Abstract
Iron deficiency impairs chlorophyll biosynthesis and chloroplast development. In leaves, most of the iron must cross several biological membranes to reach the chloroplast. The components involved in the complex internal iron transport are largely unknown. Nitric oxide (NO), a bioactive free radical, can react with transition metals to form metal-nitrosyl complexes. Sodium nitroprusside, an NO donor, completely prevented leaf interveinal chlorosis in maize (Zea mays) plants growing with an iron concentration as low as 10 μm Fe-EDTA in the nutrient solution. S-Nitroso-N-acetylpenicillamine, another NO donor, as well as gaseous NO supply in a translucent chamber were also able to revert the iron deficiency symptoms. A specific NO scavenger, 2-(4-carboxy-phenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, blocked the effect of the NO donors. The effect of NO treatment on the photosynthetic apparatus of iron-deficient plants was also studied. Electron micrographs of mesophyll cells from iron-deficient maize plants revealed plastids with few photosynthetic lamellae and rudimentary grana. In contrast, in NO-treated maize plants, mesophyll chloroplast appeared completely developed. NO treatment did not increase iron content in plant organs, when expressed in a fresh matter basis, suggesting that root iron uptake was not enhanced. NO scavengers 2-(4-carboxy-phenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide and methylene blue promoted interveinal chlorosis in iron-replete maize plants (growing in 250 μm Fe-EDTA). Even though results support a role for endogenous NO in iron nutrition, experiments did not establish an essential role. NO was also able to revert the chlorotic phenotype of the iron-inefficient maize mutants yellow stripe1 and yellow stripe3, both impaired in the iron uptake mechanisms. All together, these results support a biological action of NO on the availability and/or delivery of metabolically active iron within the plant.
Iron deficiency impairs chlorophyll biosynthesis and chloroplast development in both dicotyledonous and monocotyledonous species. Therefore, iron availability maintains a direct correlation with plant productivity. Chlorosis because of unavailability of iron in calcareous soils (high pH) is a major agricultural problem that results in diminished crop yields in an estimated 30% of calcareous soils worldwide (Mori, 1999).
Iron deficiency responses involve several physiological plant adaptations (Guerinot and Yi, 1994; Mori, 1999). Under iron deficiency, plants have evolved two separate strategies for iron acquisition. Non-graminaceous plants (strategy I) enhance acidification of the extracellular medium and increase both root ferric-reducing capacity and uptake of ferrous iron. In contrast, graminaceous plants possess the ability to secrete phytosiderophores to enhance iron uptake from soils (strategy II). However, when iron availability is under a threshold level, both strategies I and II are not sufficient to support the iron requirement for plant development, and stress symptoms become evident.
Furthermore, iron acquisition from the soil is not the only limiting step in iron use by plants. Because most of the leaf iron (80%) is located in the chloroplast, it must cross several biological membranes to arrive at its final destination. Iron is probably transported in the xylem as Fe(III)-citrate (Guerinot and Yi, 1994), and reduction of Fe(III) to Fe(II) is an essential requisite to cross the plasma membrane. The enzyme involved in this reaction is the plasma membrane-bound iron(III)-chelate reductase, whose activity seems to depend on the apoplastic pH (Kosegarten et al., 1999; González-Vallejo et al., 2000) and light (Brüggemann et al., 1993; de la Guardia and Alcántara, 1996; González-Vallejo et al., 2000). In addition, Fe(III) reduction in vivo may be aided by intermediate superoxide radical formation (Brüggemann et al., 1993), indicating that changes in the redox state of the apoplast might be involved in Fe(III) reduction. However, the features of the chemical reduction and the multistep transport of iron inside the cell and inside the chloroplast are still largely unknown. It was suggested that some steps of the internal transport system may be impaired by the iron deficiency itself (González-Vallejo et al., 2000; Larbi et al., 2001). There is also evidence that iron could be immobilized and accumulated as inactive forms in the leaf (Morales et al., 1998; Kosegarten et al., 1999), and this would explain why in many cases chlorotic leaves from iron-deficient plants have total iron concentration similar to those of iron-sufficient plants (Abadía, 1992).
Nitric oxide (NO) is a bioactive free radical implicated in a number of physiological functions, including intra- and intercellular mediation of some animal responses (Anbar, 1995). In plants, NO is involved in the signaling of growth, development, and adaptive responses to multiple stresses (Durner and Klessig, 1999; Beligni and Lamattina, 2001b) and in a number of cytotoxic and cytoprotective effects (Beligni and Lamattina, 1999a, 1999b, 2001a). Not only do plants produce significant amounts of NO, but they also respond to atmospheric NO. NO action is achieved either directly, by reaction with effector molecules or indirectly, modifying the redox state of the cell. NO can readily form complexes with transition metal ions in aqueous solutions or those present in diverse nucleophylic compounds such as metalloproteins (Stamler et al., 1992). Metal-nitrosyl complexes are formed under neutral physiological conditions and were proposed to act as a link between the different redox states of NO (Stamler et al., 1992). The Fe(III) NO complex appears to undergo a charge transfer reaction to form Fe(II) NO+ (Olson, 1981). On the basis of these chemical properties of NO, we wished to investigate whether NO is involved in iron homeostasis in plants. We evaluated the ability of NO to cope with iron deficiency symptoms in maize (Zea mays) plants and the effect of depleting endogenous NO in iron-sufficient plants. In this report, we present compelling data that reveal a novel effect of NO in plant biology, more specifically on iron nutrition. Our results support the idea that NO is closely related to iron metabolism, transport, and/or availability and, consequently, to chlorophyll biosynthesis and chloroplast development.
RESULTS
NO Induces Greening in Iron-Deficient Maize Plants
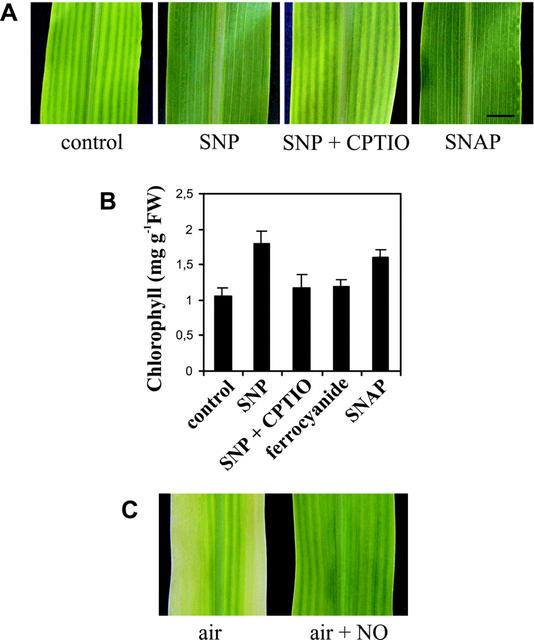
One symptom usually associated with iron deficiency is an interveinal yellowing of the leaves (Terry, 1980; Thoiron et al., 1997). Chlorophyll quantification has been widely used to estimate the effect of iron deficiency on plant metabolism (Thoiron et al., 1997). Therefore, we first evaluated the effect of NO on chlorophyll content in maize plants growing under iron-insufficient conditions (50 μm Fe-EDTA; Stocking, 1975). The younger leaves were more drastically affected by iron deficiency than the older ones. Sodium nitroprusside (SNP), an NO donor, completely prevented leaf interveinal chlorosis (Fig. 1A), producing a 70% increase in the chlorophyll content compared with control plants (Fig. 1B). An NO-specific scavenger, 2-(4-carboxy-phenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (CPTIO), was able to block the effect of the NO donor (Fig. 1, A and B). Moreover, sodium ferrocyanide, an analog of SNP that does not release NO, had no effect on the chlorophyll levels in iron-deficient plants (Fig. 1B). Another NO donor, S-nitroso-N-acetylpenicillamine (SNAP), was also able to promote greening of iron-deficient maize leaves and increased the chlorophyll content by 50% over the level found in untreated plants (Fig. 1, A and B). In another set of experiments, air was supplied with 100 μL L−1 gaseous NO inside a translucent chamber, and this allowed the reversion of chlorosis in maize plants growing under iron-deficient conditions (Fig. 1C). Although a delayed development of plants in an NO-enriched atmosphere has been reported (Wellburn, 1998), in our experimental assays, no visible alterations in maize plants occurred in the presence of the NO concentration used. Hydroponically grown maize plants (Fig. 1C, air) displayed more evident symptoms of iron deficiency than those grown in vermiculite with the same iron supply (Fig. 1A, control), probably because of iron traces in vermiculite substrate.
Figure 1.
Effect of NO on the phenotype and chlorophyll content of iron-deficient maize plants. A, Twenty-day-old maize plants were grown on vermiculite, watered with nutrient solution containing 50 μm Fe(III)-EDTA, and treated once a week with 100 μm SNP, 100 μm SNP plus 200 μm CPTIO (SNP+CPTIO), 100 μm sodium ferrocyanide, 100 μm SNAP, or untreated (control). The picture shows a section of a completely developed fourth leaf. Bar = 1 cm. B, Chlorophyll content in the fourth leaves of maize plants treated as described above. Mean values and sds were calculated from two to four independent experiments. C, Ten-day-old maize seedlings growing hydroponically in a nutrient solution containing 50 μm Fe(III)-EDTA were transferred to a translucent chamber with air (control) or air supplemented with 100 μL L−1 gaseous NO. Photographs of a section of the fourth leaves were taken 10 d after the treatment.
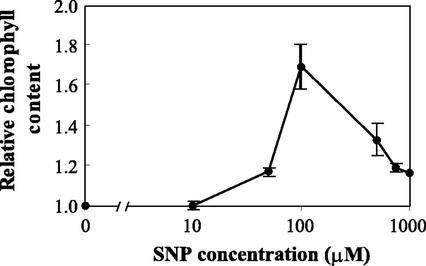
NO-mediated increase in chlorophyll content was dose dependent. Figure 2 shows the relative chlorophyll level in iron-deficient maize plants treated with increasing concentrations of SNP. Although 10 μm SNP was not effective in increasing the chlorophyll content, concentrations between 50 and 500 μm SNP were able to significantly revert the interveinal chlorosis, with 100 μm SNP being the most effective under our experimental conditions (Fig. 2). Nanomolar amounts of NO (100 nm–1 μm) are released from 100 μm SNP, as measured by the Griess reagent. At the highest concentrations of SNP assayed (from 500–1000 μm), relative chlorophyll content was lower than at 100 μm SNP. At those concentrations, chlorophyll breakdown because of NO toxicity itself is probably operating (Beligni and Lamattina, 1999b), masking the effect of NO on the reversion of iron deficiency.
Figure 2.
Dose response curve showing SNP effect on chlorophyll content in iron-deficient maize plants. Twenty-day-old maize plants were grown in vermiculite with nutrient solution containing 50 μm Fe(III)-EDTA and treated with 10, 50, 100, 500, 750, or 1,000 μm SNP. Chlorophyll content in the fourth leaves was measured. The values are expressed relative to control plants (untreated). Each point corresponds to the mean of two to four independent experiments; bars indicate the sds.
Effect of NO on Chloroplast Ultrastructure and Chloroplast-Encoded mRNAs in Iron-Deficient Maize Plants
The symptoms of iron deficiency are often associated with cytological alterations, which mainly affect the chloroplast ultrastructure and its protein composition (Stocking, 1975; Winder and Nishio, 1995; Thoiron et al., 1997). We evaluated the effect of NO on chloroplast ultrastructure in iron-deficient maize plants. Electron micrographs of mesophyll cells from iron-deficient maize plants revealed plastids with few photosynthetic lamellae and with some rudimentary grana, displaying classical features of thylakoid disorganization induced by iron deprivation (Stocking, 1975; Thoiron et al., 1997; Fig. 3A). In contrast, when iron-deficient plants were treated with NO, mesophyll chloroplasts appeared completely developed, with normal grana stacking (Fig. 3B), resembling plants growing in iron-sufficient concentrations (Stocking, 1975). In bundle sheath chloroplasts of iron-deficient plants, electron micrographs also revealed important differences between NO-treated and control plants. Control plants had chloroplasts with no detectable starch granules and increased number of plastoglobuli (Fig. 3C), whereas bundle sheath chloroplasts from NO-treated plants did not display any visible alteration (Fig. 3D).
Figure 3.
Effect of NO treatment on chloroplast ultrastructure and chloroplastic mRNA expression in iron-deficient maize plants. Twenty-day-old maize plants were grown hydroponically with nutrient solution containing 50 μm Fe(III)-EDTA, and either treated with 100 μm SNP or untreated. A through D, Transmission electron micrographs of chloroplasts from control and NO-treated iron-deficient maize plants. A and B, Mesophyll chloroplasts. C and D, Both bundle sheath and mesophyll chloroplasts. A and C, Untreated plants (control). B and D, NO-treated plants. Bar = 1 μm. Note the increased amount of internal membranes and thylakoid stacking in the plastids from NO-treated plants. M, Mesophyll cell; BS, bundle sheath cell; gr, grana; stg, starch granule; and pl, plastoglobuli. E, Northern-blot analysis of total RNA prepared from leaves of maize plants treated with 100 μm SNP or untreated (control). Each lane was loaded with 10 μg of total RNA, transferred to a nylon membrane, and hybridized with psbA or rbcL cDNA probes. The bottom panel shows ethidium bromide-stained rRNAs as loading control. F, Relative expression of psbA and rbcL genes in control and SNP-treated plants. Northern blots were scanned and corrected for loading errors with the ethidium bromide-stained rRNA.
Iron deficiency causes a marked reduction in the accumulation of chloroplastic proteins and mRNAs, whereas non-chloroplastic proteins are less affected (Spiller et al., 1987; Winder and Nishio, 1995). Therefore, we analyzed the effect of NO treatment on the steady-state levels of two mRNAs that encode major chloroplastic proteins that had been reported to decrease during iron deficiency (Spiller et al., 1987; Winder and Nishio, 1995). The chloroplast-encoded mRNAs studied were rbcL (Rubisco large subunit) and psbA (D1 protein). Northern-blot analysis revealed that the abundance of both transcripts was 75% higher in NO-treated plants than in control ones (Fig. 3, E and F). This result is in agreement with the fact that NO-treated plants had more developed chloroplasts and constitutes other evidence that NO avoids iron deficiency symptoms in maize plants.
NO-Mediated Chlorophyll Increase Is Effective at Very Low Iron Concentrations in the Nutrient Solution
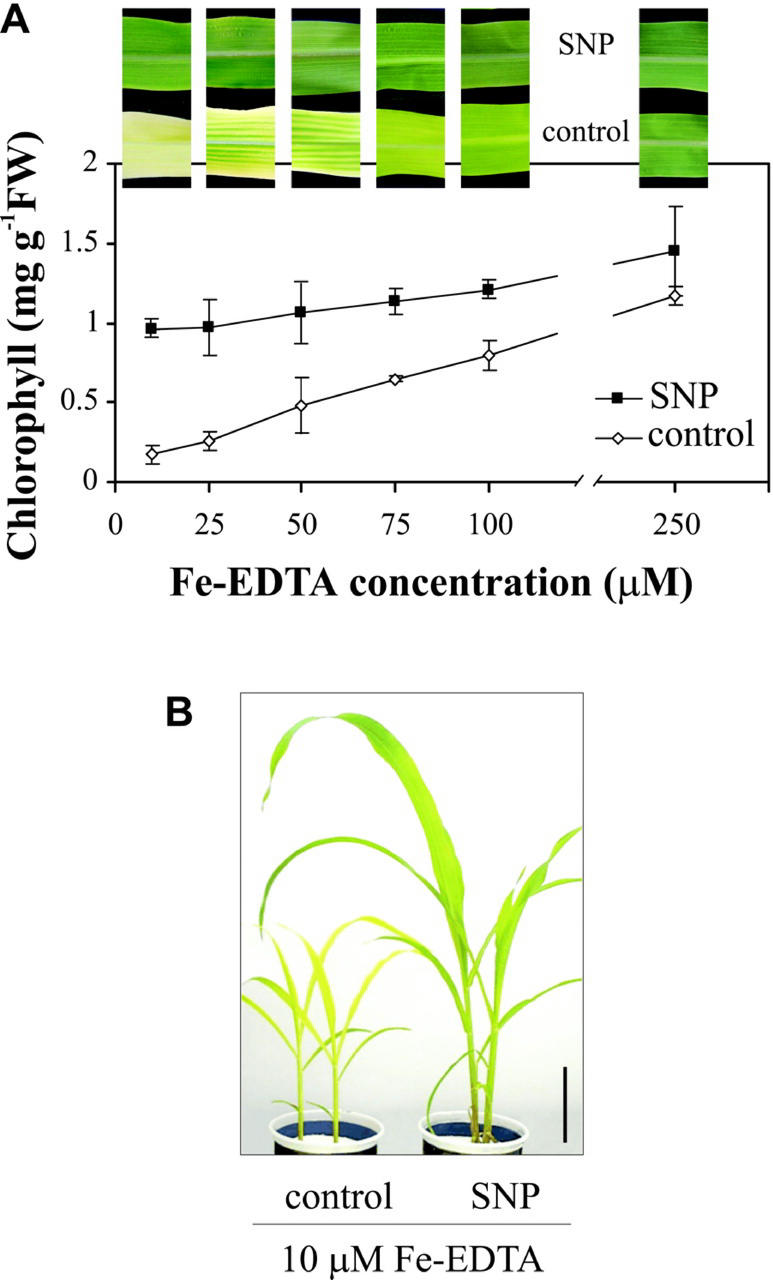
To assess the relationship between NO-mediated greening and iron availability, maize plants growing in different Fe(III)-EDTA concentrations were treated with 100 μm SNP, and chlorophyll content was analyzed. The pictures in Figure 4A show representative leaves of plants grown in Fe-EDTA concentrations ranging from 10 to 250 μm. The plot shows the effect of NO treatment on chlorophyll content. NO avoided iron deficiency chlorosis even in plants growing in an iron concentration as low as 10 μm Fe-EDTA, increasing the chlorophyll levels 5-fold relative to control plants (Fig. 4A). In leaves from untreated maize plants, chlorophyll content markedly increased with higher iron concentration in the nutrient solution. Thus, between 10 and 250 μm Fe-EDTA, chlorophyll content increased from 0.2 to 1.1 mg chlorophyll g−1 fresh weight. In NO-treated plants, chlorophyll content increased from 1 to 1.4 mg chlorophyll g−1 fresh weight for the same range of iron concentrations (Fig. 4A). The same chlorophyll content reached with 250 μm Fe-EDTA in control plants was achieved with 10 μm Fe-EDTA in NO-treated plants. Whereas plants growing under severe iron deficiency had slow growth and usually fail to complete its vegetative cycle, NO-treated plants had normal development under the same conditions (Fig. 4B). This suggests that NO enhances iron availability and/or uptake under iron-deficient conditions and, therefore, diminishes the threshold level of iron needed for maize plants to grow without iron deficiency symptoms.
Figure 4.
Effect of NO on the reversion of chlorosis in iron-deficient maize plants growing under different Fe-EDTA concentrations. Maize plants were grown hydroponically with 10, 25, 50, 75, 100, or 250 μm Fe(III)-EDTA in the nutrient solution and were either treated with 100 μm SNP once a week or untreated (control). A, Representative photographs of the fourth leaves of plants grown in different Fe-EDTA concentrations and its chlorophyll content. Each point represents the mean of two to three independent experiments; bars indicate sd. B, Phenotype of 25-d-old plants grown in 10 μm Fe-EDTA treated with 100 μm SNP or untreated. Bar = 10 cm.
NO Effect Does Not Correlate with an Increase in Iron Concentration in Maize Plants
To evaluate whether NO produces an increase in iron content inside the plant, total iron was estimated by atomic absorption spectroscopy in control and NO-treated plants growing in 50 μm Fe-EDTA. Iron concentration in leaves, stem, and roots of iron-deficient maize plants did not change upon treatment with NO (Table I, micrograms per gram fresh weight). However, the NO treatment caused a more than 2-fold increase in the fresh weight of leaves, and as a consequence, total iron content in leaves expressed as percentage of the total iron in the plant was higher in NO-treated plants than in control ones (Table I, 44% versus 27%, respectively). Overall, the fact that both green NO-treated leaves and chlorotic untreated leaves had the same iron concentration per gram of fresh matter suggests that NO action is accomplished mainly through an improvement in iron availability inside the leaf.
Table I.
Iron content in leaves, stem, and roots of control and NO-treated maize plants growing under Fe-deficient conditions
| Control
|
NO-Treated
|
|||||
|---|---|---|---|---|---|---|
| Fe | FW | Total Fe | Fe | FW | Total Fe | |
| μg g−1 FW | g | μg (%) | μg g−1 FW | g | μg (%) | |
| Leaves | 30.6 ± 6.5 | 1.25 ± 0.23 | 38.25 (27) | 28.1 ± 7.1 | 2.94 ± 0.35 | 82.61 (44) |
| Stem | 22.2 ± 7.5 | 1.37 ± 0.18 | 30.41 (22) | 17.3 ± 5.4 | 1.46 ± 0.15 | 25.25 (14) |
| Roots | 51.1 ± 12.1 | 1.39 ± 0.24 | 71.03 (51) | 54.3 ± 7.5 | 1.44 ± 0.26 | 78.19 (42) |
Twenty-day-old maize plants were grown hydroponically with 50 μm Fe(III)-EDTA, either treated with 100 μm SNP or untreated (control plants). Plants were harvested and separated into roots, stems, and leaves, and fresh weight (FW) was determined. In the different organs, iron was estimated by atomic absorption spectroscopy. Total iron in each organ is also expressed as a percentage of the total iron in the plant. Mean values and sds were calculated from two independent experiments with three replicates each.
Endogenous NO Plays a Role in Iron Availability
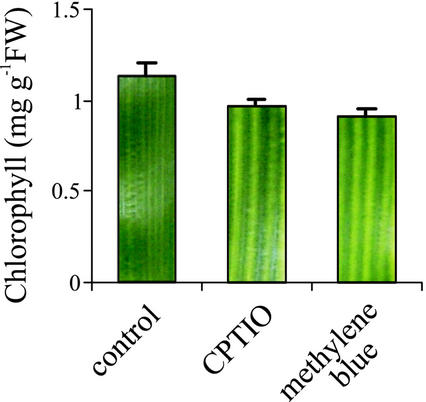
To evaluate the physiological role of NO on iron bioavailability, the effect of endogenous NO depletion on the phenotype and chlorophyll content of maize plants growing in iron-sufficient conditions was analyzed. Two NO scavengers were used: CPTIO, which reacts specifically with NO (Pfeiffer et al., 1997), and methylene blue, which inhibits NO production and/or action (Cragan, 1999). NO scavengers were previously used in whole-plant assays to block endogenous NO (Pagnussat et al., 2002). Maize plants grown in a hydroponic solution containing 250 μm Fe-EDTA were treated with CPTIO or methylene blue. Figure 5 shows that both NO scavengers render leaves with interveinal chlorosis, inducing a 20% chlorophyll decrease compared with control plants. This result indicates that endogenous NO might take part in the normal physiological process, facilitating iron availability for chlorophyll synthesis in iron-replete plants.
Figure 5.
Effect of endogenous-NO depletion on the phenotype and chlorophyll content of iron-sufficient maize plants. Maize plants grown hydroponically with 250 μm Fe(III)-EDTA in the nutrient solution were treated with 200 μm CPTIO, 100 μm methylene blue, or untreated (control plants). Chlorophyll content of the fourth leaf from 20-d-old plants is plotted; each bar shows a section of the leaf indicating the corresponding phenotype. Mean values and sds were calculated from two independent treatments.
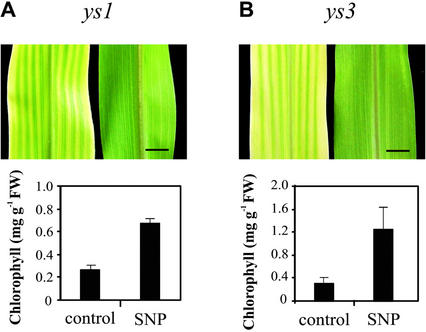
NO Reverts the Phenotype of the Iron-Inefficient Maize Mutants yellow stripe1 (ys1) and yellow stripe3 (ys3)
Maize ys1 and ys3 mutants are defective in iron acquisition mechanisms and display interveinal chlorosis even growing under iron-sufficient conditions. In ys1, iron uptake by the iron-phytosiderophore transporter is impaired (von Wirén et al., 1994; Curie et al., 2001), whereas ys3 is probably defective in phytosiderophore secretion (Motta et al., 2001). To evaluate the capacity of NO to avoid the iron deficiency phenotype when iron uptake mechanisms are altered, ys1 and ys3 maize plants were treated with 100 μm SNP. NO was able to completely revert interveinal chlorosis in leaves of both mutants (Fig. 6, A and B). In NO-treated plants, the increase of chlorophyll level was 3-fold for ys1 and 4-fold for ys3, relative to untreated ones (Fig. 6, A and B). Sodium ferrocyanide had no effect on both chlorophyll level and phenotype of maize mutants (data not shown). Again, these mutant maize plants did not present differences in iron concentration between NO-treated and untreated plants (data not shown). These results also support the idea that NO may be involved in iron availability within the plant.
Figure 6.
Effect of NO on the chlorotic phenotype of the ys1 and ys3 maize mutants. The ys1 and ys3 mutants were grown on vermiculite and watered with nutrient solution containing 100 μm Fe(III)-EDTA in the presence or the absence of 100 μm SNP. Representative photographs and chlorophyll content of the fourth leaves from 20-d-old ys1 (A) and ys3 (B) maize plants, either treated with SNP or untreated (control) are shown. Bar = 1 cm. Mean values and sds were calculated from four independent treatments.
DISCUSSION
This work presents strong evidence supporting a role for NO in plant iron nutrition. Results obtained with iron-deficient maize plants suggest that NO action should be related to iron availability inside the plant. Under iron-deficient growth conditions, NO treatment increased the chlorophyll content of leaves 5-fold over untreated plants, achieving similar chlorophyll levels to those found in maize plants growing in iron-sufficient conditions. NO-mediated chlorophyll increase was accompanied by the accumulation of transcripts encoding both the D1 protein of PSII and the Rubisco large subunit. Previous reports showed that the levels of these transcripts were reduced under iron deficiency and recovered after iron supply (Spiller et al., 1987).
The synthesis of chlorophyll and chloroplastic proteins are tightly connected with the complete development of chloroplasts. In this sense, iron deficiency is associated with cytological alterations, which mainly affect the chloroplast ultrastructure (Stocking, 1975; Spiller and Terry, 1980; Thoiron et al., 1997). In our experimental conditions, both chloroplasts from mesophyll and bundle sheath cells were severely altered by iron deficiency. Chloroplasts from mesophyll cells of iron-deficient maize leaves failed to develop normal granal stacking, as had been previously reported (Stocking, 1975; Thoiron et al., 1997). In contrast, chloroplasts from mesophyll cells of NO-treated plants developed extensive grana. Bundle sheath chloroplasts were reported to be less affected than mesophyll plastids by iron deficiency (Stocking, 1975). In our experimental conditions, bundle sheath chloroplasts displayed reduced thylakoid density, and starch granules were not detectable in control plants, whereas chloroplasts from NO-treated plants presented the same morphological characteristics observed in chloroplast from iron-sufficient plants (Stocking, 1975).
Because the NO donor SNP contains iron in its molecule (1 mol ferrocyanide mol−1 compound), one question was whether plants could be able to use iron from SNP. Even though iron concentration was the same in NO-treated and in control plants, total iron content was higher in NO-treated plants because of their bigger size. Thus, an iron mass balance calculation allowed us to confirm that in the growth solution, there was enough iron to compensate the amount contained in NO-treated plants that could come from SNP (there is about 5.4 mg of Fe in 1 L of nutrient solution containing 50 μm Fe-EDTA and less than 0.2 mg of Fe in 20-d-old SNP-treated plants). Moreover, ferrocyanide, the iron-containing residual product of SNP, did not augment chlorophyll content in iron-deficient maize plants (Fig. 1B). Besides, the effect was specific for NO because CPTIO, an NO scavenger, prevented the action of SNP. In addition, SNAP, an NO donor that does not contain iron in its molecule, also reverted iron deficiency. In another approach, gaseous NO supply in a translucent chamber was also able to partially revert chlorosis, achieving similar results to those obtained with NO donors.
Iron concentration (micrograms per gram fresh weight) in leaves, stems, and roots was similar in both NO-treated and untreated plants. However, because of the larger shoot to root ratio in NO-treated plants, almost 60% of the total iron was localized in the aerial part of NO-treated plants, compared with 50% in untreated ones. It was previously shown that iron can accumulate in large pools in the root apoplast and can be mobilized to the shoots as the plants become iron deficient. It was also suggested that this translocation of iron might be important in resistance to iron deficiency chlorosis (Longnecker and Welch, 1990). Whether NO is playing a physiological role improving iron translocation from roots to leaves is a process that remains to be studied.
The physiological relevance of NO in plant iron nutrition was assessed by treating iron-sufficient plants with compounds that react with NO or inhibit its production. Iron-replete maize plants treated with either CPTIO or methylene blue developed interveinal chlorosis in the younger leaves, whereas control plants remained green. This result suggests a physiological function for NO in iron availability under normal iron nutrition. Because the effect of the scavengers was only partial, it could not be concluded that the role accomplished by endogenous NO is critical in iron nutrition. However, the magnitude of NO blockage depends on the relative concentration of (a) the scavenger and (b) the molecules through which NO exert its effects, as well as on their respective affinity with NO. Therefore, because these points were not studied, the relevance of NO on iron nutrition cannot be precisely determined.
NO was also able to revert the phenotype of two iron-inefficient maize mutants, ys1 and ys3, both impaired in iron uptake mechanisms. The ys1 mutant holds the mutation on the protein YS1 involved in iron-phytosiderophore uptake (Curie et al., 2001), whereas ys3 is probably defective in phytosiderophore secretion (Motta et al., 2001). Therefore, NO may make the lower amount of iron taken up by the mutants more available inside the plant. The tomato (Lycopersicon esculentum) fer mutant also displays a chlorotic phenotype at normal external iron concentrations because it is incompetent to take up iron in adequate amounts (Schmidt et al., 2000). NO treatment can also revert the chlorotic phenotype of this mutant (M. Graziano and L. Lamattina, unpublished data). Interestingly, this result indicates that NO is also effective in reverting the iron deficiency chlorosis in dicotyledonous plants, which have a completely different root iron uptake system.
Iron content in leaves is usually positively correlated with the chlorophyll content. However, in our case, the levels of iron were similar in both green leaves from NO-treated plants and chlorotic leaves from untreated plants. It is known that leaves can develop chlorosis even at higher iron concentrations than those needed to render green leaves, chlorophyll biosynthesis, and chloroplast development (Kosegarten et al., 1999; González-Vallejo et al., 2000; Larbi et al., 2001). That is because an important proportion of the iron is unavailable because it remains insoluble in the apoplast of mesophyll cells. The reduction of Fe(III) in the apoplast is a prerequisite for its transport across the plasma membrane. The enzyme involved in Fe(III) reduction is the membrane Fe(III)-chelate reductase, and the reaction is under the regulation of the pH and the redox conditions of the apoplast (Brüggemann et al., 1993; Kosegarten et al., 1999). Under physiological conditions, the redox state of the apoplast is determined by several factors, among them light, which is known to activate iron reduction (Brüggemann et al., 1993; Kosegarten et al., 1999). Previous results have demonstrated the ability of NO to mediate several physiological processes in plants that are normally triggered by light (Beligni and Lamattina, 2000).
The chemistry of NO involves various redox forms, nitrosonium cation (NO+), radical nitric oxide (NO·), and nitroxyl anion (NO−). The composition of NO pools is determined by the pH and the redox potential of the microenvironment. NO appears to take part in the regulation of cellular redox homeostasis, acting either as an oxidant or as an antioxidant (Stamler et al., 1992). In addition, NO can also modulate the activity of proteins through reversible reactions with functional groups, such as heme and thiols. Overall, the redox state of NO influences its reactivity on different targets. These particular properties of NO could explain many of the biological responses in plants that have not been clearly understood yet, among them iron nutrition. A mechanism has already been described involving the oxidative activation of NO· through the binding to Fe(III), which results in the formation of NO+ and Fe(II) [Fe(III) NO· → Fe(II) NO+] (Goretski and Hollocher, 1991). Besides, the anionic form (NO−) can also react with Fe(III), analogous to the reaction of NO· with Fe(III). Thus, metal nitrosylation provides a direct link among the redox states of NO and, therefore, a link between metal redox states (Stamler et al., 1992).
Although the charge neutrality of NO· has been denoted to assume its free diffusion across cell membranes (Goretski and Hollocher, 1988), the molecular mechanisms for precise NO delivery have been recently reported and include the formation of iron-nitrosyl complexes (Gross, 2001; Pawloski et al., 2001). In animals, NO-containing compounds are viewed as means to a more rapid accomplishment of NO functions and have been reported to participate in (a) storing NO, (b) facilitating its transport, (c) prolonging its half-life, and (d) targeting it to specific effectors. Thus, it remains to be studied whether in plants, iron-nitrosyl complexes could be a way of both storing NO and transporting and delivering reduced iron within the plant cells.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds from maize (Zea mays L. cv Canner) were commercially acquired and homozygous mutants ys1 and ys3 were supplied by Maize Genetics Cooperation Stock Center (University of Illinois, Urbana). Seeds were surface sterilized in 1.8% (v/v) sodium hypochlorite, rinsed several times in distilled water, and germinated on moistened filter paper for 3 d. Seedlings were grown either in vermiculite watered with nutrient solution or hydroponically in the same aerated solution. The nutrient solution had the following composition: 5.25 mm KNO3, 7.75 mm Ca(NO3)2, 4.06 mm MgSO4, and 1.0 mm KH2PO4; micronutrients: 46 μm H3BO4, 9.18 μm MnSO4, 5.4 μm ZnSO4, 9.0 μm CuSO4, and 2.0 μm Na2MoO4. Iron was supplied as 50 μm Fe(III)-EDTA (mild iron deficiency) or in different concentrations ranging from 10 to 250 μm. The nutrient solution was adjusted to pH 5.5 and renewed once a week. Plants were grown in a growth chamber at a 60% relative humidity, 200 μmol photons m−2 s−1 of light intensity and 14 h/10 h (25°C/22°C) day/night regime. Fully expanded fourth leaves of 20-d-old plants were harvested, weighed, frozen in liquid nitrogen, and stored at −80°C for further analysis.
NO Treatments and Chemicals
SNP and SNAP were used as NO donors in a 100 μm concentration; 200 μm potassium salt of CPTIO and 100 μm methylene blue were used as NO scavengers. Sodium ferrocyanide [Na4Fe(CN)6] was used as an additional control. The solutions were supplied to plants by irrigation once a week or included in the nutrient solution at the same concentration. The amount of NO released from 100 μm NO-donor solutions was determined by the Griess reagent colorimetric kit (Cayman Chemical Company, Ann Arbor, MI), according to the manufacturer's instructions.
Gaseous NO Treatment
For the experiments with gaseous NO, the translucent chamber used in this study (60 cm front, 40 cm depth, and 60 cm high, total volume 96 L) was sealed with Teflon to minimize leaks through the walls. NO/nitrogen gas mixture (NO = 798 μL L−1, NO2 = less than 1% of NO value) was obtained from Nellcor Puritan and Bennett (Overland Park, KS). Ten-day-old hydroponic grown plantlets were transferred to the chamber and treated with 100 μL L−1 gaseous NO. The chamber was opened every 3 d, the ambient air was renewed, and 100 μL L−1 NO was newly applied. Control plants were grown in similar conditions without being treated. Leaves were photographed 10 d after the treatment.
Chlorophyll Quantification
Maize leaves (0.5 g of fresh weight) were powdered with liquid nitrogen, and pigments were extracted with 4 volumes of 80% (v/v) acetone until complete bleaching. Total chlorophyll was quantified by measuring Abs652, and its concentration was calculated as described by Arnon (1949).
Electron Microscopy
One square millimeter leaf pieces coming from the interveinal region of iron-deficient maize plants either treated with NO or untreated (controls) were fixed in 0.1 m sodium cacodylate (pH 7.4) containing 2% (w/v) glutaraldehyde for 2 h. After fixation, they were incubated in 2% (w/v) osmium tetroxide for 1 h, dehydrated in ethanol series, and embedded in Spurr's resin. Ultrathin sections (60 nm) were obtained using a Porterblum MT1 ultramicrotome and stained with uranyl acetate and lead citrate. Examination of sections was carried out using an electron microscope (Hu11C1, Hitachi, Tokyo) operating at 75 kV.
RNA Extraction and Northern-Blot Analysis
Total RNA purification was performed as described previously (Laxalt et al., 1996). For northern-blot experiments, RNAs (10 μg per lane) were electrophoresed in formaldehyde agarose gels, stained with ethidium bromide, and blotted onto Hybond N+ membranes (Amersham-Pharmacia, Rainham, UK). Probes corresponding to psbA and rbcL were labeled with [α-32P]dCTP by random priming (Amersham-Pharmacia, Rainham, UK). Prehybridization and hybridization were performed according to the manufacturer's instructions for 4 and 24 h, respectively. Filters were washed twice in 2× SSPE, 0.1% (w/v) SDS for 15 min, once in 1× SSPE, 0.1% (w/v) SDS for 30 min at 42°C, and once in 0.1× SSPE for 30 min at room temperature. Blots were exposed to autoradiographies at −80°C. The hybridization signals were quantified by densitometry and corrected according to the ethidium bromide-stained rRNA.
Iron Content Determination
Leaves, stems, and roots of maize plants grown hydroponically with 50 μm Fe-EDTA either treated or untreated with SNP 100 μm were used for total iron analysis. Roots were thoroughly washed in deionized water and dried superficially before the determination of fresh weight The samples were oven-dried at 65°C for 48 h and mineralized by wet open digestion in HNO3:H2SO4:HClO4 (5:1:1, 2–5 mL for 0.5–1.5 g fresh weight). Deionized water was added to restore the final volume to 1 mL, and total iron concentration in the digest was estimated by atomic absorption spectroscopy.
Footnotes
This work was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (grant no. PIP 0898/98), by the Agencia Nacional de Promoción Científica y Tecnológica (grant no. 6496/99), by the Fundación Antorchas, the Comisión de Investigaciones Científicas de la Provincia de Buenos Aires, and by the Universidad Nacional de Mar del Plata, Argentina.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.009076.
LITERATURE CITED
- Abadía J. Leaf responses to Fe deficiency: a review. J Plant Nutr. 1992;15:1699–1713. [Google Scholar]
- Anbar M. Nitric oxide: a synchronizing chemical messenger. Experientia. 1995;51:545–550. doi: 10.1007/BF02128740. [DOI] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts: phenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L. Nitric oxide counteracts cytotoxic processes mediated by reactive oxygen species in plant tissues. Planta. 1999a;208:337–344. [Google Scholar]
- Beligni MV, Lamattina L. Is nitric oxide toxic or protective? Trends Plant Sci. 1999b;4:299–300. doi: 10.1016/s1360-1385(99)01451-x. [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L. Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta. 2000;210:215–221. doi: 10.1007/PL00008128. [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L. Nitric oxide in plants: The history is just beginning. Plant Cell Environ. 2001a;24:267–278. [Google Scholar]
- Beligni MV, Lamattina L. Nitric oxide: a non-traditional regulator of plant growth. Trends Plant Sci. 2001b;6:508–509. doi: 10.1016/s1360-1385(01)02156-2. [DOI] [PubMed] [Google Scholar]
- Brüggemann W, Maas-Kantel K, Moog PR. Iron uptake by leaf mesophyll cells: the role of the plasma membrane-bound ferric-chelate reductase. Planta. 1993;190:151–155. [Google Scholar]
- Cragan JD. Teratogen update: methylene blue. Teratology. 1999;60:42–48. doi: 10.1002/(SICI)1096-9926(199907)60:1<42::AID-TERA12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Curie C, Panaviene Z, Loulergue C, Dellaporta S, Briat J-F, Walker EL. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature. 2001;409:346–349. doi: 10.1038/35053080. [DOI] [PubMed] [Google Scholar]
- de la Guardia MD, Alcántara E. Ferric chelate reduction by sunflower (Helianthus annuus L.) leaves: influence of light, oxygen, iron deficiency and leaf age. J Exp Bot. 1996;47:669–675. [Google Scholar]
- Durner J, Klessig DF. Nitric oxide as a signal in plants. Curr Opin Plant Biol. 1999;2:369–374. doi: 10.1016/s1369-5266(99)00007-2. [DOI] [PubMed] [Google Scholar]
- González-Vallejo EB, Morales F, Cistué L, Abadía A, Abadía J. Iron deficiency decreases the Fe(III)-chelate reducing activity of leaf protoplasts. Plant Physiol. 2000;122:337–344. doi: 10.1104/pp.122.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goretski J, Hollocher TC. Trapping of nitric oxide produced during denitrification by extracellular hemoglobin. J Biol Chem. 1988;263:2316–2323. [PubMed] [Google Scholar]
- Goretski J, Hollocher TC. Catalysis of nitrosyl transfer by denitrifying bacteria is facilitated by nitric oxide. Biochem Biophys Res Commun. 1991;175:901–905. doi: 10.1016/0006-291x(91)91650-2. [DOI] [PubMed] [Google Scholar]
- Gross SS. Targeted delivery of nitric oxide. Nature. 2001;409:577–578. doi: 10.1038/35054661. [DOI] [PubMed] [Google Scholar]
- Guerinot ML, Yi Y. Iron: nutritious, noxious, and not readily available. Plant Physiol. 1994;104:815–820. doi: 10.1104/pp.104.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosegarten HU, Hoffmann B, Mengel K. Apoplastic pH and Fe3+ reduction in intact sunflower leaves. Plant Physiol. 1999;121:1069–1079. doi: 10.1104/pp.121.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larbi A, Morales F, López-Millán AF, Gogorcena Y, Abadía A, Moog PR, Abadía J. Technical advance: reduction of Fe(III)-chelates by mesophyll leaf disks of sugar beet. Multi-component origin and effects of Fe deficiency. Plant Cell Physiol. 2001;42:94–105. doi: 10.1093/pcp/pce012. [DOI] [PubMed] [Google Scholar]
- Laxalt AM, Cassia RO, Sanllorenti PM, Madrid EA, Andreu AB, Daleo GR, Conde RD, Lamattina L. Accumulation of cytosolic glyceraldehyde-3-phosphate dehydrogenase RNA under biological stress conditions and elicitor treatments in potato. Plant Mol Biol. 1996;30:961–972. doi: 10.1007/BF00020807. [DOI] [PubMed] [Google Scholar]
- Longnecker N, Welch RM. Accumulation of apoplastic iron in plant roots: a factor in the resistance of soybeans to iron-deficiency induced chlorosis? Plant Physiol. 1990;92:17–22. doi: 10.1104/pp.92.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales F, Grasa R, Abadía A, Abadía J. Iron chlorosis paradox in fruit trees. J Plant Nutr. 1998;21:815–825. [Google Scholar]
- Mori S. Iron acquisition by plants. Curr Opin Plant Biol. 1999;2:250–253. doi: 10.1016/S1369-5266(99)80043-0. [DOI] [PubMed] [Google Scholar]
- Motta A, Basso B, Dell'Orto M, Briat J-F, Soave C. Ferritin synthesis in response to iron in the Fe-inefficient maize mutant ys3. Plant Physiol Biochem. 2001;39:461–465. [Google Scholar]
- Olson JS. Numerical analysis of kinetic ligand binding data. Methods Enzymol. 1981;76:652–667. doi: 10.1016/0076-6879(81)76149-4. [DOI] [PubMed] [Google Scholar]
- Pagnussat G, Simontachi M, Puntarulo S, Lamattina L. Nitric oxide is required for root organogenesis. Plant Physiol. 2002;129:954–956. doi: 10.1104/pp.004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawloski JR, Hess D, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409:622–626. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S, Leopold E, Hemmens B, Schmidt K, Werner ER, Mayer B. Interference of carboxy-PTIO with nitric oxide- and peroxynitrite-mediated reactions. Free Radic Biol Med. 1997;22:787–794. doi: 10.1016/s0891-5849(96)00407-8. [DOI] [PubMed] [Google Scholar]
- Schmidt W, Schikora A, Pich A, Bartels M. Hormones induce an Fe-deficiency-like root epidermal cell pattern in the Fe-inefficient tomato mutant fer. Protoplasma. 2000;213:67–73. [Google Scholar]
- Spiller S, Terry N. Limiting factors in photosynthesis: II. Iron stress diminishes photochemical capacity by reducing the number of photosynthetic units. Plant Physiol. 1980;65:121–125. doi: 10.1104/pp.65.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller SC, Kaufman LS, Thompson WF, Briggs WR. Specific mRNA and rRNA levels in greening pea leaves during recovery from iron stress. Plant Physiol. 1987;84:409–414. doi: 10.1104/pp.84.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Stocking CR. Iron deficiency and the structure and physiology of maize chloroplasts. Plant Physiol. 1975;55:626–631. doi: 10.1104/pp.55.4.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N. Limiting factors in photosynthesis: I. Use of iron stress to control photochemical capacity in vivo. Plant Physiol. 1980;65:114–120. doi: 10.1104/pp.65.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoiron S, Pascal N, Briat J-F. Impact of iron deficiency and iron re-supply during the early stages of vegetative development in maize (Zea mays L.) Plant Cell Environ. 1997;20:1051–1060. [Google Scholar]
- von Wirén N, Mori S, Marschner H, Römheld V. Iron inefficiency in maize mutant ys1 (Zea mays L. cv yellow-stripe) is caused by a defect in uptake of iron phytosiderophores. Plant Physiol. 1994;106:71–77. doi: 10.1104/pp.106.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellburn AR. Atmospheric nitrogenous compounds and ozone: Is NOx fixation by plants a possible solution? New Phytol. 1998;139:5–9. [Google Scholar]
- Winder TL, Nishio JN. Early iron deficiency stress response in leaves of sugar beet. Plant Physiol. 1995;108:1487–1494. doi: 10.1104/pp.108.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]