Abstract
The plant hormones auxin and ethylene have been shown to play important roles during root hair development. However, cross talk between auxin and ethylene makes it difficult to understand the independent role of either hormone. To dissect their respective roles, we examined the effects of two compounds, chromosaponin I (CSI) and 1-naphthoxyacetic acid (1-NOA), on the root hair developmental process in wild-type Arabidopsis, ethylene-insensitive mutant ein2-1, and auxin influx mutants aux1-7, aux1-22, and double mutant aux1-7 ein2. β-Glucuronidase (GUS) expression analysis in the BA-GUS transgenic line, consisting of auxin-responsive domains of PS-IAA4/5 promoter and GUS reporter, revealed that 1-NOA and CSI act as auxin uptake inhibitors in Arabidopsis roots. The frequency of root hairs in ein2-1 roots was greatly reduced in the presence of CSI or 1-NOA, suggesting that endogenous auxin plays a critical role for the root hair initiation in the absence of an ethylene response. All of these mutants showed a reduction in root hair length, however, the root hair length could be restored with a variable concentration of 1-naphthaleneacetic acid (NAA). NAA (10 nm) restored the root hair length of aux1 mutants to wild-type level, whereas 100 nm NAA was needed for ein2-1 and aux1-7 ein2 mutants. Our results suggest that insensitivity in ethylene response affects the auxin-driven root hair elongation. CSI exhibited a similar effect to 1-NOA, reducing root hair growth and the number of root hair-bearing cells in wild-type and ein2-1 roots, while stimulating these traits in aux1-7and aux1-7ein2 roots, confirming that CSI is a unique modulator of AUX1.
Root hairs are tip-growing, tubular-shaped outgrowths that help to anchor roots, interact with soil microorganisms, and assist in the uptake of water and nutrients (Cutter, 1978). The relatively simple and invariant cellular organization of the primary roots of Arabidopsis and the ease of isolation and characterization of mutants make it a very attractive material for studying the root hair developmental process. The first committed step for root hair development is epidermal cell specification. In many species, including Arabidopsis, the root epidermis consists of two epidermal cell types, root hair-forming trichoblast cells and hairless atrichoblast cells (Cormack, 1947, 1949; Bunning, 1951; Cutter, 1978). Within the Arabidopsis root epidermis, cells adopt distinct fates in a position-dependent manner. Epidermal cells that overlay the junction between two cortical cell files adopt a root hair cell fate, whereas the epidermal cells that contact only one cortical cell file become hairless cells (Dolan et al., 1994; Galway et al., 1994; Berger et al., 1998).
Once the immature epidermal cell adopts a root hair cell fate, it goes through characteristic changes in its shape and size (Schiefelbein, 2000). Genetic analysis revealed that the root hair initiation mutations axr2 (Wilson et al., 1990), axr3 (Leyser et al., 1996), and ctr1 (Kieber et al., 1993) exhibit changes in their response to two important plant hormones, auxin and ethylene. The root hair initiation defect of the rhd6 mutant can be suppressed by application of 1-aminocyclopropane-1-carboxylic acid (ACC; an ethylene precursor) or indole-3-acetic acid (IAA; endogenous form of auxin; Masucci and Schiefelbein, 1994), further confirming the roles of these two hormones in this process. After initiation, the root hair starts to grow through the process of tip growth. Mutants with altered responses to ethylene and auxin also show defects in root hair length (Reed et al., 1993; Okada and Shimura, 1994; Pitts et al., 1998), suggesting that these two hormones play important roles in controlling the root hair growth. Physiological experimental data with auxin, auxin transport inhibitors, and ACC further support this idea (Masucci and Schiefelbein, 1994; Okada and Shimura, 1994; Pitts et al., 1998). Collectively, these results clearly suggest that after cell specification, auxin and ethylene play indispensable roles regulating root hair morphogenesis.
We recently reported that chromosaponin I (CSI), a γ-pyronyl-triterpenoid saponin isolated from pea (Pisum sativum) and other leguminous plants (Tsurumi et al., 1991, 1992; Kudou et al., 1992, 1993; Massiot et al., 1992), specifically interacts with auxin influx carrier AUX1 (Bennett et al., 1996) and changes the response of Arabidopsis roots toward auxin and ethylene by controlling auxin uptake (Rahman et al., 2001a). Application of 60 μm CSI inhibited the auxin uptake in the roots of Arabidopsis expressing the wild-type AUX1 protein and slowed down the gravitropic response of roots. In the auxin influx mutant aux1-7, CSI conversely stimulated the uptake of auxin and partially restored the gravitropic response (Rahman et al., 2001a). We also observed that the CSI-induced change in auxin influx consequently affected the ethylene response of roots. CSI made the wild-type roots resistant to ethylene while it restored ethylene response in the ethylene-resistant mutant aux1-7 roots (Rahman et al., 2001a). In a later study, we showed that application of low concentrations of 1-naphthaleneacetic acid (NAA) restored the ethylene response in aux1-7, suggesting that the intracellular level of auxin plays an important role in regulating the ethylene response in Arabidopsis root growth (Rahman et al., 2001b).
Imhoff et al. (2000) characterized a large group of aryloxyalkylcarboxylic acids as potent inhibitors of auxin influx in suspension-cultured tobacco (Nicotiana tabacum) cells. Parry et al. (2001) recently investigated the effect of the aryloxyalkylcarboxylic acids including 1-naphthoxyacetic acid (1-NOA) on intact Arabidopsis seedlings. The authors concluded that 1-NOA was a useful auxin influx inhibitor because 1-NOA phenocopied the agravitropic aux1 root phenotype in wild type and did not show any effect on auxin efflux. Interestingly, application of 30 μm 1-NOA to wild-type roots mimicked the effect of 60 μm CSI in a root growth assay and in disrupting the root gravitropism. Although auxin and ethylene play indispensable roles during root hair development, cross talk between the two hormones (Rahman et al., 2001b) makes it difficult to resolve their independent roles. In the present paper we clarify the role of auxin by modulating its concentration in roots using the novel compounds CSI and 1-NOA.
RESULTS
Effects of CSI and 1-NOA on the Root Hair Developmental Process in Wild-Type Arabidopsis Seedlings
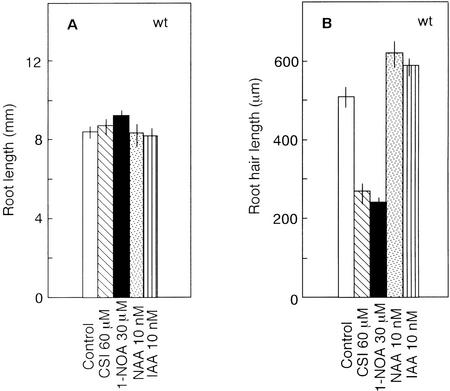
We reported earlier that application of 60 μm CSI disrupted the gravitropic response and auxin uptake in wild-type Arabidopsis roots (Rahman et al., 2001a). In the present study, we used the same concentration of CSI to see its effect on root hair developmental process in wild-type roots. Although the growth of root hairs was greatly inhibited by CSI (Figs. 1B and 3, a and b), root elongation and epidermal cell length were not inhibited (Fig. 1A; Table I). In untreated wild-type roots, approximately 40% of the epidermal cells develop root hairs, whereas in the presence of CSI, the percentage of root hair-bearing cells decreased to approximately 30% (Table I). Parry et al. (2001) showed that 30 μm 1-NOA effectively inhibited the gravitropic response of wild-type Arabidopsis roots, hence we used this concentration to observe its effect on root hair development. Interestingly, 1-NOA mimicked CSI in inhibiting the root hair growth (Figs. 1B and 3c) and root hair initiation (Table I) without altering the growth of roots. Application of 10 nm IAA or NAA, which has been shown to have little or no effect on root growth (Fig. 1A; Rahman et al., 2001b), slightly stimulated root hair elongation (0.02> P > 0.01 for NAA; 0.05> P > 0.02 for IAA; Fig. 1B) and increased the percentage of root hair-bearing cells to approximately 50% (Table I). The CSI- and 1-NOA-induced reductions in the root hair length and root hair initiation in wild type suggest that the intracellular level of auxin may play an important role for both processes.
Figure 1.
Effect of auxin, CSI, and 1-NOA on root length (A) and root hair length (B). Wild-type Arabidopsis seedlings were grown on vertical agar plates under continuous light for 3 d. Vertical bars indicate se.
Table I.
Effect of auxin, CSI, and 1-NOA on root hair formation in wild-type Arabidopsis seedlings
| Treatment | Percentage of Root Hair Cells | Epidermal Cell Length |
|---|---|---|
| % | μm | |
| Untreated wild type | 39.3 ± 1.5 | 147.37 ± 3.97 |
| Wild type + 60 μm CSI | 30.7 ± 1.7 | 167.53 ± 3.79 |
| Wild type + 30 μm 1-NOA | 33.7 ± 2.1 | 156.43 ± 1.98 |
| Wild type + 10 nm NAA | 54.3 ± 1.4 | 163.06 ± 4.07 |
| Wild type + 10 nm IAA | 54.0 ± 1.5 | 161.96 ± 4.30 |
Data are means ± se.
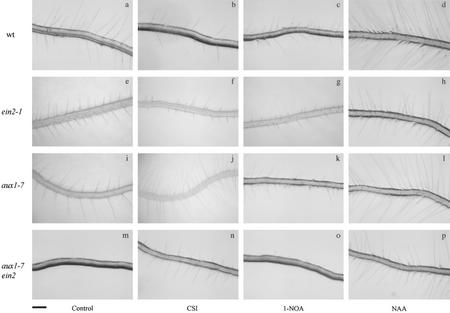
Figure 3.
Photographs showing the effect of CSI and 1-NOA on the root hair developmental process of wild-type, ein2-1, aux1-7, and aux1-7 ein2 seedlings. Arabidopsis seedlings were grown on vertical agar plates in the absence and presence of CSI, 1-NOA, or NAA under continuous light for 3 d. Concentrations of the auxin and auxin influx inhibitors were: NAA, 100 nm; CSI, 60 μm; and 1-NOA, 30 μm. Bar = 200 μm.
1-NOA and CSI Specifically Inhibit the IAA-Induced β-Glucuronidase (GUS) Expression in BA-GUS Reporter Line
The Arabidopsis BA-GUS transgenic line (BA3) encodes the auxin-responsive A and B domains of the PS-IAA4/5 promoter fused to a GUS reporter gene (Ballas et al., 1995; Oono et al., 1998). Specific GUS expression in the root elongation zone can be induced by exogenous application of auxin in this reporter line (Oono et al., 1998). By using two different auxins, IAA, which requires an uptake carrier to enter the cell, and NAA, which enters the cell mainly by diffusion (Delbarre et al., 1996; Yamamoto and Yamamoto, 1998; Marchant et al., 1999; Rahman et al., 2001a), we investigated the effect of 1-NOA and CSI on auxin influx machinery.
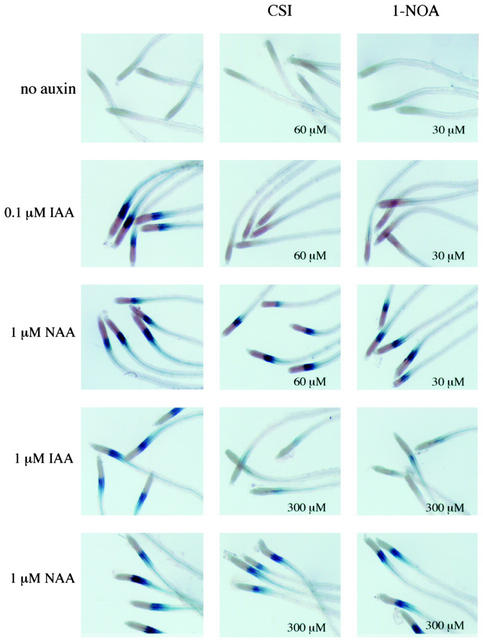
Figure 2 represents the typical effect of 1-NOA and CSI on the IAA- and NAA-induced GUS expression in the root elongation zone of BA-GUS seedlings. 1-NOA (30 μm) and 60 μm CSI completely blocked 0.1 μm IAA-induced GUS expression in these seedlings (Fig. 2, second panel), but they failed to show any effect on 1 μm NAA-induced GUS expression (Fig. 2, third panel). 1-NOA or CSI alone did not show any effect (Fig. 2, first panel). We used a 10-fold higher concentration of NAA because of the lack of response of BA-GUS transgenic line to 0.1 μm NAA. Because of a 10-fold difference in auxin concentration, one may argue that 1-NOA or CSI could not inhibit the NAA-induced GUS expression simply by the presence of a high concentration of auxin. To address this question, we investigated the effects of 1-NOA and CSI on 1 μm IAA-induced GUS expression. We found a requirement to increase the 1-NOA or CSI concentration to completely block the 1 μm IAA-induced GUS expression. A 5- to 10-fold increase in concentrations of these compounds (200–300 μm) could completely inhibit the 1 μm IAA-induced GUS expression (Fig. 2, fourth panel), whereas these concentrations did not inhibit the GUS expression induced by 1 μm NAA (Fig. 2, bottom panel). The inability of these compounds to inhibit NAA-induced GUS expression provides functional evidence that 1-NOA and CSI interfere with the auxin influx machinery of Arabidopsis roots by acting as potent auxin influx inhibitors.
Figure 2.
Histochemical analysis of GUS activity in the elongation zone of the roots of BA-GUS transgenic line. Four-day-old seedlings were incubated with 0.1 or 1.0 μm IAA or NAA supplemented with or without various concentrations of 1-NOA or CSI for 6 h. Seedlings were then stained in a buffer containing 1 mm 5-bromo-4-chloro-3-indolyl β-d-GlcUA for 18 h at 37°C in the dark.
Inhibition of Auxin Influx Blocks the Root Hair Developmental Process in Ethylene-Insensitive Mutant ein2-1
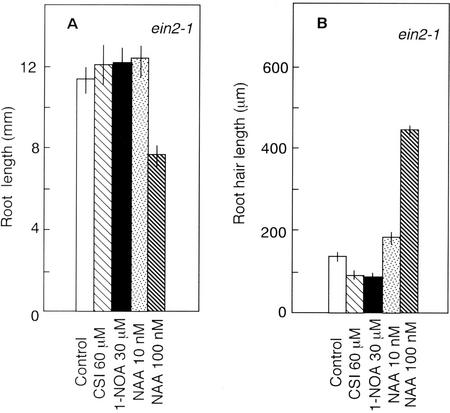
The plant hormones auxin and ethylene have been proposed to act as important regulators of root hair development (Masucci and Schiefelbein, 1996; Pitts et al., 1998), but the cross talk between these hormones (Rahman et al., 2001b) makes it difficult to separate their roles during this process. To dissect the roles of two hormones, we examined the effects of CSI and 1-NOA on the ethylene-insensitive mutant ein2-1 (Guzmán and Ecker, 1990). Untreated ein2-1 roots grew longer compared with wild-type roots (Figs. 1A and 4A), but the length of root hairs in this mutant was extremely short (Figs. 3, a and e, 1B, and 4B), as observed previously by Pitts et al. (1998). ein2-1 roots grown in the presence of CSI had fewer root hair-bearing cells and shorter root hairs compared with control (Figs. 3f and 4B). Only approximately 20% of epidermal cells formed root hairs in CSI-treated ein2-1 roots, compared with approximately 40% of untreated ein2-1 roots (Table II). 1-NOA treatment also showed similar reductions in the number of root hair-forming cells and in the length of root hairs in this mutant root (Figs. 3g and 4B; Table II). In contrast, the growth of roots and the length of mature epidermal cells were not affected by either compound (Fig. 4A; Table II). Because CSI or 1-NOA acts to block auxin influx in roots (Fig. 2), the effect of these compounds in blocking root hair initiation and elongation in ein2-1 suggests that endogenous auxin plays a critical role in both processes. Although application of 10 nm NAA to ein2-1 roots could not restore the length of root hairs to a wild-type level, a 10-fold increase in exogenous NAA (100 nm) restored root hair length to the wild-type level (Figs. 3, a and h, 1B, and 4B). The latter concentration of NAA also increased the percentage of root hair-bearing cells to approximately 50% (Table II). However, we observed reductions in root growth and in epidermal cell elongation (Fig. 4A; Table II). Our results indicate that auxin can restore root hair development in the absence of an ethylene response.
Figure 4.
Effect of auxin, CSI, and 1-NOA on root length (A) and root hair length (B). ein2-1 seedlings were grown on vertical agar plates under continuous light for 3 d. Vertical bars indicate se.
Table II.
Effect of auxin, CSI, and 1-NOA on root hair formation in ethylene-insensitive mutant ein2-1
| Treatment | Percentage of Root Hair Cells | Epidermal Cell Length |
|---|---|---|
| % | μm | |
| Untreated ein2-1 | 39.6 ± 1.7 | 173.24 ± 3.67 |
| ein2-1+ 60 μm CSI | 17.9 ± 1.6 | 173.31 ± 2.79 |
| ein2-1+ 30 μm 1-NOA | 20.0 ± 2.2 | 172.46 ± 1.95 |
| ein2-1+ 10 nm NAA | 43.3 ± 1.9 | 169.87 ± 2.63 |
| ein2-1+ 100 nm NAA | 47.0 ± 1.2 | 123.52 ± 3.52 |
Data are means ± se.
CSI Phenocopies the Wild-Type Root Hair Phenotype in the Auxin-Influx Mutant aux1-7
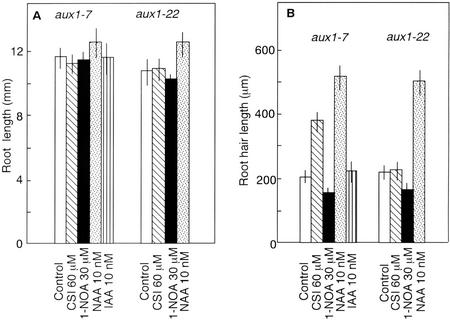
CSI has been described to exhibit opposite effects in aux1-7 mutant and wild-type roots. CSI inhibits gravitropic response, auxin influx, and ethylene-mediated growth response in wild-type roots (Rahman et al., 2001a) but stimulates all of them in aux1-7 roots (Rahman et al., 2001a). The unique effects of CSI on aux1-7 roots prompted us to investigate its effect on root hair development in this mutant. The aux1-7 mutant has a defect in auxin influx and is also resistant to ethylene (Pickett et al., 1990; Rahman et al., 2001a, 2001b). In the aux1-7 mutant root, approximately 30% of the epidermal cells formed root hairs (Table III). This value is less compared with approximately 40% of both wild-type and ein2-1 roots (Tables I and II). The root hair length of aux1-7 is also considerably shorter than that of wild type but slightly longer than ein2-1 (Figs. 3, a, e, and i, 1B, 4B, and 5B). These results suggest that the normal level of endogenous auxin or the normal response to ethylene is required for both root hair initiation and root hair elongation. Application of 60 μm CSI dramatically changed the aux1-7 root hair phenotype (Figs. 3, i and j). CSI stimulated root hair length (Fig. 5B) and also increased the percentage of root hair-bearing cells to approximately 50% (Table III) without altering root length and epidermal cell length (Fig. 5A; Table III). Application of 10 nm NAA, which has been suggested to enter into the cell mainly by diffusion (Delbarre et al., 1996), mimicked CSI treatment to rescue aux1-7 root hair development (Fig. 5B). The percentage of root hair-bearing cells in NAA-treated aux1-7 roots also increased from approximately 30% of control to approximately 50%, which is similar to that of CSI-treated roots (Table III). Application of IAA, suggested to be taken up by the uptake carrier AUX1 (Delbarre et al., 1996; Yamamoto and Yamamoto, 1998; Marchant et al., 1999; Rahman et al., 2001a), did not show any effect on root hair developmental process of aux1-7, confirming the idea that the auxin influx carrier protein is mutated in aux1-7 (Bennett et al., 1996). These results collectively suggest that CSI phenocopied the wild-type root hair phenotype by increasing the intracellular level of auxin in aux1-7 roots. In contrast, 1-NOA failed to induce any change in aux1-7 root hair phenotype (Figs. 3k and 5B; Table III).
Table III.
Effect of auxin, CSI, and 1-NOA on root hair formation in auxin influx mutants aux1-7 and aux1-22
| Treatment | Percentage of Root Hair Cells | Epidermal Cell Length |
|---|---|---|
| % | μm | |
| Untreated aux1-7 | 32.5 ± 2.3 | 179.54 ± 2.87 |
| aux1-7+ 60 μm CSI | 51.4 ± 2.8 | 175.30 ± 2.94 |
| aux1-7+ 30 μm 1-NOA | 31.0 ± 2.8 | 179.46 ± 2.68 |
| aux1-7+ 10 nm NAA | 51.0 ± 1.9 | 189.68 ± 5.06 |
| aux1-7+ 10 nm IAA | 29.0 ± 2.3 | 185.79 ± 5.70 |
| Untreated aux1-22 | 32.2 ± 2.8 | 180.56 ± 3.50 |
| aux1-22+ 60 μm CSI | 29.9 ± 1.7 | 175.81 ± 3.20 |
| aux1-22+ 30 μm 1-NOA | 33.7 ± 2.5 | 178.48 ± 4.24 |
| aux1-22+ 10 nm NAA | 44.0 ± 2.2 | 185.68 ± 3.85 |
Data are means ± se.
Figure 5.
Effect of auxin, CSI, and 1-NOA on root length (A) and root hair length (B). aux1-7 and aux1-22 seedlings were grown on vertical agar plates under continuous light for 3 d. Vertical bars indicate se.
A null allele of aux1, aux1-22 (Marchant and Bennett, 1998), exhibited a similar root hair phenotype to aux1-7, i.e. the root hair length of aux1-22 was reduced (Fig. 5B) and the percentage of root hair-bearing cells was approximately 30% (Table III). However, application of CSI failed to induce any change in the root hair phenotype in this mutant (Fig. 5B; Table III), yet NAA completely restored the root hair phenotype to wild type (Fig. 5B; Table III). These results confirm that CSI specifically interacts with AUX1 protein in regulating the auxin uptake in Arabidopsis roots and thereby controls the root hair developmental process.
Root Hair Phenotype of Double Mutant aux1-7 ein2 and the Effect of CSI
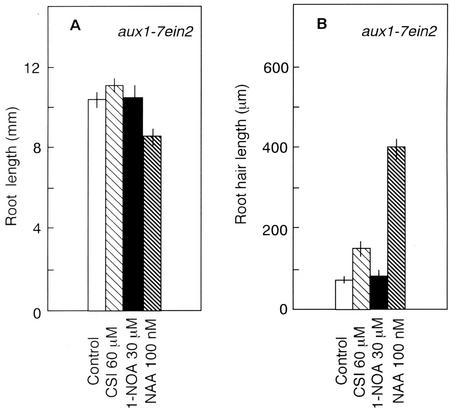
To confirm our hypothesis that auxin plays a critical role in controlling the root hair developmental process in Arabidopsis, we analyzed root hair initiation and elongation processes in the aux1-7 ein2 double mutant. The percentage of root hair-bearing cells in untreated aux1-7 ein2 double mutant was approximately 14% (Table IV), significantly less compared with the single mutants ein2-1 or aux1 (Table II, III). The root hair length of the double mutant was also significantly shorter (Figs. 3m and 6B).
Table IV.
Effect of auxin, CSI, and 1-NOA on root hair formation in aux1-7 ein2 double mutant
| Treatment | Percentage of Root Hair Cells | Epidermal Cell Length |
|---|---|---|
| % | μm | |
| Untreated aux1-7 ein2 | 13.9 ± 1.9 | 187.81 ± 2.41 |
| aux1-7 ein2+ 60 μm CSI | 27.0 ± 2.0 | 185.39 ± 1.72 |
| aux1-7 ein2+ 30 μm 1-NOA | 16.1 ± 2.1 | 182.28 ± 1.98 |
| aux1-7 ein2+ 100 nm NAA | 52.1 ± 2.7 | 138.50 ± 3.37 |
Data are means ± se.
Figure 6.
Effect of auxin, CSI, and 1-NOA on root length (A) and root hair length (B). aux1-7 ein2 seedlings were grown on vertical agar plates under continuous light for 3 d. Vertical bars indicate se.
Interestingly, CSI partially restored both root hair initiation and root hair elongation in the aux1-7 ein2 mutant. CSI increased the percentage of root hair-forming cells to approximately 27% compared with 14% of control (Table IV). A 2-fold increase in root hair length was also observed (Figs. 3, m and n, and 6B) by CSI application. Nevertheless, the effect of CSI in aux1-7 ein2 was comparatively weaker than observed for aux1-7 (Figs. 3, j and n, 5B, and 6B; Tables III and IV). In contrast to CSI, 1-NOA did not influence root hair formation or elongation in this mutant (Figs. 3o and 6B; Table IV). However, the application of 100 nm NAA completely recovered the root hair phenotype of aux1-7 ein2 double mutant to wild-type level. The percentage of root hair-bearing cells increased to approximately 50% (Table IV), and a 5-fold increase in the root hair length was also observed (Figs. 3p and 6B). Our results strongly support the idea that endogenous auxin plays a crucial role in regulating root hair developmental processes in Arabidopsis and can partially compensate for the absence of an ethylene response.
DISCUSSION
The plant hormones auxin and ethylene have been suggested to act after root hair cell specification in Arabidopsis (Masucci and Schiefelbein, 1996; for review, see Schiefelbein, 2000). The cross talk between auxin and ethylene in Arabidopsis roots (Rahman et al., 2001b) makes it difficult to understand the independent role of either hormone in root hair developmental process, as illustrated by the cross resistance of the auxin-resistant mutants (e.g. axr1, axr2, axr3, and aux1) toward ethylene. Several studies have used the ethylene biosynthetic inhibitor AVG to elucidate the role of ethylene during this developmental process (Tanimoto et al., 1995; Masucci and Schiefelbein, 1996), but no such work is available to date to determine the role of auxin. In the present paper, we dissected the role of auxin as well as ethylene during root hair development using two interesting compounds, CSI and 1-NOA.
1-NOA and CSI Are Potent Auxin Influx Inhibitors
The exogenous requirement of auxin to induce GUS expression in the root elongation zone of BA-GUS transgenic line makes it an excellent reporter to investigate the interaction of 1-NOA and CSI with the auxin influx components in Arabidopsis. Although application of 0.1 μm IAA induced the GUS expression in the BA-GUS transgenic line, 0.1 μm NAA failed to do so (data not shown). We also found a difference in the response of the transgenic line toward another auxin, 2,4-dichlorophenoxyacetic acid (2,4-D). Like NAA, at least 1 μm 2,4-D was required to induce GUS expression in the BA-GUS line (Y. Oono and A. Rahman, unpublished data). We found that 30 μm 1-NOA or 60 μm CSI completely blocked 0.1 μm IAA-induced GUS expression (Fig. 2, second panel) and that 300 μm 1-NOA or CSI was required to block 1 μm IAA-induced GUS expression (Fig. 2, fourth panel). On the other hand, these concentrations of 1-NOA and CSI were unable to block the 1 μm NAA-induced GUS expression in this line (Fig. 2, third and bottom panels). Because IAA enters the cell through an uptake carrier while NAA enters by diffusion (Delbarre et al., 1996), these results indicate that 1-NOA and CSI interfere with the auxin influx component of Arabidopsis roots. These results are also in agreement with our previous finding that CSI specifically inhibited [3H]IAA uptake in Arabidopsis roots yet failed to block [3H]NAA uptake (Rahman et al., 2001a).
Auxin Plays a Compensating Role in Root Hair Developmental Process in Arabidopsis Roots in the Absence of Ethylene
Several lines of evidence support the argument that auxin can control root hair development in the absence of an ethylene response. The first line of evidence is the root hair phenotype of ethylene-insensitive mutant ein2-1. Ethylene signaling is disrupted in ein2-1 mutant because of a mutation in the bifunctional transducer protein EIN2 (Alonso et al., 1999), which mediates an essential step in the signal propagation between CTR1 and EIN3/EIL (Roman et al., 1995; Chao et al., 1997). Even in the absence of an ethylene response, approximately 40% of ein2-1 epidermal cells form root hairs (Table II). The frequency of root hairs in ein2-1 roots is similar to that of wild-type roots (Table I). Masucci and Schiefelbein (1996) also previously reported that root hair number is not altered in ein2-1 or in another ethylene-insensitive mutant etr1-1. These results suggest that for the root hair initiation process, the absence of ethylene response can be compensated by another factor. Because auxin and ethylene have been proposed to act during root hair development (Masucci and Schiefelbein, 1996; Pitts et al., 1998), auxin represents a likely candidate as the compensating factor.
The second line of evidence is the effect of the CSI and 1-NOA on ein2-1 mutant. Both CSI and 1-NOA can act as inhibitors of auxin uptake in Arabidopsis roots (Fig. 2; Parry et al., 2001; Rahman et al., 2001a). We used these compounds to reduce the intracellular level of auxin in ein2-1 and investigated their effects on root hair initiation. As expected, we observed a significant reduction in the number of root hair-forming cells in ein2-1 roots grown in the presence of CSI or 1-NOA (Fig. 3, f and g). The frequency of root hairs was reduced to approximately 20% from approximately 40% of untreated control (Table II). These results suggest that the normal root hair initiation in the ethylene-insensitive mutant ein2-1 is attributable to auxin.
Finally, the root hair phenotype of the double mutant aux1-7 ein2 further supports the idea. If auxin plays a complementary role in the ein2-1 mutant, one can expect that in the aux1-7 ein2 double mutant, the percentage of root hair-bearing cell would be reduced compared with the ein2-1 single mutant. We observed a reduced frequency (approximately 14%) of root hair initiation in the double mutant compared with approximately 40% in ein2-1 (Fig. 3, e and m; Tables II and IV). To rule out the possibility of overlooking minute bulging, we counted the root hairs at 100× magnification and obtained identical results. This reduction in the root hair frequency in aux1-7 ein2 also confirms the function of CSI and 1-NOA as auxin influx inhibitors, because we obtained a similar reduction in the root hair frequency in CSI- or 1-NOA-treated ein2-1 roots (Tables II and IV). All of these results suggest that endogenous auxin plays a critical role for root hair initiation in the absence of an ethylene response.
The auxin influx mutant aux1, which is also ethylene resistant (Pickett et al., 1990), showed a reduced number of root hair-bearing cells compared with wild type and ein2-1 (Tables I–III). We reported previously that a reduction in the intracellular level of auxin decreased the ethylene-mediated growth response in wild-type Arabidopsis roots (Rahman et al., 2001a) and that the application of a minute concentration of NAA (10 nm) restored the ethylene response in the ethylene-resistant mutants aux1-7 and eir1-1 (Rahman et al., 2001b). In the present study, we found that 10 nm NAA restored aux1 root hair initiation (Table III). Therefore, we argue that the reduction in the frequency of the root hair-bearing cells in aux1 mutants is attributable to a reduced level of endogenous auxin and the resulting alteration in ethylene response. This argument is further supported by the observation that CSI or 1-NOA application to wild-type roots mimicked the aux1 root hair phenotype, i.e. a reduction in the frequency of root hair-forming cells (Table I). These results collectively indicate that the reduction in the root hair frequency in both aux1 mutants and CSI/1-NOA-treated wild-type seedlings is attributable to the low level of endogenous auxin and the reduced response to ethylene, which is regulated by the intracellular level of auxin.
In contrast to root hair initiation, the regulation of root hair elongation is more complex. Although ein2-1 mutant shows a normal percentage of root hair-bearing cells (Table II), the root hair length is extremely short (Figs. 3e and 4B). These results apparently could lead to a conclusion that for the root hair elongation process, auxin may not work as a compensating factor. We also found that a low level of exogenous auxin (10 nm) did not restore ein2-1 root hair length to the wild-type level (Fig. 4B), whereas, a 10-fold increase in the concentration of exogenous auxin completely recovered root hair length (Figs. 3h and 4B). Because ethylene signaling is absent in the ein2-1 mutant, the recovery of the root hair length by exogenous auxin suggests that auxin can also facilitate root hair elongation. The root hair length of the aux1-7 ein2 double mutant is significantly shorter than that of ein2-1 (Figs. 3, e and m, and 6B). We also observed the similar decrease in the root hair length in CSI- or 1-NOA-treated ein2-1 mutant (Fig. 4B). These results collectively indicate that endogenous auxin plays a significant role in root hair outgrowth of the ein2-1 mutant and partially compensates for the loss of ethylene response.
In the auxin influx mutant aux1, the root hair length was found to be significantly shorter than that of wild type (Figs. 3, a and i, 1B, and 5B) but slightly longer than ein2-1 (Figs. 3, e and i, 4B, and 5B). Application of a very low concentration (10 nm) of NAA could restore the root hair length of aux1 mutant to the wild-type level in contrast to ein2-1, which required 100 nm of NAA (Figs. 4B and 5B), suggesting that the loss of ethylene signaling makes the root less sensitive to auxin. It is also interesting to note that application of 10 nm NAA stimulated the percentage of root hair-bearing cells in both the wild-type and aux1 mutants to approximately 50%, whereas ein2-1 and aux1-7 ein2 mutants required 100 nm NAA to increase the root hair-bearing cells to that level. All of these results, along with the requirement of the higher concentration of NAA for recovering root hair growth in ein2-1and aux1-7 ein2 mutants, suggest that insensitivity in ethylene response affects auxin-driven root hair elongation and initiation processes. These results confirm that the loss of ethylene sensitivity makes the root resistant to auxin to some extent. This idea is consistent with our observation that root elongation of ein2-1 is resistant to auxin (data not shown). It has also been cited earlier as an unpublished observation of the author that both ein2 and etr1 mutants show low levels of auxin resistance (Hobbie, 1998). Later, Hobbie et al. (2000) identified ein2 alleles in the screen of 2,4-D-resistant plants. Zolman et al. (2000) found ein2-1 to be resistant to indole-butyric acid.
The root hair developmental process seems to be divided into two steps. In the first step, endogenous auxin plays a compensating role in the absence of an ethylene response as observed in ein2-1 roots, and in the second step, endogenous auxin acts together with ethylene for root hair outgrowth. A higher level of auxin is required for facilitating the latter step in the absence of ethylene signaling.
CSI: a Novel Auxin Influx Modulator in Arabidopsis Roots
CSI exhibited a unique mode of action in controlling root hair developmental process in Arabidopsis roots. Although CSI behaved like 1-NOA to inhibit both root hair initiation and root hair growth in wild-type and ein2-1 roots (Figs. 1B and 4B; Tables I and II), in aux1-7and aux1-7 ein2 roots, CSI showed completely opposite effects increasing the root hair length and the number of root hair-bearing cells (Figs. 3, j and n, 5B, and 6B; Tables III and IV), whereas 1-NOA did not show any effect on these mutant roots. These results are consistent with our previous findings that CSI partially restored the auxin influx in aux1-7 roots and restored both the gravitropic response and ethylene-induced inhibition of root growth in this mutant root (Rahman et al., 2001a). CSI was much less effective in recovering the frequency of root hair-bearing cells and root hair outgrowth in aux1-7 ein2 double mutant compared with those of aux1-7 single mutant (Figs. 3, j and n, 5B, and 6B; Tables III and IV). These results suggest that CSI-induced recovery in the root hair development of the aux1-7 mutant requires an ethylene response, highlighting the interaction between auxin and ethylene during root hair development in Arabidopsis.
We propose that CSI-induced change in the root hair phenotype of aux1-7 is mediated by restoration of auxin uptake, which consequently accelerates the response to endogenous ethylene and phenocopies the wild-type root hair phenotype. On the other hand, because of the absence of ethylene signaling in aux1-7 ein2 double mutant, CSI only partially restored the root hair phenotype (Fig. 6B; Table IV). We also observed a difference in NAA concentrations required to recover the wild-type root hair phenotype in aux1-7 and aux1-7 ein2 mutants (Table IV). For instance, 10 nm NAA increased the percentage of root hair-bearing cells to approximately 50% in aux1-7, whereas 100 nm NAA was required for the aux1-7 ein2 double mutant. A similar difference in the requirement of auxin concentration was observed for root hair growth (Figs. 5B and 6B) in these mutants. These results suggest that in the presence of ethylene signaling, a low level of auxin is enough to restore a wild-type root hair phenotype, whereas in the absence of an ethylene response, an increased level of auxin is required. The differential effect of CSI on aux1-7 and aux1-7 ein2 mutants along with the requirement of different concentrations of NAA to induce the similar changes in root hair phenotype clearly suggest that in the presence of ethylene signaling, auxin acts together with endogenous ethylene. This idea is consistent with our previous finding that auxin is a positive regulator for ethylene-mediated response in the growth of Arabidopsis roots (Rahman et al., 2001b).
In the null allele of aux1, aux1-22 (Marchant and Bennett, 1998), we could not find any effect of CSI in changing the root hair phenotype, whereas NAA completely restored the root hair phenotype to wild type (Fig. 5B; Table III). These results are consistent with our previous hypothesis that CSI specifically interacts with AUX1 protein in regulating auxin influx and thereby affecting several root developmental processes including gravitropism and the ethylene-mediated growth response (Rahman et al., 2001a). In the present paper, we show that CSI influences both the root hair initiation and root hair elongation. All of these results confirm that CSI interacts via the AUX1 protein to regulate the intracellular level of auxin in Arabidopsis roots. In the two aux1 alleles, aux1-7 and aux1-22, 1-NOA did not show any effect on root hair elongation, root hair formation, and epidermal cell elongation, indicating that AUX1 function is required for 1-NOA action. This is the first strong evidence showing that 1-NOA action requires AUX1 function.
In summary, we conclude that endogenous auxin plays a complementary role for root hair development in the absence of an ethylene response in Arabidopsis. Auxin may act as a positive regulator for the endogenous ethylene-mediated root hair growth and root hair initiation. We have also demonstrated the physiological importance of auxin influx modulators in dissecting the roles of auxin and ethylene in root hair developmental process.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
All mutant lines were derived from Arabidopsis (L.) Heynh. ecotype Columbia. Auxin-resistant mutant aux1-7 (Pickett et al., 1990), ethylene-insensitive mutant ein2-1 (Guzmán and Ecker, 1990), and double mutant aux1-7 ein2 were obtained from Arabidopsis Biological Resource Center (Ohio State University, Columbus). These mutants were propagated as described previously (Rahman et al., 2000). The AUX1 null allele aux1-22 was a kind gift from Dr. Bennett. BA-GUS transgenic Arabidopsis (BA3) line is described by Oono et al. (1998).
Buffer solution was made of 5 mm KNO3, 2 mm Ca(NO3)2, 2 mm MgSO4, 1 mm KH2PO4, and 5 mm MOPS (pH 6.6). The pH of the buffer was adjusted with KOH. Arabidopsis seeds were placed in a 2.6-cm Petri dish on filter paper (Advantec no. 2, Toyo Roshi Kaisha, Ltd., Tokyo) wetted with 300 μL of the buffer. Two or 4 d after cold treatment at 4°C under nearly saturating humidity in the dark, seeds were germinated by irradiating for 1 or 2 d with white fluorescent lamps (FL 20SS-BRN/18, Toshiba, Tokyo) at an irradiance of about 17 μmol m−2 s−1. The irradiated seeds were transferred to 1% (w/v) agar plates containing the buffer solution described above in a rectangular plastic petri dish (6 × 4 cm). 1-NOA was dissolved in dimethyl sulfoxide to make a stock solution of 0.5 m. The concentration of dimethyl sulfoxide in 30 μm 1-NOA was 0.006%. Auxin, CSI, and 1-NOA were mixed with agar medium while the temperature of agar was 45°C to 50°C. Seedlings were grown on vertically oriented agar plate at 23°C under continuous irradiation.
Chemicals
CSI was extracted from 7-d-old etiolated pea (Pisum sativum L. cv Alaska) seedlings with aqueous methanol and purified by HPLC as described previously (Tsurumi et al., 1992). The purified CSI was dried to white powder and kept under N2 at −80°C. IAA and NAA were purchased from Sigma-Aldrich (St. Louis). 1-NOA was from Aldrich Chemical Co.(Milwaukee) and toluidine blue N was from Schmidt GmbH Co. (Köngen/N, Germany). Other chemicals were from Wako Pure Chemical Industries, Ltd. (Osaka).
Morphometric Analysis
Seedlings were grown vertically as described above for 3 d. They were stained with a dilute toluidine blue (0.01%) solution and placed on a glass microscope slide under a coverslip. The number of root hairs in the 1-mm-region length at the midpoint of a root was counted under a light microscope (BX-50, Olympus, Tokyo) at 40× or 100× magnification depending on the sample type. From the midpoint of this 1-mm region, the length of 10 root hairs from each root was measured at 100× magnification. From the same zone the lengths of 10 mature epidermal cells per root were counted, and the total number of epidermal cells of this zone was calculated. Values from eight roots were used to determine the mean (± se) for percentage of root hair-bearing cells, root hair length, and epidermal cell length in each measurement. The measurement was repeated at least three to five times. P values were analyzed by Student's t test. Root hairs that grew along the surface of the agar media were photographed at 50× magnification at the longitudinal midpoint of a root by using an Axioplan (Zeiss, Welwyn Garden City, UK)/MZFLIII (Leica, Wetzlar, Germany) imaging microscope equipped with an Olympus DP-50 digital camera.
GUS Reporter Assay
GUS assay was performed as described earlier (Oono et al., 1998). In brief, 4-d-old seedlings grown on agar plate as described above were treated with IAA/NAA supplemented with or without various concentrations of 1-NOA or CSI for 6 h in germination media. Seedlings were rinsed three times with staining buffer and incubated for 18 h in staining buffer containing 1 mm 5-bromo-4-chloro-3-indolyl β-d-GlcUA at 37°C in the dark. GUS expression in the root elongation zone was observed using a Leica MZFLIII dissecting microscope equipped with an Olympus DP-50 digital camera. Images were processed with Adobe Photoshop 6.0 (Adobe Systems, Mountain View, CA).
ACKNOWLEDGMENTS
We thank Dr. Malcolm. J. Bennett of Nottingham University (Nottingham, UK) for providing us the aux1-22 seeds and for critical reading of this manuscript, Dr. Masaaki Miyamoto of Kobe University for permitting us to use the microscope, and the Arabidopsis Biological Resource Center of Ohio State University for the other mutant seeds.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010546.
LITERATURE CITED
- Alonso MJ, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- Ballas N, Wong LM, Ke M, Theologies A. Two auxin-responsive domains interact positively to induce expression of the early indole acetic acid-inducible gene PS-IAA4/5. Proc Natl Acad Sci USA. 1995;92:3483–3487. doi: 10.1073/pnas.92.8.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science. 1996;273:948–950. doi: 10.1126/science.273.5277.948. [DOI] [PubMed] [Google Scholar]
- Berger F, Hung C-Y, Dolan L, Schiefelbein J. Control of cell division in the root epidermis of Arabidopsis thaliana. Dev Biol. 1998;194:235–245. doi: 10.1006/dbio.1997.8813. [DOI] [PubMed] [Google Scholar]
- Bunning E. Uber die differenzierungsvorgange in der cruciferenwurzel. Planta. 1951;39:126–153. [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell. 1997;89:1133–1144. doi: 10.1016/s0092-8674(00)80300-1. [DOI] [PubMed] [Google Scholar]
- Cormack RGH. A comparative study of developing epidermal cells in white mustard and tomato roots. Am J Bot. 1947;34:310–314. [Google Scholar]
- Cormack RGH. The development of root hairs in angiosperms. Bot Rev. 1949;15:583–612. [Google Scholar]
- Cutter EJ. Plant Anatomy. London: Clowes & Sons; 1978. The epidermis; pp. 94–106. [Google Scholar]
- Delbarre A, Muller P, Imhoff V, Guern J. Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta. 1996;198:532–541. doi: 10.1007/BF00262639. [DOI] [PubMed] [Google Scholar]
- Dolan L, Duckett C, Grierson C, Linstead P, Schneider K, Lawson E, Dean C, Poethig S, Roberts K. Clonal relations and patterning in the root epidermis of Arabidopsis. Development. 1994;120:2465–2474. [Google Scholar]
- Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, Schiefelbein JW. The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev Biol. 1994;166:740–754. doi: 10.1006/dbio.1994.1352. [DOI] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie L, McGovern M, Hurwitz LR, Pierro A, Liu NY, Bandyopadhyay A, Estelle M. The axr6 mutants of Arabidopsis thaliana define a gene involved in auxin response and early development. Development. 2000;127:23–32. doi: 10.1242/dev.127.1.23. [DOI] [PubMed] [Google Scholar]
- Hobbie LJ. Auxin: molecular genetic approaches in Arabidopsis. Plant Physiol Biochem. 1998;36:91–102. [Google Scholar]
- Imhoff V, Muller P, Guern J, Delbarre A. Inhibitors of the carrier mediated influx of auxin in suspension-cultured tobacco cells. Planta. 2000;210:580–588. doi: 10.1007/s004250050047. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Kudou S, Tonomura M, Tsukamoto C, Shimoyamada M, Uchida T, Okubo K. Isolation and structural elucidation of the major genuine soybean saponin. Biosci Biotechnol Biochem. 1992;56:142–143. doi: 10.1271/bbb.56.142. [DOI] [PubMed] [Google Scholar]
- Kudou S, Tonomura M, Tsukamoto C, Uchida T, Sakabe T, Tamura N, Okubo K. Isolation and structural elucidation of DDMP-conjugated soyasaponins as genuine saponins from soybean seeds. Biosci Biotechnol Biochem. 1993;57:546–550. [Google Scholar]
- Leyser HMO, Pickett FB, Dharmasiri S, Estelle M. Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J. 1996;10:403–413. doi: 10.1046/j.1365-313x.1996.10030403.x. [DOI] [PubMed] [Google Scholar]
- Marchant A, Bennett MJ. The Arabidopsis AUX1 gene: a model system to study mRNA processing in plants. Plant Mol Biol. 1998;36:463–471. doi: 10.1023/a:1005961303167. [DOI] [PubMed] [Google Scholar]
- Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 1999;18:2066–2073. doi: 10.1093/emboj/18.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massiot G, Lavaud C, Benkhaled M, Le Men-Olivier L. Soyasaponin VI, a new maltol conjugate from alfalfa and soybean. J Nat Prod. 1992;55:1339–1342. [Google Scholar]
- Masucci JD, Schiefelbein JW. The rhd6 mutation of Arabidopsis alters root hair initiation through an auxin and ethylene-associated process. Plant Physiol. 1994;106:1335–1346. doi: 10.1104/pp.106.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW. Hormones act downstream of TTG and GL2 to promote root hair initiation through an auxin and ethylene-associated process. Plant Cell. 1996;8:1505–1517. doi: 10.1105/tpc.8.9.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Shimura Y. Arabidopsis. Cold Spring Harbor Laboratory Press. 1994. Modulation of root growth by physical stimuli; pp. 665–684. [Google Scholar]
- Oono Y, Chen QG, Overvoorde PJ, Köhler C, Theologies A. age mutants of Arabidopsis exhibit altered auxin-regulated gene expression. Plant Cell. 1998;10:1649–1662. doi: 10.1105/tpc.10.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G, Delbarre A, Marchant A, Swarup R, Napier R, Perrot-Rechenmann C, Bennett MJ. Novel auxin transport inhibitors phenocopy the auxin influx carrier mutation aux1. Plant J. 2001;25:399–406. doi: 10.1046/j.1365-313x.2001.00970.x. [DOI] [PubMed] [Google Scholar]
- Pickett FB, Wilson AK, Estelle M. The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol. 1990;94:1462–1466. doi: 10.1104/pp.94.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Cernac A, Estelle M. Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J. 1998;16:553–560. doi: 10.1046/j.1365-313x.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- Rahman A, Ahamed A, Amakawa T, Goto N, Tsurumi S. Chromosaponin I specifically interacts with AUX1 protein in regulating the gravitropic response of Arabidopsis roots. Plant Physiol. 2001a;125:990–1000. doi: 10.1104/pp.125.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Amakawa T, Goto N, Tsurumi S. Auxin is a positive regulator for ethylene-mediated response in the growth of Arabidopsis roots. Plant Cell Physiol. 2001b;42:301–307. doi: 10.1093/pcp/pce035. [DOI] [PubMed] [Google Scholar]
- Rahman A, Tsurumi S, Amakawa T, Soga K, Hoson T, Goto N, Kamisaka S. Involvement of ethylene and gibberellin signalings in chromosaponin I-induced cell division and cell elongation in the roots of Arabidopsis seedlings. Plant Cell Physiol. 2000;41:1–9. doi: 10.1093/pcp/41.1.1. [DOI] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red far-red light receptor phytochrome-b alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothernberg M, Ecker JR. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel loci integrated into a stress response pathway. Genetics. 1995;139:1393–1409. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW. Constructing a plant cell: the genetic control of root hair development. Plant Physiol. 2000;124:1525–1531. doi: 10.1104/pp.124.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto M, Roberts K, Dolan L. Ethylene is a positive regulator of root-hair development in Arabidopsis thaliana. Plant J. 1995;8:943–948. doi: 10.1046/j.1365-313x.1995.8060943.x. [DOI] [PubMed] [Google Scholar]
- Tsurumi S, Takagi T, Hashimoto T. A new UV-B-absorbing substance in pea seedlings. Amsterdam: Abstracts of Fourth Congress of the European Society for Photobiology; 1991. , September 1–6, 1991, Elsevier Sequoia, Lausanne, p 113. [Google Scholar]
- Tsurumi S, Takagi T, Hashimoto T. A γ-pyronyl-triterpenoid saponin from Pisum sativum. Phytochemistry. 1992;31:2435–2438. doi: 10.1016/0031-9422(92)83294-9. [DOI] [PubMed] [Google Scholar]
- Wilson A, Pickett FB, Turner JC, Estelle M. A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol Gen Genet. 1990;222:377–383. doi: 10.1007/BF00633843. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Yamamoto KT. Differential effects of 1-naphthaleneacetic acid, indole-3-acetic acid and 2,4-dichlorophenoxyacetic acid on the gravitropic response of roots in an auxin-resistant mutant of Arabidopsis, aux1. Plant Cell Physiol. 1998;39:660–664. doi: 10.1093/oxfordjournals.pcp.a029419. [DOI] [PubMed] [Google Scholar]
- Zolman BK, Yaoder A, Bartel B. Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics. 2000;156:1323–1337. doi: 10.1093/genetics/156.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]