Abstract
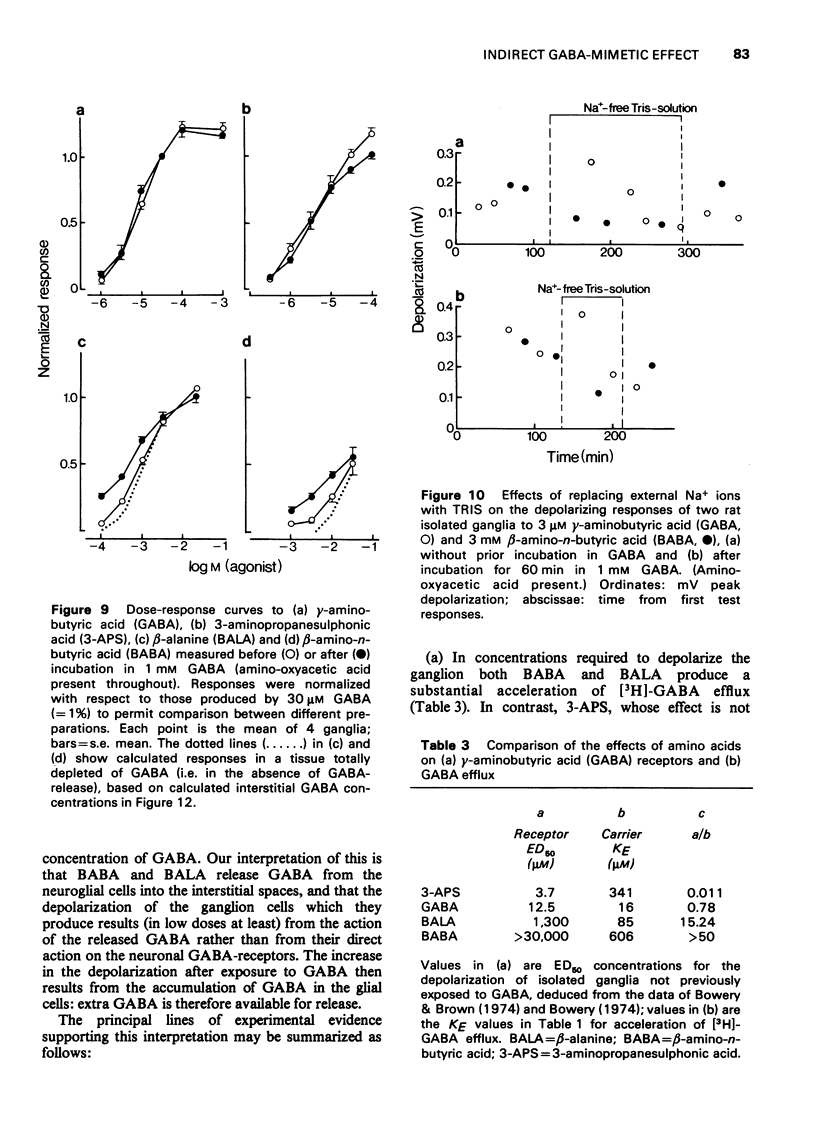
1 All experiments were performed on rat isolated desheathed superior cervical ganglia maintained in Krebs solution containing amino-oxyacetic acid (10 muM) at 25 degrees C. 2 Influx rates of gamma-amino-n-butyric acid (GABA) were measured by incubating ganglia in 0.5 muM [3H]-GABA for 30 minutes. Influx was inhibited by 50% on adding 14.3 muM unlabelled GABA, 59.2 muM beta-alanine (BALA) or 424 muM beta-amino-n-butyric acid (BABA). 3 Efflux of [3H]-GABA into non-radioactive solution superfused over ganglia previously incubated for 60 min in 1 muM [3H]-GABA was measured. The mean resting efflux rate coefficient (k) was 0.64 +/- 0.05 X 10(-3) min-1. Addition of high concentrations of unlabelled GABA, BABA or BALA to the superfusing solution increased k by (maximally) 3.6-4.3 times; half-maximal increases occurred at the following concentrations: GABA, 16 muM; BALA, 85 muM; BABA, 606 muM. Replacement of external Na+ with Li+ or TRIS increased the resting value of k and inhibited acceleration by external amino acids. Prior incubation in 1 muM [3H]-GABA with 1 mM unlabelled GABA increased resting k 1.5 times, but did not alter the peak rate coefficient produced by external amino acids. 4 Neuronal depolarization produced by the amino acids was measured with surface electrodes. Pre-incubation in 1 mM GABA for 60 min potentiated low-amplitude responses to BALA or BABA but not those to GABA or 3-aminopropanesulphonic acid (a potent agonist with low affinity for the GABA carrier). Omission of external Na+ reduced responses to BABA but increased those to GABA. 5 Incubation in 1 mM GABA for 60 min (as required to potentiate BABA or BALA actions) increased the amount of GABA in the tissue from 0.21 to 0.73 mmol/kg wet weight. Autoradiographs in which labelled GABA was used indicated that uptake into neuroglial cells was responsible for this accumulation. 6 It is suggested that: (i) BALA and BABA are substrates for the inward GABA carrier responsible for GABA entry into ganglionic glial cells; (ii) they accelerate efflux by inhibiting carrier-mediated reaccumulation of effluent GABA by the glial cells; (iii) interstitial GABA concentrations are thereby increased to a level capable of depolarizing adjacent neurones; and (iv) this, rather than direct GABA-receptor activation, accounts for the depolarization produced by low concentrations of BALA and BABA. Potentiation of their depolarizing action after pre-incubation in 1 mM GABA is suggested to result from the increased amount of intracellular GABA available for release, and is quantitatively compatible with this increase; inhibition in Na+-free solution is due to their inability to inhibit reaccumulation of GABA under these conditions. 7 A model for the action of carrier substrates is described in an Appendix. Calculations based thereon yield increments in interstitial GABA concentration in the presence of carrier substrates compatible with those determined experimentally (up to 1 muM at rest or 3.4 muM after pre-incubation in GABA).
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975 Aug;250(1):85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcom G. J., Lenox R. H., Meyerhoff J. L. Regional gamma-aminobutyric acid levels in rat brain determined after microwave fixation. J Neurochem. 1975 Apr;24(4):609–613. [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A. -Aminobutyric acid uptake by sympathetic ganglia. Nat New Biol. 1972 Jul 19;238(81):89–91. doi: 10.1038/newbio238089a0. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A., Collins J. F. Tetramethylenedisulphotetramine: an inhibitor of gamma-aminobutyric acid induced depolarization of the isolated superior cervical ganglion of the rat. Br J Pharmacol. 1975 Mar;53(3):422–424. doi: 10.1111/j.1476-5381.1975.tb07379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A. Depolarizing actions of gamma-aminobutyric acid and related compounds on rat superior cervical ganglia in vitro. Br J Pharmacol. 1974 Feb;50(2):205–218. doi: 10.1111/j.1476-5381.1974.tb08563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briel G., Neuhoff V. Microanalysis of amino acids and their determination in biological material using dansyl chloride. Hoppe Seylers Z Physiol Chem. 1972 Apr;353(4):540–553. doi: 10.1515/bchm2.1972.353.1.540. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Halliwell J. V., Scholfield C. N. Uptake of nicotine and extracellular space markers by isolated rat ganglia in relation to receptor activation. Br J Pharmacol. 1971 May;42(1):100–113. doi: 10.1111/j.1476-5381.1971.tb07090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Movements of labelled sodium ions in isolated rat superior cervical ganglia. J Physiol. 1974 Oct;242(2):321–351. doi: 10.1113/jphysiol.1974.sp010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Nicotine washout rates from isolated rat ganglia in relation to recovery from nicotine depolarization. Br J Pharmacol. 1972 May;45(1):29–36. doi: 10.1111/j.1476-5381.1972.tb09573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler R. W., Hammerstad J. P., Cornick L. R., Murray J. E. Efflux of amino acid neurotransmitters from rat spinal cord slices. I. Factors influencing the sponatenous efflux of ( 14 C)glycine and 3 H-GABA. Brain Res. 1971 Dec 24;35(2):337–355. doi: 10.1016/0006-8993(71)90479-3. [DOI] [PubMed] [Google Scholar]
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- De Belleroche J. S., Bradford H. F. Metabolism of beds of mammalian cortical synaptosomes: response to depolarizing influences. J Neurochem. 1972 Mar;19(3):585–602. doi: 10.1111/j.1471-4159.1972.tb01376.x. [DOI] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S., Krnjević K. Cortical inhibition and gamma-aminobutyric acid. Exp Brain Res. 1969;9(2):137–154. doi: 10.1007/BF00238327. [DOI] [PubMed] [Google Scholar]
- Fahn S., Côté L. J. Regional distribution of gamma-aminobutyric acid (GABA) in brain of the rhesus monkey. J Neurochem. 1968 Mar;15(3):209–213. doi: 10.1111/j.1471-4159.1968.tb06198.x. [DOI] [PubMed] [Google Scholar]
- Henn F. A., Hamberger A. Glial cell function: uptake of transmitter substances. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2686–2690. doi: 10.1073/pnas.68.11.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Johnston G. A. GABA uptake in rat central nervous system: comparison of uptake in slices and homogenates and the effects of some inhibitors. J Neurochem. 1971 Oct;18(10):1939–1950. doi: 10.1111/j.1471-4159.1971.tb09600.x. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Kelly J. S. Uptake and metabolism of gamma-aminobutyric acid by neurones and glial cells. Biochem Pharmacol. 1975 May 1;24(9):933–938. doi: 10.1016/0006-2952(75)90422-0. [DOI] [PubMed] [Google Scholar]
- Johnston G. A., Beart P. M., Curtis D. R., Game C. J., McCulloch R. M., Maclachlan R. M. Bicuculline methochloride as a GABA antagonist. Nat New Biol. 1972 Dec 13;240(102):219–220. doi: 10.1038/newbio240219a0. [DOI] [PubMed] [Google Scholar]
- Joseph M. H., Halliday J. A dansylation microassay for some amino acids in brain. Anal Biochem. 1975 Apr;64(2):389–402. doi: 10.1016/0003-2697(75)90447-9. [DOI] [PubMed] [Google Scholar]
- Kelly J. S., Dick F., Schon F. The autoradiographic localization of the GABA-releasing nerve terminals in cerebellar glomeruli. Brain Res. 1975 Feb 28;85(2):255–259. doi: 10.1016/0006-8993(75)90078-5. [DOI] [PubMed] [Google Scholar]
- Levi G., Raiteri M. Exchange of neurotransmitter amino acid at nerve endings can simulate high affinity uptake. Nature. 1974 Aug 30;250(5469):735–737. doi: 10.1038/250735a0. [DOI] [PubMed] [Google Scholar]
- Martin D. L. Kinetics of the sodium-dependent transport of gamma-aminobutyric acid by synaptosomes. J Neurochem. 1973 Aug;21(2):345–356. doi: 10.1111/j.1471-4159.1973.tb04255.x. [DOI] [PubMed] [Google Scholar]
- PURPURA D. P., GIRADO M., SMITH T. G., CALLAN D. A., GRUNDFEST H. Structure-activity determinants of pharmacological effects of amino acids and related compounds on central synapses. J Neurochem. 1959 Jan;3(3):238–268. doi: 10.1111/j.1471-4159.1959.tb12630.x. [DOI] [PubMed] [Google Scholar]
- Patel A. J., Johnson A. L., Balázs R. Metabolic compartmentation of glutamate associated with the formation of gamma-aminobutyrate. J Neurochem. 1974 Dec;23(6):1271–1279. doi: 10.1111/j.1471-4159.1974.tb12227.x. [DOI] [PubMed] [Google Scholar]
- Raiteri M., Federico R., Coletti A., Levi G. Release and exchange studies relating to the synaptosomal uptake of GABA. J Neurochem. 1975 Jun;24(6):1243–1250. doi: 10.1111/j.1471-4159.1975.tb03905.x. [DOI] [PubMed] [Google Scholar]
- Schon F., Beart P. M., Chapman D., Kelly J. S. On GABA metabolism in the gliocyte cells of the rat pineal gland. Brain Res. 1975 Mar 7;85(3):479–490. doi: 10.1016/0006-8993(75)90821-5. [DOI] [PubMed] [Google Scholar]
- Schon F., Kelly J. S. Selective uptake of (3H)beta-alanine by glia: association with glial uptake system for GABA. Brain Res. 1975 Mar 21;86(2):243–257. doi: 10.1016/0006-8993(75)90700-3. [DOI] [PubMed] [Google Scholar]
- Simon J. R., Martin D. L., Kroll M. Sodium-dependent efflux and exchange of GABA in synaptosomes. J Neurochem. 1974 Nov;23(5):981–991. doi: 10.1111/j.1471-4159.1974.tb10750.x. [DOI] [PubMed] [Google Scholar]
- Simon J. R., Martin D. L. The effects of L-2,4-diaminobutyric acid on the uptake of gamma-aminobutyric acid by a synaptosomal fraction from rat brain. Arch Biochem Biophys. 1973 Aug;157(2):348–355. doi: 10.1016/0003-9861(73)90649-8. [DOI] [PubMed] [Google Scholar]
- WILBRANDT W., ROSENBERG T. The concept of carrier transport and its corollaries in pharmacology. Pharmacol Rev. 1961 Jun;13:109–183. [PubMed] [Google Scholar]
- Walsh J. M., Bowery N. G., Brown D. A., Clark J. B. Metabolism of gamma-aminobutyric acid (GABA) by peripheral nervous tissue. J Neurochem. 1974 Jun;22(6):1145–1147. doi: 10.1111/j.1471-4159.1974.tb04350.x. [DOI] [PubMed] [Google Scholar]
- Young J. A., Brown D. A., Kelly J. S., Schon F. Autoradiographic localization of sites of (3H)gamma-aminobutyric acid accumulation in peripheral autonomic ganglia. Brain Res. 1973 Dec 7;63:479–486. doi: 10.1016/0006-8993(73)90128-5. [DOI] [PubMed] [Google Scholar]
- de Groat W. C. The actions of gamma-aminobutyric acid and related amino acids on mammalian autonomic ganglia. J Pharmacol Exp Ther. 1970 Apr;172(2):384–396. [PubMed] [Google Scholar]




