Abstract
We show that above a certain threshold concentration, ozone leads to leaf injury in tomato (Lycopersicon esculentum). Ozone-induced leaf damage was preceded by a rapid increase in 1-aminocyclopropane-1-carboxylic acid (ACC) synthase activity, ACC content, and ethylene emission. Changes in mRNA levels of specific ACC synthase, ACC oxidase, and ethylene receptor genes occurred within 1 to 5 h. Expression of the genes encoding components of ethylene biosynthesis and perception, and biochemistry of ethylene synthesis suggested that ozone-induced ethylene synthesis in tomato is under biphasic control. In transgenic plants containing an LE-ACO1 promoter-β-glucuronidase fusion construct, β-glucuronidase activity increased rapidly at the beginning of the O3 exposure and had a spatial distribution resembling the pattern of extracellular H2O2 production at 7 h, which coincided with the cell death pattern after 24 h. Ethylene synthesis and perception were required for active H2O2 production and cell death resulting in visible tissue damage. The results demonstrate a selective ozone response of ethylene biosynthetic genes and suggest a role for ethylene, in combination with the burst of H2O2 production, in regulating the spread of cell death.
The gaseous plant hormone ethylene regulates many processes during plant growth and development and is also an important mediator of plant responses to biotic and abiotic stresses (Kende, 1993; Wang et al., 2002). The first committed step in ethylene biosynthesis, the conversion of S-adenosyl Met (Ado-Met) to 1-aminocyclopropane-1-carboxylic acid (ACC) is catalyzed by ACC synthase (ACS). ACC oxidase (ACO), in turn, oxidizes ACC to ethylene. ACC can also be conjugated to biologically inactive forms. In tomato (Lycopersicon esculentum), ACS and ACO are encoded by gene families consisting of at least eight (Oetiker et al., 1997; Shiu et al., 1998) and four members (Barry et al., 1996; Nakatsuka et al., 1998), respectively. These genes show differential expression during plant growth and development, and respond differentially to various external stimuli (Rottmann et al., 1991; Lincoln et al., 1993; Barry et al., 1996, 2000; Oetiker et al., 1997; Nakatsuka et al., 1998; Tatsuki and Mori, 1999; Llop-Tous et al., 2000).
Ozone (O3) is a potent abiotic stress that induces ethylene synthesis in plants (Tingey et al., 1976; Kangasjärvi et al., 1994; Sandermann, 1996; Sandermann et al., 1998). Induction of ethylene synthesis by high O3 is rapid, and a mechanistic connection between ethylene and O3 damage has been demonstrated; when ethylene synthesis is prevented with ACS, ACO, or ethylene action inhibitors, or mutations in ethylene signaling in ozone-sensitive plants, tissue damage has been reduced accordingly (Mehlhorn and Wellburn, 1987; Bae et al., 1996; Tuomainen et al., 1997; Overmyer et al., 2000).
Ozone appears to act primarily as an elicitor of defense and damage-related processes and not directly as an oxidizing agent that damages leaf tissue (Schraudner et al., 1997; Sandermann et al., 1998; Overmyer et al., 2000; Rao and Davis, 2001; Langebartels et al., 2002). This seems to relate to the signaling function of oxygen radicals, and can be regarded as analogous to the oxidative burst in development of systemic acquired resistance and pathogen defense gene induction. The oxidative burst, corresponding to the release of reactive oxygen species (ROS) into the apoplastic space, is one of the earliest plant responses to pathogen infection (Lamb and Dixon, 1997; Dat et al., 2000). These ROS are also important components in regulating cell death in the hypersensitive response (HR), a form of programmed cell death (pcd) in plants (Levine et al., 1994; Tenhaken et al., 1995; Jabs et al., 1996; Alvarez et al., 1998).
In addition to the direct ROS formation from degradation of ozone in the apoplast, O3 also induces an oxidative burst by the plant cells. In ozone-sensitive tobacco (Nicotiana tabacum) Bel W3 (Schraudner et al., 1998), birch (Betula pendula; Pellinen et al., 1999, 2002), ozone-sensitive Arabidopsis (Rao and Davis, 1999; Overmyer et al., 2000; Wohlgemuth et al., 2002), and native plant species (Wohlgemuth et al., 2002), H2O2 (tobacco and birch) and superoxide (Arabidopsis, Malva sylvestris, and Rumex sp.) production was evident in the tissues several hours after a short ozone pulse. The ROS production in Arabidopsis, tomato, and birch was partly inhibited by the plasma membrane NADPH oxidase inhibitor diphenylene iodonium accompanied with reduced tissue damage (Pellinen et al., 1999; Rao and Davis, 1999; Overmyer et al., 2000; Wohlgemuth et al., 2002).
The similarity between ozone- and pathogen-induced plant responses suggests that they may also be mechanistically similar. Ozone responses and damage appear to be a result of deleterious triggering of pcd associated with the HR, and ethylene seems to be centrally involved in the regulation of the processes (Rao and Davis, 2001; Langebartels et al., 2002). Evidence for a regulatory role for ethylene in pcd has also been obtained during pea (Pisum sativum) carpel senescence (Orzáez and Granell, 1997), in hypoxia-induced aerenchyma formation in maize (Zea mays) root cortex (He et al., 1996), in tomato cell cultures (de Jong et al., 2002), and in maize endosperm development (Young et al., 1997).
Our previous results showed that ozone exposure rapidly increased LE-ACS2 and LE-ACO transcript levels (Tuomainen et al., 1997). LE-ACO mRNA levels were elevated already 30 min after the beginning of the stress and peaked at 1 h. However, ACO transcript levels, ACS activity, and ACC concentrations increased prior to the increase in LE-ACS2 transcript levels, suggesting that posttranscriptional regulation could be involved in the increase of ACS activity in O3-exposed tomato, or that other ACS gene family members may be induced prior to LE-ACS2. In this paper, we provide evidence that a second ACS gene, LE-ACS6, is rapidly induced by ozone prior to LE-ACS2 and that individual members of the ACO and ethylene receptor gene families are differentially regulated following ozone. The temporal pattern of gene expression suggests that ACS and ACO gene expression is induced in a biphasic fashion in response to ozone. In addition, we show that there is a close correlation in the spatial location of the ethylene synthesis, ROS accumulation, and tissue damage, and that ethylene is required for ROS accumulation and subsequent cell death in tomato.
RESULTS
Ethylene Synthesis and Cell Death in Ozone-Exposed Tomato
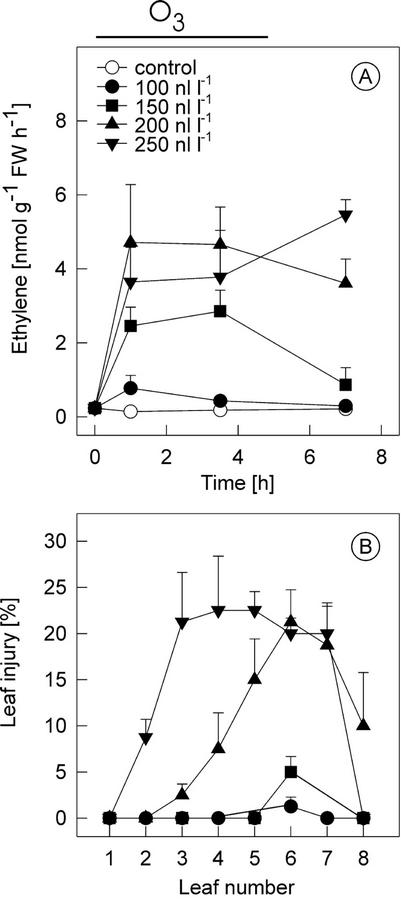
Ozone-exposed plants synthesize significant amounts of ethylene at the beginning of the exposure when a threshold level of O3, which varies between different species and cultivars, is exceeded. The O3 threshold and ethylene evolution for the tomato cv Ailsa Craig was determined by exposing plants to 0 to 250 nL L−1 O3 for up to 5 h, followed by clean air. Ethylene evolution increased in a dose-dependent manner in middle-aged leaves (three and four from the apex; Fig. 1A) and was accompanied by increased ACS activity and ACC accumulation (data not shown). In the control plants and plants exposed to 100 nL L−1 O3, no significant changes were observed in ethylene evolution, but in plants exposed to higher than 150 nL L−1, pronounced increases in ethylene evolution (Fig. 1A) were clearly visible 1.5 h after the initiation of the exposure. O3 concentrations of 200 and 250 nL L−1 caused slightly higher ethylene production. Tissue damage in leaves of different ages, visible 24 h after the exposure, was dependent on the O3 concentration (Fig. 1B). At 100 nL L−1, no damage was evident in any leaves, whereas at 200 nL L−1, the overall damage was significantly higher and the extent of damage increased from leaf three to six. When 250 nL L−1 O3 was used, most of the damage was on leaves three to five, which also showed the highest ethylene evolution (data not shown).
Figure 1.
Ozone induction of ethylene emission and tissue damage in transgenic cv Ailsa Craig (AC) tomato harboring an LE-ACO1::uidA construct. A, Dose response of ethylene evolution. cv Ailsa Craig plants were exposed to 0 to 250 nL L−1 ozone for 5 h (indicated by the line above the graph). Ethylene evolution was determined from plants collected at times shown. B, Leaf injury in ozone-exposed LE-ACO1::uidA plants treated as in A. Leaf injury was assessed 24 h later in leaves 1 to 8 from the top of the plant. Means ± se (n = 3).
The Expression of Specific LE-ACS, LE-ACO, and Ethylene Receptor Gene Family Members Is Differentially Regulated by Ozone
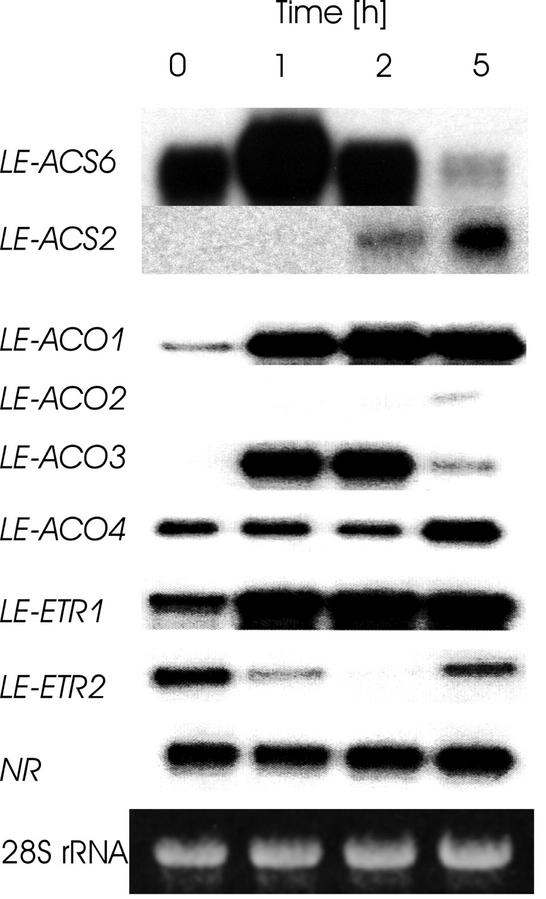
Our previous results (Tuomainen et al., 1997) showed that of the four ACS genes studied, O3 increased only LE-ACS2 transcript levels. In addition, using a generic ACC oxidase probe, pTOM13 that hybridizes to all of the ACO gene family members, we found that ACO transcript abundance increased more rapidly than LE-ACS2. Based on the published sequence (Oetiker et al., 1997), we cloned a fragment of LE-ACS6 and used it with the LE-ACS1A, LE-ACS1B, and LE-ACS2 gene-specific probes (Barry et al., 2000) in ribonuclease protection assay (RPA). LE-ACS1A expression was extremely low in leaves, transcript abundance was not altered by O3, and LE-ACS1B transcripts were below the limits of detection (data not shown). However, LE-ACS6 was up-regulated within 1 h of the beginning of the O3 treatment, declined at 2 h to the level before the treatment, and was below the initial level through 5 h. In contrast, LE-ACS2 was not induced until 2 h after the beginning of the exposure and increased through 5 h (Fig. 2).
Figure 2.
ACC synthase, ACC oxidase, and ethylene receptor gene expression in ozone-exposed leaves. Twenty micrograms of total RNA extracted from ozone-exposed (200 nL L−1 for 0–5 h) leaves of cv Ailsa Craig were hybridized with radiolabeled gene-specific probes, and RPA analysis was performed. Equal amount of RNA used in the RPAs is shown below the autoradiographs.
All four ACO gene family members were expressed in O3-treated leaves, although the expression pattern of individual members differed (Fig. 2). LE-ACO1 and LE-ACO3 transcripts increased within 1 h of the beginning of the treatment. However, whereas LE-ACO1 transcripts remained elevated throughout the duration of the experiment, LE-ACO3 transcripts showed only a transient increase, with diminishing levels by 5 h. LE-ACO4 transcripts remained unchanged until 5 h when a slight increase was observed. LE-ACO2 transcripts also showed a slight induction 5 h after the beginning of the treatment.
Expression of the ethylene receptor genes LE-ETR1, LE-ETR2, and NR (LE-ETR3) were similarly analyzed by RPA. O3 caused the transcript levels of LE-ETR1 to increase 1 h after the beginning of the exposure. Expression of NR was unaffected by O3, whereas the transcripts of LE-ETR2 decreased markedly 1 h after the beginning of the exposure, and by 2 h, the transcripts had disappeared below the detection level. However, 5 h after the beginning of the exposure, the transcripts of LE-ETR2 returned again to almost the same levels as at the beginning of the exposure.
Spatial Localization of LE-ACO1 Activation by Ozone in Transgenic ACO1 Promoter-β-Glucuronidase (GUS) Fusion Plants
To give an indication of the spatial location of ethylene synthesis in response to O3, we examined LE-ACO1 promoter-driven GUS activity in leaves of different ages in response to 250 nL L−1 O3. In terminal leaflets collected from leaves 1 to 8 during the exposure, the most prominent GUS staining was in leaves 3 and 4 (Fig. 3), which were also sensitive to O3 and showed tissue damage in these experiments (data not shown). GUS activity was visible as spots located in the interveinal tissue in close vicinity to the veins. No staining covering large, continuous leaf areas was detected. In the youngest leaves, O3 did not cause visible GUS staining in the terminal leaflet (Fig. 3) or in other leaflets (not shown). In leaves six and older, GUS activity was not detected. The oldest leaves used in these experiments with LE-ACO1::GUS plants were still fully green, had not yet started to senesce, and did not show O3 damage (data not shown).
Figure 3.
Histochemical localization of ACC oxidase activation in the leaves of ozone-exposed transgenic LE-ACO1 promoter::uidA plants. The effect of ozone (250 nL L−1 for 30 min) on GUS activity in leaves of various ages. The terminal leaflets were collected from leaves 1 through 8 counting from the top of the plant.
Localization of Ethylene Synthesis, H2O2 Production, and Tissue Damage
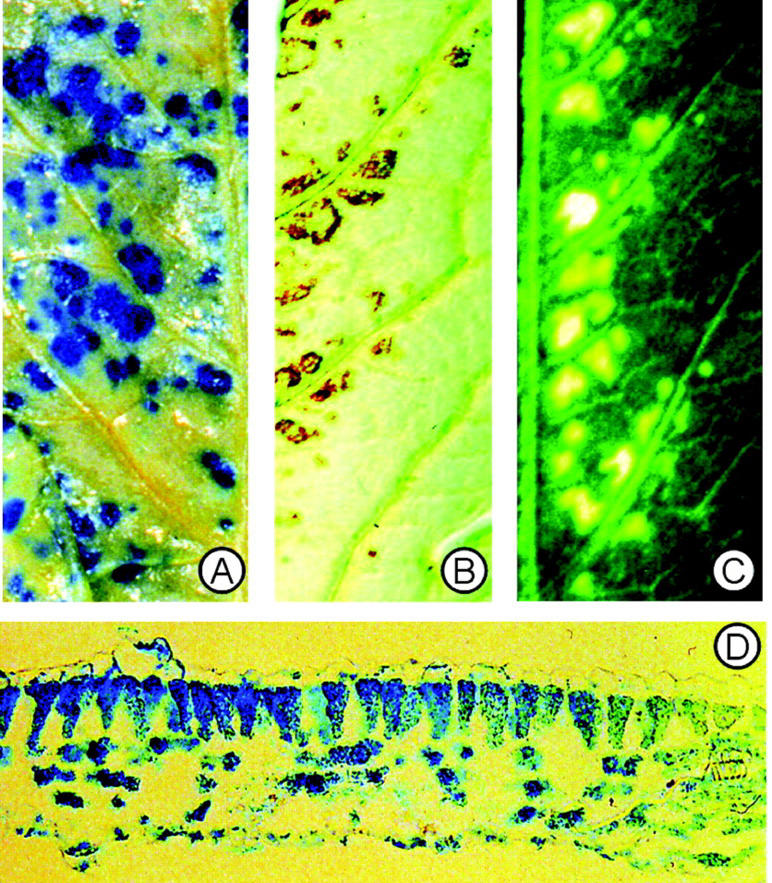
We determined histochemically the accumulation of O2−• and H2O2 in the O3-exposed plants and compared it with the location of ACC oxidase gene activation by O3. Similar to our results (Wohlgemuth et al., 2002) in various commercial tomato cultivars, superoxide-specific nitroblue tetrazolium (NBT) staining did not reveal any detectable NBT precipitation in the tomato leaves (data not shown). The positive control, an O3-sensitive Arabidopsis mutant that produces O2−• after O3 (Overmyer et al., 2000), displayed clear O2−•-dependent NBT staining under identical conditions. To study the relationship between the H2O2 accumulation and the following damage formation, one-half of a leaflet was collected for the determination of H2O2 accumulation, and the other one-half of the same leaflet was used 24 to 48 h later to determine the spatial location of cell death. Histological staining for H2O2 by 3,3′-diaminobenzidine 4 HCl (DAB; Thordal-Christensen et al., 1997) showed local accumulation of H2O2 in the vicinity of the veins at 7 h (Fig. 4B), corresponding closely to the damage pattern that was visible in the other leaflet one-half 24 to 48 h later (Fig. 4C). The GUS staining pattern at 2 h had a similar spatial pattern (Fig. 4A) as those regions showing H2O2 accumulation at 6 h (Fig. 4B), and cell death 24 to 48 h later (Fig. 4C).
Figure 4.
Cellular and tissue localization of ACC oxidase activation, H2O2 accumulation, and visible injury. Plants were exposed to 250 nL L−1 ozone for 5 h, and terminal leaflets from leaf number 2 from ozone-treated plants were analyzed for GUS activity regulated by the LE-ACO1 promoter 1 h after the beginning of the exposure (A), H2O2 accumulation 7 h after the beginning of the exposure (B), and ozone symptom localization after 24 h (C). D, Transverse section through a leaflet showing LE-ACO1 expression indicated by GUS staining at 1 h.
To determine the cellular location of the ACO1-promoter-driven GUS activity, cross-sections were cut through the GUS-positive sites of the O3-exposed leaves. The distribution of GUS staining was detectable through spongy and palisade parenchyma, but was not present in the epidermal cells (Fig. 4D). However, in the more distal regions of the spots, GUS activity was confined more to the palisade cells.
Ethylene Biosynthesis and Perception Are Required for the H2O2 Synthesis and Cell Death in Tomato
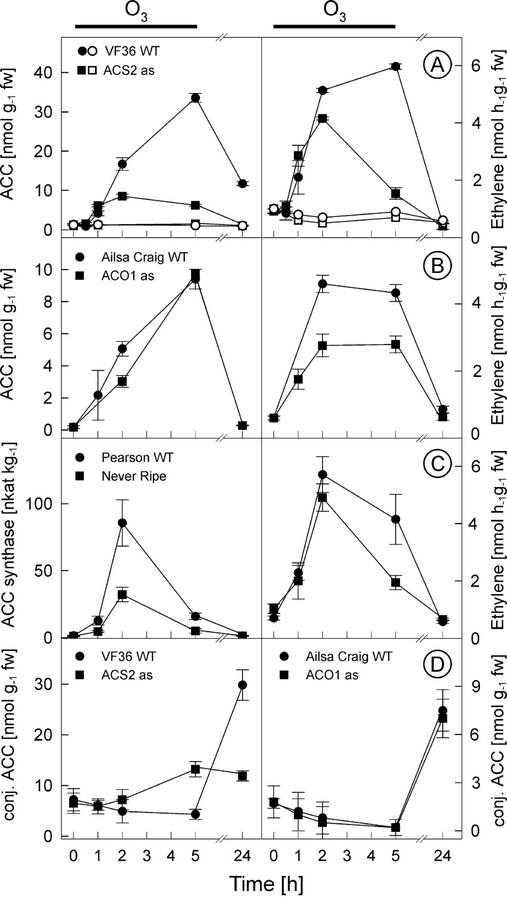
Because there was a very close correlation between the location of ethylene and H2O2 synthesis (Fig. 4) and subsequent cell death, we examined whether ethylene synthesis and perception are required for the production of H2O2 in tomato. Transgenic tomato plants harboring the LE-ACS2 gene in antisense orientation (Oeller et al., 1991) accumulated ACC and emitted ethylene during the first 2 h of O3 exposure to a similar degree as the untransformed control plants (cv VF36); however, after 2 h, ACC concentration and ethylene evolution in the antisense plants did not increase as in the wild-type plants (Fig. 5A). As a consequence, there was no difference in the O3 sensitivity between the wild-type and LE-ACS2 antisense plants (data not shown). ACC accumulation and the subsequent ethylene evolution was the result of de novo ACC synthesis because the conjugated ACC levels did not change during the exposure to such degree that release of ACC from the conjugated forms could account for the increase in ACC (Fig. 5D). However, 24 h after the exposure, the concentrations of conjugated ACC increased in cv Ailsa Craig, LE-ACO1 antisense plants, and in the VF36 wild type, but not in the LE-ACS2 antisense plants (Fig. 5D) that had also low ACC concentrations (Fig. 5A).
Figure 5.
Ethylene synthesis in ozone-exposed tomatoes deficient in ethylene biosynthesis or perception. ACC concentrations and ethylene evolution were measured from ozone-exposed (filled symbols) and clean air control (blank) tomato wild-type cv VF-36 and transgenic LE-ACS2 antisense plants (A) and from ozone-exposed cv Ailsa Craig and transgenic LE-ACO1 antisense plants (B). C, ACC synthase activity and ethylene evolution were measured from ozone-exposed cv Pearson and ethylene-insensitive Nr mutant. D, Concentrations of conjugated ACC were measured from ozone-exposed VF36, LE-ACS2 antisense (in cv VF36), cv Ailsa Craig, and LE-ACO1 antisense (in cv Ailsa Craig) tomatoes during and after the 5-h ozone exposure. Plants were treated for 5 h with 200 nL L−1 ozone (indicated with black line above the graphs) and were postcultivated in pollutant-free air. Means ± se (n = 3).
In a similar manner, transgenic tomato plants harboring the LE-ACO1 in antisense orientation (Hamilton et al., 1990) were exposed to O3. Even though these plants show highly reduced ethylene evolution in the developing fruit, in the leaves of the antisense plants, ethylene evolution was reduced by only about 50% (Fig. 5B), and the plants did not differ from the wild-type cv Ailsa Craig in their O3 sensitivity. The remaining ethylene evolution in the LE-ACO1 antisense plants was obviously over the threshold limit (Tuomainen et al., 1997) that is sufficient to stimulate lesion formation. In the LE-ACO1 antisense plants, the concentration of free ACC did not differ from the wild-type cv Ailsa Craig during the course of the experiment (Fig. 5B).
Leaves of the Never-ripe (Nr) mutant, which carries a dominant mutation in the ethylene receptor LE-ETR3 (Wilkinson et al., 1995), did not differ from the corresponding wild-type cv Pearson in O3 sensitivity. However, there was a clear difference in ACC synthase activity between cv Pearson and Nr in response to O3 (Fig. 5C). During the 2nd h of the exposure, ACS activity was so high in the cv Pearson control that Ado-Met concentration was most likely the limiting factor for ethylene synthesis because in Nr, ethylene evolution was similar as in the wild type.
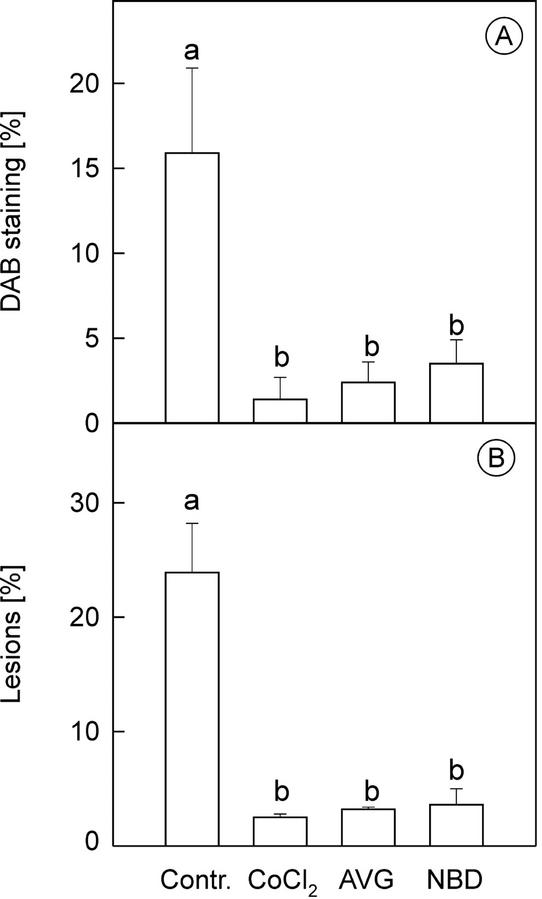
To completely block the enzymes of ethylene biosynthesis or perception in the leaves, plants were treated with the ethylene biosynthesis inhibitors aminoethoxyvinyl Gly (AVG) and Co2+, and the ethylene perception antagonist norbornadiene (NBD). Inhibition of ACS activity with AVG, ACO activity with Co2+, or ethylene perception with NBD all reduced H2O2 accumulation at 6 to 8 h significantly in O3-exposed plants (Fig. 6A), and tissue damage at 24 h was decreased accordingly (Fig. 6B). This demonstrated that ethylene synthesis and perception are required for the oxidative burst and for the progression of O3-induced lesions in the leaves to take place.
Figure 6.
The effect of inhibition of ethylene biosynthesis or perception on lesion development and H2O2 accumulation. Plants were treated before ozone exposure with the ACC synthase inhibitor AVG, ACC oxidase inhibitor CoCl2, or the ethylene antagonist NBD. Inhibitor-treated plants or untreated controls were then exposed to 250 nL L−1 ozone for 5 h. A, One-half of the leaves were harvested 7 h after the beginning of the exposure and were stained for H2O2 production with DAB. B, Cell death (indicated as the percentage lesion of the leaf area) measured after 24 h. Means ± se (n = 3). Columns with the same letter are not significantly (P < 0.05) different according to Tukey's multiple range test.
DISCUSSION
Ozone Differentially Induces Genes Involved in Ethylene Synthesis and Perception
Previous studies on tomato have shown that individual members of the ACS, ACO, and ETR gene families are differentially expressed during developmental processes and in response to external stimuli (Rottmann et al., 1991; Lincoln et al., 1993; Barry et al., 1996, 2000; Oetiker et al., 1997; Lashbrook et al., 1998; Nakatsuka et al., 1998; Tatsuki and Mori, 1999; Tieman and Klee, 1999; Ciardi et al., 2000; Llop-Tous et al., 2000). We have examined the expression of these gene families in response to a single 5-h pulse of the air pollutant O3. The changes in expression patterns seen in response to O3 can be grouped into two classes based on temporal changes in expression. Rapid changes, within 1 h of the beginning of the treatment, were seen for LE-ACS6, LE-ACO1, LE-ACO3, LE-ETR1, and LE-ETR2, and slow changes, occurring after 2 h, were seen for LE-ACS2, LE-ACO2, and LE-ACO4 (Fig. 2).
These data suggest a biphasic regulation of these genes in response to O3, and this observation is supported by measurements of ACC content and ethylene production in LE-ACS2 antisense plants (Fig. 5). ACC content and ethylene production increased rapidly in the VF36 control and LE-ACS2 antisense plants up to the first 1 to 2 h after the beginning of the treatment, after which there was a rapid decline (Fig. 5A). Based upon our expression data, it is likely that this initial increase (phase 1) was due to the rapid induction of LE-ACS6 (Fig. 2). However, 2 h after the beginning of the exposure, when LE-ACS2 was induced (Fig. 2) and the antisense effect is activated, sustained ACC accumulation and ethylene synthesis was prevented in the LE-ACS2 antisense plants. A similar pattern is seen in ACS activity in cv Pearson control and Nr mutant plants (Fig. 5C), which suggests that at around 2 h after the beginning of the O3 treatment, the increase observed in ACS activity in control plants is ethylene receptor-dependent, suggesting deficient negative feedback regulation of ACS activity in the O3-exposed Nr plants.
A biphasic relationship between LE-ACS2 and LE-ACS6 expression has previously been shown in response to wounding of tomato leaves and mature green fruit (Tatsuki and Mori, 1999). The rapid and transient induction of LE-ACS6 expression in response to wounding was followed by the later accumulation of LE-ACS2 transcripts. LE-ACS6 is regulated by negative feedback (Nakatsuka et al., 1998), which can also be deduced by comparing Figures 1 and 2, which show down-regulation of LE-ACS6 at the same time when LE-ACS2 is activated and ethylene evolution continues to increase. Together, these data suggest that a biphasic mode of ACS and possibly also ACO transcript accumulation may be a feature of stress-induced ethylene synthesis in tomato. However, it is not yet understood how the signal from these diverse input stimuli results in a common response mechanism, and more stress responses need to be investigated to see if this relationship holds true.
It is possible that biphasic control of ethylene synthesis may have evolved as a regulatory mechanism to modulate plant responses depending upon the severity of the stress encountered. For example, O3 and mechanical wounding appear to result in a biphasic induction of ACS gene expression (Fig. 2; Tatsuki and Mori, 1999). In contrast, the comparatively milder stress of touch results in the rapid induction of LE-ACS1A and LE-ACS6, but does not lead to a later higher induction of LE-ACS2 (Tatsuki and Mori, 1999). Of course, it is also possible that the differential expression of ACS and ACO genes in response to ozone treatment may occur at the cellular level and may be directly related to cell damage. The results in Figure 4 indicate that LE-ACO1 expression shows spatial specificity in response to ozone treatment. It will be of interest to determine whether cell-specific expression is shown by other members of the ACS and ACO gene families in response to ozone treatment and other stresses.
Ethylene Is Involved in the Regulation of the Degree of Ozone Damage
Ethylene emission has been shown to correlate to ozone sensitivity in several plant species. It was initially proposed that O3 could react chemically with ethylene and form radicals that in turn would damage the biological structures of the cells (Elstner et al., 1985; Mehlhorn and Wellburn, 1987). However, more recent results suggest that ethylene plays a more active role in O3 damage. Our previous results indicated reduced O3 damage when ethylene synthesis was prevented with inhibitors of ACC synthase or oxidase (Tuomainen et al., 1997). In a similar manner, use of the ethylene antagonist NBD reduced O3-induced lesion formation in tomato (Bae et al., 1996), and in Arabidopsis mutants selected for increased sensitivity to O3 (Overmyer et al., 2000), ethylene evolution was triggered during the early lesion development. Thus, existing sensitivity of the genotype to O3 (tomato and tobacco) and gain of sensitivity by mutation in resistant background (Arabidopsis; Overmyer et al., 2000) involve rapid activation of ethylene biosynthesis in the tissues that show subsequent hallmarks of pcd.
Ethylene is involved in pcd during developmental and inducible processes (He et al., 1996; Orzáez and Granell, 1997; Young et al., 1997; de Jong et al., 2002). Our results suggest that ethylene has also an intimate role in the regulation of early O3 lesion development. Disease lesion development also requires ethylene action in tomato and Arabidopsis (Bent et al., 1992; Lund et al., 1998). Together, these results suggest an active role for ethylene in regulating the spread of lesions, though the exact mechanisms of disease lesion development and the possible involvement of ROS and pcd therein are not known.
The role of ROS and Ethylene in Cell Death Signaling
Pcd is involved in several developmental and inducible processes in plants. In the HR to incompatible pathogens, which is one of the best-studied forms of pcd in plants (Dangl et al., 1996; Levine et al., 1996; Pennell and Lamb, 1997), two separate ROS bursts take place. In a similar manner, in O3-sensitive tobacco cv Bel W3, two separate O3-induced bursts were detected (Schraudner et al., 1998). The second burst was correlated in distribution and size with the lesions that appeared later, and was absent in the O3-tolerant cv Bel B. We have begun to address the spatial location of H2O2 accumulation and ethylene biosynthesis in response to O3 with the aid of transgenic plants expressing an LE-ACO1 promoter::GUS fusion (Figs. 3 and 4). The results indicated that rather than expression throughout the leaf, H2O2 accumulation and GUS expression were confined to distinct regions surrounding the vascular tissue, mainly in the parenchyma cells. This restricted expression is of interest as clearly not all cells are responding to O3 in the same way.
In O3-exposed plants, ROS formation from the degradation of O3 is not confined to a limited location as in the HR; O3 enters the substomatal cavities all over the leaf. However, ethylene synthesis, H2O2 accumulation, and the subsequent lesion development took place in clusters of cells close to the vasculature (Fig. 4). This colocalization may favor interaction of these signal molecules as it predicts that high concentrations co-occur in the same cells. In addition, the spatial location close to the veins is similar to the location of ROS generation during the HR, which is essential in the establishment of systemic resistance (Alvarez et al., 1998). In a similar manner, cell death that is preferentially localized to cells close to the vascular bundles was seen in O3-exposed tobacco (Schraudner et al., 1998), tomato, M. sylvestris, and Arabidopsis (Wohlgemuth et al., 2002). As discussed by Schraudner et al. (1998), the cells in the periveinal region might be disposed to amplify ROS production (“burst initiation sites”; Schraudner et al., 1998) and to die during the pathogenesis response, i.e. to limit pathogen spread via the vascular system.
Our results suggest an integral role for ethylene in the regulation of cell death. Ethylene seems to be involved in the regulation of cell death by amplifying a second burst of ROS production. When ethylene synthesis or perception was prevented with inhibitors, the second oxidative burst was inhibited, and accordingly, tissue damage was also reduced. In agreement with the model of the oxidative cell death cycle, originally proposed by van Camp et al. (1998) and modified by Overmyer et al. (2000), these results indicate that ethylene is intimately involved in the amplification of ROS production and regulation of cell death under oxidative stress.
MATERIALS AND METHODS
Plant Material and Conditions of Treatment
Tomato (Lycopersicon esculentum cv Ailsa Craig, cv VF36, and cv Pearson) plants were grown in pollutant-free air under a 14-h/10-h light/dark regime (at 100 μmol m−2 s−1 from 6 am to 8 pm) at 25°C/20°C as described previously (Tuomainen et al., 1997). Six- to 7-week-old plants were exposed to a single pulse of O3 (<5–300 ± 10 nL L−1) for 0.5 to 7 h (starting at 9 am). Ozone concentrations were 0, 100, 150, 200, 250, and 300 nL L−1 in dose-response experiments, and 0 and 200 nL L−1 in time course experiments. Ozone was generated by electric discharge in dry oxygen and was measured with an analyzer (CSI 3100; Messer-Griesheim, Munich, Germany), periodically calibrated as described (Langebartels et al., 1991). Control plants were cultivated in pollutant-free air in parallel chambers.
Inhibitors of ethylene biosynthesis (AVG; Sigma, St. Louis, and cobalt chloride) and perception (NBD; Sigma) were applied as described (Bae et al., 1996; Tuomainen et al., 1997).
Leaves were numbered from the apex of the plants with leaf number 2 larger than 13 cm. Analyses were routinely performed with the O3-sensitive middle-aged leaves numbers 3 to 5. Injury was scored 24 h after the onset of exposure by assessing visible leaf injury as percentage of leaf area. The data were then calibrated with a planimeter (LI3000A, LI-COR, Lincoln, NE). Gas exchange measurements were performed with a portable porometer (CQP 130a; Walz, Effeltrich, Germany) according to Langebartels et al. (1991). For biochemical analyses, leaves were immediately frozen in liquid nitrogen and were stored at −75°C.
Determination of Ethylene Production in Situ
Individual leaflets from leaves numbers 3 to 5 (approximately 0.2 g of fresh weight with the cut surface sealed with liquid paraffin) were placed adaxially on water-moistened filter papers. The papers were rolled cylindrically and placed into glass tubes, which were then sealed by silicone septa. After incubation at room temperature for 1 h in the dark, 1-mL gas samples were withdrawn with a syringe, and ethylene was analyzed according to Tuomainen et al. (1997).
Determination of ACC Contents
Leaf material was ground in liquid nitrogen and was extracted according to Langebartels et al. (1991). ACC and total ACC following acid hydrolysis (2 n HCl for 3 h at 120°C) were determined according to Lizada and Yang (1979) as described (Langebartels et al., 1991). The amount of conjugated ACC was calculated by subtracting the amount of ACC from that of total ACC.
Determination of ACC Synthase Activity
Frozen leaves (0.2 g) were ground in liquid N2 and were extracted with 0.5 mL of 100 mm EPPS [4–2(2-hydroxyethyl)-1-piperazine propane sulfonic acid] buffer, pH 8.5, containing 5 mm dl-dithiothreitol, 5 μm pyridoxal phosphate, and protease inhibitors (500 μm phenylmethylsulfonyl fluoride and 10 μm leupeptin). After the addition of water-insoluble polyvinylpolypyrrolidone (2%, w/v) and vortexing for 10 s, the extract was centrifuged at 20,000g for 10 min at 4°C. The supernatant was gel-filtered on a Sephadex G-25 column (NAP-5 column; Pharmacia, Freiburg, Germany) equilibrated with 5 mm EPPS (pH 8.5), 1 mm dl-dithiothreitol, 5 μm pyridoxal phosphate, and 500 μm phenylmethylsulfonyl fluoride. ACC synthase was assayed in glass flasks containing 0.4 mL of protein extract and final concentrations of 80 mm EPPS (pH 8.5), 20 μm pyridoxal phosphate, and 100 μm Ado-Met, in a total volume of 0.5 mL at 30°C for 2 h (Tuomainen et al., 1997). Blanks omitting Ado-Met were incubated in parallel. The reaction was stopped by addition of 100 μL of 10 mm HgCl2 on ice. ACC was converted to ethylene as described above. One milliliter of the gas phase was withdrawn by simultaneously adding 1 mL of water through a second syringe and was analyzed by gas chromatography.
Isolation of RNA, Nucleic Acid Probes, and RNase Protection Assay
Total RNA was extracted from frozen, homogenized leaf tissue as described by Chang et al. (1993). Poly(A)+ RNA was extracted from 300 to 500 μg of total RNA using the PolyATtract mRNA Isolation System IV (Promega, Madison, WI). Gene-specific probes for ACS and ACO sequences were as described previously (Barry et al., 1996, 2000; Llop-Tous et al., 2000). Gene-specific probes for the ethylene receptor genes, LE-ETR1, LE-ETR2 (Lashbrook et al., 1998), and NR (Wilkinson et al., 1995) were designed from around the 3′end of each sequence. Primer pairs were as follows: LE-ETR1, ETR1F: 5′-tagtgaatgtaggaggaaaa-3′ and ETR1R: 5′-cacataataatctattgttg-3′, generating a probe from nucleotides 2,308 to 2,621; LE-ETR2, ETR2F: 5′-cagtaaaccaaaattgtctc-3′ and ETR2R: 5′-gactgtcattgtatttttct-3′, generating a probe from nucleotides 2,324 to 2,589; and NR, NRF: 5′-taaatgacaaaaggacat-3′ and NRR: 5′-gtcaaaagctcgatgtat-3′, generating a probe from nucleotides 2,210 to 2,399. PCR products were cloned into the pCR2.1 vector (Invitrogen, San Diego). The RNase protection assay to analyze the gene-specific ACC oxidase transcript abundance was performed as described earlier (Barry et al., 1996, 2000).
GUS Activity Determination
GUS activity was localized histochemically by placing detached leaflets in the staining buffer containing 0.5 mm 5-bromo-4-chloro-3-indolyl-β-glucuronic acid (Calbiochem, La Jolla, CA) following procedure described (Blume and Grierson, 1997). After vacuum infiltration of the buffer, samples were incubated in darkness at 37°C overnight. For microscopical localization of GUS activity within the leaf, sections of stained leaves were fixed, embedded in paraffin, and transverse sections were cut through the GUS-positive spots.
H2O2 Detection by DAB Staining
Individual leaflets from middle-aged leaves were infiltrated with 0.1% (w/v) DAB, 10 mm MES (pH 6.5; Thordal-Christensen et al., 1997) or 0.1% (w/v) NBT, 10 mm sodium azide, and 50 mm potassium phosphate (pH 6.4; Jabs et al., 1996). Leaves were incubated in the light for 30 min and were then cleared in ethanol for 2 d at room temperature in the dark (Wohlgemuth et al., 2002).
Statistical Analysis
All experiments were conducted in a completely randomized design with three replicates for each treatment. When indicated, the Tukey multiple range test was used to test for differences among treatment means (at P = 0.05; Statgraphics software; STSC, Rockville, MD).
ACKNOWLEDGMENTS
We thank Dr. Sakis Theologis for the seeds of VF36 and ACS2 antisense line A11.1, and Dr. Neil Olszewski for the seeds of Pearson and Never-ripe. Ms. Anu Miettinen is acknowledged for her skillful assistance in microscope sections, Lucia Gössl and Rosina Ludwig in biochemical analysis, and Renate Kreitmeyer for growing of the plants.
Footnotes
This work was supported by the Scientific Council of Research of Environment and Natural Resources in Finland (grant nos. 33200 and 8822), by the Finnish Centre of Excellence Program (2000–2005), by the European Union (grant no. FAIR-CT97–3493, TOMSTRESS), by Bayerisches Staatsministerium für Landesentwicklung und Umweltfragen, by Deutsche Forschungsgemeinschaft (grant no. SFB 607), and by the Biotechnology and Biological Sciences Research Council (grant no. 42/P09465).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.009712.
LITERATURE CITED
- Alvarez ME, Pennell RI, Meijer P-J, Ishikawa A, Dixon RA, Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;92:773–784. doi: 10.1016/s0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- Bae GY, Nakajima N, Ishizuka K, Kondo N. The role in ozone phytotoxicity of the evolution of ethylene upon induction of 1-aminocyclopropane-1-carboxylic acid synthase by ozone fumigation in tomato plants. Plant Cell Physiol. 1996;37:129–134. [Google Scholar]
- Barry CS, Blume B, Bouzayen M, Cooper W, Hamilton AJ, Grierson D. Differential expression of the 1-aminocyclopropane-1-carboxylate oxidase gene family of tomato. Plant J. 1996;9:525–535. doi: 10.1046/j.1365-313x.1996.09040525.x. [DOI] [PubMed] [Google Scholar]
- Barry CS, Llop-Tous MI, Grierson D. The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol. 2000;123:979–986. doi: 10.1104/pp.123.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent AF, Innes RW, Ecker JR, Staskawicz BJ. Disease development in ethylene-insensitive Arabidopsis thaliana infected with virulent and avirulent Pseudomonas and Xanthomonas pathogens. Mol Plant-Microbe Interact. 1992;5:372–378. doi: 10.1094/mpmi-5-372. [DOI] [PubMed] [Google Scholar]
- Blume B, Grierson D. Expression of ACC oxidase promoter-GUS fusions in tomato and Nicotiana plumbaginifolia regulated by developmental and environmental stimuli. Plant J. 1997;12:731–746. doi: 10.1046/j.1365-313x.1997.12040731.x. [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney C. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep. 1993;11:113–116. [Google Scholar]
- Ciardi JA, Tieman DM, Lund ST, Jones JB, Stal RE, Klee HJ. Response to Xanthomonas campestris pv. Vesicatoria in tomato involves regulation of ethylene receptor gene expression. Plant Physiol. 2000;123:81–92. doi: 10.1104/pp.123.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl JL, Dietrich RA, Richberg MH. Death don't have no mercy: Cell death programs in plant-microbe interactions. Plant Cell. 1996;8:1793–1807. doi: 10.1105/tpc.8.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dat J, Vandenabeele S, Vranová E, Van Montagu M, Van Breusegem F. Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci. 2000;57:779–795. doi: 10.1007/s000180050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstner EF, Osswald W, Youngman RJ. Basic mechanisms of pigment bleaching and loss of structural resistance in spruce (Picea abies) needles: advances in phytomedical diagnostics. Experientia. 1985;41:591–597. [Google Scholar]
- Hamilton AJ, Lycett GW, Grierson D. Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature. 1990;346:284–287. [Google Scholar]
- He C-J, Morgan PW, Drew MC. Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. Plant Physiol. 1996;112:463–472. doi: 10.1104/pp.112.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs T, Dietrich RA, Dangl JL. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science. 1996;273:1853–1856. doi: 10.1126/science.273.5283.1853. [DOI] [PubMed] [Google Scholar]
- de Jong AJ, Yakimova ET, Kapchina VM, Woltering EJ. A critical role for ethylene in hydrogen peroxide release during programmed cell death in tomato suspension cells. Planta. 2002;214:537–545. doi: 10.1007/s004250100654. [DOI] [PubMed] [Google Scholar]
- Kangasjärvi J, Talvinen J, Utriainen M, Karjalainen R. Plant defense systems induced by ozone. Plant Cell Environ. 1994;17:783–794. [Google Scholar]
- Kende H. Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:283–307. [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Langebartels C, Kerner K, Leonardi S, Schraudner M, Trost M, Heller W, Sandermann H. Biochemical plant responses to ozone: differential induction of polyamine and ethylene biosynthesis in tobacco. Plant Physiol. 1991;95:882–889. doi: 10.1104/pp.95.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langebartels C, Schraudner M, Heller W, Ernst D, Sandermann H. Oxidative stress and defense reactions in plants exposed to air pollutants and UV-B radiation. In: Inzé D, Van Montagu M, editors. Oxidative Stress in Plants. London: Taylor and Francis; 2002. pp. 105–135. [Google Scholar]
- Lashbrook CC, Tieman DM, Klee HJ. Differential regulation of the tomato ETR gene family throughout plant development. Plant J. 1998;15:243–252. doi: 10.1046/j.1365-313x.1998.00202.x. [DOI] [PubMed] [Google Scholar]
- Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb C. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr Biol. 1996;6:427–437. doi: 10.1016/s0960-9822(02)00510-9. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Lincoln JE, Campbell AD, Oetiker J, Rottmann WH, Oeller PW, Shen NF, Theologis A. LE-ACS4, a fruit ripening and wound-induced 1-aminocyclopropane-1-carboxylate synthase gene of tomato (Lycopersicon esculentum) J Biol Chem. 1993;268:19422–19430. [PubMed] [Google Scholar]
- Lizada C, Yang SF. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979;100:140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Llop-Tous I, Barry CS, Grierson D. Regulation of ethylene biosynthesis in response to pollination in tomato flowers. Plant Physiol. 2000;123:971–978. doi: 10.1104/pp.123.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S, Stall R, Klee H. Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell. 1998;10:371–382. doi: 10.1105/tpc.10.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn H, Wellburn AR. Stress ethylene formation determines plant sensitivity to ozone. Nature. 1987;327:417–418. [Google Scholar]
- Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A. Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol. 1998;118:1295–1305. doi: 10.1104/pp.118.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeller PW, Min-Wong L, Taylor LP, Pike DA, Theologis A. Reversible inhibition of tomato fruit senescence by antisense RNA. Science. 1991;254:437–439. doi: 10.1126/science.1925603. [DOI] [PubMed] [Google Scholar]
- Oetiker JH, Olson DC, Shiu Y, Yang SF. Differential induction of seven 1-aminocyclopropane-1-carboxylate synthase genes by elicitor in suspension cultures of tomato. Plant Mol Biol. 1997;34:275–286. doi: 10.1023/a:1005800511372. [DOI] [PubMed] [Google Scholar]
- Orzáez D, Granell A. DNA fragmentation is regulated by ethylene during carpel senescence in Pisum sativum. Plant J. 1997;11:137–144. [Google Scholar]
- Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann H, Kangasjärvi J. The ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell. 2000;12:1849–1862. doi: 10.1105/tpc.12.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellinen R, Palva T, Kangasjärvi J. Subcellular localization of ozone- induced hydrogen peroxide production in birch (Betula pendula) leaf cells. Plant J. 1999;20:349–356. doi: 10.1046/j.1365-313x.1999.00613.x. [DOI] [PubMed] [Google Scholar]
- Pellinen RI, Korhonen M-S, Tauriainen AA, Palva ET, Kangasjärvi J. Hydrogen peroxide activates cell death and defense gene expression in birch. Plant Physiol. 2002;130:549–560. doi: 10.1104/pp.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell RI, Lamb C. Programmed cell death in plants. Plant Cell. 1997;9:1157–1168. doi: 10.1105/tpc.9.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Davis KR. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J. 1999;17:603–614. doi: 10.1046/j.1365-313x.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- Rao MV, Davis KR. The physiology of ozone-induced cell death. Planta. 2001;213:682–690. doi: 10.1007/s004250100618. [DOI] [PubMed] [Google Scholar]
- Rottmann WH, Peter GF, Oeller PW, Keller JA, Shen NF, Nagy BP, Taylor LP, Campbell AD, Theologis A. 1-Aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senescence. J Mol Biol. 1991;222:937–961. doi: 10.1016/0022-2836(91)90587-v. [DOI] [PubMed] [Google Scholar]
- Sandermann H. Ozone and plant health. Annu Rev Phytopathol. 1996;34:347–366. doi: 10.1146/annurev.phyto.34.1.347. [DOI] [PubMed] [Google Scholar]
- Sandermann H, Ernst D, Heller W, Langebartels C. Ozone: an abiotic elicitor of plant defence reactions. Trends Plant Sci. 1998;3:47–50. [Google Scholar]
- Schraudner M, Langebartels C, Sandermann H. Changes in the biochemical status of plants cells induced by the environmental pollutant ozone. Physiol Plant. 1997;100:274–280. [Google Scholar]
- Schraudner M, Moeder W, Wiese C, van Camp W, Inzé D, Langebartels C, Sandermann H. Ozone-induced oxidative burst in the ozone biomonitor plant, tobacco Bel W3. Plant J. 1998;16:235–245. doi: 10.1046/j.1365-313x.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- Shiu OY, Oetiker JH, Yip WK, Yang SF. The promoter of LE-ACS7, an early flooding-induced 1-aminocyclopropane-1-carboxylate synthase gene of the tomato, is tagged by a Sol3 transposon. Proc Natl Acad Sci USA. 1998;95:10334–10339. doi: 10.1073/pnas.95.17.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuki M, Mori H. Rapid and transient expression of 1-aminocyclopropane-1-carboxylate synthase isogenes by touch and wound stimuli in tomato. Plant Cell Physiol. 1999;40:709–715. doi: 10.1093/oxfordjournals.pcp.a029597. [DOI] [PubMed] [Google Scholar]
- Tenhaken R, Levine A, Brisson LF, Dixon RA, Lamb C. Function of the oxidative burst in hypersensitive disease resistance. Proc Natl Acad Sci USA. 1995;92:4158–4163. doi: 10.1073/pnas.92.10.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. [Google Scholar]
- Tieman DM, Klee HJ. Differential expression of two novel members of the tomato ethylene-receptor family. Plant Physiol. 1999;120:165–172. doi: 10.1104/pp.120.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingey DT, Standley C, Field RW. Stress ethylene evolution: a measure of ozone effects on plants. Atmos Environ. 1976;10:969–974. doi: 10.1016/0004-6981(76)90204-3. [DOI] [PubMed] [Google Scholar]
- Tuomainen J, Betz C, Kangasjärvi J, Ernst D, Yin ZH, Langebartels C, Sandermann H., Jr Ozone induction of ethylene emission in tomato plants: regulation by differential transcript accumulation for the biosynthetic enzymes. Plant J. 1997;12:1151–1162. [Google Scholar]
- van Camp W, Van Montagu M, Inzé D. H2O2 and NO: redox signals in disease resistance. Trends Plant Sci. 1998;3:330–334. [Google Scholar]
- Wang KL-C, Li H, Ecker JR. Ethylene biosynthesis and signaling networks. Plant Cell. 2002;14:S131–S151. doi: 10.1105/tpc.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ. An ethylene-inducible component of signal transduction encoded by Never-ripe. Science. 1995;270:1807–1809. doi: 10.1126/science.270.5243.1807. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth H, Mittelstrass K, Kschieschan S, Bender J, Weigel HJ, Overmyer K, Kangasjärvi J, Langebartels C, Sandermann H. Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant Cell Environ. 2002;25:717–726. [Google Scholar]
- Young TE, Gallie DR, DeMason DA. Ethylene-mediated programmed cell death during maize endosperm development of wild-type and shrunken2 genotypes. Plant Physiol. 1997;115:737–751. doi: 10.1104/pp.115.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]