to a battery of housekeeping genes of carbohydrate metabolism, we observed changes in hexose transporter, plasma membrane H+-ATPase, ZmMADS1, and 14-3-3 proteins. Reduction or deficiency in 14-3-3 protein levels in all three major cellular sites (amyloplasts [starch], mitochondria, and cytosol) in male-sterile relative to male-fertile genotypes are of potential interest because of interorganellar communication in this CMS system. Further, the levels of hexose sugars were significantly reduced in male-sterile as compared with male-fertile tissues, not only at “early” and “late” stages but also at an earlier point during meiosis. Collectively, these data suggest that combined effects of both reduced sugars and their reduced flux in starch biosynthesis along with a strong possibility for altered redox passage may lead to the observed temporal changes in gene expressions, and ultimately pollen sterility.
Several excellent reviews on male gametophyte development in plants (Mascarenhas, 1989; Bewley et al., 2000) and maize (Zea mays) in particular (Bedinger, 1992; McCormick, 1993) have been written recently and provide overviews of events from meiosis to mature pollen development. In brief, haploid gametes as tetrads are encased in a callose wall and are well nourished through the sporophytic cell layer, tapetum. The role of the tapetum in pollen development is recently elaborated (Liu et al., 2001). Release of single, free microspores from each tetrad is achieved by callase secreted from the tapetal cells, which degenerate and lead to the symplastic isolation of microspores from the mother plant. All nourishments for developing microspores are transported presumably from the nutrient-rich locular fluid inside the anthers. Most importantly, symplastic discontinuity requires that the individual microspores be programmed with appropriate signals or at least be activated for major functions, including the two mitotic divisions, intracellular vacuolar biogenesis, and several metabolic changes such as starch biosynthesis.
Starch biosynthesis during the final phases of pollen maturation is critical not only because starch is a reserve source of energy for pollen germination but it also serves as a checkpoint of pollen maturity. Very often, pollen maturation appears to be prematurely terminated if starch levels remain lower than a certain threshold point as evident from several genetically controlled male-sterile mutants, including the S-type cytoplasmic male sterility (S-CMS) studied here, where pollen inviability is associated with starch deficiency (Wen and Chase, 1999a). In fact, Lee et al. (1980) noted in a comparative ultrastructural analysis of fertile and sterile pollen development in S-CMS system in maize that pollen collapses during the starch accumulation phase. The S-CMS trait is maternally inherited. Plants with normal (N) cytoplasm produce fertile plants independent of the Rf genes. As the name implies, female fertility in CMS plants is unaffected. The nuclear gene that restores fertility to S-CMS plants is Rf3. Fertility restoration in S-CMS plants is gametophytic in nature; i.e. the individual pollen is fertile or sterile based on its Rf3 or rf3 genotype, respectively. Starch deposition is also controlled gametophytically; fertile pollen are starch positive and sterile pollen are starch deficient (for review, see Laughnan and Gabay-Laughnan, 1983; Wen and Chase, 1999a).
Various aspects of starch biosynthesis, including biochemical, physiological, and molecular genetics, are well analyzed in another similar storage sink, developing seed. Suc, the long distance sugar of transport, is unloaded at the base of the seed in the pedicel through phloem termini. Its entrance into basal endosperm cells in maize is believed to be mediated by a plasma membrane (PM)-associated Suc transporter (Aoki et al., 1999), and thereafter a rapid hydrolysis by endosperm-specific cell wall invertase (Cheng et al., 1996). As with the microspores, basal endosperm cells are also symplastically discontinuous from the maternal pedicel because there are no plasmodesmatal connections between these two cell layers. Indirect evidence suggests that Suc unloading and its initial metabolism in endosperm is through a futile cycle of Suc turnover reactions because both Suc synthesis and Suc cleavage enzymes are localized to this part of the endosperm (Chourey et al., 1995; Cheng and Chourey, 1999). Subsequent metabolism of Suc in starch biosynthesis is mediated by several housekeeping enzymes, including hexokinase (HXK), phosphoglucomutase (PGM), UDPG pyrophosphorylase, ADPG pyrophosphorylase (AGPase), and granule-bound starch synthase (GBSS). Correlated increases in these enzyme activities in developing endosperm coincident with starch biosynthesis were analyzed previously and described extensively (Tsai et al., 1970; for review, see Nelson and Pan, 1995).
Despite much knowledge on starch biosynthesis in developing seed, very little is known about developing pollen, except our recent limited studies in sorghum (Sorghum bicolor; Datta et al., 2001). Our objective here is to obtain expression profiles of genes related to sugar transport, metabolism, and its utilization in starch biosynthesis. Two specific stages in developing pollen are of interest: the “early” stage, before or during pollen mitosis I (PM-I), where no starch is detected; and “late” stage, which is an active starch-filling phase in immature pollen (see “Materials and Methods” for details). Three very similar genotypes in lineage-related background of the Mo-17 inbred line are examined: S-CMS male-sterile starch-deficient line, S, rf3rf3; and two male-fertile genotypes, N, rf3rf3 (a normal, maintainer) and S, Rf3rf3, the fertility-restored F1 hybrid. The F1 heterozygote segregates in a 1:1 ratio for fertile and sterile pollen. We also report sugar analyses on these samples to better understand the possible basis of starch deficiency and the observed changes in gene expression in male-sterile plants.
RESULTS
RNA Profiles of Genes Involved in Sugar Metabolism and Starch Biosynthesis
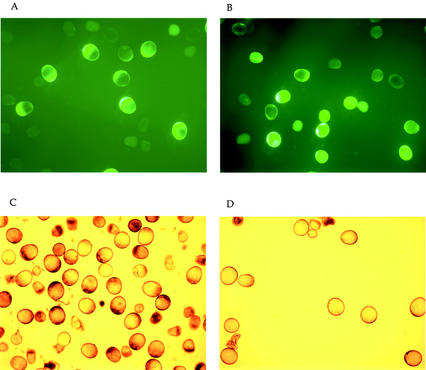
Figure 1 shows immature pollen of male-fertile (N, rf3rf3) and -sterile (S, rf3rf3) genotypes of “late” stage stained with a vital stain, FDA and I2KI, as described in “Materials and Methods.” The male-fertile samples from greenhouse-grown plants were collected when a majority of the cells were I2KI positive (Fig. 1C), whereas male-sterile samples were collected approximately 48 h before the collapsed pollen stage described by Wen and Chase (1999). There was no detectable difference in fluorescence in male-fertile and -sterile samples by the FDA stain (Fig. 1, A and B). Fluorescein-positive cells were considered metabolically alive because they were able to hydrolyze FDA to release fluorescent fluorescein into the cytoplasm through intracellular esterases. The I2KI pattern (Fig. 1, C and D), however, was different; there was starch accumulation in the male-fertile but not in the male-sterile samples. The samples of “early” stage, collected 5 to 7 d before the “late” stage, showed no difference between male-fertile and -sterile genotypes either by FDA or by the I2KI stain (data not shown). As expected, both samples were metabolically alive and were lacking in starch.
Figure 1.
Staining of immature pollen at the “late” stage with fluorescein diacetate (FDA) for cell viability (A and B) and I2KI for starch (C and D). A and C, Samples from male-fertile genotypes; B and D, male-sterile genotypes.
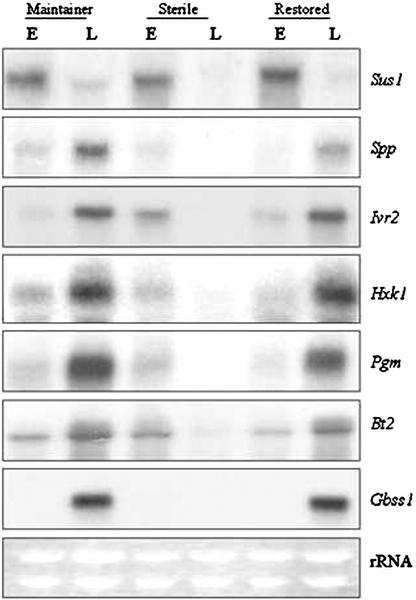
Figure 2 shows the comparative RNA profiles of several key metabolic genes in two male-fertile genotypes and one CMS male-sterile genotype at “early” and “late” stages of developing pollen in maize. The biochemical reactions catalyzed by each enzyme are shown in Table II. The Sus1-encoded Suc synthase (SuSy), of all the genes in Figure 2, is unique because it is the only enzyme that catalyzes a reversible reaction with Suc, and it was also temporally the first transcript in high steady-state abundance, at the “early” phase relative to the “late,” in all three genotypes. Three SuSy genes, Sh1, Sus1, and Sus2, have been described in maize (Carlson et al., 2002); however, no transcripts were detected using Sh1 or Sus2 cDNA probes (data not shown). Suc 6-phosphate phosphohydrolase (SPP) catalyzes the final step in Suc synthesis subsequent to the formation of Suc-6 phosphate by Suc phosphate synthase (SPS). Spp profile showed much lower steady-state levels at the “early” stage in all three genotypes; thereafter, a significant increase was seen at the “late” stage in the male-fertile genotypes coincident with the starch-filling phase. No such temporal increase in Spp RNA levels was seen in male-sterile immature pollen. Although SPP and SPS are known to act in a sequential fashion and as a complex (Lunn et al., 2000, and refs. therein), we did not detect SPS transcripts in any of our samples (data not shown).
Figure 2.
RNA gel-blot analyses showing expression patterns of genes, shown on right, in developing pollen. Each lane consists of 20 μg of total RNA isolated from “early” (E) and “late” (L) stages of developing pollen from maintainer, N, rf3rf3; male-sterile, S, rf3rf3; and F1-restored hybrid, S, Rf3rf3. The same blot or parallel blots run under identical conditions were hybridized with various 32P-labeled cDNA probes. Ethidium bromide-stained rRNA bands are shown as loading controls.
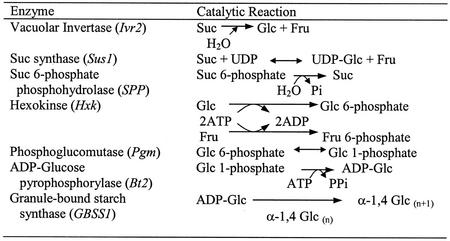
Table II.
Reactions catalyzed by metabolic enzymes involved in starch biosynthesis
The corresponding transcripts are indicated in brackets.
Invertases are critical in the irreversible cleavage of Suc to hexose sugars. Two soluble or vacuolar invertase genes, Ivr1 and Ivr2, have been described in maize (Xu et al., 1996; Carlson and Chourey, 1999). Both fertile lines showed temporal increases in steady-state abundance of Ivr2 RNA during transition from “early” to “late” phase; the male-sterile samples, in contrast, showed greatly reduced levels at the “late” relative to the “early” stage. Cell wall invertases' (Incw1 and Incw2; Taliercio et al., 1999) transcripts were undetectable in all six samples (data not shown). Hxk and Pgm genes that encode HXK and PGM, respectively, also showed nearly the same expression pattern as the Spp and Ivr2 genes in all samples. The formation of ADP-Glc by AGPase is the first committed step in the transfer of Glc into starch biosynthesis. In maize, AGPase is a heteromeric protein, encoded by two nonallelic genes, Sh2 and Bt2 (Bae et al., 1990; Bhave et al., 1990). Similar steady-state levels of Bt2 transcripts were detected at the “early” stage in all samples, but the increases seen at “late” stage in male-fertile genotypes were not seen in the male-sterile samples. The Bt2 transcripts were detected only under reduced stringency wash conditions (see “Materials and Methods”), indicating that a Bt2 paralog was expressed in immature pollen. We did not detect any Sh2 transcripts (data not shown); presumably, the Sh2 paralog in pollen is significantly divergent from the endosperm gene. Gbss1, encoded by the Waxy gene in maize, constitutes the final step in which the Glc moiety of ADP-Glc is transferred to the nonreducing end of the starch molecule inside the starch granule (for review, see Nelson and Pan, 1995). Although undetectable at the “early” stage in all samples, Gbss1 RNA was seen in great abundance at the “late” stage in fertile but not in the sterile samples. We used ethidium bromide-stained rRNA bands as gel-loading controls. Similar band intensities of the two rRNAs confirmed uniform loading in all our samples (Fig. 2).
RNA Level Profiles of Putative Regulatory and Transport Genes
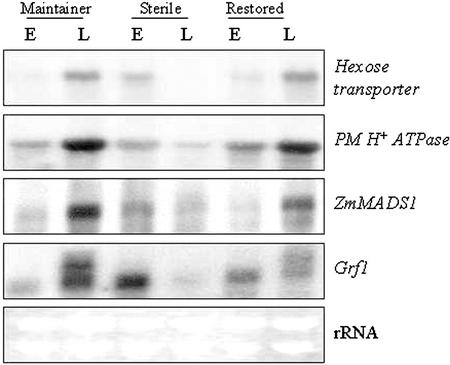
Figure 3 shows the RNA profiles of a hexose transporter, PM H+-ATPase, and genes corresponding to MADS box and 14-3-3 proteins, ZmMADS1 and Grf1, respectively. Two expressed sequence tag (EST) clones, one each for hexose transporter and PM H+-ATPase, were obtained from the Zea mays Database (http://zmdb.iastate.edu) based on their sequence identities with the corresponding rice (Oryza sativa) clones (Table I). Both hexose transporter and PM H+-ATPase genes showed qualitatively similar expression profiles in male fertile (higher levels at “late” relative to “early”) and sterile (higher levels at “early” relative to “late”) at the two stages of development. In contrast to hexose transporter, no hybridization was seen with our maize Suc transporter, Sut1, clone (Aoki et al., 1999; R. Datta and P.S. Chourey, unpublished data) to these RNA samples (data not shown).
Figure 3.
RNA gel-blot (20 μg of total RNA lane−1) analyses showing expression patterns of transporters (hexose transporter and PM H+-ATPase) and putative regulatory (ZmMADS1 and Grf1) genes in developing pollen at early (E) and late (L) stages from maintainer, male-sterile, and F1-restored genotypes. The same blot or parallel blots run under identical conditions were hybridized as described in “Materials and Methods.”
Table I.
Probes used in RNA- and protein-blot analyses
| Gene | GenBank No. | Probe
|
Source of cDNA | Reference | |
|---|---|---|---|---|---|
| cDNA | Antibody | ||||
| Soluble Invertase2 | U31451 | Ivr2 | – | Reverse transcriptase (RT)-PCRa | Xu et al. (1996) |
| Suc synthase | L22296 | Sus1 | SS2 | cDNA library | Gupta et al. (1988) |
| Suc 6-phosphate phosphohydrolase | AF283564 | SPP | – | Genomic library | Lunn et al. (2000) |
| Hexokinase | AF372831 | Hxk1 | – | RT-PCRb | Wu et al. (2001) |
| Phosphoglucomutase | U89342 | Pgm2 | – | cDNA library | Manjunath et al. (1998) |
| ADP-Glc pyrophosphorylase | S72425 | Bt2 | BT2 | cDNA library | Bae et al. (1990) |
| Granule-bound starch synthase | AF079261 | Gbss1 | – | RT-PCRc | Mason-Gamer et al. (1998) |
| GF14 (14-3-3) | S77133 | Grf1 | 14-3-3 | Genomic library | de Vetten and Ferl (1994) |
| ZmMADS1 | AF112148 | ZmMADS1 | – | RT-PCRd | Heuer et al. (2000) |
| α-Tubulin (TUA) | X15704 | – | TUA | – | Datta and Chourey (2001) |
| Hexose transporter | AB052885 | hexose transporter | – | Maize database EST (accession no. AI834551) | Toyofuku et al. (2000) |
| PM H+-ATPase | U09989 | H+ATPase (Mha1) | – | Maize database EST (accession no. AW056178) | Jin and Bennetzen (1994) |
The Ivr2 cDNA clone was amplified from mature maize pollen RNA with the forward primer 5′-CTCACCAACTGGACCAAGTACGA and reverse primer 5′-CCATGTAGTCGTGGTTGTATGACG.
The Hxk1 cDNA clone was amplified from 20-d after pollination maize endosperm RNA using primers designed from the rice Hxk1 sequence in GenBank, with the forward primer 5′-AGGTGAAATTGTAAGGAGGGT and the reverse primer 5′-ATCTGCATGCTTGACGGCCACT.
The GBSS1 cDNA clone was amplified from 20-d after pollination maize endosperm RNA using primers designed from the published maize GBSS1 sequence, with the forward primer 5′-TACATCGCCGTGAAGTACGACGTG and the reverse primer 5′-GGTCGATCGATCTTGGCGCCCT.
The ZmMADS1 cDNA clone was amplified from late-stage maize pollen RNA with the forward primer 5′-GCAGATGAAGCGAATAGAGAACCC and the reverse primer 5′-GGCTTGCATCTCGATCTCCACACT.
Two MADS box genes specific to microsporogenesis, ZmMADS1 and ZmMADS2, have been described in maize (Heuer et al., 2000). An RT-PCR derived cDNA clone of ZmMADS1 (Table I) detected low levels of transcripts at the “early” stage in all three samples. There was a large increase in the steady-state levels of ZmMADS1 RNA at the “late” stage in the male-fertile lines, but not in the male-sterile line, which in fact showed a slight decrease in these transcript levels.
The 14-3-3 proteins encoded by the Grf genes were recently proposed to have a regulatory role in starch accumulation (Sehnke et al., 2001). All three lines here at the “early” stage showed similar steady-state levels of the Grf1 transcripts, but the “late” stage profiles for fertile and sterile immature pollen were highly divergent. In particular, the fertile samples showed both increased levels and an additional transcript; the male-sterile samples, however, showed significant reduction in Grf1 RNA to undetectable levels.
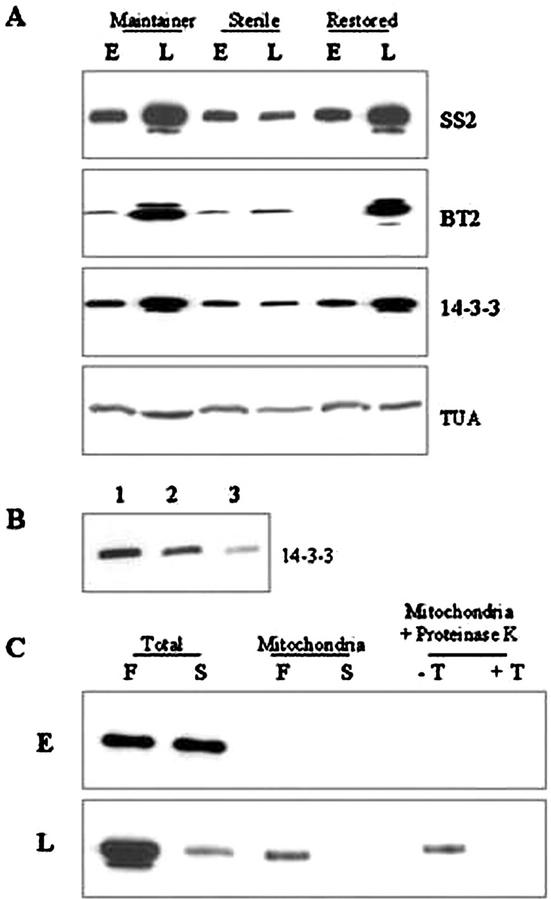
Immunoblot Profiles of SS2, AGPase, 14-3-3, and TUA
All three lines, regardless of male-fertile or -sterile trait, showed abundant levels of SuSy protein, SS2, at “early” stage, but greatly decreased levels at “late” stage of developing pollen (Fig. 2). These observations were in agreement with the RNA profile (Fig. 2). AGPase subunit, BT2 protein, showed a reverse pattern; low levels at “early” and much higher levels at “late” in starch-filling samples from fertile plants. Male-sterile plants showed only low levels of BT2 protein at both stages of developing pollen. The temporal pattern of 14-3-3 proteins was similar to the BT2 protein, greatly increased levels at “late” stage relative to “early” in male-fertile samples, and much reduced levels at both stages in male-sterile plants. Also, an additional, slightly lower Mr isoform appeared at the “late” stage of pollen maturation that was absent in the “early” stage. This isoform was not detectable in the male-sterile line. TUA, a gel-loading control, was at uniform levels in all the three lines during both “early” and “late” stages of pollen maturation (Fig. 4).
Figure 4.
A, Immunoblots showing SS2, BT2, 14-3-3, and TUA proteins in developing pollen at early (E) and late (L) stages of developing pollen from the genotypes as shown above. Antisera dilutions were done as described in “Materials and Methods.” SS2, Each lane contains 2.5 μg of total protein; BT2, 2 μg of protein; 14-3-3, 0.5 μg of protein; TUA, 5 μg of protein. B, Immunoblot showing 14-3-3 proteins in starch granules of developing pollen. Starch granules were extracted from late-stage immature pollen from the maintainer genotype. Thermolysin digestion of 1.0 μg of total protein was done as described in “Materials and Methods.” Lane 1, Starch granules without thermolysin treatment; lane 2, thermolysin-treated starch granules; lane 3, thermolysin digestion of proteins after release from isolated starch granules. C, Immunoblot showing 14-3-3 proteins in mitochondria. Total proteins (1 μg lane−1) or proteins from isolated mitochondria (25 μg lane−1) from developing pollen at “early” (E) and “late” (L) stages in male-fertile maintainer (F) and -sterile (S) lines were used. Isolated mitochondria were subjected to proteinase K digestion in the presence of Triton X-100 (+T) or absence (−T). Protease protection assay and antisera dilutions were done as described in “Materials and Methods.”
The 14-3-3 proteins were also bound to starch granules in immature pollen in the male-fertile maintainer line (Fig. 3B), as first shown by Sehnke et al. (2001) in starch granules from Arabidopsis leaves and maize endosperm. Figure 4B shows that 14-3-3 proteins were not digested by thermolysin treatment in pregelatinized starch granules, but were susceptible to such treatment in post-gelatinized starch granules (lanes 2 and 3, respectively). No extractable starch was available for these analyses in male-sterile immature pollen. A recent demonstration of 14-3-3 proteins in mitochondria in barley (Hordeum vulgare) seedlings (Bunney et al., 2001) prompted us to examine mitochondrial extracts from developing pollen (Fig. 4C). Although no 14-3-3 proteins were detected in “early” samples of either genotype, a clear positive signal was seen in mitochondria from “late” samples in fertile, albeit at much reduced levels relative to that seen in the total (soluble) fraction. Importantly, no 14-3-3 proteins were seen in mitochondrial extracts from the male-sterile plants. To demonstrate that the 14-3-3 proteins were not a cytosolic contamination or a nonspecific association with the organelle, protease protection assays were performed according to Bunney et al. (2001). Figure 4C shows that mitochondrial 14-3-3 protein is protected from proteinase K treatment in the absence of the detergent Triton X-100. However, dissolution of the mitochondrial membrane using Triton X-100 resulted in complete degradation of the protein. Thus, as in barley seedlings, 14-3-3 proteins were inside the mitochondria of fertile immature pollen.
Carbohydrate Profile during Pollen Maturation
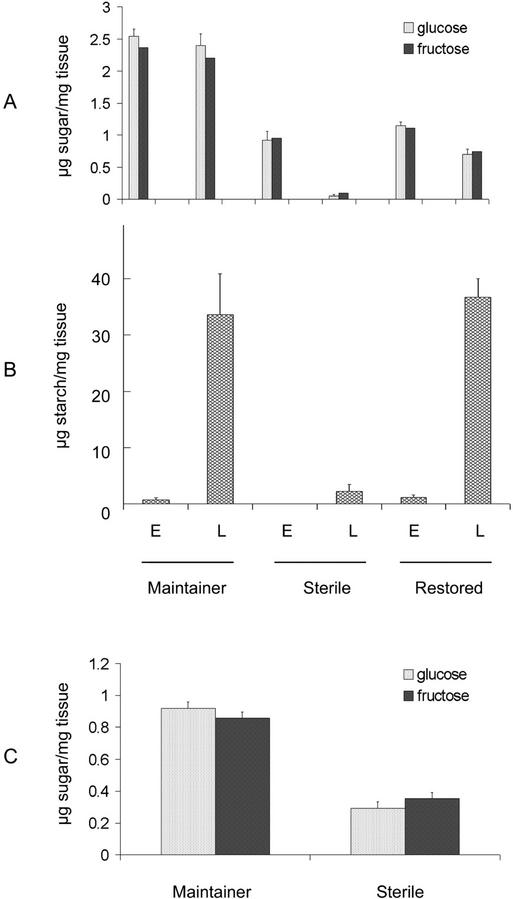
To better understand the possible basis of starch deficiency in male-sterile immature pollen and large changes in gene expression described in the previous sections, we examined aliquots of the same tissue samples for relative levels of carbohydrates, mainly Glc, Fru, Suc, and starch (Fig. 5). Significant differences were observed between male-fertile and -sterile lines. Specifically, the highest levels of hexose sugars were seen in the maintainer line at both “early” and “late” stages. Nearly the same levels of hexoses were also seen in the F1 fertility-restored line at “early” stage, which was followed by a significant reduction at “late” stage. In contrast, male-sterile plants showed greatly reduced levels of hexoses at the “early” stage, which was further reduced to barely detectable levels at the “late” stage. Remarkably, there was no detectable Suc at either stage in all three genotypes (data not shown). As for starch levels, although the fertile lines showed very low levels at the “early” stage, a massive increase (>30-fold) was seen by the “late” stage (Fig. 5B). Both fertile lines showed nearly the same level of starch, but extremely low levels of starch were detected in the male-sterile plants. As a follow-up on hexose levels, we also examined Glc, Fru, and Suc levels 5 to 7 d before the “early” stage, which coincided with the dyad phase of meiosis, in both male-fertile maintainer and male-sterile plants. Although no Suc was detected, Glc and Fru levels in male-sterile dyads were only at one-third the level as compared with the male-fertile samples (Fig. 5C).
Figure 5.
Sugar and starch level profiles during pollen maturation. Glc, Fru (A), and starch (B) levels were assayed during “early” (E) and “late” (L) stages of developing pollen in maintainer, male-sterile, and F1-restored lines as described in “Materials and Methods.” Glc and Fru levels were also assayed in meiotic microspores harvested 5 to 7 d before the “early” stage in maintainer male-fertile and -sterile line (C). The data presented are averages of three independent experiments (±se).
DISCUSSION
We report three major observations in developing pollen during a metabolic transition that initiates, among other changes, a rapid phase of starch biosynthesis in the final phases of pollen maturation: (a) A large number of genes showed temporal changes in their expression during a transition from no starch (“early”) to an active starch-filling (“late”) phase in two genotypes (N, r3frf3 and S, Rf3rf3) that yield normal fertile pollen; (b) No such temporal changes were seen during the same developmental phase in immature pollen in the starch-deficient male-sterile (S, rf3rf3) line; and (c) Sugar profiles of microspores and immature pollen showed much reduced levels of various carbohydrates in male-sterile relative to male-fertile plants.
Temporal Changes in the Expression of Carbohydrate Genes
It is significant that transcript profiles of all genes in Figures 2 through 4 showed temporal changes during pollen maturation. These results, although very interesting, were not unexpected because several of the genes included here have been shown previously to be associated with Suc metabolism and/or Suc to starch conversion reactions in another major sink tissue, the developing maize endosperm. Whether or not the very same genes as in endosperm or their paralogs are expressed in immature pollen is unknown. Also not known, until this study, was whether Suc or hexoses enter the maize microspores. Cumulative evidence (see below) suggests that hexoses may enter the microspores. Regardless, we observed changes in the expression of several genes encoding enzymes that are active in both Suc synthesis (SPP and SuSy) and Suc cleavage (vacuolar invertase and SuSy) reactions. However, our sugar analyses did not detect any Suc in these tissues, indicating that either the Suc levels were below our detection limits, or the synthesized Suc was transient in nature, possibly to fuel a futile cycle of Suc synthesis and cleavage reactions. Such a futile cycle is often implicated in regulatory controls in Suc metabolism in various sink tissues, including developing endosperm (see above) and other sink tissue in diverse plant systems (for review, see Nguyen-Quoc and Foyer, 2001).
Among Suc-metabolizing enzymes, SuSy plays a prominent role in providing both substrate and precursors for cellulose, callose, and starch biosynthesis in plants. In a previous study, we observed high levels of SuSy protein, SS2, in callase-secreting tapetal cells in maize (Chourey and Miller, 1995). Our recent cellular level observations (data not shown) show no difference between S-CMS and male fertile for the SS2 signal in tapetal cells (K. Chamusco and P.S. Chourey, unpublished data). Sus1-encoded mRNA and the SS2 protein were at a higher level at the “early” stage compared with the “late” stage (Figs. 2 and 4), and there was no detectable difference between male-fertile (starch-positive) and -sterile (starch-deficient) microspores and immature pollen samples. We suggest that SuSy, as in developing endosperm (Chourey et al., 1998), may play only a minor role in starch biosynthesis in these cells. We speculate that SuSy may provide UDP-Glc for the synthesis of callose that is essential for cell plate formation after PM-I. Reversible phosphorylation of SuSy protein is known to modulate its reversible reaction of this enzyme (for review, see Winter and Huber, 2000). Thus, SuSy along with SPS and SPP enzymes may also catalyze Suc synthesis for intracellular transient storage of Suc in vacuoles, which are formed just before starch biosynthesis (Bedinger, 1992). One of the main functions of vacuolar invertases (Ivr2) is the rapid mobilization of vacuolar Suc to hexose through irreversible hydrolysis and the control of hexose to Suc ratios in a sink tissue. Higher steady-state levels of Ivr2 and other downstream genes, Hxk1, Bt2, and Gbss1, in male-fertile but not in male-sterile genotypes were consistent with their previously demonstrated roles of Suc utilization in starch biosynthesis.
Developmental transition from early to late stage was also associated with coordinated up-regulation of hexose transporter, PM H+-ATPase, and Grf1 genes (Fig. 3). Such changes were consistent with potential roles of these genes in the transport of extracellular sugars from the nutrient rich locular fluid to the developing pollen. As seen here in maize, an Arabidopsis hexose transporter, AtSUT2, was also described in freshly released microspores from tetrads soon after tapetal degeneration (Truenit et al., 1999). Hexose transporters are anchored in the PM and as symporters, function in the uptake of hexose, often released from Suc hydrolysis by cell wall invertase in various sink tissues (for review, see Lalonde et al., 1999). Correlated temporal changes in the expression of PM H+-ATPase gene here suggest that sugar transport from the locular fluid to the microspores is an energy-dependent process. Reduced translocation of sugars to sink tissues and impaired male fertility in tobacco (Nicotiana plumbaginifolia) plants upon cosuppression of PM H+-ATPase gene are in agreement with such a role (Zhao et al., 2000). Significantly, phosphorylation-dependent activation of PM H+-ATPase leading to greater influx of nutrients is dependent on its interaction with 14-3-3 proteins (Jahn et al., 1997). Increases in the levels of 14-3-3 RNA and proteins are also consistent with the proposed function; however, given that 14-3-3 proteins regulate a wide range of enzyme activities and metabolism (for review, see Chung et al., 1999; Finnie et al., 1999), it must also influence several other reactions, as discussed below.
The 14-3-3 proteins were also detected inside starch granules and mitochondria from immature pollen (Fig. 4, B and C), as first described in starch granules of Arabidopsis leaves and maize endosperm (Sehnke et al., 2001) and barley leaf mitochondria (Bunney et al., 2001). Metabolic significance of the localization of 14-3-3 proteins inside starch granules is not clear, except that it might be critical in reversible phosphorylation reactions of various amyloplastic target proteins (see also Sehnke et al., 2001). Greatly reduced levels of 14-3-3 (Grf1) RNA and proteins in sterile tissues relative to fertile are probably because of the lack of starch granules in male-sterile immature pollen (a similar control is also evident in the lack of Wx-encoded Gbss1 RNA in the starch-deficient male-sterile genotype; Fig. 2). Mitochondrial 14-3-3 proteins were detected in only our “late” samples from fertile plants; metabolic significance of this temporal change is unclear. Bunney et al. (2001) show copurification of 14-3-3 proteins with ATP synthase complex and suggest their role in the regulation of ATP synthase. Obviously, the lack of 14-3-3 proteins in mitochondria of immature pollen of sterile plants would have serious implications in energy metabolism, including the changes in mitochondrial gene expression described by Wen and Chase (1999a, 1999b).
The most unexpected observation among the genes tested here is the temporal changes in the expression of the MADS-box gene, ZmMADS1, first described by Heuer et al. (2000). MADS box genes are usually associated with meristem and organ identity and developmental functions. However, this phase also coincides with PM-I, an asymmetric cell division that yields a larger vegetative cell with a strong metabolic sink and a small generative cell (Mascarenhas, 1989). It is possible that up-regulation of ZmMADS1 gene is associated with cellular fate determination or with metabolic switching as shown recently with the Rin (Ripening-Inhibitor) locus that encodes MADS box protein, LeMADS-RIN, which controls fruit ripening in tomato (Lycopersicon esculentum; Vrebalov et al., 2002).
Sugar Profiles, Metabolic Sensing, and Gene Expression
Results from sugar analyses (Fig. 5) showed only the hexoses, Glc and Fru, and no detectable Suc in any of our samples. In tobacco, Goetz et al. (2001) have shown a critical role of cell wall invertase in tapetal cells before their degeneration. Thus, it is possible that, as in tobacco, hexose sugars are the main source of carbon that is transported into the developing maize pollen. Further, greatly reduced levels of hexose sugars in starch-deficient male-sterile relative to the -fertile samples suggests that starch deficiency may result from impaired sugar uptake/transport to the microspores. Alternatively, reduced resource utilization may regulate the capacity for lesser sugar uptake in the male-sterile line. Consistent with this possibility are the data that show reductions not only in the microspores where starch synthesis is not yet initiated, but also at the dyad stage during meiosis, nearly 5 to 7 d before our “early” stage (Fig. 5C). To the best of our knowledge, this is the earliest (most upstream) detectable change between male-sterile and -fertile genotypes during male gametophyte development. There was also a major disparity in sugar utilization, especially in the net levels of starch accumulation in these genotypes. For example, although male-sterile samples at the “early” stage show only approximately 50% less hexoses than the F1-restored fertile hybrid (Fig. 5), starch levels were far more reduced in immature pollen of sterile than fertile plants (net levels of 2.2 and 36.7 μg starch mg tissue−1, respectively). Clearly, much reduced flux of sugars in starch accumulation was evident in male-sterile than -fertile samples. Much recent data from various plant species indicate that sugars, in particular their metabolism rather than the actual levels, can act as signaling molecules in the control of gene expression (for review, see Sheen et al., 1999; Smeekens, 2000). These changes in gene expression in fertile and sterile samples are in agreement with metabolic sensing in the regulation of genes described here. How such sensing may occur and regulate gene expression, especially in symplastically isolated free microspores and immature pollen, is unknown.
Regulation by the Rf3 Gene
Large changes in gene expression in male-fertile genotypes, S, Rf3rf3 and N, rf3rf3, as compared with the male-sterile genotype, S, rf3rf3, suggest that the Rf3 gene action is epistatic to not only the genes shown in Figures 2 through 4, but also to the maternal genes that differentiate the S from the N cytoplasm. How the Rf3 gene acting upstream regulates such diverse functions is not known, and is a major challenge for further studies. Mackenzie and McIntosh (1999, and refs. therein) have provided an extensive survey and a general discussion on nuclear-cytoplasmic interactions in various plant systems. Among several possibilities, they suggest an important role for redox passage and metabolic exchange during interorgannellar communication within the cell. Oswald et al. (2001) have shown very recently that a blockage in plastidial photosynthetic electron flux (redox state) prevents increase in transcription levels of several nuclear-encoded photosynthetic genes. In our study here, alterations in expressions of PM H+-ATPase, Glc transporter, and Grf1 genes (14-3-3 proteins) that influence C metabolism and mitochondrial functions would significantly alter overall cellular redox states in sterile relative to fertile immature pollen. Most importantly, the changes in the levels of Grf1 expressions in male-sterile relative to -fertile lines are of significant importance because 14-3-3 proteins, well-known metabolic regulators, are present in the nucleus (Bihn et al., 1997), the chloroplast/amyloplast (Sehnke et al., 2000), and the mitochondria where they are observed to be associated with ATP synthases in a phosphorylation-dependent manner (Bunney et al., 2001). Thus, it is logical to suggest that the 14-3-3 proteins, by their presence in all three cellular sites and their ability to regulate intracellular localization of transcription factors (for recent review, see Eckerdt, 2001), may have a direct role in signaling or in coordination of various functions discussed above, including pollen fertility/sterility.
MATERIALS AND METHODS
Plant Material
Three genotypes, one each of male-fertile (N, rf3rf3) S-CMS (S, rf3rf3), and male-fertile F1-restored hybrid (S, Rf3rf3) in a lineage-related background of the Mo-17 inbred line (Wen and Chase, 1999a) used in this study were grown in a greenhouse at approximately 27°C.
Microspore and Immature Pollen RNA Preparation
The “early” samples were collected from tassels harvested before their emergence from the flag leaf and may represent a mixture of unicellular microspores and bicellular immature pollen before any detectable starch accumulation. Immature pollen, or “late,” samples were collected 6 to 7 d later from fully emerged tassels, predominantly of starch-filling immature pollen in fertile lines, approximately 48 h before anthesis. In the sterile line, the immature pollen do not deposit starch but remain metabolically active at the late vacuolated stage, with few collapsed pollen. Samples were also collected 5 to 7 d before the “early” stage, representing the dyad stage, for sugar analysis. Microspore and immature pollen samples were isolated according to Bedinger and Edgerton (1990) and with modifications as described (Wen and Chase, 1999a). Before their use in RNA and protein extractions, freshly harvested samples were tested cytologically for starch by staining with 1% (w/v) I2-KI and with a vital stain, FDA, for metabolic viability (Widholm, 1972). Microspores and immature pollen samples were routinely examined immediately using an Optiphot-2 fluorescent microscope with a fluorescein isothiocyanate filter to detect fluorescein (ex 450 nm, em 520 nm, Nikon, Tokyo). Fluorescein-positive cells were considered to be metabolically active. In addition to FDA, we also used another vital stain, Evans blue, which selectively stains dead cells. Although the Evans blue data are not shown, results from both of these tests for cell viability were always in good agreement. The isolated microspore and pollen of desired stages were frozen in liquid nitrogen and stored at −80°C until use for RNA, protein, and sugar analyses.
RNA-Blot Analyses
RNA from microspores and pollen was isolated as previously described by Wen and Chase (1999a). In brief, collected microspores/pollen samples were concentrated by centrifugation and ground in a small mortar with Trizol reagent (Life Technologies, Gaithersburg, MD) followed by two chloroform extractions. RNA was precipitated using isopropanol. Total RNA samples (20 μg), as quantified by GeneQuant II (Pharmacia Biotech, Kalamazoo, MI), were glyoxylated and loaded in 1.2% (w/v) agarose gels (Ausubel et al., 1993). Uniform loading was confirmed by ethidium bromide staining of the gels. The gels were blotted onto Nytran membranes (Schleicher & Schull, Keene, NH) for 3 to 4 h. Before hybridization, the blot was UV cross-linked and prehybridized in 50 mm PIPES buffer (pH 6.5) containing 100 mm NaCl, 50 mm sodium phosphate (pH 6.5), 1 mm EDTA, and 5% (w/v) ultrapure SDS at 65°C for 1 h. All probes were prepared from maize (Zea mays) cDNA clones as listed in Table I. All RT-PCR-generated cDNA clones were sequenced by automatic sequencing for authenticity before their use (Applied Biosystems, Foster City, CA) by the University of Florida DNA Sequencing Core facility.
Probes were labeled using the BRL (Life Technologies) Random Primers DNA labeling kit. Hybridizations were done using the prehybridization buffer with 3 × 106 counts mL−1 of 32P-labeled probe at 65°C overnight in a shaking water bath. The hybridized blots were rinsed twice in low-stringency washes consisting of 6× SSC solution (1× SSC is 0.15 m NaCl and 0.015 m sodium citrate) supplemented with 5 mm sodium phosphate (pH 6.5), 5 mm EDTA, and 5% (w/v) SDS for 20 min each. This was followed by two high-stringency washes in 0.2× SSC, 5 mm EDTA (pH 8), 5 mm sodium phosphate (pH 6.5), and 1% (w/v) SDS. For reduced stringency washes that were limited to the Bt2 probe (Fig. 2), the high-stringency washes were done in 1× SSC instead of 0.2× SSC, and the temperature was reduced to 55°C. The blots were exposed to x-ray film in between two intensifying screens for 1 to 3 d depending on the transcript abundance. For repeated hybridizations, the blots were stripped by using 50% (w/v) formamide and 6× SSPE (1× SSPE is 150 mm NaCl, 10 mm sodium phosphate [pH 7.4], and 1 mm EDTA) at 65°C for 30 min, followed by washing for 10 min in 2× SSPE at 65°C. All RNA-blot experiments were repeated three times to ensure consistent data.
Isolation of Starch Granules
Starch granules from the “late” stage of pollen development were isolated as described by Echt and Schwartz (1981). The starch granules were vacuum dried and extracted in SDS-PAGE sample buffer (20 μL buffer mg dry weight−1) consisting of 62.5 mm Tris-HCl (pH 6.8), 10% (w/v) glycerol, 2% (w/v) SDS, 5% (w/v) 2-mercaptoethanol, 10 mm EDTA, and 10 mm EGTA with protease inhibitors (20 μg mL−1 leupeptin, 10 μg mL−1 aproteinin, 20 μg mL−1 pepstatin, and 1 mm phenylmethylsulfonyl fluoride) by heating in a boiling water bath for 10 min. After cooling to room temperature, the slurry was centrifuged and the supernatant was subjected to SDS-PAGE and immunoblotting. Thermolysin digestion of starch granules was done according to Mu-Forster et al. (1996). Protein extraction from the starch granules was done as described above.
Isolation of Mitochondria
Freshly isolated “early” and “late” stage pollen was used for isolation of mitochondria according to Chase and Pring (1986). Protease protection assay was done according to Bunney et al. (2001). The mitochondrial samples were boiled in SDS-PAGE sample buffer and used for SDS-PAGE and immunoblotting as described below.
SDS-PAGE and Immunoblot Analyses
Frozen aliquots of “early” and “late” samples of microspores and pollen were ground in liquid nitrogen and then extracted in SDS-PAGE sample buffer. Samples were boiled for 5 min. Protein concentrations were determined using the Bio-Rad (Hercules, CA) protein assay, with bovine serum albumin as a standard. The samples were separated on 7.5% (w/v) or 10% (w/v) SDS-polyacrylamide gels according to Laemmli (1970) using the Bio-Rad Mini Protean II apparatus. The gels were blotted onto nitrocellulose membranes (Schleicher & Schull) using the Bio-Rad Mini Trans-Blot apparatus. Appropriate dilutions of the available primary (Table I) and secondary antibodies were done so as to maximize signal specificities and minimize background staining. The following primary antibody dilutions were made: SS2 (Gupta et al., 1988), 1:6,000 (w/v); BT2 (Bae et al., 1990), 1:4,000 (w/v); 14-3-3 (de Vetten and Ferl, 1994), 1:2,000 (w/v); and TUA (TUA monoclonal antibody, catalog no. N356, Amersham, Piscataway, NJ), 1:2,000 (w/v). Dilutions of secondary antibodies were 1:5,000 (w/v) for anti-mouse (SS2, GRF and TUA) or anti-rabbit (BT2). Antibody detection was done with enhanced chemiluminescent substrate (Pierce Super Signal, Rockford, IL) following the manufacturer's instructions. All protein blots were repeated three times to ensure consistent results.
Sugar Analyses
Soluble sugars were extracted from frozen microspore samples using hot ethanol as described by Kerr et al. (1984) to separate soluble sugars from starch. After centrifugation, the pellet was used for starch analysis. The supernatant was treated with activated charcoal to remove ethanol soluble materials that might have interfered with the assays, and used for Glc Suc, and Fru analyses in microtiter plates (Kerr et al., 1985). Starch analysis was done by amyloglucosidase digestion as described by Rufty and Huber (1983).
ACKNOWLEDGMENTS
Excellent technical assistance from Ms. Melanie Cash in cloning Hxk and Gbss1cDNA fragments and Dr. Richard Wheeler for the sugar assays is gratefully acknowledged. We thank Drs. Robert J. Ferl for GRF clones and 14-3-3 antibodies, L. Curtis Hannah for Sh2 and Bt2 clones and AGPase antibodies and Julia Bailey-Serres for the Pgm clone, Christine D. Chase for seeds of the foundation stocks of Mo-17 inbred lines, and Daryl R. Pring for many helpful discussions. Technical advice from Dr. Daryl R. Pring about the isolation of mitochondria and from Dr. C.D. Chase about the isolation of microspores and immature pollen is greatly appreciated. In addition, we thank Drs. Daryl R. Pring and Earl W. Taliercio for critical reading of the manuscript. We also acknowledge the services of the DNA Sequencing Core laboratory of the Interdisciplinary Center for Biotechnology Research of the University of Florida.
Footnotes
This work was a cooperative investigation of the U.S. Department of Agriculture-Agricultural Research Service and the Institute of Food and Agricultural Science, University of Florida, and was supported in part by the U.S. Department of Agriculture-National Research Initiative Competitive Grants Program (grant no. 98–35301–6135 to P.S.C.) This paper is Florida Agricultural Experiment Journal Series no. R–08668.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.006908.
LITERATURE CITED
- Aoki N, Hirose T, Takahashi S, Ono K, Ishimaru K, Ohsugi R. Molecular cloning and expression analysis of a gene for a sucrose transporter in maize (Zea mays L.) Plant Cell Physiol. 1999;40:1072–1078. doi: 10.1093/oxfordjournals.pcp.a029489. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1993. [Google Scholar]
- Bae JM, Giroux M, Hannah L. Cloning and characterization of the Brittle-2 gene of maize. Maydica. 1990;35:317–322. [Google Scholar]
- Bedinger P. The remarkable biology of pollen. Plant Cell. 1992;4:879–887. doi: 10.1105/tpc.4.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedinger P, Edgerton MD. Developmental staging of maize microspores reveals a transition in developing microspore proteins. Plant Physiol. 1990;92:474–479. doi: 10.1104/pp.92.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley TD, Hempel FD, McCormick S, Zambryski P. Reproductive development. In: Buchanan B, Gruissem W, Jones R, editors. Biochemistry and Molecular Biology of Plants. Rockville, MD: American Society of Plant Physiologists; 2000. pp. 988–1043. [Google Scholar]
- Bhave MR, Lawrence S, Barton C, Hannah LC. Identification and molecular characterization of Shrunken-2 cDNA clones of maize. Plant Cell. 1990;2:581–588. doi: 10.1105/tpc.2.6.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihn EA, Paul AL, Wang SW, Erdos GW, Ferl RJ. Localization of 14-3-3 proteins in the nuclei of Arabidopsis and maize. Plant J. 1997;12:1439–1445. doi: 10.1046/j.1365-313x.1997.12061439.x. [DOI] [PubMed] [Google Scholar]
- Bunney TD, van Walraven HS, de Boer AH. 14-3-3 protein is a regulator of the mitochondrial and chloroplast ATP synthase. Proc Natl Acad Sci USA. 2001;98:4249–4254. doi: 10.1073/pnas.061437498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SJ, Chourey PS. A re-evaluation of the relative roles of two invertases, INCW2 and IVR1 in developing maize kernels and other tissues. Plant Physiol. 1999;121:1025–1035. doi: 10.1104/pp.121.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SJ, Chourey PS, Helentjaris T, Datta R. Gene-expression studies on developing kernels of maize sucrose synthase (SuSy) mutants show evidence for a third SuSy gene. Plant Mol Biol. 2002;49:15–29. doi: 10.1023/a:1014457901992. [DOI] [PubMed] [Google Scholar]
- Chase CD, Pring DR. Properties of the linear N1 and N2 plasmid-like DNAs from mitochondria of cytoplasmic male-sterile Sorghum bicolor. Plant Mol Biol. 1986;6:53–64. doi: 10.1007/BF00021306. [DOI] [PubMed] [Google Scholar]
- Cheng W-H, Chourey PS. Genetic evidence that invertase-mediated release of hexoses is critical for appropriate carbon partitioning and normal seed development in maize. Theor Appl Genet. 1999;98:485–495. [Google Scholar]
- Cheng W-H, Taliercio EW, Chourey PS. The Miniature1 seed locus of maize encodes a cell wall invertase required for normal development of endosperm and maternal cells in the pedicel. Plant Cell. 1996;8:971–983. doi: 10.1105/tpc.8.6.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourey PS, Cheng W-H, Taliercio EW, Im K. Genetic aspects of sucrose-metabolizing enzymes in developing maize seed. In: Madore MA, Lucas WJ, editors. Carbon Partitioning and Source-Sink Interaction in Plants. Current Topics in Plant Physiology. Vol. 13. Rockville, MD: American Society of Plant Physiologists; 1995. pp. 239–245. [Google Scholar]
- Chourey PS, Miller ME. On the role of sucrose synthase in cellulose and callose biosynthesis in plants. In: Pontis HG, Salerno GL, Echeverria E, editors. Current Topics in Plant Physiology. Vol. 14. Rockville, MD: American Society of Plant Physiologists; 1995. pp. 80–87. [Google Scholar]
- Chourey PS, Taliercio EW, Carlson SJ, Ruan YL. Genetic evidence that the two isozymes of sucrose synthase present in developing maize endosperm are critical, one for cell wall integrity and the other for starch biosynthesis. Mol Gen Genet. 1998;259:88–96. doi: 10.1007/s004380050792. [DOI] [PubMed] [Google Scholar]
- Chung H-J, Sehnke PC, Ferl RJ. The 14-3-3 proteins: cellular regulators of plant metabolism. Trends Plant Sci. 1999;4:67–371. doi: 10.1016/s1360-1385(99)01462-4. [DOI] [PubMed] [Google Scholar]
- Datta R, Chourey PS. Sugar-regulated control of α-tubulin in maize cell-suspension culture. Plant Cell Rep. 2001;20:262–266. [Google Scholar]
- Datta R, Chourey PS, Pring DR, Tang HV. Gene-expression analysis of sucrose-starch metabolism during pollen maturation in cytoplasmic male-sterile and fertile lines of sorghum. Sex Plant Reprod. 2001;14:127–134. [Google Scholar]
- de Vetten NC, Ferl RJ. Two genes encoding GF14 (14-3-3) proteins in Zea mays. Plant Physiol. 1994;106:1593–1604. doi: 10.1104/pp.106.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echt CS, Schwartz D. Evidence for the inclusion of controlling elements within the structural gene at the Waxy locus of maize. Genetics. 1981;99:275–284. doi: 10.1093/genetics/99.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerdt NA. Transcription factors dial 14-3-3 for nuclear shuttle. Plant Cell. 2001;13:2385–2389. doi: 10.1105/tpc.13.11.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnie C, Borch J, Collinge DB. 14-3-3 proteins: eukaryotic regulatory proteins with many functions. Plant Mol Biol. 1999;40:545–554. doi: 10.1023/a:1006211014713. [DOI] [PubMed] [Google Scholar]
- Goetz M, Godt DE, Guivarch A, Kahmann U, Chriqui D, Roitsch T. Induction of male-sterility by metabolic engineering of the carbohydrate supply. Proc Natl Acad Sci USA. 2001;98:6522–6527. doi: 10.1073/pnas.091097998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M, Chourey PS, Burr B, Still PE. cDNAs of two non-allelic Sucrose Synthase genes in maize: cloning, expression, characterization and molecular-mapping of Sucrose Synthase2 gene. Plant Mol Biol. 1988;10:215–224. doi: 10.1007/BF00027398. [DOI] [PubMed] [Google Scholar]
- Heuer S, Lorz H, Dresselhaus T. The MADS box gene ZmMADS2 is specifically expressed in maize pollen and during maize pollen tube growth. Sex Plant Reprod. 2000;13:21–27. [Google Scholar]
- Jahn T, Fuglsang AT, Olsson A, Bruntrup IM, Collinge DB, Volkmann D, Sommarin M, Palmgren MG, Larsson C. The 14-3-3 protein interacts directly with the C-terminal region of the plant plasma membrane H+-ATPase. Plant Cell. 1997;9:1805–1814. doi: 10.1105/tpc.9.10.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Bennetzen JL. Integration and non-random mutation of a plasma membrane proton ATPase gene fragment within the BSI retroelement of maize. Plant Cell. 1994;6:1177–1186. doi: 10.1105/tpc.6.8.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr PS, Huber SC, Israel DW. Effect of N-source on soybean leaf sucrose phosphate synthase, starch formation and whole plant growth. Plant Physiol. 1984;75:483–488. doi: 10.1104/pp.75.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr PS, Rufty TW, Huber SC. Changes in non-structural carbohydrates in different parts of soybean (Glycine max [L.] Merr.) plants during a light/dark cycle and in extended darkness. Plant Physiol. 1985;78:576–581. doi: 10.1104/pp.78.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lalonde S, Boles E, Hellmann H, Barker L, Patrick JW, Frommer WB, Ward JM. The dual function of sugar carriers: transport and sugar sensing. Plant Cell. 1999;11:707–726. doi: 10.1105/tpc.11.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughnan JR, Gabay-Laughnan S. Cytoplasmic male-sterility in maize. Annu Rev Genet. 1983;17:27–48. doi: 10.1146/annurev.ge.17.120183.000331. [DOI] [PubMed] [Google Scholar]
- Lee SLJ, Earle ED, Gracen VE. The cytology of pollen abortion in S cytoplasmic male sterile corn anthers. Am J Bot. 1980;67:237–245. [Google Scholar]
- Liu F, Cui X, Horner HT, Weiner H, Schnable PS. Mitochondrial aldehyde dehydrogenase activity is required for male fertility in maize. Plant Cell. 2001;13:1063–1078. doi: 10.1105/tpc.13.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JE, Ashton AR, Hatch MD, Heldt HW. Purification, molecular cloning and sequence analysis of Su-6F-phosphate phosphohydrolase from plants. Proc Natl Acad Sci USA. 2000;97:12914–12919. doi: 10.1073/pnas.230430197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie S, McIntosh L. Higher plant mitochondria. Plant Cell. 1999;11:571–586. doi: 10.1105/tpc.11.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath S, Lee C-HK, Van Winkle P, Bailey-Serres J. Molecular and biochemical characterization of cytosolic phosphoglucomutase in maize. Expression during development and in response to oxygen deprivation. Plant Physiol. 1998;117:997–1006. doi: 10.1104/pp.117.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas JP. The male gametophyte of flowering plants. Plant Cell. 1989;1:657–664. doi: 10.1105/tpc.1.7.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason-Gamer RJ, Weil CF, Kellogg EA. Granule-bound starch synthesis: structure, function and phylogenic utility. Mol Biol Evol. 1998;15:1658–1673. doi: 10.1093/oxfordjournals.molbev.a025893. [DOI] [PubMed] [Google Scholar]
- McCormick S. Male gametophyte development. Plant Cell. 1993;5:1265–1275. doi: 10.1105/tpc.5.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu-Forster C, Huang R, Powers JR, Harriman RW, Knight M, Singletary GW, Keeling PL, Wasserman BP. Physical association of starch biosynthetic enzymes with starch granules of maize endosperm. Plant Physiol. 1996;111:821–829. doi: 10.1104/pp.111.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson O, Pan D. Starch synthesis in maize endosperm. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:475–496. [Google Scholar]
- Nguyen-Quoc B, Foyer CH. A role for futile-cycles involving invertase and sucrose synthase in sucrose metabolism in tomato fruit. J Exp Bot. 2001;52:881–889. doi: 10.1093/jexbot/52.358.881. [DOI] [PubMed] [Google Scholar]
- Oswald O, Martin T, Dominy PJ, Graham IA. Plastid redox state and sugars: interactive regulators of nuclear-encoded photosynthetic gene expression. Proc Natl Acad Sci USA. 2001;98:2047–2052. doi: 10.1073/pnas.021449998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty TW, Huber SC. Changes in starch formation and activities of sucrose phosphate synthase and cytoplasmic fructose-1,6-bisphosphatase in response to source-sink alterations. Plant Physiol. 1983;72:474–480. doi: 10.1104/pp.72.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehnke PC, Chung HG, Wu K, RJ. Regulation of starch accumulation by granule-associated plant 14-3-3 proteins. Proc Natl Acad Sci USA. 2001;98:765–770. doi: 10.1073/pnas.021304198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehnke PC, Henry R, Cline K, Ferl RJ. Interaction of a plant 14-3-3 protein with the signal peptide of a thylakoid-targeted chloroplast precursor protein and the presence of 14-3-3 isoforms in the chloroplast stroma. Plant Physiol. 2000;122:235–241. doi: 10.1104/pp.122.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J, Zhou L, Jang J-C. Sugars as signaling molecules. Curr Opin Plant Biol. 1999;2:410–418. doi: 10.1016/s1369-5266(99)00014-x. [DOI] [PubMed] [Google Scholar]
- Smeekens S. Sugar induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:49–81. doi: 10.1146/annurev.arplant.51.1.49. [DOI] [PubMed] [Google Scholar]
- Taliercio EW, Kim JY, Mahe A, Shanker S, Choi J, Cheng W-H, Prioul J-L, Chourey PS. Isolation, Characterization and expression analyses of two cell wall invertase genes in maize. J Plant Physiol. 1999;155:197–204. [Google Scholar]
- Toyofuku K, Kasahara M, Yamaguchi J. Characterization and expression of monosaccharide transporter (OsMSTs) in rice. Plant Cell Physiol. 2000;41:940–947. doi: 10.1093/pcp/pcd016. [DOI] [PubMed] [Google Scholar]
- Truenit E, Stadler R, Baier K, Sauer N. A male gametophyte-specific monosaccharide transporter in Arabidopsis. Plant J. 1999;17:191–201. doi: 10.1046/j.1365-313x.1999.00372.x. [DOI] [PubMed] [Google Scholar]
- Tsai CY, Salamini F, Nelson OE. Enzymes of carbohydrate metabolism in the developing endosperm of maize. Plant Physiol. 1970;46:299–306. doi: 10.1104/pp.46.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmnabham V, White R, Medrano D, Drake R, Schuch W, Giovannoni J. A MADS-Box gene necessary for fruit ripening at the tomato Ripening-Inhibitor (Rin) locus. Science. 2002;296:343–346. doi: 10.1126/science.1068181. [DOI] [PubMed] [Google Scholar]
- Wen L, Chase CD. Mitochondrial gene expression in developing male gametophytes of male-fertile and S male-sterile maize. Sex Plant Reprod. 1999a;11:323–330. [Google Scholar]
- Wen L, Chase CD. Pleiotropic effects of a nuclear restorer-of-fertility locus on mitochondrial transcripts in male-fertile and S male-sterile maize. Curr Genet. 1999b;35:521–526. doi: 10.1007/s002940050448. [DOI] [PubMed] [Google Scholar]
- Widholm JM. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972;47:189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]
- Winter H, Huber SC. Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Crit Rev Plant Sci. 2000;19:31–67. doi: 10.1080/10409230008984165. [DOI] [PubMed] [Google Scholar]
- Xu J, Avigne WT, McCarty DR, Koch KE. A similar dichotomy of sugar modulation and developmental expression affects both paths of sucrose metabolism: evidence from a maize invertase gene family. Plant Cell. 1996;8:1209–1220. doi: 10.1105/tpc.8.7.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Dielen V, Kinet J-M, Boutry M. Cosuppression of a plasma membrane H+-ATPase isoform impairs sucrose translocation, stomatal opening, plant growth, and male fertility. Plant Cell. 2000;12:535–546. doi: 10.1105/tpc.12.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]