Abstract
Most organisms form protein-rich, linear, ladder-like structures associated with chromosomes during early meiosis, the synaptonemal complex. In Schizosaccharomyces pombe, linear elements (LinEs) are thread-like, proteinacious chromosome-associated structures that form during early meiosis. LinEs are related to axial elements, the synaptonemal complex precursors of other organisms. Previous studies have led to the suggestion that axial structures are essential to mediate meiotic recombination. Rec10 protein is a major component of S. pombe LinEs and is required for their development. In this report we study recombination in a number of rec10 mutants, one of which (rec10-155) does not form LinEs, but is predicted to encode a truncated Rec10 protein. This mutant has levels of crossing over and gene conversion substantially higher than a rec10 null mutant (rec10-175) and forms cytologically detectable Rad51 foci indicative of meiotic recombination intermediates. These data demonstrate that while Rec10 is required for meiotic recombination, substantial meiotic recombination can occur in rec10 mutants that do not form LinEs, indicating that LinEs per se are not essential for all meiotic recombination.
SEXUAL reproduction in most eukaryotes involves haploid gamete cells fusing to form a diploid cell, which, in metazoans, is the progenitor for the multiple cells found in the different tissues of the body and the germ line. The generation of the haploid gametes is dependent upon a specialized cell division known as meiosis, in which a diploid cell undergoes a single genome replication event followed by two successive rounds of chromosome segregation. The first meiotic chromosome segregation event involves a complex series of interactions between homologous chromosomes, culminating in a physical conjoining via the formation of genetic recombination intermediates that ensure correct segregation of homologs at meiosis I.
In most organisms, proteinacious structures, known as axial elements (AEs), form on chromosomes during meiotic prophase. AEs are the precursors to a synaptic structure, the synaptonemal complex (SC), which forms between homologs. The function of the SC remains poorly understood (Zickler and Kleckner 1999; Bishop and Zickler 2004). The fission yeast, Schizosaccharomyces pombe, is a member of a unique group of organisms that do not form fully mature SCs, although S. pombe does form cytologically distinct linear elements (LinEs) that exhibit similarities to AEs (Bähler et al. 1993; Kohli and Bähler 1994; Lorenz et al. 2004). The LinE structures of S. pombe and the AEs of Saccharomyces cerevisiae have conserved common features and the key structural components in the two organisms, Rec10 (S. pombe) and Red1 (S. cerevisiae), exhibit some amino acid conservation (Lorenz et al. 2004). This structural conservation and the absence of a visible SC in S. pombe strongly suggest that AEs serve some function other than providing a precursory platform for SC formation; they may function by contributing to the establishment of chromosomal mechanical forces of compaction, which have been proposed to regulate meiotic recombination (Blat et al. 2002; Kleckner et al. 2004). Furthermore, AEs (and possibly LinEs) appear to play a critical role in directing interhomolog recombination, precluding intersister recombination events (Schwacha and Kleckner 1997; Thompson and Stahl 1999), possibly by providing a platform for Hop1 protein-mediated dimerization of the Mek1 kinase, which, like Red1 and Hop1, is required to promote interhomolog recombination (Niu et al. 2005).
Although LinE formation is rec10+ dependent (Molnar et al. 2003; Lorenz et al. 2004), the precise function(s) of Rec10 and LinEs remains unclear. rec10 mutants have been isolated in a genetic screen for mutants that are defective in meiotic recombination, indicating that Rec10 is required for this process (Ponticelli and Smith 1989; De Veaux et al. 1992). However, until now the question of whether or not LinEs are essential for meiotic recombination remained unanswered (Loidl 2006). Further detailed analysis of one particular rec10 mutant, rec10-109, indicated that Rec10 functions to regulate recombination in a region-specific fashion (De Veaux and Smith 1994; Krawchuk et al. 1999). This is consistent with the observation that S. cerevisae Red1 null mutants are defective for recombination at some, but not all loci (Rockmill and Roeder 1990). The regional specificity of the rec10-109 mutant shows that Rec10 regulates recombination in the middle region of chromosomes, a pattern similar to that observed for the meiosis-specific cohesins, Rec8 and Rec11, although residual LinEs have been observed in this mutant, suggesting that it is not totally defective in LinE function (De Veaux and Smith 1994; Krawchuk et al. 1999; Parisi et al. 1999; Lorenz et al. 2004). The similar regional pattern of recombination in the rec10-109 and cohesion mutants indicate that there is an intimate functional association between meiotic cohesins and LinEs. However, recent studies demonstrate that Rec10 is required to regulate recombination more widely throughout the genome, indicating that the middle region regulation pattern exposed by the study of rec10-109 is allele specific (Ellermeier and Smith 2005; this study).
The lack of a cytologically distinct SC in S. pombe makes it an exceptionally amenable system in which to reveal functions of SC components in addition to their role in the immediate juxtapositioning of homologous chromosomes. In this report we address one of the central questions relating to LinE function. We demonstrate that while loss of LinEs correlates with a reduction in recombination throughout the genome, substantial crossing over remains in the absence of LinEs, indicating that LinEs are not essential for all recombination. This also demonstrates that Rec10 has functions distinct from its role in LinE formation. Finally, we demonstrate that LinE-defective cells have a regional bias in the relative reduction of gene conversion events and that at some loci gene conversions are more severely affected than crossovers in the associated intervals. This feature of Rec10 is discussed in parallel with its role in crossing over.
MATERIALS AND METHODS
S. pombe strains:
A list of strains employed in this study and their genotypes is provided in Table 1. Culture media and strain storage were as described by Moreno et al. (1991). Construction of plasmid pYL167 was previously described by Lin and Smith (1995) and it was transformed into S. pombe as described by Moreno et al. (1991).
TABLE 1.
Strains used in this study
| Straina | Genotype | Source |
|---|---|---|
| BP11 | h− ade6-M26 | McFarlane collection |
| BP85 | h− lys7-1 | McFarlane collection |
| BP86 | h+ lys7-2 | McFarlane collection |
| BP265 | h− lys4-95 | McFarlane collection |
| BP372 | h− ade6-M26 rec10-144 | This study |
| GP746 (BP404) | h− ura1-61 | G. R. Smith |
| GP2020 (BP409) | h− ade6-704 | G. R. Smith |
| BP581 | h− ade6-M26 rec10-155:LEU2+ leu1-32 | This study |
| BP670 | h+ ade6-M26 his4-239 | This study |
| BP691 | h− ade6-L52 lys4-95 | This study |
| BP692 | h− ade6-L52 lys4-95 rec10-144 | This study |
| BP693 | h+ ade6-M26 his4-239 rec10-144 | This study |
| BP771 | h+ ade6-M26 leu2-120 | This study |
| BP772 | h− ade6-L52 lys7-1 | This study |
| BP805 | h+ ade6-M26 leu2-120 rec10-144 | This study |
| BP823 | h+ ade6-L52 tps16-23 | This study |
| BP856 | h+ ade6-L52 tps16-23 rec10-144 | This study |
| BP862 | h+ ade6-L52 tps16-23 rec10-155:LEU2+ leu1-32 | This study |
| BP863 | h− ade6-L52 lys7-1 rec10-144 | This study |
| BP874 | h+ ade6-M26 lys7-2 rec10-144 | This study |
| BP880 | h− ade6-M26 arg3-124 leu1-32 rec10-155∷LEU2+ | This study |
| BP888 | h− ade6-M26 arg3-124 | This study |
| BP890 | h+/h− ade6-M210/ade6-M216 rec10-155∷LEU2+/rec10-155∷LEU2+ leu1-32/leu1-32 | This study |
| BP892 | h+ ade6-L52 pro2-1 leu1-32 rec10-155∷LEU2+ | This study |
| BP896 | h+ ade6-L52 pro2-1 | This study |
| BP904 | h+ his4-239 leu1-32 rec10-155∷LEU2+ | This study |
| BP905 | h+ ade6-M26 lys7-2 | This study |
| BP915 | h− lys4-95 leu1-32 rec10-155∷LEU2+ | This study |
| BP926 | h+ his4-239 | This study |
| BP968 | h+ pro1-1 leu1-32 | This study |
| BP970 | h− arg4-55 leu1-32 rec10-155∷LEU2+ | This study |
| BP971 | h+ ade8-106 rec10-155∷LEU2+ | This study |
| BP972 | h− arg4-55 | This study |
| BP974 | h+ ade8-106 | This study |
| BP981 | h+ pro1-1 leu1-32 rec10-155∷LEU2+ | This study |
| BP1012 | h+ lys7-2 rec10-155∷LEU2+ leu1-32 | This study |
| BP1013 | h− lys7-1 rec10-155∷LEU2+ leu1-32 | This study |
| BP1027 | h+/h− ade-M210/ade6-M216 | This study |
| BP1045 | h− ura1-61 rec10-175∷kanMX6 | This study |
| BP1046 | h+ ura1-171 rec10-175∷kanMX6 | This study |
| BP1047 | h+ ura1-171 | This study |
| BP1048 | h− ura1-171 | This study |
| BP1049 | h+ ura1-61 | This study |
| BP1100 | h+ ura1-61 leu1-32 rec10-155∷LEU2+ | This study |
| BP1102 | h− ura1-171 leu1-32 rec10-155∷LEU2+ | This study |
| BP1148 | h+ rad32Δ∷ura4+ ura4-D18 leu1-32 | This study |
| BP1149 | h− rad32Δ∷ura4+ ura4-D18 leu1-32 | This study |
| BP1150 | h+ rad32Δ∷ura4+ ura4-D18 leu1-32 rec10-155∷LEU2+ ade6-704 | This study |
| BP1151 | h− rad32Δ∷ura4+ ura4-D18 leu1-32 rec10-155∷LEU2+ ade6-704 | This study |
| BP1152 | h+ ade6-704 | This study |
| BP1154 | h+ leu1-32 rec10-155∷LEU2+ ade6-704 | This study |
| BP1155 | h− leu1-32 rec10-155∷LEU2+ ade6-704 | This study |
| BP1156 | h− rec10-175∷kanMX6 ura4-D18 ade6-704 | This study |
| BP1157 | h+ rec10-175∷kanMX6 ura4-D18 ade6-704 | This study |
| BP1172 | h+/h− ade-M210/ade6-M216 rec10-175∷kanMX6/rec10-175∷kan MX6 | This study |
| BP1182 | h+ leu1-32 ura1-61 rec10-175∷kanMX6 (pYL167) | This study |
| BP1183 | h+ leu1-32 ura1-61 rec10-175∷kanMX6 (pSP1) | This study |
| BP1184 | h− leu1-32 ura1-171 rec10-175∷kanMX6 (pYL167) | This study |
| BP1185 | h− leu1-32 ura1-171 rec10-175∷kanMX6 (pSP1) | This study |
| BP1191 | h− rec10-175∷kanMX6 rad32Δ∷ura4+ ura4-D18 ade6-704 | This study |
| BP1192 | h+ rec10-175∷kanMX6 rad32Δ∷ura4+ ura4-D18 ade6-704 | This study |
| BP1193 | h+ leu1-32 lys4-95 ura4-D18 rec10-175∷kanMX6 (pYL167) | This study |
| BP1194 | h+ leu1-32 lys4-95 ura4-D18 rec10-175∷kanMX6 (pSP1) | This study |
| BP1195 | h− leu1-32 his4-95 ura4-D18 rec10-175∷kanMX6 (pYL167) | This study |
| BP1196 | h− leu1-32 his4-95 ura4-D18 rec10-175∷kanMX6 (pSP1) | This study |
Full genealogies are available upon request.
Meiotic crosses:
Cultures were grown in yeast extract liquid (YEL), supplemented with 100 mg/ml adenine, to a density of ∼2.5 × 107 cells/ml. A total of 600 μl of each strain to be mated were added to a sterile microfuge tube, pulse centrifuged, and aspirated. Cell pellets were washed with 1 ml sterile dH2O and finally resuspended in 20 μl dH2O. Suspensions were spotted onto fully supplemented synthetic sporulation media (SPA) plates and incubated at 30° for 3–4 days. After incubation, sporulating cells were scrapped into a microfuge tube containing 1 ml of 0.6% β-glucuronidase (Sigma, St. Louis)/dH2O solution and incubated for 16 hr at 25°. After incubation spores were harvested and resuspended in 30% ethanol and incubated at room temperature for no longer than 5 min. Suspensions were then centrifuged and aspirated dry and cell pellets were resuspended in 1 ml sterile dH2O.
Determination of recombination frequencies:
Intragenic recombination frequencies were determined as previously described (Pryce et al. 2005). For ura1 and lys7 intragenic recombination uracil- and lysine-deficient media were employed, respectively.
To determine intergenic recombination frequencies using prototrophic markers, serial dilutions of spore suspensions were plated onto yeast extract agar (YEA) plates to a colony density of ∼50–100 colonies per plate. These were then replica plated onto nitrogen base agar (NBA) plates with and without appropriate supplements to permit the counting of double auxotrophs and prototrophs. The intergenic recombination frequency is the summed values of double prototrophs and double auxotrophs as a percentage of viable spores. To determine the intergenic recombination frequency between ade6 and tps16, serial dilutions of spore suspensions were plated onto YEA plates to a density of ∼50–100 colonies per plate and incubated at 25°. Plates were then replica plated onto fresh YEA plates and incubated at 37° (the tps16-23 restrictive temperature) and recombinants were scored.
Recombination frequencies were used to determine the genetic distance (centimorgans) by employing Haldane's mapping function [genetic distance (centimorgans) = −50 ln(1 − 2R), where R = the total fraction of recombinant spores among all spores analyzed].
Microscopical preparation and staining:
Meiotic time courses and nuclear spreads were carried out as previously described (Lorenz et al. 2004). In short, cells from sporulating cultures were freed of cell walls by enzymatic treatment, applied to a microscopic slide, opened up with a detergent, and fixed with paraformaldehyde. Primary antibodies [1:50 mouse monoclonal antibody against recombinant Rad51 protein (NeoMarkers, Fremont, CA) and 1:400 polyclonal rabbit antibody directed against amino acids 670–684 of Rec10] were applied together under a coverslip for overnight at room temperature. Secondary FITC-conjugated anti-mouse and Cy3-conjugated anti-rabbit antibodies were applied for ∼4 hr at room temperature. Finally, the slides were mounted in Vectashield antifading agent (Vector Laboratories, Burlingame, CA) supplemented with 1 μg/ml 4′, 6-diamidino-2-phenylindole (DAPI) as a DNA-specific counterstain. Fluorescent signals were detected with a Zeiss Axioskop 2 Plus epifluorescence microscope equipped with an Axiocam HR camera. Black and white images were assigned false color and merged with Axiovision 3.1 software.
RESULTS
Rec10 is required for regulation of recombination throughout the genome:
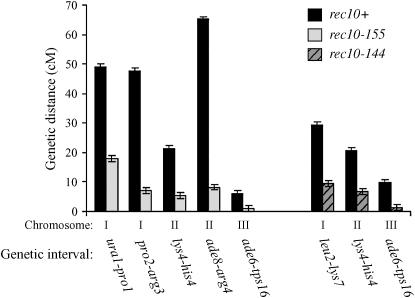
Rec10-controlled recombination in the middle regions of S. pombe chromosomes has been demonstrated by genetic analysis using rec10-109, a mutant allele generated by chemical mutagenesis (De Veaux and Smith 1994; Krawchuk et al. 1999). However, LinEs form in the rec10-109 mutant during meiosis at the appropriate time, albeit with an altered morphological profile, and may retain some function for activation of recombination (Lorenz et al. 2004). Another allele of rec10, rec10-144, has recently been characterized, which resides in a separate complementation group to rec10-109 and is a chemically induced missense mutation (Pryce et al. 2005). We noted that crossovers in the leu2–lys7 interval (see Figure 1 for location) on chromosome I were reduced in rec10-144 homozygous zygotic crosses, relative to the rec10+ control (Figure 2). Recombination in this interval was previously reported to be unaltered in rec10-109 homozygous crosses (De Veaux and Smith 1994). Our data using rec10-144 indicate that Rec10 is influencing recombination in intervals outside the previously reported middle regions (De Veaux and Smith 1994). We examined this further by looking at another interval, his4–lys4, on chromosome II (Figure 1), for which recombination was also previously reported to be rec10 independent (De Veaux and Smith 1994). Crossover levels in the his4–lys4 interval were also reduced relative to the rec10+ control in the rec10-144 mutant, indicative of a more extensive, genomewide requirement for Rec10 function (Figure 2). Recombination in the ade6–tps16 interval, situated close to the centromere of chromosome III, was reduced in the rec10-144 mutant to a level similar to that previously reported for the rec10-109 mutant (Figure 2) (De Veaux and Smith 1994).
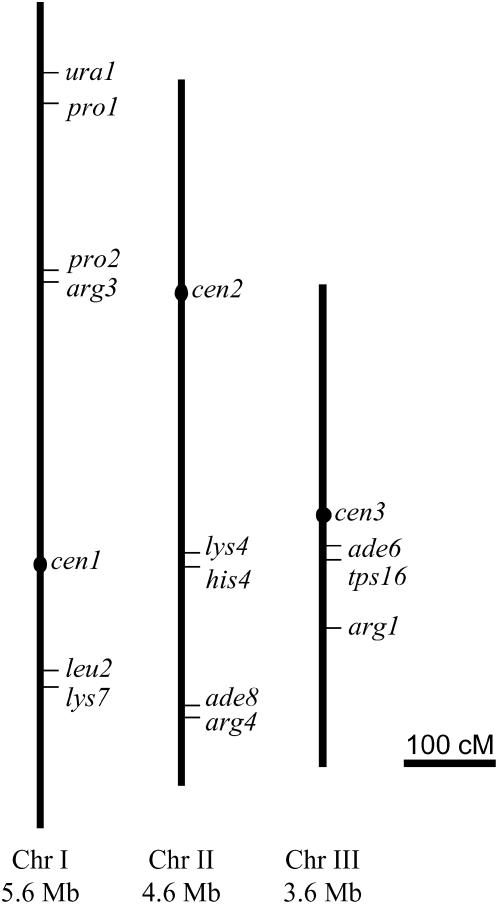
Figure 1.—
Schematic genetic map of the S. pombe genome showing the approximate positions of the markers employed in this study. S. pombe has three chromosomes varying in size (chromosome I, 5.6 Mb; chromosome II, 4.6 Mb; chromosome III, 3.5 Mb). The positions of the three centromeres are marked (cen1, cen2, cen3). The bar is 100 cM based on 0.16 cM/kb (Young et al. 2002).
Figure 2.—
Intergenic meiotic recombination is reduced in the rec10-144 and rec10-155 mutants. Intergenic meiotic recombination was measured in rec10+, rec10-155, and rec10-144 meioses. Different intervals were measured on each chromosome (see Figure 1 for positions), some of which exhibit little or no reduction in the previously studied rec10-109 allele (De Veaux and Smith 1994). Recombination levels were reduced at each interval in both mutants, indicating a role for Rec10 throughout the genome. Bars represent 95% confidence intervals. Pairwise comparison of rec10+ vs. rec10-144 or rec10-155 using Student's t-test gave a P-value of <0.01 in all cases; n ≥ 4 in all cases.
Although the rec10-144 mutant exhibits defects in meiotic recombination at noncentral intervals (see above), this mutant forms LinEs, albeit with a morphological profile very different from the wild type, so may retain some LinE functions (Pryce et al. 2005). To explore the question of whether or not LinEs are required for recombination we used an insertion inactivation mutant of rec10, rec10-155 (Lin and Smith 1995), which does not form any detectable LinEs by electron microscopy (Molnar et al. 2003) or immunocytochemistry (Lorenz et al. 2006). The rec10-155 allele is predicted to encode a truncated Rec10 protein that has lost the C-terminal 103 amino acids containing the homology to the Red1 helical region, proposed to be required for Red1 homo-oligomerization (Hollingsworth and Ponte 1997; Woltering et al. 2000; Lorenz et al. 2006). We measured crossover frequencies in a number of intervals in the rec10-155 mutant, some of which appear to retain rec+ levels of recombination in the rec10-109 mutant (De Veaux and Smith 1994). In all cases crossing over was reduced (Figure 2), demonstrating that Rec10 is required to control crossing over more extensively throughout the genome than previously suggested (De Veaux and Smith 1994; Krawchuk et al. 1999).
Recent work has indicated that gene conversions can occur via a crossover-independent pathway (reviewed in Bishop and Zickler 2004; Heyer 2004; Hollingsworth and Brill 2004). In the rec10-109 mutant, gene conversion levels at the lys7 and ura1 loci have been reported to be indistinguishable from rec10+ levels, although data from only one experiment were reported (De Veaux and Smith 1994). To address whether the requirement for Rec10 throughout the genome is specific for crossovers, we also tested gene conversion frequencies at the lys7 and ura1 loci. lys7 gene conversion levels were greatly reduced in two-factor crosses of rec10-144 and rec10-155 mutants. Gene conversion at ura1 was reduced marginally in the rec10-155 mutant (Table 2). We also measured conversion frequency at the ade6 locus in the rec10-155 mutant and found this to be consistent with the previously reported reduction at this centromere-proximal locus (Lin and Smith 1995). These results indicate that Rec10 plays a role in regulating both crossing over and gene conversion throughout the genome and not only in restricted regions, as previously reported. This finding corroborates a similar finding made recently by Ellermeier and Smith (2005), who employed a rec10Δ mutant. Moreover, there is a differential requirement for a function of Rec10 for controlling gene conversions; this function is lost in the rec10-155 mutant (see discussion).
TABLE 2.
Intragenic gene conversion frequencies for two-factor rec10-155 and rec10-144 homozygous crosses
| rec10 allele | Plasmid | Locus | Mean recombination frequencya | Fold reductionb |
|---|---|---|---|---|
| rec10+ | — | ade6c | 297 ± 114 | — |
| rec10-155 | — | ade6 | 1.2 ± 0.3 | 247 |
| rec10+ | — | lys7d | 14 ± 10 | — |
| rec10-144 | — | lys7 | 0.3 ± 0.2 | 47 |
| rec10-155 | — | lys7 | 0.4 ± 0.4 | 35 |
| rec10+ | — | ura1e | 212 ± 51 | — |
| rec10-155 | — | ura1 | 35 ± 5 | 6 |
| rec10-175 | — | ura1 | 3.0 ± 0.5 | 70f |
| rec10-175 | pSP1g | ura1 | 1.8 ± 1.2 | 118 |
| rec10-175 | pYL167g | ura1 | 24 ± 10 | 9 |
Number of prototrophs per 106 viable spores ± 95% confidence interval; n ≥ 4 in all cases.
Student's t-test P-values from pairwise comparisons of rec10+ vs. rec10− gave values of P ≤ 0.01 in all cases.
Alleles used: ade6-M375 × ade6-52.
Alleles used: lys7-1 × lys7-2.
Alleles used: ura1-61 × ura1-171.
This was the lowest fold reduction obtained in this study for ura1 intragenic recombination. Other mean data values resulted in fold reductions as high as 155-fold.
pYL167 is the plasmid carrying the rec10-155 allele (Lin and Smith 1995). pSP1 is a vector control containing no rec10 cloned DNA.
Significant levels of genetic recombination occur in the absence of LinEs:
While we demonstrated that Rec10 is required for recombination at all loci tested, we observed only relatively minor reductions in intergenic recombination. In addition, intragenic recombination at the ura1 locus was reduced only slightly in the rec10-155 mutant. Recently Ellermeier and Smith (2005) observed much greater levels of reduction in inter- and intragenic recombination at all loci/intervals tested with a rec10 null mutant (rec10-175; ≥40-fold in all cases). This includes loci at which we observed only relatively minor reductions. For example, the rec10-155 mutation results in a 2-fold reduction in intergenic recombination at the ura1–pro1 interval on chromosome I, while the rec10-175 null mutation gives a 42-fold reduction at this interval (Ellermeier and Smith 2005). We confirmed the differences between rec10-155 and rec10-175 by measuring pro1–ura1 intergenic recombination and ura1 intragenic recombination for both mutants under identical conditions (Table 2; data not shown). Table 3 shows the relative reductions in recombination observed between rec10-155 and rec10-175 mutants (data for the rec10-109 region-specific allele are also shown for comparison). These differences indicate that the truncated Rec10-155 protein retains the ability to mediate high levels of crossing over and region-specific gene conversion, while failing to form LinEs.
TABLE 3.
Comparison of levels of reduction for three rec10 mutants
| Fold reduction in recombination frequencya
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Intergenic
|
Intragenic: | |||||||
| rec10 allele | LinE status | pro1–ura1 | pro2–arg3 | his4–lys4 | ade8–arg4 | ade6–tps16 | ade6–arg1 | ura1 |
| rec10-109b | Partial | 1.0 | 1.6 | 0.8 | NA | 14.6 | NA | 0.7 |
| rec10-155c | None | 2.1 | 4.7 | 3.4 | 6.2 | 5.6 | NA | 6.1 |
| rec10-175d | None | 42.0 | NA | 64.0 | NA | NA | >96.0 | 70.7 |
Relative to rec10+: These reductions are derived from relative reductions in recombination frequency and not genetic distances (in the case of this study the recombination frequencies were the same values employed to calculate genetic distances; see Figure 1).
Derived from De Veaux and Smith (1994).
Derived from this study.
Derived from this study and Ellermeier and Smith (2005).
To explore the recombination proficiency of the rec10-155 allele further we introduced a plasmid (pYL167; Lin and Smith 1995) encoding the truncated rec10-155 allele into the rec10-175 strain. pYL167 elevates recombination in the rec10-175 null mutant to levels comparable to those in the rec10-155 mutant (Table 2; data not shown).
Further evidence for the occurrence of recombination in the rec10-155 mutant:
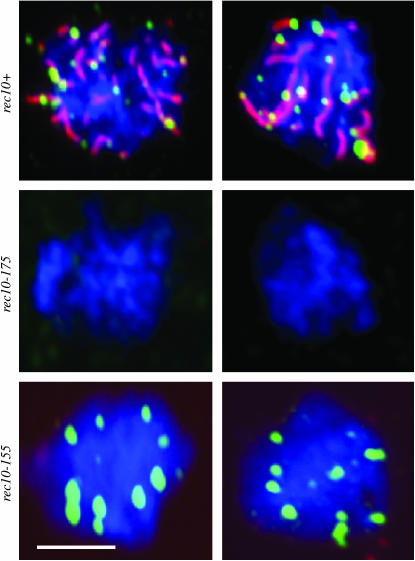
It has been proposed that—in accordance with evidence from other organisms—recombination in S. pombe is initiated by the formation of DNA double-strand breaks (DSBs) (Cervantes et al. 2000). Such lesions in the DNA, which appear as fragmented DNA in physical assays, are not detected in the rec10-175 mutant (Ellermeier and Smith 2005). Moreover, while Rad51 associates with meiotic recombination-initiating lesions in S. pombe (Grishchuk et al. 2004), no Rad51 foci are observed in rec10-175 cells traversing meiosis (Lorenz et al. 2006). The failure to observe either DNA fragmentation or Rad51 foci indicates that initiation of meiotic recombination does not take place in the rec10-175 mutant (Ellermeier and Smith 2005; Lorenz et al. 2006). This is consistent with the recombination analysis of the rec10-175 mutant in which genetic recombination is at a level similar to that of mutants defective in the initiation of meiotic recombination (Ellermeier and Smith 2005). Since our data indicated that recombination is being initiated to significant levels in the absence of LinEs (see above), we predicted that Rad51 foci should be measurable at a significant level in the absence of LinEs in the rec10-155 mutant. To test this we induced homozygous rec10-155 h+/h− diploid cells to traverse meiosis by transferring them to sporulation medium and employed immunocytochemistry to determine whether Rad51 foci could be observed. Rad51 foci form in the rec10-155 mutant with a high frequency (Figure 3), confirming that Rad51-dependent recombination is occurring. Higher-resolution temporal comparison of the rec10-155 mutant and the wild type is not possible due to the fact that rec10-155 diploids traverse meiosis more rapidly (data not shown) and LinEs are missing as specific meiotic landmarks. However, the number of Rad51 foci detected in the rec10-155 mutant was similar to the number observed in rec10+ meioses, albeit with a different temporal profile (data not shown).
Figure 3.—
Rad51 foci are present in meiotic cells devoid of LinEs. Examples of meiotic nuclei from diploid rec10+ (LinEs and Rad51 foci), rec10-175 (no LinEs and no Rad51 foci), and rec10-155 (no LinEs and Rad51 present) are shown. rec10-155 mutants do not form any LinEs detectable by electron microscopic analysis of silver-stained nuclear spreads (Molnar et al. 2003), so the loss of staining is not likely to be due to epitope loss. Blue is DAPI, Red is Rec10 (LinEs), and green is Rad51. Bar, 5 μm.
Ellermeier and Smith (2005) further demonstrated that recombination-initiating lesions are not generated to any significant level in the rec10-175 mutant by showing that the rec10-175 mutation could rescue the low spore production of a rad32Δ mutant. Rad32 is required to process recombination-initiating lesions. If such lesions are formed in the absence of Rad32 they cannot be properly processed and there is a low viable spore yield (Tavassoli et al. 1995). However, if no initiating lesions form then the viable spore yield is elevated to levels consistent with random segregation of the three S. pombe chromosomes, i.e., ∼12% (see Ellermeier and Smith 2005 for further details). If recombination-initiating lesions are generated in rec10-155 cells, it follows that the rec10-155 mutation should not rescue the poor spore viability of the rad32Δ mutant to the same extent as does the rec10-175 mutation. Table 4 shows that this is indeed the case, indicating that lesions that require Rad32 processing, most likely DSBs, are being formed in the rec10-155 mutant.
TABLE 4.
rad32Δ reduced spore viability is not rescued by the rec10-155 mutation
| Spore viability (%)a
|
||
|---|---|---|
| Genotype | rad32+ | rad32Δ |
| rec10+ | 76.5 ± 9.34 | 0.7 ± 0.3b |
| rec10-155 | 29.1 ± 9.2 | 1.0 ± 0.4 |
| rec10-175 | 37.5 ± 10.4 | 14.4 ± 3.0 |
n ≥ 4 in all cases; values are ± SD.
Similar to values previously reported for the rad32Δ mutant (Tavassoli et al. 1995).
Collectively these data indicate that the Rec10-155 protein is capable of mediating significant levels of recombination in the absence of LinEs.
DISCUSSION
The discovery that Rec10 is a central component of LinEs in S. pombe, and shares features in common with S. cerevisiae Red1, suggests that LinEs have functional parallels with the SC of other organisms or at least with SC axial precursor structures (Lorenz et al. 2004). However, the exact role of these structures remains unclear (Loidl 2006; Wells et al. 2006). Whether or not recombination can occur outside the context of LinEs has developed into a central question. Here we provide data that answer this question, but unravel a more complex picture.
Rec10 controls recombination throughout the genome:
Previous work using the chemically induced rec10 mutant, rec10-109, demonstrated that recombination in strains carrying this allele was defective only in middle regions of each of the three chromosomes (De Veaux and Smith 1994; Krawchuk et al. 1999). Early on during this study we found that loci/intervals that retained rec10+ levels of recombination in the rec10-109 mutant exhibited reductions in both the rec10-144 and rec10-155 mutants. In fact, we observed some degree of recombination loss at all loci/intervals tested, implying that Rec10 is needed, to some extent, for the regulation of recombination throughout the genome. This conclusion corroborates that recently made by Ellermeier and Smith (2005), who observed genomewide recombination reductions in a rec10Δ null mutant (rec10-175). Null mutants of the meiosis-specific cohesion genes, rec8 and rec11, result in region-specific reductions in recombination in the central regions of chromosomes as does rec10-109 (Parisi et al. 1999; Ellermeier and Smith 2005). This suggests that the rec10-109 mutant is defective in some function of Rec10 that is intimately associated with the meiotic cohesins and, further, would imply Rec10 having more than one functional role (also see below). It is possible that in central chromosomal regions it functions, in part, to modulate meiosis-specific cohesion dynamics. In the more distal regions it might partner with the Rad21–Psc3 cohesin complex, although there is not yet direct experimental evidence to support this.
This and other studies have now found that various rec10 mutants exhibit different phenotypes, which go some way to demonstrate that Rec10 has more than one function. In the interest of clarity Table 5 lists the mutants that have been characterized in detail to date.
TABLE 5.
Characteristics of the well-studied mutant alleles of rec10
| rec10 allele | Mutation | DSB proficient? | Crossover proficiency | Gene conversion proficiency | ade6–M26 hot spot proficiency | LinE status | Other |
|---|---|---|---|---|---|---|---|
| rec10-175a | Full open reading frame deletion | No | Noneb | Noneb | Noneb | Nonec | — |
| rec10-109d | Two point mutationsa G526A (V176I) and G533A (G178D)e | Levels of DSBs diminishedf | Regionalgh | Regionalgh | Partialg | Abnormal LinEs formedci | Complements rec10-144jk. Slightly temperature sensitive for recombination at some regionsa |
| rec10-144jk | Single point mutationj G2180A (G727E)e | Not determined | Limited reduction at all intervals testedl | Limited reduction at all intervals testedjl | Reduced hot spot activityj | Limited abnormal LinEs formedjm | Complements rec10-109jk |
| rec10-155n | Insertion inactivation mutant; presumed C-terminal truncation of last 103 amino acidsn | Not determined | Limited reduction at all intervals testedl | Limited reduction at all intervals testedln | Reduced hot spot activityo | Noneimp | — |
Other rec10 mutant alleles have been isolated/generated and sequenced (De Veaux et al. 1992; Ellermeier and Smith 2005; our unpublished data), but they have undergone only limited analysis to date and are not listed here.
None equates to the levels observed for a rec12 null mutant.
As measured by immunocytochemistry.
Numbered from the start of the open reading frame.
Lorenz et al. (2004).
This study.
As determined by immunocytochemistry and electron microscopy.
D. W. Pryce and R. J. Mc Farlane (unpublished observation).
Rec10 has a LinE-independent function in mediating recombination:
The rec10-155 mutant does not form LinEs. This has been determined by immunocytochemistry (Lorenz et al. 2004); this is not simply an artifact, due to loss of antibody epitope recognition, as LinEs cannot be detected in this mutant by electron microscopy (Molnar et al. 2003). However, we have shown that relative to the rec10Δ null mutant (rec10-175) there is substantial recombination occurring throughout the genome in the rec10-155 mutant. Furthermore, a clone carrying the rec10-155 allele is capable of restoring significant levels of recombination to the rec10Δ null mutant. This clearly demonstrates that while Rec10 protein is essential for meiotic recombination, LinEs are not, and at best LinEs only enhance meiotic recombination. Moreover, while the rec10-155 mutant is defective in recombination, we cannot dismiss the possibility that LinEs have no function in regulating interhomolog meiotic recombination to any degree and that the reduced recombination levels observed in this allele are caused by other functions of Rec10 being impaired due to the truncation of the Rec10 protein and not due to the loss of LinEs per se.
The rec10-155 allele encodes a truncated Rec10 protein that has lost the C-terminal 103-amino-acids (aa) domain (Lin and Smith 1995) that contains homology to the helical region of Red1 (Hollingsworth and Ponte 1997; Lorenz et al. 2004). This domain is proposed to be required for Red1 homo-oligomerization (Woltering et al. 2000). Its loss in S. pombe might explain the failure of this mutant to develop LinEs (Molnar et al. 2003; Lorenz et al. 2004). Limited in silico analysis of the truncated Rec10-155 protein did not identify any obvious features that may provide clues to the function of Rec10 essential for meiotic recombination.
It might be argued that the truncated Rec10-155 protein goes undetected by immunostaining with the antibody that is directed to aa 670–684 of the 791-aa wild-type Rec10 (Lorenz et al. 2004). However, rec10-155 mutants do not form any LinEs detectable by electron microscopic analysis of silver-stained nuclear spreads either (Molnar et al. 2003), so the loss of staining is not likely to be due to epitope loss. On the other hand, we cannot dismiss the possibility that tiny Rec10 dots go undetected both by electron microscopy (due to background silver grains) and by immunostaining (due to a weakened affinity).
A disparity between gene conversions and crossovers in the rec10-155 mutant:
An unexpected feature of recombination in the rec10-155 mutant is the greater reduction in gene conversion relative to the reduction in crossovers at some intervals; for example, at the ade6 locus gene conversions are reduced 247-fold in the rec10-155 mutant (Table 1), while crossovers in the associated ade6–tps16 interval are reduced only ∼6-fold (Figure 2). At the present time, there is no clear explanation for this observation. If conversions at ade6 occurred without high levels of associated crossing over, this observation could be accounted for; i.e., rec10-155 is defective in the conversions at ade6 that have little associated crossing over. However, Cromie et al. (2005) recently demonstrated that there are high levels of crossovers associated with conversions at ade6, dismissing the proposal that there is a greater reduction in conversions at ade6 than crossovers in the rec10-155 mutant because there is limited crossing over associated with ade6 gene conversions. A similar, but less dramatic, observation has also been made for mutants defective in the meiosis-specific cyclin Rem1, where a reduction in gene conversions is observed (∼4-fold) at ade6, but no reduction in crossing over in the adjacent ade6–arg1 interval (Malapeira et al. 2005).
Another explanation for this observation might be that there is increased marker coconversion in the rec10-155 mutant in two-factor crosses at some loci (for example, ade6), resulting in a reduction in measurable gene conversions. However, there is no other evidence to support mechanistic models based on elevated marker coconversion, such as extended gene conversion tracts in the rec10-155 mutant resulting in elevated coconversions.
LinEs and crossing over:
It has been proposed that axial proteins, such as Red1 in S. cerevisiae, function to introduce a compaction stress into the chromosomal structure that facilitates crossing over and might play a role in genetic interference (Blat et al. 2002; Kleckner et al. 2004), the latter not being apparent in S. pombe (Munz 1994). Our data are not inconsistent with LinEs generating a recombinogenic compaction stress that may enhance crossing over to above the level observed in the rec10-155 mutant.
In conclusion, we demonstrate that Rec10 has more than one distinct function. Rec10 is required for DSB formation (Cervantes et al. 2000; Ellermeier and Smith 2005), but we show that with the loss of the C-terminal domain of Rec10 substantial levels of recombination occur despite the fact that LinEs do not form. Some function of Rec10 is differentially required for regulating gene conversions with some loci being more dependent upon this function than others; whether this function is linked to LinE function or not remains unresolved. Finally, we can conclude that LinEs are not absolutely essential for all programmed meiotic recombination. LinEs have been implicated in other processes, such as interhomolog pairing (Bähler et al. 1993), but loss of pairing might be an indirect effect of mutating rec10. Furthermore, LinEs hold Hop1 in place, which in turn may play a role in recombination partner choice (Loidl 2006). It remains to be determined whether or not LinEs are dispensable for all programmed meiotic recombination.
Acknowledgments
We offer special thanks to Gerry Smith and Chad Ellermeier for strains, communicating results prior to publication, and for permission to use the rec10-175 mutant prior to publication. We thank other members of the North West Cancer Research Fund Institute for critical review of this manuscript. This work was supported, in part, by Biotechnology and Biological Sciences Research Council Committee studentship no. 99/B1/G05482, Wellcome Trust grant no. 057317, and Austrian Science Fund grant no. P18186. J.L.W. and D.W.P. were partly supported by the North West Cancer Research Fund.
References
- Bähler, J., T. Wyler, J. Loidl and J. Kohli, 1993. Unusual nuclear structures in meiotic prophase of fission yeast: a cytological analysis. J. Cell Biol. 121: 241–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, D. K., and D. Zickler, 2004. Early decision: meiotic crossover interference prior to stable strand exchange and synapsis. Cell 117: 9–15. [DOI] [PubMed] [Google Scholar]
- Blat, Y., R. U. Protacio, N. Hunter and N. Kleckner, 2002. Physical and functional interactions among basic chromosome organizational features govern early steps of meiotic chiasma formation. Cell 111: 791–802. [DOI] [PubMed] [Google Scholar]
- Cervantes, M. D., J. A. Farah and G. R. Smith, 2000. Meiotic DNA breaks associated with recombination in S. pombe. Mol. Cell 5: 883–888. [DOI] [PubMed] [Google Scholar]
- Cromie, G. A., C. A. Rubio, R. W. Hyppa and G. R. Smith, 2005. A natural meiotic DNA break site in Schizosaccharomyces pombe is a hotspot of gene conversion, highly associated with crossing over. Genetics 169: 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veaux, L. C., and G. R. Smith, 1994. Region-specific activators of meiotic recombination in Schizosaccharomyces pombe. Genes Dev. 8: 203–210. [DOI] [PubMed] [Google Scholar]
- De Veaux, L. C., N. A. Hoagland and G. R. Smith, 1992. Seventeen complementation groups of mutations decreasing meiotic recombination in Schizosaccharomyces pombe. Genetics 130: 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier, C., and G. R. Smith, 2005. Cohesins are required for meiotic DNA breakage and recombination in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 102: 10952–10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishchuk, A. L., R. Kraehenbuehl, M. Molnar, O. Fleck and J. Kohli, 2004. Genetic and cytological characterization of the RecA-homologous proteins Rad51 and Dmc1 of Schizosaccharomyces pombe. Curr. Genet. 44: 317–328. [DOI] [PubMed] [Google Scholar]
- Heyer, W. D., 2004. Recombination: Holliday junction resolution and crossover formation. Curr. Biol. 14: R56–R58. [DOI] [PubMed] [Google Scholar]
- Hollingsworth, N. M., and S. J. Brill, 2004. The Mus81 solution to resolution: generating meiotic crossovers without Holliday junctions. Genes Dev. 18: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth, N. M., and L. Ponte, 1997. Genetic interactions between HOP1, RED1 and MEK1 regulate assembly of axial element components during meiosis in the yeast Saccharomyces cerevisiae. Genetics 147: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner, N., D. Zickler, G. H. Jones, J. Dekker, R. Padmore et al., 2004. A mechanical basis for chromosome function. Proc. Natl. Acad. Sci. USA 101: 12592–12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli, J., and J. Bähler, 1994. Homologous recombination in fission yeast: absence of crossover interference and synaptonemal complex. Experientia 50: 295–306. [DOI] [PubMed] [Google Scholar]
- Krawchuk, M. D., L. C. De Veaux and W. P. Wahls, 1999. Meiotic chromosome dynamics dependent upon the rec8(+), rec10(+) and rec11(+) genes of the fission yeast Schizosaccharomyces pombe. Genetics 153: 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y., and G. R. Smith, 1995. Molecular cloning of the meiosis-induced rec10 gene of Schizosaccharomyces pombe. Curr. Genet. 27: 440–446. [DOI] [PubMed] [Google Scholar]
- Loidl, J., 2006. S. pombe linear elements: the modest cousins of synaptonemal complexes. Chromosoma 115: 260–271. [DOI] [PubMed] [Google Scholar]
- Lorenz, A., J. L. Wells, D. W. Pryce, M. Novatchkova, F. Eisenhaber et al., 2004. S. pombe meiotic linear elements contain proteins related to synaptonemal complex components. J. Cell Sci. 117: 3343–3351. [DOI] [PubMed] [Google Scholar]
- Lorenz, A., A. Estreicher, J. Kohli and J. Loidl, 2006. Meiotic recombination proteins localize to linear elements in Schizosaccharomyces pombe. Chromosoma 115: 330–340. [DOI] [PubMed] [Google Scholar]
- Malapeira, J., A. Moldón, E. Hidalgo, G. R. Smith, P. Nurse et al., 2005. A meiosis-specific cyclin regulated by splicing is required for proper progression through meiosis. Mol. Cell. Biol. 25: 6330–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar, M., E. Doll, A. Yamamoto, Y. Hiraoka and J. Kohli, 2003. Linear element formation and their role in meiotic sister chromatid cohesion and chromosome pairing. J. Cell Sci. 116: 1719–1731. [DOI] [PubMed] [Google Scholar]
- Moreno, S., A. Klar and P. Nurse, 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194: 795–823. [DOI] [PubMed] [Google Scholar]
- Munz, P., 1994. An analysis of interference in the fission yeast Schizosaccharomyces pombe. Genetics 137: 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, H., L. Wan, B. Baumgartner, D. Schaefer, J. Loidl et al., 2005. Partner choice during meiosis is regulated by Hop1-promoted dimerization of Mek1. Mol. Biol. Cell 16: 5804–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi, S., M. J. Mc Kay, M. Molnar, M. A. Thompson, P. J. Van Der Spek et al., 1999. Rec8p, a meiotic recombination and sister chromatid cohesion phosphoprotein of the Rad21p family conserved from fission yeast to humans. Mol. Cell. Biol. 19: 3515–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponticelli, A. S., and G. R. Smith, 1989. Meiotic recombination-deficient mutants of Schizosaccharomyces pombe. Genetics 123: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce, D. W., A. Lorenz, J. B. Smirnova, J. Loidl and R. J. Mc Farlane, 2005. Differential activation of M26-containing meiotic recombination hot spots in Schizosaccharomyces pombe. Genetics 170: 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockmill, B., and G. S. Roeder, 1990. Meiosis in asynaptic yeast. Genetics 126: 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacha, A., and N. Kleckner, 1997. Interhomolog bias during meiotic recombination: meiotic functions promote a highly differentiated interhomolog-only pathway. Cell 90: 1123–1135. [DOI] [PubMed] [Google Scholar]
- Tavassoli, M., M. Shayeghi, A. Nasim and F. Z. Watts, 1995. Cloning and characterization of the Schizosaccharomyces pombe rad32 gene: a gene required for repair of double strand breaks and recombination. Nucleic Acids Res. 23: 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, D. A., and F. W. Stahl, 1999. Genetic control of recombination partner preference in yeast meiosis. Isolation and characterization of mutants elevated for meiotic unequal sister-chromatid recombination. Genetics 153: 621–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, J. L., D. W. Pryce and R. J. Mc Farlane, 2006. Homologous chromosome pairing in Schizosaccharomyces pombe. Yeast 23: 977–989. [DOI] [PubMed] [Google Scholar]
- Woltering, D., B. Baumgartner, S. Bagchi, B. Larkin, J. Loidl et al., 2000. Meiotic segregation, synapsis and recombination checkpoint functions require physical interaction between the chromosomal proteins Red1p and Hop1p. Mol. Cell. Biol. 20: 6646–6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, J. A., R. W. Schreckhise, W. W. Steiner and G. R. Smith, 2002. Meiotic recombination remote from prominent DNA break sites in S. pombe. Mol. Cell 9: 253–263. [DOI] [PubMed] [Google Scholar]
- Zickler, D., and N. Kleckner, 1999. Meiotic chromosomes: integrating structure and function. Annu. Rev. Genet. 33: 603–754. [DOI] [PubMed] [Google Scholar]