Abstract
Transposons are very valuable tools for genetic manipulation. However, the number of transposable elements that have been suitably adapted for experimental use is insufficient and the spectrum of heterologous hosts in which they have been deployed is restricted. To date, only transposons from animal hosts have been utilized in heterologous animal species and transposons of plant origin have been used in plant genetics. There has been no experimental evidence that any of the known elements could transpose in hosts belonging to both kingdoms. Here we demonstrate that the maize Dissociation (Ds) element is capable of effective Activator (Ac) transposase-mediated transposition in the zebrafish Danio rerio, yielding remarkable germline transmission rates. In addition, mammalian cells were also found to be conducive to Ds transposition. Furthermore, we demonstrate that nuclear localization of Ac transposase is essential for genomic Ds transposition. Our results support the hypothesis that Ac/Ds elements do not rely on host-specific factors for transposition and that host factors involved in their mobility mechanism are widely conserved. Finally, even in vertebrate cells, the Ac/Ds system displays accurate transposition, large-fragment carrying capacity, high transposition frequencies, efficient germline transmission, and reporter gene expression, all of which are advantageous for various genetic applications and animal biotechnology.
TRANSPOSABLE element families are widespread among all living organisms, suggesting certain versatility of their transposition mechanisms. Some elements can effectively transpose in heterologous species (Ac/Ds, mariner, piggyBac, etc.), whereas others favor a specific host environment. For example, transposition of the Drosophila P element is markedly suppressed outside the drosophilid family (Handler et al. 1993). Involvement of host factors in transposition has been previously demonstrated (Makris et al. 1990; Handler et al. 1993; Staveley et al. 1995; Beall and Rio 1996), leading to the perception that distinct host factors are largely responsible for the limited success rate of heterologous transposition. It has been generally assumed that a transposable element should have a higher chance for successful transfer in taxonomically close species. Partly driven by this assumption, traditionally only transposons of animal origin were utilized in heterologous animals (Ivics et al. 1997; Fadool et al. 1998; Raz et al. 1998; Kawakami et al. 2000; Fischer et al. 2001; Horie et al. 2001; Davidson et al. 2003; Ding et al. 2005) and plant transposons were used exclusively in plants (Haring et al. 1991). To date, none of the known elements was successfully used in both plant and animal hosts.
The maize Ac/Ds elements were the first transposable elements discovered over half a century ago by Barbara McClintock (McClintock 1948; McClintock 1951). They belong to the large hAT family (hobo from Drosophila, Ac from maize, Tam3 from snapdragon) of “cut-and-paste” transposons. The Ac is an autonomous element—it carries a transposase gene enclosed between the cis-required terminal sequences that contain 11-bp imperfect terminal repeats. The transposase induces excision of the element at the ends of the terminal repeats and transposition into a new genomic location. The Ds element also contains the terminal repeats and the cis-required sequences but does not carry the transposase gene. It can be trans-activated only in the presence of the Ac element or Ac transposase.
Several features, including accurate cut-and-paste mechanism of transposition, small size of cis-required sequences (∼600 bp of minimal Ds), large cargo-insert capacity, reasonably high transposition frequency, moderate copy number, and preferential insertion in transcribed regions and in 5′ regions of genes have made the Ac/Ds elements particularly amenable for genetic studies (Parinov et al. 1999; Kolesnik et al. 2004; Kuromori et al. 2004). Moreover, Ac/Ds elements have been successfully utilized in many heterologous plant species, although the activity in different plant hosts varied considerably. Previously, a genetic screen identified mutants displaying increased levels of Ac/Ds activity in Arabidopsis, suggesting that the host factors were responsible for variations in activity in different plant hosts (Jarvis et al. 1997). Nevertheless, Ds transposition catalyzed by a modified Ac transposase was demonstrated in Saccharomyces cerevisiae (Weil and Kunze 2000), indicating that plant specific factors were not necessary for transposition. We tested the Ac/Ds elements in animals using a model vertebrate, the zebrafish Danio rerio, and also a human cell line.
Zebrafish is a good model vertebrate for several reasons such as high fecundity, external fertilization and development, transparency of the embryos, and low maintenance, cost, and space requirements. It is also a very convenient system for testing the viability of new transposable elements from heterologous species. Microinjections of DNA and RNA are routinely performed in every zebrafish laboratory (hundreds to thousands of eggs can be injected in a few hours). The injected RNA is short lived in the embryo, obviating the need for additional markers and steps to get rid of transposase. Besides, zebrafish is an important developmental model and it would greatly benefit from introduction of a new transposon-based methodology.
Here we demonstrate that in zebrafish Ac/Ds elements are highly active and maintain many properties that are essential for an effective transgenesis and mutagenesis system.
MATERIALS AND METHODS
Plasmid constructs:
The construct containing EGFP (CLONTECH, Palo Alto, CA) with a 2.25-kb promoter of the keratin 8 (krt8) gene (GenBank accession no. AF440690) was obtained from Zhiyuan Gong (The National University of Singapore). The 3.1-kb krt8:EGFP fragment was subcloned into a 0.6-kb miniDs construct (Weil and Kunze 2000).
The NLS-TPase containing a nuclear localization sequence (NLS) was amplified by PCR from the pWL80 plasmid (Weil and Kunze 2000) using the primers Ac5′-1-CCAAAGAAGAAGCGTAAGGTAGAAATGGCTATTGTTCATGAACCACA and Ac3-GTATCGATAAGCTTGATATCGAATTCC. The product was used as a template in the secondary PCR using primers Ac5′-2-CGCGGATCCGCCACCATGGGTCCTCCAAAGAAGAAGCGTAAGGTAG and Ac3-GTATCGATAAGCTTGATATCGAATTCC. The product, which contained a nuclear localization sequence (MGPPKKKRKVE) fused to a truncated Ac TPase103–807 and Kozak sequence was digested with BamHI and cloned into the BglII site of the pSP64T vector (Krieg and Melton 1984). The NLSK5E-TPase was generated fortuitously during cloning of the NLS-TPase construct because of a (A to G) mismatch in the Ac5′-2 primer CGCGGATCCGCCACCATGGGTCCTCCAgAGAAGAAGCGTAAGGTAG. To produce the NoNLS construct, we removed the NLS sequence using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) and primers CTCAACTTTGGCAGATCCGCCACCATGGCTATTGTTCATGAACCACAACC and GGTTGTGGTTCATGAACAATAGCCATGGTGGCGGATCTGCCAAAGTTGAG.
To produce the NLS-TPase-EGFP and NLSK5E-TPase-EGFP fusion constructs, we amplified NLS-TPase and NLSK5E-TPase fragments by PCR using primers AGAGGGATCCAGCTCAGAATAAACGCTCAAC and AGAGACCGGTCCTGGAGAGGAGCCACTTGCTA and cloned the fragments in AgeI and BamHI sites of the krt8-EGFP plasmid (Gong et al. 2002). To produce NLS-EGFP and NLSK5E-EGFP constructs, we deleted the Ac TPase103–807 open reading frame (ORF) sequence from the NLS-TPase-EGFP and NLSK5E-TPase-EGFP constructs using the QuikChange site-directed mutagenesis kit (Stratagene) and primers AGAAGAAGCGTAAGGTAGAAATGGTGAGCAAGGGCGAGGAGC and GCTCCTCGCCCTTGCTCACCATTTCTACCTTACGCTTCTTCT.
To produce a plasmid construct carrying the NLSK5E-TPase ORF under the CMV promoter used for human cell transfection, we amplified the NLSK5E-TPase fragment by PCR using primers Ac5Bam: GCGCGGATCCATACGATTTAGGTGACACTATAG and Ac3Not: CGATCGATGCGGCCGCCTTGGCTAACATAAGAAG and cloned it into the BamHI and NotI restriction sites of the pEGFP-N1 plasmid (CLONTECH).
All TPase constructs were verified by sequencing through the entire TPase ORF, promoter regions, and the 3′-UTR sequences. In each case, several independent clones were sequenced and only constructs without mismatches in the TPase sequences were used for injections. We did not test the TPase-EGFP, NLSK5E-TPase-EGFP, and NLS-TPase-EGFP fusion proteins for transposase activity.
RNA synthesis and injections:
The TPase plasmids were linearized downstream of the poly(A) tail with the BamHI restriction enzyme and used for generating capped mRNA in vitro with the Message Machine SP6 kit (Ambion, Austin, TX). The products were purified using the RNeasy mini kit (QIAGEN, Hilden, Germany). A total of 5–10 pg of plasmid DNA was co-injected with 25–50 pg of in vitro-synthesized transposase mRNA into zebrafish embryos (yolk center) at the one- to two-cell stage. Injected embryos were raised and maintained according to established protocols (Westerfield 1995).
Analysis of Ds excision:
To detect excision events, we designed two primers complementary to sequences at the donor site that flanked the 3.7-kbp Ds. PCR was performed without extension and with a short annealing time to prevent amplification of the long donor product. Under these conditions (94° for 30 sec; 55° for 10 sec for 35 cycles), only a 120-bp Ds-excision product could be amplified but not the 3.8-kbp donor site (the 3.7-kbp Ds plus the 120-bp surrounding vector) even when present in excess. Products were resolved using 1.8% agarose gels. The bands were cut from the gel, purified using QIAquick gel extraction kit (QIAGEN), and sequenced using ABI cycle sequencer (PE Applied Biosystems, Foster City, CA).
Analyses of Ds flanking sequences:
Thermal asymmetric interlaced (TAIL)–PCR was performed as described previously (Liu and Whittier 1995; Parinov et al. 2004) using the following set of primers: Ds5′-1 CCGTTTACCGTTTTGTATATCCCG; Ds5′-2 CGTTCCGTTTTCGTTTTTTACC; Ds5′-3 CGGTCGGTACGGGATTTTCC; Ds3′-1 CGATTACCGTATTTATCCCGTTCG; Ds3′-2 CCGGTATATCCCGTTTTCG; Ds3′-3 GAAATTGAAAACGGTAGAGGT; AD-1 WGTGNAGNANCANAGA; AD-2 WCAGNTGWTNGTNCTG; AD-3 STTGNTASTNCTNTGC; AD-4 NCASGAWAGNCSWCAA.
Products of the secondary and tertiary reactions were resolved on 1.8% agarose gel. Individual bands from “band shift” pairs were cut from the gel and purified using the QIAquick gel extraction kit (QIAGEN), and sequenced with Ds5′-3 and Ds3′-3 primers using the ABI cycle sequencer (PE Applied Biosystems).
Southern blot hybridization:
Genomic DNA from pooled zebrafish embryos was phenol extracted and digested using the EcoRI restriction endonuclease that cut the Ds at a unique site. The digested genomic DNA was fractionated by gel electrophoresis, transferred to positively charged nylon membrane (Roche Applied Science) by capillary blotting (Sambrook et al. 1989), and crosslinked by UV irradiation. The DNA probe for EGFP was labeled with digoxigenin (Roche Applied Science) using a PCR DIG synthesis kit. We used DIG EasyHyb, DIG wash, and block buffer set for hybridization, an anti-DIG alkaline phosphatase conjugate antibody and CDP–Star chemiluminescent substrate (Roche Applied Science) to detect the hybridized probe. Hybridization and detection were carried out according to the manufacturer's instructions.
Transfection of HEK293 cell line:
Human embryonic kidney cells, HEK293 (ATCC no. CRL-1573), at a density of 2.5 × 105 cells/6-well plate were seeded in 2 ml Dulbecco's Modified Eagle's Medium (DMEM)/10% fetal bovine serum (FBS) and grown in 5% CO2 for 24 hr prior to transfection. Cells were cotransfected with 0.5 μg of each plasmid (pDs-CMV-EGFP-Ub-Bsd and pCMV-NLSK5E-TPase). The GenePorter2 transfection reagent (Genlantis, San Diego, CA) was used for transfection according to the manufacturer's instructions. Twenty-four hours after transfection, cells were diluted to single-cell density and seeded in 96-well plates in DMEM/10% FBS containing 10 μg/ml blasticidin (Invitrogen, San Diego). Antibiotic selection of the resistant colonies was continued for 6 days. After selection, the medium was changed to DMEM/10% FBS and EGFP-positive colonies were grown until confluence on 6-well plates. DNA from the cells was obtained by phenol/chloroform extraction followed by ethanol precipitation. Flanking sequences were obtained by TAIL–PCR.
RESULTS
Experimental design:
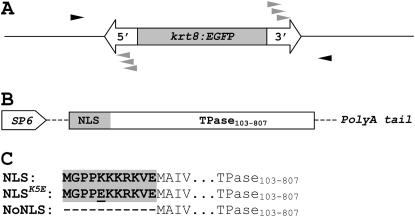
To produce Ds insertions in the zebrafish genome, we designed a two-component system consisting of a donor construct with a nonautonomous Ds element and a messenger RNA encoding a modified Ac transposase (see materials and methods). The Ds element harbored the EGFP gene regulated by the zebrafish 2.25-kb keratin 8 (krt8) promoter (Gong et al. 2002) between the 5′- and 3′-end cis-required sequences (Weil and Kunze 2000) (Figure 1A). The second construct harboring the coding sequence of the truncated Ac transposase (TPase103–807) (Houba-Herin et al. 1990), fused to a synthetic nuclear localization signal analogous to that of the SV40 large T antigen, was also generated (Figure 1B). The coding sequence of this chimeric NLS-TPase103–807 fusion (referred to as NLS-TPase hereafter) was cloned into the pSP64T plasmid (Krieg and Melton 1984) containing the SP6 promoter for in vitro transcription. This plasmid also contained the 5′- and 3′-UTRs of the Xenopus β-globin gene and a dA32 tail. Two additional TPase constructs were made (Figure 1, B and C; see materials and methods for details): one containing only the TPase103–807 sequence without NLS (NoNLS-TPase) and another containing an amino acid substitution (K to E) at the fifth position of the NLS (NLSK5E-TPase).
Figure 1.—
Construct design. (A) Ds donor construct carrying 3.1-kbp reporter fragment (EGFP gene under the zebrafish keratin 8 promoter), inserted between the 5′- and 3′-Ds cis-sequences (250 and 370 bp, respectively). Solid arrowheads indicate the primers for excision PCR; shaded arrowheads, specific primers for TAIL–PCR. (B) TPase construct containing SP6 promoter for in vitro transcription and the coding sequence for the truncated Ac transposase (TPase103–807) fused to a synthetic nuclear localization signal. Dashed lines represent the 5′- and 3′-UTRs of the Xenopus β-globin gene. (C) N-terminal amino acid sequences of the NLS-, NLSK5E-, and NoNLS-TPase. NLS signals are shown in boldface type and are shaded.
Co-injection of Ds donor construct with TPase mRNA causes specific Ds excision:
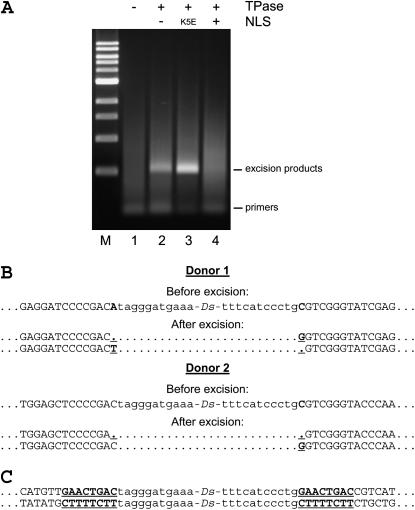
In vitro transcribed, capped, and polyadenylated TPase mRNA was microinjected, together with nonlinearized Ds donor plasmid, into zebrafish embryos at the one-cell stage. The embryos were incubated for 10 hr and their genomic DNA was extracted for analysis by excision PCR with primers flanking the Ds sequence (see materials and methods). We detected the excision products only in embryos injected with both the Ds construct and the TPase mRNA (NLSK5E-TPase or NoNLS-TPase), whereas control embryos injected with the Ds construct alone did not produce PCR fragments of the expected size (Figure 2A). Surprisingly, the NLS-TPase failed the excision assay, while the NLSK5E-TPase produced the highest yield of excision products. The NoNLS-TPase required more RNA to induce excision at a level similar to the NLSK5E-TPase. These experiments were repeated at least three times for each TPase variant using independent mRNA preparations. On the basis of excision data we selected the NLSK5E-TPase as the most productive variant and further used it in the majority of our experiments. Sequencing of the PCR-amplified excision derivatives confirmed that excision occurred specifically at the Ds termini, confirming transposition. The excision products contained a mixture of various excision-repair events resulting in overlapping sequences beginning at the junction of the Ds and the adjacent vector. We observed dominant sequences in the excision products from two vectors with different Ds flanking sequences, indicative of preferential excision-repair outcomes (Figure 2B, supplemental Figure 1 at http://www.genetics.org/supplemental/). These predominant footprints contained deletion of a flanking nucleotide immediately adjacent to one Ds terminus and a change or deletion of a flanking nucleotide at the other Ds end. Predominant Ds excision footprints have been previously reported (Scott et al. 1996; Weil and Kunze 2000).
Figure 2.—
TPase-specific excision and insertion of the Ds element (A) Ds excision assay. Zebrafish embryos were injected with the Ds DNA construct alone (lane 1) or the Ds DNA construct co-injected with NoNLS-, NLSK5E-, and NLS-TPase mRNA (lanes 2, 3, and 4, respectively). DNA was isolated from injected embryos at 10 hpf and subjected to PCR using primers flanking the Ds donor site. M, 1-kb DNA ladder (New England Biolabs, Hitchin, UK). (B) Predominant excision footprints from two different donor vectors. Missing or changed nucleotides of the flanking donor vector are underlined. (C) Representative examples of sequences flanking the Ds insertion sites from two different transgenic F1 fish, demonstrating transposition. For simplicity, 2 of 28 nonredundant flanking DNA sequences matching publicly available zebrafish genomic fragments are shown. Ds end sequences are shown in lowercase type; flanking sequences are shown in uppercase type. The classic 8-bp direct target duplication is shown in boldface and underlined.
Nuclear localization signals affect intracellular localization and aggregation of the Ac TPase:
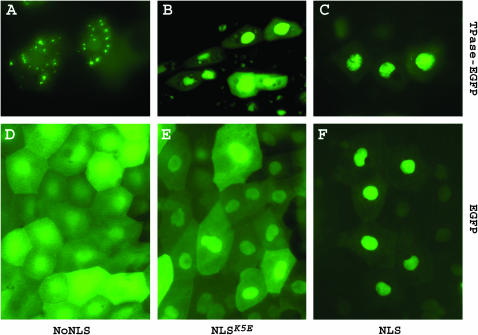
To examine the effects of different NLSs on intracellular localization, we produced the C-terminal EGFP fusion constructs krt8:TPase–EGFP for all three TPases (NoNLS-, NLS-, and NLSK5E-TPase). The krt8 promoter drives expression in a single-cell epithelial layer with large flat cells, which allow observation of intracellular localization in live embryos. The krt8:TPase-EGFP constructs were microinjected into zebrafish embryos at the one-cell stage and EGFP fluorescence was observed at 12–24 hours postfertilization (hpf). High expression levels of the TPase-GFP fusion proteins were toxic for the cells and embryos. Injection of 15 pg of plasmid caused lethality in >50% of embryos by 24 hr of development and the surviving embryos were mostly devoid of krt8-specific EGFP expression. Nevertheless, for each construct we found a small proportion of EGFP-positive cells that retained their epithelial shape, allowing observation of the intracellular localization of the EGFP-tagged TPases (Figure 3, A–C). The NoNLS-TPase-EGFP localized mainly to the cytoplasm, whereas the NLS-TPase-EGFP and NLSK5E-TPase-EGFP proteins were predominantly nuclear. Both NoNLS-TPase-EGFP and NLS-TPase-EGFP showed a strong tendency to form aggregates in the cytoplasm and nucleus, respectively (Figure 3, A–C), that resembled the Activator TPase aggregates reported in plants (Heinlein et al. 1994; Boehm et al. 1995). In contrast, the NLSK5E rarely formed aggregates even at visibly higher expression levels. To confirm whether both NLS and NLSK5E are functional in zebrafish cells, we analyzed subcellular localization of NLS-EGFP and NLSK5E-EGFP fusion proteins in similar experiments (Figure 3, D–F). We observed a gradual increase in the nucleus/cytoplasm distribution ratio of NoNLS–EGFP, NLSK5E-EGFP, and NLS-EGFP, respectively, with maximal nuclear accumulation of NLS-EGFP.
Figure 3.—
Effects of different NLS sequences on the intracellular localization of TPase. (A–C). Subcellular localization of the GFP-tagged versions of NoNLS-TPase, NLSK5E-TPase, and NLS-TPase zebrafish epithelial cells. Photographs were overexposed to highlight the cellular outline. (D–F) Intracellular localization of NoNLS-, NLSK5E-, and NLS-EGFP fusion proteins in zebrafish epithelial cells.
Modified Ac transposase induces high rates of Ds insertions in the germline:
The embryos injected with Ds donor plasmid and TPase mRNAs were raised to adulthood and outcrossed to wild-type fish. We did not preselect embryos on the basis of either intensity or abundance of the GFP signal; all injected embryos were raised to adulthood regardless of GFP expression levels. Approximately 60% of founders (F0) injected with NLS-TPase or NLSK5E-TPase produced F1 embryos with GFP fluorescence (Table 1). The number of EGFP-positive embryos among the progeny was also remarkable: ∼10% of positive founders produced progeny containing >50% of EGFP-positive embryos. One striking example was an individual F0 fish that produced 100% EGFP-positive progeny. Since we observed EGFP fluorescence at 4 days postfertilization, this expression was unlikely maternal (Parinov et al. 2004). Interestingly, individuals within a single clutch very often displayed distinct expression patterns. Such high F1 segregation ratios suggest high efficiency and early developmental time of transposition. Founders injected with NoNLS-TPase produced a significantly lower transgenesis rate (Table 1). We did not observe any EGFP-positive offspring in the control population injected with the Ds construct alone (n = 21; integration of circular DNA is not very efficient in zebrafish).
TABLE 1.
Ds transgenesis rates of different TPase variants
| No NLS | NLSK5E | NLS | |
|---|---|---|---|
| F0 screened | 26 | 91 | 20 |
| F0 producing GFP-positive F1 | 2 | 52 | 12 |
| Transgenesis and expression rate | 8% | 57% | 60% |
| Highest F1 GFP ratio | 14/67 (21%) | 133/138 (96%) | 250/250 (100%) |
Transgenesis rates are calculated as the percentage of founders producing EGFP-positive offspring. Since we measure only the transgenic offspring that express EGFP, these rates are likely underestimated. The final row shows the highest rates of EGFP-positive embryos among the F1 progeny. These are cumulative data from four independent experiments using NLSK5E-TPase and two experiments with NoNLS-TPase.
Integration of the Dissociation element into the zebrafish genome:
We isolated DNA sequences flanking Ds insertions in the F1 fish using thermal asymmetric interlaced PCR TAIL–PCR (Liu and Whittier 1995). Twenty-eight nonredundant flanking sequences perfectly matched zebrafish nucleotide sequences in the GenBank or Ensembl databases. In each instance the match started from the first nucleotide adjacent to Ds 5′- or 3′-termini. Moreover, the Ds insertions were flanked by the classic 8-bp direct duplication of the target site, typically accompanying Ds insertions in plants (Figure 2C). Therefore, Ds integrated into the zebrafish genome through a specific TPase-mediated transposition mechanism. In a small number (n = 6) of F1 families, we isolated flanking sequences corresponding to the original Ds donor vector, which were, however, accompanied by additional nonvector flanking sequences in the same F1 fish. Of 28 identified Ds insertion sites, 21 were found within genes (mainly in introns), suggestive of a potential preference for actively transcribed regions.
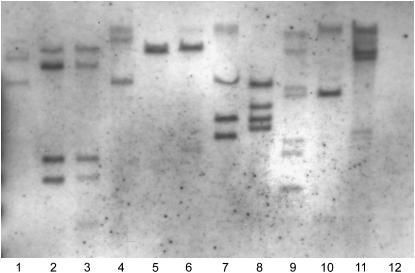
Southern blot hybridization with EGFP-specific probe revealed predominantly multiple insertions in the genome of individual F1 fish (Figure 4). The copy number ranged from one to seven or more insertions per F1 fish with an average of four insertions. Different F1 fish from the same family (descendants from the same F0 founder) often harbored distinct insertions (Figure 4, lanes 1–4).
Figure 4.—
Evaluation of the Ds copy number in F1 fish. To avoid sacrificing the F1 fish, we analyzed DNA from pooled F2 embryos. In each case, a single F1 fish was outcrossed to a wild-type fish and DNA from 12 pooled randomly selected EGFP-positive F2 embryos was used for Southern blot analysis. The DNA samples were digested with EcoRI and hybridized with DIG-labeled EGFP probe. (Lanes 1–4) Progeny of four different F1 fish that originated from the same founder (F0). (Lanes 5–11) Progeny of F1 fish that originated from different F0 founders. (Lane 2) GFP negative control.
Retransposition of the Ds elements integrated in the genome:
To confirm transposition of the Ac/Ds, we remobilized the genomic Ds insertions in the offspring of the F1 fish heterozygous for a single Ds insertion. TPase mRNA was injected into F2 embryos with EGFP expression in the skin epithelia and in the gut (Figure 5). Approximately 95% of the EGFP-positive embryos injected with NLS-TPase (n = 85) or NLSK5E-TPase (n = 72) exhibited novel EGFP expression that appeared mosaically in various organs, including the brain, spinal cord, muscles, heart, liver, gonadal region, etc. (Figure 5). The novel expression patterns can be attributed to the enhancer-trap effect created by reinsertion of the Ds element to the new genomic locations (Parinov et al. 2004). Novel EGFP patterns were not observed in control embryos injected with mRNA encoding the Tol2 transposase (Kawakami et al. 2000), whose recognition sequence is distinct from that of the Ds sequence. Interestingly, injection of NoNLS-TPase resulted in a much lower rate of novel GFP expression (9 of 168 injected EGFP-positive embryos) in comparison to embryos injected with NLS-TPase or NLSK5E-TPase. The novel mosaic patterns induced by NoNLS-TPase were typically simpler, usually affecting only a single cluster of the same cell type. These results confirm that the TPase requires nuclear localization for genomic transposition.
Figure 5.—
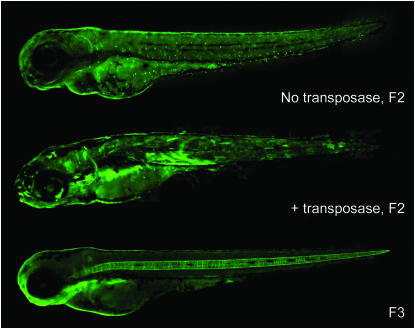
Retransposition of genomic Ds insertions. (Top) Control-injected transgenic fish (F2) with a single heterozygous Ds insertion in the genome exhibiting weak and uniform EGFP expression in the skin epithelia and the gut. (Middle) Example of a fish carrying the same Ds insertion and injected with NLSK5E-TPase mRNA, demonstrating novel EGFP expression in the brain, spinal cord, ears, muscles, gonadal region, and variegated mosaic expression in the skin. (Bottom) Example of a novel expression pattern found in the F3. Expression in the notochord is absent in the control. The dotted pattern in the skin of control fish (top) is not detected in the F3.
The F2 embryos injected with NLSK5E-TPase or control Tol2 transposase mRNA were raised to maturity and outcrossed to wild-type fish. All progeny (F3) of the control-injected transgenic F2 fish continued to express EGFP in the parental pattern that segregated with the expected 1:1 ratio. We found F3 embryos with novel expression patterns in the offspring of 10 of 13 F2 founders (77% germline transmission) injected with NLSK5E-TPase (Figure 5; Table 2). Sequences amplified from the F3 embryos carrying such new expression patterns revealed novel Ds insertion sites that were not present in the original fish line (F1). Hence, the modified Ac transposase is clearly capable of effectively transposing not only the Ds carried by the vector construct supplied via pan-embryonic injection, but also the Ds elements stably integrated into the zebrafish nuclear genome. Importantly, reinserted Ds copies are transmitted to the next generation.
TABLE 2.
Retransposition and loss of the integrated Ds
| F2 parent | GFP+/GFP− in F3 | New GFP patterns | GFP segregation remarks |
|---|---|---|---|
| 1 | 144/151 | — | 1:1 |
| 2 | 70/78 | +1 | 1:1 |
| 3 | 118/130 | +1 | 1:1 |
| 4 | 175/93 | +3 | Ds copies increase |
| 5 | 120/187 | +2 | Loss of Ds |
| 6 | 55/314 | — | Loss of Ds |
| 7 | 56/170 | +1 | Loss of Ds |
| 8 | 41/122 | +1 | Loss of Ds |
| 9 | 225/201 | +1 | 1:1 |
| 10 | 74/253 | +2 | Loss of Ds |
| 11 | 165/172 | — | 1:1 |
| 12 | 126/124 | +2 | 1:1 |
| 13 | 153/134 | +1 | 1:1 |
Transgenic embryos (F2) carrying a single heterozygous Ds insert in their genome were injected with NLSK5E-TPase, raised to maturity, and outcrossed to wild-type fish. The number of novel expression patterns and GFP segregation ratios in F3 are shown.
It is possible that the real number of retranspositions was higher since we detected only the insertions that generated new distinguishable EGFP expression patterns. We also noticed that, in comparison to the enhancer-trap construct carrying the 0.5-kb krt8 promoter that we used in a previous study (Parinov et al. 2004), the Ds construct with the 2.25-kb krt8 promoter used here was markedly less effective for enhancer trapping.
In addition, we frequently observed altered EGFP segregation ratios following remobilization of Ds transposons integrated into the genome (Table 2). Of the 13 injected F2 founders, 1 produced >50% EGFP-positive progeny, significantly higher than expected from an outcross of a founder heterozygous for a single-copy transgene. This suggests an increase in Ds copy number. Five injected F2 founders produced significantly <50% EGFP-positive progeny, indicating partial loss of the Ds. Germinal excision without concomitant Ds reinsertion has been previously reported in plants (Grevelding et al. 1992). Taken together, we detected TPase activity in the germline of 11 of 13 (85%) F2 founders injected with NLSK5E-TPase by observing the presence of a novel GFP expression pattern and/or by altered segregation ratios in their offspring (F3).
Ds can transpose in human cells:
We also tested the ability of Ds to transpose in human cells. We used a plasmid DNA construct containing NLSK5E-TPase under the regulation of the CMV promoter. The Ds construct contained pCMV:EGFP and the blasticidin-resistance gene as selection markers. Both plasmids (containing the Ac and Ds components, respectively) were cotransfected into the human embryonic kidney cell line HEK293 and selected on blasticidin. DNA from the harvested cells was extracted, analyzed by TAIL–PCR, and sequenced to identify TPase-mediated Ds integrations in the human genome. We have successfully obtained flanking sequences that perfectly matched human genome sequences beginning with the first nucleotide immediately adjacent to the Ds 5′- or 3′-termini (supplemental Figure 2 at http://www.genetics.org/supplemental/). In one case, a Ds insertion was flanked by the classic 8-bp direct duplication of the target site that typically accompanies hAT transposons. Therefore, the intracellular environment of human cells also supports Ds transposition.
DISCUSSION
Versatility of Ac/Ds transposition:
Transposons of the hAT superfamily are widespread among eukaryotes, including plants, animals, and fungi. There have been multiple reports of successful transposition of the hAT members in heterologous species, reflecting remarkable adaptation and flexibility of their transposition mechanism (Baker et al. 1986; Weil and Kunze 2000; Ishikawa et al. 2002; Kawakami and Noda 2004; Kolesnik et al. 2004). On the basis of sequence conservation between distant hAT members it was previously hypothesized that horizontal transmission between distinct kingdoms occurred during their evolution (Calvi et al. 1991). However, more recent and accurate analysis did not find any evidence of trans-kingdom horizontal transfer in the evolution of this ancient superfamily (Rubin et al. 2001). The inability to transpose in a very different environment, possibly due to lack of the specific factors involved in the transposition mechanism or due to unspecific suppression, may be one of the reasons preventing horizontal transmission between distant phylogenetic groups. Here we show that the Ds element from plants can effectively transpose in the genome of animal cells supplied with transposase carrying some modifications. This suggests that inactivation of the transposition mechanism in distant hosts is not a critical factor per se that restricts trans-kingdom transfer of at least one member of the hAT family.
Our data show high frequency of Ds transpositions for plasmid-to-genome transpositions as well as for retranspositions within the genome. In addition, these transpositions give rise to multiple insertions and transmit through the germline. This suggests that animal cells have the necessary factors required to operate in concert with Ac transposase and do not harbor factors that suppress Ac/Ds transposition. This supports the hypothesis that Ac transposase is self-sufficient and does not rely on additional proteins for Ds excision and insertion, whereas the host factors participate mainly in the repair of the excision (Yu et al. 2004) and target sites. This is in agreement with the recent demonstration that purified transposase from another hAT element, Hermes, can catalyze DNA cleavage and transposon-target end-joining reactions in vitro without the help of additional proteins (Zhou et al. 2004). Thus, the Ac/Ds and other members of the hAT superfamily may have a wider host range than previously thought. This may also apply to other families of transposable elements.
Effects of nuclear localization on TPase functions:
In plants, the Ac TPase is nuclear localized and a truncation of the first 102 N-terminal amino acids, which contain a strong nuclear localization signal and severely reduce nuclear transport (Heinlein et al. 1994; Boehm et al. 1995). However, the truncated TPase103–807 reportedly produced even higher Ds excision rates compared to full-length Ac TPase in a transient Ds excision assay in Petunia protoplasts cotransfected with Ds and TPase constructs (Houba-Herin et al. 1990; Becker et al. 1992; Kunze et al. 1993).
Our results clearly demonstrate that adding a nuclear localization signal to the truncated TPase103–807 (NoNLS-TPase) results in higher plasmid-to-genome transposition and genomic retransposition rates in zebrafish.
Surprisingly, we did not detect Ds excision using the exclusively nuclear localized NLS-TPase, whereas NoNLS-TPase yielded the expected products in the transient Ds excision assay. Importantly, the excision PCR (see materials and methods) detects only the Ds donor constructs that have undergone at least two processes: (i) excision of the Ds element by the TPase and (ii) subsequent end-joining of the external vector DNA. The plasmids from which the Ds excised but failed to repair the excision sites are not detected by this method. We hypothesize that after Ds excision in the cytoplasm, the external vector DNA undergoes fairly accurate end-joining (presumably followed by amplification), resulting in generation of predominantly uniform products. The presence of DNA ligases in the cytoplasm and the concatenation and amplification of microinjected linear exogenous DNA were previously reported (Soderhall and Lindahl 1975; Prigent et al. 1987; Marini et al. 1988). In the nucleus, the vector DNA from which the Ds excised may be processed differently (e.g., distinct repair mechanism, nuclease degradation). Thus, the Ds excisions generated by NoNLS-TPase and the cytoplasmic pool of NLSK5E-TPase yield excision PCR fragments of the expected size, but excision products generated exclusively in the nucleus by NLS-TPase are not detected by the excision PCR. It is also possible that the amount of the Ds donor plasmid that enters the nucleus and subsequently undergoes excision and repair is insufficient for detection.
In our transient excision assay we analyze DNA from the embryos at 10 hpf, but most excision events likely occur during early development, including the cleavage stages where intact nuclei exist for short periods. Therefore, additional evidence is required to show that nuclear localization of the transposase enzyme affects accessibility to the substrate at that stage.
Furthermore, NoNLS- and NLS-TPases form strong aggregates, whereas the K5E substitution in NLSK5E-TPase reduces such aggregation and may increase the effective concentration of active NLSK5E-TPase. This may explain the slightly higher activity of NLSK5E-TPase compared to NoNLS-TPase in the transient excision assay.
Since the full-length Ac TPase protein is reportedly nuclear localized in insect cells (Hauser et al. 1988), an animal-specific NLS may be unnecessary for effective transposase function in animal cells if unmodified wild-type Ac transposase were used instead of the truncated TPase103–807. Furthermore, the higher activity of TPase103–807 compared to wild-type Ac TPase observed in transient assays in Petunia protoplasts (Houba-Herin et al. 1990; Becker et al. 1992; Kunze et al. 1993) could likely be attributed to the assay design and the intracellular localization of the TPase103–807, rather than to its enzymatic activity. This is further supported by the fact that no difference between TPase103–807 and full-length Ac-TPase was reported in transgenic Arabidopsis plants carrying stably integrated Ds and Ac elements in the genome (Grevelding et al. 1992). Thus, the truncation in TPase103–807 may not be essential for enzymatic activity of the transposase. Future experiments can test if wild-type Ac transposase is efficient in animal cells.
Ac/Ds transposon system as a new tool for animal genetics and biotechnology:
Transposable elements revolutionized genetic research in several model organisms, most notably Drosophila and Arabidopsis (Spradling et al. 1995; Parinov and Sundaresan 2000). However, in vertebrate genetics this area is largely unexplored. Transposons are currently the easiest, the fastest, and the most inexpensive transformation tools used for producing transgenic animals. Retroviral techniques can reportedly generate a higher integration rate that is advantageous for insertional mutagenesis (Gaiano et al. 1996). Both transposons and retroviruses are far less effective than ENU as mutagenes, but they greatly simplify cloning of the tagged genes. However, retroviral vectors have several constraints on construct design, and producing high-titer viruses is a difficult time- and resources-consuming task and requires specialized facilities. Hence their application is limited to only a few laboratories. Most importantly, unlike mutations introduced by ENU or retroviruses, transposon insertions can be reactivated in the presence of transposase, allowing mutagenesis of closely linked genes and inducible phenotype reversal experiments (Tower et al. 1993; Preston et al. 1996; Smith et al. 1996; Machida et al. 1997).
Our results suggest that the Ac/Ds system has several advantages over other transposable elements currently utilized in fish and other vertebrates. In spite of using larger cargo fragments within the Ds, the transgenesis rates that we obtained were at least equal to that reported for Tol2 and Sleeping Beauty transposons (both of fish origin) in zebrafish (Davidson et al. 2003; Kawakami et al. 2004). We found that ∼60% of founders injected with Ds construct and TPase mRNA harbor insertions in the germline. Since we measure only the transgenic offspring that express EGFP, it is likely to be an underestimate of the true transgenesis rate. Moreover, the genomic Ds insertions could be easily remobilized, producing remarkable germline transmission rate (at least 77%). Even in plants such a high germinal Ds transposition rate was rarely achieved, suggesting that animal cells are equally or perhaps even more conducive for Ds transposition than plant cells. In addition, in zebrafish, Ds frequently produced multiple insertions, a feature that can be an asset for insertional mutagenesis. Furthermore, using the assays reported here, we easily produced transgenic fish carrying large 6.5-kb cargo fragments containing multiple genes (5 of 12 founders; data not shown). We have not yet determined the upper limit for the size of the cargo DNA fragment that can be transposed. Finally, introducing a new effective transposon system will allow for the independent use of many different elements in the same host and should minimize the problem of insertion preference (Hacker et al. 2003).
We anticipate that the Ac/Ds system will be widely used for generating transgenic animals and foresee its extensive use in various functional genomics efforts, including insertional mutagenesis, gene- and enhancer-trapping, activation tagging, and gene therapy (Largaespada 2003; Miskey et al. 2005).
Acknowledgments
We are grateful to Clifford Weil and Reinhard Kunze for providing the original miniDs and Ac-ORF plasmids and for sharing unpublished data. We thank Zhiyuan Gong for providing the krt8 promoter-EGFP construct. We thank Karuna Sampath for constructive suggestions and help in editing the manuscript. This research was supported by intramural funds from the Temasek Life Sciences Laboratory, Singapore.
References
- Baker, B., J. Schell, H. Lörz and N. Fedoroff, 1986. Transposition of the maize controlling element “Activator” in tobacco. Proc. Natl. Acad. Sci. USA 83: 4844–4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall, E. L., and D. C. Rio, 1996. Drosophila IRBP/Ku p70 corresponds to the mutagen-sensitive mus309 gene and is involved in P-element excision in vivo. Genes Dev. 10: 921–933. [DOI] [PubMed] [Google Scholar]
- Becker, D., R. Lutticke, M. Li and P. Starlinger, 1992. Control of excision frequency of maize transposable element Ds in Petunia protoplasts. Proc. Natl. Acad. Sci. USA 89: 5552–5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm, U., M. Heinlein, U. Behrens and R. Kunze, 1995. One of three nuclear localization signals of maize Activator (Ac) transposase overlaps the DNA-binding domain. Plant J. 7: 441–451. [DOI] [PubMed] [Google Scholar]
- Calvi, B. R., T. J. Hong, S. D. Findley and W. M. Gelbart, 1991. Evidence for a common evolutionary origin of inverted repeat transposons in Drosophila and plants: hobo, Activator, and Tam3. Cell 66: 465–471. [DOI] [PubMed] [Google Scholar]
- Davidson, A. E., D. Balciunas, D. Mohn, J. Shaffer, S. Hermanson et al., 2003. Efficient gene delivery and gene expression in zebrafish using the Sleeping Beauty transposon. Dev. Biol. 263: 191–202. [DOI] [PubMed] [Google Scholar]
- Ding, S., X. Wu, G. Li, M. Han, Y. Zhuang et al., 2005. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell 122: 473–483. [DOI] [PubMed] [Google Scholar]
- Fadool, J. M., D. L. Hartl and J. E. Dowling, 1998. Transposition of the mariner element from Drosophila mauritiana in zebrafish. Proc. Natl. Acad. Sci. USA 95: 5182–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, S. E., E. Wienholds and R. H. Plasterk, 2001. Regulated transposition of a fish transposon in the mouse germ line. Proc. Natl. Acad. Sci. USA 98: 6759–6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiano, N., M. Allende, A. Amsterdam, K. Kawakami and N. Hopkins, 1996. Highly efficient germ-line transmission of proviral insertions in zebrafish. Proc. Natl. Acad. Sci. USA 93: 7777–7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, Z., B. Ju, X. Wang, J. He, H. Wan et al., 2002. Green fluorescent protein expression in germ-line transmitted transgenic zebrafish under a stratified epithelial promoter from keratin8. Dev. Dyn. 223: 204–215. [DOI] [PubMed] [Google Scholar]
- Grevelding, C., D. Becker, R. Kunze, A. von Menges, V. Fantes et al., 1992. High rates of Ac/Ds germinal transposition in Arabidopsis suitable for gene isolation by insertional mutagenesis. Proc. Natl. Acad. Sci. USA 89: 6085–6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker, U., S. Nystedt, M. P. Barmchi, C. Horn and E. A. Wimmer, 2003. piggyBac-based insertional mutagenesis in the presence of stably integrated P elements in Drosophila. Proc. Natl. Acad. Sci. USA 100: 7720–7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler, A. M., S. P. Gomez and D. A. O'Brochta, 1993. A functional analysis of the P-element gene-transfer vector in insects. Arch. Insect Biochem. Physiol. 22: 373–384. [DOI] [PubMed] [Google Scholar]
- Haring, M. A., C. M. Rommens, H. J. Nijkamp and J. Hille, 1991. The use of transgenic plants to understand transposition mechanisms and to develop transposon tagging strategies. Plant Mol. Biol. 16: 449–461. [DOI] [PubMed] [Google Scholar]
- Hauser, C., H. Fusswinkel, J. Li, C. Oellig, R. Kunze et al., 1988. Overproduction of the protein encoded by the maize transposable element Ac in insect cells by a baculovirus vector. Mol. Gen. Genet. 214: 373–378. [DOI] [PubMed] [Google Scholar]
- Heinlein, M., T. Brattig and R. Kunze, 1994. In vivo aggregation of maize Activator (Ac) transposase in nuclei of maize endosperm and Petunia protoplasts. Plant J. 5: 705–714. [DOI] [PubMed] [Google Scholar]
- Horie, K., A. Kuroiwa, M. Ikawa, M. Okabe, G. Kondoh et al., 2001. Efficient chromosomal transposition of a Tc1/mariner-like transposon Sleeping Beauty in mice. Proc. Natl. Acad. Sci. USA 98: 9191–9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houba-Herin, N., D. Becker, A. Post, Y. Larondelle and P. Starlinger, 1990. Excision of a Ds-like maize transposable element (Ac delta) in a transient assay in Petunia is enhanced by a truncated coding region of the transposable element Ac. Mol. Gen. Genet. 224: 17–23. [DOI] [PubMed] [Google Scholar]
- Ishikawa, N., Y. Johzuka-Hisatomi, K. Sugita, H. Ebinuma and S. Iida, 2002. The transposon Tip100 from the common morning glory is an autonomous element that can transpose in tobacco plants. Mol. Genet. Genomics 266: 732–739. [DOI] [PubMed] [Google Scholar]
- Ivics, Z., P. B. Hackett, R. H. Plasterk and Z. Izsvak, 1997. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 91: 501–510. [DOI] [PubMed] [Google Scholar]
- Jarvis, P., F. Belzile, T. Page and C. Dean, 1997. Increased Ac excision (iae): Arabidopsis thaliana mutations affecting Ac transposition. Plant J. 11: 907–919. [PubMed] [Google Scholar]
- Kawakami, K., and T. Noda, 2004. Transposition of the Tol2 element, an Ac-like element from the Japanese medaka fish Oryzias latipes, in mouse embryonic stem cells. Genetics 166: 895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami, K., A. Shima and N. Kawakami, 2000. Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proc. Natl. Acad. Sci. USA 97: 11403–11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami, K., H. Takeda, N. Kawakami, M. Kobayashi, N. Matsuda et al., 2004. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell 7: 133–144. [DOI] [PubMed] [Google Scholar]
- Kolesnik, T., I. Szeverenyi, D. Bachmann, C. S. Kumar, S. Jiang et al., 2004. Establishing an efficient Ac/Ds tagging system in rice: large-scale analysis of Ds flanking sequences. Plant J. 37: 301–314. [DOI] [PubMed] [Google Scholar]
- Krieg, P. A., and D. A. Melton, 1984. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 12: 7057–7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze, R., U. Behrens, U. Courage-Franzkowiak, S. Feldmar, S. Kuhn et al., 1993. Dominant transposition-deficient mutants of maize Activator (Ac) transposase. Proc. Natl. Acad. Sci. USA 90: 7094–7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromori, T., T. Hirayama, Y. Kiyosue, H. Takabe, S. Mizukado et al., 2004. A collection of 11 800 single-copy Ds transposon insertion lines in Arabidopsis. Plant J. 37: 897–905. [DOI] [PubMed] [Google Scholar]
- Largaespada, D. A., 2003. Generating and manipulating transgenic animals using transposable elements. Reprod. Biol. Endocrinol. 1: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. G., and R. F. Whittier, 1995. Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25: 674–681. [DOI] [PubMed] [Google Scholar]
- Machida, C., H. Onouchi, J. Koizumi, S. Hamada, E. Semiarti et al., 1997. Characterization of the transposition pattern of the Ac element in Arabidopsis thaliana using endonuclease I-SceI. Proc. Natl. Acad. Sci. USA 94: 8675–8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris, J. C., P. L. Nordmann and W. S. Reznikoff, 1990. Integration host factor plays a role in IS50 and Tn5 transposition. J. Bacteriol. 172: 1368–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini, N. J., L. D. Etkin and R. M. Benbow, 1988. Persistence and replication of plasmid DNA microinjected into early embryos of Xenopus laevis. Dev. Biol. 127: 421–434. [DOI] [PubMed] [Google Scholar]
- McClintock, B., 1948. Mutable loci in maize. Carnegie Inst. Washington Year Book 47: 155–169. [PubMed] [Google Scholar]
- McClintock, B., 1951. Chromosome organization and genic expression. Cold Spring Harbor Symp. Quant. Biol. 16: 13–47. [DOI] [PubMed] [Google Scholar]
- Miskey, C., Z. Izsvak, K. Kawakami and Z. Ivics, 2005. DNA transposons in vertebrate functional genomics. Cell. Mol. Life Sci. 62: 629–641. [DOI] [PubMed] [Google Scholar]
- Parinov, S., and V. Sundaresan, 2000. Functional genomics in Arabidopsis: large-scale insertional mutagenesis complements the genome sequencing project. Curr. Opin. Biotechnol. 11: 157–161. [DOI] [PubMed] [Google Scholar]
- Parinov, S., M. Sevugan, D. Ye, W. C. Yang, M. Kumaran et al., 1999. Analysis of flanking sequences from dissociation insertion lines: a database for reverse genetics in Arabidopsis. Plant Cell 11: 2263–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parinov, S., I. Kondrichin, V. Korzh and A. Emelyanov, 2004. Tol2 transposon-mediated enhancer trap to identify developmentally regulated zebrafish genes in vivo. Dev. Dyn. 231: 449–459. [DOI] [PubMed] [Google Scholar]
- Preston, C. R., J. A. Sved and W. R. Engels, 1996. Flanking duplications and deletions associated with P-induced male recombination in Drosophila. Genetics 144: 1623–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigent, C., D. Maniey, J. Lefresne, D. Epel, J. Signoret et al., 1987. Changes in the catalytic properties of DNA ligases during early sea urchin development. Dev. Biol. 124: 281–286. [DOI] [PubMed] [Google Scholar]
- Raz, E., H. G. van Luenen, B. Schaerringer, R. H. Plasterk and W. Driever, 1998. Transposition of the nematode Caenorhabditis elegans Tc3 element in the zebrafish Danio rerio. Curr. Biol. 8: 82–88. [DOI] [PubMed] [Google Scholar]
- Rubin, E., G. Lithwick and A. A. Levy, 2001. Structure and evolution of the hAT transposon superfamily. Genetics 158: 949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., E. F. Fritsch and T. Maniatis, 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Scott, L., D. LaFoe and C. F. Weil, 1996. Adjacent sequences influence DNA repair accompanying transposon excision in maize. Genetics 142: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D., Y. Yanai, Y. G. Liu, S. Ishiguro, K. Okada et al., 1996. Characterization and mapping of Ds-GUS-T-DNA lines for targeted insertional mutagenesis. Plant J. 10: 721–732. [DOI] [PubMed] [Google Scholar]
- Soderhall, S., and T. Lindahl, 1975. Mammalian DNA ligases. Serological evidence for two separate enzymes. J. Biol. Chem. 250: 8438–8444. [PubMed] [Google Scholar]
- Spradling, A. C., D. M. Stern, I. Kiss, J. Roote, T. Laverty et al., 1995. Gene disruptions using P transposable elements: an integral component of the Drosophila genome project. Proc. Natl. Acad. Sci. USA 92: 10824–10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staveley, B. E., T. R. Heslip, R. B. Hodgetts and J. B. Bell, 1995. Protected P-element termini suggest a role for inverted-repeat-binding protein in transposase-induced gap repair in Drosophila melanogaster. Genetics 139: 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower, J., G. H. Karpen, N. Craig and A. C. Spradling, 1993. Preferential transposition of Drosophila P elements to nearby chromosomal sites. Genetics 133: 347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil, C. F., and R. Kunze, 2000. Transposition of maize Ac/Ds transposable elements in the yeast Saccharomyces cerevisiae. Nat. Genet. 26: 187–190. [DOI] [PubMed] [Google Scholar]
- Westerfield, M., 1995. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish. University of Oregon Press, Eugene, OR.
- Yu, J., K. Marshall, M. Yamaguchi, J. E. Haber and C. F. Weil, 2004. Microhomology-dependent end joining and repair of transposon-induced DNA hairpins by host factors in Saccharomyces cerevisiae. Mol. Cell. Biol. 24: 1351–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, L., R. Mitra, P. W. Atkinson, A. B. Hickman, F. Dyda et al., 2004. Transposition of hAT elements links transposable elements and V(D)J recombination. Nature 432: 995–1001. [DOI] [PubMed] [Google Scholar]