Abstract
We have previously identified mutant alleles of genes encoding two Rab proteins, Ypt3 and Ryh1, through a genetic screen using the immunosuppressant drug FK506 in fission yeast. In the same screen, we isolated gdi1-i11, a mutant allele of the essential gdi1+ gene encoding Rab GDP-dissociation inhibitor. In gdi1-i11, a conserved Gly267 was substituted by Asp. The Gdi1G267D protein failed to extract Rabs from membrane and Rabs were depleted from the cytosolic fraction in the gdi1-i11 mutant cells. Consistently, the Gdi1G267D protein was found mostly in the membrane fraction, whereas wild-type Gdi1 was found in both the cytosolic and the membrane fraction. Notably, overexpression of spo20+, encoding a phosphatidylcholine/phosphatidylinositol transfer protein, rescued gdi1-i11 mutation, but not ypt3-i5 or ryh1-i6. The gdi1-i11 and spo20-KC104 mutations are synthetically lethal, and the wild-type Gdi1 failed to extract Rabs from the membrane in the spo20-KC104 mutant. The phosphatidylinositol-transfer activity of Spo20 is dispensable for the suppression of the gdi1-i11 mutation, suggesting that the phosphatidylcholine-transfer activity is important for the suppression. Furthermore, knockout of the pct1+ gene encoding a choline phosphate cytidyltransferase rescued the gdi1-i11 mutation. Together, our findings suggest that Spo20 modulates Gdi1 function via regulation of phospholipid metabolism of the membranes.
IN all eukaryotic cells, Rab family small GTPases (Rabs) form the largest branch of the small GTPase superfamily (Takai et al. 2001). In mammals, Rabs define a family of almost 70 proteins that play critical roles in the trafficking of vesicles that mediate transport between compartments of the exocytic and endocytic pathways (Pfeffer 2001, 2005). Like Ras, Rabs act as molecular switches, cycling between an active GTP-bound state and an inactive GDP-bound state. Thus, transport vesicles bear Rabs with bound GTP; concomitant with or after membrane fusion, Rabs are converted into their GDP-bound states. In this manner, target membranes acquire vesicle-derived Rabs in their GDP-bound conformations (Pfeffer et al. 1995).
We have previously identified two Rab family small GTPases, Ypt3/Its5 and Ryh1/Its6, in the fission yeast Schizosaccharomyces pombe through a genetic screen using the immunosuppressant drug FK506, a specific inhibitor of calcineurin (Cheng et al. 2002; He et al. 2006). In the same genetic screen, we also isolated a mutant of the apm1+ gene that encodes a homolog of the mammalian μ1A subunit of the clathrin-associated adaptor protein-1 complex and that is implicated in the Golgi/endosome function (Kita et al. 2004). This prompted us to further screen immunosuppressant-sensitive mutants to identify molecules involved in membrane trafficking.
Here, we report the identification and characterization of its11-1/gdi1-i11, a new addition to the series of immunosuppressant- and temperature-sensitive mutants. The gdi1+ gene encodes a protein that is highly similar to the mammalian Rab GDP-dissociation inhibitor (GDI) (Sasaki et al. 1990) and to Saccharomyces cerevisiae GDI1/SEC19 (Garrett et al. 1994). GDI is a Rab-specific regulator that plays a key role in the recycling of Rabs (Ullrich et al. 1993). GDI binds and releases Rabs in the presence of GDP but not GTP, which is thought to be a key biochemical function of GDI in retrieving Rabs from target membranes. GDI can retrieve a broad spectrum of Rabs from the membrane following bilayer fusion. In the cytosol, GDI binds Rab in the GDP-bound form. The heterodimeric complex of GDI and Rab–GDP serves as a cytosolic reservoir for delivering Rab to the newly formed vesicles, where it becomes activated to the GTP-bound form (Luan et al. 1999). Thus, GDI plays a key role in the Rab recycling by retrieving prenylated Rabs from their fusion targets in membranes, maintaining Rabs as a soluble cytosolic complex and delivering Rabs to specific membrane-bound compartments for vesicle formation. In mammals, the importance of GDI function is reflected in the findings that mutations in the gene encoding the human GDI result in X-linked nonspecific mental retardation (D'Adamo et al. 1998) and that Gdi1-deficient mice show impairment in associative memory and social behavior (D'Adamo et al. 2002). In S. cerevisiae, GDI1 is an essential gene, and depletion of Gdi1p in vivo leads to loss of the soluble pool of Sec4p and inhibition of protein transport at multiple stages of the secretory pathway (Garrett et al. 1994).
The structural and mutational analysis of GDI has been focused on the regions called sequence conserved regions (SCRs). SCR1 and SCR3B, forming a compact structural unit at the apex of GDI, were reported to be implicated for Rab binding in vitro and in vivo (Schalk et al. 1996; Luan et al. 1999). In addition to Rab binding, domain II, which consists of part of SCR2 and SCR3A, is required for both Rab extraction and Rab loading (Gilbert and Burd 2001). In a study by Luan et al. (2000), the double mutation in SCR3B and SCR3A (R248A-R226A or R248A-Y227A) caused a defect in the association with the membrane.
In this study of fission yeast, we identified a novel mutant allele of the gdi1+ gene (gdi1-i11), containing an amino acid change at codon 267 (Gly to Asp), which is located outside the SCRs. The Gdi1G267D protein failed to extract Rabs from the membrane, yet maintained the ability to bind Rabs. To further delineate Gdi1 function, we screened for dosage-dependent suppressors of the gdi1-i11 mutant and isolated the spo20+ gene. spo20+ is an essential gene, which encodes a phosphatidylcholine/phosphatidylinositol transfer protein (PITP) of the Sec14 family (Nakase et al. 2001). Further study indicated that the gdi1-i11 and spo20-KC104 mutations are synthetically lethal. Consistently, the wild-type Gdi1 failed to extract Rabs from the membrane in the spo20-KC104 mutant, indicating that Spo20 is necessary for Gdi1 to efficiently extract Rabs. We also provide evidence suggesting that the phosphatidylcholine-transfer activity, but not the phosphatidylinositol-transfer activity, is the mechanism of suppression of the gdi1-i11 mutation by Spo20. To the best of our knowledge, this article provides the first evidence suggesting that PITP modulates Gdi1 function via regulation of lipid metabolism.
MATERIALS AND METHODS
Strains, media, and genetic and molecular biology methods:
Strains used in this study are listed in Table 1. The complete medium YPD and the minimal medium EMM have been described previously (Toda et al. 1996). Standard genetic and recombinant-DNA methods (Moreno et al. 1991) were used except where noted. FK506 was provided by Fujisawa Pharmaceutical (Osaka, Japan).
TABLE 1.
S. pombe strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| HM123 | h− leu1-32 | Our stock |
| KP162 | h− leu1-32 ypt3-i5 | Our stock |
| HM528 | h+ his2 | Our stock |
| KP928 | h+ his2 leu1-32 ura4-D18 | Our stock |
| KP1248 | h− leu1-32 ura4-294 | Our stock |
| KP1259 | h+leu1-32 ura4-294 nmt1GFP-ypt3∷ura4+ | Our stock |
| KP1892 | h− leu1-32 gdi1-i11 | This study |
| KP1893 | h+ his2 leu1-32 gdi1-i11 | This study |
| KP2035 | h− leu1-32 ura4-294 nmt1GFP-syb1∷ura4+ | Our stock |
| KP2439 | h− leu1-32 ura4-294 gdi1 nmt1GFP-syb1∷ura4+ | This study |
| KP2454 | h+ his2 leu1-32 ura4-D18 gdi1-i11 | This study |
| KP2580 | h− leu1-32 ura4-D18 ryh1∷ura4+ | Our stock |
| KP2591 | h−/h+ leu1-32/leu1-32 ura4-D18/ura4-D18 his2/+ ade6-M210/ade6-M216 gdi1+/gdi1∷ura4+ | This study |
| KP2678 | h−leu1-32 ura4-D18 spo20-KC104 | Nakase et al. (2001) |
| KP2881 | h+leu1-32 ura4 spo20-KC104 nmt1GFP-ypt3∷ura4+ | This study |
| KP2902 | h− leu1-32 pct1∷ura4+ | This study |
| KP2913 | h− leu1-32 ura4-D18 gdi1-i11 pct1∷ura4+ | This study |
Isolation of the its11-1/gdi1-i11 mutant and cloning of the gdi1+ gene:
The its11-1/gdi1-i11 mutant was isolated in a screen of cells that had been mutagenized with nitrosoguanidine as described previously (Zhang et al. 2000). To clone its11+, the its11-1 mutant (KP1892) was grown at 27° and transformed with an S. pombe genomic DNA library constructed in the vector pDB248 (Beach et al. 1982). Leu+ transformants were replica plated onto YPD plates at 36° and the plasmid DNA was recovered from transformants that showed plasmid-dependent rescue. These plasmids complemented both the immunosuppressant sensitivity and the temperature sensitivity of the its11-1 mutant. By DNA sequencing, the suppressing plasmids were identified to contain the gdi1+ gene (SPAC22H10.12c).
To investigate the relationship between the cloned gdi1+ gene and its11-1 mutant, linkage analysis was performed as follows. The entire gdi1+ gene was subcloned into the pUC-derived plasmid containing the S. cerevisiae LEU2 gene and integrated by homologous recombination into the genome of the wild-type strain HM123. The integrant was mated with the its11-1 mutant. The resulting diploid was sporulated, and tetrads were dissected. A total of 30 tetrads were dissected. In all cases, only parental ditype tetrads were found, indicating allelism between the gdi1+ gene and the its11-1 mutation (data not shown).
Cloning of the spo20+ gene:
To screen for dosage-dependent suppressors of its11-1, the its11-1 mutant (KP1892) was transformed with an S. pombe genomic DNA library, and Leu+ transformants were replica plated onto YPD plates at 30°. By Southern blot analysis, the suppressing plasmids fell into two classes, with one class containing the gdi1+ gene, and the other class containing the spo20+ gene (SPBC3H8.10). No other gene was isolated in this screening.
Tagging of the gdi1+ gene:
The gdi1+ gene was amplified by PCR with genomic DNA of wild-type cells as a template. The sense primer was 5′-CGC GGA TCC ATG GAT GAG GAA TAT GAT GTT ATA GTC C-3′, and the antisense primer was 5′-CGC GGA TCC TTA TTG ATC TTC CAT TTT GGG-3′. The amplified product containing the gdi1+ gene was digested with BamHI, and the resulting fragment was subcloned into BlueScriptSK (+) (Stratagene. La Jolla, CA).
For ectopic expression of proteins, we used the thiamine-repressible nmt1 promoter (Maundrell 1993). Expression was repressed by the addition of 4 μm thiamine to EMM. To express GST–Gdi1, Gdi1 was tagged at its N terminus with GST. GST-Gdi1G267D was made in the same way except that the genomic DNA was from gdi1-i11 mutant cells. Genes either tagged or untagged were subcloned into the pREP1 vector to express the gene (Maundrell 1993).
Gene deletion:
A one-step gene disruption by homologous recombination was performed (Rothstein 1983). The gdi1∷ura4+ disruption was constructed as follows. The BamHI fragment containing the gdi1+ gene was subcloned into the BamHI site of BlueScriptSK (+). Then, a BamHI fragment containing the ura4+ gene was inserted into the BglII site of the previous construct. The fragment containing the disrupted gdi1+ gene was transformed into diploid cells. Stable integrants were selected on medium lacking uracil, and disruption of the gene was checked by genomic Southern hybridization and tetrad analysis. The pct1+ gene (SPCC1827.02c) was disrupted by the insertion of the ura4+ gene at the HincII site, using a one-step gene disruption by homologous recombination as described above.
Microscopy:
Methods in light microscopy, such as fluorescence microscopy and differential interference contrast microscopy, were performed as described (Kita et al. 2004). FM4-64 labeling and staining of vacuoles with Lucifer Yellow (LY) were performed as described (Kita et al. 2004). Septa were visualized by adding 1 μl of 1 mg/ml Calcofluor (Sigma, St. Louis) to a 10- to 20-μl aliquot of cell suspension. Conventional electron microscopy was performed as described (Kita et al. 2004).
Assays and miscellaneous methods:
Tetrad analysis (Zhang et al. 2000), expression and detection of GFP-Pho1 (Cheng et al. 2002), and cell extract preparation and immunoblot analysis (Sio et al. 2005) were performed as previously described. Quantification of the immunoblot data was performed by ImageJ software (http://rsb.info.nih.gov/ij/). Antibody to fission yeast Cdc4 protein was prepared by immunizing the rabbit with purified Cdc4 protein and was used for the detection of endogenous Cdc4 as a loading control.
RESULTS
Isolation of the its11-1 mutant:
To identify proteins that function in membrane trafficking, we searched for mutants that are sensitive to the immunosuppressive drug FK506 and isolated the its11-1 mutant (for immunosuppressant and temperature sensitive). As shown in Figure 1A, its11-1 mutants grew equally as well as the wild-type cells at 27°. However, its11-1 mutant cells could not grow at 36° nor could they grow on YPD containing FK506 at the permissive temperature, whereas wild-type cells grew normally (Figure 1A). As predicted, no double mutant was obtained at any temperature by the genetic cross between its11-1 and calcineurin deletion (Δppb1), indicating that its11-1 and Δppb1 are synthetically lethal (data not shown).
Figure 1.—
Mutation in the its11+/gdi1+ gene causes immunosuppressant- and temperature-sensitive phenotypes. (A) The immunosuppressant and temperature sensitivities of the its11-1/gdi1-i11 mutant cells. Cells transformed with the multicopy vector pDB248 or the vector containing the gdi1+ gene were streaked onto each plate containing YPD and YPD plus 0.5 μg/ml FK506, and then incubated for 4 days at 27° and for 3 days at 36°, respectively. (B) Alignment of protein sequences of S. pombe Gdi1 with related proteins from human and S. cerevisiae. Sequence alignment was performed using the Clustal W program. Asterisks indicate identical amino acids. Arrow indicates the mutation site in the glycine267 of Gdi1, which, when mutated to aspartic acid, resulted in immunosuppressant- and temperature-sensitive function in Gdi1. (C) Schematic of the SCRs and where the gdi1-i11 mutation resides. Star indicates the mutation site.
The its11-1/gdi1-i11 is an allele of the gdi1+ gene that encodes a homolog of mammalian GDP-dissociation inhibitor:
The its11+ gene was cloned by complementation of the temperature-sensitive growth defect of the its11-1 mutant (Figure 1A, YPD36°, +gdi1+). The its11+ gene also complemented the immunosuppressant sensitivity of the its11-1 mutant (Figure 1A, YPD27° +FK506, +gdi1+). Nucleotide sequencing of the cloned DNA fragment revealed that its11+ is identical to the gdi1+ gene (SPAC22H10.12c), which encodes a protein of 440 amino acids that is highly similar to human α-GDI (249/440, 56.59% identity) and S. cerevisiae Gdi1p (272/440, 61.82% identity) (Figure 1B). Linkage analysis was performed (see materials and methods) and results indicated the allelism between the gdi1+ gene and the its11-1 mutation. We therefore renamed its11-1 as gdi1-i11. The its11-1 mutant is the only allele of gdi1+ that was identified in the screen of mutants that show sensitivity to the immunosuppressant.
To identify the mutation in the gdi1-i11 allele, genomic DNA from the gdi1-i11 mutant was isolated, and the full-length coding region of the gdi1-i11 gene was sequenced. The G-to-A nucleotide substitution caused a highly conserved glycine to be altered to an aspartic acid residue at amino acid position 267 (Figure 1B, arrow). We therefore refer to the protein product of the gdi1-i11 gene as Gdi1G267D. It should be noted that Gly267Asp is a novel mutation located outside the SCRs, known to be required for many aspects of GDI function (Figure 1C).
Gdi1 is essential for cell viability:
To determine the effect of complete loss of gdi1+ function, the gdi1+ gene was knocked out in a wild-type diploid strain by homologous recombination using the ura4+ gene as a marker (Rothstein 1983). Southern blotting of the genomic DNA of one such transformant confirmed that the wild-type gdi1+ gene had been replaced by the derivative containing the ura4+ insertion (data not shown). Tetrad analysis indicated that each ascus consisted of two viable and two inviable spores and that all viable spores failed to grow in EMM lacking uracil (data not shown). Microscopic observation of nonviable meiotic progeny showed that these spores germinated but ceased growth soon thereafter. Therefore, the gdi1+ gene is essential for vegetative cell growth and viability.
Rabs failed to distribute in the cytosolic fraction in the gdi1-i11 mutant cells:
To determine which activity of Rab GDI is affected in the gdi1-i11 mutant, we examined the distribution of several Rabs by performing immunoblot analyses of the cytosolic and membrane fractions obtained from wild-type and the gdi1-i11 mutant cells. In wild-type cells, Ypt3, Ryh1, and Ypt2 predominantly localized to the membrane fraction and were also found in the cytosolic fraction [Figure 2, wild type (wt)]. In gdi1-i11 mutant cells, the distribution of the three Rabs changed from cytosol to membrane (Figure 2, gdi1-i11). The distribution of Cdc4 as a loading control remained unchanged (Figure 2, Cdc4). Thus, the gdi1-i11 mutation affects the activity of Gdi1 in extracting Rabs from the membrane.
Figure 2.—
Rabs failed to distribute in the cytosolic fraction in the gdi1-i11 mutant cells. Wild-type cells and gdi1-i11 mutant cells transformed with each of the indicated GFP-Rab were grown in EMM containing 4 μm thiamine for 12 hr. Cells were lysed in the absence of detergent and the resulting extracts were centrifuged at 100,000 × g for 70 min to generate membrane and cytosolic fractions. Equal aliquots were treated with antibodies to GFP. Endogenous Cdc4 was used as a loading control and was immunoblotted using anti-Cdc4 antibodies. C, cytosolic fraction; M, membrane fraction.
The Gdi1G267D protein failed to extract Rabs from the membrane, yet maintained the ability to bind Rabs:
Glycine267 of the Gdi1 protein is highly conserved from fission yeast to humans. This prompted us to investigate the functional significance of the Gdi1G267D mutation. To investigate whether Gdi1G267D protein is affected in its ability to bind Rabs, we expressed GST, GST-tagged Gdi1, or GST-tagged Gdi1G267D protein in GFP-Ypt3 integrated cells and performed GST pull-down experiments. Expression of GST-Gdi1 fully suppressed the phenotypes of the gdi1-i11 mutation, indicating that the fusion protein is functional (data not shown). As shown in Figure 3A, GST-Gdi1G267D interacted with GFP-Ypt3 as efficiently as GST-Gdi1. GST-Gdi1G267D also interacted with GFP-Ryh1 (data not shown). The results suggest that Gdi1G267D protein is not affected in its ability to bind Rabs, consistent with our finding that Gly267 is located outside SCR1 and SCR3B.
Figure 3.—
The characterization of the Gdi1G267D protein. (A) Coprecipitation of Ypt3 with GST-Gdi1G267D. GFP-Ypt3-integrated cells expressing pREP1-GST (control), pREP1-GST-Gdi1, or pREP1-GST-Gdi1 G267D were grown in EMM medium in the absence of thiamine for 20 hr. The proteins were extracted in the presence of detergent. GST-Gdi1, GST-Gdi1G267D, and GST were precipitated by glutathione beads, washed extensively, subjected to SDS–PAGE, and immunoblotted using anti-GFP or anti-GST antibodies. (B) Distribution of Ypt3 and Ryh1 between membrane and cytosolic fractions after wild-type Gdi1 and Gdi1G267D overexpression. GFP-Ypt3- and GFP-Ryh1-integrated cells harboring pREP1-GST-Gdi1 or pREP1-GST-Gdi1G267D were grown in EMM containing 4 μm thiamine for 12 hr. The cells were collected and the fractionation experiment was performed as described in Figure 2. Equal aliquots were treated with anti-GFP antibodies to detect Ypt3 and Ryh1 and with anti-GST antibodies to detect Gdi1. C, cytosolic fraction; M, membrane fraction. (C) Distribution of overexpressed wild-type Gdi1 or Gdi1G267D in cytosolic and membrane fractions in wild-type cells. Wild-type cells harboring pREP1-GST-Gdi1 or pREP1-GST-Gdi1G267D were grown in EMM containing 4 μm thiamine for 20 hr. The cells were collected and the fractionation experiment was performed as described in Figure 2. (D) Quantifications of the distribution of Rabs and GDIs (coexpressed with GFP-Ypt3) between cytosolic and membrane fractions. The panels in Figure 3B were quantitated using ImageJ software.
Furthermore, to investigate the ability of the Gdi1G267D protein to extract Rabs from membranes, we expressed the GST-fused wild-type gdi1+ gene, the gdi1G267D gene, or the control GST vector in strains chromosomally expressing nmt1-GFP-Ypt3 or nmt1-GFP-Ryh1 and performed the fractionation experiments. Fractions from the cells were probed with the anti-GST antibody to detect Gdi1 and with the anti-GFP antibody to detect Ypt3 and Ryh1. As shown in Figure 3, B and D, overexpression of wild-type Gdi1 resulted in a significant increase in the amount of Ypt3 and Ryh1 detected in the cytosolic fraction (+Gdi1), whereas overexpression of the Gdi1G267D protein failed to alter the distribution of these Rabs between the cytosolic and membrane fractions (+Gdi1G267D). Notably, the Gdi1G267D protein was detected in the membrane fraction, while it was almost negligible in the cytosolic fraction (Figure 3, B and D, +Gdi1G267D), whereas wild-type Gdi1 was found in both the cytosolic and the membrane fraction (Figure 3, B and D, +Gdi1). To exclude the possibility that the overexpression of Rabs might affect the distribution of Gdi1, we expressed the wild-type Gdi1 or Gdi1G267D alone in wild-type cells and examined the distribution. Again, the Gdi1G267D protein was not detected in the cytosolic fraction in wild-type cells (Figure 3C).
The gdi1-i11 mutant cells are defective for secretion:
The function and genetic interactions between two Rabs and Gdi1 as described above prompted us to examine the role of Gdi1 in the secretory pathway, as these two Rabs are involved in Golgi/endosome membrane trafficking. For this, we examined the ability of gdi1-i11 mutant cells to secrete S. pombe leader (SPL)-GFP, because this fusion protein is secreted through the same pathway as Pho1 acid phosphatase (Cheng et al. 2002).
As shown in Figure 4, at the permissive temperature there was no marked decrease in the amount of secreted SPL-GFP in the growth medium (gdi1-i11, lane 1). However, when shifted to 33° for 4 hr, the secretion of SPL-GFP was almost abolished in the gdi1-i11 mutant (gdi1-i11, lane 2), whereas no remarkable change was seen in wild-type cells (wt, lane 2). These results clearly demonstrate a defect in the secretory pathway associated with the gdi1-i11 mutant. On the other hand, there was no dramatic change of secreted SPL-GFP when the gdi1-i11 mutant was treated with FK506 for 8 hr (gdi1-i11, lane 4). This suggests that calcineurin is not directly involved in the regulation of the secretory pathway. As calcineurin plays a key role in maintaining cell wall integrity by regulating the enzymes involved in cell wall synthesis, impairment of both Gdi1 and calcineurin, the two key players in cell wall synthesis, might explain the synthetic lethal interaction.
Figure 4.—
Defects in membrane traffic in the gdi1-i11 mutant cells. (A) Defective secretion of SPL-GFP to the growth medium in gdi1-i11 mutants. Wild-type cells and gdi1-i11 mutant cells expressing SPL-GFP were cultured at 27° or 33° for 4 hr or at 27° with or without the addition of FK506 for 8 hr. Proteins were separated by SDS–PAGE and analyzed by Western blotting using anti-GFP antibody. Extra: the trichloroacetic-acid precipitate of supernatant of 5 × 106 cells; Intra: cell extract of 1 × 106 cells. (B) Time-course analysis of LY internalization. Wild-type (wt) and gdi1-i11 mutant cells were incubated in the medium containing Lucifer yellow (5 mg/ml) and the washed cells were viewed under a fluorescence microscope (LY) and a differential interference contrast microscope. Bar, 10 μm. (C) Electron microscopic analysis of gdi1-i11 mutant cells grown at 33° for 4 hr. (D) The boxed regions (a, b, and c) in C are enlarged. (a) Putative post-Golgi vesicles around Golgi structures. Bar, 1 μm. (b) An abnormal membranous structure of a Berkeley body. Bar, 0.5 μm. (c) Fragmented vacuoles in gdi1-i11 mutant. Bar, 1 μm. (d) Abnormal electron-dense membranous structures closely connected to the Golgi structure. Bar, 1 μm.
Fluid-phase uptake of Lucifer Yellow is impaired in gdi1-i11 mutant cells:
To assess the effect of the gdi1-i11 mutation on the endocytic pathway, we used the membrane-impermeable fluorescence dye LY to monitor the fluid-phase endocytosis (Riezman 1985). Wild-type cells and gdi1-i11 mutant cells were incubated in LY at permissive temperature. At the indicated periods, aliquots were washed extensively to remove excess LY and the cells were viewed under Nomarski differential interference contrast and fluorescence microscope. LY clearly labeled the vacuoles of wild-type cells after a 30-min incubation (Figure 4B, wt, 30 and 60 min). However, the gdi1-i11 mutant cells showed only weak staining of the cell wall (Figure 4B, gdi1-i11, 15 and 30 min) and small internal structures (Figure 4B, gdi1-i11, 60 min), indicating that fluid-phase endocytosis is significantly impaired in gdi1-i11 mutant cells even at the permissive temperature. It should be noted that, in ypt3-i5 and ryh1-i6 mutant cells, no defect was detected in endocytosis (data not shown), indicating that Gdi1 has other Rab partner(s) functioning in the endocytic pathway.
Abnormal findings in gdi1-i11 mutant cells as observed in electron micrographs:
As noted, gdi1-i11 mutant cells exhibited various defects in membrane trafficking and this prompted us to perform electron microscopy analysis. Wild-type cells and gdi1-i11 mutant cells were grown at the permissive temperature of 27°, and then each culture was divided into two portions. One portion was maintained at 27°, while the remaining portion was shifted to 33° for 4 hr. Cells were then fixed and examined by electron microscopy. At 27°, the morphology of gdi1-i11 mutant cells was almost indistinguishable from wild-type cells, except that fragmented vacuoles were often observed (data not shown). Upon temperature upshift to 33° for 4 hr, the appearance of gdi1-i11 mutant cells was clearly distinct from that of wild-type cells (Figure 4C). First, the accumulation of putative large post-Golgi vesicles ranging from 100 to 150 nm in size (intensely stained after permanganate fixation) was observed around the Golgi structures [Figure 4D(a)]. These large vesicles were often observed in gdi1-i11 mutant cells (>50%), whereas they were rarely observed in the wild-type cells. Second, the abnormal membranous structure, which may be a Berkeley body that represents abnormal Golgi membrane, was also observed [Figure 4D(b)]. Third, the abnormal electron-dense membranous structures that were ∼500 nm in diameter were observed and were closely connected to the Golgi structures [Figure 4D(d)]. These structures were negligible in wild-type cells. Abnormal membranous structures related to the Golgi structures were observed in gdi1-i11 mutant cells (∼3%), whereas they were seldom observed in the wild-type cells. Fourth, almost all the gdi1-i11 mutant cells showed fragmented vacuoles [Figure 4D(c)], whereas the wild-type cells showed larger vacuoles (data not shown). As observed in the electron micrographs, it should be noted that the abnormal findings in gdi1-i11 mutant cells are similar to those that we previously reported in ypt3-i5 mutant cells (Cheng et al. 2002). Thus, these findings are consistent with our data that Ypt3 accumulated in membrane fraction in gdi1-i11 mutant (Figure 2) and our finding that Gdi1 plays an important role in recycling of Ypt3.
Gdi1 shows genetic interactions with the Sec14-like PITP Spo20:
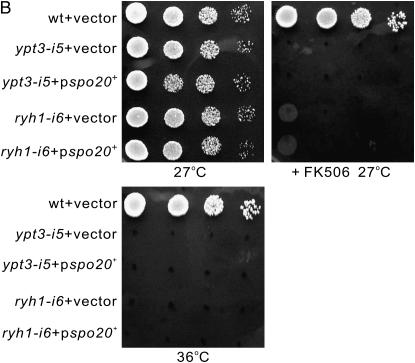
To identify proteins that function together with Gdi1, or that bypass the requirement for Gdi1, we performed a screen for gene dosage-dependent suppressors that could rescue the growth defect of the gdi1-i11 mutants at the nonpermissive temperature of 30°. This led to the isolation of several clones containing the spo20+ gene. As shown in Figure 5A, the overexpression of the spo20+ gene suppressed the growth defect of the gdi1-i11 mutants at 30°, but not at 33°. The spo20+ gene fully suppressed the immunosuppressant sensitivity of the gdi1-i11 mutants.
Figure 5.—
The isolation of spo20+ as a specific dosage-dependent suppressor of the gdi1-i11 mutant cells. (A and B) Overexpression of Spo20-suppressed phenotypes of the gdi1-i11 mutant, but not those of the ypt3-i5 or ryh1-i6 mutant. The indicated cells transformed with the control vector or spo20+ were spotted onto each plate as indicated. Cells were spotted in serial 10-fold dilutions starting with OD660 = 0.3 of log-phase cells (5 μl). (C) Overexpression of Spo20, but not of other Sec14 family members, suppressed the phenotypes of the gdi1-i11 mutant. The gdi1-i11 mutants expressing control vector, SPBC23B6.04, SPBC3H8.02, SPBC365.01, SPBC589.09, and spo20+ were spotted onto the indicated plates and then incubated for 4 days at 27° and 30°, respectively. (D) The gdi1-i11 mutant is synthetically lethal with the spo20-KC104 mutant. Tetrad analysis used progeny derived from crossing KP1893 (h+ his2 leu1-32 gdi1-i11) with KP2678 (h− leu1-32 ura4-D18 spo20-KC104). Colonies grown on YPD at 27 ° were replica plated onto various plates as indicated. Colonies that failed to grow on YPD at 33° and at 36° were predicted to be gdi1-i11 (gdi1), and those that grew on YPD at 33° and grew very slowly at 36° were predicted to be spo20-KC104 (spo20). The rest of the colonies that grew at either of the temperatures were predicted to be wild-type cells (wt). Spores that failed to grow were predicted to be gdi1-i11spo20-KC104 double mutants (D).
We also investigated whether the overexpression of the spo20+ gene could suppress the growth defects of ypt3-i5 and ryh1-i6 mutants. As shown in Figure 5B, overexpression of the spo20+ gene failed to suppress the temperature sensitivity and immunosuppressant sensitivity of these Rab mutants. Thus, Spo20 overexpression suppressed phenotypes of gdi1-i11 mutants, but not those of ypt3-i5 and ryh1-i6 mutants, indicating that Spo20 specifically suppressed the growth defects of gdi1-i11 mutants.
In fission yeast, the spo20+ gene encodes Sec14-like PITP (Nakase et al. 2001), which thus far contains five members, including spo20+. Then, we examined the ability of each of these gene members to suppress the temperature sensitivity and immunosuppressant sensitivity of the gdi1-i11 mutants. The results clearly showed that overexpression of Spo20, but not of other Sec14 family members, suppressed the phenotypes of the gdi1-i11 mutants (Figure 5C), indicating that the suppression of the phenotypes of the gdi1-i11 mutant was specifically caused by the overexpression of Spo20.
To further examine the genetic interaction between Gdi1 and Spo20, we crossed the gdi1-i11 mutant with the spo20-KC104 mutant and performed tetrad analysis (Figure 5D). The spo20-KC104 mutant is a missense allele of the essential spo20+ gene that shows a temperature-sensitive growth defect and is defective in cell separation at the restrictive temperature (Nakase et al. 2001). The results showed that no double mutant was obtained, indicating that gdi1-i11 and spo20-KC104 mutations are synthetically lethal and therefore that Spo20 activity is essential for the viability of gdi1-i11 mutants. Together, these data are indicative of a highly specific functional relationship between Gdi1 and Spo20.
Spo20 suppressed the defective membrane trafficking of the gdi1-i11 mutant cells:
To determine if overexpression of Spo20 could rescue the membrane-trafficking defects of the gdi1-i11 mutants, first we examined the effect of Spo20 overexpression on the abnormal localization of GFP-fused Syb1, the synaptobrevin in fission yeast (Edamatsu and Toyoshima 2003). As the secretory vesicle SNARE, Syb1 would be expected to cycle between the cell surface and the endocytic pathway. In gdi1-i11 mutants harboring vector, GFP-Syb1 failed to localize to the cell ends and accumulated as large dots in the cytosol as compared with wild-type cells (Figure 6A). While in gdi1-i11 mutants harboring spo20+, GFP-Syb1 was visible at the cell ends and the accumulation of the large dots in the cytosol was greatly improved (Figure 6A, +spo20+). This result indicated that Spo20 overexpression suppressed the defective GFP-Syb1 localization of gdi1-i11 mutant.
Figure 6.—
Spo20 suppressed the defective phenotypes associated with gdi1-i11 mutants. (A) Spo20 partially suppressed the defective localization of GFP-Syb1 in the gdi1-i11 mutant cells. The gdi1-i11 mutant cells expressing chromosome-borne GFP-Syb1 were transformed with the control vector, gdi1+, or spo20+ and were examined under the fluorescence microscope. (B) Spo20 suppressed the defective vacuole fusion in the gdi1-i11 mutant cells. The gdi1-i11 mutant cells transformed with each of the indicated plasmids were grown in YPD medium at 27°. Cells were harvested, labeled with FM4-64 fluorescent dye for 60 min (see materials and methods), and then resuspended in water and examined by fluorescence microscopy. Photographs were taken after 60 min. Bar, 10 μm. (C) Spo20 suppressed the defective cytokinesis in the gdi1-i11 mutant cells. The gdi1-i11 mutant cells expressing control vector, spo20+, or gdi1+ were shifted to the restrictive temperature (33°) for 6 hr, or FK506 was added for 6 hr and incubated at 27° and then stained with Calcofluor to visualize cell wall and septum. Bar, 10 μm. (D) Percentage of cells forming a division septum at each time point in the gdi1-i11 mutant cells expressing control vector, spo20+, or gdi1+ after the shift to 33° (left) or the addition of FK506 at 27° (right). Values are the average of three independent experiments with 500 cells counted for each time point. Standard deviations between experiments were <10%.
Second, we examined the effect of Spo20 overexpression on vacuole fusion, as fragmented vacuoles were observed in the electron micrographs [Figure 4D(c)]. FM4-64 is a vital fluorescent dye that is internalized in living cells and then accumulates at the vacuoles (Vida and Emr 1995). When the cells were labeled with FM4-64 for 60 min, tiny vacuoles were observed in the gdi1-i11 mutants (Figure 6B, −H2O, +vector), while big vacuoles were observed in wild-type cells (Figure 6B, −H2O, +gdi1+). In a study by Bone et al. (1998), hypotonic stress causes a dramatic fusion of vacuoles in S. pombe. When cells were collected, washed, and resuspended in water for 60 min, the wild-type cells had evidently large vacuoles that resulted from vacuole fusion (Figure 6B, +H2O, +gdi1+), while vacuoles remained small and numerous in gdi1-i11 mutants (Figure 6B, +H2O, +vector). In contrast, gdi1-i11 mutants transformed with spo20+ contained large vacuoles Figure 6B, +H2O, +spo20+), indicating that overexpression of Spo20 partially but clearly suppressed the defective vacuole fusion of gdi1-i11 mutants.
Finally, we examined the effect of Spo20 overexpression on defective cytokinesis. Cells grown to midlog phase at 27° in liquid YPD medium were subjected to a temperature upshift or to the medium containing FK506 at 27°. Upon shifting to 33° for 6 hr, the frequency of septated cells in gdi1-i11 mutant cells significantly increased to 46% (Figure 6, left panels in C and D, +vector), whereas the septation index of wild-type cells remained unchanged (Figure 6, left panels in C and D, +gdi1+). Strikingly, the magnitude of the defect in gdi1-i11 mutant cells was even more pronounced after the addition of FK506 for 6 hr, because nearly 66% of gdi1-i11 mutant cells were septated (Figure 6, right panels in C and D, +vector) as compared with 34% of wild-type cells that were septated (Figure 6, right panels in C and D, +gdi1+). As shown in Figure 6, C and D, Spo20 overexpression partially suppressed the defect in cytokinesis at the restrictive temperature of 33°. Notably, Spo20 overexpression almost completely suppressed the defective cytokinesis caused by FK506 treatment.
Phosphatidylinositol-transfer activity is dispensable for the suppression of the phenotypes of gdi1-i11 mutants:
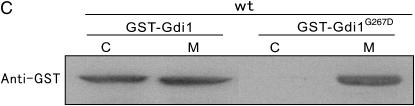
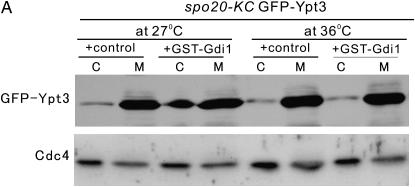
To provide insight into the mechanism by which Spo20 overexpression suppresses the phenotypes of gdi1-i11 mutants, we examined whether Spo20 influences the ability of Gdi1 to extract Rab from the membrane. The spo20-KC104 mutant cells integrated with GFP-Ypt3, harboring control vector or pREP1-GST-Gdi1, were cultured in EMM containing 4 μm thiamine for 8 hr, and then each culture was divided into two portions. One portion was maintained at 27°, while the remaining portion was shifted to 36° for 4 hr. The cells were collected and the fractionation experiment was performed. Notably, in spo20-KC104 mutant cells, Gdi1 failed to extract Ypt3 from membrane when shifted to 36°, although it maintains the ability at the permissive temperature of 27° (Figure 7A). In wild-type cells, Gdi1 maintains its ability to extract Ypt3 from membrane even at 36° (data not shown). Thus, Spo20 is necessary for facilitating Gdi1 to extract Rab from membrane.
Figure 7.—
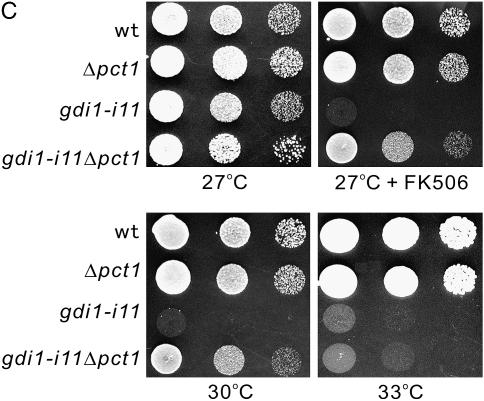
Spo20 modulates Gdi1 function via regulation of phospholipid metabolism. (A) Spo20 affects extraction of Rab from membrane. The spo20-KC104 mutant cells integrated with GFP-Ypt3 harboring control vector or pREP1-GST-Gdi1 were cultured in EMM containing 4 μm thiamine for 8 hr, and then each culture was divided into two portions. One portion was maintained at 27°, while the remaining portion was shifted to 36° for 4 hr. The cells were collected and the fractionation experiment was performed as described in Figure 2. Endogenous Cdc4 was used as a loading control and was immunoblotted using anti-Cdc4 antiserum. C, cytosolic fraction; M, membrane fraction. (B) phosphatidylinositol-transfer activity of Spo20 is dispensable for the suppression of the gdi1-i11 mutant phenotype. The gdi1-i11 mutants expressing the control vector, Spo20K60AK234A, Spo20K60A, Spo20K234A, or wild-type Spo20 were spotted onto the plates as indicated and then incubated for 4 days at 27° and 30°, respectively. (C) The growth defect of the gdi1-i11 mutant was partially rescued by pct1 deletion. Wild-type, gdi1-i11 mutant, Δpct1, and gdi1-i11Δpct1 cells were spotted onto each plate as indicated and then incubated for 4 days at 27° or 30° and for 3 days at 33°, respectively.
In a study by Phillips et al. (1999) cytosol from Sec14K66A- or Sec14K239A-expressing strains exhibited a reduced phosphatidylinositol-transfer activity, and Sec14K66AK239A-expressing strains exhibited no detectable phosphatidylinositol-transfer activity, but normal phosphatidylcholine-transfer activity. To examine whether phosphatidylinositol-transfer activity is necessary for suppression of the gdi1-i11 mutant, we created three mutants, namely Spo20K61A, Spo20K234A, and Spo20K61AK234A, on the basis of an analogy to budding yeast Sec14. As shown in Figure 7B, all three mutant versions of Spo20 suppressed FK506- and temperature-sensitive phenotypes of the gdi1-i11 mutant, and the suppression was the same as that of wild-type Spo20. The result suggests that phosphatidylinositol-transfer activity is dispensable for the suppression of the phenotypes of gdi1-i11 mutants.
Deletion of the pct1+ gene encoding a homolog of choline phosphate cytidyltransferase suppressed the phenotypes of gdi1-i11 mutants:
Sec14p, budding yeast homolog of Spo20, plays a key role in regulating inositol and choline phospholipid metabolism (Huijbregts et al. 2000; Li et al. 2000). The phosphatidylcholine- and phosphatidylinositol-bound forms of Sec14p are proposed to independently regulate inositol and choline phospholipid metabolism (Kearns et al. 1998). Phosphatidylcholine-bound Sec14p is hypothesized to downregulate flux through the diacylglycerol-utilizing citidine 5′-diphosphocholine (CDP-choline) pathway for phosphatidylcholine biosynthesis via inhibiting the activity of the PCT1 gene encoding choline phosphate cytidyltransferase, a rate-limiting enzyme. Assuming that overexpression of Spo20 may suppress the phenotypes of the gdi1-i11 mutant by inhibiting Pct1 activity, we examined whether the pct1 deletion can rescue the phenotypes of gdi1-i11 mutants. As expected, the growth defect of the gdi1-i11 mutant was rescued by pct1 deletion (Figure 7C) to the same extent as that of Spo20 overexpression (Figure 5A), thus providing further evidence that Spo20 may affect Gdi1 function via modulating phosphatidylcholine metabolism.
DISCUSSION
Our study highlights the functional significance of the highly conserved Gly267 located outside the SCRs of Gdi1. More importantly, we discovered a genetic and functional interaction between Gdi1 and genes encoding proteins involved in phospholipid metabolism.
Highly conserved Gly267 is important for Gdi1 function in vivo:
Structural and mutational analyses of Gdi1 have focused on the SCRs. SCR1 and 3B were implicated for Rab binding in vitro and in vivo (Schalk et al. 1996; Luan et al. 1999). In S. cerevisiae, two mutants were reported to be defective in extracting Rabs from membrane. One mutant contained multiple mutations located in domain II, which consists of part of SCR2 and SCR3A (Gilbert and Burd 2001), and the other mutant contained mutations in both SCR3B and SCR3A (Luan et al. 2000). However, the gdi1-i11 single substitution Gly267Asp mutation is located outside the SCRs and the overexpression of Gdi1G267D protein failed to increase the cytosolic pool of Rabs, thus indicating a defect in extracting Rabs from membranes. However, the Gdi1G267D protein maintains the ability to bind Rabs.
Remarkably, the cytosolic Gdi1G267D protein dramatically decreased, yet maintained the ability to associate with the membrane. This is the first report of an amino acid change located outside SCRs affecting the intracellular distribution of Gdi1, and this might be a novel regulatory mechanism of Gdi1 function. It may not be merely the charge of the aspartic acid residue that altered the intracellular Gdi1 distribution; instead, the mutation may cause a conformational change by introducing a bulkier group than the wild-type glycine.
Functional link between Gdi1 and Sec14-like phosphatidylinositol transfer protein Spo20:
Here, we identified Spo20 as a dosage-dependent suppressor of the gdi1-i11 mutation. Overexpression of Spo20 also suppressed the defects in membrane trafficking, including abnormal Syb1 localization, defective vacuole fusion, and defective cytokinesis.
How does the overexpression of Spo20 suppress the phenotypes of gdi1-i11 mutants? Spo20, similar to S. cerevisiae Sec14p, regulates the Golgi secretory function in fission yeast (Nakase et al. 2001). Thus, it might be that Spo20 suppressed phenotypes of gdi1-i11 by promoting the membrane trafficking from Golgi. However, Spo20 overexpression suppressed the growth defects of gdi1-i11 mutants, but not those of ypt3-i5 or ryh1-i6 mutants that demonstrate defects in Golgi membrane trafficking, indicating that the suppression by Spo20 is highly specific to gdi1-i11 mutants and not caused by its effects on Golgi trafficking.
To address the role of Gdi1 and its regulation by Spo20, we examined whether the phosphatidylinositol-transfer activity of Spo20 is required for suppression of gdi1-i11. Surprisingly, our results showed that phosphatidylinositol-transfer activity is dispensable for the suppression of gdi1-i11 phenotypes, suggesting that the mechanisms of suppression include phosphatidylcholine-transfer activity of Spo20. Consistently, the ability to suppress gdi1-i11 is unique to Spo20 that was reported to exhibit both phosphatidylinositol- and phosphatidylcholine-transfer activity (Nakase et al. 2001) and is not shared by other PITP-encoding genes, which were reported to exhibit only phosphatidylinositol-transfer activity in budding yeast.
To further investigate whether Spo20 modulates Gdi1 function, we examined Rab membrane extraction by Gdi1 in spo20-KC104 mutant cells. The wild-type Gdi1 was able to extract Rabs from the membrane in the wild-type background, but failed to extract them in the spo20-KC104 mutant at the restrictive temperature. Thus, the combination of mutations in spo20-KC104 and gdi1-i11, by causing severe reduction of Rab extraction from membranes, would disrupt membrane trafficking mediated by Rabs. This might explain the synthetic lethality between spo20-KC104 and gdi1-i11. According to the study by Fovick and Novick (2000), a synthetic lethality can be interpreted as indicating that both genes function in the same essential pathway if the mutation causes partial loss-of-function in cases where single null mutations are lethal. As single null mutations in gdi1+ and spo20+ are lethal, the synthetic lethal interaction between gdi1-i11 and spo20-KC104 indicates that these genes function in the same essential pathway.
As mentioned above, phosphatidylcholine-transfer activity of Spo20 may be important in that it modulates the lipid composition of the membrane structure, and this in turn affects the ability of Gdi1 to extract Rab. Consistent with these findings, deletion of the pct1+ gene that encodes a homolog of choline phosphate cytidyltransferase, a rate-limiting enzyme in the diacylglycerol-utilizing CDP-choline pathway for phosphatidylcholine biosynthesis, rescued the gdi1-i11 mutation to the same extent as that of Spo20 overexpression. This further strengthens our hypothesis that Spo20 exerts its effect on the gdi1-i11 mutant through regulation of phospholipid metabolism.
Acknowledgments
We thank Takashi Toda, Mitsuhiro Yanagida, Chikashi Shimoda, Kaoru Takegawa, and the National Bio-Resource Project for providing strains and plasmids; Susie O. Sio for critical reading of the manuscript; Hideyuki Mukai for helpful suggestions; and Fujisawa Japan for gifts of FK506. This work was supported by the 21st Century Center of Excellence Program, the Asahi Glass Foundation, the Uehara Memorial Foundation, and research grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Beach, D., M. Piper and P. Nurse, 1982. Construction of a Schizosaccharomyces pombe gene bank in a yeast bacterial shuttle vector and its use to isolate genes by complementation. Mol. Gen. Genet. 187: 326–329. [DOI] [PubMed] [Google Scholar]
- Bone, N., J. B. Millar, T. Toda and J. Armstrong, 1998. Regulated vacuole fusion and fission in Schizosaccharomyces pombe: an osmotic response dependent on MAP kinases. Curr. Biol. 8: 135–144. [DOI] [PubMed] [Google Scholar]
- Cheng, H., R. Sugiura, W. Wu, M. Fujita, Y. Lu et al., 2002. Role of the Rab GTP-binding protein Ypt3 in the fission yeast exocytic pathway and its connection to calcineurin function. Mol. Biol. Cell 13: 2963–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Adamo, P., A. Menegon, N. C. Lo, M. Grasso, M. Gulisano et al., 1998. Mutations in GDI1 are responsible for X-linked non-specific mental retardation. Nat. Genet. 19: 134–139. [DOI] [PubMed] [Google Scholar]
- D'Adamo, P., H. Welzl, S. Papadimitriou, D. B. Raffaele, C. Tiveron et al., 2002. Deletion of the mental retardation gene Gdi1 impairs associative memory and alters social behavior in mice. Hum. Mol. Genet. 11: 2567–2580. [DOI] [PubMed] [Google Scholar]
- Edamatsu, M., and Y. Y. Toyoshima, 2003. Fission yeast synaptobrevin is involved in cytokinesis and cell elongation. Biochem. Biophys. Res. Commun. 301: 641–645. [DOI] [PubMed] [Google Scholar]
- Finger, F. P., and P. Novick, 2000. Synthetic interactions of the post-Golgi sec mutations of Saccharomyces cerevisiae. Genetics 156: 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett, M. D., J. E. Zahner, C. M. Cheney and P. J. Novick, 1994. GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J. 13: 1718–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, P. M., and C. G. Burd, 2001. GDP dissociation inhibitor domain II required for Rab GTPase recycling. J. Biol. Chem. 276: 8014–8020. [DOI] [PubMed] [Google Scholar]
- He, Y., R. Sugiura, Y. Ma, A. Kita, L. Deng et al., 2006. Genetic and functional interaction between Ryh1 and Ypt3: two Rab GTPases that function in S. pombe secretory pathway. Genes Cells 11: 207–221. [DOI] [PubMed] [Google Scholar]
- Huijbregts, R. P., L. Topalof and V. A. Bankaitis, 2000. Lipid metabolism and regulation of membrane trafficking. Traffic 1: 195–202. [DOI] [PubMed] [Google Scholar]
- Kearns, B. G., J. G. Alb, Jr and V. Bankaitis, 1998. Phosphatidylinositol transfer proteins: the long and winding road to physiological function. Trends Cell Biol. 8: 276–282. [DOI] [PubMed] [Google Scholar]
- Kita, A., R. Sugiura, H. Shoji, Y. He, L. Deng et al., 2004. Loss of Apm1, the μ1 subunit of the clathrin-associated adaptor-protein-1 complex, causes distinct phenotypes and synthetic lethality with calcineurin deletion in fission yeast. Mol. Biol. Cell 15: 2920–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., S. M. Routt, Z. Xie, X. Cui, M. Fang et al., 2000. Identification of a novel family of nonclassic yeast phosphatidylinositol transfer proteins whose function modulates phospholipase D activity and Sec14p-independent cell growth. Mol. Biol. Cell 11: 1989–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan, P., W. E. Balch, S. D. Emr and C. G. Burd 1999. Molecular dissection of guanine nucleotide dissociation inhibitor function in vivo. Rab-independent binding to membranes and role of Rab recycling factors. J. Biol. Chem. 274: 14806–14817. [DOI] [PubMed] [Google Scholar]
- Luan, P., A. Heine, K. Zeng, B. Moyer, S. E. Greasely et al., 2000. A new functional domain of guanine nucleotide dissociation inhibitor (α-GDI) involved in Rab recycling. Traffic 1: 270–281. [DOI] [PubMed] [Google Scholar]
- Maundrell, K., 1993. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123: 127–130. [DOI] [PubMed] [Google Scholar]
- Moreno, S., A. Klar and P. Nurse 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194: 795–823. [DOI] [PubMed] [Google Scholar]
- Nakase, Y., T. Nakamura, A. Hirata, S. M. Routt, H. B. Skinner et al., 2001. The Schizosaccharomyces pombe spo20+ gene encoding a homologue of Saccharomyces cerevisiae Sec14 plays an important role in forespore membrane formation. Mol. Biol. Cell 12: 901–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer, S. R., 2001. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11: 487–491. [DOI] [PubMed] [Google Scholar]
- Pfeffer, S. R., 2005. Structural clues to Rab GTPase functional diversity. J. Biol. Chem. 280: 15485–15488. [DOI] [PubMed] [Google Scholar]
- Pfeffer, S. R., A. B. Dirac-Svejstrup and T. Soldati 1995. Rab GDP dissociation inhibitor: putting rab GTPases in the right place. J. Biol. Chem. 270: 17057–17059. [DOI] [PubMed] [Google Scholar]
- Phillips, S. E., B. Sha, L. Topalof, Z. Xie, J. G. Alb et al., 1999. Yeast Sec14p deficient in phosphatidylinositol transfer activity is functional in vivo. Mol. Cell 4: 187–197. [DOI] [PubMed] [Google Scholar]
- Riezman, H., 1985. Endocytosis in yeast: several of the yeast secretory mutants are defective in endocytosis. Cell 40: 1001–1009. [DOI] [PubMed] [Google Scholar]
- Rothstein, R. J., 1983. One-step gene disruption in yeast. Methods Enzymol. 101: 202–211. [DOI] [PubMed] [Google Scholar]
- Sasaki, T., A. Kikuchi, S. Araki, Y. Hata, M. Isomura et al., 1990. Purification and characterization from bovine brain cytosol of a protein that inhibits the dissociation of GDP from and the subsequent binding of GTP to smg p25A, a ras p21-like GTP-binding protein. J. Biol. Chem. 265: 2333–2337. [PubMed] [Google Scholar]
- Schalk, I., K. Zeng, S. K. Wu, E. A. Stura, J. Matteson et al., 1996. Structure and mutational analysis of Rab GDP-dissociation inhibitor. Nature 381: 42–48. [DOI] [PubMed] [Google Scholar]
- Sio, S. O., T. Suehiro, R. Sugiura, M. Takeuchi, H. Mukai et al., 2005. The role of the regulatory subunit of fission yeast calcineurin for in vivo activity and its relevance to FK506 sensitivity. J. Biol. Chem. 280: 12231–12238. [DOI] [PubMed] [Google Scholar]
- Takai, Y., T. Sasaki and T. Matozaki 2001. Small GTP-binding proteins. Physiol. Rev. 81: 153–208. [DOI] [PubMed] [Google Scholar]
- Toda, T., S. Dhut, G. Superti-Furga, Y. Gotoh, E. Nishida et al., 1996. The fission yeast pmk1+ gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol. Cell. Biol. 16: 6752–6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich, O., H. Stenmark, K. Alexandrov, L. A. Huber, K. Kaibuchi et al., 1993. Rab GDP dissociation inhibitor as a general regulator for the membrane association of rab proteins. J. Biol. Chem. 268: 18143–18150. [PubMed] [Google Scholar]
- Vida, T. A., and S. D. Emr 1995. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128: 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., R. Sugiura, Y. Lu, M. Asami, T. Maeda et al., 2000. Phosphatidylinositol 4-phosphate 5-kinase Its3 and calcineurin Ppb1 coordinately regulate cytokinesis in fission yeast. J. Biol. Chem. 275: 35600–35606. [DOI] [PubMed] [Google Scholar]