Abstract
The endoplasmic reticulum (ER) body is a characteristic structure derived from ER and is referred to as a proteinase-sorting system that assists the plant cell under various stress conditions. Fluorescent ER bodies were observed in transgenic plants of Arabidopsis expressing green fluorescent protein fused with an ER retention signal. ER bodies were widely distributed in the epidermal cells of whole seedlings. In contrast, rosette leaves had no ER bodies. We found that wound stress induced the formation of many ER bodies in rosette leaves. ER bodies were also induced by treatment with methyl jasmonate (MeJA), a plant hormone involved in the defense against wounding and chewing by insects. The induction of ER bodies was suppressed by ethylene. An electron microscopic analysis showed that typical ER bodies were induced in the non-transgenic rosette leaves treated with MeJA. An experiment using coi1 and etr1-4 mutant plants showed that the induction of ER bodies was strictly coupled with the signal transduction of MeJA and ethylene. These results suggested that the formation of ER bodies is a novel and unique type of endomembrane system in the response of plant cells to environmental stresses. It is possible that the biological function of ER bodies is related to defense systems in higher plants.
Soluble proteins with a KDEL or HDEL sequence at their C terminus are retained within the endoplasmic reticulum (ER; Denecke et al., 1992). Green fluorescent protein (GFP) with an ER retention signal (GFP-HDEL) has been shown to exhibit characteristic fluorescence in transgenic Arabidopsis (Haseloff et al., 1997; Ridge et al., 1999; Hawes et al., 2001). Rod-shaped GFP-fluorescent structures (5 μm long and 0.5 μm wide) are observed in epidermal cells of Arabidopsis seedlings expressing GFP-HDEL. Electron microscopic studies revealed that the structures had a fibrous pattern inside and that they were derived from ER and were surrounded with ribosomes (Hayashi et al., 2001). We proposed to call them the “ER bodies” (Hayashi et al., 2001). Electron microscopic studies showed that ER bodies developed in the epidermal cells of the non-transgenic Arabidopsis seedlings. Therefore, ER bodies are not artificial structures by overexpression of the transgene. Similar characteristic structures have been reported in the cells of various organs of Brassicaceae plants (Bonnett and Newcomb, 1965; Iversen, 1970b), which are related to Arabidopsis. Their biological function, however, has not been elucidated, so the structures have been referred to as “mystery organelles” (Gunning, 1998).
Three ER-derived compartments with specific functions have been identified in plant cells. Precursor accumulating vesicles that were found in the maturing seeds of pumpkin (Cucurbita maxima) mediate the direct transport of the precursors of storage proteins from ER into protein storage vacuoles (Hara-Nishimura et al., 1998). Two other types of ER-derived compartments, KV and ricinosome, have been shown to accumulate a Cys proteinase with an ER retention signal, KDEL. KV accumulates a vacuolar proteinase, SH-EP, responsible for breakdown of the seed storage proteins of mung bean (Vigna mungo; Toyooka et al., 2000). KV was proposed to mediate the protein mobilization in the cotyledon cells of germinated seeds (Toyooka et al., 2000). Ricinosome accumulates the same type of proteinase, Cys-EP, which is activated during senescence of castor bean (Ricinus communis) endosperm (Schmid et al., 2001). Ricinosome was suggested to be involved in programmed cell death in plant cells (Schmid et al., 2001). These compartments found in storage organs have diameters of 0.2 to 0.5 μm. The ER bodies with a characteristic shape and size (about 5 μm long) are completely distinct from the other ER-derived compartments. This implies that the ER bodies have a novel biological function.
Immunocytochemical analysis showed that ER bodies contain two Cys proteinases, RD21 and γVPE (Hayashi et al., 2001). Both RD21 and γVPE are vacuolar proteinases that are induced by environmental stresses (Koizumi et al., 1993; Kinoshita et al., 1999; Yamada et al., 2001). Electron microscopic studies revealed the fusion of ER bodies with lytic vacuoles. When seedlings are stressed with a concentrated salt solution, leading to death of the epidermal cells, ER bodies start to fuse with each other and with the vacuoles, thereby mediating the delivery of the precursor of proteinases directly to the vacuoles (Hayashi et al., 2001). Environmental stresses are known to affect signal transduction and the expression of various genes in plant cells. However, the effects of stresses on the intracellular membrane systems in plant cells have not been determined. Plant cells probably modulate the membrane systems to cope with environmental stresses. We previously suggested that ER bodies are a proteinase-sorting system that assists plant cells under various stress conditions (Hayashi et al., 2001).
In this study, we found that wound stress and treatment with methyl jasmonate (MeJA) induced many ER bodies in rosette leaves, which had no ER bodies under normal conditions. This means that environmental stresses regulate the development of ER bodies. Our findings indicate that the biological function of ER bodies is related to MeJA and wound stress, and that ER bodies are dynamic endomembrane structures that assist the plant cells under stress conditions.
RESULTS
Specific Distribution of ER Bodies in Epidermal Cells of Whole Seedlings of Arabidopsis
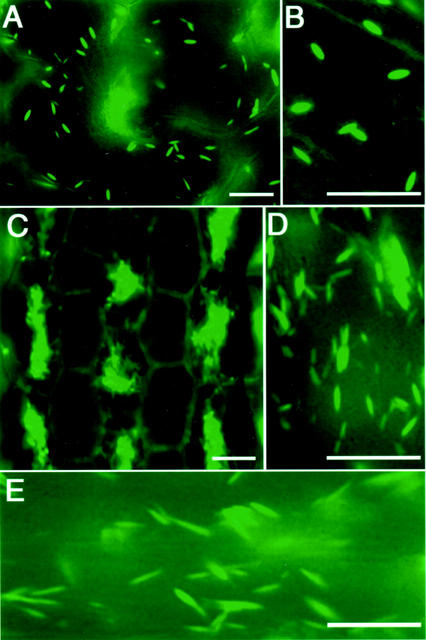
Transgenic Arabidopsis plants expressing GFP-HDEL exhibited fluorescent ER bodies of rod-shaped structures (0.5 μm diameter × 5 μm long) in the epidermal cells of cotyledons, as shown in Figure 1, A and B. In addition, a stable fluorescence on the ER network throughout the cells was detected (Fig. 1B). The hypocotyls of the seedlings showed the characteristic distribution of ER bodies (Fig. 1, C and D). Cells with a lot of ER bodies and cells with a few ER bodies lined up alternately in the hypocotyls (Fig. 1C). ER bodies found in the roots of the seedlings were longer than the others (Fig. 1E).
Figure 1.
Fluorescent images of epidermal cells of seedlings of transgenic Arabidopsis plants expressing GFP-HDEL. Arabidopsis plants were transformed with a p35S::sp-gfp-hdel gene encoding the signal peptide of pumpkin 2S albumin and GFP followed by a 12-amino acid sequence including an ER retention signal, HDEL (Mitsuhashi et al., 2000). Six-day-old (B and C) and 12-d-old (A, D, and E) seedlings were inspected with a fluorescence microscope. ER bodies (0.5-μm diameter × approximately 5 μm long) and the fluorescent ER network were found in the epidermal cells of cotyledons (A and B), hypocotyls (C and D), and roots (E) of the seedlings. Bars = 20 μm.
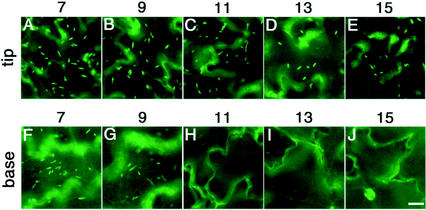
Next, we examined whether the development of ER bodies is affected by the growth stage. We investigated the developmental change in the number of ER bodies of the cotyledons during senescence. Figure 2 shows fluorescent images of the epidermal cells of 7-, 9-, 11-, 13-, and 15-d-old cotyledons. The top panels show the tip part of each cotyledon and the bottom panels show the basal parts. Cotyledons at d 7 and 9 had many ER bodies in the epidermal cells (Fig. 2, A, B, F, and G). The number of ER bodies was rapidly reduced in the basal part of the cotyledons at d 11. The basal parts of the cotyledons had no fluorescent ER bodies, but they did have fluorescent ER networks (Fig. 2H). In contrast to the rapid reduction of the number of ER bodies in the basal part, ER bodies were still detected in the tip part of the cotyledons at d 15 (Fig. 2E). This means that the disappearance of the ER bodies progresses from the basal part to the tip of the cotyledon during senescence of the tissues. The development of ER bodies depended on the growth stage of the cotyledons.
Figure 2.
The number of ER bodies in the epidermal cells of the cotyledons decreased during senescence. The tip part (A–E) and the basal part (F–J) of the cotyledons were inspected with a fluorescence microscope. The number of days after germination is indicated at the top. Disappearance of ER bodies started from the basal part of the cotyledons. The tip part (A–E) had ER bodies even at 15 d after germination, whereas the basal part (F–J) lost them in the 11-d-old cotyledons. Bars = 20 μm.
Induction of ER Bodies by Wound Stress and Treatment with MeJA in Rosette Leaves, Which Have No ER Bodies under Normal Conditions
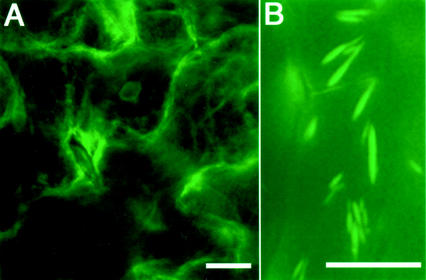
In contrast to the seedlings, which had well-developed ER bodies, the rosette leaves of the 28-d-old transgenic plants had no ER bodies at all (Fig. 3A). On the other hand, many ER bodies were found in the root cells of the plants (Fig. 3B). The ER bodies in root cells of the plants were similar in shape to those found in the root cells of the seedlings (Fig. 1E) but a little different in shape from that in cotyledons and hypocotyls (Fig. 1, A–D). These results mean that ER bodies develop organ specifically in mature plants.
Figure 3.
Fluorescent images of rosette leaves and roots of transgenic mature plants. A, Typical ER bodies were not found in epidermal cells of rosette leaves of 28-d-old plants, although fluorescent ER network was observed. B, On the other hand, ER bodies were present in the root cells even at this growth stage (27-d-old plants). Bars = 20 μm.
Next, we examined whether the formation of ER bodies is inducible. We succeeded in induction of ER bodies in rosette leaves, which have no ER bodies under normal conditions. When the detached rosette leaves were wounded with a toothpick as shown in Figure 4A, many ER bodies were induced in the leaves 44 to 66 h after wounding (Fig. 4, B and C). The development of ER bodies was limited to the peripheral regions around the wound site. This suggests that ER bodies play a role in cells that are most exposed to stresses. The induction of ER bodies depended on the way in which the leaves were wounded (data not shown). The penetration of the leaves was effective in inducing ER bodies. Squeezing with tweezers did not induce the ER bodies around the wounded site. This implies that the induction of ER bodies depends on the way in which rosette leaves are wounded.
Figure 4.
Induction of ER bodies in rosette leaves by wounding. Detached rosette leaves were wounded with toothpicks at six sites on one leaf (A) and were floated in water. Fluorescent images of the wounded sites were obtained 48 h (B) and 66 h (C) later. Induction of ER bodies was detected around the wounded sites. Bars = 20 μm.
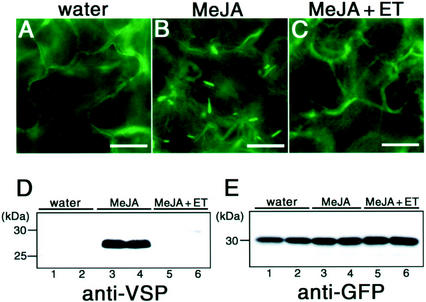
Jasmonic acid (JA) and MeJA are plant hormones that mediate plant defenses against wounding and chewing by insects (McConn et al., 1997; Wasternack and Parthier, 1997). JA is known to be accumulated in wounded leaves (Reymond et al., 2000). To clarify the effect of plant hormones, rosette leaves of the transgenic plants were floated on either water or a solution of 50 μm MeJA for 37 to 38 h and inspected with a fluorescence microscope. Figure 5, A and B, shows that ER bodies were induced in the rosette leaves treated with MeJA, although no ER bodies were found in the rosette leaves treated with water instead of MeJA. The induction by MeJA was observed throughout the rosette leaves (data not shown). The shape of the induced ER bodies was similar to that of ER bodies found in the cotyledons of seedlings (Fig. 1, A and B).
Figure 5.
Induction of ER bodies in rosette leaves treated with MeJA. Fluorescent images of rosette leaves treated with water (A), 50 μm MeJA (B), and 50 μm MeJA plus 20 μL L−1 ethylene (ET; C). Rosette leaves were floated in the hormone solutions and inspected with a fluorescence microscope 37 to 38 h later. A, The rosette leaves had no ER bodies. Water had no effect on the ER network or the development of ER bodies. B, The development of ER bodies in the rosette leaves was induced by MeJA. C, The induction of ER bodies by MeJA was suppressed by ethylene. Bars = 20 μm. Extracts were prepared from the transgenic leaves treated with water, MeJA, and MeJA plus ethylene (ET) for 36 h. The extracts were subjected to SDS-PAGE and immunoblotted with anti-VSP antibodies (D) or anti-GFP antibodies (E). Lanes 1 and 2, Water treatment; lanes 3 and 4, 50 μm MeJA treatment; lanes 5 and 6, 50 μm MeJA plus 20 μL L−1 ET. The molecular mass is given on the left in kilodaltons.
MeJA has been reported to have synergistic or antagonistic functions with another plant hormone, ethylene (Xu et al., 1994; Penninckx et al., 1998; Rojo et al., 1999). Ethylene had no ability to induce ER bodies in the rosette leaves (data not shown). To elucidate the effect of ethylene on the induction of ER bodies by MeJA, the rosette leaves of the transgenic plants were floated on a MeJA solution in the airtight box containing 20 μL L−1 ethylene gas. Figure 5C shows that no ER bodies were induced in the rosette leaves treated with MeJA plus ethylene. This means that ethylene suppressed the effect of MeJA. MeJA and ethylene have an antagonistic effect on the induction of ER bodies. These results showed that the biological roles of ER bodies are related to MeJA and ethylene.
As a control for chemical treatments, we checked the expression pattern of vegetative storage protein (VSP) in rosette leaves after the chemical treatment. It had already been reported that VSP mRNA was induced by MeJA and suppressed by ethylene (Rojo et al., 1999). We prepared the protein extracts from rosette leaves after treatment with water, MeJA, and MeJA plus ethylene and then performed an immunoblot analysis with anti-VSP antibodies (Fig. 5D). Rosette leaves treated with water hardly contained VSP (Fig. 5D, lanes 1 and 2). However, a large amount of VSP was induced by MeJA treatment (Fig. 5D, lanes 3 and 4). The induction was suppressed by ethylene (Fig. 5D, lanes 5 and 6). The results are consistent with the previous report (Rojo et al., 1999). These results showed that the chemical treatments were successfully performed. The induction pattern of VSP was the same as that of ER bodies (Fig. 5, A–C). It raised the possibility that VSP was localized in the induced ER bodies. We performed immunocytochemistry using anti-VSP antibodies. However, no positive signal of VSP was detected in ER bodies (data not shown; discussed below).
Some studies have reported that the overproduction of proteins with an ER retention signal resulted in the formation of ER-derived structures (Denecke et al., 1992; Wandelt et al., 1992; Crofts et al., 1999). We examined the effect of the GFP-HDEL overexpression on the induction of ER bodies. The amount of GFP in the rosette leaves after the treatments with water, MeJA, and MeJA plus ethylene was monitored (Fig. 5E). The expression pattern of GFP was different from the induction pattern of ER bodies (Fig. 5, A–C). No significant increase in the GFP expression level was detected after MeJA treatment. This means that overexpression of GFP-HDEL did not result in the induction of ER bodies in rosette leaves.
Induction of ER Bodies Was Observed in Rosette Leaves of Non-Transgenic Plants
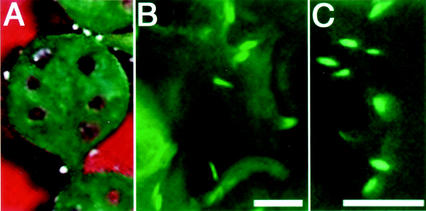
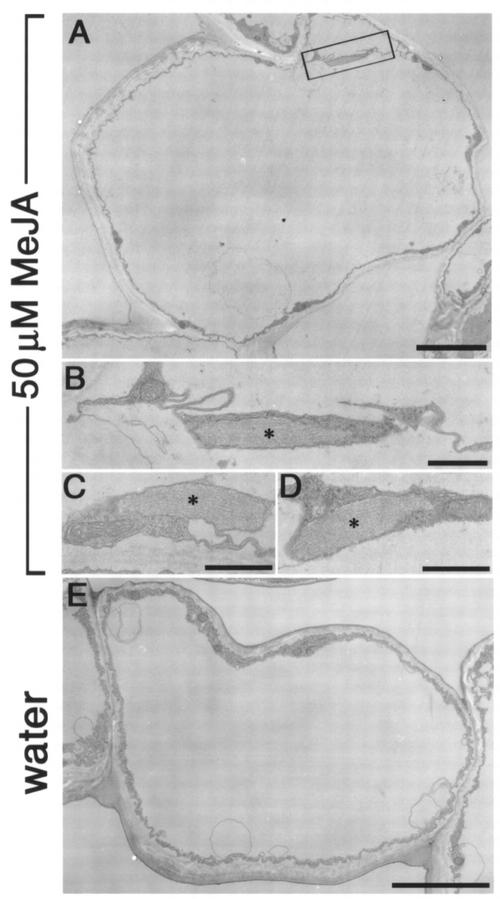
Next, we examined whether the induction of ER bodies by MeJA is observed in non-transgenic rosette leaves. We performed an electron microscopic analysis with the epidermal cells of wild type rosette leaves treated with MeJA. Ultrastructural analysis revealed that MeJA-treated rosette leaves contained ER bodies in the epidermal cells (Fig. 6, A–D). The induction of ER bodies was limited in the epidermal cells (data not shown). ER bodies had a characteristic fibrous pattern inside (Fig. 6, B–D). The ultrastructural shapes of induced ER bodies were similar with the ER bodies in young seedlings (Hayashi et al., 2001). Water-treated rosette leaves has no detectable ER bodies in epidermal cells (Fig. 6E). These results confirmed that MeJA-treatment induced ER bodies in rosette leaves and that the induction was not resulted from the overexpression of GFP-HDEL.
Figure 6.
Electron micrographs showing that rosette leaves of non-transgenic Arabidopsis induced ER bodies when treated with MeJA. A, Rod-shaped ER bodies were induced in the epidermal cells of rosette leaves treated with MeJA. Bar = 5 μm. B, An ER body with a characteristic fibrous pattern in the boxed area of A is magnified. Bar = 1 μm. C and D, ER bodies were often detected in the treated tissues. Asterisks indicate the ER body. Bar = 1 μm. E, No ER body was detected in the epidermal cells of wild-type rosette leaves treated with water. Bar = 5 μm.
Binding Protein (BiP) Expression Is Not Responsible for the Induction of ER Bodies
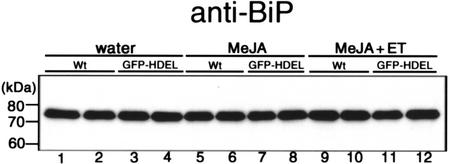
Lumenal BiP, a member of the Hsp70 family, is an authentic ER-resident protein. Induction of BiP expression was observed when plant was under stress condition by treatment with fungal cell wall-degrading enzymes (Jelitto-Van Dooren et al., 1999). We examined whether the content of BiP is increased by MeJA treatment (Fig. 7). We performed an immunoblot analysis with anti-BiP antibodies. Treatment with MeJA (Fig. 7, lanes 5–8) or MeJA plus ethylene (Fig. 7, lanes 9–12) did not affect the BiP expression level in rosette leaves compared with the control experiment with water treatment (Fig. 7, lanes 1–4). Expression level of BiP was almost same between the wild type plants and the transgenic plants. This means that BiP expression is not responsible for the induction of ER bodies in the rosette leaves.
Figure 7.
An immunoblot showing BiP content of rosette leaves was not affected by chemical treatments. Extracts were prepared from the wild-type (Wt) and transgenic (GFP-HDEL) leaves treated with water, 50 μm MeJA, and 50 μm MeJA plus 20 μL L−1 ethylene (ET) for 36 h. The extracts were subjected to SDS-PAGE and immunoblotted with anti-BiP antibodies. Lanes 1 and 2, Wild type after water treatment; lanes 3 and 4, transgenic plants after water treatment; lanes 5 and 6, wild type treated with MeJA; lanes 7 and 8, transgenic plants treated with MeJA; lanes 9 and 10, wild type treated with MeJA plus ET; lanes 11 and 12, transgenic plants treated with MeJA plus ET.
MeJA Does Not Induce ER Bodies in Rosette Leaves of coi1 Mutant Plants That Are Insensitive to Exogenous MeJA
MeJA induced ER bodies in rosette leaves, and ethylene suppressed the induction by MeJA. This raises a question of whether ER bodies are induced in the rosette leaves of mutant plants that are related to these plant hormones. To answer this question, we examined crosses between transgenic plants expressing GFP-HDEL and two Arabidopsis mutants (coi1 and etr1-4) that exhibit altered expression of MeJA- and wound-induced genes (Xie et al., 1998; Rojo et al., 1999). coi1 mutant plants are insensitive to exogenous MeJA (Feys et al., 1994; Xie et al., 1998). etr1-4 mutant plants are insensitive to ethylene (Chang et al., 1993). After crossing the plants, we isolated mutant plants that had the p35S::sp-gfp-hdel gene. The rosette leaves of each mutant plant expressing GFP-HDEL were treated with MeJA and/or ethylene and inspected with a fluorescence microscope. Table I summarizes the results obtained. When rosette leaves of coi1 mutant plants were treated with MeJA, ER bodies was not induced. Exogenous MeJA had no effect on the MeJA-insensitive mutants. Treatment of rosette leaves of the etr1-4 mutant plants with MeJA induced ER bodies even in the presence of ethylene. These results indicated that the induction of ER bodies was strictly coupled with the signal transduction of MeJA and ethylene.
Table I.
Induction of ER bodies in MeJA- and ethylene-related mutants
| MeJA | MeJA + ET | ET | |
|---|---|---|---|
| Wta | + | − | − |
| etr1-4 | + | + | − |
| coi1 | − |
Two MeJA- or ethylene-related mutants (coi1 and etr1-4) were crossed with transgenic plants expressing GFP-HDEL. Rosette leaves of the mutants were treated with MeJA or ethylene (ET), and were inspected with a fluorescence microscope. +, ER bodies were induced throughout the rosette leaves. −, No ER body was induced.
Wt, Transgenic plants expressing GFP-HDEL.
DISCUSSION
ER bodies exhibit unique distribution in Arabidopsis plants. They were well distributed in the whole young seedlings (Fig. 1) but not in the rosette leaves (Figs. 3A and 5A). We found that the wound stress and MeJA treatment induced many ER bodies in the rosette leaves of the transgenic Arabidopsis expressing GFP fused with an ER retention signal (Figs. 4 and 5B). The shape of the induced ER bodies was similar to that of ER bodies in the young seedlings. It was reported that the overproduction of a foreign protein with an ER retention signal resulted in the formation of some ER-derived structures (Denecke et al., 1992; Wandelt et al., 1992; Crofts et al., 1999). However, the amount of GFP in the rosette leaves did not change after induction of ER bodies (Fig. 5E). In addition, ER body can be observed in young cotyledons (Hayashi et al., 2001) and MeJA-treated rosette leaves (Fig. 6) of non-transgenic plants. Thus, the formation of ER bodies is not attributable to the overproduction of GFP-HDEL.
Many ER bodies were induced in the rosette leaves when treated with MeJA (Fig. 5B). MeJA is a plant hormone that mediates plant defenses against wounding and chewing by insects (McConn et al., 1997; Wasternack and Parthier, 1997). The induction of ER bodies was also observed in the wounded leaves (Fig. 4). These results suggest that the induced ER bodies in the rosette leaves have a defense function against chewing insects and other wound stresses.
We previously reported that two stress-inducible proteinases, RD21 and γVPE, are localized in the ER bodies (Hayashi et al., 2001). This implies that the development of ER bodies is linked with environmental stresses. The cotyledons, especially their epidermal cells, which are most sensitive to environmental stresses in the plant life, had a large number of ER bodies. We postulated that ER bodies are responsible for plant defense against stresses (Hayashi et al., 2001).
It is not clear that the induced ER bodies in the rosette leaves have the same function of ER bodies in the young seedlings. ER bodies in the cotyledons accumulated the precursor of vacuolar Cys proteinases, RD21 and γVPE (Hayashi et al., 2001). Exogenous MeJA did not induce the expression of γVPE (Kinoshita et al., 1999) or RD21 (K. Yamada and I. Hara-Nishimura, unpublished data). Thus, these proteins are not the materials in the induced ER bodies in rosette leaves. It is possible that the induced ER bodies in the rosette leaves have different functions and contents from those of ER bodies in young seedlings.
To clarify the function of ER bodies at the molecular level, we need to know the main materials in the ER bodies. The main materials can be expected to exhibit the same induction pattern as the ER bodies. They will namely be induced by MeJA and suppressed by ethylene. It has been shown that wound stress or MeJA treatment induces a number of genes, including genes for pathogen-related proteins, proteinase inhibitors, and VSP (Wasternack and Parthier, 1997). VSP especially was induced by MeJA and suppressed by ethylene (Fig. 5D), as described by Rojo et al. (1999). The induction pattern was the same as that of ER bodies. However, an immunocytochemistry with anti-VSP antibodies showed that no positive signal of VSP was detected in ER bodies (data not shown). In contrast, the vacuole was stained with the anti-VSP antibodies. This result was consistent with the previous study (Franceschi et al., 1983). Therefore, VSP was not the main material in the ER bodies.
A KDEL-tailed Cys proteinase was reported to be the dominant protein in the ricinosome, which is an ER-derived structure in endosperm of castor bean (Schmid et al., 2001). It is possible that abundant production of authentic KDEL-tailed proteins contribute to the formation of ER bodies. A novel type of myrosinase, Pyk10, from Arabidopsis was recently reported to have an ER retention signal, KDEL (Nitz et al., 2001). Myrosinase is a thioglycosidase that catalyzes the hydrolysis of glucosinolates (Bones and Rossiter, 1996; Rask et al., 2000). ER body-like structures, “dilated cisternae,” have been reported to exist, mainly in the Brassicaceae family (Iversen, 1970b; Behnke and Eschlbeck, 1978). Several attempts have been made to correlate the presence of dilated cisternae with myrosinase (Iversen, 1970a). However, no direct evidence for such a correlation has been presented (Thangstad et al., 1990, 1991). It is unknown whether Pyk10 has myrosinase activity or not. However, one of the homologs of Pyk10 is suggested to be related with defense responses against herbivorous insects (Stotz et al., 2000). Pyk10 is one of the most increased proteins during seed germination (Gallardo et al., 2001). In the young seedlings, Pyk10 is a major protein in cotyledon, hypocotyl, and roots but not in the rosette leaves (data not shown). It is possible that abundant production of Pyk10 contributes to the formation of ER bodies in the young seedlings. Further studies are necessary to elucidate the characterization of Pyk10 at the subcellular level.
MATERIALS AND METHODS
Growth Conditions of Arabidopsis Plants
Arabidopsis (ecotype Columbia) was transformed with a chimeric gene encoding SP-GFP-HDEL as described previously (Mitsuhashi et al., 2000). Seeds of the transgenic plants were sown on soil or onto 0.5% (w/v) Gellan Gum (Wako, Tokyo) and Murashige and Skoog medium and were grown at 22°C under continuous light conditions.
Fluorescence Microscopy
Various organs of the transgenic plants were mounted in water on glass coverslips. The specimens were examined with a fluorescence microscope (Axiophot, Carl Zeiss, Jena, Germany) using a filter set (BP450-490 excitation filter, FT510 dichroic mirror, and BP515-565 barrier filter, Carl Zeiss). The GFP fluorescent images were analyzed with Photoshop software (Adobe Systems, Tokyo).
Chemical and Wound Treatments
The rosette leaves from 15- or 16-d-old plants were floated on 50 μm MeJA solution and incubated at 22°C under continuous light conditions. To determine the response to ethylene, the floated leaves were transferred to an airtight box containing 20 μL L−1 of the ethylene gas. The rosette leaves were inspected with a fluorescence microscope at 37 to 38 h after the treatments.
For wound treatment, the first and second rosette leaves of 16-d-old plants were wounded at six sites per leaf with a toothpick. The wounded leaves were floated in water and incubated at 22°C under continuous light conditions. The rosette leaves were inspected with a fluorescence microscope at 48 to 66 h after treatments.
Preparation of Specific Antibodies
An expressed sequence tag clone encoding VSP (ATTS1295) was obtained from Arabidopsis Biological Resource Center. A cDNA encoding VSP was inserted into the pET32 vector (Novagen, Madison, WI). The fusion protein with a His-tag was synthesized in Escherichia coli BL21(DE3) cells and was purified with a Ni2+ column. The purified fusion protein was injected into a rabbit subcutaneously with complete Freund's adjuvant. After 3 weeks, two booster injections with incomplete adjuvant were given at 7-d intervals. One week after the booster injections, blood was drawn and the antibodies were prepared. We used antibodies against GFP (BD Biosciences Clontech, Palo Alto, CA). Anti-BiP antibodies were described previously (Hatano et al., 1997).
Immunoblot Analysis
Extracts were prepared from leaves treated with MeJA or ethylene for 36 h. One leaf after chemical treatment was homogenized in 200 μL of extraction buffer (100 mm Tris-HCl, pH 6.8, 2% [w/v] SDS, 40% [v/v] glycerol, and 2% [v/v] 2-mercaptoethanol). Five microliters of the extracts was subjected to SDS-PAGE and transferred electrophoretically to a GVHP membrane (0.22 μm; Nihon Millipore, Tokyo). The blotted membrane was thoroughly dried for blocking. The membrane was incubated in Tris-buffered saline (pH 7.5) plus 0.05% (v/v) Tween 20 with the specific antibodies for 1 h. Dilutions of the antibodies were as follows; anti-VSP (1:2,000 [v/v]), anti-GFP (1:10,000 [v/v]), and anti-BiP (1:10,000 [v/v]). Horseradish peroxidase-conjugated goat antibodies against rabbit IgG (Amersham Japan, Tokyo) were diluted (1:5,000 [v/v]) to be used as second antibodies. Immunodetection was performed with an ECL kit (Amersham Japan) according to the manufacturer's directions.
Ultrastructural Analysis
The rosette leaves treated with MeJA or water were vacuum-infiltrated with a fixative that consisted of 4% (w/v) paraformaldehyde and 1% (v/v) glutaraldehyde in 0.05 m cacodylate buffer (pH 7.4). The tissues were then cut into slices with a razor blade and treated for another 2 h with freshly prepared fixative. Procedures for ultrastructural studies were essentially the same as those described previously (Hara-Nishimura et al., 1993), except that the material was postfixed with 1% (w/v) osmium tetroxide in 0.1 m cacodylate buffer (pH 7.4) at 4°C for 2 h. Ultrathin sections were cut with a diamond knife on a Reichert ultramicrotome (Reichert, Leica, Heidelberg) and mounted on copper grids. The sections were stained with 4% (w/v) uranyl acetate and lead citrate. After staining, sections were examined with a transmission electron microscope (model 1200EX, JEOL, Tokyo) at 80 kV.
Transformation of the Mutants (coi1 and etr1-4) with the p35S::sp-gfp-hdel Gene
Arabidopsis mutant coi1 was donated by Dr. John G. Turner (University of East Anglia, Norwich, UK). The mutant etr1-4 was provided by Arabidopsis Biological Resource Center. The transgenic plants with the homozygous gene (p35S::sp-gfp-hdel) were crossed with each of two mutants (coi1 and etr1-4). We isolated the coi1 mutants that exhibited GFP fluorescence from F2 progeny plants. The coi1 mutants exhibited male sterility, which was used to isolate these mutants. To examine ER bodies in the etr1-4 mutants, we used F1 progeny that are heterozygous for the etr1-4, which is a dominant mutation (Bleecker et al., 1988).
ACKNOWLEDGMENTS
We thank Dr. John G. Turner (University of East Anglia, Norwich, UK) for his kind donation of Arabidopsis mutant coi1. We also thank Dr. Niwa (University of Shizuoka, Japan) for his kind donation of the modified GFP gene with a strong fluorescence.
Footnotes
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grants-in-Aid for Scientific Research nos. 10182102, 12138205, and 12304049) and by the Japan Society for the Promotion of Science (postdoctoral fellowship no. 14001468 to R.M.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.009464.
LITERATURE CITED
- Behnke H-D, Eschlbeck G. Dilated cisternae in Capparales: an attempt towards the characterization of a specific endoplasmic reticulum. Protoplasma. 1978;97:351–363. [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Bones AM, Rossiter JT. The myrosinase-glucosinolate system, its organization and biochemistry. Physiol Plant. 1996;97:194–208. [Google Scholar]
- Bonnett HT, Jr, Newcomb EH. Polyribosomes and cisternal accumulations in root cells of radish. J Cell Biol. 1965;27:423–432. doi: 10.1083/jcb.27.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Crofts AJ, Leborgne-Castel N, Hillmer S, Robinson DG, Phillipson B, Carlsson LE, Ashford DA, Denecke J. Saturation of the endoplasmic reticulum retention machinery reveals anterograde bulk flow. Plant Cell. 1999;11:2233–2248. doi: 10.1105/tpc.11.11.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke J, De Rycke R, Botterman J. Plant and mammalian sorting signals for protein retention in the endoplasmic reticulum contain a conserved epitope. EMBO J. 1992;11:2345–2355. doi: 10.1002/j.1460-2075.1992.tb05294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJF, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to bacterial pathogen. Plant Cell. 1994;6:751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi VR, Wittenbach VA, Giaquinta RT. Paraveinal mesophyll of soybean leaves in relation to assimilate transfer and compartmentation. Plant Physiol. 1983;72:586–589. doi: 10.1104/pp.72.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo K, Job C, Groot SPC, Puype M, Demol H, Vandekerckhove J, Job D. Proteomic analysis of Arabidopsis seed germination and priming. Plant Physiol. 2001;126:838–848. doi: 10.1104/pp.126.2.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning BES. The mystery organelles in Arabidopsis expressing GFP. Trend Plant Sci. 1998;3:417. [Google Scholar]
- Hara-Nishimura I, Shimada T, Hatano K, Takeuchi Y, Nishimura M. Transport of storage proteins to protein-storage vacuoles is mediated by large precursor-accumulating vesicles. Plant Cell. 1998;10:825–836. doi: 10.1105/tpc.10.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura I, Takeuchi Y, Inoue K, Nishimura M. Vesicle transport and processing of the precursor to 2S albumin in pumpkin. Plant J. 1993;4:793–800. doi: 10.1046/j.1365-313x.1993.04050793.x. [DOI] [PubMed] [Google Scholar]
- Haseloff J, Siemering KR, Prasher DC, Hodge S. Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci USA. 1997;94:2122–2127. doi: 10.1073/pnas.94.6.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano K, Shimada T, Hiraiwa N, Nishimura M, Hara-Nishimura I. A rapid increase in the level of binding protein (BiP) is accompanied by synthesis and degradation of storage proteins in pumpkin cotyledons. Plant Cell Physiol. 1997;38:344–351. doi: 10.1093/oxfordjournals.pcp.a029172. [DOI] [PubMed] [Google Scholar]
- Hawes C, Saint-Jore C, Martin B, Zheng H-Q. ER confirmed as the location of mystery organelles in Arabidopsis plants expressing GFP. Trends Plant Sci. 2001;6:245–246. doi: 10.1016/s1360-1385(01)01980-x. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Yamada K, Shimada T, Matsushima R, Nishizawa N, Nishimura M, Hara-Nishimura I. A proteinase-storing body that prepares for cell death or stresses in the epidermal cells of Arabidopsis. Plant Cell Physiol. 2001;42:894–899. doi: 10.1093/pcp/pce144. [DOI] [PubMed] [Google Scholar]
- Iversen T-H. Cytochemical localization of myrosinase (β-thioglucosidase) in root tips of Sinapis alba. Protoplasma. 1970a;71:451–466. [Google Scholar]
- Iversen T-H. The morphology, occurrence, and distribution of dilated cisternae of the endoplasmic reticulum in tissues of plants of the Cruciferae. Protoplasma. 1970b;71:467–477. [Google Scholar]
- Jelitto-Van Dooren EWM, Vidal S, Denecke J. Anticipating endoplasmic reticulum stress: a novel early response before pathogenesis-related gene induction. Plant Cell. 1999;11:1935–1943. doi: 10.1105/tpc.11.10.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Yamada K, Hiraiwa N, Nishimura M, Hara-Nishimura I. Vacuolar processing enzyme is up-regulated in the lytic vacuoles of vegetative tissues during senescence and under various stressed conditions. Plant J. 1999;19:43–53. doi: 10.1046/j.1365-313x.1999.00497.x. [DOI] [PubMed] [Google Scholar]
- Koizumi M, Yamaguchi-Shinozaki K, Tsuji H, Shinozaki K. Structure and expression of two genes that encode distinct drought-inducible cysteine proteinases in Arabidopsis thaliana. Gene. 1993;129:175–182. doi: 10.1016/0378-1119(93)90266-6. [DOI] [PubMed] [Google Scholar]
- McConn M, Creelman RA, Bell E, Mullet JE, Browse J. Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA. 1997;94:5473–5477. doi: 10.1073/pnas.94.10.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi N, Shimada T, Mano S, Nishimura M, Hara-Nishimura I. Characterization of organelles in the vacuolar-sorting pathway by visualization with GFP in tobacco BY-2 cells. Plant Cell Physiol. 2000;41:993–1001. doi: 10.1093/pcp/pcd040. [DOI] [PubMed] [Google Scholar]
- Nitz I, Berkefeld H, Puzio PS, Grundler FMW. Pyk10, a seedling and root specific gene and promoter from Arabidopsis thaliana. Plant Sci. 2001;161:337–346. doi: 10.1016/s0168-9452(01)00412-5. [DOI] [PubMed] [Google Scholar]
- Penninckx IAMA, Thomma BPHJ, Buchala A, Metraux J-P, Broekaert WF. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell. 1998;10:2103–2113. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask L, Andreasson E, Ekbom B, Eriksson S, Pontoppidan B, Meijer J. Myrosinase: gene family evolution and herbivore defense in Brassicaceae. Plant Mol Biol. 2000;42:93–113. [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell. 2000;12:707–720. doi: 10.1105/tpc.12.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge RW, Uozumi Y, Plazinski J, Hurley UA, Williamson RE. Developmental transitions and dynamics of the cortical ER of Arabidopsis cells seen with green fluorescent protein. Plant Cell Physiol. 1999;40:1253–1261. doi: 10.1093/oxfordjournals.pcp.a029513. [DOI] [PubMed] [Google Scholar]
- Rojo E, Leon J, Sanchez-Serrano JJ. Cross-talk between wound signalling pathways determines local versus systemic gene expression in Arabidopsis thaliana. Plant J. 1999;20:135–142. doi: 10.1046/j.1365-313x.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- Schmid M, Simpson DJ, Sarioglu H, Lottspeich F, Gietl C. The ricinosomes of senescing plant tissue bud from the endoplasmic reticulum. Proc Natl Acad Sci USA. 2001;98:5353–5358. doi: 10.1073/pnas.061038298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz HU, Pittendrigh BR, Kroymann J, Weniger K, Fritsche J, Bauke A, Mitchell-Olds T. Induced plant defense responses against chewing insects: Ethylene signaling reduces resistance of Arabidopsis against cotton worm but not diamondback moth. Plant Physiol. 2000;124:1007–1017. doi: 10.1104/pp.124.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangstad OP, Evjen K, Bones A. Immunogold-EM localization of myrosinase in Brassicaceae. Protoplasma. 1991;161:85–93. [Google Scholar]
- Thangstad OP, Iversen T-H, Slupphaug G, Bones A. Immunocytochemical localization of myrosinase in Brassica napus L. Planta. 1990;180:245–248. doi: 10.1007/BF00194003. [DOI] [PubMed] [Google Scholar]
- Toyooka K, Okamoto T, Minamikawa T. Mass transport of proform of a KDEL-tailed cysteine proteinase (SH-EP) to protein storage vacuoles by endoplasmic reticulum-derived vesicle is involved in protein mobilization in germinating seeds. J Cell Biol. 2000;148:453–463. doi: 10.1083/jcb.148.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandelt CI, Khan MRI, Carig S, Schroeder HE, Spencer D, Higgins TJV. Vicilin with carboxy-terminal KDEL is retained in the endoplasmic reticulum and accumulates to high levels in the leaves of transgenic plants. Plant J. 1992;2:181–192. doi: 10.1046/j.1365-313x.1992.t01-41-00999.x. [DOI] [PubMed] [Google Scholar]
- Wasternack C, Parthier B. Jasmonate-signalled plant gene expression. Trend Plant Sci. 1997;2:302–307. [Google Scholar]
- Xie D-X, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280:1091–1094. doi: 10.1126/science.280.5366.1091. [DOI] [PubMed] [Google Scholar]
- Xu Y, Chang P-FL, Liu D, Narasimhan ML, Raghothama KG, Hasegawa PM, Bressan RA. Plant defense genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell. 1994;6:1077–1085. doi: 10.1105/tpc.6.8.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Matsushima R, Nishimura M, Hara-Nishimura I. A slow maturation of a cysteine protease with a granulin domain in the vacuoles of senescing Arabidopsis leaves. Plant Physiol. 2001;127:1626–1634. [PMC free article] [PubMed] [Google Scholar]