Abstract
Transgenic maize (Zea mays) plants were generated with a construct harboring a maize caffeic acid O-methyltransferase (COMT) cDNA in the antisense (AS) orientation under the control of the maize Adh1 (alcohol dehydrogenase) promoter. Adh1-driven β-glucuronidase expression was localized in vascular tissues and lignifying sclerenchyma, indicating its suitability in transgenic experiments aimed at modifying lignin content and composition. One line of AS plants, COMT-AS, displayed a significant reduction in COMT activity (15%–30% residual activity) and barely detectable amounts of COMT protein as determined by western-blot analysis. In this line, transgenes were shown to be stably integrated in the genome and transmitted to the progeny. Biochemical analysis of COMT-AS showed: (a) a strong decrease in Klason lignin content at the flowering stage, (b) a decrease in syringyl units, (c) a lower p-coumaric acid content, and (d) the occurrence of unusual 5-OH guaiacyl units. These results are reminiscent of some characteristics already observed for the maize bm3 (brown-midrib3) mutant, as well as for COMT down-regulated dicots. However, as compared with bm3, COMT down-regulation in the COMT-AS line is less severe in that it is restricted to sclerenchyma cells. To our knowledge, this is the first time that an AS strategy has been applied to modify lignin biosynthesis in a grass species.
Lignins are complex phenolic polymers present in all vascular plants. They provide rigidity to conducting xylem elements and fiber cells. Lignins are composed of C6C3 units, principally p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) units, and are present in various proportions according to botanical, physiological, and cytological criteria (Lewis and Yamamoto, 1990). Throughout the plant kingdom, grass lignins appear to be particularly specialized because they contain not only H, G, and S units, but also additional p-hydroxycinnamic units such as p-coumaric and ferulic acids (Higuchi et al., 1967). Ferulic acid may be ester linked to wall polysaccharides and/or ether linked to G units, thereby forming bridges between lignins and polysaccharides (Jacquet et al., 1995), whereas p-coumaric acid is primarily ester linked to S lignin units in lignified walls (Ralph et al., 1994; Grabber et al., 1996).
Lignification in dicotyledons has been extensively studied and most of the known lignin biosynthetic genes have been employed in genetic engineering experiments (for review, see Grima-Pettenati and Goffner, 1999). There are almost no molecular data on lignification in grasses (Collazo et al., 1992; McAlister et al., 1998; Pichon et al., 1998; Selman-Housein et al., 1999; Spangenberg et al., 2001). Although lignification in grass species is likely to share a high degree of similarity to other angiosperms, the aforementioned structural specificity of grass cell walls may also involve a certain degree of grass-specific regulatory mechanisms. An in-depth knowledge of lignification in Graminaeae is of utmost importance because lignins are one of the main limiting factors affecting forage digestibility in ruminants (Jung and Deetz, 1993). Although it is clear-cut that lignin content negatively affects the enzymatic degradability of cell wall polysaccharides, the impact of lignin structure on this important parameter is poorly understood. It is difficult to establish a clear relationship between digestibility and wall composition such as S to G ratio or p-coumaric and ferulic acid content.
Four naturally occurring mutant lines of maize (Zea mays), bm1, bm2, bm3, and bm4, exhibit a reduced lignin content, a modified S to G ratio, and increased digestibility (for review, see Barriere and Argillier, 1993). These mutants are called bm (brown-midrib) mutants because they exhibit a characteristic reddish-brown pigmentation of lignified tissues. There has been long-standing incentive to used bm mutations in breeding programs, but bm genotypes are frequently associated with a lower field standability (Cherney et al., 1991). Genes corresponding to both bm1 and bm3 mutations have been identified. The bm1 mutation affected the cinnamyl alcohol dehydrogenase gene (Halpin et al., 1998), whereas bm3 maize is altered in the COMT gene (Vignols et al., 1995; Morrow et al., 1997). In the past, bm mutants have been useful to study lignin biosynthesis and its impact on cell wall digestibility. Nowadays, genetically engineered maize with altered gene expression provides an alternative means to study the impact of lignin content, structure, and distribution on forage digestibility. Until recently, techniques to disrupt gene expression in maize were not routinely available. However, recent progress on maize transformation has allowed us to envisage down-regulation of cell wall genes (Komari et al., 1998; Frame et al., 2002).
With the objectives of using genetic engineering to improve lignin profiles in maize, we generated transgenic maize lines with reduced COMT expression using RNA antisense (AS) technology. This gene has been extensively studied and AS strategies have been successful in different dicot species: tobacco (Nicotiana tabacum; Dwivedi et al., 1994; Atanassova et al., 1995), poplar (Populus tremula × P. alba; Van Doorsselaere et al., 1995; Lapierre et al., 1999; Jouanin et al., 2000), aspen (Populus tremuloides Michx; Tsai et al., 1998), and alfalfa (Medicago sativa; Guo et al., 2001a). In maize, the cDNA encoding caffeate O-methyl-transferase was originally isolated from a root cDNA library (Collazo et al., 1992). The corresponding enzyme appears to be encoded by a single gene. In situ hybridization and COMT promoter analysis revealed that COMT was expressed in the vascular system of roots and leaves of young plantlets (Capellades et al., 1996).
In this study, a transgenic approach was applied to down-regulate COMT gene expression in maize. One line with severely reduced COMT gene expression exhibited dramatically altered lignin content and composition in addition to improved digestibility. Because the biochemical alterations caused by transgenesis were less pronounced than for the bm3 mutant, these results illustrate that genetic engineering is a promising approach to improve maize performance.
RESULTS
Cell Specificity of the Maize Adh1 (Alcohol Dehydrogenase 1) Promoter
Stable expression of the maize Adh1 promoter fused to the β-glucuronidase (GUS) reporter gene has been mainly studied in transgenic rice (Oryza sativa; Kyozuka et al., 1991, 1994). Although Fromm et al. (1990) reported the regeneration of maize plantlets containing the GUS reporter gene under the control of the full-length maize Adh1 promoter, precise expression patterns were not described. To examine if this promoter could be suitable to down-regulate COMT in lignifying tissues of maize, we first generated a series of independent transformants by microparticle bombardment using the pBAR-GUS plasmids described in Figure 1A (Fromm et al., 1990). Four independent R1 progeny exhibited detectable levels of GUS activity in 20- to 30-d-old plants. In all transformants, GUS expression was specifically associated with the vascular system in roots, leaves, and internodes (Fig. 2). In roots, GUS staining was restricted to the protoxylem cells of the stele (Fig. 2C). Cross sections of the basal part of the stalk enabled us to examine GUS expression in nodes, internodes, and rolled leaves. In the nodal region, GUS staining was intense in vascular bundles, mainly in cells surrounding the protoxylem and protophloem (Fig. 2, A and B). In the first leaves surrounding the internodes, vascular strands were at the early stage of differentiation and GUS activity was detected in differentiating sclerenchyma fibers and cells surrounding the protoxylem (Fig. 2D). In mature leaves, GUS activity was observed in both small and large vascular strands (Fig. 2, E–G). However, in the latter case, staining was restricted to phloem and companion cells. The vascular tissue-specific expression of the maize Adh1 promoter indicated that it was suitable to drive COMT transgene expression in lignifying tissues.
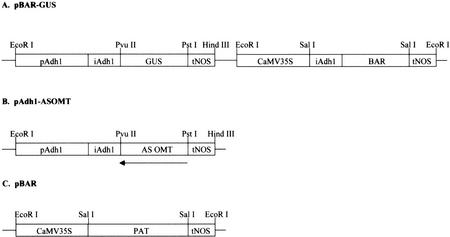
Figure 1.
pBAR-GUS (A), pAdh1-ASOMT (B), and pBAR (C) constructs used for maize transformation. pAdh1, Promoter of maize Adh1 gene; iAdh1, first intron of Adh1 maize gene; CaMV35S, cauliflower mosaic virus (CaMV) 35S RNA promoter; PAT or BAR, coding sequence of phosphinotricin acetyltransferase gene conferring resistance to phosphinotricin (bialaphos [Basta]); Tnos, terminator of the nopaline synthase gene; AS OMT, partial sequence (850 bp) of the maize COMT in AS orientation; GUS, coding sequence of uidA gene.
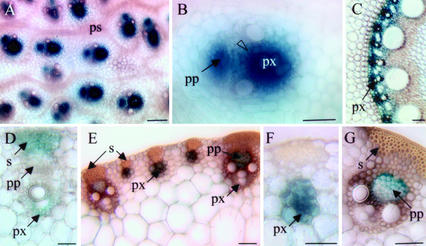
Figure 2.
GUS expression in different organs of 30-d-old transgenic maize transformed with the Adh1 promoter construct (pBAR-GUS; Fig. 1A). A and B, Cross section of the nodal region of maize stem. GUS staining is restricted to vascular bundles (A) and more precisely in cells surrounding the protoxylem (B, open arrowhead). C, Root cross sections. GUS activity is mainly located in protoxylem cells. D through G, Cross sections of rolled leaves around the internodes. In proximal leaves (youngest), GUS activity is located in differentiating sclerenchyma cells and in cells surrounding the protoxylem (D). In distal (older) leaves, small and large vascular strands exhibit GUS activity (E). Higher magnifications indicate GUS activity in protoxylem cells of small vascular strands (F) and in phloem and companion cells of large vascular strands (G). px, Protoxylem; s, sclerenchyma; pp, protophloem; ps, procambial strands. Magnification bar = 50 μm (A–C) and 100 μm (D–G).
Generation and Characterization of a Significantly Down-Regulated COMT Line in Maize
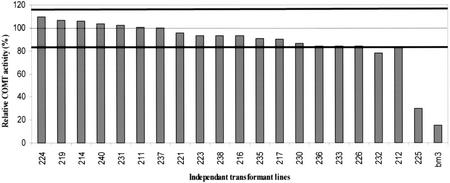
An 850-bp fragment of the maize COMT cDNA (Collazo et al., 1992) was placed under the control of the maize Adh1 promoter (Fig. 1B). This construct was co-introduced into maize by particle acceleration with a plasmid containing the pat gene (Fig. 1C). Twenty independent R2 transformants were obtained and screened for COMT activity using caffeic acid as substrate (Fig. 3). COMT activity was measured in young, rolled leaves and piled-up internodes of 20-d-old transformants (4–5-leaf stage) and compared with the mean activity calculated for a population of 12 untransformed plants. Among the 20 transformants, one COMT-AS line (225) exhibited 30% residual activity. COMT down-regulation was even more pronounced in internodes at the flowering stage (100 d after sowing) with a residual activity of only 15% (data not shown). This reduction was genetically stable because it was also observed in lines resulting from an independent backcross of the R1 progeny. The bm3 mutant exhibited even lower residual COMT activity than in the COMT-AS line grown under the same conditions (Fig. 3). No overall differences in growth and development (growth rate, height at flowering, and internode length) of the COMT-AS line were observed relative to the control.
Figure 3.
COMT activity in COMT AS transformants. Enzyme activity using caffeic acid as substrate was measured in 20-d-old R2 transformants and the bm3 mutant, and expressed as a percentage of the mean value of the control population (dashed horizontal line). The two solid lines indicated the sd of the control population. COMT activity values of each transgenic line (histogram) represents the mean of three plants and two activity measurements per plant.
Because it has been shown recently in other species that the preferred substrate of COMT was 5-OH coniferaldehyde and 5-OH coniferyl alcohol (Chen et al., 1999, 2001; Osakabe et al., 1999; Inoue et al., 2000; Li et al., 2000; Parvathi et al., 2001), we tested COMT activity vis-à-vis these substrates in the COMT-AS line. A strong decrease in activity was also observed for both substrates as compared with control plants, indicating that both 5-OH coniferaldehyde and 5-OH coniferyl alcohol are also substrates for the down-regulated COMT enzyme in maize (data not shown).
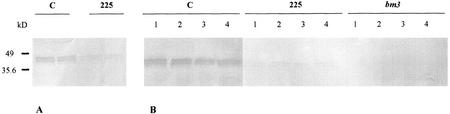
To correlate COMT protein content with results obtained for enzyme activity, western blots using maize anti-COMT antibodies were performed on rolled leaf tissue of 20-d-old plants (Fig. 4A) and internodes at the flowering stage (Fig. 4B). The COMT protein content in the COMT-AS line was significantly lower at both stages of development as compared with controls. In the bm3 mutant, COMT protein is undetectable. Thus, the reduction in COMT protein content at both developmental stages is in good agreement with enzyme activity data.
Figure 4.
Determination of COMT protein content in the COMT-AS line, bm3 mutant, and control line by western-blot analysis. Total protein was extracted from 20-d-old plants (A) and at the flowering stage (B), separated by SDS-PAGE, blotted to nitrocellulose membranes, and probed with polyclonal antisera raised against recombinant maize COMT protein. C, Control line; 225, COMT-AS line, bm3, bm3 mutant; 1 through 4, internode number from the bottom to the top of the plant.
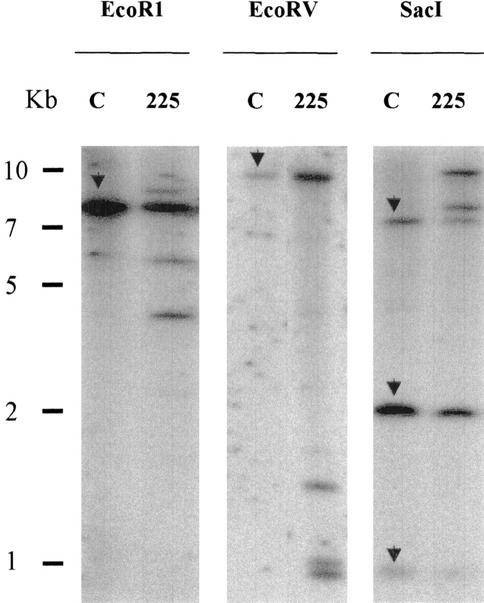
To determine transgene copy number in the COMT-AS line, Southern blots were performed (Fig. 5). Results indicated that two to three copies of the AS COMT construct were integrated into the COMT-AS line. These copies were inserted at one locus as determined by the segregation of Basta resistance. The same transgene integration pattern was observed in progeny from two independent backcrosses, indicating that the transgenes were stably integrated into the plant genome and transmitted to the progeny (data not shown).
Figure 5.
Determination of COMT transgene copy number in COMT-AS line by Southern-blot analysis. Ten micrograms of genomic DNA was cut by EcoRI, EcoRV, or SacI restriction enzymes and transferred to nylon membranes. Blots were probed with full-length COMT sequence. C, Control line; 225, COMT-AS line. The endogenous COMT gene is indicated by arrows in lane C. AS transgenes correspond to additional bands detected in lanes 225.
Down-Regulation of COMT Alters Lignin Profiles and p-Hydroxycinnamic Ester Content in Maize
The consequences of COMT down-regulation on lignification were evaluated using different chemical methods. Lignin content of internodes, leaves, and whole plants was determined by the Klason procedure on R2 progeny of two independent backcrosses of R1 transformants and the bm3 mutant (Table I). Lignin content was 25% to 30% lower in the two AS COMT progenies and the bm3 mutant as compared with controls. Lignin structure was investigated by thioacidolysis, which is an analytical degradation method that proceeds by cleavage of labile β-O-4 bonds (Lapierre et al., 1986; Table II). The total amount and relative frequency of the H, G, S, and 5-OH-G monomers cleaved by thioacidolysis provides an estimate of the amount and composition of these units that are uniquely β-O-4 linked. Both the COMT-AS line and the bm3 mutant exhibited lower thioacidolysis yields (Table II) as compared with controls. Because these data are expressed as a percentage of Klason lignin content, it may be deduced that down-regulating COMT increased the relative amounts of resistant interunit bonds in lignins.
Table I.
Klason lignin of the COMT-AS line, bm3 mutant, and control line at the flowering stage
| Line | I 1 + 2 | I 3 + 4 | I 5 + 6 | Leaves | Whole Plants |
|---|---|---|---|---|---|
| Control | 9.45 | 8.8 | 7.69 | 10.13 | 8.76 |
| Line 225a | 6.78 | 7.13 | 5.88 | 8.06 | 6.23 |
| Line 225b | 6.12 | 6.69 | 6.23 | 8.6 | 6.54 |
| bm3 | 8.27 | 6.72 | 5.68 | 6.66 | nda |
Internodes (I) were annotated 1 to 6 from the bottom to the top and pooled two by two for analysis. 225a and 225b are R2 progeny from two independent backcrosses of R1 transformants. The data are expressed as weight percentage of the extract-free sample. They represent the mean of two independent assays with individual values varying from the mean by <1.5%. The values reported for the whole plants were obtained from a second set of plants.
nd, Not determined.
Table II.
Relative frequency and total yield of lignin-derived monomers recovered from the thioacydolyis of the COMT-AS line, bm3 mutant, and control line at the flowering stage
| Line and Samples | Relative Frequency of Thioacydolysis Monomers
|
Thioacydolysis Yield | |||
|---|---|---|---|---|---|
| H | G | S | 5-OH-G | ||
| mol % | μmol g−1 KLa | ||||
| Control | |||||
| I 1 + 2 | 2 | 40.7 | 56.7 | 0.6 | 790 |
| I 3 + 4 | 1.9 | 48.3 | 49.4 | 0.4 | 690 |
| I 5 + 6 | 2.1 | 53.6 | 43.8 | 0.4 | 610 |
| Leaves | 4 | 60.2 | 35.1 | 0.7 | 320 |
| Whole plant | 3.2 | 56.0 | 40.5 | 0.3 | ndb |
| Line 225a | |||||
| I 1 + 2 | 1.7 | 56.2 | 35.8 | 6.3 | 610 |
| I 3 + 4 | 1.7 | 65.4 | 27.6 | 5.3 | 490 |
| I 5 + 6 | 2 | 70.4 | 25.5 | 2.1 | 520 |
| Leaves | 2.9 | 73.4 | 20.7 | 2.9 | 280 |
| Whole plant | 2.4 | 70.1 | 20.4 | 7.1 | nd |
| Line 225b | |||||
| I 1 + 2 | 1.6 | 58.3 | 33.4 | 6.7 | 600 |
| I 3 + 4 | 1.8 | 67.2 | 25.5 | 5.6 | 450 |
| I 5 + 6 | 1.9 | 70.8 | 22.3 | 5 | 500 |
| Leaves | 2.7 | 72 | 21.8 | 3.6 | 295 |
| Whole plant | 2.5 | 68.7 | 24.4 | 4.4 | nd |
| bm3 | |||||
| I 1 + 2 | 1 | 76.4 | 7.7 | 14.9 | 630 |
| I 3 + 4 | 1.4 | 81.1 | 5.2 | 12.3 | 540 |
| I 5 + 6 | 1.9 | 85.1 | 3.7 | 9.3 | 580 |
| Leaves | 2.3 | 88.7 | 1.5 | 7.5 | 210 |
Internodes (I) were annotated 1 to 6 from the bottom to the top. H, p-Hydroxyphenyl; G, guaiacyl; S, syringyl; 5-OH-G, 5-hydroxyguaiacyl. 225a and 225b are R2 progeny from two independent backcrosses of R1 transformants. Each measurement represents the mean of two assays with individual values varying by <3.5% from the mean. The values reported for the whole plants were obtained from a second set of plant culture.
KL, Klason lignin.
nd, Not determined.
The most striking difference between COMT-AS and control lines is the 2-fold decrease in S units. The reduction of S units in the COMT-AS line is intermediary between the bm3 mutant and controls. Concomitant with a decrease in S units, we also observed a significant increase in 5-OH-G units, which is a well-characterized effect of COMT down-regulation in various mutant and transgenic dicot species. Interestingly, the severe decrease in S units was compensated by an increase in G units. Although lignins in leaves exhibited greater amounts of G units and resistant interunit bonds as indicated by low thioacidolysis yield, the data obtained in leaves were similar to the data for internodes. In agreement with our current knowledge of the temporal variation of the types of lignin subunits, the amount of G units decreased with plant age (internodes 5 + 6 versus 1 + 2). In both lines, H units were detected in trace amounts, but their relative frequency was also slightly reduced as a result of COMT down-regulation.
Because grasses are known to contain sizable amounts of wall-bound p-coumaric and ferulic acids, a mild alkaline hydrolysis was performed to evaluate the impact of COMT down-regulation on these components (Table III). A 30% decrease in esterified p-coumaric acid content was observed in the COMT-AS line. Because p-coumaric acid is primarily esterified to S residues (Grabber et al., 1996), this reduction may be an indirect effect of the reduced amount of S units in the COMT-AS line. On the contrary, the COMT-AS line exhibited an increase in ferulic acid content. Together, the structural features observed in the COMT-AS line are reminiscent of those previously reported for the bm3 mutant, but to a lesser extent.
Table III.
Recovery yields of p-coumaric (PC) and ferulic (FE) acids from the alkaline hydrolysis of the COMT-AS line, bm3 mutant, and control line at the flowering stage
| Line and Samples | PC | FE |
|---|---|---|
| Control | ||
| I 1 + 2 | 12.4 | 7.6 |
| I 2 + 3 | 6.0 | 5.7 |
| Whole plant | 8.0 | 8.6 |
| Line 225a | ||
| I 1 + 2 | 8.8 | 8.1 |
| I 2 + 3 | 4.6 | 6.5 |
| Whole plant | 5.6 | 10.0 |
| Line 225b | ||
| I 1 + 2 | 7.6 | 7.8 |
| I 2 + 3 | 4.7 | 6.7 |
| Whole plant | 5.4 | 10.5 |
| bm3 | ||
| I 1 + 2 | 5.7 | 7.8 |
| I 2 + 3 | 4.2 | 8.6 |
225a and 225b are R2 progeny from two independent backcrosses of R1 transformants. Data are expressed as mg g−1 extract-free internodes or whole plants and represent the mean of two independent experiments with individual values varying by <3% from the mean. The values reported for the entire plants were obtained from a second set of plant culture.
COMT Down-Regulation Causes a brown-midrib Phenotype and Altered Histochemical Staining
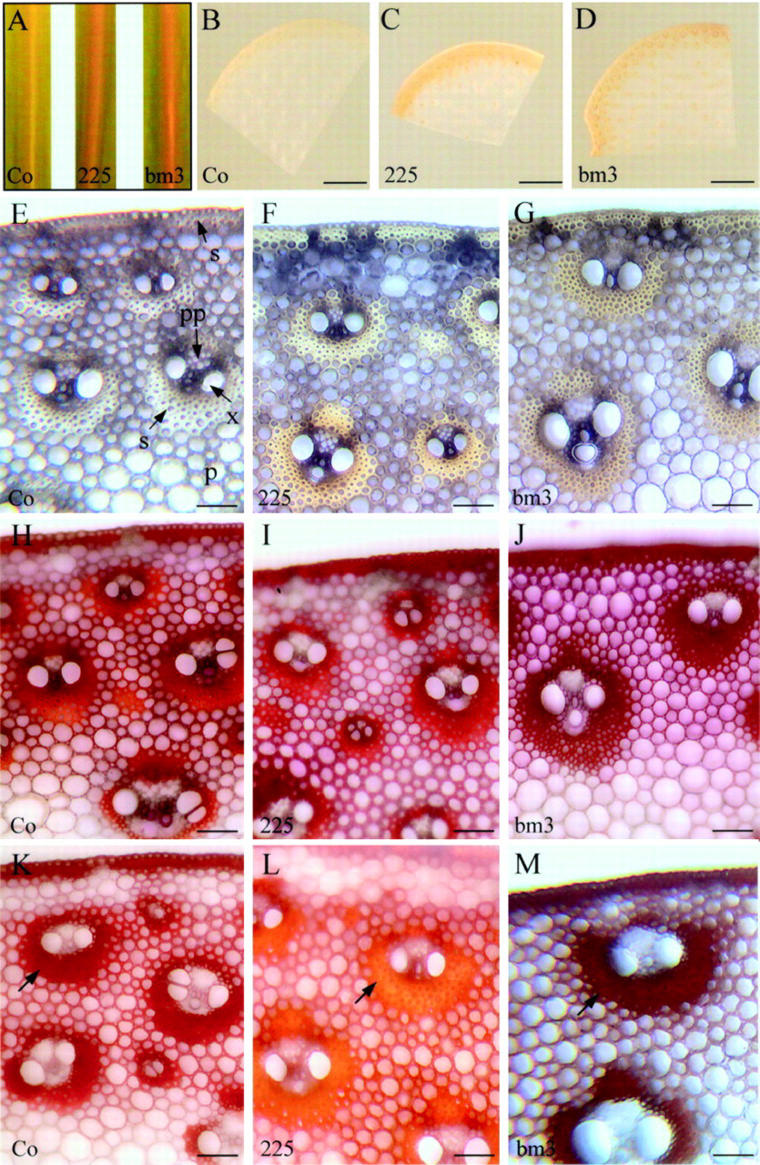
Starting at the 5- to 6-leaf stage, the COMT-AS line displayed a reddish-brown coloration of the leaf midrib, similar to that one observed in the bm3 mutant (Fig. 6A). This coloration was also observed in internodes at the flowering stage (Fig. 6, B–D). A more detailed microscopic analysis indicated that this coloration was mainly associated with sclerenchyma cells adjacent to the epidermis and surrounding the vascular bundles (Fig. 6, E–G). In the COMT-AS line, the coloration varied from yellow to brown depending on the bundle, whereas in the bm3 mutant, the coloration is clearly brown (Fig. 6, F and G, respectively).
Figure 6.
Phenotypical and histochemical characterization of COMT-AS and bm3 lines. A, Brown coloration of the leaf midrib in the COMT-AS line and bm3 mutant compared with the yellowish coloration of the leaf midrib of untransformed control plants at 5- to 6-leaf stage. B through D, Stereomicroscopic observations of transverse sections of internodes. E through M, Light microscopy observations of transverse sections of internodes, in the absence of staining (E–G) or stained with Wiesner (H–J) or Maüle (K–M) reagent. Note the orangey coloration of sclerenchyma in COMT-AS line (arrow) in the presence of reagent (L). Co, Control line; 225, COMT-AS line; bm3, bm3 mutant. Magnification bar = 5 mm (B–D) or 100 μm (E–M).
Internodes were then stained with Wiesner reagent, which specifically reacts with cinnamaldehyde side chains in lignins. No significant differences were observed for either the COMT-AS line or the bm3 as compared with controls (Fig. 6, H–J). The Maüle reagent, which enables the distinction between S and G units, revealed striking differences between the COMT-AS line, bm3, and controls (Fig. 6, K–M). In the control, all lignified tissues including xylem, sclerenchyma, and lignified parenchyma between vascular bundles exhibited a red coloration that is diagnostic of S units. In the COMT-AS line, lignified parenchyma cells stained red, similar to controls. However, the sclerenchyma cells surrounding vascular bundles displayed an overall orangey appearance. This alteration in Maüle staining indicated a sclerenchyma-specific reduction of S units in the COMT-AS line. However, in the bm3, both sclerenchyma and parenchyma between bundles displayed a dark-brown coloration, indicating the absence of S units in both of these cell types.
Despite changes in lignin content and composition, the overall histological organization of xylem elements and sclerenchyma fiber cells was unaltered in the COMT-AS line and bm3 internodes.
Down-Regulation of COMT Is Beneficial for Digestibility in Maize
To evaluate the effect of COMT down-regulation on forage digestibility, the COMT-AS line was further evaluated for neutral detergent fiber (NDF) content, which provides a measure of the total cell wall content and for in vitro NDF digestibility (IVNDFD). The COMT-AS line was more digestible than controls as indicated by its higher IVNDFD value (84% versus 77%, respectively). In this study, the COMT-AS line displayed a similar increase in IVNDFD values to those reported for the bm3 mutant (Mechin et al., 2000).
DISCUSSION
With the aim of modifying lignification via genetic manipulation of lignin biosynthetic enzymes, it is of great interest to target transgenes in a tissue-specific manner. In angiosperms, most of the AS strategies to modify lignin profiles have employed strong, constitutive promoters such as CaMV 35S. In some cases, tissue-specific promoters of lignification genes such as cinnamate 4-hydroxylase (Meyer et al., 1998) and Phe ammonia-lyase (Guo et al., 2001a) also have been used successfully to drive transgene expression. In cereals, much less information concerning promoter activity is available. Transgenic experiments have been mainly performed using maize ubiquitin (Christensen and Quail, 1996), rice actin (McElroy et al., 1991), maize alcohol dehydrogenase 1 (Fromm et al., 1990), and, more recently, maize streak geminivirus (Mazithulela et al., 2000) promoters. In maize, the efficiency of these promoters has only been reported in transient expression assays and their in-depth expression patterns have never been described. For the first time, to our knowledge, we have provided a detailed description of stable Adh1 promoter expression in maize. The fact that the maize Adh1 promoter was highly active in both lignifying vascular tissue and sclerenchyma cells demonstrates its suitability for targeting AS COMT transgene expression to modify lignin synthesis.
That said, among the 20 transformants containing the pAdh1-ASOMT construct, only one line exhibited significantly reduced COMT expression. This may be because of multiple-copy transgene insertion often observed when using biolistic transformation methods (Register et al., 1994). Multiple copies are generally inserted at one locus, increasing the probability of recombination events that subsequently lead to the insertion of inactivated partial constructs. Alternatively, the introduction of homologous promoter sequences (in this case, maize Adh1) could lead to methylation of chromatin in the region where the transgene is inserted, and, thus, cause transgene extinction (Iyer et al., 2000). In the future, it will be important to identify and characterize suitable promoters to drive transgene expression for specific purposes in grass species. The complete sequencing of the rice genome will undoubtedly provide an excellent source of new promoters to complete this gap.
Several studies have been reported on the effects of down-regulation of COMT activity on lignin content and composition in dicotyledonous plants (Dwivedi et al., 1994; Ni et al., 1994; Atanassova et al., 1995; Van Doorsselaere et al., 1995; Tsai et al., 1998; Lapierre et al., 1999; Jouanin et al., 2000; Guo et al., 2001a). In dicots, transgenic plants with 10% to 50% residual COMT activity exhibited similar modifications in lignin structure: a decrease in S unit content and incorporation of 5-OH-G units. A reduction in lignin content has also been observed, but only in a limited number of cases in which COMT activity was close to zero (Jouanin et al., 2000; Guo et al., 2001a). In grass species, transgenic approaches have thus far not been used to down-regulate expression of lignification genes. In this study, an 85% reduction in COMT activity at the flowering stage was sufficient to cause a decrease in lignin content.
As is the case in dicots, the down-regulation of COMT in maize also caused a marked decrease in S unit content. Although the involvement of COMT in S unit synthesis has been known for some time, it is only recently that this enzyme could be accurately situated in the lignin biosynthetic pathway. In elegant biochemical studies of recombinant COMT protein (Humphreys et al., 1999; Li et al., 2000), it was shown that the preferred substrate of COMT in dicot angiosperms is not caffeic acid as expected, but rather 5-hydroxyconiferyl aldehyde and 5-hydroxyconiferyl alcohol, indicating that S unit synthesis occurs at the aldehyde and alcohol level and not at the acid level as previously predicted. Evidence provided here also indicates that COMT from maize is also capable of methylating 5-hydroxyconiferyl aldehyde and 5-hydroxyconiferyl alcohol because reduced activity in the COMT-AS line was also observed with these substrates. These findings further support data recently reported by Chen et al. (2001) showing that there is no significant differences in substrate specificity between O-methyltransferases from tall fescue (Festuca arundinacea), a grass, and alfalfa, a dicot. Together, these data suggest strong similarities between S unit synthesis in grasses and dicots. The production of recombinant COMT from maize should allow us to precisely determine its substrate specificity.
To further examine the changes in lignin content and structure in the COMT-AS line, histochemical analyses were performed. In maize internodes, it is known that despite the obvious anatomical differences between lignified sclerenchyma and parenchyma cells (i.e. wall thickness), there is little difference in their overall phenolic content and composition (Chesson et al., 1997; Joseleau and Ruel, 1997). Here, by using the Adh1 promoter, we directed the down-regulation of COMT specifically in sclerenchyma cells as revealed by Maüle staining. On the contrary, transposon-induced disruption of COMT in the bm3 mutant led to the absence of S units in both cell types. The fact that the reduction of COMT activity was restricted to sclerenchyma, whereas the bm3 mutant was affected in all lignifying tissues, most likely explains why the reduction of S unit content observed in the COMT-AS line was less severe than in the bm3 mutant.
Although the involvement of COMT in methylating S unit precursors of lignins has been clearly established, the methylating enzyme(s) leading to ferulic acid synthesis remains unclear. If ferulic acid were the direct end product of COMT-mediated methylation of caffeic acid, one would have expected that COMT down-regulation would result in a decrease in ferulic acid content. Surprisingly, we have shown here that down-regulation of COMT had little to no effect on ferulic acid content. The ferulic acid content was even slightly higher in the COMT-AS line and the bm3 mutant as compared with the control. Thus, the question remains as to know how ferulic acid is synthesized in maize. Several hypotheses may be suggested. First, a genetically distinct COMT, with no sequence similarity to the COMT studied here, could be responsible for ferulic acid synthesis. This hypothesis is conceivable if multiple COMT genes existed in the maize genome. Several genes encoding COMT have been characterized in alfalfa (Gowri et al., 1991) and tobacco (Pellegrini et al., 1993). In wheat (Triticum aestivum), it has been suggested that an “early” COMT may be involved in the synthesis of ferulic acid for esterification of cell wall arabinoxylans, whereas a “late” COMT may be involved in the synthesis of ferulic and sinapic acids for lignin biosynthesis (Lam et al., 1996). To date, only one gene encoding COMT has been described in maize, but large-scale expressed sequence tag databases are now becoming available (Gai et al., 2000) and will allow for an exhaustive search of COMT genes in maize. Second, it is conceivable that ferulic acid originates from the hydrolysis of feruloyl-CoA via a putative feruloyl esterase (Sancho et al., 1999). One argument in favor of this hypothesis is that tobacco down-regulated for cinnamoyl-CoA reductase, which uses feruloyl-CoA as a substrate, exhibited higher amounts of wall-bound hydroxycinnamic acids (Piquemal et al., 1998). This would imply that caffeoyl-CoA 3-O-methyltransferase, which is an essential enzyme in feruloyl-CoA synthesis, is also involved, albeit indirectly, in ferulic acid synthesis. Third, the fact that the amount of ferulic acid is unchanged in the COMT-AS line may also be a consequence of the lower lignin content, which in turn facilitates ferulic acid release by mild alkaline hydrolysis (Grabber et al., 1998).
Beyond understanding phenylpropanoid metabolism in grasses, transgenesis is a powerful tool to create genetic variability and study the effect of cell wall modifications on forage digestibility. To date, conflicting results have been reported concerning the digestibility of transgenic plants and mutants down-regulated for COMT gene expression. COMT down-regulated tobacco with a decrease in S to G ratio and no change in lignin content exhibited improved digestibility (Bernard-Vailhé et al., 1996; Sewalt et al., 1997). Similarly, the down-regulation of COMT in transgenic alfalfa (Guo et al., 2001b) and a tropical pasture legume, Stylosanthes humilis (Rae et al., 2001) also led to improved digestibility. In contrast, the total absence of S units in the Arabidopsis ferulate 5-hydroxylase mutant did not alter cell wall digestibility (Jung et al., 1999). In maize, a decrease in COMT activity, either by an AS strategy or in the bm3 mutant, led to improved digestibility. It is well established that one of the major mechanisms limiting forage cell wall degradation is the lack of physical access of wall polysaccharides to hydrolytic enzymes because of steric hindrance (Jung and Deetz, 1993). It is likely that the down-regulation of COMT alters the overall cell wall organization via modified lignin-polysaccharide interactions in a way that walls are more readily accessible to bacterial enzymes. Beyond digestibility, other agronomically important traits of the COMT-AS line are currently being evaluated to determine if these plants characterized by intermediary COMT down-regulation may be better adapted than bm3 under field conditions.
MATERIALS AND METHODS
Generation of AS Vectors
The pBAR-GUS (Fig. 1A) plasmid contains the Adh1 promoter region and the first intron fused to the gusA reporter gene. For the pAdh1-ASOMT construct (Fig. 1B), the pMC1 vector containing the maize (Zea mays) COMT cDNA (Collazo et al., 1992) was used to PCR amplify an 850-bp COMT fragment using two synthetic oligonucleotides: 5′-CTGCTGGAGGTGCTGCAGAAG-3′ (plus strand) and 5′CTCCTTGCCCCCGGGGTTGTG-3′ (minus strand) containing PstI and SmaI restriction sites (underlined). The PCR product was subcloned into the pGEMT vector and the clone was excised using PstI-SmaI. The insert was then cloned in the AS orientation upstream of the terminator of the nopaline synthase gene in the PstI-SmaI site in the pNOS vector to generate the pASOMT-NOS vector. A 1.75-kb EcoRI/PvuII fragment spanning the AdhI1 promoter and the first Adh1 intron isolated from the pBAR-GUS vector was cloned upstream from the ASOMT sequence in an EcoRI/SmaI site of the pASOMT-NOS vector resulting in pAdh1-ASOMT (I. Nadaud, unpublished data). The pBAR construct (Fig. 1C) contains a CaMV 35S promoter-pat gene fusion.
Maize Transformation
Transformation experiments were performed either with pBAR-GUS alone for GUS histochemical assays or pAdh1-ASOMT and pBAR together for AS experiments. Maize callus (B73 × A188 derivates) were bombarded with a particle delivery system similar to the one described by Finer et al. (1992). Four hours before bombardment, calli were transferred to modified N6 (Chu, 1978; Amstrong and Green, 1985) medium containing 0.2 m mannitol and 0.2 m sorbitol for osmotic treatment. Supercoiled plasmid DNA were precipitated on tungsten microprojectiles. For plasmid coprecipitation, equal amounts of each plasmid DNA (2.5 μg) were used. The bombarded tissues were incubated on osmotic medium for an additional 16 h at 27°C in the dark and then transferred to selective medium containing 5 mg L−1 Basta. Stable transformants were selected by transferring calli and, subsequently, plantlets to selective medium. Primary transformants were transferred to the greenhouse, tested with Basta in a paint assay, and backcrossed. R2 progeny were selected for single locus integration on Basta. Southern-blot analysis of Basta-resistant transformants confirmed the presence of both the phosphinotricin acetyltransferase and AS COMT portions of the construct (data not shown).
Analysis of gusA Expression
Histological GUS assays were performed according to Jefferson et al. (1987) using a solution containing 1 mm 5-bromo-4-chloro-3-indolyl-β-glucuronic acid and 100 mm sodium phosphate buffer (pH 7.0). Leaves and internodes from 20- and 30-d-old-plants were cut into thin sections with a razor blade. Plant material was fixed in 0.5% (w/v) paraformaldehyde and 100 mm potassium phosphate buffer (pH 7.0) under vacuum and incubated in staining solution for 4 to 12 h at 37°C. After staining, sections were conserved in 70% (v/v) ethanol.
COMT Activity Assays
All transformants and untransformed controls were analyzed for COMT activity using caffeic acid as described by Atanassova et al. (1995). Rolled leaves and young internodes from 20-d-old transformants and controls grown under greenhouse conditions were harvested. Samples were ground in liquid nitrogen and extracted in 100 mm sodium phosphate buffer (pH 7.5) containing 5% (w/v) polyvinylpolypyrrolidone and 10 mm dithiothreitol. After centrifugation, 40 μL of protein extract was added to 1 mL of phosphate sodium buffer containing 3 mm caffeic acid, 10 mm dithiothreitol, 0.6 mm tritiated S-adenosyl-l-Met (13 μCi μmol−1, Amersham, Buckinghamshire, UK), and 50 μm unlabeled S-adenosyl-l-Met and incubated for 1 h at 37°C. COMT assays were also performed using 5-OH coniferylaldehyde (30 μm) or 5-OH coniferyl alcohol (30 μm) as substrates. The reaction was stopped with 100 μL of 9 n H2SO4. Radiolabeled ferulic acid was extracted in 5 mL of scintillation solution (OCS, Amersham), and the radioactivity was counted in a 1900TR scintillation counter (Hewlett-Packard, Palo Alto, CA). The protein content was determined according to Bradford (1976) using the Bio-Rad reagent (Bio-Rad Laboratories, Hercules, CA).
Southern- and Northern-Blot Analysis
For Southern blots, DNA was isolated from young leaves according to Dellaporta et al. (1983). Ten micrograms of genomic DNA was digested with restriction enzymes, run on agarose gel, and transferred to Hybond N+ membranes (Amersham) using the alkaline blotting procedure according to manufacturer's recommendations. For northern blots, RNA was prepared from rolled leaves, roots, and collar with Extract-all (Laboratories Eurobio, Les Ulis, France). Ten micrograms of total RNA was run on agarose gels containing formaldehyde and transferred to Hybond N+ membranes according to standard protocols (Sambrook et al., 1989). All blots were hybridized with radiolabeled COMT cDNA overnight in 3× SSC, 0.5% (w/v) SDS, and 0.1% (w/v) skimmed milk powder at 65°C. Filters were washed consecutively in 3× SSC/0.5% (w/v) SDS, 0.3× SSC/0.5% (w/v) SDS, and 0.1× SSC/0.5% (w/v) SDS at 65°C for 15 min each.
Western-Blot Analysis
Ten micrograms of protein (the same extracts used for COMT activity assays) were run on 10% (w/v) SDS-PAGE gels. Proteins were electroblotted onto nitrocellulose membranes (Amersham). Membranes were blocked in Tris-buffered saline (pH 8), Tween 20 0.1% (v/v) (TTBS) (pH 8) containing 30 mg mL−1 of polyvinylpolypyrrolidone, overnight at 4°C. Membranes were incubated in maize polyclonal anti-COMT serum (dilution 1/1,000 [v/v]) for 1h. After three washes with TTBS (pH 8, 10 min each), membranes were incubated with an alkaline phosphatase-conjugated goat anti-rabbit antibody. Alkaline phosphatase detection was performed with nitroblue tetrazolium and 5-bromo-4-chloro indoxyl phosphate according to the manufacturer's recommendations (Bio-Rad).
Histochemical Staining of Lignins
Leaf and stem sections were hand-cut with a razor blade from 100-d-old plants grown in the greenhouse. Wiesner and Maüle reactions were performed according to standard protocols (Nakano and Meshitsuka, 1992). Sections were observed using an inverted microscope (Leitz DMRIBE, Leica Microsystems, Wetzlar, Germany). Images were registered using a CCD camera (Color Coolview, Photonic Science, Milham, UK) and treated by image analysis (Image PRO-Plus, Media Cybernetics, Silver Spring, MD).
Lignin Analysis
Whole plants at the flowering stage were lyophilized at harvest and ground to a fine powder. Lignin analyses were performed on extract-free cell wall residue. The lignin content was estimated by the Klason procedure (Whitting et al., 1981). The lignin monomeric composition was determined by thioacidolysis followed by gas chromatography-mass spectrometry of lignin-derived monomer trimethylsilyl derivatives (Lapierre et al., 1986). The determination of p-hydroxycinnamic esters linked to lignin was performed by mild alkaline hydrolysis according to Jacquet et al. (1995).
NDF and Digestibility Measurements
Whole plant samples were dried in an oven at 65°C. After drying, samples were ground with a hammer mill and passed through a 1-mm screen. NDF was estimated according to Goering and Van Soest (1970) and the in vitro dry matter digestibility (IVDMD) was estimated according to the enzymatic solubility of Aufrère and Michalet-Doreau (1983). The digestibility IVNDFD was computed assuming that the non-NDF part of plant material was completely digestible according to Struik (1983) and Dolstra and Medema (1990), and the formula used was:
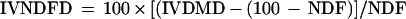 |
ACKNOWLEDGMENTS
The authors are grateful to Fréderic Legée (Laboratoire de Chimie Biologique, Institut National Agronomique, Grignon, France) for performing the Klason lignin analysis. We gratefully acknowledge Dr. Richard Dixon (Plant Biology Division, Samuel Roberts Noble Foundation, Ardmore, OK) for the receipt of the 5-OH-coniferylaldehyde and the 5-OH-coniferyl alcohol substrates. pBARGUS plasmid was kindly provided by Michael Fromm (Plant Sciences, Monsanto Company, St. Louis) and pBAR plasmid was provided by Peter Eckes (Biology Research Center, Hoechst AG, Frankfurt). We also thank Philippe Ranocha (Signaux et Messages Cellulaires chez les Végétaux, Unité Mixte de Recherche, Centre National de la Recherche Scientifique–Université Paul Sabatier 5546, Pôle de Biotechnologie Végétale, Tolosan, France) for critically reading our manuscript.
Footnotes
This work was supported by the Génoplante Program, by the Institut National de la Recherche Agronomique, by the Centre National de la Recherche Scientifique, and by the European program, COPOL (grant no. QLK5–CT–2000–01493).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.012237.
LITERATURE CITED
- Amstrong CL, Green CE. Establishment and maintenance of friable, embryogenic maize callus and the involvement of l-proline. Planta. 1985;164:207–214. doi: 10.1007/BF00396083. [DOI] [PubMed] [Google Scholar]
- Atanassova R, Favet N, Martz F, Chabbert B, Tollier MT, Monties B, Fritig B, Legrand M. Altered lignin composition in transgenic tobacco expressing O-methyltransferase sequences in sense and antisense orientation. Plant J. 1995;8:465–477. [Google Scholar]
- Aufrère J, Michalet-Doreau B. EEC Seminar on Feeding Value of By-Products and Their Use by Beef Cattle, Gontrode. 1983. In vivo digestibility and prediction of digestibility of some by-products; pp. 27–29. [Google Scholar]
- Barriere Y, Argillier O. Brown-midrib genes of maize, a review. Agronomie. 1993;13:865–876. [Google Scholar]
- Bernard-Vailhé MA, Migné C, Cornu A, Maillot MP, Grenet E, Besle JM, Atanassova R, Mertz F, Legrand M. Effect of modification of the O-methyltransferase activity on cell wall composition, ultrastructure and degradability of transgenic tobacco. J Sci Food Agric. 1996;72:385–391. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Capellades M, Torres MA, Bastisch I, Stiefel V, Vignols F, Bruce WB, Peterson D, Puigdomenech P, Rigau J. The maize caffeic acid O-methyltransferase gene promoter is active in transgenic tobacco and maize plant tissues. Plant Mol Biol. 1996;31:307–322. doi: 10.1007/BF00021792. [DOI] [PubMed] [Google Scholar]
- Chen F, Kota P, Blount JW, Dixon RA. Chemical syntheses of caffeoyl and 5-OH coniferyl aldehydes and alcohols and determination of lignin O-methyltransferase activities in dicot and monocot species. Phytochemistry. 2001;58:1035–1042. doi: 10.1016/s0031-9422(01)00391-0. [DOI] [PubMed] [Google Scholar]
- Chen F, Yasuda S, Fukushima K. Evidence for a novel biosynthetic pathway that regulates the ratio of syringyl to guaiacyl residues in lignin in the differentiating xylem of Magnolia kobus DC. Planta. 1999;207:597–603. [Google Scholar]
- Cherney JH, Cherney DJR, Akin DE, Axtell JD. Potential of brown-midrib, low-lignin mutants for improving forage quality. Adv Agron. 1991;46:157–198. [Google Scholar]
- Chesson A, Provan GJ, Russell W, Scobbie L, Chabbert B, Monties B. Characterisation of lignin from parenchyma and sclerenchyma cell walls of the maize internode. J Sci Food Agric. 1997;73:10–16. [Google Scholar]
- Christensen AH, Quail PH. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res. 1996;5:213–218. doi: 10.1007/BF01969712. [DOI] [PubMed] [Google Scholar]
- Chu CC. Proceedings of Symposium on Plant Tissue Culture, Peking, May 25–30, 1978. Science Press, Peking. 1978. The N6 medium and its applications to anther culture of cereal crops; pp. 43–50. [Google Scholar]
- Collazo P, Montoliu L, Puigdomenech P, Rigau J. Structure and expression of the lignin O-methyltransferase gene from Zea mays L. Plant Mol Biol. 1992;20:857–867. doi: 10.1007/BF00027157. [DOI] [PubMed] [Google Scholar]
- Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: version II. Plant Mol Biol Rep. 1983;1:19–21. [Google Scholar]
- Dolstra O, Medema JM. An effective screening method for improvement of cell-wall digestibility in forage maize. In: Hinterholzer J, editor. Proceedings of the 15th EUCARPIA Congress Maize-Sorghum, Austria: Baden; 1990. pp. 258–270. [Google Scholar]
- Dwivedi UN, Campbell WH, Yu J, Datla RSS, Bugos RC, Chiang VL, Podila GK. Modification of lignin biosynthesis in transgenic Nicotiana through expression of an antisense O-methyltransferase gene from Populus. Plant Mol Biol. 1994;26:61–71. doi: 10.1007/BF00039520. [DOI] [PubMed] [Google Scholar]
- Finer J, Vain P, Jones M, Mc Mullen M. Development of the particle inflow gun for DNA delivery to plant cells. Plant Cell Rep. 1992;11:323–328. doi: 10.1007/BF00233358. [DOI] [PubMed] [Google Scholar]
- Frame BR, Shou H, Chikwamba RK, Zhang Z, Xiang C, Fonger TM, Pegg SE, Li B, Nettleton DS, Pei D et al. Agrobacterium tumefaciens-mediated transformation of maize embryos using a standard binary vector system. Plant Physiol. 2002;129:13–22. doi: 10.1104/pp.000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M, Morrish F, Armstrong C, Willians R, Thomas J, Klein T. Inheritance and expression of chimeric genes in the progeny of transgenic maize plants. Biotechnology. 1990;8:833–839. doi: 10.1038/nbt0990-833. [DOI] [PubMed] [Google Scholar]
- Gai X, Lal S, Xing L, Brendel V, Walbot V. Gene discovery using the maize genome database ZmDB. Nucleic Acids Res. 2000;28:94–96. doi: 10.1093/nar/28.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering HK, Van Soest PJ. Agricultural Handbook 379, U.S. Department of Agriculture-Agricultural Research Service. U.S. Washington, DC: Government Printing Office; 1970. Forage fiber analysis (apparatus, reagents, procedures, and some applications) pp. 19–20. [Google Scholar]
- Gowri G, Bugos RC, Campbell WH, Maxwell CA, Dixon RA. Stress responses in alfalfa (Medicago sativa L.): X. Molecular cloning and expression of S-adenosyl-l-methionine:caffeic acid 3-O-methyltransferase, a key enzyme of lignin biosynthesis. Plant Physiol. 1991;97:7–14. doi: 10.1104/pp.97.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabber JH, Quideau S, Ralph J. p-Coumaroylated syringyl units in maize lignin: implications for β-ether cleavage by thioacidolysis. Phytochemistry. 1996;43:1189–1194. [Google Scholar]
- Grabber JH, Ralph J, Hatfield RD. Ferulate cross-links limit the enzymatic degradation of synthetically lignified primary walls of maize. J Agric Food Chem. 1998;46:2609–2614. [Google Scholar]
- Grima-Pettenati J, Goffner D. Lignin genetic engineering revisited. Plant Sci. 1999;145:51–65. [Google Scholar]
- Guo D, Chen F, Inoue K, Blount J, Dixon R. Downregulation of caffeic acid 3-O-methyltransferase and caffeoyl CoA 3-O-methyltransferase in transgenic alfalfa. Impacts on lignin structure and implications for the biosynthesis of G and S lignin. Plant Cell. 2001a;13:73–88. doi: 10.1105/tpc.13.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Chen F, Wheeler J, Winder J, Selman S, Peterson M, Dixon R. Improvement of in-rumen digestibility of alfalfa forage by genetic manipulation of lignin O-methyltransferases. Transgenic Res. 2001b;10:457–464. doi: 10.1023/a:1012278106147. [DOI] [PubMed] [Google Scholar]
- Halpin C, Holt K, Chojecki J, Oliver D, Chabbert B, Monties B, Edwards K, Barakate A, Foxon GA. Brown-midrib maize (bm1): a mutation affecting the cinnamyl alcohol dehydrogenase gene. Plant J. 1998;14:545–553. doi: 10.1046/j.1365-313x.1998.00153.x. [DOI] [PubMed] [Google Scholar]
- Higuchi T, Ito Y, Shimada M, Kawamura I. Chemical properties of milled wood lignin of grasses. Phytochemistry. 1967;6:1551–1556. [Google Scholar]
- Humphreys JM, Hemm MR, Chapple C. New routes for lignin biosynthesis defined by biochemical characterization of recombinant ferulate 5-hydroxylase, a multifunctional cytochrome P450-dependent monooxygenase. Proc Natl Acad Sci USA. 1999;96:10045–10050. doi: 10.1073/pnas.96.18.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Parvathi K, Dixon R. Substrate preferences of caffeic acid/5-hydroxyferulic acid 3/5-O-methyltransferase in developing stems of alfalfa (Medicago sativa L.) Arch Biochem Biophys. 2000;375:175–182. doi: 10.1006/abbi.1999.1674. [DOI] [PubMed] [Google Scholar]
- Iyer LM, Kumpatla SP, Chandrasekharan MB, Hall TC. Transgene silencing in monocots. Plant Mol Biol. 2000;43:323–346. doi: 10.1023/a:1006412318311. [DOI] [PubMed] [Google Scholar]
- Jacquet G, Pollet B, Lapierre C. New ether-linked ferulic acid-coniferyl alcohol dimers identified in grass straws. J Agric Food Chem. 1995;43:2746–2751. [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseleau JP, Ruel K. Study of lignification by noninvasive techniques in growing maize internodes. An investigation by Fourier transform infrared cross-polarization-magic angle spinning 13C-nuclear magnetic resonance spectroscopy and immunocytochemical transmission electron microscopy. Plant Physiol. 1997;114:1123–1133. doi: 10.1104/pp.114.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanin L, Goujon T, De Nadaï V, Martin MT, Mila I, Vallet C, Pollet B, Yoshinaga A, Chabbert B, Petit-Conil M et al. Lignification in transgenic poplars with extremely reduced caffeic acid O-methyltransferase activity. Plant Physiol. 2000;123:1363–1373. doi: 10.1104/pp.123.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJG, Deetz DA. Cell wall lignification and degradability. In: Jung HG, Buxton DR, Hatfield RD, Ralph J, editors. Forage Cell Wall Structure and Digestibility. WI: Madison; 1993. pp. 315–346. [Google Scholar]
- Jung HJG, Ni WT, Chapple C, Meyer K. Impact of lignin composition on cell-wall degradability in an Arabidopsis mutant. J Sci Food Agric. 1999;79:922–928. [Google Scholar]
- Komari T, Hiei Y, Ishida Y, Kumashiro T, Kubo T. Advances in cereal gene transfer. Curr Opin Plant Biol. 1998;1:161–165. doi: 10.1016/s1369-5266(98)80019-8. [DOI] [PubMed] [Google Scholar]
- Kyozuka J, Fujimoto H, Izawa T, Shimamoto K. Anaerobic induction and tissue-specific expression of maize Adh1 promoter in transgenic rice plants and their progeny. Mol Gen Genet. 1991;228:40–48. doi: 10.1007/BF00282445. [DOI] [PubMed] [Google Scholar]
- Kyozuka J, Olive M, Peacock WJ, Dennis ES, Shimamoto K. Promoter elements required for developmental expression of the maize Adh1 gene in transgenic rice. Plant Cell. 1994;6:799–810. doi: 10.1105/tpc.6.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TBT, Iiyama K, Stone BA. Caffeic acid: O-methyltransferases and the biosynthesis of ferulic acid in primary cell walls of wheat seedlings. Phytochemistry. 1996;41:1507–1510. [Google Scholar]
- Lapierre C, Rolando C, Monties B. Thioacidolysis of poplar lignins: identification of monomeric syringyl products and characterisation of guaiacyl syringyl fractions. Holzforschung. 1986;40:113–118. [Google Scholar]
- Lapierre C, Pollet B, Petit Conil M, Toval G, Romero J, Pilate G, Leple JC, Boerjan W, Ferret V, De Nadai V et al. Structural alterations of lignins in transgenic poplars with depressed cinnamyl alcohol dehydrogenase or caffeic acid O-methyltransferase activity have an opposite impact on the efficiency of industrial kraft pulping. Plant Physiol. 1999;119:153–163. doi: 10.1104/pp.119.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis NG, Yamamoto E. Lignin: occurrence, biogenesis and biodegradation. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:455–496. doi: 10.1146/annurev.pp.41.060190.002323. [DOI] [PubMed] [Google Scholar]
- Li L, Popko JL, Umezawa T, Chiang VL. 5-hydroxyconiferyl aldehyde modulates enzymatic methylation for syringyl monolignol formation, a new view of monolignol biosynthesis in angiosperms. J Biol Chem. 2000;275:6537–6545. doi: 10.1074/jbc.275.9.6537. [DOI] [PubMed] [Google Scholar]
- Mazithulela G, Sudhakar D, Heckel T, Mehlo L, Christou P, Davies JW, Boulton MI. The maize streak virus coat protein transcription unit exhibits tissue-specific expression in transgenic rice. Plant Sci. 2000;155:21–29. doi: 10.1016/s0168-9452(99)00256-3. [DOI] [PubMed] [Google Scholar]
- McAlister FM, Jenkins CLD, Watson JM. Sequence and expression of a stem-abundant caffeic acid O-methyltransferase cDNA from perennial ryegrass (Lolium perenne) Aust J Plant Physiol. 1998;25:225–235. [Google Scholar]
- McElroy D, Blowers AD, Jenes B, Wu R. Construction of expression vectors based on the rice actin 1 (Act1) 5′ region for use in monocot transformation. Mol Gen Genet. 1991;231:150–160. doi: 10.1007/BF00293832. [DOI] [PubMed] [Google Scholar]
- Mechin V, Argillier O, Menanteau V, Barriere Y, Mila I, Pollet B, Lapierre C. Relationship of cell wall composition to in vitro cell wall digestibility of maize inbred line stems. J Sci Food and Agric. 2000;80:574–580. [Google Scholar]
- Meyer K, Shirley AM, Cusumano JC, Bell-Lelong DA, Chapple C. Lignin monomer composition is determined by the expression of a cytochrome P450-dependent monooxygenase in Arabidopsis. Proc Natl Acad Sci USA. 1998;95:6619–6623. doi: 10.1073/pnas.95.12.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow MP, Mascia P, Self KP, Altschuler M. Molecular characterization of a brown midrib deletion mutation in maize. Mol Breed. 1997;3:351–357. [Google Scholar]
- Nakano J, Meshitsuka G. The detection of lignin. In: Dence CW, Lin SY, editors. Methods in Lignin Chemistry. New York: Springer-Verlag; 1992. pp. 23–61. [Google Scholar]
- Ni W, Paiva NL, Dixon RA. Reduced lignin in transgenic plants containing a caffeic acid O-methyltransferase antisense gene. Transgenic Res. 1994;3:120–126. [Google Scholar]
- Osakabe K, Tsao CC, Li L, Popko JL, Umezawa T, Carraway DT, Smeltzer RH, Joshi CP, Chiang VL. Coniferyl aldehyde 5-hydroxylation and methylation direct syringyl lignin biosynthesis in angiosperms. Proc Natl Acad Sci USA. 1999;96:8955–8960. doi: 10.1073/pnas.96.16.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvathi K, Chen F, Guo D, Blount JW, Dixon RA. Substrate preferences of O-methyltransferases in alfalfa suggest new pathways for 3-O-methylation of monolignols. Plant J. 2001;25:193–202. doi: 10.1046/j.1365-313x.2001.00956.x. [DOI] [PubMed] [Google Scholar]
- Pellegrini L, Geoffroy P, Fritig B, Legrand M. Molecular cloning and expression of a new class of ortho-diphenol-O-methyltransferases induced in tobacco leaves by infection or elicitor treatment. Plant Physiol. 1993;103:509–517. doi: 10.1104/pp.103.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichon M, Courbou I, Beckert M, Boudet AM, Grima-Pettenati J. Cloning and characterization of two maize cDNAs encoding cinnamoyl-CoA reductase (CCR) and differential expression of the corresponding genes. Plant Mol Biol. 1998;38:671–676. doi: 10.1023/a:1006060101866. [DOI] [PubMed] [Google Scholar]
- Piquemal J, Lapierre C, Myton K, Schuch W, Grima-Pettenati J, Boudet AM. Down-regulation of cinnamoyl-CoA reductase induces significant changes of lignin profiles in transgenic tobacco plants. Plant J. 1998;13:71–83. [Google Scholar]
- Rae AL, Manners JM, Jones RJ, McIntyre CL, Lu DY. Antisense suppression of the lignin biosynthetic enzyme, caffeate O-methyltransferase, improves in vitro digestibility of the tropical pasture legume, Stylosanthes humilis. Aust J Plant Physiol. 2001;28:289–297. [Google Scholar]
- Ralph J, Hatfield RD, Quideau S, Helm RF, Grabber JH, Jung HJG. Pathway of p-coumaric acid incorporation into maize lignin as revealed by NMR. J Am Chem Soc. 1994;116:9448–9456. [Google Scholar]
- Register JC, 3rd, Peterson DJ, Bell PJ, Bullock WP, Evans IJ, Frame B, Greenland AJ, Higgs NS, Jepson I, Jiao S et al. Structure and function of selectable and non-selectable transgenes in maize after introduction by particle bombardment. Plant Mol Biol. 1994;25:951–961. doi: 10.1007/BF00014669. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sancho AI, Faulds CB, Bartolome B, Williamson G. Characterization of feruloyl esterase activity in barley. J Sci Food Agric. 1999;79:447–449. [Google Scholar]
- Selman-Housein G, Lopez MA, Hernandez D, Civardi L, Miranda F, Rigau J, Puigdomenech P. Molecular cloning of cDNAs coding for three sugarcane enzymes involved in lignification. Plant Sci. 1999;143:163–171. [Google Scholar]
- Sewalt VJH, Ni W, Jung HJG, Dixon R. Lignin impact on fiber degradation: increased enzymatic digestibility of genetically engineered tobacco (Nicotiana tabacum) stems reduced in lignin content. J Agric Food Chem. 1997;45:1977–1983. [Google Scholar]
- Spangenberg GC, Lidget AJ, Heath RL, McInnes RL, Lynch DP, inventors (2001) Cloning and sequences of enzymes of lignin biosynthesis from perennial ryegrass and their use in modification of lignin biosynthesis in plant. International Publication Number WO/01/95702A1.
- Struik PC. Physiology of forage maize (Zea mays L.) in relation to its productivity. PhD thesis. The Netherlands: Agricultural University of Wageningen; 1983. [Google Scholar]
- Tsai CJ, Popko JL, Mielke MR, Hu WJ, Podila GK, Chiang VL. Suppression of O-methyltransferase gene by homologous sense transgene in quaking aspen causes red-brown wood phenotypes. Plant Physiol. 1998;117:101–112. doi: 10.1104/pp.117.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doorsselaere J, Baucher M, Chognot E, Chabbert B, Tollier MT, Petit-Conil M, Leple JC, Pilate G, Cornu D, Monties B et al. A novel lignin in poplar trees with a reduced caffeic acid 5-hydroxyferulic acid O-methyltransferase activity. Plant J. 1995;8:855–864. [Google Scholar]
- Vignols F, Rigau J, Torres MA, Capellades M, Puigdomenech P. The brown midrib3 (bm3) mutation in maize occurs in the gene encoding caffeic acid O-methyltransferase. Plant Cell. 1995;7:407–416. doi: 10.1105/tpc.7.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitting P, Favis BD, St-Germain F, Goring D. Fractional separation of middle lamella and secondary wall tissue from spruce wood. J Wood Chem Technol. 1981;1:29–42. [Google Scholar]