Abstract
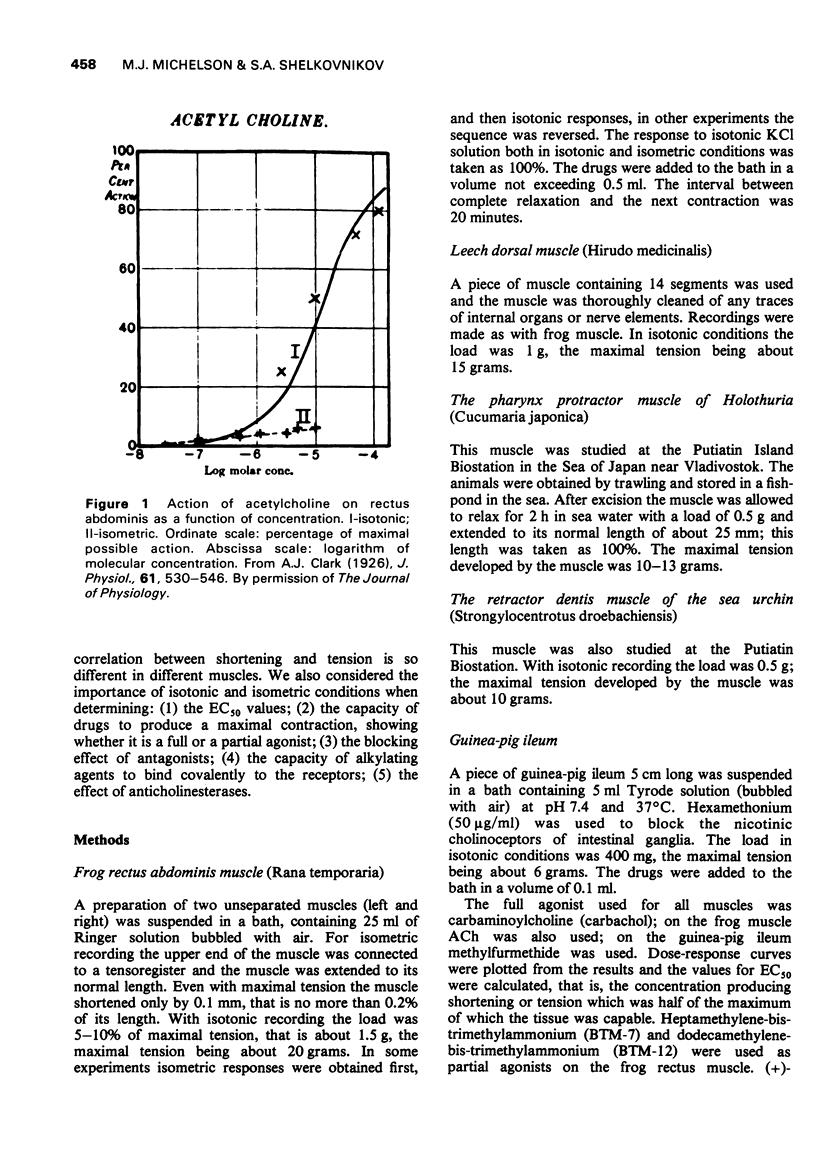
1 With isotonic recording the percentage of muscle shortening as compared with the maximal possible shortening, and with isometric recording the percentage of developed tension were determined. In relatively 'thick' muscles, such as dorsal leech muscle, frog rectus abdominis or protractor pharynx of holothuria (0.3-0.8 mm thick), the concentrations of a full agonist (carbachol) producing a given percentage of tension, (e.g. 50%) are about 5 times greater than the concentrations, producing the same percentage of shortening. In 'thin' muscles the difference between the percentage of shortening and tension is either small (retractor dentis of the sea urchin, 0.1 mm thick, response to carbachol) or absent (guinea-pig ileum, 0.06 mm thick, responses to methylfurmethide). The possible mechanism underlying this difference is discussed. 2 With partial agonists (dodecamethonium and heptamethonium) the fractional tension of the frog rectus abdominis is always less than the fractional shortening and the correlation between shortening and tension is the same as in the case of full agonists. 3 The blocking activity of (+)-tubocurarine on the frog rectus abdominis is the same in isotonic and in isometric conditions. 4 On the frog rectus abdominis the alkylating agent, decamethonium mustard, does not produce any 'parallel shift' of the dose-response curve for carbachol, the only result of alkylation being a decrease in maximal response, which is more pronounced in isometric than in isotonic conditions. The degree of decrease is in accordance with the correlation between percentage of shortening and percentage of tension in the absence of alkylating agent. Probably this muscle does not possess any 'spare receptors'. 5 On the frog muscle the dose-isometric response curve for acetylcholine (ACh) is shifted toward greater concentration about 33-fold as compared with the dose-isotonic response curve but after the inhibition of cholinesterases the shift is only about 6-fold. The same shift (5-fold) is observed for carbachol, which is not hydrolysed by cholinesterases. The results with ACh are due to the fact, that after cholinesterase inhibition the sensitivity to ACh increases in isotonic conditions only 13-fold, but in isometric conditions it increases 71-fold. Probably under isometric conditions, when the muscle remains in the extended state, the rate of hydrolysis of ACh is much greater than under isotonic conditions when the muscle is shortened during contraction.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brookes N., Mackay D. Rate of onset and offset of neuromuscular block in the isolated rat diaphragm. Br J Pharmacol. 1971 Feb;41(2):339–343. doi: 10.1111/j.1476-5381.1971.tb08034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSAPO A. Dependence of isometric tension and isotonic shortening of uterine muscle on temperature and on strength of stimulation. Am J Physiol. 1954 Jun;177(3):348–354. doi: 10.1152/ajplegacy.1954.177.3.348. [DOI] [PubMed] [Google Scholar]
- Clark A. J. The reaction between acetyl choline and muscle cells. J Physiol. 1926 Aug 6;61(4):530–546. doi: 10.1113/jphysiol.1926.sp002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Tattersall M. L. Rapid histamine assays: a method and some theoretical considerations. Br J Pharmacol. 1970 Jan;38(1):241–252. doi: 10.1111/j.1476-5381.1970.tb10353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill E. W., Rang H. P. An alkylating derivative of benzilylcholine with specific and long-lasting parasympatholytic activity. Mol Pharmacol. 1966 Jul;2(4):284–297. [PubMed] [Google Scholar]
- KRNJEVIC K., MITCHELL J. F. Diffusion of acetylcholine in agar gels and in the isolated rat diaphragm. J Physiol. 1960 Oct;153:562–572. doi: 10.1113/jphysiol.1960.sp006555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittag T. W., Ehrenpreis S., Patrick P. Some properties of cholinesterases in intact guinea-pig ileum in vitro. Arch Int Pharmacodyn Ther. 1971 Jun;191(2):270–278. [PubMed] [Google Scholar]
- NICKERSON M. Receptor occupancy and tissue response. Nature. 1956 Sep 29;178(4535):697–698. doi: 10.1038/178697b0. [DOI] [PubMed] [Google Scholar]
- PATON W. D., RANG H. P. THE UPTAKE OF ATROPINE AND RELATED DRUGS BY INTESTINAL SMOOTH MUSCLE OF THE GUINEA-PIG IN RELATION TO ACETYLCHOLINE RECEPTORS. Proc R Soc Lond B Biol Sci. 1965 Aug 24;163:1–44. doi: 10.1098/rspb.1965.0058. [DOI] [PubMed] [Google Scholar]
- Waud D. R. Pharmacological receptors. Pharmacol Rev. 1968 Jun;20(2):49–88. [PubMed] [Google Scholar]


