Abstract
The chemosensory complexes in Escherichia coli are localized predominantly in large aggregates at one or both of the cell poles, however, neither the role of the polar localization nor the role of the clustering is understood. In E. coli, the two classes of chemoreceptors or transducers, high- and low-abundance, differ in their ability to support chemotaxis when expressed as the sole chemoreceptor type in the cell. In this study, we examined both the contribution of individual chemoreceptors to polar clustering and the ability of each chemoreceptor type to cluster in the absence of all others. We found that polar clustering of methyl-accepting chemotaxis proteins (MCPs) is not dependent on any one chemoreceptor type. Remarkably, when expressed individually at similar levels, the chemoreceptors display differential clustering abilities. The high-abundance transducers cluster at the cell pole almost as well as do the MCPs in cells expressing all four species, whereas the low-abundance transducers, although polar, are not particularly clustered. CheA and CheW distributions in strains expressing only one chemoreceptor type coincide with MCP localization, indicating that the low-abundance chemoreceptors are competent for ternary complex formation but are defective in aggregation. These studies reveal that, in contrast to our previous model, polarity of the chemoreceptors is independent of clustering, suggesting that the polar localization of the chemoreceptors is not simply caused by diffusion limitations on large protein aggregates.
It has been well established that proper function and regulation of many eukaryotic proteins and protein complexes require specific subcellular localization of these proteins. Recent studies have demonstrated that many bacterial proteins are also sequestered to distinct cellular locations. For example, a number of chemotaxis, partitioning, cell division, and regulatory proteins in Escherichia coli, Caulobacter crescentus, and Bacillus subtilis are preferentially localized at the cell poles (see ref. 1). These studies suggest that the end of the bacterial cell may provide a unique microenvironment, distinct from that of the rest of the cell, and demonstrate that organization of the bacterial cell is highly complex.
The polar clustering of bacterial chemoreceptor complexes is particularly intriguing because the function of the localization is not obvious. E. coli cells often have a single large cluster of chemoreceptors at one pole of the cell (2) and yet can swim in either direction (3). It has been proposed that the clustering of the chemoreceptors plays a critical role in amplification of the chemotactic signal (2, 4), although no experimental evidence in support of this hypothesis has been obtained thus far.
In E. coli, the methyl-accepting chemotaxis proteins (MCPs) or transducers are membrane-bound proteins that form a ternary complex with two soluble proteins, the histidine kinase CheA and the coupling protein CheW. Maximal polar clustering of each protein component requires the presence of the other two (2), indicating that aggregation requires the formation of the ternary complex. The methyltransferase CheR and methylesterase CheB interact with the ternary complex, however, their presence is not required for clustering (5). Although the role of chemoreceptor complex clustering is unknown, chemoreceptor complexes have been found in C. crescentus (6) and Rhodobacter sphaeroides (7), suggesting that clustering of the ternary complexes plays an essential role in all bacterial chemotactic signaling.
In E. coli, there are two classes of chemoreceptors, the high-abundance transducers (Tar and Tsr) and the low-abundance transducers (Tap and Trg). In wild-type cells, the high-abundance transducers are 10 times more abundant than are the low-abundance transducers (8, 9). When expressed alone, the high-abundance transducers can support chemotaxis, whereas the low-abundance transducers cannot, even when they are expressed at levels comparable to those of Tar or Tsr (10, 11). If coexpressed with a high-abundance chemoreceptor, however, the low-abundance chemoreceptors can mediate the chemotaxis signal transduction cascade (10, 11). Recently, it has been demonstrated that the low-abundance transducer Trg is not significantly defective in CheA kinase activation in vitro (12). Addition of the CheR-binding site from Tsr was sufficient to allow Trg-mediated chemotaxis in the absence of the other chemoreceptors, suggesting that the inability of Trg to function alone was attributable to a difference in methylation state (13). However, addition of the CheR-binding site to Tap did not restore Tap-mediated chemotaxis (11). These conflicting observations suggest that ability to support chemotaxis may be more complex than variation in methyl-accepting activity.
Differences in the clustering potentials of receptors may contribute to their specific abilities to support chemotaxis. It is possible that only the high-abundance chemoreceptors are capable of facilitating clustering, whereas the low-abundance chemoreceptors only cluster maximally when Tar and/or Tsr are present to “nucleate” clusters. It is equally possible that the high-abundance transducers have a greater clustering potential than do the low-abundance transducers. To investigate whether any one specific MCP type is responsible for mediating clustering of the others, we examined the localization patterns of the chemoreceptors in strains lacking one or more MCPs. We demonstrate that removal of any one chemoreceptor species has no effect on the polarity or clustering of the remaining MCPs in the cell. To assess the ability of each type of transducer to localize to the poles and cluster, we examined the intracellular distribution of single MCP species in the absence of all others. We show that each MCP type is capable of localizing to the polar membranes, but only the high-abundance transducers are clustered. The lack of clustering of the low-abundance transducers does not appear to be caused by a defect in ternary complex formation because CheA and CheW distributions in these strains parallel MCP localization. Furthermore, the polarity of the low-abundance transducers in the absence of clustering implies that polarity is independent from clustering. These observations strongly suggest that the E. coli cell poles are physiologically distinct from the lateral edges of the cell.
Materials and Methods
Bacterial Strains and Plasmids.
E. coli strains UU1250 (Δmcp, Δaer), RP2361 (Δtar), RP5700 (Δtsr), and RP3525 (Δtap) are previously unpublished strains that were kindly provided by Sandy Parkinson (University of Utah, Salt Lake City) and are derivatives of chemotactically wild-type RP437 (14). E. coli strain CP177 (Δtrg; ref. 15) and plasmids pCT1 (13) and pGB1 (10), which contain tsr and trg, respectively, under the tac promoter, were a gift from Jerry Hazelbauer (Washington State University, Pullman). Plasmids pMK113 (16) and pSW2 (11), which contain tar and tap, respectively, under the tac promoter, were a gift from Mike Manson (Texas A & M University, College Station).
Growth Conditions.
Overnight cultures of cells were grown in tryptone broth (TB; 1% tryptone/0.5% NaCl), supplemented with 100 μg/ml ampicillin when appropriate. Cultures were diluted 1:50 into fresh TB and grown to an OD600 of 0.5–0.8 before fixation. Because Trg expression from pGB1 was low in the absence of induction, these cultures were diluted 1:50 into fresh TB, grown to an OD600 of 0.4, and then induced with 50 μM isopropyl β-d-thiogalactoside before fixation. The expression levels of each of the individual transducers in UU1250, used in this study, were comparable to one another and to the full complement of MCPs in wild-type cells, as indicated by the total number of membrane particles per section (Table 1).
Table 1.
Spatial distribution of chemoreceptors
| Background strain | Chromosome-encoded MCPs | Plasmid-encoded MCPs | Total no. of particles | No. of total particles in cytoplasm | Membrane particles
|
Membrane particles per section | No. of polar clusters | Size of polar clusters | No. of lateral clusters | Size of lateral clusters | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polar particles
|
Lateral particles
|
||||||||||||
| % | % in clusters | % | % in clusters | ||||||||||
| RP437 | All | None | 1,385 | 44 | 86 | 90 | 14 | 53 | 8.4 | 104 | 10 ± 0.5 | 15 | 6.5 ± 0.7 |
| RP2361 | Tsr, Tap, Trg | None | 1,016 | 74 | 89 | 69* | 11 | 14 | 2.9 | 76 | 7.6 ± 0.5 | 3 | 4.7 ± 0.7 |
| RP5700 | Tar, Tap, Trg | None | 1,448 | 75 | 85 | 68* | 15 | 39 | 4.3 | 96 | 8.3 ± 0.5 | 11 | 7.0 ± 1.0 |
| RP3525 | Tsr, Tar, Trg | None | 1,142 | 50 | 88 | 77* | 12 | 42 | 6.8 | 77 | 9.5 ± 0.6 | 10 | 5.6 ± 0.9 |
| CP177 | Tsr, Tar, Tap | None | 1,191 | 34 | 94* | 78* | 6 | 40 | 7.2 | 89 | 9.5 ± 0.5 | 4 | 7.2 ± 1.1 |
| RP2361 | Tsr, Tap, Trg | Tar | 1,538 | 40 | 88 | 91 | 12 | 48 | 9.4 | 105 | 11 ± 0.6 | 14 | 6.0 ± 0.6 |
| RP2361 | Tsr, Tap, Trg | Tsr | 2,091 | 55 | 86 | 87 | 14 | 52 | 12.7 | 134 | 11 ± 0.7 | 23 | 6.6 ± 0.4 |
| RP5700 | Tar, Tap, Trg | Tsr | 2,023 | 229 | 85 | 86 | 15 | 25 | 11.2 | 119 | 11 ± 0.6 | 10 | 6.4 ± 0.8 |
| RP5700 | Tar, Tap, Trg | Tar | 1,125 | 59 | 89 | 82* | 11 | 54 | 6.7 | 88 | 8.8 ± 0.5 | 11 | 5.6 ± 0.8 |
| UU1250 | None | Tsr | 1,187 | 26 | 90 | 74* | 10 | 13 | 7.3 | 81 | 9.6 ± 0.6 | 3 | 5.0 ± 0.6 |
| UU1250 | None | Tar | 985 | 153 | 79 | 74* | 21 | 2.3 | 5.2 | 55 | 8.9 ± 0.5 | 1 | 4 |
| UU1250 | None | Tap | 1,487 | 141 | 83 | 54* | 17 | 1.8 | 8.4 | 96 | 6.3 ± 0.2 | 1 | 4 |
| UU1250 | None | Trg | 1,583 | 40 | 71* | 24* | 29 | 4.8 | 9.6 | 51 | 5.2 ± 0.3 | 4 | 5.5 ± 0.8 |
For each strain, 160 longitudinal cell sections were scored except for RP2361 (Δtar) and RP5700 (Δtsr), for which 320 cells were considered. Data for cluster size are expressed as mean ± SEM. Percentages of polarity and clustering in all strains were compared to wild-type values by χ2 analysis, and those with values significantly different from RP437 have been marked with an asterisk.
Cell Fixation and Embedding Conditions.
Cells were fixed with 3% formaldehyde and 0.1% glutaraldehyde in phosphate buffer (30 mM Na2PO4, pH 7.0) for 1 to 2 h on ice, and then washed three times with phosphate buffer. Samples were incubated in 1% sodium metaperiodate for 15 min, washed with phosphate buffer, and quenched with 50 mM NH4Cl for 15 min. Finally, the cells were washed with water, dehydrated in a graded ethanol series, and embedded in LR White resin (Electron Microscopy Sciences, Fort Washington, PA) in gelatin capsules at 45–47°C for 2 days, after which 70- to 90-nm sections were cut and placed on “sticky” nickel grids (17).
Immunoelectron Microscopy.
All solutions for antibody reactions were made with ddH2O and were filtered through a 0.22-μm filter (ISC BioExpress, Kaysville, UT) before the addition of antibodies. Primary antibodies were diluted (1:500 for Tsr and CheA; 1:25 for CheW) in PBS + Tween 20 (PBST; 140 mM NaCl/2 mM KCl/8 mM Na2HPO4/1.5 mM KH2PO4/0.05% Tween 20) with 2% BSA. Diluted anti-MCP, anti-CheA, and anti-CheW were preadsorbed for 20 min with acetone powders (18) prepared from strains KO607 (Δmcp) (19), RBB382 (ΔcheA) (20), and RP1078 (ΔcheW) (21), respectively. The grids were blocked in PBST + BSA for 15–30 min in a humidity chamber and then incubated in diluted, preadsorbed primary antibody for 1–2 h. The grids were washed three times with PBST, blocked, and incubated for 1–2 h in a 1:30 dilution of goat anti-rabbit IgG conjugated to 12 nm of colloidal gold particles (Jackson ImmunoResearch). The grids were washed three times with PBST and once with ddH2O, and then stained with a 1% uranyl acetate solution. Samples were examined on a Philips CM10 electron microscope at 60 kV. The anti-Tsr antibody was raised against the highly conserved domain of Tsr (22) and crossreacts with all four MCPs in E. coli. Spatial distributions of chemoreceptors were scored as previously described (2). Briefly, we defined an MCP cluster as a core group of at least 4 gold particles no farther than 20 nm apart, plus all particles within 40 nm of the core. Because the CheA and CheW antibodies used in this study resulted in a lower average number of particles per section in wild-type cells, our criterion for scoring gold particles as a cluster with these antibodies was lowered to ≥3 gold particles.
Results
Polar Clustering of the Chemoreceptors Is Not Mediated by Any One Specific Transducer.
To address whether the polar clustering of the MCPs was mediated specifically by a single chemoreceptor type, we examined the intracellular positioning of the MCPs in E. coli strains lacking one of the four chemoreceptors. To do this, we used an antibody raised against the highly conserved domain of Tsr (22) that crossreacts with all four E. coli MCPs (data not shown). In wild-type cells, 86% of the chemoreceptors, detected by an anti-Tsr antibody, were found at the cell poles, and the majority of those gold particles (90%) were in one or more clusters. In the absence of any single chemoreceptor type, the polarity of the remaining MCPs was similar to that seen in wild-type cells (Table 1), but the levels of clustering were slightly reduced. In cells that lacked a low-abundance transducer, 77–78% of the polar particles were clustered, whereas in cells that lacked a high-abundance transducer, 68–69% of the polar particles were in clusters (Table 1). The total number of gold particles associated with the membrane was slightly reduced in Δtap and Δtrg strains but significantly reduced in Δtar and Δtsr strains. In addition, the size of the polar clusters was similar to that observed in wild type in Δtap and Δtrg strains but slightly reduced in Δtar and Δtsr strains (Table 1).
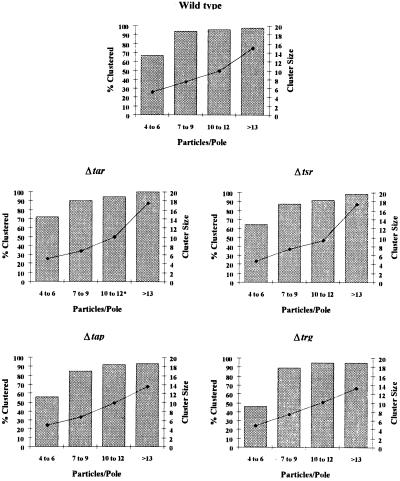
We were concerned that inclusion of a high proportion of cell sections with fewer than 4 gold particles in a data set could diminish the observed level of clustering, as the gold particles in these sections could not be scored as being in clusters regardless of their level of association. To determine whether the reduction in the number of polar clusters in the MCP deletion strains was a consequence of the lower total number of chemoreceptors in the cell, we examined the clustering potential of each strain, restricting our analysis to only those cell poles with ≥4 gold particles. In wild-type cells, poles containing a low number of gold particles (4–6) had 68% of those gold particles in clusters. Wild-type cell poles with 7 or more gold particles had >90% of those particles in clusters and usually only 1 cluster was found at the cell end. The size of the clusters increased as the total number of polar particles increased. All four of the single-chemoreceptor-deletion mutants displayed a profile of percentage clustering and average size of clusters similar to that seen in wild type (Fig. 1), indicating that the apparent decrease in clustering initially observed is the result of lower numbers of total gold particles scored. Therefore, we conclude that removal of any one MCP type does not affect the potential of the remaining transducers to cluster.
Figure 1.
Clustering potential of the single-deletion mutants. Cell poles containing at least 4 particles were scored separately to examine the frequency of clustering at poles with sufficient particles to cluster. Poles were sorted by particle number (abscissa), and then the percentage of particles clustered (gray bars, left ordinate) and mean cluster size (black diamonds, right ordinate) were determined. A minimum of 10 cell poles was included in each category, except where noted by an asterisk (*). We examined 160 cell sections of RP437 (wild type), RP3525 (Δtap), and CP177 (Δtrg), and 320 cell sections of RP2361 (Δtar) and RP5700 (Δtsr).
To further confirm that the apparent reduction in clustering seen in Δtar and Δtsr strains was a consequence of the lower total number of chemoreceptors in the cell, we expressed a high-abundance transducer from a plasmid in these strains to achieve total MCP levels comparable to wild-type levels (Table 1). In Δtar cells, expression of plasmid-borne Tar or Tsr resulted in wild-type polarity (88 and 86%), clustering (91 and 87%), and polar cluster size (11 and 11 gold particles). Similarly, expression of Tsr in a Δtsr strain resulted in wild-type polar clustering (85% polar, 86% clustered, and size of 11 gold particles). Expression of Tar in Δtsr cells resulted in wild-type polarity (89%) but in slightly reduced polar clustering (82%) and smaller clusters (8.8). The lower level of polar clustering, however, is likely attributable to the total lower signal observed under the induction conditions used (6.7 gold particles per membrane as compared with 8.4 gold particles in wild type). Examination of the clustering potential of all of these strains revealed that in sections with ≥4 gold particles, the profiles of clustering percentage and cluster size were similar to those of wild type (data not shown). Thus, wild-type levels of polar clustering are observed in cells lacking either of the high-abundance transducers.
The Low-Abundance Transducers, When Present as Sole Transducers, Are Polar but Not Clustered.
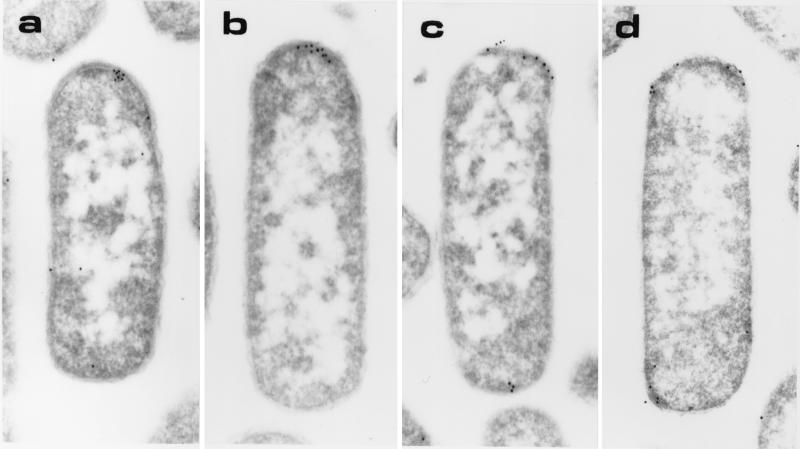
We next examined the potential of each of the MCPs to cluster at the cell poles when expressed as the sole chemoreceptor type in the cell. To do this, we expressed each individual chemoreceptor from a plasmid in a strain that lacks all E. coli chemoreceptors. Protein expression levels were adjusted so that the amount of each single transducer expressed was comparable to the wild-type level of all four MCPs together. The intracellular distribution of the expressed MCP was detected by immunoelectron microscopy. When expressed as the sole chemoreceptor type in the cell, each of the four MCPs was found predominantly at the cell poles (Table 1 and Fig. 2). In cells expressing Tar, Tsr, or Tap, the percentage of gold particles at the cell poles was comparable to that of cells expressing all of the chemoreceptors (79–90%), whereas in cells expressing only Trg, there was a modest reduction in polarity (71%). These data suggest that polarity may be an inherent feature of each chemoreceptor; the individual MCPs are capable of polar localization in the absence of the other MCP types.
Figure 2.
Intracellular localization of MCPs. (a–d) Shown are representative immunoelectron micrographs of longitudinal sections of cells expressing Tar (UU1250 + pMK113) (a), Tsr (UU1250 + pCT1) (b), Tap (UU1250 + pSW2) (c), or Trg (UU1250 + pGB1) (d). Polyclonal antibody to Tsr was diluted to 1:500 and detected by using goat anti-rabbit colloidal gold conjugate.
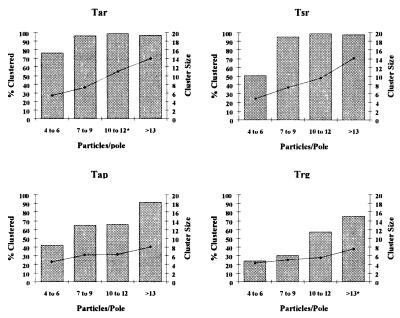
The clustering patterns of the high- and low-abundance transducers were remarkably different. The high-abundance transducers Tar and Tsr displayed a reduction in the percentage of polar clusters relative to that of wild type (74% vs. 90%), but the size of the polar clusters was comparable to that of wild type (Table 1). In contrast, cells expressing either of the low-abundance transducers displayed a significant reduction both in the number of polar clusters and in the size of those clusters. In cells expressing only Tap, 54% of the polar particles were in clusters with an average size of 6.3. Cells expressing only Trg localized only 24% of the particles at the cell poles in clusters, and the size of these clusters was approximately half that found in wild-type cells (5.2 and 10 particles, respectively).
To assess whether the reduction in percentage of polar particles clustered was, in fact, a reduction in the clustering potential of the receptor types, we examined the clustering profiles of the individual MCPs in cell sections with sufficient polar gold particles to score as a cluster (≥4 gold particles). The clustering potential of cells expressing only Tar or Tsr was equivalent to that of cells containing all four MCPs in both the level of clustering and the size of the clusters (compare Figs. 1 and 3). In cell sections with ≥7 gold particles at one pole, ≥90% of the gold particles were clustered, and cell poles usually had only one cluster. In contrast, the polar gold particles found in strains expressing only the low-abundance transducers were less clustered, and the size of the clusters also was reduced (Fig. 3). Moreover, multiple smaller clusters were seen in these cells, resulting in a lower average cluster size.
Figure 3.
Clustering potential of the individual MCPs. Cell poles containing at least 4 particles were scored separately to examine the frequency of clustering at poles with sufficient particles to cluster. Poles were sorted by particle number (abscissa), and then the percentage of particles clustered (gray bars, left ordinate) and mean cluster size (black diamonds, right ordinate) were determined. A minimum of 10 cell poles was included in each category except where noted by an asterisk (*). We examined 320 cell sections of UU1250 transformed with the following chemoreceptors (plasmid indicated in parentheses): Tar (pMK113), Tsr (pCT1), Tap (pSW2), and Trg (pGB1).
The Distributions of CheA and CheW Parallel That of the Transducers.
We recently have demonstrated that although high-level polar clustering of MCPs requires CheA, a significant level of CheA-independent and, therefore, ternary complex-independent aggregation of the MCPs occurs (23). This observation raises the possibility that either (i) the clustering potential of each chemoreceptor is greatly enhanced in the presence of CheA, or (ii) there are differences in the requirements for CheA in the clustering of the four different chemoreceptors. In order to determine whether CheA was similarly associated with each MCP, we examined the intracellular distribution of CheA in cells expressing each of the chemoreceptors individually.
In wild-type cells, 77% of the gold particles were membrane-associated and 77% of these were at the cell poles with 42% in clusters (Table 2). The average size of the CheA polar clusters was small (3.6), reflecting the low but specific signal. In the absence of all membrane-bound MCPs, the majority of the gold particles was found in the cytoplasm (60%). Of those particles found within 20 nm of the cell membrane, there was no polar bias (26% polar) nor was there any significant clustering. Only one cluster of 3 particles was observed in the 320 sections examined, which likely represents a random event. Thus, as reported previously (2), membrane association, polarity, and clustering of CheA in E. coli absolutely requires the presence of MCPs.
Table 2.
Spatial distribution of CheA
| Background strain | Chromosome-encoded MCPs | Plasmid-encoded MCPs | Total no. of particles | No. of total particles in cytoplasm | Membrane particles
|
Membrane particles per section | No. of polar clusters | Size of polar clusters | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Polar particles
|
Lateral particles
|
||||||||||
| % | % in clusters | % | % in clusters | ||||||||
| RP437 | All | None | 847 | 193 | 77 | 42 | 23 | 12 | 2.0 | 59 | 3.6 ± 0.1 |
| UU1250 | None | None | 417 | 254 | 26* | 6.7* | 74 | 0 | 0.5 | 1 | 3.0 |
| UU1250 | None | Tsr | 700 | 108 | 83 | 39 | 17 | 6.1 | 1.9 | 50 | 4.7 ± 0.3 |
| UU1250 | None | Tar | 587 | 186 | 74 | 38 | 26 | 8.5 | 1.2 | 27 | 4.0 ± 0.2 |
| UU1250 | None | Tap | 672 | 86 | 80 | 35 | 20 | 10 | 1.8 | 45 | 3.7 ± 0.1 |
| UU1250 | None | Trg | 572 | 109 | 78 | 25* | 22 | 9.5 | 1.4 | 27 | 3.3 ± 0.1 |
For each strain, 320 longitudinal cell sections were scored except for RP437, for which 160 cells were considered. Data for cluster size are expressed as mean ± SEM. Percentages of polarity and clustering in all strains were compared to wild-type values by χ2 analysis, and those with values significantly different from RP437 have been marked with an asterisk.
The intracellular distribution of CheA was determined in Δmcp cells expressing a single chemoreceptor type. In cells expressing any one of the chemoreceptors, the majority of the gold particles were membrane-associated (68–87%), and these were predominantly localized at the cell poles at levels similar to those seen in wild-type cells (74–83%; Table 2), indicating that CheA interacted with each of the different chemoreceptors in vivo. Cells that expressed Tsr, Tar, or Tap also exhibited wild-type CheA clustering levels (35–38%; Table 2). In cells expressing Trg, the majority of the CheA was at the cell poles, however, there was a significant reduction in the level of clustering (25%). Thus, CheA was membrane-associated in a pattern similar to that of the individual MCPs, suggesting that in vivo ternary complex formation was not compromised in these strains.
To further examine ternary complex formation in the strains expressing single transducer types, we also examined the intracellular distribution of CheW. In wild-type cells, approximately half (51%) of the gold particles were associated with the membrane, 68% of which were localized to the pole with 37% in clusters. In strains lacking membrane-bound MCPs, the majority of gold particles (72%) were cytoplasmic. In the absence of the MCPs, there was no polar bias of those particles within 20 nm of the cell membrane (29% at the pole), and there was no significant clustering. Thus, membrane association, polar localization, and clustering of CheW require the presence of the MCPs, as previously reported (2).
In the Δmcp strains expressing individual receptor species, the distribution of CheW followed that of the MCPs. In cells expressing only Tsr, Tap, or Trg, the majority (67–70%) of gold particles was associated with the membrane and polarly localized (66–78%). CheW exhibited wild-type clustering levels in strains expressing only the high-abundance transducer Tsr (41% of polar particles were clustered). Clustering was reduced, however, in strains expressing only low-abundance transducers Tap or Trg (23% and 27%, respectively). Thus, it appears that the distribution of CheW mirrors that of both CheA and the MCPs, suggesting that ternary complex formation is intact within strains expressing single transducer types.
Discussion
Our long-term goal is to elucidate the relationship between chemotaxis and clustering of the chemoreceptors. To date, we had examined only the immunolocalization of all of the MCPs in the presence of all others. In this paper, we have examined both the requirement for each transducer in the polar clustering of the others and the ability of each chemoreceptor, when expressed individually, to cluster at the cell poles.
The deletion of any one chemoreceptor population had no effect on the polar clustering of the remaining chemoreceptors, indicating that no single chemoreceptor type was required for the polar clustering of the other three. In wild-type E. coli cells, the high-abundance transducers are approximately 10 times more prevalent than are the low-abundance transducers (8, 9), and thus removal of one of these receptor genes results in an overall reduction in MCP content. When we restricted our analysis to cell poles with at least 4 gold particles to compensate for the reduction in the total number of membrane particles per cell, we observed no decrease in the overall level of clustering. Wild-type levels of polar clustering also were seen in these strains when the total level of MCPs was restored to that seen in wild-type cells by expression of either Tar or Tsr from a plasmid.
When expressed individually to levels comparable to that of all four MCPs in wild-type cells, the high-abundance transducers (Tar and Tsr) cluster well at the cell poles, whereas the low-abundance transducers (Tap and Trg) were polar but not particularly clustered. The lack of maximal polar clustering in cells expressing either Tap or Trg was not simply caused by a reduction in the number of gold particles at the cell poles, because the clustering profiles of the low-abundance transducers showed a marked reduction in both the number of clusters observed and in their size when compared with either wild-type cells or cells expressing only Tar or Tsr (Fig. 3). Clearly, high-abundance transducers have a greater clustering potential than do low-abundance transducers, although all four transducers display slightly different abilities to cluster. These data may, in part, explain why the low-abundance chemoreceptors fail to mediate chemotactic signaling when present as the sole MCP type in the cell. Perhaps clustering is necessary for chemotaxis and these low-abundance transducers are not efficiently clustered in the absence of high-abundance chemoreceptors.
In cells expressing any single MCP species, both polarity and clustering of CheA and CheW mirror the localization patterns of the expressed MCPs. Because CheA and CheW are cytoplasmic proteins that are only found at the cell membrane when complexed with MCPs, these data suggest that ternary complexes form even in cells expressing only one MCP type. Because the low-abundance transducers are reduced in their polar clustering but not in their ability to recruit CheA or CheW to the cell pole, ternary complexes must form independently of clusters. Moreover, the recruitment of CheA and CheW to the cell poles by each of the MCP types suggests that, in vivo, the individual chemoreceptor types are equally competent to form ternary complexes. These data are consistent with the in vitro studies demonstrating that Tsr and Trg each are capable of stimulating the CheA kinase activity (12). Taken together, these data indicate that all four MCP types have similar affinities for CheA and CheW, supporting a model in which the inability of the low-abundance transducers alone to mediate chemotaxis is not attributable to their inability to interact with CheA or CheW.
We have demonstrated that Tap and Trg are localized to the cell poles and, yet, are not clustered well (Table 1). This is the first example of chemoreceptors preferentially localized to the cell poles in the absence of high levels of clustering and raises the question of how nonaggregated proteins could be specifically restricted to the cell poles. To date, we have favored a model in which the large size of the MCP aggregates impedes their random diffusion throughout the membrane. The chemoreceptor complexes would be restricted to the ends of the cells either by steric hindrance because of the curvature at the cell ends or because the old pole of the cell is a location where little insertion of newly synthesized membrane proteins occurs (J.R.M., unpublished data). We have shown here that either Tap or Trg proteins, as sole chemoreceptors, are polar but not in large clusters. Thus, it is unlikely that polarity of these MCPs is a result of diffusion limitations. Recently, a number of partitioning, cell division, and regulatory proteins in E. coli (24), C. crescentus (25, 26), and B. subtilis (27, 28) also have been shown to be preferentially localized at the cell poles. These studies have relied on the use of green fluorescent protein fusions, and therefore the physical juxtaposition of these proteins and the polar membrane have not been determined unequivocally. The specific polar localization of all of these proteins, however, suggests that the bacterial cell poles provide a unique microenvironment distinct from the rest of the cell.
Although the relationship between the clustering of MCPs detected by immunoelectron microscopy and the physical interactions between the MCPs within the cell is unknown, it is likely that the clustering of colloidal gold particles reflects a close association between the MCPs themselves, as differences in clustering were observed in strains expressing different MCP types. Recently, it has been shown that Tsr crystals are composed of trimers of Tsr dimers (29), demonstrating a higher-order interaction between Tsr molecules. Furthermore, it is likely that Tar is capable of mediating higher-order complexes because, in vitro, a cytoplasmic Tar fragment forms higher-order structures with CheA and CheW (30). It will be interesting to see whether these higher-order structures are formed with all chemoreceptors in vitro or are limited to the high-abundance transducers, as would be predicted from our study.
Based on the evidence to date, we propose that once at the poles, the low-abundance transducers are recruited into clusters by the high-abundance transducers. Perhaps the high-abundance transducers have a high affinity for any transducer type, whereas the low-abundance transducers only have a high affinity for high-abundance transducers. Clearly an unambiguous identification of individual transducers in the context of the others is necessary to test this hypothesis.
Acknowledgments
We thank Sandy Parkinson, Mike Manson, and Jerry Hazelbauer for generous gifts of strains, antibodies, and plasmids; and Susan Sullivan and Robert Bender for critical reading of this manuscript. We also want to thank members of the Maddock laboratory for their generous help collecting the data. This work was supported in part by Grant GM55133 from the National Institutes of Health.
Abbreviation
- MCP
methyl-accepting chemotaxis protein
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.130195397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.130195397
References
- 1.Shapiro L, Losick R. Cell. 2000;100:89–98. doi: 10.1016/s0092-8674(00)81686-4. [DOI] [PubMed] [Google Scholar]
- 2.Maddock J R, Shapiro L. Science. 1993;259:1717–1723. doi: 10.1126/science.8456299. [DOI] [PubMed] [Google Scholar]
- 3.Berg H C, Turner L. Proc Natl Acad Sci USA. 1995;92:477–479. doi: 10.1073/pnas.92.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray D, Levin M D, Morton-Firth C J. Nature (London) 1998;393:85–88. doi: 10.1038/30018. [DOI] [PubMed] [Google Scholar]
- 5.Lybarger S R, Maddock J R. J Bacteriol. 1999;181:5527–5529. doi: 10.1128/jb.181.17.5527-5529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alley M R, Maddock J R, Shapiro L. Genes Dev. 1992;6:825–836. doi: 10.1101/gad.6.5.825. [DOI] [PubMed] [Google Scholar]
- 7.Harrison D M, Skidmore J, Armitage J P, Maddock J R. Mol Microbiol. 1999;31:885–892. doi: 10.1046/j.1365-2958.1999.01226.x. [DOI] [PubMed] [Google Scholar]
- 8.Hazelbauer G L, Engstrom P, Harayama S. J Bacteriol. 1981;145:43–49. doi: 10.1128/jb.145.1.43-49.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hazelbauer G L, Engstrom P. J Bacteriol. 1981;145:35–42. doi: 10.1128/jb.145.1.35-42.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng X, Baumgartner J W, Hazelbauer G L. J Bacteriol. 1997;179:6714–6720. doi: 10.1128/jb.179.21.6714-6720.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weerasuriya S, Schneider B M, Manson M D. J Bacteriol. 1998;180:914–920. doi: 10.1128/jb.180.4.914-920.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnakov A N, Barnakova L A, Hazelbauer G L. J Bacteriol. 1998;180:6713–6718. doi: 10.1128/jb.180.24.6713-6718.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng X, Lilly A A, Hazelbauer G L. J Bacteriol. 1999;181:3164–3171. doi: 10.1128/jb.181.10.3164-3171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parkinson J S, Houts S E. J Bacteriol. 1982;151:106–113. doi: 10.1128/jb.151.1.106-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park C, Hazelbauer G L. J Bacteriol. 1986;167:101–109. doi: 10.1128/jb.167.1.101-109.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardina P, Conway C, Kossman M, Manson M. J Bacteriol. 1992;174:1528–1536. doi: 10.1128/jb.174.5.1528-1536.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright R, Rine J. Methods Cell Biol. 1989;31:473–512. doi: 10.1016/s0091-679x(08)61624-6. [DOI] [PubMed] [Google Scholar]
- 18.Harlow E, Lane D. Antibodies : A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. p. 633. [Google Scholar]
- 19.Oosawa K, Mutoh N, Simon M I. J Bacteriol. 1988;170:2521–2526. doi: 10.1128/jb.170.6.2521-2526.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourret R B, Hess J F, Simon M I. Proc Natl Acad Sci USA. 1990;87:41–45. doi: 10.1073/pnas.87.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Parkinson J S. Proc Natl Acad Sci USA. 1989;86:8703–8707. doi: 10.1073/pnas.86.22.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ames P, Parkinson J S. J Bacteriol. 1994;176:6340–6348. doi: 10.1128/jb.176.20.6340-6348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skidmore J M, Ellefson D D, McNamara B P, Couto M M P, Wolfe A J, Maddock J R. J Bacteriol. 2000;182:967–973. doi: 10.1128/jb.182.4.967-973.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raskin D M, DeBoer P A J. Proc Natl Acad Sci USA. 1999;96:4971–4976. doi: 10.1073/pnas.96.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohl D A, Gober J W. Cell. 1997;88:675–684. doi: 10.1016/s0092-8674(00)81910-8. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs C, Domian I J, Maddock J R, Shapiro L. Cell. 1999;97:111–120. doi: 10.1016/s0092-8674(00)80719-9. [DOI] [PubMed] [Google Scholar]
- 27.Marston A L, Errington J. Mol Cell. 1999;4:673–682. doi: 10.1016/s1097-2765(00)80378-0. [DOI] [PubMed] [Google Scholar]
- 28.Quisel J D, Lin D C, Grossman A D. Mol Cell. 1999;4:665–672. doi: 10.1016/s1097-2765(00)80377-9. [DOI] [PubMed] [Google Scholar]
- 29.Kim K K, Yokota H, Kim S-H. Nature (London) 1999;400:787–792. doi: 10.1038/23512. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Levit M, Lurz R, Surette M G, Stock J B. EMBO J. 1997;16:7231–7240. doi: 10.1093/emboj/16.24.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]