Abstract
Boron (B)-deficient pumpkin (Cucurbita moschata Duchesne) plants exhibit reduced growth, and their tissues are brittle. The leaf cell walls of these plants contain less than one-half the amount of borate cross-linked rhamnogalacturonan II (RG-II) dimer than normal plants. Supplying germanium (Ge), which has been reported to substitute for B, to B-deficient plants does not restore growth or reduce tissue brittleness. Nevertheless, the leaf cell walls of the Ge-treated plants accumulated considerable amounts of Ge. Dimeric RG-II (dRG-II) accounted for between 20% and 35% of the total RG-II in the cell walls of the second to fourth leaves from Ge-treated plants, but only 2% to 7% of the RG-II was cross-linked by germanate (dRG-II-Ge). The ability of RG-II to form a dimer is not reduced by Ge treatment because approximately 95% of the monomeric RG-II generated from the walls of Ge-treated plants is converted to dRG-II-Ge in vitro in the presence of germanium oxide and lead acetate. However, dRG-II-Ge is unstable and is converted to monomeric RG-II when the Ge is removed. Therefore, the content of dRG-II-Ge and dRG-II-B described above may not reflect the actual ratio of these in muro. 10B-Enriched boric acid and Ge are incorporated into the cell wall within 10 min after their foliar application to B-deficient plants. Foliar application of 10B but not Ge results in an increase in the proportion of dRG-II in the leaf cell wall. Taken together, our results suggest that Ge does not restore the growth of B-deficient plants.
B is a micronutrient that is required for the normal growth of seed plants. The results of numerous studies have shown that B deficiency results in altered cell wall structure together with the formation of small irregularly shaped cells (Brown and Hu, 1997; Matoh, 1997). Most of B in the cell wall is present as a borate diol diester that cross-links two chains of rhamnogalacturonan II (RG-II; Matoh et al., 1993; Ishii and Matsunaga, 1996; Kobayashi et al., 1996; O'Neill et al., 1996; Kaneko et al., 1997). Borate ester cross-linking of RG-II has been shown to decrease the wall porosity of suspension-cultured Chenopodium album cells (Fleischer et al., 1999) and to control cell wall thickness in pumpkin (Cucurbita moschata Duchesne) tissues (Ishii et al., 2001). Taken together, these results demonstrate that a physiologically important function of B is to cross-link cell wall pectins and to regulate the mechanical and biological properties of the wall (O'Neill et al., 2001).
The ability of germanium (Ge) to substitute for B in plant growth is of interest because borate and germanate both form cyclic diesters with cis-diols (Loomis and Durst, 1991). McIlrath and Skok (1966) proposed that Ge does not substitute for B in the plant because the appearance of B-deficient symptoms in sunflower (Helianthus annuus) plants are delayed but not prevented by treatment. Brown and Jones (1972) obtained the same results in tomato (Lycopersicon esculentum) plants as McIlrath and Skok did. They hypothesized that Ge might displace B in B-sufficient tissue and thus allow the B to become available to deficient tissue. In contrast, Loomis and Durst (1991) claimed that Ge does replace B because B-deficient suspension-cultured carrot (Daucus carota) cells grew, albeit at a slower rate than B-sufficient cells, when Ge was added to the culture medium. Kobayashi et al. (1997) have shown that in vitro, a RG-II dimer is formed when germanic acid is reacted with monomeric RG-II (mRG-II). However, there have been no reports, to our knowledge, on the formation of Ge cross-linked dimeric RG-II (dRG-II-Ge) in muro and the affects of Ge treatment on the mechanical properties of plant tissue.
We now show that B-deficient pumpkin plants treated with Ge accumulate Ge in their cell walls. Nevertheless, Ge treatment did not restore the growth of these B-deficient plants. The petioles of the second leaves of Ge-treated and B-deficient plants had comparable mechanical properties. endo-Polyga-lacturonase (EPG) solubilized similar amounts of RG-II from the second to fourth leaves of Ge-treated and B-deficient plants. mRG-II accounted for between 60% and 85% of the total RG-II in the EPG-soluble material from B-deficient and Ge-treated plants. A small amount (2%–7%) of the total RG-II was shown to be present in the form of dRG-II-Ge in the walls of Ge-treated plants. However, the dRG-II-Ge dimer is much less stable than the dRG-II-B dimer. Thus, the ratio of dRG-II-Ge and dRG-II-B in the EPG-solublized material may not reflect the amounts of the Ge and B cross-linked RG-II dimers in muro.
RESULTS
Ge Contents of Leaves from Pumpkin Plant Grown in the Presence of Ge
Pumpkin seeds were germinated on rock fiber and grown for 7 d in Hoagland liquid medium containing 10 μm boric acid. One-third of the plants were transferred to Hoagland medium containing 25 μm B (B-treated) and grown for 7 d. One-third of the plants were transferred to Hoagland medium without Ge and B (B-deficient). The B content of the B-deficient medium was minimized by adding the borate-binding ion-exchange resin IRA743 to the growth medium, and the plants were grown in the presence of the resin for 7 d. The remaining one-third of the plants was transferred to medium containing the borate-binding resin to remove the residual B. After 1 d, the resin was removed, and then germanium oxide (28 or 140 μm Ge) was added. The plants were then grown for an additional 6 d. The fresh weights of the second, third, and fourth leaves from the Ge-treated and B-deficient plants were comparable, but somewhat less than those of the corresponding leaves of the B-treated plants (Table I). Between 70% and 90% of the Ge in the leaves of the Ge-treated plants was present in the alcohol-insoluble residue (AIR; Table I) and thus is assumed to be present in the cell wall. The amounts of Ge in pumpkin leaves (94–260 and 460-1290 μg Ge g−1 dry weight, 28 and 140 μm Ge supply, respectively; Table I) are somewhat higher than the amount of Ge (14 and 70–140 μg Ge g−1 dry weight, 10 μm and 1 mm Ge supply, respectively) that is present in suspension-cultured carrot cells grown in the presence of Ge (Loomis and Durst, 1991). Such differences may be attributable to the increased uptake of Ge by a transpiring tissue such as a pumpkin plant compared with the non-transpiring cultured carrot cells.
Table I.
The affect of germanium oxide and boric acid treatments on the fresh weight, Ge and B contents, and proportion of dimeric RG-II in the leaves of B-deficient pumpkin plants
| Treatment | Leaf No. | Fresh Leaf Wta | Leaf Bb | AIR Bb | Leaf Geb | AIR Geb | dRG-II-B in Total RG-IIc | dRG-II-Ge in Total RG-IIc |
|---|---|---|---|---|---|---|---|---|
| mg | μg g−1 dry leaf | % | ||||||
| 28 μm Ge | Cotyledon | 690 ± 40 | 55 ± 3 | 26 ± 2 | 94 ± 4 | 77 ± 6 | 94 | 1 |
| 1st leaf | 740 ± 50 | 26 ± 2 | 15 ± 1 | 240 ± 10 | 190 ± 20 | 68 | 2 | |
| 2nd leaf | 470 ± 50 | 11 ± 1 | 9 ± 3 | 260 ± 40 | 210 ± 30 | 33 | 2 | |
| 3rd leaf | 140 ± 40 | 11 ± 1 | 7 ± 1 | 170 ± 10 | 120 ± 10 | 23 | 2 | |
| 4th leaf | 20 ± 10 | 11 ± 2 | 10 ± 1 | 120 ± 20 | 96 ± 6 | 18 | 2 | |
| 140 μm Ge | Cotyledon | 680 ± 30 | 80 ± 7 | 37 ± 2 | 460 ± 20 | 380 ± 2 | 92 | 3 |
| 1st leaf | 860 ± 90 | 49 ± 7 | 27 ± 3 | 1,240 ± 20 | 990 ± 20 | 80 | 6 | |
| 2nd leaf | 470 ± 100 | 17 ± 3 | 10 ± 2 | 1,290 ± 30 | 1,000 ± 30 | 15 | 7 | |
| 3rd leaf | 120 ± 60 | 16 ± 2 | 8 ± 1 | 1,160 ± 50 | 920 ± 30 | 15 | 5 | |
| 4th leaf | 20 ± 7 | 17 ± 2 | 6 ± 1 | 780 ± 10 | 720 ± 20 | 16 | 4 | |
| 25 μm B | Cotyledon | 710 ± 50 | 71 ± 2 | 38 ± 4 | 0 | 0 | 90 | 0 |
| 1st leaf | 850 ± 70 | 73 ± 3 | 40 ± 5 | 0 | 0 | 85 | 0 | |
| 2nd leaf | 720 ± 80 | 48 ± 2 | 22 ± 3 | 0 | 0 | 90 | 0 | |
| 3rd leaf | 220 ± 50 | 35 ± 2 | 14 ± 1 | 0 | 0 | 90 | 0 | |
| 4th leaf | 30 ± 8 | 33 ± 8 | 15 ± 4 | 0 | 0 | 85 | 0 | |
| 0 μm B | Cotyledon | 780 ± 50 | 55 ± 5 | 27 ± 3 | 0 | 0 | 95 | 0 |
| 1st leaf | 740 ± 60 | 25 ± 1 | 15 ± 2 | 0 | 0 | 90 | 0 | |
| 2nd leaf | 540 ± 60 | 8 ± 1 | 6 ± 1 | 0 | 0 | 40 | 0 | |
| 3rd leaf | 150 ± 50 | 5 ± 1 | 2 ± 1 | 0 | 0 | 10 | 0 | |
| 4th leaf | 10 ± 20 | 5 ± 1 | 6 ± 1 | 0 | 0 | 20 | 0 | |
Plants were grown for 7 d in Hoagland medium containing 10 μm boric acid. One-third of the plants were then transferred to B-free Hoagland medium containing borate-binding resin RA743, grown for 1 d, and then the resin was removed and germanium oxide (28 or 140 μm) was added. The plants were grown for 6 d in the presence of Ge. One-third of the plants were transferred to Hoagland medium containing 25 μm boric acid. One-third of the plants were transferred to B-free Hoagland medium containing borate-binding resin IRA743. The plants were then grown for 7 d.
Fresh leaf wts are the mean value ± se (n = 18).
The Ge and B contents of the leaves and AIR were determined by ICP-MS. The values are means of three replicates and se are shown. AIR Ge and AIR B were calculated from the Ge and B content in AIR determined by ICP-MS and the yield of AIR.
The relative proportions of dRG-II-B and dRG-II-Ge were determined by SEC with RI and SEC/ICP-MS analyses of the material solubilized by EPG treatment of the AIR. The values are average from two individual experiments.
The second to fourth leaves from the Ge-treated and B-deficient plants contained discernible amounts of B (see Table I). Nevertheless, the amounts of B in these tissues were not sufficient to allow normal growth. The second to fourth leaves from the B-de-ficient plants contained less B than the leaves of the Ge-treated plants (Table I). The Ge-treated plants were grown for 1 d in the presence of the borate-binding resin to reduce the B content of the medium, whereas the B-deficient plants were grown for 7 d in the presence of the borate-binding resin. The IRA743 resin was removed before the addition of Ge because this ion-exchange material absorbs both Ge and B. We assume that a 24 h treatment with IRA743 is not sufficient to completely remove all the B from the growth medium. The leaves of 140 μm Ge-treated plants contained more B than the leaves of the 28 μm Ge-treated plants. Nevertheless, the AIR B contents of the 28 and 140 μm Ge-treated plants were similar, suggesting that the walls have a limited capacity to bind Ge (Table I).
Mechanical Properties of Ge-Grown Plant Petioles
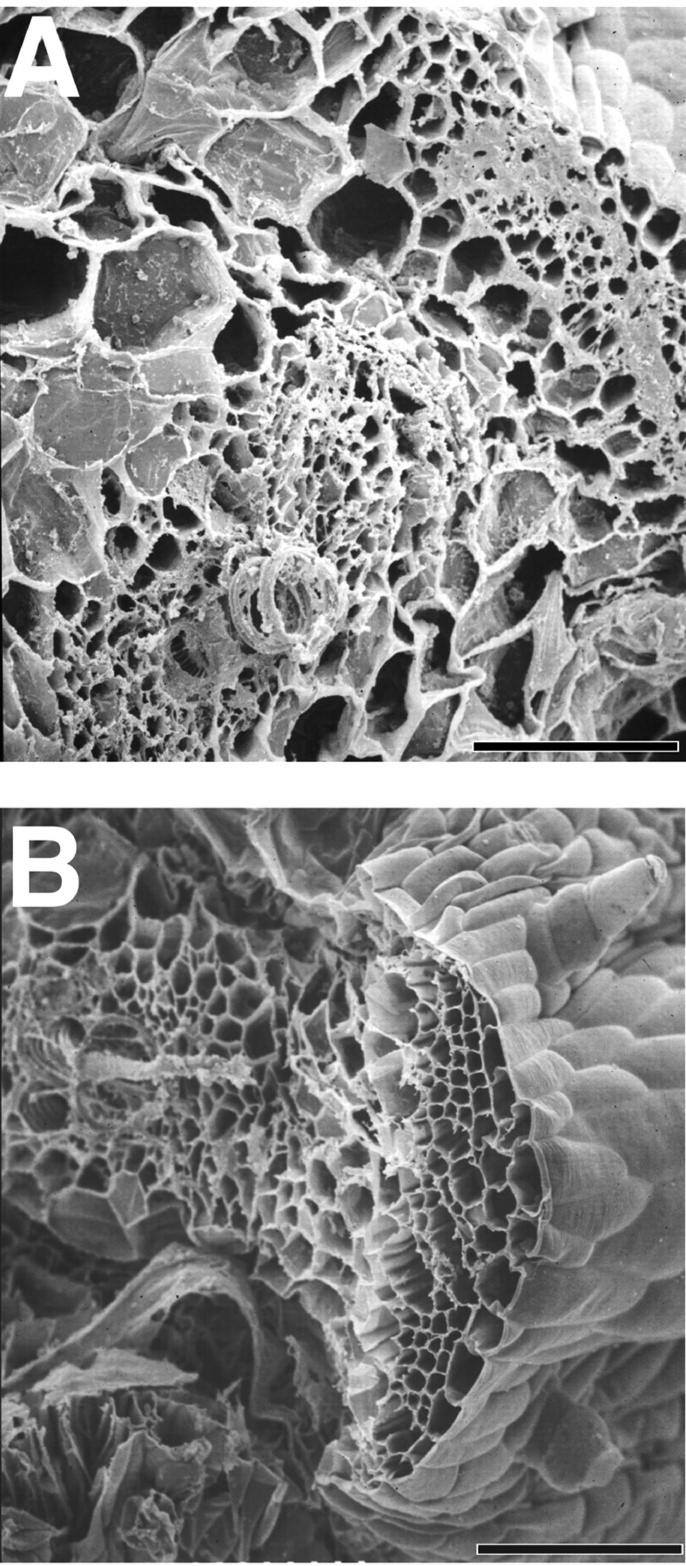
During routine handling of the Ge-treated and B-deficient plants, we observed that their petioles were more fragile than their B-treated counterparts. Thus, we used a three-point bending test to estimate the mechanical strength of second leaf petiole segments from Ge- and B-treated and B-deficient plants (Table II). The strength of the petiole is estimated from its maximum bending stress, whereas the brittleness is indicated by the maximum bending strain. The maximum bending stress of petioles from Ge- and B-treated and B-deficient plants were comparable. However, discernible differences were observed in the maximum bending strain of the petioles (Table II). The petioles of the Ge-treated and B-deficient plants have comparable bending strains, but the values are about a third of that of the petioles from B-treated plants. The petioles from the Ge-treated and the B-deficient plants broke at the maximum bending stress point, whereas the petioles from B-sufficient plants bent but did not break. Scanning electron microscopy of the broken ends indicated that the fractures occurred within the walls and that cell separation at the middle lamella did not occur (Fig. 1). These results are consistent with the notion that B deficiency results in an increase in tissue brittleness. The Arabidopsis mur 1 mutant has stems that are more brittle than their wild-type counterparts (Reiter et al., 1993), and this is believed to result from incomplete borate ester cross-linking of RG-II in mur 1 plants (O'Neill et al., 2001).
Table II.
The effects of Ge and B on the maximum bending stress and strain of the petiole of the second leaf of pumpkin
| Treatment | Stress (ςmax) | Strain (Smax) |
|---|---|---|
| N mm−2 | % | |
| 28 μm Ge | 0.87 ± 0.03a | 10.7 ± 0.64a |
| 25 μm B | 0.75 ± 0.03 | 30.0 ± 12 |
| 0 μm B and 0 μm Ge | 0.63 ± 0.03 | 10.1 ± 0.23 |
The values are means ± se (n = 7).
Figure 1.
Scanning electron micrographs of the broken petiole ends from the second leaf of B-deficient and Ge-treated (28 μm) pumpkin plants. A, Petiole from Ge-treated plant; B, petiole from B-deficient plant. Scale bar = 100 μm.
Characterization of the RG-II from the Leaf Cell Walls of Ge-Treated Pumpkin Plants
The AIRs generated from the cotyledons and from the first to fourth leaves of the Ge- and B-treated and B-deficient pumpkin were saponified and then treated with EPG to release RG-II together with RG-I and oligogalacturonides. The ratio of dimeric RG-II (dRG-II) and mRG-II in the EPG-soluble material was determined by size-exclusion chromatography (SEC) with refractive index detector (RI; Fig. 2A). The dRG-II accounted for at least 85% of the total RG-II in the cotyledon and in the first to fourth leaves of the B-treated plants but for only 10% to 25% of the RG-II in the third and fourth leaves of the Ge-treated and the B-deficient plants (Table I). These results show that RG-II is present mainly as a monomer in the leaves of B-deficient plants, irrespective of whether they have been treated with Ge. Such a result is consistent with the report that mRG-II is the predominant form of RG-II in the walls of B-deficient plants (Fleischer et al., 1999; Ishii et al., 2001).
Figure 2.
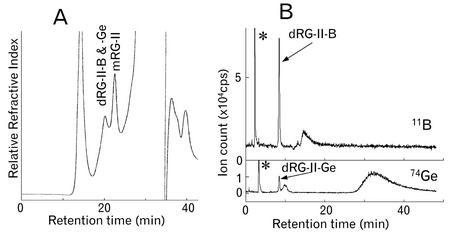
SEC/RI and SEC/ICP-MS of the material solubilized by EPG treatment of the AIR from the third leaves of Ge-treated (Ge, 28 μm) pumpkin plants. A, SEC/RI profile obtained using a Superdex-75 column with ammonium formate (pH 5.0, 50 mm) buffer. The Superdex-75 column was calibrated with sugar beet (Beta vulgaris) dRG-II-B (approximately 9.4 kD) and sugar beet mRG-II (approximately 4.7 kD), which have retention times at 21.0 and 23.2 min, respectively. Pullulans of 23.7 and 5.8 kD eluted at 15.8 and 22.4 min, respectively. The column included volume was 31.8 min using Glc. B, The 11B and 74Ge profiles obtained by SEC/ICP-MS. A Diol-120 column (details in “Material and Methods”) and ammonium formate (pH 6.5, 200 mm) buffer was used to separate the EPG-soluble material. dRG-II-B (approximately 9.4 kD) and boric acid were eluted at 8.5 and 14.9 min, respectively. The broad peak at 10 min was not identified. *, Peaks come from standard solution of boric acid and Ge oxide for calibration.
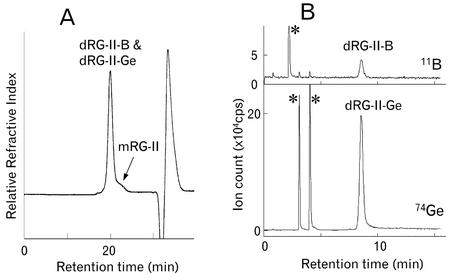
Kobayashi et al. (1997) have reported that the RG-II dimer formed in vitro by treating mRG-II with germanic acid and is much less stable than its borate cross-linked counterpart. We confirmed this observation by treating mRG-IIs generated from 28 μm Ge-treated plants and from red wine in vitro with germanium oxide in the presence of lead acetate. Approximately 95% of the mRG-II was converted under these conditions to a product that had a molecular mass of approximately 10 kD, suggesting that dRG-II-Ge had been formed (Fig. 3, A and B). However, approximately 80% of the dRG-II-G was converted back to mRG-II when the reaction mixture was dialyzed to remove the Ge and the lead. In contrast, dRG-II-B is stable when the borate and lead are removed by dialysis.
Figure 3.
SEC/RI and SEC/ICP-MS of the product formed by reacting mRG-II in vitro with germanium oxide in the presence of lead acetate. A, SEC/RI profile using a Superdex-75 column with ammonium formate (pH 5.0, 50 mm) buffer. mRG-II was eluted at 23.2 min as indicated with an arrow. B, The 11B and 74Ge profiles obtained by SEC/ICP-MS. A Diol-120 column and ammonium formate (pH 6.5, 200 mm) buffer was used to separate RG-II. See the legend to Figure 2 for further details.
To determine whether the walls of Ge-treated plants contained dRG-II-Ge, the EPG soluble material from the AIR of these plants was analyzed by SEC in combination with inductively coupled plasma mass spectrometer (ICP-MS). Most of the RG-II dimer that was present existed in its borate cross-linked form (Fig. 2B). A peak corresponding to Ge co-eluted with the RG-II dimer, and we estimated that dRG-II-Ge accounted for between 1% and 10% of the total RG-II present in the walls of the Ge-treated plants (Table I). However, these values may not reflect the actual ratios of dRG-II-Ge and dRG-II-B in muro because the dRG-II-Ge dimer is much less stable than dRG-II-B. The EPG-soluble material from Ge-treated plants contained a broad Ge peak that elutes between 30 and 35 min (Fig. 2B). The Ge peaks may correspond to Ge released by the conversion of dRG-II-Ge to mRG-II and from Ge that was nonspecifically bound to cell wall polymers. A small broad peak of Ge eluted at about 10 min was not identified.
Uptake of Ge and 10B and the Formation of dRG-II in B-Deficient Plants
Previous studies have shown that dRG-II-B is rapidly formed when B-deficient C. album cells are treated with boric acid (Fleischer et al., 1999). Thus, we compared the affects of short-term B and Ge treatments on the dRG-II contents of 7-d-old B-deficient pumpkin plants. When these B-deficient plants were grown for 22 h with 10B-enriched boric acid (95% [w/w] 10B, 25 μm), the walls of the third leaf contained 17 μg B g−1 dry weight (Ishii et al., 2001). This corresponds to 8-fold more B than is present in the walls of the untreated plants. The ratio of dRG-II-10B to total RG-II was 0.8, showing that dRG-II-10B had formed in muro (Ishii et al., 2001). In contrast, when the B-deficient plants were grown in the presence of Ge (28 μm) for 18 h in the present study, the dRG-II content of the walls from the second leaves did not increase, and dRG-II-Ge was not detected by SEC/ICP-MS, even though these walls contained 57 μg Ge g−1 dry weight.
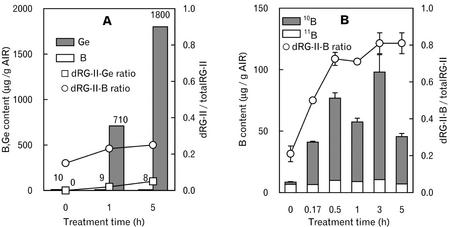
Foliar application of B to marginally B-deficient plants has been shown to cause an increase in the B content of the leaf to which it is applied for up to 24 h (Bellaloui et al., 1999). Thus, we compared the affects on RG-II when B and Ge were applied to the third leaf of B-deficient pumpkin plants. The 10B content of the walls increased within 10 min after foliar application of 10B (20 mm 10B-enriched boric acid), and within 30 min, dRG-II-B accounted for almost 80% of the RG-II (Fig. 4B). Foliar application of Ge (14 mm germanium oxide) resulted in a rapid accumulation of Ge in the cell walls but did not result in a quantitatively significant increase in the amounts of dRG-II-Ge (Fig. 4A). These results show that B and Ge are rapidly taken up from the leaf surface but that only B is effective in promoting the formation of a stable RG-II dimer.
Figure 4.
The uptake of Ge and 10B by the third leaves of B-deficient pumpkin plants and formation of dRG-II-Ge and dRG-II-10B after the foliar application of germanium oxide (14 mm Ge) and 10B-enriched boric acid (20 mm B). A, The Ge and B contents and the ratio of dRG-II-Ge, dRG-II-B, and mRG-II in the AIR after foliar application of Ge. The mean of the two independent experiments. B, The 10B and 11B contents and ratio of dRG-II-B and mRG-II in the AIR after foliar application of 10B-enriched boric acid. The B and Ge contents in the AIR were determined by ICP-MS. The ratio of dRG-II (dRG-II-Ge and dRG-II-B) and mRG-II was determined by SEC/RI. The values are means of three replicates ± se (n = 3). ○, dRG-II-B; □, dRG-II-Ge.
DISCUSSION
The possibility that Ge can substitute for B in plant growth and development has been a subject of debate (McIlrath and Skok, 1966; Loomis and Durst, 1991). B-deficient carrot cells have been reported to grow albeit more slowly than B-sufficient cells, when Ge is added to the growth medium (Loomis and Durst, 1991). However, such a result does not necessarily mean that Ge is substituting for B, because suspe-nsion-cultured C. album cells (Fleischer et al., 1998) and carrot cells (Fleischer, 2000) grow in the absence of B provided that they are maintained in their logarithmic growth phase. Our data have shown that Ge treatment does not restore the growth of B-deficient pumpkin plants and that the petioles of the Ge-treated plants are as fragile as their B-deficient counterparts. Small amounts of the Ge cross-linked RG-II dimer were detected, but the low stability of this dimer prevents its quantitation.
Our results confirm that dRG-II-Ge does form in the presence of Ge in vitro and show that dRG-II-Ge is converted to the monomer when the Ge is removed by dialysis. The inability of Ge to effectively substitute for B in the formation of a stable RG-II dimer in vitro, and most probably in muro, may be attributable in large part to the differences in the dimensions and geometry of borate and germanate (Loomis and Durst, 1991). The covalent single-bond radius of Ge (0.122 nm) is somewhat larger than B (0.088 nm; Dean, 1979). B is tetrahedral (coordination no. = 4) in anionic cyclic borate-diol diesters, whereas Ge is octahedral (coordination no. = 6) in germanate diol diesters (Lavigne et al., 1968). A germanate diester is likely to have a longer bond length than a borate diester. Thus, a germanate cross-link may be weaker than a borate cross-link, and its shape would be different from the borate. Isolated mRG-II, which has a molecular mass of approximately 5 kD, is likely to be more flexible than wall-bound RG-II, which itself is covalently linked to homogalacturonan (Ishii and Matsunaga, 2001). Such differences in flexibility may further reduce the ability of Ge to form a stable cross-link between two mRG-II molecules in muro. It is also possible that in muro two adjacent mRG-II molecules adopt a conformation that favors the formation of borate (Ishii et al., 1999) rather than germanate esters.
Brown and Jones (1972) have suggested that in B-deficient tomato plants, Ge may replace B in those tissues that contain sufficient B and that the released B is then moved to the B-deficient tissues. Our data do not support this hypothesis. We and others (Kobayashi et al., 1997) have shown that dRG-II-B is far more stable than dRG-II-Ge. Thus, it is unlikely that Ge can displace B in dRG-II-B to form dRG-II-Ge in muro.
In summary, we have shown that the leaves of Ge-treated pumpkin plants accumulate Ge in their walls, although this does not result in the restoration of the growth of B-deficient plants. A pectic network in muro that is cross-linked by Ge is unlikely to be sufficiently stable to restore the mechanical properties of the walls to normal levels. We conclude that Ge does not substitute for B in cross-linking cell wall.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Pumpkin (Cucurbita moschata Duchesne cv Tokyo-Kabocha) plants were grown using a modification of the procedure previously described (Ishii et al., 2001). Seeds were germinated on rock fiber (Nittobo Co., Tokyo) for 7 d at 25°C with a 12-h daylength. The plants were then transferred to 2 L of one-quarter-strength Hoagland solution (Hoagland and Arnon, 1950) supplemented with 10 μm B. The plants were grown for 7 d. One-third of the plants (18 plants) was transferred to one-quarter-strength Hoagland solution (2 L) with 25 μm B. One-third of the plants (18 plants) was transferred to one-quarter-strength Hoagland solution (2 L) without B and containing the borate-binding ion-exchange resin IRA743 (2 mL, Organo Co., Tokyo) to remove the residual B in the medium. The plants were grown for 1 d, the resin was removed, and 1,000 μg L−1 Ge solution (4 mL for 28 μm or 20 mL for 140 μm) was then added, and the plants were grown for a additional 6 d. The remaining one-third of the plants (18 plants) was transferred to one-quarter-strength Hoagland solution (2 L) without B and Ge and containing IRA-743 (2 mL) to minimize the B content of the B-deficient medium. The plants were grown for 7 d in the presence of the resin. After 7 d, the plants were harvested as previously described (Ishii et al., 2001). Ge standard solution (Ge2O solution, 1,000 μg L−1; 14 mm Ge for atomic absorption spectrometry was purchased from Wako Chemical Co. [Osaka]). Isotopic B (95% [w/w] 10B and 5% [w/w] 11B, Tosho Tsusho, Tokyo) as boric acid was used as a tracer for uptake.
The plants grown without B and Ge and in the presence of the borate-binding ion-exchange resin for 7 d were used for foliar treatment with Ge and 10B-enriched B. Aqueous solutions of 10B-enriched (95% [w/w] 10B) boric acid (B, 20 mm solution) or germanium oxide (Ge, 14 mm) were applied to the surface of the third leaves using a brush. The leaves were harvested over a period of 5 h, and the AIR was prepared as described previously (Ishii et al., 2001).
Physical Measurement
Petiole segments (5 cm) cut from the second leaves of Ge-treated (28 μm Ge), B-treated (25 μm B), and B-deficient plants were used immediately for the three-point bending tests using a Auto-GRAPH, AG-10 kN 1 (Shimadzu, Kyoto). A mean value for petiole diameter was obtained by measuring the diameter at each end of the segment with a caliper. For bending tests, segments were placed on two supports separated by a distance of 20 mm. A pushing probe with a one-eighth-inch radius was then lowered until it was in contact with the petiole. The crosshead was then lowered at 10 mm min−1. Measurement was made on two different days, and at least 10 petioles from each treated plant were tested. Maximum bending stress (ςmax, N mm−2) and maximum bending strain (Smax, %) were calculated with Trapezium software as follows:
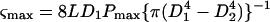 |
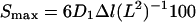 |
where L is the distance between edges (20 mm), D1 and D2 are the outer and inner diameters of the petiole (millimeters), Pmax is the maximum load (newtons), and Δl is the bending strain (millimeters).
Preparation of the AIR from Pumpkin Leaves and Solubilization of RG-II from AIR
AIR was prepared, saponified, and then treated with EPG to release RG-II, RG-I, and oligogalacturonides (Ishii et al., 2001). The EPG-soluble fraction was directly analyzed without dialysis to SEC/RI and SEC/ICP-MS.
Preparation of Ge-Containing dRG-II in Vitro
The EPG digests of the Ge-treated pumpkin (28 μm Ge supply) were fractioned using a Superdex-75 HR 10/30 column (Amersham Biosciences AB, Uppsala), and the fraction containing dRG-II was collected (Ishii et al., 2001). The dimer (1.0 mg) was treated with 0.1 n HCl for 1 h at room temperature to completely hydrolyze the borate ester, dialyzed (1-kD cut-off) for 24 h against water, and freeze-dried. mRG-II from the Ge-treated pumpkin (0.5 mg) and wine mRG-II (1 mg) was treated with 1.4 mm germanium oxide (20 μL) and 5 mm lead acetate (10 μL) in 50 mm potassium phthalate buffer (pH 3.4, 100 μL) for 16 h. A portion of the reaction mixture was directly subjected to SEC/RI and SEC/ICP-MS. The residual reaction mixture was dialyzed and then freeze-dried as described above.
Analytical Methods
SEC/RI was performed with a liquid chromatography system (Gulliver PU-980 pump, JASCO, Hachioji, Japan) and RI (model RID-10A, Shimadzu, Kyoto) connected to a Superdex-75 HR 10/30 column (Amersham Biosciences AB) eluted at 0.6 mL min−1 with 50 mm ammonium formate, pH 5.3, as described (Ishii and Matsunaga, 1996). The mRG-II and dRG-II-B in the EPG digests were confirmed by comparing their retention time with those of the authentic mRG-II and dRG-II-B from sugar beet (Beta vulgaris) and red wine. SEC/ICP-MS was performed with a Diol-120 column (8 × 300 mm, YMC Co., Kyoto) connected to the ICP source of a MS (SII SPQ 9000, Seiko Instruments Inc., Chiba, Japan). The column was eluted at 1 mL min−1 with 200 mm ammonium formate, pH 6.5. The MS was operated to selectively detect 10B, 11B, and 74Ge. The B and Ge content and 10B abundance of the AIR were determined by the ICP-MS.
Anatomical Determination
Petioles were fixed for 2 h at room temperature in 2.5 mm sodium phosphate, pH 6.8, containing 2.5% (v/v) glutaraldehyde. The petioles were then washed for 10 min with the same buffer. The petioles were dehydrated by passage through an ethanol series, and the ethanol then replaced with iso-amyl acetate. The tissue was then dried in a critical-point dryer (HCP-2, Hitachi, Tokyo). Finally, the tissue surface was coated with platinum/palladium in an ion-sputtering system (E-102, Hitachi), and the tissue was then examined with a scanning electron microscope (S-2500, Hitachi).
ACKNOWLEDGMENTS
We thank Malcolm A. O'Neill (Complex Carbohydrate Research Center, University of Georgia, Athens) for critical reading of the manuscript. Masako Ishikawa (Forestry and Forest Products Research Institute) is acknowledged for growing the pumpkin plants and preparing the manuscript.
Footnotes
This work was supported by the Ministry of Agriculture, Forestry, and Fisheries (Japan; grant no. BDP–02–II–1–5).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.009514.
LITERATURE CITED
- Bellaloui N, Brown PH, Dandekar AM. Manipulation of in vivo sorbitol production alters boron uptake and transport in tobacco. Plant Physiol. 1999;119:735–741. doi: 10.1104/pp.119.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Jones WE. Effect of germanium on utilization of boron in tomato (Lycopersicon esculentum Mill.) Plant Physiol. 1972;49:651–653. doi: 10.1104/pp.49.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PH, Hu H. Does boron play only a structural role in the growing tissues of higher plants? Plant Soil. 1997;196:211–215. [Google Scholar]
- Dean AD, editor. Lange's Handbook of Chemistry. Ed 12. New York: McGraw-Hill; 1979. [Google Scholar]
- Fleischer A. Effects of boron on primary cell walls of plant cells. PhD thesis. Germany: Humboldt-Universitaet zu Berlin; 2000. [Google Scholar]
- Fleischer A, O'Neill MA, Ehwald R. The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol. 1999;121:829–838. doi: 10.1104/pp.121.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer A, Titel C, Ehwald R. The boron requirement and cell wall properties of growing- and stationary-phase suspension-cultured Chenopodium album L. cells. Plant Physiol. 1998;117:1401–1410. doi: 10.1104/pp.117.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. California Agricultural Experiment Station Circular 347. Berkeley: The College of Agriculture, University of California; 1950. pp. 1–39. [Google Scholar]
- Ishii T, Matsunaga T. Isolation and characterization of a boron-rhamnogalacturonan II complex from cell walls of sugar beet pulp. Carbohydr Res. 1996;284:1–9. [Google Scholar]
- Ishii T, Matsunaga T. Rhamnogalacturonan II is covalently cross-linked to homogalacturonan. Phytochemistry. 2001;57:969–974. doi: 10.1016/s0031-9422(01)00047-4. [DOI] [PubMed] [Google Scholar]
- Ishii T, Matsunaga T, Hayashi N. Formation of rhamnogalacturonan II-borate dimer in pectin determines cell wall thickness of pumpkin tissue. Plant Physiol. 2001;126:1698–1705. doi: 10.1104/pp.126.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Matsunaga T, Pellerin P, O'Neill MA, Darvill A, Albersheim P. The plant cell wall polysaccharide rhamnogalacturonan II self-assembles into a covalently cross-linked dimer. J Biol Chem. 1999;274:13098–13104. doi: 10.1074/jbc.274.19.13098. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Ishii T, Matsunaga T. A boron-rhamnogalacturonan-II complex from bamboo shoots cell walls. Phytochemistry. 1997;44:243–248. [Google Scholar]
- Kobayashi M, Matoh T, Azuma J. Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol. 1996;110:1017–1020. doi: 10.1104/pp.110.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Ohno K, Matoh T. Boron nutrition of cultured tobacco BY-2 cells: II. Characterization of the boron-polysaccharide complex. Plant Cell Physiol. 1997;38:676–683. [Google Scholar]
- Lavigne AA, Tancrede JM, Pike RM. Coordination compounds of germanium. Coordination Chem Rev. 1968;3:497–508. [Google Scholar]
- Loomis WD, Durst RW. Boron and cell wall. Curr Top Plant Biochem Physiol. 1991;10:149–179. [Google Scholar]
- Matoh T. Boron in plant cell walls. Plant Soil. 1997;193:59–70. [Google Scholar]
- Matoh T, Ishigaki K, Ohno K, Azuma J. Isolation and characterization of a boron-polysaccharide complex from radish roots. Plant Cell Physiol. 1993;34:639–642. [Google Scholar]
- McIlrath WJ, Skok J. Substitution of germanium for boron in plant growth. Plant Physiol. 1966;41:1209–1212. doi: 10.1104/pp.41.7.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill M, Eberhard S, Albersheim P, Darvill AG. Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science. 2001;294:846–849. doi: 10.1126/science.1062319. [DOI] [PubMed] [Google Scholar]
- O'Neill M, Warrenfeltz D, Kates K, Pellerin P, Doco T, Darvill AG, Albersheim P. Rhamnogalacturonan II, a pectic polysaccharide in the walls of growing plant cells, forms a dimer that is covalently cross-linked by a borate ester. J Biol Chem. 1996;271:22923–22930. doi: 10.1074/jbc.271.37.22923. [DOI] [PubMed] [Google Scholar]
- Reiter W-D, Chapple CCS, Somerville CR. Altered growth and cell walls in a fucose-deficient mutant of Arabidopsis. Science. 1993;261:1032–1035. doi: 10.1126/science.261.5124.1032. [DOI] [PubMed] [Google Scholar]