Abstract
The inactive X chromosome of female mammals displays several properties of heterochromatin including late replication, histone H4 hypoacetylation, histone H3 hypomethylation at lysine-4, and methylated CpG islands. We show that cre-Lox-mediated excision of 21 kb from both Xist alleles in female mouse fibroblasts led to the appearance of two histone modifications throughout the inactive X chromosome usually associated with euchromatin: histone H4 acetylation and histone H3 lysine-4 methylation. Despite these euchromatic properties, the inactive X chromosome was replicated even later in S phase than in wild-type female cells. Homozygosity for the deletion also caused regions of the active X chromosome that are associated with very high concentrations of LINE-1 elements to be replicated very late in S phase. Extreme late replication is a property of fragile sites and the 21-kb deletions destabilized the DNA of both X chromosomes, leading to deletions and translocations. This was accompanied by the phosphorylation of p53 at serine-15, an event that occurs in response to DNA damage, and the accumulation of γ-H2AX, a histone involved in DNA repair, on the X chromosome. The Xist locus therefore maintains the DNA stability of both X chromosomes.
X-INACTIVATION in female mammals is the formation of heterochromatin throughout one of two X chromosomes early in development (Gartler and Riggs 1983). X-inactivation requires a region called the X-inactivation center (Xic) (Lyon 1996). Physical homologous association of the two copies of the Xic has been proposed to trigger X-inactivation (Marahrens 1999) and such an association has recently been shown to mark the onset of X-inactivation (Bacher et al. 2006; Xu et al. 2006). The X-linked Xist gene, which resides in the Xic (Brown et al. 1991), plays a central role in the subsequent heterochromatin formation (Penny et al. 1996) and Xist knockout mice die early in embryogenesis due to a failure to undergo X-inactivation (Marahrens et al. 1997). Xist encodes an untranslated RNA that is expressed from the inactive X chromosome (Xi) but not from the active X chromosome (Xa) (Brockdorff et al. 1991; Brown et al. 1991). The Xist RNA is quite stable and colocalizes exclusively with the Xi (Brown et al. 1992; Clemson et al. 1996). In addition to the role of Xist, the spread of X-inactivation correlates with high concentrations of LINE-1 elements on the X chromosome (Lyon 1998). Accordingly, X-linked genes that escape X-inactivation are found in regions with reduced concentrations of LINE-1 sequence (Bailey et al. 2000). In cells deficient for the DNA methyltransferase Dnmt3b, the DNA of LINE-1 elements on the Xi, but not on the Xa, is hypomethylated (Hansen 2003) and X-inactivation is either incomplete or not fully maintained (Hansen et al. 2000).
Another feature that distinguishes the Xi from the Xa and from autosomes is that it is replicated later in S phase (Taylor 1968; Taylor and Miner 1968). The replication timing of the Xi reflects a general trend where later replication times are associated with gene repression and early replication with transcriptional competence (Gilbert 2002). The available evidence indicates that the same replication origins are utilized on the active and inactive X chromosomes (Cohen et al. 2003; Gomez and Brockdorff 2004), thus suggesting that the replication timing differences between the two X chromosomes stem from the times in S phase that their origins are activated. While in female human cells the Xi is replicated much later in S phase than the Xa (Priest et al. 1967), the Xi is not replicated nearly as late in S phase in female mouse cells as in human cells (Evans et al. 1965; Galton and Holt 1965; Tiepolo et al. 1967). This has led to the Xi in mouse cells being distinguished by its absence of label incorporation in early S phase rather than by its being disproportionately replicated late in S phase (Nesbitt and Gartler 1970). Nevertheless, there is always a consistent trend of the mouse Xi displaying more label incorporation late in S phase than the Xa in both primary and transformed female fibroblasts (Diaz-Perez et al. 2005).
The protein composition of the Xi also distinguishes it from other chromosomes. The histone H2A homolog, macrohistone H2A, is present along the length of the Xi but not the Xa (Costanzi and Pehrson 1998). In addition, nearly all of the nucleosomes of the Xi are hypoacetylated at the N-terminal tail of histone H4 (Jeppesen and Turner 1993). Histone tail acetylation is a widespread characteristic of euchromatin and histone deacetylation is a general characteristic of heterochromatin (Jenuwein and Allis 2001). Furthermore the nucleosomes of the Xi are methylated at histone H3 lysine-9 (Peters et al. 2001; Chadwick and Willard 2004) or lysine-27 (Plath et al. 2003; Chadwick and Willard 2004), and both are histone modifications associated with heterochromatin. Methylation at H3 lysine-4, a euchromatic histone modification that appears to be mutually exclusive to lysine-9 methylation, is conspicuously absent from the Xi (Boggs et al. 2001). Yet another feature that distinguishes the Xi from other chromosomes is that it is associated with high concentrations of the BRCA1 protein that associates with XIST RNA (Ganesan et al. 2002). In BRCA1-deficient cells, XIST RNA, macroH2A, and H3 lysine-9 methylation all failed to concentrate on the Xi (Ganesan et al. 2002, 2004).
In addition to its role in X-inactivation, BRCA1 functions as a tumor suppressor that plays a role in cell cycle checkpoints, in multiple types of DNA repair, and in the maintenance of genome stability (Scully and Livingston 2000; Welcsh et al. 2000; Narod and Foulkes 2004). Stalled DNA replication forks as well as various types of DNA damage, including UV damage, cause the ataxia-telangiectasia-mutated and Rad3-related (ATR) kinase to phosphorylate various targets including BRCA1 (Tibbetts et al. 2000), p53 at serine-15 (Tibbetts et al. 1999), and H2AX (to produce γ-H2AX) (Ward and Chen 2001; Ward et al. 2004). Double-strand breaks cause the related ataxia-telangiectasia mutated (ATM) kinase to phosphorylate many of the same targets including BRCA1 (Cortez et al. 1999; Gatei et al. 2000), p53 at serine-15 (Banin et al. 1998; Canman et al. 1998; Khanna et al. 1998), and histone H2AX to produce γ-H2AX (Burma et al. 2001). γ-H2AX associates with the Xi in the absence of experimentally incurred DNA damage, but this is restricted to late S phase (Chadwick and Lane 2005). The phosphorylation of p53 stabilizes and activates the protein, which signals for either cell cycle arrest or apoptosis (Attardi 2005). γ-H2AX has been proposed to recruit additional proteins to sites of DNA damage (Bassing and Alt 2004). Deficiency in either ATR or ATM disturbs the maintenance of X-inactivation (Ouyang et al. 2005).
Excision of the transcribed Xist allele from the Xi leads to the loss of the Xist RNA and absence of macroH2A from the Xi (Csankovszki et al. 1999) and to a destabilization of X chromosomal gene silencing (Csankovszki et al. 2001) but does not abolish late replication (Csankovszki et al. 1999) or result in an acetylated Xi (Csankovszki et al. 1999). The transcribed Xist allele, therefore, functions in cis to maintain a subset of the features of the Xi heterochromatin. Excision of 21 kb from the nontranscribed Xist locus of the Xa results in the Xa being replicated later in S phase (Diaz-Perez et al. 2005). Both Xist alleles therefore display biological activity. Here we show that element(s) at both copies of the Xist gene control the chromatin structure of the Xi and influence the replication time of both X chromosomes. Xist deficiency furthermore destabilizes both X chromosomes, leading to deletions and translocations, the phosphorylation of p53 at serine-15, and the increased association of the DNA repair/genome maintenance protein γ-H2AX with the Xi. Xist deletions therefore reveal trans-interactions that occur subsequent to the initiation of X-inactivation.
MATERIALS AND METHODS
Fibroblasts and growth conditions:
All of the mice used in this study had a 129 genetic background. Mouse primary fibroblasts were obtained from wild-type 129 mice and also from crosses involving previously described mouse strains (Csankovszki et al. 1999, 2001) by trypsinization of 13-day embryos, culture, and immortalization with SV40 T-antigen (Jat et al. 1986). Three immortalized fibroblast cell lines were obtained from three E13.5 mouse embryos (one embryo per cell line), in which 21 kb of sequence at the Xist locus were flanked by Lox sites (floxed) on both the Xa and Xi (XaXist-floxXiXist-flox). Three additional immortalized fibroblast cell lines were obtained from three wild-type 129 embryos (XaXist-WTXiXist-WT). The three XaXist-floxXiXist-flox cell lines (XaXist-floxXiXist-flox-1, -2, and -3) and three XaXist-WTXiXist-WT cell lines (XaXist-WTXiXist-WT-1, -2, and -3) were infected with adenovirus expressing cre recombinase and GFP (Tan et al. 1999) and plated out in 24-well plates at less than one cell per well (limiting dilution) to recover clonal cell lines from each progenitor line. GFP expression was used to identify infected cells. Starting from the fibroblasts that arise during the limiting dilution procedure, each clonal cell line was passaged five times. During this passaging, lines that were homozygous for the 21-kb deletion (XaXist-Δ21-kbXiXist-Δ21-kb-1.1, -2.1, and -3.1) and one line that was heterozygous for the floxed Xist allele were identified using PCR. RNA FISH for Xist transcript was used to determine that, in the heterozygous line, the deletion was on the Xi (XaXist-floxXiXist-Δ21-kb-1.1) (not shown). After the aforementioned five passages, the three clonal XaXist-Δ21-kbXiXist-Δ21-kb cell lines and the three clonal XaXist-WTXiXist-WT cell lines (from six different embryos) were each subjected to a BrdU pulse (see below) and metaphase spread chromosomes were prepared. These spreads were analyzed for evidence of chromosomal deletions and translocations using chromosome paint, BrdU immunostaining, and spectral karyotyping (see below). Note that the three XaXist-Δ21-kbXiXist-Δ21-kb (-1.1, -2.1, and -3.1) and three XaXist-WTXiXist-WT (-1.1, -2.1, and -3.1) cell lines used in the analysis for deletions and translocations were generated using identical procedures. Primary XaXist-Δ21-kbXiXist-Δ21-kb did not grow well enough to perform immunostaining or replication timing experiments; we are exploring approaches to remedy this.
In addition, cell lines XaXist-floxXiXist-flox-1, -2, and -3 were infected with adenovirus expressing only GFP and limiting dilution was used to recover clonal cell lines XaXist-floxXiXist-flox-1.1, -2.1, and -3.1 using the same procedure and the same number of passages as was used to obtain lines XaXist-Δ21-kbXiXist-Δ21-kb-1.1, -2.1, and -3.1. Two additional cell lines used in this study were obtained by infecting fibroblasts that were heterozygous for the Xist-flox allele with adenovirus expressing cre recombinase and GFP and using limiting dilution, PCR, and RNA FISH to recover and identify clonal cell lines that carried the 21-kb deletion on the Xi (XaXist-WTXiXist-Δ21-kb-1.1 and XaXist-WTXiXist-Δ21-kb-2.1). Finally, three XaXist-flox,Hprt-Δ XiXist-flox,Hprt-WT cell lines and derivative XaXist-Δ21-kb,Hprt-Δ XiXist-Δ21-kb,Hprt-WT cells were obtained using the same procedure, except that a series of additional mouse matings were first performed to enable the production of fibroblasts that also included a published Hprt deletion (Hooper et al. 1987) on the Xa. The generation of the Hprt-heterozygous cell lines is described in detail elsewhere (J. L. Salstrom, C. Wang, C. Wang, D. Dutta, S. Zeitlin, G. Csankovszki, C. D. Eller, S. Diaz-Perez, J. Wang, A. Chess, S. Huang, B. Kaltenboeck and Y. Marahrens, unpublished data).
A large proportion of the immortalized cells in each culture contained either three or four X chromosomes. Limiting dilution was also used to obtain clonal cell lines containing predominantly two X chromosomes and an approximately diploid number of chromosomes. To this end, the same three immortalized XaXist-floxXiXist-flox (-1, -2, and -3) cell lines described in the previous paragraph were infected with adenovirus expressing cre recombinase and/or GFP (Tan et al. 1999), and limiting dilution was used to recover numerous clonal cell lines from each progenitor line. PCR was used to identify XaXist-Δ21-kbXiXist-Δ21-kb cell lines and metaphase spread chromosomes and flow cytometry was used to identify XaXist-Δ21-kbXiXist-Δ21-kb and XaXist-floxXiXist-flox lines that were derived from diploid cells. A number of these diploid cell lines were subjected to X chromosome paint to confirm the presence of two X chromosomes. We chose six approximately diploid cell lines containing two X chromosomes each that were derived from three embryos, with lines XaXist-Δ21-kbXiXist-Δ21-kb-1.2 and XaXist-floxXiXist-flox-1.2 from the same embryo, XaXist-Δ21-kbXiXist-Δ21-kb-2.2 and XaXist-floxXiXist-flox-2.2 from the same embryo, and XaXist-Δ21-kbXiXist-Δ21-kb-3.2 and XaXist-flox XiXist-flox-3.2 from the same embryo. Using the identical procedure, approximately diploid cell lines containing two X chromosomes each were also derived from the XaXist-WTXiXist-Δ21-kb and XaXist-floxXiXist-Δ21-kb cells (XaXist-WTXiXist-Δ21-kb-1.2 and XaXist-floxXiXist-Δ21-kb-1.2). The diploid lines thus established were all used at equally low passage numbers because they all became increasingly tetraploid with extended passaging. Diploid XaXist-flox,Hprt-Δ XiXist-flox,Hprt-WT and XaXist-Δ21-kb,Hprt-Δ XiXist-Δ21-kb,Hprt-WT cells were also obtained.
Infection of fibroblasts with adenovirus expressing cre recombinase and/or adenovirus expressing GFP (Tan et al. 1999) was performed at 50 multiplicities of infection (MOI) in 6-cm-diameter dishes with 106 cells with virus in 200 μl of Dulbecco modified Eagle's minimum essential medium (DMEM) with 5% fetal bovine serum (FBS) at 37° for 1 hr followed by the addition of 3.0 ml of DMEM with10% FBS. Primary and transformed mouse fibroblast cell lines were grown in DMEM supplemented with 10% fetal bovine serum (GIBCO, Grand Island, NY), penicillin (100 μg/ml), and streptomycin (100 μg/ml). The PCR primers 5′ LoxF (5′-TTT CTG GTC TTT GAG GGC AC-3′), 5′ LoxR (5′-ACC CTT GCC TTT TCC ATT TT-3′), and Xint3R (5′-CAC TGG CAA GGT GAA TAG CA-3′) were used to identify the Xist-flox (612-bp PCR product), Xist-Δ21kb (513 bp), and Xist-WT (427 bp) alleles. XaXist-Δ21kbXiWT fibroblasts were distinguished from XaWTXiXist-Δ21kb fibroblasts using RNA FISH against the Xist RNA (see below).
RNA FISH:
Fibroblasts were grown on coverslips for 24 hr and then fixed in 4% formaldehyde for 15 min at room temperature (RT). The cells were permeabilized in PBS containing 0.5% Triton-X for 5 min on ice and washed in PBS and 2× SSC. RNA FISH hybridization was carried out as previously described (Spector and Goldman 1998.). The Xist probe was labeled by nick translation with biotin-21-dUTP. After overnight hybridization at 37° posthybridization washes were done as previously described (Spector and Goldman 1998). The probe was detected with a 500-fold dilution of avidin-FITC (Jackson ImmunoResearch, Westgrove, PA) at RT for 1 hr. The nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI).
Two-color DNA FISH:
A combination probe, composed of a mixture of seven different small probes, was used to identify the inactive X chromosome. This combination probe, henceforth referred to as 6.8-kb probe, contains PCR products of sizes (in base pairs) 412, 605, 609, 850, 1011, 1560, and 1755. Each of these PCR products was amplified separately using Taq Polymerase (Promega, Madison, WI) from a region extending up to 14.7 kb upstream of the 5′ end of exon 2 of the Hprt gene and that is deleted in the Hprt-Δ allele used in this study (Thompson et al. 1989). The mouse BAC RP23-412J16 [purchased from Invitrogen (Carlsbad, CA)] served as template for the PCR reactions. The sequences of primers are: GCA AGC ATA AGG ACC AGA GC (412R), TTC CAC AAG AAA TAT TAC ACA AAA CA (412L), CCT AAC CAT TGA GCC GTC TT (605R), GGT CTC TGA ACT ACC AAT TGC AC (605L), GCA ATG ACA AAT GTT TTG TGG (609R), TGC TTA TTA GCA CAA GAC CTC AAG (609L), ATC ACC CTA TTC CCA GTG GA (850R), GCA GAT GAT AAG CTA TCC TTG AGA (850L), CAT CAC TGA GTC TTG CTG GTT T (1011R), CAA TTT AGG GGA AGG AAG CA (1011L), TGG TAG CTG GGC ATA AAA GC (1560R), AAT GGG AGA AAA GGC AGG AT (1560L), CAG GAA AGG GTG TGT GTG TG (1755R), and TAC GCT CTG GCA GTT TTC AA (1755L). The PCR products were gel extracted using the Wizard PCR Preps DNA purification system (Promega). To mark both the active and the inactive X chromosomes, mouse BAC RP23-298N24 (obtained from Invitrogen) was used to obtain a second probe. For fluorescent probe preparations, 1 mg of DNA was direct labeled with either FluorX-dCTP (for the whole BAC probe) or Cy3-dCTP (for the Xi-specific 6.8-kb combination probe), using the Nick Translation kit (Amersham Biosciences, Arlington Heights, IL). To prepare the 6.8-kb probe, equimolar concentrations of the seven different probes were mixed together, to a total DNA content of 1 mg, for labeling. Labeled probes were purified using NucAway Spin columns (Ambion, Austin, TX) and precipitated with 40 μg mouse Cot-1 DNA, 100 μg salmon sperm, and 100 μg tRNA; washed in 75% ethanol followed by 100% ethanol; and resuspended in 100 μl hybridization buffer (50% formamide, 10% dextran sulfate, 1× SSC). Cells were treated for FISH as described previously (Singh et al. 2003). Briefly, cells were fixed with 3:1 methanol–acetic acid, dropped on poly-l-lysine-coated slides (Sigma, St. Louis) in a humid chamber, and denatured for 2 min at 69°–72° in 70% formamide/2× SSC. An 18-ml aliquot of the two probes (12 μl of 6.8-kb probe, 6 ml of BAC probe) was prehybridized (90° for 5 min, followed by 10 min at 37°) and then hybridized overnight with cells at 37°. Next, cells were washed three times with 50% formamide/2× SSC at 42° followed by three washes with 1× SSC also at 42°. The following washes were done at room temperature: 1× SSC (10 min), 4× SSC (5 min), 4× SSC/0.1% Tween-20 (5 min), and 4× SSC (5 min). The cells were mounted in Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingame, CA) to counterstain the nuclei. Cells were viewed with a Nikon E600 fluorescent microscope. Images were captured with a CCD camera using SPOT Advanced software.
Quantitative determination of Xist mRNA:
Quantitative determination of Xist mRNA was performed by one-step reverse transcription (RT) fluorescence resonance energy transfer (FRET) real-time PCR of 1:100 diluted poly(A) RNA samples in a Lightcycler modeled after the duplex PCR approach described earlier (Wang et al. 2004). Xist primers (muXISTmRNAUP, 5′-CCC TAC ATC AAA GTA GGA GAA AAG CTG CTG-3′; muXISTmRNADN, 5′-GAA GGG TAA TAT TTG GTA GAT GGC ATT GTG T-3′) transversed the boundaries of exons 4 and 5 and of exons 5 and 6, respectively, and the FRET probes (muXISTFLU, 5′-CCT AGC TTC TGG AGA GAG AAC CAA ATA GAG-6-FAM-3′; muXISTBOD, 5′-Bodipy 630/650-AGA ATG GCT TCC TCG AAG GTC AGT GC-Phosphate-3′) detected exon 5. Thermal cycling conditions of this PCR were 30 min reverse transcription at 55°, followed by 2 min denaturation at 95°, followed by thermal cycling: 6 times at 95°, 0 sec/68°, 12 sec/72°, 8 sec; 9 times at 95°, 0 sec/66°, 12 sec/72°, 8 sec; 3 times at 95°, 0 sec/64°, 12 sec/72°, 8 sec; 25 times at 95°, 0 sec/56°, 12 sec followed by fluorescence acqusition/72°, 10 sec. Quantitative standards were produced by PCR amplification with dTTP, gel purification, and quantification of the fragment by Pico-Green assay (Invitrogen). Reaction chemistry was as published (Wang et al. 2004). The internal autosomal reference gene transcript (porphobilinogen deaminase, PBGD) was amplified from undiluted poly(A) RNA in a separate reaction following the duplex PCR protocol as described (Wang et al. 2004). All analyte transcript concentrations are expressed as copies per PBGD reference transcripts.
Fluorescent immunostaining for BrdU in metaphase chromosome spreads:
BrdU (30 μm) was added to asynchronous actively growing fibroblasts at 80% confluence. Several BrdU pulse lengths were performed on multiple cell lines and these data were used to determine that 4.5 hr is the appropriate duration of BrdU incorporation for each replication-timing experiment (data not shown). Exponentially growing asynchronous fibroblasts were cultured for 4.5 hr in the presence of BrdU and 0.050 μg/ml Colcemid (Life Technologies, Grand Island, NY) was added 1 hr before harvesting. Cell suspensions were incubated 13–15 min in 0.4% KCl at 37° followed by fixation with 3:1 methanol:acetic acid. Metaphase spreads were prepared by dropping the BrdU-treated cells onto coverslips followed by DNA denaturation in 70% formamine/2× SSC at 73° for 2 min. Following preincubation with blocking buffer (1× PBS, 10% FBS, 0.2% Tween 20), incorporated BrdU was detected using 1:20 dilution in blocking solution of monoclonal anti-BrdU antibody (Sigma) followed by 1:150 dilution in blocking solution of Texas-Red anti-mouse antibody (Jackson ImmunoResearch) in blocking buffer. Images were captured using Quips mFISH software (Vysis, Adelphia, NJ). The individual colors of a recorded image were stored separately by the Vysis Quips mFISH software and the representation of each color in the final image was adjusted using the software setting of the gain for that color. The BrdU incorporation studies were not done simultaneously with Xist RNA FISH because the Xist RNA is lost from the Xi during mitosis. The Xist RNA signal that can be seen on the Xi in early mitotic cells is very fragile and the treatments that occur during the BrdU incorporation assay caused the Xi to lose Xist RNA signal.
Spectral karyotyping:
The spectral karyotyping (SKY) probe mixture (Applied Spectral Imaging) was applied according to the manufacturer's recommendations for metaphase chromosome spreads (MCSs) prepared as described for the BrdU incorporation assay. Chromosomal aberrations were quantified using an Olympus BX-61 microscope equipped with an Applied Spectral Imaging interferometer and 40× and 63× objectives, driven by a desktop computer with SKY acquisition and analysis software.
Quantitation of incorporated BrdU:
X chromosome paints (Cambio, Cambridge, UK) were used to identify the X chromosomes and the X chromosome displaying the higher level of BrdU incorporation within a spread was always assumed to be the inactive one. Images obtained using Quips mFISH were transferred to NIH image (http://rsb.info.nih.gov/nih-image) and the numbers of pixels occupied by the X chromosomes (DAPI) and by fluorescently labeled BrdU (Texas Red) were then calculated for each MCS. Fisher's exact test was used to compare the percentages of X chromosomes displaying BrdU incorporation between different cell lines. Box plots were used to visualize the distributions of BrdU measurements across categorical groupings. Box plots labeled “% BrdU signal” represent measurements of the number of pixels of BrdU signal on a chromosome divided by the number of pixels of DAPI signal occupied by the same chromosome multiplied by 100. NIH Image was also used to record the intensity of each pixel. “BrdU area × intensity” represents the % BrdU signal multiplied by the average intensity of the pixels representing BrdU. The statistical analyses were performed using the software package R (Ihaka and Gentleman 1996), which can be downloaded from http://cran.r-project.org/. Differences in measurements were tested across categorical groupings using the Kruskal–Wallis test (Kruskal 1964) and the P-values obtained from this test are displayed above the corresponding box plots.
X chromosome paint:
A total of 10 μl of mouse X chromosome-specific biotinylated probe (Cambio) were used to detect the X chromosomes by fluorescent in situ hybridization (DNA FISH) to ethanol-dehydrated cells according to manufacturer's instructions. The probe was detected using streptavidin–FITC (Jackson ImmunoResearch) and anti-streptavidin FITC (Vector Laboratories) at 1:50 dilution each in blocking solution (1× PBS, 10% human serum, 0.05% Tween 20). The chromosomes were counterstained with DAPI and viewed with a Leica DMR fluorescent microscope. Images were captured with Quips mFISH software (Vysis).
Determining sequence composition across the X chromosome:
We identified the types and positions of repetitive sequences from the RepeatMasker output provided by the UCSC genome browser (http://genome.ucsc.edu). For successive 1-Mb intervals, we then obtained a value for each repeat type representing the percentage of the 1-Mb sequence occupied by that repeat type.
Histone immunolabeling and whole-chromosome paint:
Indirect immunofluorescence with anti-acetyl-histone H4 antibody (Serotec, Oxford) and histone H3 (trimethyl-K4) antibody (Abcam) was carried out on asynchronous cultures. Fibroblasts were grown at 80% confluence, Colcimid (0.05 μg/ml) was added for 1 hr, and fibroblasts were harvested and then swollen in 0.4% KCl at 37° for 13 min. Fibroblasts were dropped onto coverslips. Cell membranes were solubilized by immersion in KCM buffer [120 mm KCl, 20 mm NaCl, 10 mm Tris–HCl, pH 8, 0.5 mm EDTA, 0.1% (v/v) Triton X-100] in a petri dish (35 × 10 mm). The coverslips were transferred to blocking solution (10% FBS in KCM) at 37° for 1 hr and then incubated with 20 μl of 1:10 of anti-acetylated H4 (Serotec) or 1:20 anti-methH3-K4 (Abcam) in a humidified chamber for 2 hr at RT or overnight at 4°. The coverslips were then washed three times for 5 min in KCM and transferred to blocking solution and incubated at 37° for 1 hr. Primary antibody was detected with anti-rabbit Texas Red (Jackson ImmunoResearch) at 1:100 dilution in blocking solution and incubated for 1 hr at RT. The coverslips were washed three times with KCM buffer and fixed at RT with 4% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA) in KCM for 20 min and washed three times with KCM at RT. To detect the X chromosomes, the coverslips were incubated two times in methanol:acetic acid (1:3 v/v) at −20° for 20 min each. DNA was denatured with 70% formamide/2× SSC at 73° for 8 min, dehydrated, and then hybridized to 10 μl of the biotinylated probe (Cambio) according to the manufacturer's instructions. The probe was detected using streptavidin–FITC (Jackson ImmunoResearch) and anti-streptavidin FITC (Vector Laboratories) at 1:50 dilution each in blocking solution (1× PBS, 10% human serum, 0.05% Tween 20). The chromosomes were counterstained with DAPI and viewed with a Leica DMR fluorescent microscope. Images were captured with Quips mFISH software (Vysis).
Western analysis of p53:
Proteins from whole-cell extracts were separated by sodium dodecyl sulfate (SDS) polyacrylimide gel electrophoresis (PAGE), transferred to a charged PVDF membrane, and blocked with 5% nonfat milk in TBST (20 mm Tris pH 7.4, 150 mm NaCl, 0.05% Tween-20). Primary and secondary antibody incubations were performed in 5% nonfat milk in TBST. Proteins were detected using the ECL Plus Western blotting detection reagent (GE Healthcare, Piscataway, NJ). Primary reagents included: rabbit anti-p53-Ser15 (Cell Signaling Technology, Beverly, MA), rabbit anti-p53(CM5) (Vector Laboratories), mouse anti-β actin (Sigma), and mouse anti-ATM-Ser1981 (Rockland, Gilbersville, PA). Donkey anti-rabbit IgG-horseradish peroxidase (HRP) (GE Healthcare) and sheep anti-goat IgG-HRP (GE Healthcare) were used as the secondary antibodies.
BRCA1, γ-H2AX, and MeCP2 immunolabeling:
Fibroblasts were grown at 80% confluence and dropped onto coverslips. The cells were fixed with 2% of paraformaldehyde in PBS for 5 min and washed three times with 1× PBS. The cells were incubated in permeabilized solution (1× PBS, 0.5% Triton X-100) for 10 min at 4° and washed three times with KCM buffer [120 mm KCl, 20 mm NaCl, 10 mm Tris–HCl, pH 8, 0.5 mm EDTA, 0.1% (v/v) Triton X-100]. The cells were transferred to blocking solution (10% FBS in KCM) at 37° for 1 hr and then incubated with anti-Brca1 1:2.5 dilution at 37° for 2 hr. The coverslips were washed three times with KCM buffer and blocking again at 37° for 30 min. The Brca1 antibody was detected with anti-mouse Texas Red at 1:150 dilution and incubated at room temperature for 1 hr and then washed three times and the process repeated for histone γH2AX (Upstate Biotechnology, Charlottesville, VA) that was detected using anti-rabbit FITC. The immunodetections were also performed in the reverse order (Brca1 last) with similar results. In other experiments, the γ-H2AX antibody (Upstate Biotechnology) was detected using either anti-rabbit FITC or anti-rabbit Texas Red. In yet other experiments, MeCP2 antibody (Upstate Biotechnology) was detected using anti-rabbit FITC and in a subset of experiments the process was repeated using Brca1 antibody detected with anti-mouse Texas Red. Finally, the cells were fixed with 4% paraformaldehyde in KCM for 15 min and washed three times with KCM. The nuclei were counterstained with DAPI and viewed with a Leica DMR fluorescent microscope. Images were captured with Quips mFISH software (Vysis).
Simultaneous immunolabeling of histone H3 trimethyl-lysine 4 and DNA FISH for sequence flanking the Xist gene:
We detected histone H3 (trimethyl-K4) using a rabbit antibody to H3 (Abcam), followed by a goat antibody to rabbit conjugated with Texas Red (Jackson ImmunoResearch). The immunodetection of histone was combined with DNA FISH as described (Brown et al. 2001) with the following modifications: we labeled the P1 clone pπJL1 (Lee et al. 1999) with digoxigenin by nick translation (Dig–Nick translation mix; Roche, Indianapolis) and detected the label with sheep anti-Digoxigenin-Fluorecein (Roche) and rabbit anti-sheep Fluorecein (Vector Laboratories).
Simultaneous immunolabeling of γ-H2AX and DNA FISH for sequence flanking the Xist gene:
Biotinylated dUTP-labeled DNA FISH probes were prepared by nick translation from BAC DNA and P1 DNA, both of which include the Xist gene and flanking DNA sequence. Cell lines XaXist-Δ21-kbXiXist-Δ21-kb-1.1 and XaXist-Δ21-kbXiXist-Δ21-kb-2.1 were maintained in DMEM containing 10% FBS and seeded on coverslips 1 day before the experiment. Cells on coverslips were fixed with 4% paraformaldehyde in PBS for 10 min followed by 5 min permeabilization with 0.5% Triton X-100 at room temperature. Primary antibody recognizing γ-H2AX (Upstate Biotechnology) was applied for 1 hr. Cells were washed with PBS three times before incubation with secondary antibody that was conjugated with FITC (Jackson ImmunoResearch Laboratories). Cells were then fixed again with methanol:acetic acid (3:1) at −20° for 40 min followed by dehydration in 70, 90, and 100% ethanol for 3 min each. Cells were denatured in 70% formamide/2× SSC at 85° for 30 min, cooled down with cold 70% ethanol, and dehydrated with 90 and 100% ethanol. The probe derived from BAC DNA or from P1 DNA was simultaneously denatured at 75° for 10 min in a hybridization mixture (DNA probe, Cot1DNA, hybridization buffer, and 50% formamide) and prehybridized at 37° for 20 min. The prehybridized probe was applied to the pretreated coverslip and incubated at 37° overnight. After a series of stringent washes, avidin–Texas Red was added to the coverslip and incubated for 1 hr. Signal was visualized using a Nikon Eclipse E800 microscope equipped with a SenSys cooled CCD camera (Photometrics, Tucson, AZ). Images were captured using Metamorph image acquisition software (Universal Imaging, Downingtown, PA).
RESULTS
Histone H4 acetylation and histone H3 lysine-4 methylation accumulate on the inactive X chromosome when 21 kb are excised from both copies of the Xist gene:
To examine the role of the Xist locus (Figure 1A, top) in the maintenance of chromatin structure, three female immortalized murine embryonic fibroblast (MEF) cell lines were obtained, from three different E13.5 embryos, in which 21 kb of sequence at the Xist gene were flanked by Lox sites (Figure 1A, middle) on both the Xa and the Xi (lines XaXist-floxXiXist-flox-1, XaXist-floxXiXist-flox-2, and XaXist-floxXiXist-flox-3). The three lines were treated with adenovirus expressing cre recombinase and GFP and were also infected with adenovirus expressing only GFP. Limiting dilution was used to obtain clonal cells that were homozygous for the 21-kb deletion (XaXist-Δ21-kbXiXist-Δ21-kb) (Figure 1A, bottom) or were XaXist-floxXiXist-flox (from the adeno-GFP infections). The cell lines were chosen such that each pair of cell lines with the same number was derived from the same embryo (e.g., lines XaXist-floxXiXist-flox-1.1 and XaXist-Δ21-kbXiXist-Δ21-kb-1.1 are derived from GFP-adenovirus and cre-adenovirus infections of line XaXist-floxXiXist-flox-1, respectively). Using the same procedure, MEF lines XaXist-Δ21-kbXiXist-WT-1.1, XaXist-WTXiXist-Δ21-kb-1.1, and XaXist-Δ21-kbXiXist-WT-1.1 were obtained. PCR (Figure 1A, right) and FISH (see below) were used to determine the genotypes of the cell lines (see materials and methods).
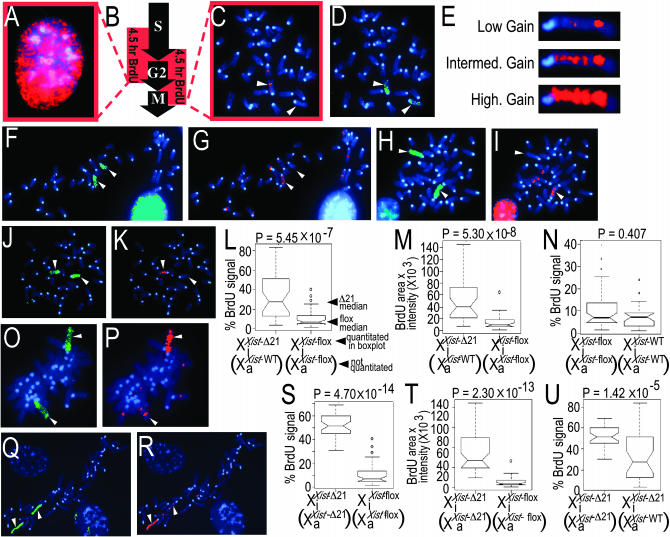
Figure 1.—
Accumulation of histone H4 acetylation and histone H3 lysine-4 methylation on the inactive X chromosome in XaXist-Δ21-kbXiXist-Δ21-kb cells. (A) Map of the Xist-WT, Xist-flox, and Xist-Δ21-kb alleles (left) and identification of the three alleles by PCR genotyping (right) (see materials and methods for details regarding genotyping). (B–G, J, and K) Chromosomes were immunostained using an antibody that recognizes acetylated lysines on histone H4 or an antibody against trimethylated lysine-4 on histone H3 and were also stained with DAPI and subjected to X chromosome paint or DNA FISH using an X chromosome-specific probe as indicated. Arrowheads mark X chromosomes. X chromosome paint hybridized throughout the X chromosome and also to the centromeres of all chromosomes in MCS. (B) XaXist-floxXiXist-flox MCSs fluorescently immunostained for acetylated lysines on histone H4 (Texas Red, red): (a) XaXist-floxXiXist-flox-1; (b) XaXist-floxXiXist-flox-2; (c) XaXist-floxXiXist-flox-2. The XaXist-floxXiXist-flox-1 MCS was also subjected to X chromosome paint (green) (a, right ). (C) XaXist-Δ21-kbXiXist-Δ21-kb MCSs immunostained for acetylated lysines on histone H4 (Texas Red, red): (a) XaXist-Δ21-kbXiXist-Δ21-kb-1.1, (b) XaXist-Δ21-kbXiXist-Δ21-kb-2.1, and (c) XaXist-Δ21-kbXiXist-Δ21-kb-3.1. MCS from a XaXist-Δ21-kbXiXist-WT-1 cell (D) and a XaXist-WTXiXist-Δ21-kb-1 cell (E) immunostained for acetylated lysines on histone H4 (FITC, green). (F) MCSs from XaXist-floxXiXist-flox cells immunostained for methylated lysine-4 on histone H3 (red): (a) XaXist-floxXiXist-flox-1; (b) XaXist-floxXiXist-flox-2; (c) XaXist-floxXiXist-flox-3. The XaXist-floxXiXist-flox-1 MCS was also subjected to X chromosome paint (green) (a, right). (G) MCSs from XaXist-Δ21-kbXiXist-Δ21-kb cells immunostained for methylated lysine-4 on histone H3 (red): (a) XaXist-Δ21-kbXiXist-Δ21-kb-1.1; (b) XaXist-Δ21-kbXiXist-Δ21-kb-2.1; (c) XaXist-Δ21-kbXiXist-Δ21-kb-3.1. The XaXist-Δ21-kbXiXist-Δ21-kb-1.1 MCS was also subjected to X chromosome paint (green) (a, right). Xist RNA FISH against a XaXist-WTXiXist-WT cell (H) and a XaXist-Δ21-kbXiXist-Δ21-kb cell (I). XaXist-flox, Hprt-ΔXiXist-flox, Hprt-WT (J) and XaXist-Δ21-kb,Hprt-ΔXiXist-Δ21-kb,Hprt-WT (K) MEFs from two different mouse matings (a and b) were subjected to DNA FISH using a P1-derived probe that recognizes the X chromosome and simultaneously stained with DAPI (top, blue) and immunostained for trimethylated lysine-4 on histone H3 (bottom, red). (L) XaXist-Δ21-kb,Hprt-ΔXiXist-Δ21-kb,Hprt-WT MEFs were subjected to DNA FISH using a probe that recognizes DNA sequence that is exclusively on the inactive X chromosome (red) and a probe that recognizes both X chromosomes (green). The XaXist-Δ21-kb,Hprt-ΔXiXist-Δ21-kb,Hprt-WT MEFs “a” and “b” represented in K and L are derived from the XaXist-flox,Hprt-ΔXiXist-flox,Hprt-WT MEFs “a” and “b” respectively represented in J via exposure to cre recombinase.
We examined the state of histone acetylation in these cell lines by fluorescence immunostaining of MCSs. Similar to previously published results for wild-type female cells (Jeppesen and Turner 1993), the Xi was readily distinguishable from the other chromosomes due to its severe hypoacetylation of histone H4 in MCSs from cell lines XaXist-floxXiXist-flox-1, -2, and -3 (Figure 1B) and in MCSs from lines XaXist-floxXiXist-flox-1.1, -2.1, and -3.1 (not shown). The Xi was also hypoacetylated on histone H4 in MCSs from XaXist-Δ21-kbXiXist-WT cells (Figure 1D), XaXist-WTXiXist-Δ21-kb cells (Figure 1E), and XaXist-floxXiXist-Δ21-kb cells (not shown) in agreement with previously published results (Csankovszki et al. 1999). In contrast, in MCSs from the three XaXist-Δ21-kbXiXist-Δ21-kb cell lines (1.1, 2.1, and 3.1), an inactive X chromosome could no longer be distinguished from the other chromosomes on the basis of hypoacetylation (Figure 1C).
The Xi was also severely hypomethylated at lysine 4 of histone H3 in MCSs from the cell lines XaXist-floxXiXist-flox-1, -2, and -3 (Figure 1F) and in the cell lines XaXist-floxXiXist-flox-1.1, 2.1, and 3.1 (not shown), as was previously reported for wild-type female cells (Boggs et al. 2001). In contrast, in MCSs from all three XaXist-Δ21-kbXiXist-Δ21-kb cell lines (1.1, 2.1, and 3.1), a Xi could not be distinguished from the other chromosomes on the basis of hypomethylation (Figure 1G). RNA FISH revealed that, in contrast to the Xist RNA seen in XaXist-WTXiXist-WT cells (Figure 1H), no XIST RNA signal whatsoever was seen in XaXist-Δ21-kbXiXist-Δ21-kb cells (Figure 1I). To determine whether the excision of 21 kb from both Xist alleles causes the Xi to be lost from cells, we examined XaXist-Δ21-kbXiXist-Δ21-kb cell lines from three embryos that carried a deletion at the Hprt locus (Hooper et al. 1987) on the Xa, while the Hprt locus on the Xi was intact. DNA FISH for a region of the X chromosome in combination with immunofluorescence indicated that one of the two X chromosomes was hypomethylated at lysine-4 on histone H3 in XaXist-flox, Hprt-ΔXiXist-flox, Hprt-WT cells (Figure 1J), indicating that the Hprt deletion did not disrupt H3 lysine-4 hypomethylation on the Xi. In contrast, an X chromosome that was hypomethylated at H3 lysine-4 was absent from in the corresponding XaXist-Δ21-kb,Hprt-ΔXiXist-Δ21-kb,Hprt-WT cells (Figure 1K), as expected. Two-color DNA FISH was performed to verify the presence of the inactive X chromosome in the XaXist-Δ21-kb,Hprt-ΔXiXist-Δ21-kb,Hprt-WT cells. A Cy3-labeled (red) combination probe, comprising a mixture of seven separate probes to the deleted region of Hprt, was used to identify the inactive X chromosome (see materials and methods for details). A FluorX (green)-labeled mouse BAC (RP23-298N24) was used as a probe to mark both the inactive and the active X chromosomes. Cells, hybridized with both these probes together, when examined confirmed the presence of the inactive X chromosome. For one predominantly diploid XaXist-Δ21-kb,Hprt-ΔXiXist-Δ21-kb,Hprt-WT cell line, 94 of 100 cells examined showed red hybridization signal in their nuclei (Figure 1L, a). Similarly, 96 of 100 cells showed a red hybridization dot in another XaXist-Δ21-kb,Hprt-ΔXiXist-Δ21-kb,Hprt-WT cell line derived from a different embryo (Figure 1L, b). For both cell lines, most cells had one red dot near one of the two green dots. Also, we occasionally observed cells (∼5%) that were tetraploid for the X chromosome—having four green dots and two red dots near two of the green dots (not shown). We conclude that the Xi is still present in cells that have excised 21 kb of Xist sequence from both the Xa and the Xi and the homozygous deletion of Xist sequence results in the Xi acquiring H3 lysine-4 methylation and H4 acetylation along its length. Additional lines of evidence that indicate that the Xi is retained in XaXist-Δ21-kbXiXist-Δ21-kb cells are presented below.
The dramatic chromatin changes observed on the inactive X chromosome in XaXist-Δ21-kbXiXist-Δ21-kb cells raised the question of whether Xist RNA was expressed from the 3′ undeleted portion of the Xist gene of either X chromosome in XaXist-Δ21-kbXiXist-Δ21-kb cells. To determine whether a truncated Xist RNA was expressed, quantitative determination of Xist mRNA was performed by RT–FRET real-time PCR using PCR primers that transversed the boundaries of exons 4, 5, and 6, respectively, and a FRET probe that recognized exon 5. The internal autosomal reference gene transcript used was PBGD (Wang et al. 2004). In two XaXist-WTXiXist-WT cell lines, the Xist mRNA/PBGD mRNA ratios were 720.3 and 699.1. In contrast, the Xist mRNA/PBGD mRNA ratios in two XaXist-Δ21-kbXiXist-Δ21-kb cell lines were 0.0 and 0.0 because only PBGD mRNA, but no Xist RNA, was detected in these samples. A comprehensive and detailed analysis of Xist RNA levels in cells bearing various Xist genotypes will be published elsewhere (J. L. Salstrom, C. Wang, C. Wang, A. Datta, S. Zeitlin, G. Csankovszki, C. D. Eller, S. Diaz-Perez, J. Wang, A. Chess, S. Huang, B. Kaltenboeck and Y. Marahrens, unpublished data).
Altered replication time on the inactive X chromosome in response to 21-kb deletions:
Since the Xi had acquired two euchromatic properties (H4 acetylation and H3 lysine-4 methylation) in XaXist-Δ21-kbXiXist-Δ21-kb cells we predicted that the deletions would also cause the Xi to replicate earlier in S phase since euchromatin generally replicates earlier in S phase than heterochromatin (Gilbert 2002). To determine if this was the case, we purified approximately diploid lines XaXist-Δ21-kbXiXist-Δ21-kb-1.2, -2.2, and -3.2 from cre-adenovirus-infected XaXist-floxXiXist-flox progenitor lines (1, 2, and 3) using limiting dilution. We also purified predominantly diploid lines XaXist-floxXiXist-flox-1.2, -2.2, and -3.2 from GFP-adenovirus-infected XaXist-floxXiXist-flox progenitor lines (1, 2, and 3). Each pair of diploid cell lines with the same first number was derived from the same embryo (e.g., lines XaXist-floxXiXist-flox-1.2 and XaXist-Δ21-kbXiXist-Δ21-kb-1.2 are derived from line XaXist-floxXiXist-flox-1). Using the same procedure, the predominantly diploid lines XaXist-Δ21-kbXiXist-WT-1.2, XaXist-WTXiXist-Δ21-kb-1.2, and XaXist-Δ21-kbXiXist-WT-1.2 MEF lines were also obtained. All diploid lines in this study were used at a low passage number (with respect to limiting dilution) in our analyses because they increasingly accumulated cells that had lost their diploid character with repeated passages.
The predominantly diploid cell lines were subjected to a replication-timing assay that uses fluorescence immunostaining to detect chromosomal regions that incorporated BrdU late in S phase. Cells were pulse labeled with BrdU for 4.5 hr and metaphase chromosome spreads were prepared. Cells that were in mid-S phase at the onset of BrdU addition (Figure 2B) incorporated BrdU in mid- and late S phase but did not reach mitosis in the 4.5-hr interval (Figure 2A) and therefore were not represented among the MCSs. Cells that were in late S phase at the onset of BrdU addition (Figure 2B) incorporated BrdU in late S phase, reached mitosis in the 4.5-hr interval, and the incorporated BrdU was detected in the MCSs (Figure 2C). Identification of X chromosomes using X chromosome paint (Figure 2D) revealed that one of the two X chromosomes in female cells consistently displayed more BrdU signal than the other X chromosome and more signal than most or all autosomes. Although the mouse Xi does not replicate nearly as late in S phase in mouse cells as in human cells, it nevertheless replicates later in S phase than the active X chromosome (Evans et al. 1965; Galton and Holt 1965; Tiepolo et al. 1967). We consequently inferred that the later replicating X chromosome in our female cultures was the Xi and that the earlier replicating X chromosome was the Xa. The level of BrdU signal that was recorded in a photograph of an X chromosome in a MCS depended, in part, on the gain setting of the red channel of the mQuips Vysis software that was used to display the BrdU signal. At low gain, only the fluorescent signal representing incorporated BrdU that exceeds a high intensity threshold is recorded in the image of an X chromosome (Figure 2E, top). At intermediate gain, the fluorescent BrdU signal exceeding an intermediate intensity threshold is recorded in the image (Figure 2E, middle). At high gain, even small amounts of incorporated BrdU will be displayed among the pixels representing the BrdU signal (Figure 2E, bottom). To normalize the BrdU signal, all photographs of metaphase chromosome spreads were taken at a standardized “low-gain” setting where only five autosomes display BrdU signal and one of these five autosomes displays only one pixel of BrdU. This method of normalizing the gain using BrdU signal on autosomes was previously used to show that portions of the Xa replicate later in S phase if 21 kb are deleted from the Xist locus of the Xa and the method is described in more detail in this earlier study (Diaz-Perez et al. 2005). In addition, only MCSs displaying two X chromosomes were considered. At the measurements taken at low gain, all three clonal XaXist-flox XiXist-flox fibroblast lines (1.2, 2.2, and 3.2) displayed modest BrdU signal on one X chromosome and either very little (Figure 2, C and D) or no (Figure 2, F and G) BrdU signal on the other X chromosome and therefore closely resembled the signal levels seen in XaXist-WTXiXist-WT MCSs (Diaz-Perez et al. 2005). Contrary to expectation, more BrdU signal (late replication) was observed on the inactive X chromosome in XaXist-WTXiXist-Δ21-kb-1.2 cells (Figure 2, H–K) and XaXist-floxXiXist-Δ21-kb-1.2 cells (not shown) than in the XaXist-floxXiXist-flox cells. Quantitation of the BrdU signal on the Xi in 40 XaXist-WTXiXist-Δ21-kb spreads and 40 XaXist-floxXiXist-flox spreads using NIH IMAGE software revealed significantly more BrdU signal on the XiXist-Δ21-kb than on the XiXist-flox by percentage of area displaying BrdU signal (P = 5.45 × 10−7) (Figure 2L) or when multiplying this area by the average intensity of the signal (P = 5.30 × 10−8) (Figure 2M). In contrast, no significant difference was detected on the Xa in the same spreads regardless of whether percentage of area occupied by BrdU signal (P = 0.655) or area times intensity (P = 0.377) was considered (box plots not shown). However, this quantitation was from a single XaXist-WTXiXist-Δ21-kb cell line and should therefore be considered preliminary. No significant difference in BrdU incorporation levels on the inactive X chromosome was seen when comparing XaXist-floxXiXist-flox cells to XaXist-WTXiXist-WT cells (P = 0.407) (Figure 2N), indicating that the presence of the Lox sites in the absence of the 21-kb deletion did not have a measurable effect on replication time. The Xi therefore may replicate overall later in S phase when it is harboring the 21-kb deletion. Furthermore, heterozygosity for the Xist deletion causes the X chromosome harboring the deletion to be replicated later than normal in S phase but does not affect the replication time of the wild-type X chromosome, regardless of whether the mutation is on the Xa (Diaz-Perez et al. 2005) or on the Xi.
Figure 2.—
Deletion of 21 kb from the Xist gene on the Xa and the Xi cause the Xi to be replicated later in S phase. Actively growing MEF cultures were exposed to BrdU for 4.5 hr, and MCSs were prepared and immunostained using anti-BrdU (red), hybridized to X-chromosome paint (green), and counterstained using DAPI (blue). In all MCSs, the active X chromosome is always assumed to be the X chromosome displaying the lower proportion of BrdU incorporation and the two X chromosomes are marked by arrowheads. In each photograph of a MCS displaying BrdU, the gain has been adjusted such that five autosomes display BrdU incorporation. (A–D) XaXist-floxXiXist-flox-1.2: (A) interphase cell; (B) possible times in the cell cycle that the indicated fibroblasts were inferred to be exposed to BrdU; (C and D) XaXist-floxXiXist-flox-1.2 MCS. (E) XiXist-flox from an XaXist-floxXiXist-flox MCS displayed at low gain (top), intermediate gain (middle), and high gain (bottom). Only the most intense BrdU signal is visible at a standardized low-gain setting that was used throughout (see text). (F and G) XaXist-floxXiXist-flox-3.2. (H and I) XaXist-WTXiXist-Δ21-1.2. (J and K) XaXist-WTXiXist-Δ21-1.2. (L and M) Quantitation of BrdU signal on the Xi in 40 XaXist-floxXiXist-flox and 40 XaXist-WTXiXist-Δ21 MCSs (>10 MCSs from each of the lines) summarized in box plots that display percentage of BrdU signal on the Xi, which represents the number of pixels of BrdU signal divided by the number of pixels of DAPI signal multiplied by 100 (L) or percentage of BrdU signal multiplied by average intensity of the BrdU signal (M). (N) Quantitation of BrdU signal in 40 XaXist-floxXiXist-flox MCSs (>10 MCSs from each of the three lines) and 17 XaXist-WTXiXist-WT MCSs (7 and 10 MCSs from each of two lines) summarized in box plots that display percentage of BrdU signal on the Xi. (O and P) XaXist-Δ21XiXist-Δ21-1.2 MCS. (Q and R) XaXist-Δ21XiXist-Δ21-3.2 MCS. (S and T) Quantitation of BrdU signal in the Xi from 40 XaXist-floxXiXist-flox and 40 XaXist-Δ21XiXist-Δ21 spreads (>10 MCSs from each of the six lines) using box plots that display percentage of BrdU signal (S) and percentage of BrdU signal multiplied by average intensity of the BrdU signal for the Xi (T). (U) Box plots that compare the Xi BrdU signal between 40 XaXist-WTXiXist-Δ21 spreads and 40 XaXist-Δ21XiXist-Δ21 spreads. P-values were obtained using the Kruskal–Wallis test (Kruskal 1964).
The effect of deleting the 21 kb from both Xist alleles was next investigated using the same assay. The earlier replicating Xa displayed more BrdU signal in spreads from the three XaXist-Δ21-kbXiXist-Δ21-kb lines (Figure 2, O–R) than in the XaXist-floxXiXist-flox cell lines (Figure 2, C, D, F, and G), in agreement with earlier results obtained using XaXist-Δ21-kbXiXist-WT cells (Diaz-Perez et al. 2005). In XaXist-Δ21-kbXiXist-Δ21-kb MCSs the Xi also displayed higher levels of BrdU incorporation (Figure 2, O–R) than in XaXist-floxXiXist-flox cell lines (Figure 2, C, D, F, and G). This difference was readily apparent when 40 inactive X chromosomes were quantitated from each cell line (Figure 2, S and T). We conclude that excision of 21 kb from the Xist gene of the Xi causes the Xi to be replicated later in S phase. Finally, preliminary data indicated that the Xi displayed a significantly higher proportion of incorporated BrdU when 21 kb was deleted from both Xist alleles (Figure 2, O–R and U) than when the deletion was exclusively on the Xi in XaXist-WTXiXist-Δ21-kb-1.2 (Figure 2, H–K and U) and XaXist-floxXiXist-Δ21-kb-1.2 cells (not shown). This suggested that elements at both Xist alleles may influence the extent of BrdU signal on the Xi in the assay for late replication.
Relationship between the pattern of late S-phase BrdU incorporation and the concentration of LINE-1 sequence on the XaXist-Δ21-kb of XaXist-Δ21-kbXiXist-Δ21-kb cells:
When the XaXist-Δ21-kbXiXist-Δ21-kb MCSs were examined at a higher gain than in Figure 2, the Xi displayed BrdU signal throughout its length (Figure 3, A and B) while the Xa displayed four to six regions of concentrated BrdU signal in 33 of the 40 XaXist-Δ21-kbXiXist-Δ21-kb-2.2 MCSs examined (Figure 3, A–C). This pattern was also prevalent on the Xa in XaXist-Δ21-kbXiXist-Δ21-kb -1.2 and -3.2 cells (not shown). Four of the five regions displaying late replication on the Xa were found to correspond to the four regions along the Xa that are most heavily enriched for LINE-1 elements (Figure 3D). The fifth region on the Xa that displayed late replication was the pericentromeric region for which the genome sequence was unavailable. In contrast to this reproducible pattern of BrdU signal seen on the Xa in XaXist-Δ21-kbXiXist-Δ21-kb cells, a consistent pattern was not readily apparent on the Xa in wild-type cells or when 21 kb was deleted only from one Xist allele (not shown) (Diaz-Perez et al. 2005). Therefore, although deletion of 21 kb exclusively from the Xist allele on the Xa causes the Xa to be replicated later in S phase (Diaz-Perez et al. 2005), the excision of 21 kb from both Xist copies further altered the replication timing in a manner that resulted in the regions with the highest concentrations of LINE-1 elements on the Xa being replicated later in S phase.
Figure 3.—
Relationship between the pattern of late S-phase BrdU incorporation and the concentration of LINE-1 sequence on the XaXist-Δ21-kb of XaXist-Δ21-kbXiXist-Δ21-kb MCSs. Images of the 40 XaXist-Δ21-kbXiXist-Δ21-kb spreads used in Figure 2 were obtained at a gain that was higher than that used in Figure 2. Red, BrdU; blue, DAPI stain of chromosomal DNA; green, X chromosome paint. (A and B) Active and inactive X chromosomes of a XaXist-Δ21-kbXiXist-Δ21-kb-2.2 spread displaying X chromosome paint (A) and BrdU signal (B). (C) Fourteen active X chromosomes from XaXist-Δ21-kbXiXist-Δ21-kb-2.2 spreads displaying BrdU signal. (D) Graph displaying the concentration of LINE-1 elements along the X chromosome superimposed on an image of a Xa displaying BrdU signal. The graph was generated using a Loess curve applied to RepeatMasker output provided by the UCSC genome browser. The DNA sequence of the centromere and pericentromeric region were not available and the coordinates along the X chromosome (x-axis) were measured starting at the centromere-proximal starting point of the available DNA sequence.
Evidence that the 21-kb Xist deletions destabilize the DNA of both X chromosomes:
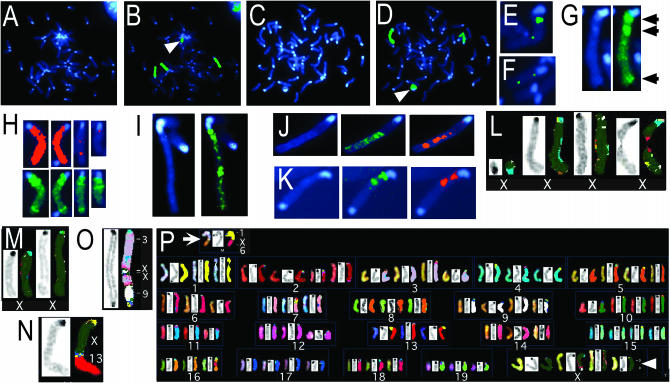
During our preliminary analyses we had encountered numerous XaXist-Δ21-kbXiXist-Δ21-kb MCSs displaying evidence of deletions or translocations involving the X chromosome. Such spreads were excluded from subsequent analyses of histone modifications and BrdU incorporation. To investigate the influence of the 21-kb deletion in the Xist gene on the incidence of deletions and translocations, it was necessary to prepare three XaXist-WTXiXist-WT MEF cell lines using the identical procedure as was used to produce XaXist-Δ21-kbXiXist-Δ21-kb cell lines. To this end, three XaXist-WTXiXist-WT MEF cell lines were obtained from three E13.5 129 embryos using the same procedure as was used to produce the three XaXist-floxXiXist-flox lines. All six lines were infected with adenovirus expressing cre recombinase and GFP and subjected to limiting dilution, and derivative cell lines were expanded from infected (GFP-expressing) cells using equal numbers of passages (see materials and methods). The cell lines were subjected to the replication-timing assay used for Figure 2 to distinguish the active and inactive X chromosomes. Inspection of MCSs from three XaXist-WTXiXist-WT cell lines derived from cre-adenovirus-infected cells revealed evidence for X chromosome deletions or translocations in 0 of 60 MCSs. Similarly, 0 of 68 MCSs from XaXist-floxXiXist-flox cells showed signs of aberrations in the X chromosome. In contrast, we saw evidence for deletions or translocations in 14 of 84 spreads (16.6%) from cultures of the three XaXist-Δ21-kbXiXist-Δ21-kb cell lines (1.1, 2.1, and 3.1). One of the most common abnormalities was a small fragment of the X chromosome (Figure 4, A and B). We also saw evidence of a ring X chromosome (Figure 4, C and D) and small portions of the X chromosome integrated into autosomes (Figure 4, E and F) but were not confident of the latter findings because the signal could be due to X chromosome paint hybridizing to autosomal material. By reducing the gain, the X chromosome paint was seen to display a characteristic pattern along the X chromosome that was highly reproducible among wild-type spreads (Figure 4G). Using this pattern as a guide, we observed that a frequent abnormality was the truncation of a Xi (not shown) or a Xa (Figure 4H) due to the loss of the centromere-distal tip of the X chromosome. We also observed a chromosome that appeared to be a duplication of the X chromosome (Figure 4I), an X chromosome that seemed to be linked to an autosome (Figure 4J), and what appeared to be dicentric chromosomes where a portion (Figure 4K) or all (not shown) of the chromosome was derived from the X chromosome.
Figure 4.—
Evidence that deletion of 21 kb from the Xist gene destabilizes the X chromosome. MCSs from fibroblasts were either subjected to an assay that identifies X chromosomes using X chromosome paint (B and D–K, green) and late replicating regions by fluorescent immunostaining for BrdU (H, J, and K, red) or subjected to spectral karyotyping (SKY) (L–P). Chromosomes from XaXist-Δ21-kbXiXist-Δ21-kb MCSs are shown, except in G, which shows an X chromosome from a wild-type MCS. Evidence for small X chromosomal fragments (A–D and L), X chromosomal DNA within autosomes [E, F, O, and P (arrow)], truncated X chromosomes (H and M), X chromosomal duplication (I), X–autosome translocations (J, K, and N), and a dicentric X chromosome (P, arrowhead) is shown. (P) Full karyotype of a XaXist-Δ21-kbXiXist-Δ21-kb MCS where each chromosome is represented three times with SKY hybridization (left), DAPI (middle), and computer-classified color (right). Note that SKY cannot identify the origin of centromeric/pericentromeric regions (oriented at the top of each chromosome), leading to frequent discolorations that do not represent translocations. The software also displays discolored regions at the edges (left or right sides) of chromosomes, which is a staining artifact rather than translocated material.
To confirm these findings and to determine whether autosomes in the XaXist-Δ21-kbXiXist-Δ21-kb cell lines also displayed abnormalities, SKY (Liyanage et al. 1996) was performed on spreads from cell lines XaXist-Δ21-kbXiXist-Δ21-kb-1.1 and XaXist-Δ21-kbXiXist-Δ21-kb-1.3. In agreement with the X chromosome paint, the size of the X chromosome varied within spreads, reflecting deletions or rearrangements of the X chromosome (Figure 4, L and M). In some metaphases structural rearrangements involving the X were identified. These included a simple nonreciprocal translocation involving chromosomes X and 13 (Figure 4N) and a dicentric structure resulting from fusion between two X chromosomes (Figure 4P, arrowhead). Involvement of the X in more complex rearrangements was also suggested by SKY. These included a dicentric chromosome involving segments of chromosomes 3 and 9 flanking two or more small segments of X (Figure 4O) and a translocation linking portions of chromosomes 1 and 6 with a small segment of X chromosome material at the junction (Figure 4P, arrow). Because the MCSs displaying specific chromosomal anomalies were not examined as clonal cell lines, no material exists to allow confirmation of the complex rearrangements, so it is a formal possibility that the X signal arose from intermixing between two SKY colors at the junction. A tally of the definitive simple rearrangements and deletions provided clear evidence of X chromosome instability in XaXist-Δ21-kbXiXist-Δ21-kb MCSs: in total, 13/80 karyotypes (16%) analyzed by SKY displayed structural abnormalities involving the X chromosome. In the same spreads, 0/80, 1/80, and 1/80 karyotypes displayed abnormalities involving chromosomes 3, 4, and 5, respectively, and did not involve the X chromosome. The abnormal chromosome 4 was a small fragment and the abnormal chromosome 5 was slightly shorter than normal and not a definitive abnormality.
Phosphorylation of p53 and H2AX and localization of γ-H2AX to the Xi in cells carrying the 21-kb deletion:
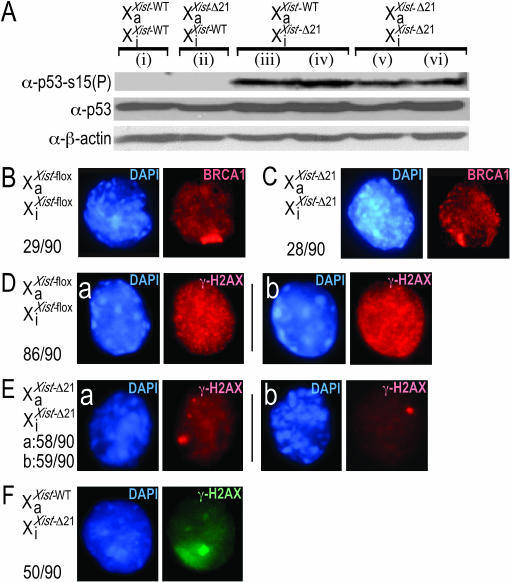
The frequent rearrangements suggested the presence of DNA damage on the X chromosome that might have arisen from replication stress. DNA damage would cause the ATR (Tibbetts et al. 1999) and/or ATM (Banin et al. 1998; Canman et al. 1998; Khanna et al. 1998) protein kinases to phosphorylate the p53 protein at serine-15. To determine whether the 21-kb deletion is associated with the phosphorylation of the p53, extracts were prepared from independent cell lines of each of the following genotypes: XaXist-WTXiXist-WT, XaXist-Δ21-kbXiXist-WT, XaXist-WTXiXist-Δ21-kb, and XaXist-Δ21-kbXiXist-Δ21-kb. Western blots using these extracts revealed elevated levels of serine-15 phosphorylated p53 protein in all cell lines that carried the XiXist-Δ21-kb compared to XaXist-WTXiXist-WT cells (Figure 5A) while overall levels of p53 remained approximately the same (Figure 5A). Although the simplest explanation is that p53 is phosphorylated by ATR, we acknowledge that ATM (Paull et al. 2000; Burma et al. 2001; Stiff et al. 2004) or another kinase might play a role.
Figure 5.—
Phosphorylation of p53 and H2AX and increased localization of γ-H2AX to the Xi in cells carrying the 21-kb deletion in the Xist gene. (A) p53 is phosphorylated on serine-15 in cells that carry the Xist-Δ21-kb allele on the Xi. Western blot probed for p53 phosphorylated at serine-15 (top), for total p53 (middle), and for β-actin (loading control, bottom) in extracts from XaXist-WTXiXist-WT-1.2 (i), XaXist-Δ21-kbXiXist-WT-1.2 (ii), XaXist-WTXiXist-Δ21-kb-1.1 (iii), XaXist-WTXiXist-Δ21-kb-2.1 (iv), XaXist-Δ21-kbXiXist-Δ21-kb-2.2 (v), and XaXist-Δ21-kbXiXist-Δ21-kb-3.2 (vi) cells. (B–F) Brca1 or γ-H2AX immunolocalization in female cells using FITC (green) or Texas Red (red) conjugated antibodies. Blue, DAPI. (B and C) Brca1 signal in XaXist-Δ21-kbXiXist-Δ21-kb cells: (B) XaXist-flox XiXist-flox-1.2 cell displaying DAPI (left) and Brca1 (right); (C) XaXist-Δ21-kbXiXist-Δ21-kb-1.2 cell displaying DAPI (left), and Brca1 (right). (D–F) γ-H2AX displays a concentrated signal in the majority of XaXist-Δ21-kbXiXist-Δ21-kb and XaXist-WTXiXist-Δ21-kb cells but only in 4.4% of XaXist-floxXiXist-flox cells: (D) a XaXist-floxXiXist-flox-1.2 cell (a) and a XaXist-floxXiXist-flox-2.2 cell (b) displaying DAPI (left side for each) and γ-H2AX (right side for each); (E) a XaXist-Δ21-kbXiXist-Δ21-kb-1.2 cell (a) and a XaXist-Δ21-kbXiXist-Δ21-kb-2.2 cell (b) displaying DAPI (left) and γ-H2AX (right); (F) a XaXist-WTXiXist-Δ21-kb-1.2 cell displaying DAPI (left) and γ-H2AX (right). Note that a higher gain was used in D than in E for the γ-H2AX images to show that the signal representing the γ-H2AX domain was not present at reduced intensity in D.
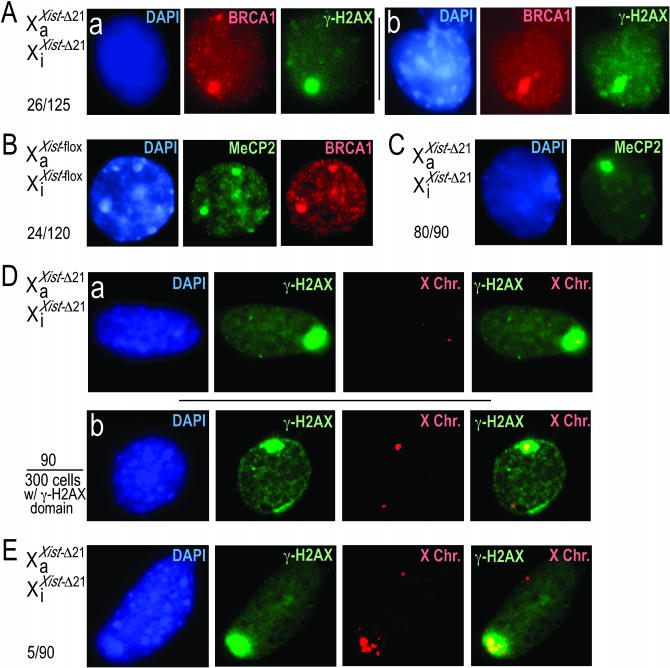
Brca1 localizes to the Xi, associates with Xist RNA, fosters the Xist RNA signal at the Xi, and is involved in X-inactivation (Ganesan et al. 2002, 2004). Brca1 is also involved in the maintainance of genome stability (Moynahan et al. 2001; Weaver et al. 2002; Bruun et al. 2003). Our finding that Xi DNA is unstable in XaXist-Δ21-kbXiXist-Δ21-kb cells raised the issue of whether the Brca1 signal is still present in XaXist-Δ21-kbXiXist-Δ21-kb cells. Brca1 has been reported to concentrate on the Xi primarily in S-phase cells (Ganesan et al. 2002; Chadwick and Lane 2005). Immunostaining of XaXist-floxXiXist-flox cells for Brca1 revealed a large domain of concentrated Brca1 protein (Figure 5B) in 29/90 XaXist-floxXiXist-flox cells (32.2%) and in 24/90 XaXist-WTXiXist-WT cells (26.7%). XaXist-Δ21-kbXiXist-Δ21-kb cells displayed the concentrated Brca1 signal (Figure 5C) in 28/90 cells (31.1%), suggesting that loss of the Xist sequence (including Xist RNA) did not affect the concentration of Brca1 to the Xi.
The delayed replication times and DNA instability on both X chromosomes in XaXist-Δ21-kbXiXist-Δ21-kb cells may be due to replication stress. A number of expressed fragile sites are known to be replicated late in S phase (Hansen et al. 1993; Wang et al. 1999; Hellman et al. 2000; Arlt et al. 2003) and the ATR kinase maintains fragile site stability (Casper et al. 2002). Replication stress triggers the ATR kinase to phosphorylate the histone H2A variant H2AX to produce γ-H2AX and also causes DNA instability (Ward et al. 2004). Immunofluorescence failed to detect large regions of concentrated γ-H2AX resembling the Brca1 signal in the three XaXist-floxXiXist-flox cell lines (Figure 5D) or in XaXist-WTXiXist-WT cells (not shown) except in a low proportion of cells (4/90 XaXist-floxXiXist-flox cells and 4/90 XaXist-WTXiXist-WT cells), consistent with a report that γ-H2AX associates with the Xi in wild-type cells exclusively in late S phase (Chadwick and Lane 2005). In contrast, a region of γ-H2AX concentration was observed in 58/90 (64%) and 59/90 (65%) in two XaXist-Δ21-kbXiXist-Δ21-kb cell lines examined (Figure 5E, a and b, respectively) and in 50/90 (56%) XaXist-WTXiXist-Δ21-kb cells (Figure 5F). Twenty-six of 125 (21%) XaXist-Δ21-kbXiXist-Δ21-kb cells displaying the γ-H2AX signal also displayed a Brca1 signal, a marker of the Xi (Ganesan et al. 2002) (Figure 6A). In an attempt to identify a heterochromatic feature other than Brca1 or late replication that was retained by the Xi in XaXist-Δ21-kbXiXist-Δ21-kb cells we also considered MeCP2, which binds methylated DNA (Meehan et al. 1992) but for which localization to the hypermethylated Xi has not been addressed. We found a distinct domain of concentrated MeCP2 in 83/90 (92.5%) of diploid (not shown) and tetraploid (not shown) XaXist-WTXiXist-WT cells. Twenty-four of 120 (20%) of XaXist-WTXiXist-WT cells displaying a MeCP signal also displayed a Brca1 signal (Figure 6B). XaXist-WTXiXist-Δ21-kb (not shown) and XaXist-Δ21-kbXiXist-Δ21-kb (Figure 6C) cells retained the MeCP2 signal in approximately the same proportion of cells (84/90, 93.5% and 80/90, 88.9% respectively), indicating that the 21-kb deletions did not notably affect the intense localization of MeCP2. Since MeCP2 is not an established marker of the Xi these data did not conclusively show that the γ-H2AX signal localized to the X chromosome.
Figure 6.—
Concentrated γ-H2AX signal observed in XaXist-Δ21-kbXiXist-Δ21-kb cells localizes to the inactive X chromosome. (A) γ-H2AX signal colocalizes with the Brca1 signal in XaXist-Δ21-kbXiXist-Δ21-kb cells: (a) a XaXist-Δ21-kbXiXist-Δ21-kb-1.2 cell displaying DAPI (left), Brca1 (center), and γ-H2AX (right); (b) a XaXist-Δ21-kbXiXist-Δ21-kb-2.2 cell displaying DAPI (left), Brca1 (center), and γ-H2AX (right). (B) A concentrated MeCP2 signal in XaXist-floxXiXist-flox cells colocalizes with the Brca1 signal. (C) A concentrated MeCP2 signal is retained in XaXist-Δ21-kbXiXist-Δ21-kb cells. (D) The concentrated γ-H2AX signal in XaXist-Δ21-kbXiXist-Δ21-kb cells colocalizes with X chromosomal DNA: a XaXist-Δ21-kbXiXist-Δ21-kb-1.2 cell (a) and a XaXist-Δ21-kbXiXist-Δ21-kb-2.2 cell (b) display, in each case, DAPI (left), γ-H2AX (center left ), DNA FISH signal recognizing the X chromosome (center right), and the γ-H2AX and DNA FISH signals merged (right). (E) Example of a rare XaXist-Δ21-kbXiXist-Δ21-kb cell where the DNA FISH signal recognizing the Xic region on the X chromosome and colocalizing with the γ-H2AX domain is greatly enlarged, suggesting multiple duplications involving the Xic; a XaXist-Δ21-kbXiXist-Δ21-kb-2.2 cell is shown. The fractions represent the proportion of the cells displaying a γ-H2AX or a MeCP2 signal that simultaneously display a colocalized BRCA1 or X-chromosome-specific DNA FISH signal.
To definitively determine whether the γ-H2AX signal in XaXist-Δ21-kbXiXist-Δ21-kb cells colocalized with the X chromosome, DNA FISH specific to the Xic (a region on the X chromosome that encompasses the Xist gene) was performed in conjunction with immunostaining for γ-H2AX. A total of 300 XaXist-Δ21-kbXiXist-Δ21-kb cells displaying the large γ-H2AX signal (as reported earlier in Figure 5E) were scored for the additional presence of a superimposed Xic DNA FISH signal. Ninety of the 300 XaXist-Δ21-kbXiXist-Δ21-kb cells displaying the large γ-H2AX signal (30.0%) displayed a superimposed Xic DNA FISH signal (Figure 6D). Interestingly, 5 of these 90 XaXist-Δ21-kbXiXist-Δ21-kb cells displayed one greatly enlarged Xic signal that always colocalized with the γ-H2AX domain, suggesting that multiple duplications of Xic sequence had occurred (Figure 6E). In addition to the 90 cells displaying superimposed signals, 47 of the 300 cells (16%) displayed a γ-H2AX signal that touched but did not encompass the Xic DNA FISH signal (not shown), bringing the overall γ-H2AX–Xic association to 46% among cells displaying a large γ-H2AX signal. The high proportion of cells with immediately adjacent γ-H2AX and DNA FISH signals may be due to γ-H2AX being associated with a subset of the X chromosome that does not include the Xic. Finally, 44 of the 300 cells (15%) showed a γ-H2AX domain that was clearly separate from the Xic DNA FISH signals (not shown). The remaining cells could not be scored with confidence due to uneven signals, uncertain signal number, or absence of DNA FISH signal. A total of 19.5% of all XaXist-Δ21-kbXiXist-Δ21-kb cells displayed a clear γ-H2AX signal that encompassed (90/703) or touched (47/703) a clear Xic DNA FISH signal. We conclude that the X chromosome instability, delayed replication timing, and p53 phosphorylation that is brought on by excision of 21 kb from both Xist alleles are accompanied by an increase in γ-H2AX on the X chromosome.
We consider the aforementioned colocalization numbers to be very conservative because, when γ-H2AX immunostaining was combined with X chromosome-specific DNA FISH, the γ-H2AX signal appeared to be degraded in a subset of cells and cells with degraded γ-H2AX signal were not scored as “superimposed” or “immediately adjacent”. [In contrast >90% (654/703) of all cells displayed DNA FISH signals, so FISH was relatively unaffected.] Consistent with the idea that degradation reduced our percentages, γ-H2AX/DNA FISH colocalization was markedly higher when only the 36 cells (of the 300 cells) with the most pronounced γ-H2AX domains were considered. Among these 36 cells, 19 (53%) had the Xic DNA FISH signal encompassed by the γ-H2AX signal, 9 (25%) displayed directly adjacent γ-H2AX and Xic DNA FISH signals, and 8 (22%) showed separated γ-H2AX and DNA FISH signals. However, the increased colocalization of X-chromosome-specific DNA FISH signal with the 36 most pronounced γ-H2AX domains should be interpreted with caution as the level of colocalization involving these γ-H2AX domains may not be representative of all γ-H2AX domains but may reflect a qualitatively distinct subpopulation that exhibits higher colocalization for another reason(s). For example, they may represent cells in a particular portion of the cell cycle that are characterized by resistance to signal degradation and γ-H2AX domain colocalization with the Xic. Cells with spacially distinct γ-H2AX and Xic DNA FISH signals may contain X chromosomes that have lost the Xic region (a plausible explanation since the Xic appears to be unstable; Figure 6E). In addition, some γ-H2AX domains may be associated with autosomes. For example, there is evidence that 8–23% of autosomal genes are subject to a random but coordinated inactivation process that resembles X-inactivation (Gimelbrant and Chess 2006). It is not known whether any autosome contains a higher proportion of inactivated genes, like the X chromosome. Since in Xist-WT cells γ-H2AX associates with the Xi exclusively in late S phase (Chadwick and Lane 2005), we cannot exclude the possibility that γ-H2AX can also transiently form a strong signal on an autosome that has a high abundance of inactivated genes.
DISCUSSION
We show that in female mouse cells, the excision of 21 kb from the Xist gene of both the Xa and the Xi (XaXist-Δ21-kbXiXist-Δ21-kb) resulted in the appearance of two histone modifications throughout the Xi that are generally associated with euchromatin: histone H4 acetylation and methylation on lysine-4 of histone H3. Despite the appearance of these euchromatic histone modifications, the inactive X chromosome of XaXist-Δ21-kbXiXist-Δ21-kb cells displayed abundant DNA replication that was very late in S phase and stood in contrast with the moderately late replication that normally predominates on the Xi in mouse cells (Nesbitt and Gartler 1970). The active X chromosome of XaXist-Δ21-kbXiXist-Δ21-kb cells also displayed a shift to later replication that was predominantly in chromosomal regions associated with high concentrations of LINE-1 elements. The X chromosomes of XaXist-Δ21-kbXiXist-Δ21-kb cells were unstable and prone to deletions and translocations. The X chromosome instability was accompanied by the phosphorylation of p53 at serine-15 and an increase in the proportion of cells that bear a high concentration of γ-H2AX on the X chromosome. These findings are summarized in Table 1
TABLE 1.
Summary of the properties of MEFs bearing the 21-kb deletion in the Xist gene
| Property | XaXist-WTXiXist-WT | XaXist-floxXiXist-flox | XaXist-WTXiXist-Δ21-kb | XaXist-floxXiXist-Δ21-kb | XaXist-Δ21-kbXiXist-WT | XaXist-Δ21-kbXiXist-Δ21-kb |
|---|---|---|---|---|---|---|
| Xist transcription | Yes | Yesa | Nob | Noa | Yesb | No |
| Xi H4 hypoacetylation | Yes | Yes | Yesc | Yesc | Yesc | No |
| Xi H3-lys4 hypomethylation | Yes | Yes | ND | ND | ND | No |
| Xi replication time | Late | Late | Very lated | Very lated | Latee | Very very late |
| X deletions or translocations | None | None | (few)f | (few)f | ND | Many |
| p53-ser15 phosphorylation | Low | Low | Elevatedd | Elevatedd | Low | Elevated |
| γ-H2AX signal | Infrequent | Infrequent | Frequentd | Frequentd | ND | Frequent |
| MeCP2 signal | Yes | Yes | Yesd | Yesd | ND | Yes |
| Brca1 signal | Yes | Yes | Yesd | Yesd | ND | Yes |
S. Diaz-Perez and Y. Marahrens (unpublished data).
Preliminary evidence for a slight increase in H4 acetylation near the telomeres has been obtained (S. Diaz-Perez and Y. Marahrens, unpublished data).
Data were obtained from only one XaXist-WTXiXist-Δ21-kb and one XaXist-floxXiXist-Δ21-kb cell line; however, the XaXist-WTXiXist-Δ21-kb and XaXist-floxXiXist-Δ21-kb cell lines produced very similar results.
Preliminary evidence indicates an altered pattern of DNA replication (S. Diaz-Perez and Y. Marahrens, unpublished results).
Preliminary data (S. Diaz-Perez and Y. Marahrens, unpublished results).
We previously showed that the nontranscribed Xist allele on the Xa has biological activity as the 21-kb Xist deletion altered Xa replication timing in cis; however, this deletion did not significantly affect the overall replication timing of the Xi in trans (Diaz-Perez et al. 2005). Here we show that the nontranscribed Xist allele on the Xa has more far-reaching biological activities that were not evident in heterozygous cells due to redundancy with the transcribed Xist allele on the Xi. We show that element(s) at the nontranscribed Xist allele on the Xa (possibly promotor elements) function in trans to help maintain hypoacetylation on histone H4 and also to control the replication timing of the Xi. Under the assumption that the later replicating X chromosome was the Xi, the Xi was much later replicating in XaXist-Δ21-kbXiXist-Δ21-kb cells than in cells without the deletion on the Xa. Deletions at both Xist alleles also resulted in a highly distinctive replication-timing pattern on the other X chromosome (in all likelihood the Xa): the LINE-1-rich regions were replicated later in S phase than the rest of the X chromosome. This pattern, which was not apparent in heterozygous or wild-type cells, provides a link between the Xist gene and LINE-1 elements that had previously been proposed to play a role in X-inactivation (Lyon 1998) and had also been proposed to interact with the Xist locus by heterochromatin association (Marahrens 1999). LINE-1 elements display the heterochromatic property of DNA methylation on both the Xa and the Xi in wild-type cells (Hansen 2003). Our findings therefore indicate that both Xist alleles function in cis and in trans to influence the state of heterochromatic regions on both X chromosomes. Our data reveal nonredundant cis- and trans-effects by the two alleles on replication time as the double deletion caused both X chromosomes to display altered replication times and/or patterns that were distinct from the times and/or patterns in heterozygous or wild-type cells. In contrast, the two Xist alleles were redundant in the control of histone H4 deacetylation since the presence of either Xist deletion resulted in little or no H4 acetylation while the double deletion resulted in H4 acetylation appearing throughout the Xi.
What are possible explanations/mechanisms for the delayed replication timing of the Xi in the doubly deleted cells? A protein has been reported that increases replication fork progression through chromatin and also helps resolve paused forks (Szyjka et al. 2005). Other studies have identified chromatin-associated proteins that also maintain chromosome stability by preventing the stalling and collapse of replication forks (Krings and Bastia 2004; Kai et al. 2005; Sommariva et al. 2005). The abnormal chromatin in XaXist-Δ21-kbXiXist-Δ21-kb cells may impede the association or function of such proteins. Another possibility is that the XaXist-Δ21-kbXiXist-Δ21-kb condition increases the amount of DNA damage that arises on the X chromosome, perhaps by fostering a chromatin structure that either is refractory to DNA repair or renders repetitive sequences unstable and prone to DNA damage. Increased DNA damage would not only slow replication but also lead to the observed X chromosome instability.
The late-shifted replication of the two X chromosomes in XaXist-Δ21-kbXiXist-Δ21-kb cells was associated with an increased incidence of deletions and translocations involving the X chromosome, p53 phosphorylation at serine-15, and the appearance of high levels of phosphorylated histone H2AX (γ-H2AX) on the Xi. Unusually late replication and a predisposition to form deletions and translocations are also properties of rare (Hansen et al. 1993; Wang et al. 1999; Hellman et al. 2000) and common (Arlt et al. 2003) fragile sites. Rare fragile sites are caused by triplet repeat expansions (Sutherland 2003) while common fragile sites are broader regions (with poorly defined sequence determinants) whose fragility can be induced (or “activated”) by the partial inhibition of DNA replication using DNA polymerase inhibitors such as aphidicolin (Arlt et al. 2003). Activated fragile sites are therefore thought to be regions that cause DNA polymerase to stall and a subset of these stalling events escapes cell cycle checkpoints leading to chromosome breaks (Arlt et al. 2003). Aphidicolin treatment has been associated with the appearance of numerous γ-H2AX foci (Musio et al. 2005), which may be associating not only with double-strand breaks but also with stalled replication forks (Ward et al. 2004). An attractive possibility therefore is that the deletion of 21 kb from both Xist gene copies creates an abnormal chromatin structure on the X chromosome that causes DNA polymerases to stall, resulting in the widespread accumulation of γ-H2AX, delayed DNA replication, and the appearance of fragile sites. Interestingly, both Brca1 (Ganesan et al. 2002) and Atr (Ouyang et al. 2005) have been implicated in the control of the heterochromatin of the Xi and the disruption of either Brca1 (Arlt et al. 2004) or Atr (Casper et al. 2002) activity has been reported to lead to the appearance of common fragile sites throughout the human genome. As chromatin is increasingly implicated in the maintenance of genome stability and in DNA repair and BRCA1 associates with XIST RNA, it is not inconceivable that a common pathway to maintain X chromosome stability may involve Xist, Brca1, and Atr.
Cells homozygous for the Xist deletion retained the Brca1 signal while loss of Brca1 has previously been reported to lead to loss of Xist RNA signal (Ganesan et al. 2002), suggesting that Brca1 functions upstream of Xist. Consistent with this are the similar effects of Brca1 and Xist deficiencies on the Xi: loss of macroH2A (Csankovszki et al. 1999; Ganesan et al. 2002), altered replication timing (Ganesan et al. 2002), altered H3 methylation (Ganesan et al. 2002), and DNA instability (Narod and Foulkes 2004). The proportion of cells that displayed a BRCA1 signal on the X chromosome was previously reported to vary considerably among human cell lines (Ganesan et al. 2002) and may be particularly high in MEFs. Differences in species and cell type likely play a role in these differences. It should be noted that in human cells the BRCA1 signal on the Xi is restricted to late S phase when the Xi is replicated (Chadwick and Lane 2005). In mouse cells, the inactive X chromosome begins replicating much earlier in S phase (Evans et al. 1965; Galton and Holt 1965; Tiepolo et al. 1967). Brca1 may associate with the Xi while it replicates and the extension of the Brca1 signal to a broader portion of the cell cycle in MEFs may, at least in part, reflect the replication of the Xi over a larger time interval. We should also point out that, in mouse, the Xist RNA signal associated with the Xi persists into metaphase (Lee and Jaenisch 1997; Duthie et al. 1999) while in humans the RNA signal disappears at the onset of mitosis (Clemson et al. 1996). In MEFs, Brca1 association with the Xi might also be extended to additional sections of the cell cycle.
Our finding that an intense MeCP2 signal that colocalizes with the Brca1 signal is retained in XaXist-Δ21-kbXiXist-Δ21-kb cells suggests that DNA methylation is retained on the Xi. Indeed, we have found that DNA methylation plays an important role in gene silencing on the inactive X chromosome in XaXist-Δ21-kbXiXist-Δ21-kb cells (J. L. Salstrom, C. Wang, C. Wang, A. Datta, S. Zeitlin, G. Csankovszki, C. D. Eller, S. Diaz-Perez, J. Wang, A. Chess, S. Huang, B. Kaltenboeck and Y. Marahrens, unpublished results). Interestingly, observations in fungi (Tamaru and Selker 2001), plants (Jackson et al. 2002), and mammals (Lehnertz et al. 2003) indicate that H3 lysine-9 methylation drives DNA methylation. H3 lysine-4 methylation arises throughout the inactive X chromosome in XaXist-Δ21-kbXiXist-Δ21-kb cells and this modification is thought to be mutually exclusive to H3 lysine-9 methylation (Boggs et al. 2001). Our data therefore suggest that, in XaXist-Δ21-kbXiXist-Δ21-kb cells, DNA methylation is uncoupled from histone H3 lysine-9 methylation. Tiling arrays previously revealed an incomplete correspondence between H3 lysine-9 methylation and DNA methylation in plants (Lippman et al. 2004).
Our findings raise the issue of how the trans-effects that we detect between the Xa and Xi are achieved. We had previously invoked Occam's razor to propose that the initiation of X-inactivation is triggered by the transient homologous pairing of the two copies of the Xic (Marahrens 1999). In support of this model, transient homologous pairing of the Xic has recently been demonstrated to mark the initiation of X-inactivation (Bacher et al. 2006; Xu et al. 2006). Our findings that deletions at the Xist locus result in trans-effects are most parsimonious with a model in which the loss of Xist from both alleles alters the frequency or the consequences of homologous pairing. An alternative explanation is that the Xist deletions cause the undeleted 3′ portion of the Xist gene to be transcribed in XaXist-Δ21-kbXiXist-Δ21-kb cells that, in contrast to wild-type Xist RNA, function in trans. However, we find that the 3′ undeleted portion of the Xist-Δ21 allele is not transcribed from either the Xa or the Xi. Homologous pairing could not be detected in wild-type MEFs in asynchronous cells (Xu et al. 2006). Although this may be due to pairing being restricted to a small portion of the cell cycle in wild-type cells such as late S phase (LaSalle and Lalande 1996), we wonder whether the loss of Xist may also increase the homologous pairing of the Xa and Xi. Interestingly, there is evidence that H3 lysine-4 methylation is required for homologous pairing during male meiosis (Hayashi et al. 2005). It will be interesting to see whether homologous pairing of the X chromosomes is increased in XaXist-Δ21-kbXiXist-Δ21-kb cells and whether such an increase occurs at many positions along the X chromosomes and requires the H3 lysine-4 methylation that arises on the Xi in these cells.
Acknowledgments
We thank Shridar Ganesan and David M. Livingston for their generous gift of anti-Brca1 antibody. Adenoviruses expressing cre recombinase (Tan et al. 1999) and GFP were kindly provided by Carol Eng and Arnold J. Berk. We thank Elaine Wong for generating some of the primary fibroblasts, Moira Regelson for performing statistical analysis, Jeannie T. Lee (Department of Molecular Biology, Massachusetts General Hospital) for providing the Xist P1 mouse clone pπJL1, and Barbara Panning (Department of Biochemistry and Biophysics, University of California, San Francisco) for providing a Xist-containing BAC. This work was supported by a March of Dimes Basil O'Connor Starter Scholar research award (5-FY99-819) (Y.M.), a seed grant from the University of California, Los Angeles, Jonsson Cancer Center Foundation (Y.M.), and the National Institutes of Health [R01 HD41451:01 (Y.M.) and R01CA107300 (M.A.T.)].
References
- Arlt, M. F., A. M. Casper and T. W. Glover, 2003. Common fragile sites. Cytogenet. Genome Res. 100: 92–100. [DOI] [PubMed] [Google Scholar]
- Arlt, M. F., B. Xu, S. G. Durkin, A. M. Casper, M. B. Kastan et al., 2004. BRCA1 is required for common-fragile-site stability via its G2/M checkpoint function. Mol. Cell. Biol. 24: 6701–6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi, L. D., 2005. The role of p53-mediated apoptosis as a crucial anti-tumor response to genomic instability: lessons from mouse models. Mutat. Res. 569: 145–157. [DOI] [PubMed] [Google Scholar]
- Bacher, C. P., M. Guggiari, B. Brors, S. Augui, P. Clerc et al., 2006. Transient colocalization of X-inactivation centres accompanies the initiation of X inactivation. Nat. Cell Biol. 8: 293–299. [DOI] [PubMed] [Google Scholar]
- Bailey, J. A., L. Carrel, A. Chakravarti and E. E. Eichler, 2000. Molecular evidence for a relationship between LINE-1 elements and X chromosome inactivation: the Lyon repeat hypothesis. Proc. Natl. Acad. Sci. USA 97: 6634–6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banin, S., L. Moyal, S. Shieh, Y. Taya, C. W. Anderson et al., 1998. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 28: 1674–1677. [DOI] [PubMed] [Google Scholar]
- Bassing, C. H., and F. W. Alt, 2004. H2AX may function as an anchor to hold broken chromosomal DNA ends in close proximity. Cell Cycle 3: 149–153. [DOI] [PubMed] [Google Scholar]
- Boggs, B. A., P. Cheung, E. Heard, D. L. Spector, A. C. Chinault et al., 2001. Differentially methylated forms of histone H3 show unique association patterns with inactive human X chromosomes. Nat. Genet. 30: 73–76. [DOI] [PubMed] [Google Scholar]
- Brockdorff, N., A. Ashworth, G. F. Kay, P. Cooper, S. Smith et al., 1991. Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nature 351: 329–331. [DOI] [PubMed] [Google Scholar]
- Brown, C. J., A. Ballabio, J. L. Rupert, R. G. Lafreniere, M. Grompe et al., 1991. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 349: 38–44. [DOI] [PubMed] [Google Scholar]
- Brown, C. J., B. D. Hendrich, J. L. Rupert, R. G. Lafreniere, Y. Xing et al., 1992. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell 71: 527–542. [DOI] [PubMed] [Google Scholar]
- Brown, K. E., S. Amoils, J. M. Horn, V. J. Buckle, D. R. Higgs et al., 2001. Expression of alpha- and beta-globin genes occurs within different nuclear domains in haemopoietic cells. Nat. Cell Biol. 3: 602–606. [DOI] [PubMed] [Google Scholar]
- Bruun, D., A. Folias, Y. Akkari, Y. Cox, S. Olson et al., 2003. siRNA depletion of BRCA1, but not BRCA2, causes increased genome instability in Fanconi anemia cells. DNA Repair 2: 1007–1013. [DOI] [PubMed] [Google Scholar]
- Burma, S., B. P. Chen, M. Murphy, A. Kurimasa and D. J. Chen, 2001. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276: 42462–42467. [DOI] [PubMed] [Google Scholar]
- Canman, C. E., D. S. Lim, K. A. Cimprich, Y. Taya, K. Tamai et al., 1998. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281: 1677–1679. [DOI] [PubMed] [Google Scholar]
- Casper, A. M., P. Nghiem, M. F. Arlt and T. W. Glover, 2002. ATR regulates fragile site stability. Cell 111: 779–789. [DOI] [PubMed] [Google Scholar]
- Chadwick, B. P., and T. F. Lane, 2005. BRCA1 associates with the inactive X chromosome in late S-phase, coupled with transient H2AX phosphorylation. Chromosoma 114: 432–439. [DOI] [PubMed] [Google Scholar]
- Chadwick, B. P., and H. F. Willard, 2004. Multiple spacially distinct types of facultative heterochromatin on the human inactive X chromosome. Proc. Natl. Acad. Sci. USA 101: 17450–17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemson, C. M., J. A. McNeil, H. F. Willard and J. B. Lawrence, 1996. XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure. J. Cell Biol. 132: 259–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, S. M., B. P. Brylawski, M. Cordeiro-Stone and D. G. Kaufman, 2003. Same origins of DNA replication function on the active and inactive human X chromosomes. J. Cell Biochem. 88: 923–931. [DOI] [PubMed] [Google Scholar]
- Cortez, D., Y. Wang, J. Qin and S. J. Elledge, 1999. Requirement of ATM-dependent phosphorylation of Brca1 in the DNA damage response to double-strand breaks. Science 286: 1162–1166. [DOI] [PubMed] [Google Scholar]
- Costanzi, C., and J. R. Pehrson, 1998. Histone macroH2A1 is concentrated in the inactive X chromosome of female mammals. Nature 393: 599–601. [DOI] [PubMed] [Google Scholar]
- Csankovszki, G., B. Panning, B. Bates, J. R. Pehrson and R. Jaenisch, 1999. Conditional deletion of Xist disrupts histone macroH2A localization but not maintenance of X inactivation. Nat. Genet. 22: 323–324. [DOI] [PubMed] [Google Scholar]
- Csankovszki, G., A. Nagy and R. Jaenisch, 2001. Synergism of Xist RNA, DNA methylation, and histone hypoacetylation in maintaining X chromosome inactivation. J. Cell Biol. 153: 773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Perez, S., Y. Ouyang, V. Perez, R. Cisneros, M. Regelson et al., 2005. Element(s) at the non-transcribed Xist locus of the active X chromosome control chromosomal replication timing in the mouse. Genetics 171: 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie, S. M., T. B. Nesterova, E. J. Formstone, A. M. Keohane, B. M. Turner et al., 1999. Xist RNA exhibits a banded localization on the inactive X chromosome and is excluded from autosomal material in cis. Hum. Mol. Genet. 8: 195–204. [DOI] [PubMed] [Google Scholar]
- Evans, H. J., C. E. Ford, M. F. Lyon and J. Gray, 1965. DNA replication and genetic expression in female mice with morphologically distinguishable X chromosomes. Nature 206: 900–903. [DOI] [PubMed] [Google Scholar]
- Galton, M., and S. F. Holt, 1965. Asynchronous replication of the mouse sex chromosomes. Exp. Cell Res. 37: 111–116. [DOI] [PubMed] [Google Scholar]
- Ganesan, S., D. P. Silver, R. A. Greenberg, D. Avni, R. Drapkin et al., 2002. BRCA1 supports XIST RNA localization on the inactive X chromosome. Cell 111: 393–405. [DOI] [PubMed] [Google Scholar]
- Ganesan, S., D. P. Silver, R. Drapkin, R. Greenberg, J. Feunteun et al., 2004. Association of BRCA1 with the inactive X chromosome and XIST RNA. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359: 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartler, S. M., and A. D. Riggs, 1983. Mammalian X-chromosome inactivation. Annu. Rev. Genet. 17: 155–190. [DOI] [PubMed] [Google Scholar]
- Gatei, M., S. P. Scott, I. Filippovitch, N. Soronika, M. F. Lavin et al., 2000. Role for ATM in DNA damage-induced phosphorylation of BRCA11. Cancer Res. 60: 3299–3330. [PubMed] [Google Scholar]
- Gilbert, D. M., 2002. Replication timing and transcriptional control: beyond cause and effect. Curr. Opin. Cell Biol. 14: 377–383. [DOI] [PubMed] [Google Scholar]
- Gimelbrant, A. A., and A. Chess, 2006. An epigenetic state associated with areas of gene duplication. Genome Res. 16: 723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, M., and N. Brockdorff, 2004. Heterochromatin on the inactive X chromosome delays replication timing without affecting origin usage. Proc. Natl. Acad. Sci. USA 101: 6923–6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, R. S., 2003. X inactivation-specific methylation of LINE-1 elements by DNMT3B: implications for the Lyon repeat hypothesis. Hum. Mol. Genet. 12: 2559–2567. [DOI] [PubMed] [Google Scholar]
- Hansen, R. S., T. K. Canfield, M. M. Lamb, S. M. Gartler and C. D. Laird, 1993. Association of fragile X syndrome with delayed replication of the FMR1 gene. Cell 73: 1403–1409. [DOI] [PubMed] [Google Scholar]
- Hansen, R. S., R. Stoger, C. Wijmenga, A. M. Stanek, T. K. Canfield et al., 2000. Escape from gene silencing in ICF syndrome: evidence for advanced replication time as a major determinant. Hum. Mol. Genet. 9: 2575–2587. [DOI] [PubMed] [Google Scholar]
- Hayashi, K., K. Yoshida and Y. Matsui, 2005. A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature 438: 374–378. [DOI] [PubMed] [Google Scholar]
- Hellman, A., A. Rahat, S. W. Scherer, A. Darvasi, L. C. Tsui et al., 2000. Replication delay along FRA7H, a common fragile site on human chromosome 7, leads to chromosomal instability. Mol. Cell. Biol. 20: 4420–4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper, M. L., K. Hardy, A. Handyside, S. Hunter and M. Monk, 1987. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature 326: 292–295. [DOI] [PubMed] [Google Scholar]
- Ihaka, R., and R. Gentleman, 1996. R: a language for data analysis and graphics. J. Comput. Graph. Stat. 5: 299–314. [Google Scholar]
- Jackson, J. P., A. M. Lindroth, X. Cao and S. E. Jacobsen, 2002. Control of CpNpG DNA methylation by the KRYPTONITE histone H3 methyltransferase. Nature 416: 556–560. [DOI] [PubMed] [Google Scholar]
- Jat, P. S., C. L. Cepko, R. C. Mulligan and P. A. Sharp, 1986. Recombinant retroviruses encoding simian virus 40 large T antigen and polyomavirus large and middle T antigens. Mol. Cell. Biol. 6: 1204–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein, T., and C. D. Allis, 2001. Translating the histone code. Science 293: 1074–1080. [DOI] [PubMed] [Google Scholar]
- Jeppesen, P., and B. M. Turner, 1993. The inactive X chromosome in female mammals is distinguished by a lack of histone H4 acetylation, a cytogenetic marker for gene expression. Cell 74: 281–289. [DOI] [PubMed] [Google Scholar]
- Kai, M., M. N. Boddy, P. Russell and T. S. Wang, 2005. Replication checkpoint kinase Cds1 regulates Mus81 to preserve genome integrity during replication stress. Genes Dev. 19: 919–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna, K. K., K. E. Keating, S. Kozlov, S. Scott, M. Gatei et al., 1998. ATM associates with and phosphorylates p53: mapping the region of interaction. Nat. Genet. 20: 398–400. [DOI] [PubMed] [Google Scholar]
- Krings, G., and D. Bastia, 2004. swi1- and swi3-dependent and independent replication fork arrest at the ribosomal DNA of Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 101: 14085–14090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruskal, J. B., 1964. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 29: 1–27. [Google Scholar]
- LaSalle, J. M., and M. Lalande, 1996. Homologous association of oppositely imprinted chromosomal domains. Science 272: 725–728. [DOI] [PubMed] [Google Scholar]
- Lee, J. T., and R. Jaenisch, 1997. Long-range cis effects of ectopic X-inactivation centres on a mouse autosome. Nature 386: 275–279. [DOI] [PubMed] [Google Scholar]
- Lee, J. T., N. Lu and Y. Han, 1999. Genetic analysis of the mouse X inactivation center defines an 80-kb multifunction domain. Proc. Natl. Acad. Sci. USA 96: 3836–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnertz, B., Y. Ueda, A. A. Derijck, U. Braunschweig, L. Perez-Burgos et al., 2003. Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr. Biol. 13: 1192–1200. [DOI] [PubMed] [Google Scholar]
- Lippman, Z., A. V. Gendrel, M. Black, M. W. Vaughn, N. Dedhia et al., 2004. Role of transposable elements in heterochromatin and epigenetic control. Nature 430: 471–476. [DOI] [PubMed] [Google Scholar]
- Liyanage, M., A. Coleman, S. du Manoir, T. Veldman, S. McCormack et al., 1996. Multicolour spectral karyotyping of mouse chromosomes. Nat. Genet. 14: 312–315. [DOI] [PubMed] [Google Scholar]
- Lyon, M. F., 1996. Pinpointing the centre. Nature 379: 116–117. [DOI] [PubMed] [Google Scholar]
- Lyon, M. F., 1998. X-chromosome inactivation: a repeat hypothesis. Cytogenet. Cell Genet. 80: 133–137. [DOI] [PubMed] [Google Scholar]
- Marahrens, Y., 1999. X-inactivation by chromosomal pairing events. Genes Dev. 13: 2624–2632. [DOI] [PubMed] [Google Scholar]
- Marahrens, Y., B. Panning, J. Dausman, W. Strauss and R. Jaenisch, 1997. Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev. 11: 156–166. [DOI] [PubMed] [Google Scholar]
- Meehan, R. R., J. D. Lewis and A. P. Bird, 1992. Characterization of MeCP2, a vertebrate DNA binding protein with affinity for methylated DNA. Nucleic Acids Res. 20: 5085–5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynahan, M. E., T. Y. Cui and M. Jasin, 2001. Homology-directed dna repair, mitomycin-c resistance, and chromosome stability is restored with correction of a Brca1 mutation. Cancer Res. 61: 4842–4850. [PubMed] [Google Scholar]
- Musio, A., C. Montagna, T. Mariani, M. Tilenni, M. L. Focarelli et al., 2005. SMC1 involvement in fragile site expression. Hum. Mol. Genet. 14: 525–533. [DOI] [PubMed] [Google Scholar]
- Narod, S. A., and W. D. Foulkes, 2004. BRCA1 and BRCA2: 1994 and beyond. Nat. Rev. Cancer 4: 665–676. [DOI] [PubMed] [Google Scholar]
- Nesbitt, M. N., and S. M. Gartler, 1970. Replication of the mouse sex chromosomes early in the S period. Cytogenetics 9: 212–221. [DOI] [PubMed] [Google Scholar]
- Ouyang, Y., J. Salstrom, S. Diaz-Perez, S. Nahas, Y. Matsuno et al., 2005. Inhibition of Atm and/or Atr disrupts gene silencing on the inactive X chromosome. Biochem. Biophys. Res. Commun. 337: 875–880. [DOI] [PubMed] [Google Scholar]
- Paull, T. T., E. P. Rogakou, V. Yamazaki, C. U. Kirchgessner, M. Gellert et al., 2000. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10: 886–895. [DOI] [PubMed] [Google Scholar]
- Penny, G. D., G. F. Kay, S. A. Sheardown, S. Rastan and N. Brockdorff, 1996. Requirement for Xist in X chromosome inactivation. Nature 379: 131–137. [DOI] [PubMed] [Google Scholar]
- Peters, A. H., J. E. Mermoud, D. O'Carroll, M. Pagani, D. Schweizer et al., 2001. Histone H3 lysine 9 methylation is an epigenetic imprint of facultative heterochromatin. Nat. Genet. 30: 77–80. [DOI] [PubMed] [Google Scholar]
- Plath, K., J. Fang, S. K. Mlynarczyk-Evans, R. Cao, K. A. Worringer et al., 2003. Role of histone H3 lysine 27 methylation in X inactivation. Science 300: 131–135. [DOI] [PubMed] [Google Scholar]
- Priest, J. H., J. E. Heady and R. E. Priest, 1967. Delayed onset of replication of human X chromosomes. J. Cell Biol. 35: 483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully, R., and D. M. Livingston, 2000. In search of the tumour-suppressor functions of brca1 and brca2. Nature 408: 429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, N., F. A. Ebrahimi, A. A. Gimelbrant, A. W. Ensminger, M. R. Tackett et al., 2003. Coordination of the random asynchronous replication of autosomal loci. Nat. Genet. 33: 339–341. [DOI] [PubMed] [Google Scholar]
- Sommariva, E., T. K. Pellny, N. Karahan, S. Kumar, J. A. Huberman et al., 2005. Schizosaccharomyces pombe Swi1, Swi3, and Hsk1 are components of a novel S-phase response pathway to alkylation damage. Mol. Cell. Biol. 25: 2770–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector, D., and R. Goldman, 1998. In situ hybridization to RNA, pp. 114–117 in Essential from Cells: A Laboratory Manual, edited by D. Spector and R. Goldman. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Stiff, T., M. O'Driscoll, N. Rief, K. Iwabuchi, M. Lobrich et al., 2004. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 64: 2390–2396. [DOI] [PubMed] [Google Scholar]
- Sutherland, G. R., 2003. Rare fragile sites. Cytogenet. Genome Res. 100: 77–84. [DOI] [PubMed] [Google Scholar]
- Szyjka, S. J., C. J. Viggiani and O. M. Aparicio, 2005. Mrc1 is required for normal progression of replication forks throughout chromatin in S. cerevisiae. Mol. Cell 19: 691–707. [DOI] [PubMed] [Google Scholar]
- Tamaru, H., and E. Selker, 2001. A histone H3 methyltransferase controls DNA methylation in Neurospora crassa. Nature 414: 277–283. [DOI] [PubMed] [Google Scholar]
- Tan, B. T., L. Wu and A. J. Berk, 1999. An adenovirus-Epstein-Barr virus hybrid vector that stably transforms cultured cells with high efficiency. J. Virol. 73: 7582–7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, J. H., 1968. Rates of chain growth and units of replication in DNA of mammalian chromosomes. J. Mol. Biol. 31: 579–594. [DOI] [PubMed] [Google Scholar]
- Taylor, J. H., and P. Miner, 1968. Units of DNA replication in mammalian chromosomes. Cancer Res. 9: 1810–1814. [PubMed] [Google Scholar]
- Thompson, S., A. R. Clarke, A. M. Pow, M. L. Hooper and D. W. Melton, 1989. Germ line transmission and expression of a corrected HPRT gene produced by gene targeting in embryonic stem cells. Cell 56: 313–321. [DOI] [PubMed] [Google Scholar]
- Tibbetts, R. S., K. M. Brumbaugh, J. M. Williams, J. N. Sarkaria, W. A. Cliby et al., 1999. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 13: 152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts, R. S., D. Cortez, K. M. Brumbaugh, R. Scully, D. Livingston et al., 2000. Functional interactions between BRCA1 and the checkpoint kinase ATR during genotoxic stress. Genes Dev. 14: 2989–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiepolo, L., M. Fraccaro, M. Hulten, J. Lindsten, A. Mannini et al., 1967. Timing of sex chromosome replication in somatic and germ-line cells of the mouse and the rat. Cytogenetics 6: 51–66. [DOI] [PubMed] [Google Scholar]
- Wang, C., D. Gao, A. Vaglenov and B. Kaltenboeck, 2004. One-step real-time duplex reverse transcription PCRs simultaneously quantify analyte and housekeeping gene mRNAs. Biotechniques 36: 508–519. [DOI] [PubMed] [Google Scholar]
- Wang, L., J. Darling, J. S. Zhang, H. Huang, W. Liu et al., 1999. Allele-specific late replication and fragility of the most active common fragile site, FRA3B. Hum. Mol. Genet. 8: 431–437. [DOI] [PubMed] [Google Scholar]
- Ward, I. M., and J. Chen, 2001. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 276: 47759–47762. [DOI] [PubMed] [Google Scholar]
- Ward, I. M., K. Minn and J. Chen, 2004. UV-induced ataxia-telangiectasia-mutated and Rad3-related (ATR) activation requires replication stress. J. Biol. Chem. 279: 9677–9680. [DOI] [PubMed] [Google Scholar]
- Weaver, Z., C. Montagna, X. Xu, T. Howard, M. Gadina et al., 2002. Mammary tumors in mice conditionally mutant for Brca1 exhibit gross genomic instability and centrosome amplification yet display a recurring distribution of genomic imbalances that is similar to human breast cancer. Oncogene 21: 5097–5107. [DOI] [PubMed] [Google Scholar]
- Welcsh, P. L., K. N. Owens and M. C. King, 2000. Insights into the functions of BRCA1 and BRCA2. Trends Genet. 16: 69–74. [DOI] [PubMed] [Google Scholar]
- Xu, N., C. L. Tsai and J. T. Lee, 2006. Transient homologous chromosome pairing marks the onset of X inactivation. Science 311: 1149–1152. [DOI] [PubMed] [Google Scholar]