Abstract
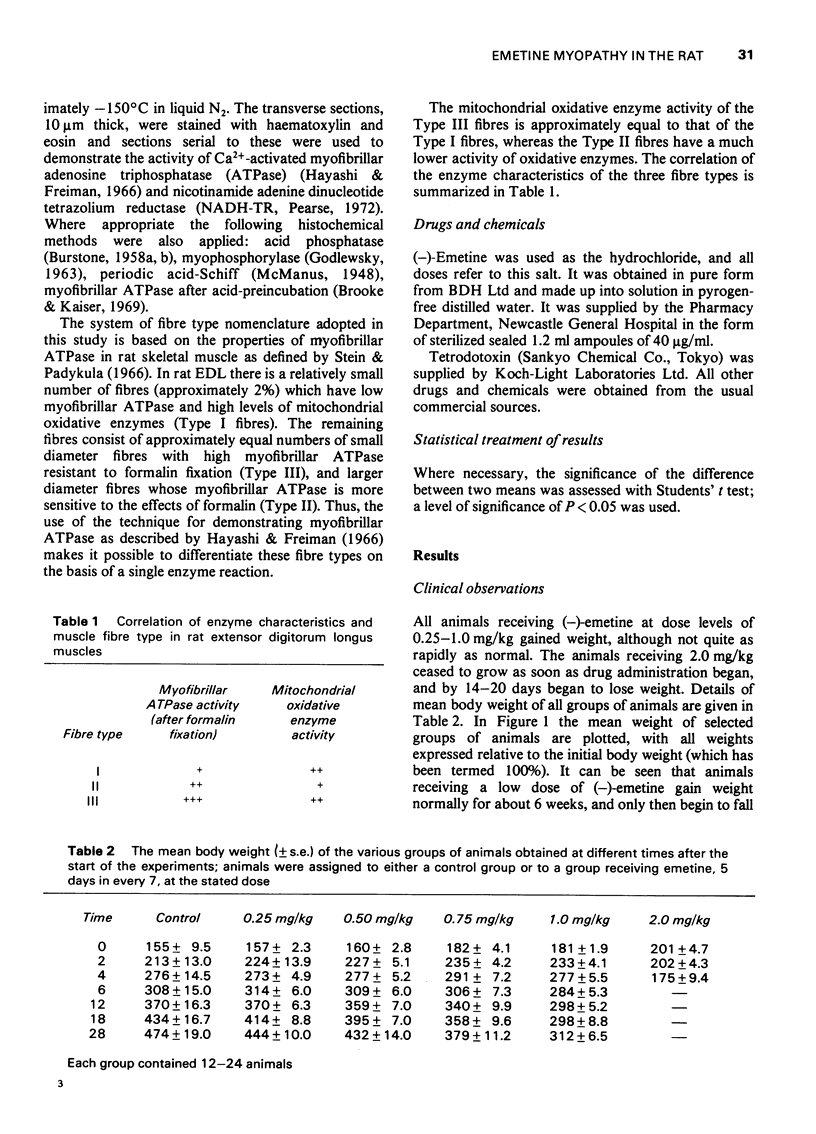
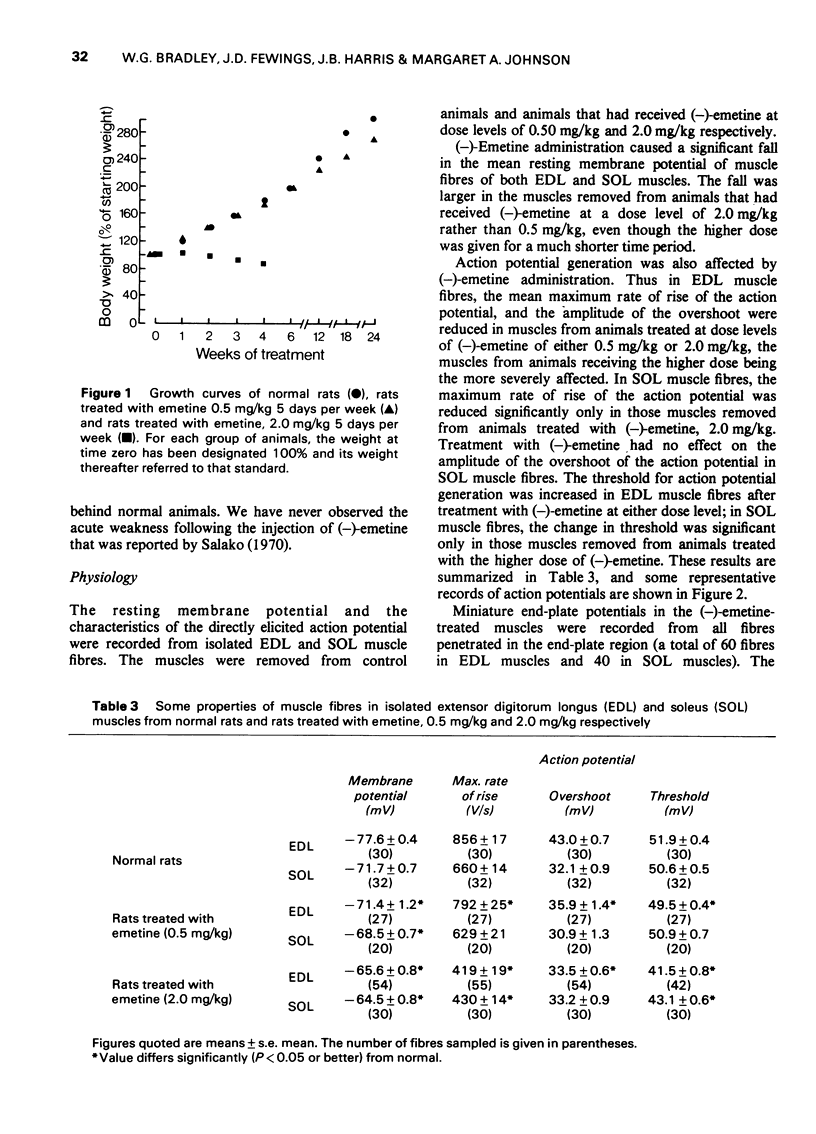
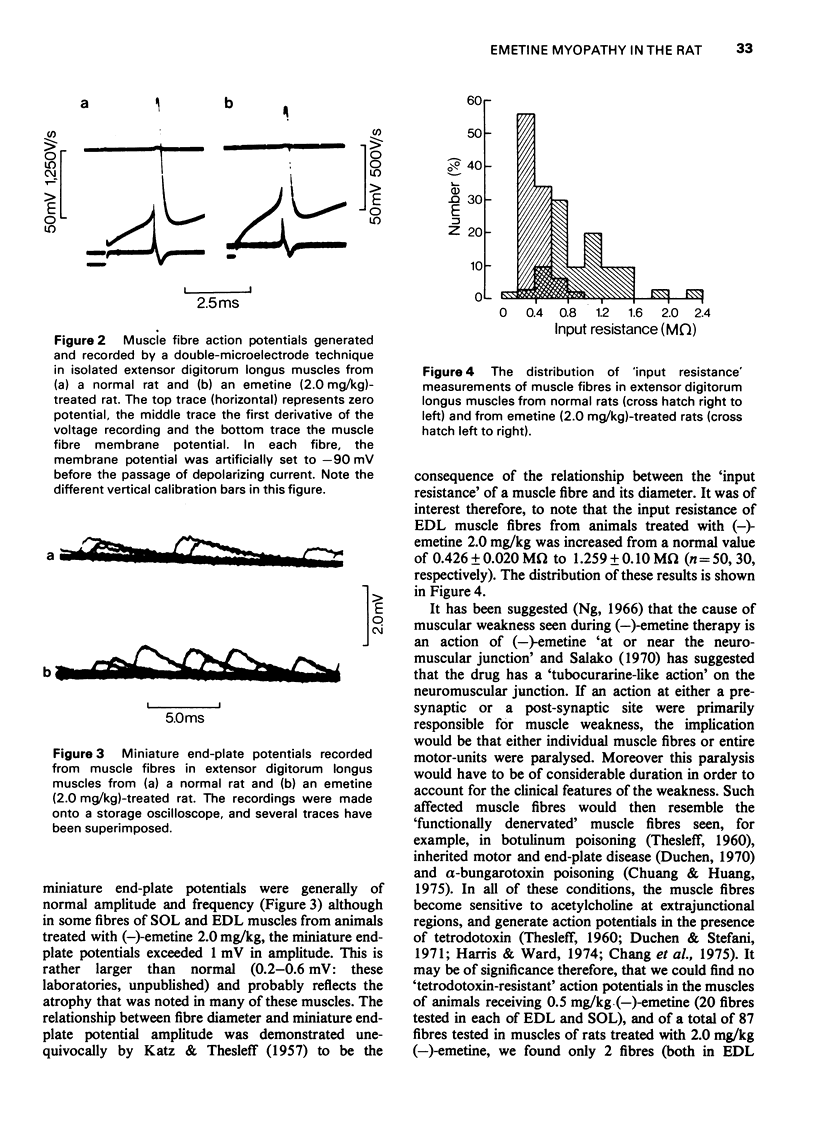
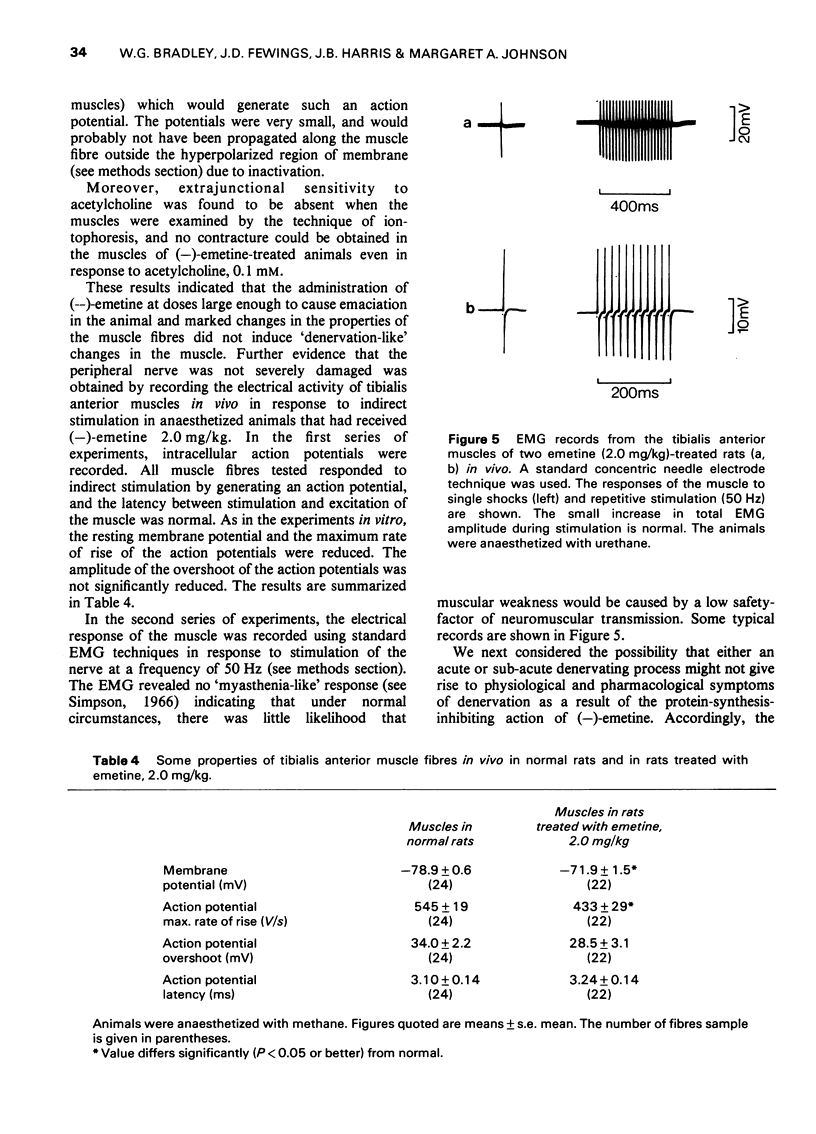
1 (-)Emetine (0.25-2.0 mg/kg i.p.) was administered to rats for up to 220 days. 2 At doses of 1.0 mg/kg or less, the animals continued to gain weight but more slowly than the untreated control animals. The physiological changes in the muscles from these animals were minimal; there was a small reduction in both the resting membrane potential and in the maximum rate of rise of the action potential. There was no atrophy or loss of muscle fibres although in the occasional muscle, hyaline fibres, necrotic fibres and split fibres were observed. There was a focal loss of myofibrillar adenosine triphosphatase (ATPase) and nicotinamide adenine dinucleotide tetrazolium reductase (NADH-TR) in Type II and Type III fibres, but no such loss in Type I fibres. 3 The animals receiving 2.0 mg/kg of (-)emetine gained weight slowly for up to 20 days but then rapidly lost weight and by 30 days they were weak and emaciated. The muscles from these animals were severly atrophied and the total muscle wet weight was reduced by almost 20%. 4 The strength of the muscles from these animals was measured in vitro using direct stimulation. They were weaker than normal both in absolute terms and when expressed in terms of tension developed/unit wet weight. 5 There was no evidence of either functional or structural denervation but surgically denervated muscles from animals in this group were indistinguishable from denervated muscles from normal rats. 6 Severe structural damage was obvious in the fibres of both extensor digitorum longus and soleus. Necrotic, hyaline and splitting fibres were common and the focal loss of myofibrillar ATPase and NADH-TR activity was extensive and occurred in Type I fibres as well as in Type II and Type II fibres. 7 It is concluded that the muscular weakness induced by (-)-emetine is due to a direct effect on the muscle fibres and that this occurs at a subcellular level. There is no evidence that functional or structural denervation plays any role in the aetiology of emetine myopathy in the rat.
Full text
PDF

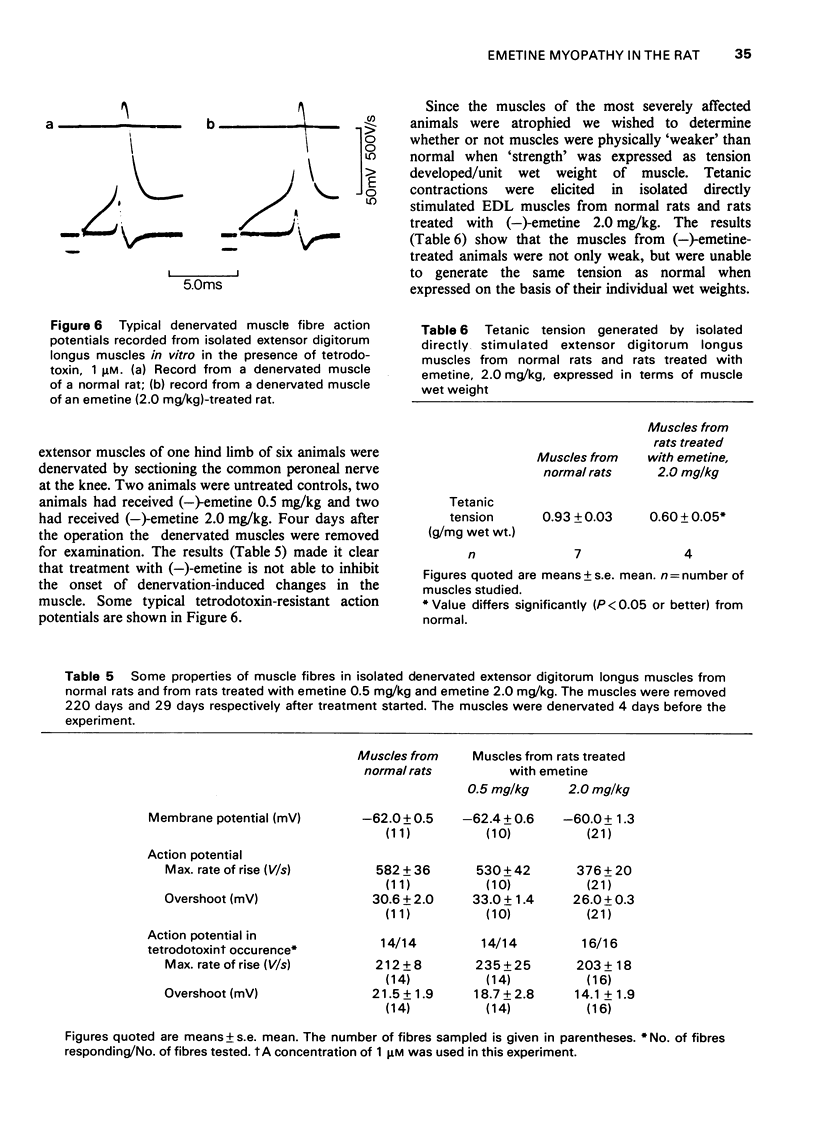





Images in this article
Selected References
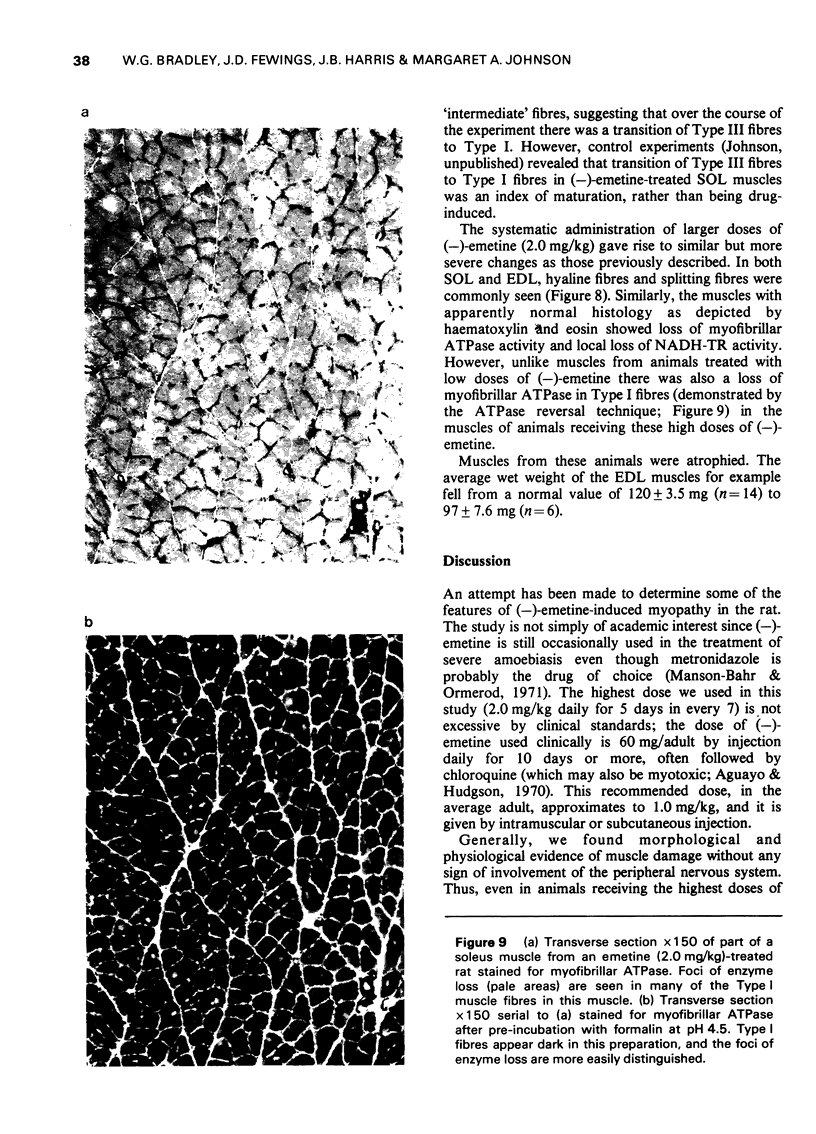
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo A. J., Hudgson P. Observations on the short-term effects of chloroquine on skeletal muscle. An experimental study in the rabbit. J Neurol Sci. 1970 Oct;11(4):301–325. doi: 10.1016/0022-510x(70)90080-8. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Warnick J. E., Tasse J. R., Sansone F. M. Effects of vinblastine and colchicine on neural regulation of the fast and slow skeletal muscles of the rat. Exp Neurol. 1972 Dec;37(3):607–634. doi: 10.1016/0014-4886(72)90103-3. [DOI] [PubMed] [Google Scholar]
- BURSTONE M. S. Histochemical comparison of naphthol AS-phosphates for the demonstration of phosphatases. J Natl Cancer Inst. 1958 Mar;20(3):601–615. [PubMed] [Google Scholar]
- BURSTONE M. S. Histochemical demonstration of acid phosphatases with naphthol AS-phosphates. J Natl Cancer Inst. 1958 Sep;21(3):523–539. [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Some comments on the histochemical characterization of muscle adenosine triphosphatase. J Histochem Cytochem. 1969 Jun;17(6):431–432. doi: 10.1177/17.6.431. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Chuang S. T., Huang M. C. Effects of chronic treatment with various neuromuscular blocking agents on the number and distribution of acetylcholine receptors in the rat diaphragm. J Physiol. 1975 Aug;250(1):161–173. doi: 10.1113/jphysiol.1975.sp011047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duane D. D., Engel A. G. Emetine myopathy. Neurology. 1970 Aug;20(8):733–739. doi: 10.1212/wnl.20.8.733. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Stefani E. Electrophysiological studies of neuromuscular transmission in hereditary 'motor end-plate disease' of the mouse. J Physiol. 1971 Jan;212(2):535–548. doi: 10.1113/jphysiol.1971.sp009340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen L. W., Strich S. J. An hereditary motor neurone disease with progressive denervation of muscle in the mouse: the mutant 'wobbler'. J Neurol Neurosurg Psychiatry. 1968 Dec;31(6):535–542. doi: 10.1136/jnnp.31.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grampp W., Harris J. B., Thesleff S. Inhibition of denervation changes in skeletal muscle by blockers of protein synthesis. J Physiol. 1972 Mar;221(3):743–754. doi: 10.1113/jphysiol.1972.sp009780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman A. P. Inhibitors of protein biosynthesis. V. Effects of emetine on protein and nucleic acid biosynthesis in HeLa cells. J Biol Chem. 1968 Aug 10;243(15):4089–4094. [PubMed] [Google Scholar]
- Grollman A. P. Structural basis for inhibition of protein synthesis by emetine and cycloheximide based on an analogy between ipecac alkaloids and glutarimide antibiotics. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1867–1874. doi: 10.1073/pnas.56.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. B. The resting membrane potential of fibres of fast and slow twitch muscles in normal and dystrophic mice. J Neurol Sci. 1971 Jan;12(1):45–52. doi: 10.1016/0022-510x(71)90250-4. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Ward M. R. A comparative study of "denervation" in muscles from mice with inherited progressive neuromuscular disorders. Exp Neurol. 1974 Jan;42(1):169–180. doi: 10.1016/0014-4886(74)90015-6. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Freiman D. G. An improved method of fixation for formalin-sensitive enzymes with special reference to myosin adenosine triphosphatase. J Histochem Cytochem. 1966 Aug;14(8):577–581. doi: 10.1177/14.8.577. [DOI] [PubMed] [Google Scholar]
- JOSEFSSON J. O., THESLEFF S. Electromyographic findings in experimental botulinum intoxication. Acta Physiol Scand. 1961 Feb-Mar;51:163–168. doi: 10.1111/j.1748-1716.1961.tb02124.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. K., Donahue J. D., Jondorf W. R. Effect of (minus)-emetine on liver microsomal protein synthesis in partially hepatectomized rats. Xenobiotica. 1971 Mar;1(2):131–141. doi: 10.3109/00498257109044385. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llados F., Zapata P. Effects of blockers of protein synthesis upon fibrillation due to denervation. Acta Physiol Lat Am. 1974;24(1):48–56. [PubMed] [Google Scholar]
- Manson-Bahr P. E., Ormerod W. E. Amoebic and bacillary dysentery and the enteric fevers. Practitioner. 1971 Aug;207(238):154–163. [PubMed] [Google Scholar]
- Miller H. H., Jondorf W. R. The subcellular distribution of (plus or minus)-2,3-dehydroemetine and (-)-emetine in rat liver and changes in hepatic lipid content after treatment of rats with (plus or minus)-2,3-dehydroemetine. J Pharm Pharmacol. 1970 Sep;22(9):659–663. doi: 10.1111/j.2042-7158.1970.tb12749.x. [DOI] [PubMed] [Google Scholar]
- Murphy M. L., Bulloch R. T., Pearce M. B. The correlation of metabolic and ultrastructural changes in emetine myocardial toxicity. Am Heart J. 1974 Jan;87(1):105–108. doi: 10.1016/0002-8703(74)90397-4. [DOI] [PubMed] [Google Scholar]
- Ng K. K. Blockade of adrenergic and cholinergic transmissions by emetine. Br J Pharmacol Chemother. 1966 Nov;28(2):228–237. doi: 10.1111/j.1476-5381.1966.tb01889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. I. Quantitative aspects. Acta Physiol Scand. 1971 Apr;81(4):557–564. doi: 10.1111/j.1748-1716.1971.tb04932.x. [DOI] [PubMed] [Google Scholar]
- STEIN J. M., PADYKULA H. A. Histochemical classification of individual skeletal muscle fibers of the rat. Am J Anat. 1962 Mar;110:103–123. doi: 10.1002/aja.1001100203. [DOI] [PubMed] [Google Scholar]
- Salako L. A. Effects of emetine on neuromuscular transmission. Eur J Pharmacol. 1970;11(3):342–348. doi: 10.1016/0014-2999(70)90010-5. [DOI] [PubMed] [Google Scholar]
- Simpson J. A. Applied electrophysiology in nerve and muscle disease. Disorders of neuromuscular transmission. Proc R Soc Med. 1966 Oct;59(10):993–998. doi: 10.1177/003591576605901024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THESLEFF S. Supersensitivity of skeletal muscle produced by botulinum toxin. J Physiol. 1960 Jun;151:598–607. doi: 10.1113/jphysiol.1960.sp006463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS G. A. Experimental hepatic amoebiasis and its application to chemotherapeutic studies. Br J Pharmacol Chemother. 1959 Dec;14:488–492. doi: 10.1111/j.1476-5381.1959.tb00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]





