Abstract
Oxygen deprivation has a role in the pathology of many human diseases. Thus it is of interest in understanding the genetic and cellular responses to hypoxia or anoxia in oxygen-deprivation-tolerant organisms such as Caenorhabditis elegans. In C. elegans the DAF-2/DAF-16 pathway, an IGF-1/insulin-like signaling pathway, is involved with dauer formation, longevity, and stress resistance. In this report we compared the response of wild-type and daf-2(e1370) animals to anoxia. Unlike wild-type animals, the daf-2(e1370) animals have an enhanced anoxia-survival phenotype in that they survive long-term anoxia and high-temperature anoxia, do not accumulate significant tissue damage in either of these conditions, and are motile after 24 hr of anoxia. RNA interference was used to screen DAF-16-regulated genes that suppress the daf-2(e1370)-enhanced anoxia-survival phenotype. We identified gpd-2 and gpd-3, two nearly identical genes in an operon that encode the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase. We found that not only is the daf-2(e1370)-enhanced anoxia phenotype dependent upon gpd-2 and gpd-3, but also the motility of animals exposed to brief periods of anoxia is prematurely arrested in gpd-2/3(RNAi) and daf-2(e1370);gpd-2/3(RNAi) animals. These data suggest that gpd-2 and gpd-3 may serve a protective role in tissue exposed to oxygen deprivation.
OXYGEN deprivation is a critical factor in several medical problems, including myocardial infarction, pulmonary disease and dysfunction, anemia, blood loss, suffocation, sleep apnea, stroke, and resistance of solid tumor cells to radiation and chemotherapy (Semenza 2000). The detrimental effects of oceanic “dead zone” regions, in which oxygen levels are reduced or depleted due to natural and/or human activities, are another concern (Diaz 2001). Thus, health and environmental issues associated with oxygen deprivation are economically relevant challenges. Animal model systems are used to understand the molecular and physiological response that organisms, tissues, and cells have to oxygen deprivation. Yet, to date, oxygen-deprivation protective or tissue repair mechanisms are not completely understood (Hochachka et al. 1997; Hermes-Lima and Zenteno-Savin 2002; Lahiri et al. 2005).
For many vertebrates, including humans, prolonged exposure to severe oxygen deprivation results in cellular death. However, the reduction or absence of oxygen is not always fatal to organisms; some survive days or even years of hypoxia or anoxia. For example, the harbor seal (Phoca vitulina), turtle (Chrysemys picta belli), brine shrimp (Artemia franciscana), zebrafish (Danio rerio), fruit fly (Drosophila melanogaster), and nematode (Caenorhabditis elegans) have adapted to survive oxygen deprivation (Foe and Alberts 1985; Hochachka et al. 1993; Hand 1998; Padilla et al. 2002; Fuson et al. 2003). Thus, it is likely that various oxygen-deprivation protective mechanisms exist in metazoans.
Identifying the molecular and genetic responses of oxygen-deprivation-tolerant organisms to hypoxia or anoxia has greatly increased our understanding of the mechanisms involved with oxygen-deprivation sensing and survival (Haddad et al. 1997; Haddad 1998; Wingrove and O'Farrell 1999; Jiang et al. 2001; Ghabrial et al. 2003; Nystul et al. 2003; Gray et al. 2004; Morton 2004). For example, analysis of the hypoxia-inducing factor 1 (HIF-1) and the HIF-prolyl hydroxylase signal transduction pathway demonstrated that aspects of oxygen sensing are conserved between vertebrates and invertebrates (Epstein et al. 2001; Maxwell et al. 2001; Semenza 2001). Studies of the C. elegans hif-1(ia04) mutant demonstrated that hif-1 is required for embryos and larvae to survive hypoxia (Jiang et al. 2001; Treinin et al. 2003). However, the hif-1 is not required for the adult animal to survive hypoxia (1% O2) or for the embryo to survive anoxia (Jiang et al. 2001; Padilla et al. 2002; Shen and Powell-Coffman 2003). Together, these studies suggest that several genetic pathways are involved with oxygen-deprivation response and survival and that these genetic pathways are dependent upon the developmental state of the organism and the level of available oxygen.
Wild-type C. elegans exposed to anoxia (<0.001 kPa O2) in laboratory culture and temperature (20°) conditions enters a reversible state of suspended animation. In the anoxic environment, embryonic and postembryonic development arrests. Furthermore, the postembryonic animal stops feeding, becomes immobile, and does not lay eggs. After reexposure to a normoxic environment, the nematodes proceed with development. C. elegans at all stages of development survive at least 1 day of anoxia with a viability of ∼90% (Van Voorhies and Ward 2000; Padilla et al. 2002; Hajeri et al. 2005). The dauer larvae, a developmentally arrested larval stage, survive several days of anoxia, making this stage the most resistant to anoxia (Anderson 1978; Padilla et al. 2002). The long-term anoxia survival phenotype of dauer larvae is consistent with its ability to survive stress. Yet, further studies are needed to understand the molecular mechanism that the dauer larvae use to survive anoxia.
Given that the dauer larvae have an enhanced anoxia survival phenotype, it is of interest to investigate the relationship of anoxia survival and the genetic pathways involved with dauer formation. One of the dauer regulatory pathways includes the daf-2 and daf-16 genes, which encode the insulin/IGF-1 receptor-like protein and a fork-head transcription factor, respectively (Larsen et al. 1995; Kimura et al. 1997; Riddle and Albert 1997; Gems et al. 1998). In the daf-2(e1370) animal, which has a mutation in the insulin receptor kinase domain, the DAF-16 protein is translocated to the nucleus. Phenotypes observed in the daf-2(e1370) animal include constitutive dauer formation at increased temperature, alteration in gene expression, increased longevity, resistance to a variety of stresses, and changes in metabolism (Kenyon et al. 1993; Vanfleteren and De Vreese 1996; Kimura et al. 1997; Ogg et al. 1997; Gems et al. 1998; Lee et al. 2003; McElwee et al. 2003, 2004; Munoz 2003; Murphy et al. 2003; Van Voorhies 2003; Burnell et al. 2005). DNA microarray studies determined that genes involved with stress response and metabolism are upregulated in the daf-2 mutant. However, it is not known how gene expression and metabolic changes are involved with the mechanism by which the daf-2 mutant animal survives stress.
A study in which researchers screened for genetic mutants that survive hypoxia at a high temperature (20 hr of 0.3% O2, 28°) identified a mutation in the daf-2 gene, thus providing the first direct genetic evidence that the daf-2/daf-16 insulin-like signaling pathway is involved with oxygen deprivation survival in C. elegans (Scott et al. 2002). In this report we compared the phenotypes of the daf-2(e1370) and wild-type animals exposed to long-term anoxia (defined here as ≥3 days of <0.001 kPa O2, 20°) as well as to high-temperature anoxia (defined here as 1 day of <0.001 kPa O2 at 28°). We determined that, unlike wild-type animals, the daf-2(e1370) animals survive long-term anoxia and high-temperature anoxia. To identify genes involved with the daf-2(e1370) high-temperature anoxia survival phenotype, we used RNA interference (RNAi) to decrease the expression of specific genes upregulated in the daf-2(e1370) strain. We determined that gpd-2 and gpd-3, two nearly identical genes in an operon that encode the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH), are required for response to anoxia. Here, we suggest that gpd-2 and gpd-3 are involved with cell and tissue protection during anoxia exposure. The reduction of expression of other glycolytic genes by RNAi does not result in phenotypes similar to that of daf-2(e1370);gpd-2/3(RNAi), suggesting that it may not be the mere flux through glycolysis that is required for anoxia survival.
MATERIALS AND METHODS
Strains and growth conditions:
The wild-type Bristol strain (N2) and mutant strains were cultured using NGM plates seeded with Escherichia coli (OP50) and raised at 20° as described (Sulston and Hodgkin 1988). Synchronized populations of animals were obtained by allowing embryos, obtained from hypochlorite-treated adults, to grow to specific developmental stages. Anatomical markers such as gonad morphology or the hours after L4 molt were used to determine the developmental stage of the animal. Wild-type or daf-2(e1370) adult animals were collected at ∼21–25 or 17–21 hr after the L4-to-adulthood molt, respectively. The following genetic strains were obtained from either the Caenorhabditis elegans Genetics Center or colleagues: daf-2(e1370)III, daf-16(m26)I, daf-2(e1370)III, daf-16(mu86)I, daf-16(m26)I, and PD4792(mIs11 IV).
Oxygen-deprivation experiments:
For all experiments, the nematodes were exposed to an anoxic environment using the BioBag type A environmental chamber as previously described (Becton and Dickinson, Cockeysville, MD; Padilla et al. 2002). The anoxia exposure time (1–5 days) and the temperature conditions (20° or 28°) are noted for each experiment. For the purpose of these studies, we refer to long-term anoxia as ≥3 days of <0.001 kPa O2, at 20° and high-temperature anoxia as 1 day of <0.001 kPa O2 at 28°.
Anoxia survival assays:
Nematodes either at the L2 larval stage or at the young adult hermaphrodite stage were assayed for anoxia survival. The L2 larvae were collected ∼24 hr after newly hatched L1 were placed on seeded plates. Due to different developmental progression rates between the wild-type and daf-2(e1370) animals, the actual time to reach adulthood varied between the two strains. Therefore, we collected adult wild-type and daf-2(e1370) animals ∼16.5–25 hr after the L4-to-adulthood molt. The adult hermaphrodites assayed were at a developmental stage where their offspring consisted of embryos and newly hatched L1 larvae. Nematodes were exposed to anoxia as described above and 24-hr postanoxic animals were examined for survival by visual inspection for movement or reaction to a platinum wire. To determine if anoxia-exposed animals had an uncoordinated phenotype, the animals were inspected for abnormal movement or inability to move along the agar surface. The data collected for nematode survival and phenotype analysis included at least three independent experiments consisting of ∼50–150 worms/experiment.
RNA interference assays:
We used RNAi to inhibit the expression of several genes, including genes upregulated by DAF-16 and genes predicted to encode a glycolytic enzyme (Lee et al. 2003; Murphy et al. 2003). Briefly, the daf-2(e1370), or N2 synchronized L1 larvae, were collected and grown to adulthood on NGM (200 μg/ml ampicillin, 12.5 μg/ml tetracycline, and 0.5 mg/ml IPTG) seeded with the E. coli strain for RNAi of a specified gene. The E. coli strains were developed by the J. Ahringer laboratory and obtained from the MRC Geneservice (Cambridge, UK) (Timmons et al. 2001; Kamath et al. 2003). The bacterial clones used for RNAi of gpd-2 and gpd-3 were K10B3.8 or K10B3.7, respectively. Throughout most of these experiments we used the K10B3.8 clone and refer to the RNAi experiment as gpd-2/3(RNAi). The long-term anoxia survival phenotype observed for daf-2(e1370);gpd-2/3(RNAi) was more pronounced when the bacteria were grown as follows: Bacteria were plated on LB plates supplemented with 200 μg/ml ampicillin (amp) and 12.5 μg/ml tetracycline (tet). From this plate a single colony was grown in 1 ml of LB amp/tet (200 μg/ml ampicillin, 12.5 μg/ml tetracycline) for 6–8 hr and 15 μl of this culture was used to inoculate 5 ml of LB amp/tet liquid, which was grown for 4 hr before seeding onto NGM–IPTG agar plates. These NGM–IPTG plates were placed at 37° for 24 hr and then transferred to 15° or 20° for 24–48 hr before L1 larvae were placed onto the plates. L1 larvae were grown on the RNAi food to adulthood. The adult nematodes were exposed to anoxia and assayed for viability as described above. The F01F1.12(RNAi) and F25H5.3(RNAi) results in embryo lethality or larvae arrest, respectively. Therefore, for these RNAi experiments, the daf-2(e1370) animals were grown to the L4 stage on control food for 72 hr and transferred to the appropriate RNAi food for 48 hr, and then these adults were assayed for high-temperature anoxia survival. As a control, we grew the E. coli strain, which has no insert in the plasmid, on identical NGM–IPTG plates to account for any differences attributable to the vector.
Motility assays of nematodes exposed to anoxia:
The wild-type, gpd-2/3(RNAi), daf-2(e1370), daf-2(e1370);gpd-2/3(RNAi), daf-16(m26)I, and daf-16(mu86)I adult hermaphrodites were used to compare and analyze nematode motion in anoxia. Nematode strains were placed on separate NGM plates in the same anoxia BioBag chamber to ensure that the samples were treated equally. To assay animal motility during anoxia exposure, nematodes were observed (through the anoxia BioBag) over a 4.5-min period at 30-sec intervals every hour for the time indicated in each experiment. Motility was assayed and images were obtained using a Zeiss M2Bio stereoscope and the Openlab software 3.17 imaging software (Improvision, Lexington, MA). Nematodes were maintained at 20° and were at only room temperature during image collection, which took <20 min for each sampling. For the wild type, gpd-2/3(RNAi), daf-2(e1370), and daf-2(e1370);gpd-2/3(RNAi) comparisons, a worm was scored “positive” for movement if the nematode displayed forward or reverse motion along the agar surface. Data include at least three independent experiments. For further analysis of worm motility during anoxia exposure, nematodes were observed as described above except the images were collected at 5-sec intervals over a 4.5-min period. The collected images were imported into QuickTime 7 Pro (Apple Computer) for visualization.
Nomarski microscopy analysis:
Nematodes were exposed to normoxic or anoxic conditions and allowed to recover in air for the time indicated for each experiment. The animals were placed on a 2.5% agar pad containing 10 mm sodium azide and covered with a coverslip. Animals were also analyzed without the use of sodium azide to verify that this chemical did not contribute factors to the analysis of the phenotype. For each experiment at least three independent evaluations were done. Microscopy was conducted using a motorized Zeiss Axioscope with a ×100 objective lens and imaging Openlab software 3.17. Images were prepared using Adobe Photoshop CS (Adobe Systems).
Identification of putative glycolytic genes in C. elegans:
We identified C. elegans genes homologous to mammalian glycolytic genes using BLASTP analysis and Wormbase. Briefly, the primary sequences of human glycolytic genes were obtained using the NCBI protein database and the C. elegans ortholog(s) was identified using the NCBI and Wormbase BLASTP program.
Real time–quantitative PCR assays:
Real time–quantitative PCR assay (RT–qPCR) was used to assay RNAi efficiency as well as to compare the gpd-2/3 transcript level in daf-2 and wild-type animals. Briefly, animals were placed in pureZOL (Bio-Rad, Hercules, CA), frozen in liquid nitrogen, and then thawed at 37°. This freeze/thaw process was repeated three times before RNA was extracted using the Aurum Fatty and Fibrous Tissue RNA extraction kit (Bio-Rad). RNA quality and quantity were determined using the Experion system (Bio-Rad). RT–qPCR was conducted using the iScript One-step RT–PCR kit (Bio-Rad) of which 10 ng of total RNA was loaded into each reaction. Reactions were normalized against a nonvariable control gene, F23B2.13 (Link et al. 2003). The ΔCT with a reference gene method was used to calculate fold decrease or increase in transcript (Real-time PCR Applications Guide, Bio-Rad). All experiments were from at least four independent experiments and each experiment was performed in triplicate.
RESULTS
The daf-2(e1370) animal survives long-term anoxia and high-temperature anoxia:
Wild-type adult hermaphrodites survive 1 day of anoxia at 20°, yet the survival rate dramatically decreases when the animals are exposed to long-term anoxia (≥3 days, 20°) (Van Voorhies and Ward 2000; Padilla et al. 2002). When considering the developmental stage of C. elegans, the dauer larvae have the highest long-term anoxia (>3 days) survival rate (Anderson 1978; Padilla et al. 2002). Unlike wild-type animals, the daf-2(e1370) animals raised to adulthood at 20° and then transferred to high-temperature hypoxia (0.3% O2, 28°) have a high survival rate (Scott et al. 2002). To determine if daf-2(e1370) and wild-type animals have different anoxia survival rates, both strains were exposed to anoxia at 20°. Wild-type and daf-2(e1370) animals were collected as L2 larvae or gravid adults and exposed to 1, 3, 4, or 5 days of anoxia at 20°. Figure 1 shows that wild-type L2 larvae and adult animals had a high survival rate when exposed to 1 day of anoxia; however, the survival rate decreased when exposed to 3 days of anoxia and the animals did not survive 4 or 5 days of anoxia. In comparison, the daf-2(e1370) larvae and adult animals survived long-term anoxia exposures at a significantly higher rate in comparison to wild-type animals. The daf-2(e1370) adults survived 5 days of anoxia better than the L2 larvae (P < 0.001). Given that the DAF-16 transcription factor is translocated to the nucleus in the daf-2(e1370) animal, we wanted to determine if the daf-2(e1370) long-term anoxia phenotype was mediated by DAF-16. The daf-16(m26);daf-2(e1370) adults and larvae animals exposed to 5 days of anoxia at 20° had an average viability of 1.65 ± 2.71% (n = 353) and 3.32 ± 5.04 (n = 458), respectively, suggesting that DAF-16 mediates the long-term anoxia survival phenotype.
Figure 1.—
The survival rate of daf-2(e1370) and wild-type animals exposed to long-term anoxia at 20°. Wild-type L2 larvae, wild-type adults, daf-2(e1370) L2 larvae, and daf-2(e1370) adults were exposed to 1, 3, 4, and 5 days of anoxia at 20°. The data shown represent at least three independent experiments with a total of at least 150 animals. Error bars represent the standard deviations.
To determine if an increase in temperature affected the daf-2(e1370) and wild-type anoxia survival rate, L2 larvae and adult animals were exposed to 1 day of anoxia at 28°. Figure 2 shows that the wild-type larvae and adults had a reduced survival rate when exposed to anoxia at 28°, indicating that the combined stress of high temperature and anoxia is detrimental to wild-type animals. In comparison, the daf-2(e1370) larvae and adults had a high survival rate when exposed to anoxia at 28°. These data further support the finding that wild-type animals but not the daf-2(e1370) animals are sensitive to oxygen deprivation (hypoxia and anoxia) at increased temperatures (Scott et al. 2002). To determine if DAF-16 mediates high-temperature anoxia survival, the daf-16(m26);daf-2(e1370) animals were exposed to 1 day of anoxia at 28°. Figure 2 shows that the daf-16(m26);daf-2(e1370) animals had a significantly decreased viability when exposed to high-temperature anoxia, indicating that DAF-16 mediates the high-temperature anoxia survival phenotype.
Figure 2.—
The survival rate of daf-2(e1370), wild-type, and daf-16(m26); daf-2(e1370); animals exposed to high-temperature (28°) anoxia. Wild-type L2 larvae, daf-2(e1370) L2 larvae, daf-16(m26); daf-2(e1370) L2 larvae, wild-type adults, daf-2(e1370) adults, and daf-16(m26); daf-2(e1370) adults were exposed to 1 day of anoxia at 28°. The data shown represent at least three independent experiments with a total of >150 nematodes. Error bars represent the standard deviations.
Wild-type animals but not the daf-2(e1370) animals accumulate tissue damage in long-term anoxia and high-temperature anoxia exposure:
To assay for anoxia-induced tissue damage, the wild-type and daf-2(e1370) adult animals were exposed to prolonged anoxia or high-temperature anoxia and examined using DIC Nomarski microscopy. We focused our analysis on the tissue at the anterior head region of the nematode however the entire animal was evaluated. Figure 3 shows that the wild-type and daf-2(e1370) animals exposed to 1 day of anoxia at 20° had no significant tissue damage in comparison to normoxic controls. However, the few wild-type animals that survived 3 days of anoxia at 20° showed notable tissue damage (100%, n = 6). Specifically, there was an increase in cavities in the tissue surrounding the pharynx, a loss of pharynx structure, and an overall bent morphology in the head region (Figure 3). In comparison, most (77.8%, n = 18) of the daf-2(e1370) animals exposed to 5 days of anoxia at 20° did not accumulate such tissue damage (Figure 3). To further characterize the morphological defects of wild-type animals that survive 3 days of anoxia, we used strain PD4792(mIs11 IV) in which GFP is expressed in cytoplasmic pharyngeal muscle and nuclear gut (multi-construct array containing myo-2∷GFP, pes-10∷GFP, and gut∷GFP). Due to their large size, we evaluated gut cells in the few PD4792 animals that survived 3 days of anoxia and determined that these cells had abnormalities that included cavities within the cells or cellular loss (data not shown). Both wild-type and daf-2(e1370) adult animals survived and had normal pharynx structure when exposed to 1 day of normoxia at 28°. However, the majority (83%, n = 6) of the few wild-type animals that survived anoxia at 28° showed extensive tissue damage, including multiple cavities, abnormal pharynx structure, and a bent head, whereas the majority (83.3%, n = 12) of the daf-2(e1370) animals did not display such tissue abnormalities (Figure 3). These data suggest that daf-2(e1370) animals have an altered physiologic state that either prevents or minimizes the tissue damage associated with long-term anoxia or high-temperature anoxia exposure.
Figure 3.—
Tissue morphology of wild-type and daf-2(e1370) animals exposed to long-term anoxia (20°) and high-temperature anoxia (28°). The anterior head region of wild-type and daf-2(e1370) adult hermaphrodites was examined using DIC microscopy. (A) The animals were exposed to normoxia; to 1 day, 3 days (wild type) or 1 day, 5 days [daf-2(e1370)] of anoxia at 20°; or to 1 day of normoxia or anoxia at 28°. Animals exposed to anoxia were allowed to recover in air for 1–4 hr before images were obtained. Animals representative of each genotype and condition are shown. Bar, 50 μm. (B) Enlarged image of a wild-type adult hermaphrodite exposed to 3 days of anoxia. (C) Enlarged image of wild-type adult hermaphrodite exposed to 1 day of anoxia at 28°. In B and C, the arrow points to a cavity and the open line is drawn along the lumen. Bar, 50 μm.
During examination of the morphological differences between wild-type and daf-2(e1370) animals it was noted that the daf-2(e1370) animals appeared thinner in comparison to wild type. Results indicate that the distance across the terminal bulb of the pharynx for the wild-type and daf-2(e1370) adult animals exposed to normoxia, averaged 56.8 ± 2.04 μm (n = 10) and 51.3 ± 1.33 μm (n = 10), respectively (P < 0.001). These data suggest that the daf-2(e1370) animals are slightly thinner in comparison to wild-type adults. It is not known if such anatomical distances between daf-2(e1370) animals and wild-type animals have a role in anoxia response and survival.
The daf-2(e1370) animals are motile while exposed to anoxia at 20°:
It was previously shown that wild-type animals become immobile if exposed to 24 hr of anoxia at 20°, but after several hours of reexposure to normoxia, the animals proceeded with movement in a manner indistinguishable from untreated nematodes (Van Voorhies and Ward 2000; Padilla et al. 2002). We hypothesized that if daf-2(e1370) animals have an altered physiologic state that mediates anoxia survival, the animals could have additional phenotypes, such as altered motility when exposed to anoxia. We used time-lapse microscopy to compare the motility of wild-type and daf-2(e1370) animals exposed to anoxia for specific time periods. Briefly, wild-type and daf-2(e1370) adult animals were placed into anoxia at 20° and visualized using a stereomicroscope for 4.5 min every hour for 10 hr and again at 16 and 24 hr. An animal's motility was scored positive if the animal displayed forward or reverse motion along the agar surface. Results indicate that both the wild-type and daf-2(e1370) animals are motile during the transition time (time 0) from a normoxic to an anoxic environment (Figure 4; supplemental Figures 1 and 2 at http://www.genetics.org/supplemental/). However, the motility of wild-type adults decreased after 2 hr of anoxia exposure and continued to decrease with time. Approximately 5% of the wild-type animals are motile after 8 hr of anoxia exposure and completely arrest after 16 hr of anoxia exposure. In comparison, the daf-2(e1370) adults exposed to anoxia also display a decrease in motility, yet the percentage of worms that remain motile is significantly higher in comparison to wild-type animals. A substantial number of the daf-2(e1370) adults were still moving after 8 hr of anoxia exposure. After 24 hr of anoxia exposure all of the wild-type animals arrest motility, yet several of the daf-2(e1370) continue to remain motile. Quick Time movies further demonstrate the lack of motility for the wild-type animals and the presence of motility for the daf-2(e1370) animals exposed to 24 hr of anoxia (supplemental Figures 3 and 4 at http://www.genetics.org/supplemental/). The daf-2(e1370) animals were motile after 24 hr of anoxia exposure (supplemental Figure 4 at http://www.genetics.org/supplemental/), but they appeared to be moving slower in comparison to the animals that were exposed to anoxia for less time (supplemental Figure 2 at http://www.genetics.org/supplemental/).
Figure 4.—
The motility of wild-type and daf-2(e1370) animals exposed to anoxia at 20°. Wild-type and daf-2(e1370) adult hermaphrodites were exposed to 1 day of anoxia at 20° and time-lapse microscopy was used to analyze motility while exposed to anoxia. Time 0 is when the oxygen sensor (reazurin) in the anoxia chamber indicated that no oxygen was detected in the environmental chamber. The data shown represent six independent experiments. A minimum of 50 animals was used for each experiment, of which a range of 10–44 animals was in the microscope's field of view during the motility assay. Error bars represent the standard deviations. There is a significant difference (P < 0.001) between wild-type and daf-2(e1370) for the data points time 16 and 24.
The gpd-2/3 genes predicted to encode the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase are required for the daf-2(e1370) animals' anoxia survival phenotypes:
The long-term anoxia and high-temperature anoxia survival phenotypes of daf-2(e1370) animals were suppressed in daf-16(m26);daf-2(e1370) animals, providing evidence that DAF-16 regulates a physiologic state advantageous for anoxia survival. The genes regulated by DAF-16 in early adulthood have been identified by others using microarrays (Lee et al. 2003; Murphy et al. 2003). To gain a greater understanding of the role that the daf-2/daf-16 pathway plays with anoxia survival, we used RNAi to screen through the DAF-16 upregulated genes to identify suppressors of the daf-2(e1370) high-temperature anoxia survival phenotype. This screen led to the identification of the K10B3.8 clone for gpd-2.
In C. elegans, gpd-2 encodes one of four predicted GAPDHs and, on the basis of protein homology, is predicted to reversibly catalyze the following reaction in glycolysis: glyceraldehyde-3-phosphate + NAD+ + Pi ↔ 1,3-bisphosphoglycerate + NADH + H+ GPD-2 is expressed at the highest levels during postembryonic development, primarily in the actin-containing A and I zones of body-wall muscle (Lee et al. 1992). The gpd-2 gene is part of an operon including gpd-3 and mai-1, which, respectively, encode another GAPDH isoform and a protein with homology to human mitochondrial ATPase inhibitor (Huang et al. 1989; Blumenthal and Gleason 2003). Predicted protein analysis of GPD-2 and GPD-3, using ClustalW (1.82) multiple alignment software, indicates that the proteins are 99% identical. Analysis of the predicted open reading frame sequence indicates that there is 97% identity between gpd-2 and gpd-3; therefore, using either the K10B3.8 clone specific for gpd-2 or the K10B3.7 clone specific for gpd-3 will likely cause a reduction in both the gpd-2 and the gpd-3 gene product. Throughout most of these experiments we used the K10B3.8 clone; however, we also used the K10B3.7 clone to verify results. We refer to the RNAi experiments as gpd-2/3(RNAi).
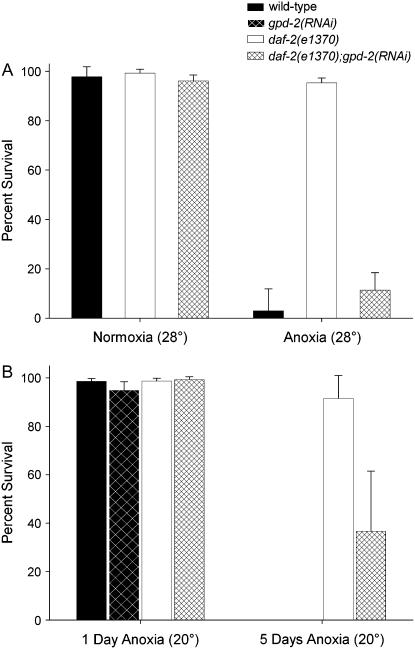
Figure 5A shows that similar to wild type and unlike daf-2(e1370), the daf-2(e1370);gpd-2/3(RNAi) adult animals did not survive 1 day of high-temperature anoxia. Use of bacterial clone K10B3.7 specific for gpd-3 resulted in similar phenotypes in that the daf-2(e1370);gpd-2/3(RNAi) animals did not survive high-temperature anoxia (n = 50). The daf-2(e1370);gpd-2/3(RNAi) animals had no detectable normoxic phenotype at 28°. The daf-2(e1370);mai-1(RNAi) animal did not result in a reduced high-temperature anoxia survival phenotype, indicating that this gene, which is in the gpd-2/3 operon, was not involved with anoxia survival (data not shown). Another gene regulated by DAF-16, lys-7, encodes a putative enzyme homologous to an antimicrobial lysozyme of the protozoan parasite Entamoeba histolytica. The daf-2(e1370);lys-7(RNAi) animals survived high-temperature anoxia (91.3%, n = 240). Together, these data suggest that gpd-2 and gpd-3 are required for the daf-2(e1370) high-temperature anoxia survival phenotype and that not all genes upregulated by DAF-16 are required for high-temperature anoxia survival.
Figure 5.—
The survival rate of gpd-2/3(RNAi) and daf-2(e1370);gpd-2/3(RNAi) adult animals exposed to anoxia. (A) Wild-type, daf-2(e1370), and daf-2(e1370);gpd-2/3(RNAi) adult hermaphrodites were exposed to 1 day of normoxia or anoxia at 28°. (B) Wild-type, daf-2(e1370), gpd-2/3(RNAi), and daf-2(e1370);gpd-2/3(RNAi) adult hermaphrodites were exposed to 1 or 5 days of anoxia at 20°. There is a significant difference between daf-2(e1370) and daf-2(e1370);gpd-2/3(RNAi) adult hermaphrodites exposed to 5 days of anoxia (P < 0.001). The data shown represent at least five independent experiments with a total of ∼50 animals for each independent experiment. Error bars represent the standard deviations.
Previously, we found that wild-type animals exposed to 1 day of anoxia at 20° maintained a high survival rate and resumed normal processes such as movement and eating after reexposure to normoxia (Padilla et al. 2002). Figure 5B shows that gpd-2/3(RNAi) animals exposed to 24 hr of anoxia at 20°, like wild-type animals, also survived 1 day of anoxia at 20° (94.6 ± 4.1%; n = 146) (Figure 5B). However, unlike wild-type animals, 52 ± 3.8% of the gpd-2/3(RNAi) animals analyzed 24 hr after anoxia treatment had an uncoordinated phenotype in that they did not move along the agar plate normally or displayed only head movements. The daf-2(e1370);gpd-2/3(RNAi) animals also survived 1 day of anoxia at 20°, yet, unlike gpd-2/3(RNAi) animals, the daf-2(e1370);gpd-2/3(RNAi) animals did not show an uncoordinated phenotype after anoxia exposure. Figure 5B shows that, unlike daf-2(e1370), the survival rate of daf-2(e1370);gpd-2/3(RNAi) animals exposed to long-term anoxia was reduced in comparison to daf-2(e1370). Furthermore, of the daf-2(e1370);gpd-2/3(RNAi) animals that survived long-term anoxia, ∼16% had an uncoordinated phenotype in that they did not move normally or were unable to move along the agar surface after the anoxia treatment. Together these data suggest that gpd-2 and gpd--3 have a functional role in anoxia response and survival.
The gpd-2/3(RNAi) and daf-2(e1370);gpd-2/3(RNAi) animals accumulate tissue damage due to anoxia exposure:
Given that gpd-2/3(RNAi) animals display anoxia-induced uncoordinated phenotypes, we wanted to determine if tissue damage occurred in the gpd-2/3(RNAi) or daf-2(e1370);gpd-2/3(RNAi) animals exposed to anoxia. We used DIC Nomarski microscopy to evaluate the animals after anoxia treatment and focused our tissue evaluation at the anterior head region; however, the entire animal was also analyzed. Figure 6A shows that the gpd-2/3(RNAi) animals exposed to normoxic conditions had normal tissue morphology. However, many (77.8%, n = 9) of the gpd-2/3(RNAi) animals had an abnormal pharynx structure after 1 day of anoxia exposure (Figure 6, A and C). In comparison, the wild-type animals, exposed to anoxia for 1 day at 20°, did not have abnormal pharynx morphology (see Figure 3).
Figure 6.—
The tissue morphology of the gpd-2/3(RNAi) and daf-2(e1370);gpd-2/3(RNAi) adult animals exposed to long-term anoxia and high-temperature anoxia. The anterior head region of gpd-2/3(RNAi) and daf-2(e1370);gpd-2/3(RNAi) adult animals was examined using DIC microscopy. (A) gpd-2/3(RNAi) animals were exposed to normoxia or to 1 day of anoxia at 20° and allowed to recover in air for 4 or 24 hr (as indicated). (B) daf-2(e1370);gpd-2/3(RNAi) animals were exposed to normoxia (20°), 1 day of anoxia (20°), 5 days of anoxia (20°), 1 day of normoxia (28°), or 1 day of anoxia (28°). Animals exposed to anoxia were allowed to recover in air for 4 or 24 hr of anoxia (as indicated) before analysis. (C) An enlarged image of gpd-2/3(RNAi) animals exposed to 1 day of anoxia at 20° is shown. The open line is drawn along the lumen and an arrow points to a region in the isthmus that is abnormal. (D) An enlarged image of daf-2(e1370);gpd-2/3(RNAi) animals exposed to 1 day of anoxia at 28°. (E) An enlarged image of daf-2(e1370);gpd-2/3(RNAi) animals exposed to 5 days of anoxia at 20° and then allowed to recover in normoxia for 36 hr. For C, the solid arrow points to a bend in the pharynx. For D, the solid arrow points to cavities surrounding the pharynx and the open arrow points to cavities within the pharynx tissue. For C and E, the open line is drawn along the lumen. Bar, 50 μm.
Figure 6B shows daf-2(e1370);gpd-2/3(RNAi) animals exposed to various anoxia treatments. The daf-2(e1370);gpd-2/3(RNAi) animals exposed to 1 day of anoxia at 20° displayed normal tissue structure. The daf-2(e1370);gpd-2/3(RNAi) animals that survived 5 days of anoxia and displayed an uncoordinated phenotype had tissue abnormalities (100%, n = 10) (Figure 6, B and E). Of the few daf-2(e1370);gpd-2/3(RNAi) animals that survived anoxia at 28°, the majority (85.7%, n = 14) had extensive damage to the tissue (Figure 6, B and D). The enlarged image of the anterior view of a representative daf-2(e1370);gpd-2/3(RNAi) animal that survived 1 day of anoxia at 28° demonstrates that it accumulates large cavities in the pharynx and the tissue surrounding the pharynx (Figure 6D). These findings further support the idea that gpd-2 and gpd-3 have a role in anoxia survival and response.
gpd-2 and gpd-3 are required for motility during brief periods of anoxia:
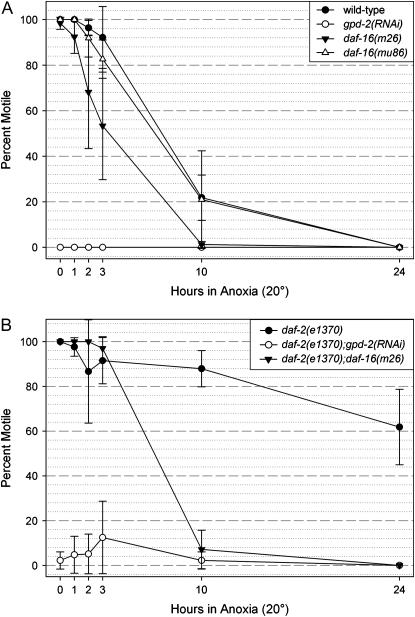
In this study, we show that both wild-type and daf-2(e1370) animals remained motile during the transition from normoxia to anoxia; however, by 24 hr of anoxia exposure the wild-type animals had arrested motility yet a significant percentage of the daf-2(e1370) animals displayed some motility (see Figure 4 and supplemental Figures 1–4 at http://www.genetics.org/supplemental/). We wanted to determine if RNAi of gpd-2 and gpd-3 influenced the motility of the daf-2(e1370) or wild-type animals exposed to anoxia. The animals were placed into anoxia at 20° and were visualized over a 4.5-min period every hour for 3 hr and again at 10 and 24 hr. An animal's motility was scored “positive” if it displayed forward or reverse motion along the agar surface. Figure 7 and supplemental Figures 5 and 6 at http://www.genetics.org/supplemental/ show, respectively, that the majority of the gpd-2/3(RNAi) and daf-2(e1370);gpd-2/3(RNAi) adult animals immediately arrested motility after the transition time (time 0) from a normoxic environment to an anoxic environment. The only detectable movement observed for these animals was head movement. The gpd-2/3(RNAi) and daf-2(e1370);gpd-2/3(RNAi) L1 larvae animals remained motile (supplemental Figures 5 and 6 at http://www.genetics.org/supplemental/). The wild-type and daf-2(e1370) animals fed control bacteria that contain the vector without the gpd-2/3 insert remained motile for several hours after exposure to anoxia, which is consistent with our results shown in Figure 4 and supplemental Figures 1 and 2 at http://www.genetics.org/supplemental/ in which the animals were fed the OP50 E. coli strain. Unlike daf-2(e1370) animals, the majority of daf-2(e1370);gpd-2/3(RNAi) adult animals remained immobile during exposure to anoxia (Figure 7B). The daf-2(e1370);gpd-2/3(RNAi) or gpd-2/3(RNAi) animals exposed to normoxic conditions are motile and did not display an uncoordinated phenotype (supplemental Figures 7 and 8 at http://www.genetics.org/supplemental/). These findings suggest that gpd-2/3 function is required for adult animals to remain motile while initially exposed to anoxia.
Figure 7.—
The motility of gpd-2/3(RNAi) and daf-2(e1370);gpd-2/3(RNAi) animals exposed to anoxia at 20°. (A)Wild-type, gpd-2/3(RNAi), daf-16(mu86), and daf-16(m26) adult animals were exposed to 1 day of anoxia at 20°. There is a significant difference in the motility of gpd-2/3(RNAi) and wild-type animals exposed to ≤3 hr of anoxia (P < 0.001). (B) daf-2(e1370), daf-2(e1370);gpd-2/3(RNAi), and daf-16(m26);daf-2(e1370) adult animals exposed to 1 day of anoxia at 20°. There is a significant difference between the motility daf-2(e1370);gpd-2/3(RNAi) and daf-2(e1370) animals exposed to ≤3 hr of anoxia (P < 0.001) as well as daf-2(e1370) and daf-16(m26);daf-2(e1370) adult animals exposed to 24 hr of anoxia (P < 0.001). For A and B, time-lapse microscopy is used to assay animal motility in anoxia. Time 0 is when the anoxia sensor in the anoxia bag indicates that no oxygen is detected. The data shown represent at least three independent experiments with a minimum of 50 animals for each experiment, of which a range of 6–43 animals was in the microscope's field of view during the motility assay time point. Error bars represent the standard deviations.
Given that DAF-16 is a regulator of gpd-2/3 expression (Lee et al. 2003; Murphy et al. 2003), we wanted determine how the motility of animals was affected by mutations in daf-16. Figure 7A shows that like wild type, and unlike gpd-2/3(RNAi), the daf-16(m26) and daf-16(mu86) animals remained motile for the first few hours of anoxia exposure. Figure 7B shows that, like daf-2(e1370), and unlike daf-2(e1370);gpd-2/3(RNAi) animals, the daf-16(m26);daf-2(e1370) remained motile for the first few hours of anoxia exposure. These data suggest that a factor in addition to DAF-16 regulates gpd-2/3 expression. Furthermore, unlike daf-2(e1370), all of the daf-16(m26);daf-2(e1370) animals had arrested motility after 24 hr of anoxia exposure, thus indicating that the daf-2(e1370) animals ability to remain motile after 24 hr of anoxia is regulated through daf-16.
RNAi of predicted glycolytic genes did not result in a reduced capacity for the daf-2(e1370) animals to survive high-temperature anoxia:
There are several possible reasons why RNAi of gpd-2 and gpd-3 results in an anoxia phenotype. For example, it is possible that disruption of glycolysis leads to a reduction of cellular ATP or that the lack of GPD-2/3 function leads to a reduction of 1,3-bisphosphoglycerate or NADH or to a buildup of glyceraldehyde-3-phosphate or NAD+. To further investigate these possibilities, we used RNAi to reduce the expression of several gene products predicted to be involved with glycolysis. Glycolysis uses 10 enzymatic reactions to convert glucose into pyruvate, and in C. elegans there are several genes, on the basis of homology, that are predicted to be involved with the glycolytic pathway. We used RNAi to reduce the expression of specific predicted glycolytic genes in the daf-2(e1370) animal to determine if this resulted in reduced survival of high-temperature anoxia similar to that seen in the daf-2(e1370);gpd-2/3(RNAi) animal. Results indicate that, unlike RNAi of gpd-2/3, RNAi of predicted glycolytic genes did not result in a significantly reduced capacity for the daf-2(e1370) animals to survive high-temperature anoxia (Table 1).
TABLE 1.
High-temperature anoxia phenotype of the daf-2(e1370);glycolytic gene(RNAi)
| Metabolic enzyme (sequence name, gene) | % survival anoxia at 28° (% ±SD) | Predicted enzymatic activity |
|---|---|---|
| Hexokinase | Glucose + ATP → glucose-6-phosphate + ADP | |
| F14B4.2 | 100.0 ± 0 | |
| H25P06.1 | 99.0 ± 1.4 | |
| Y77E11A.1 | 84.0 ± 12.4 | |
| Glucose phosphate isomerase | Glucose-6-phosphate → fructose-6-phosphate | |
| Y87G2A.8, gpi-1 | 100.0 ± 0 | |
| Phosphofructokinase | Fructose-6-phosphate → fructose-1,6-bisphosphate | |
| Y71H10A.1 | 89.9 ± 11.3 | |
| C50F4.2 | 98.0 ± 2.0 | |
| Fructose-1,6-bisphosphate aldolase | Fructose-1,6-bisphosphate → glyceraldehyde-3-phosphate, dihydroxyacetone phosphate | |
| F01F1.12 | 99.1 ± 1.5 | |
| T05D4.1 | 93.6 ± 5.5 | |
| Triose phosphate isomerase | Dihydroxyacetone phosphate → glyceraldehyde-3-phosphate | |
| Y17G7B.7, tpi-1 | 99.0 ± 1.2 | |
| Glyceraldehyde-3-phosphate dehydrogenase | Glyceraldehyde-3-phosphate + NAD + Pi → 1,3-bisphosphoglycerate + NADH | |
| T09F3.3, gpd-1 | 95.3 ± 5.1 | |
| K10B3.8, gpd-2 | 36.7 ± 16.8* | |
| K10B3.7, gpd-3 | 6.0 ± 6.0* | |
| F33H1.2, gpd-4 | 98.6 ± 1.5 | |
| Phosphoglycerate kinase | 1,3-Bisphosphoglycerate + ADP → 3-phosphoglycerate + ATP | |
| T03F1.3, pgk-1 | 100 ± 0 | |
| Phosphoglycerate mutase | 3-Phosphoglycerate → 2-phosphoglycerate | |
| F53B6.7 | 98.7 ± 2.3 | |
| R07G3.5 | 95.5 ± 5.3 | |
| F55A11.11 | 96.8 ± 5.6 | |
| T07F12.1 | 100 ± 0 | |
| F09C12.8 | 100 ± 0 | |
| Enolase | 2-Phosphoglycerate → phosphoenolpyruvate + H2O | |
| T21B10.2, enol-1 | 94.3 ± 6.4 | |
| Pyruvate kinase | Phosphoenolpyruvate + ADP → pyruvate + ATP | |
| F25H5.3 | 99.3 ± 1.5 | |
| ZK593.1 | 98 ± 1.6 | |
| Control vector | 98.4 ± .9 |
The daf-2(e1370) animal was fed a specific bacterial clone to inhibit the gene expression of interest. For all data sets, three independent experiments of at least 50 worms were tested. * P < 0.001, as determined by ANOVA or Student's t-test.
The majority of the predicted glycolytic enzymes are encoded by several genes, and therefore it is possible that redundancy could contribute to the lack of a detectable anoxia phenotype by RNAi. To further analyze the role that glycolysis may play in anoxia response and survival, we focused our phenotype analysis on the RNAi of specific glycolytic genes that do not contain a paralog in the genome. BLASTP analysis indicates that the E-value for the proteins most similar to glucose phosphate isomerase, phosphoglycerate kinase, or enolase is 2.9, 2.6, or 4.0, respectively, indicating that the genes encoding these glycolytic proteins do not have high homology to another gene in the genome. Therefore, the genes that encode glucose phosphate isomerase (gpi-1, Y87G2A.8), phosphoglycerate kinase (T03F1.3, henceforth referred to as pgk-1), and enolase (T21B10.2, henceforth referred to as enol-1) were further analyzed. We did not include tpi-1 (Y17G7B.7), which does not contain a paralog, in our analysis because the product of this enzyme, glyceraldehyde-3-phosphate, is produced by either TPI-1 or aldolase.
In glycolysis, GPI-1 functions upstream of GPD-2 and GPD-3, whereas PGK-1 and ENOL-1 function downstream of GPD-2 and GPD-3. Further phenotype analysis of gpi-1(RNAi), pgk-1(RNAi), and enol-1(RNAi) animals indicates that these animals do not have the anoxia-induced motility arrest phenotype that gpd-2/3(RNAi) animals do have (data not shown). To verify the efficiency of the RNAi experiments, we used RT–qPCR. Table 2 shows the relative reduction of mRNA for gpd-2, gpd-3, gpi-1, pgk-1, and enol-1. Results indicate that RNAi was effective in significantly reducing the mRNA for these specific genes, yet the only strain that showed an anoxia phenotype was gpd-2/3(RNAi) (Tables 1 and 2). These data indicate that wild-type levels of gpi-1, pgk-1, and enol-1 mRNA are not required for anoxia response and survival.
TABLE 2.
Efficiency of RNAi experiments determined by RT–qPCR
| Strain | mRNA assayed | Transcript fold decreasea |
|---|---|---|
| daf-2(e1370);gpd-2/3(RNAi) | gpd-2/3 | 9.67 |
| daf-2(e1370);gpi-1(RNAi) | gpi-1 | 16.40 |
| daf-2(e1370);enol-1(RNAi) | enol-1 | 12.91 |
| daf-2(e1370);pgk-1(RNAi) | pgk-1 | 7.91 |
For all RNAi experiments, the level of transcript was significantly reduced in comparison to the control strain (P < 0.05, as determined by the Mann–Whitney U-test).
Transcript level was determined relative to the daf-2(e1370) control strain.
Thus far, our data suggest that gpd-2 and gpd-3 are specifically required for anoxia response and survival. To test the hypothesis that an increased rate of anoxia survival correlates with an increased level of gpd-2/3 transcript, we analyzed the anoxia survival rate and the gpd-2/3 mRNA levels for different daf-2 alleles. We found that the daf-2(m579) animal survives high-temperature anoxia and long-term anoxia, albeit at a reduced rate in comparison to daf-2(e1370), yet the daf-2(e1371) and daf-2(m596) animals do not survive anoxia at a significant level (Table 3). We used RT–qPCR to compare the levels of gpd-2/3 mRNA in wild type to those of daf-2(e1370), daf-2(m579), daf-2(e1371), or daf-2(m596) animals. We determined that daf-2(e1370) had the highest level of gpd-2/3 transcript increase relative to wild type (Table 3). In comparison, the gpd-2/3 mRNA levels in wild-type, daf-2(e1371), daf-2(m579), and daf-2(m596) were not significantly different, as determined by the Mann–Whitney U-test. The gpd-2/3 mRNA levels in daf-2(m579) animals averaged a 1.91-fold increase compared to wild type, yet the range, from four independent experiments, was 0.98–4.23, suggesting that the gpd-2/3 mRNA level varies in the daf-2(m579) strain. In conclusion, our data indicate that the daf-2 allele with the highest rate of anoxia survival, daf-2(e1370), has the highest level of gpd-2/3 mRNA increase relative to wild type.
TABLE 3.
Transcript level of gpd-2/3 in daf-2 alleles relative to the control
| Strain | High-temperature anoxia survival rate | Long-term anoxia survival rate | Transcript fold increasea |
|---|---|---|---|
| daf-2(e1370) | 95.2 ± 3.3 (n = 150) | 91.6 ± 9.4 (n = 150) | 3.36b |
| daf-2(e1371) | 4.5 ± 5.3 (n = 200) | 0.0 ± 0.0 (n = 200) | 1.09 |
| daf-2(m596) | 0.0 ± 0.0 (n = 200) | 0.0 ± 0.0 (n = 200) | 1.05 |
| daf-2(m579) | 77.6 ± 17.7 (n = 300) | 75.3 ± 32.1 (n = 300) | 1.91 |
Transcript level was determined relative to the wild-type control strain.
P < 0.005, determined by the Mann–Whitney U-test.
DISCUSSION
The use of animal model systems to understand the response that organisms have to oxygen deprivation is important for understanding many human health issues. The objective of this study was to use C. elegans to gain a greater understanding of oxygen-deprivation survival mechanisms. In this study, we found that, unlike wild type, the daf-2(e1370) animal survives and does not display tissue damage when exposed to long-term anoxia and high-temperature anoxia. The daf-2(e1370) high-temperature anoxia survival phenotype is suppressed by daf-16(m26) and gpd-2/3(RNAi), but not by RNAi of other glycolytic genes. Furthermore, gpd-2/3(RNAi) animals are very sensitive to anoxia exposure, as seen by arrest of movement when exposed to brief periods of anoxia, supporting the idea that glyceraldehyde-3-phosphate dehydrogenase has a vital role in anoxia response and survival in adult C. elegans.
Genotypes and anoxia survival phenotypes:
Phenotype analysis of the daf-2(e1370) animal suggests an altered physiological or metabolic state that contributes to long-term anoxia and high-temperature anoxia survival. The factor(s) influencing anoxia survival may or may not be more prevalent in the daf-2(e1370) adult than in the daf-2(e1370) L2 larvae. Thus, even though the daf-2(e1370) adult has a higher survival rate in 5 days of anoxia in comparison to daf-2(e1370) larvae, this may be due to the fact that the larvae not only need to respond to the damage that results from anoxia, but also must reinitiate development upon return to normoxia, whereas the adults do not have to deal with the energy-requiring process of developmental progression. In both the daf-2(e1370) L2 larva and the adult, the ability to survive high-temperature anoxia or long-term anoxia is dependent on DAF-16, indicating that the genetic pathway best known for dauer formation and longevity is also involved with enhanced anoxia response and survival.
Use of RNAi to screen the genes upregulated via DAF-16 in the daf-2(e1370) animal identified gpd-2 and gpd-3 as suppressors of the daf-2(e1370) high-temperature anoxia survival phenotype. The gpd-2/3 genes encode the highly conserved enzyme GAPDH, which catalyzes the oxidation and phosphorylation of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate in glycolysis (Huang et al. 1989; Lee et al. 1992). We determined by RNAi that 17 other glycolytic genes, including those that encode phosphoglycerate kinase and pyruvate kinase, which catalyze reactions that produce ATP, did not suppress the daf-2(e1370) high-temperature anoxia survival phenotype. RNAi of glycolytic genes that do not have paralogs in the genome (gpi-1, enol-1, pgk-1) did not result in anoxia phenotypes. This suggests that the gpd-2/3 products are specifically required for the anoxia survival phenotype observed in daf-2(e1370) animals.
We hypothesize that the increased expression of gpd-2 and gpd-3 in the daf-2(e1370) animal has a role in the ability to survive anoxia. Theoretically, an increase in gpd-2/3 expression could result in increased ratios of 1,3-bisphosphoglycerate to glyceraldehyde-3-phosphate; ATP to ADP, and/or a NADH to NAD+. Perhaps an increase in ATP/ADP ratios provides the necessary energy for survival of long-term anoxia or high-temperature anoxia. Alternatively, consider that there is increasing evidence that the accumulation of glyceraldehyde-3-phosphate can glycate proteins, which will lead to deleterious effects within cells (Hipkiss 2006). Thus, another interpretation as to why daf-2(e1370) animals survive long-term anoxia or high-temperature anoxia is that, in comparison to wild type, there is a lower concentration of glyceraldehyde-3-phosphate available to glycate proteins and the lower level of such altered proteins has a beneficial effect on an organism exposed to anoxia.
Other daf-2 alleles analyzed indicate that, in addition to daf-2(e1370), the daf-2(m579) animal survives long-term anoxia and high-temperature anoxia, albeit at a slightly reduced rate. However, the daf-2(e1371) and daf-2(m596) animals do not survive these anoxic conditions, thus demonstrating that some but not all daf-2 mutants have the enhanced anoxia survival phenotype. We found by RT–qPCR that the daf-2(e1370) animals have an increased level of gpd-2/3 mRNA, in comparison to wild type, indicating that an increased level of gpd-2/3 mRNA correlates with anoxia survival phenotypes. The levels of gpd-2/3 mRNA in daf-2(m579) animals appeared to have a variable range from similar to wild type to an increased level in comparison to wild type, which may explain the large standard deviation observed for the survival rate of these animals exposed to high-temperature anoxia.
Others have analyzed the survival rate of high-temperature hypoxia for various daf-2 alleles and determined that daf-2(e1370) and daf-2(m579) animals survive high-temperature hypoxia, that only about half of the daf-2(m596) animals survive, and that the majority of the daf-2(e1371) animals do not survive (Scott et al. 2002). The fact that daf-2(m579) and daf-2(m596) animals have a higher survival rate when exposed to high-temperature hypoxia in comparison to high-temperature anoxia or long-term anoxia supports the idea that there could be different requirements for surviving anoxia in comparison to hypoxia.
Anoxia-induced tissue damage:
The wild-type animal has an overall normal tissue structure, moves normally, and is able to produce offspring after exposure to 1 day of anoxia at 20°. Some of the gpd-2/3(RNAi) animals exposed to 1 day of anoxia have tissue damage and an uncoordinated phenotype, suggesting that gpd-2 and gpd-3 have a role in maintaining tissue structure and function when exposed to 1 day of anoxia. Unlike the gpd-2/3(RNAi) animals, the daf-2(e1370);gpd-2/3(RNAi) animals exposed to 1 day of anoxia at 20° displayed normal tissue structure. One interpretation of these results is that GPD-2/3 may not be the only gene product involved with tissue structure maintenance within the daf-2(e1370) animal.
Of the few wild-type animals that survive long-term anoxia or high-temperature anoxia, the majority accumulates extensive tissue damage, including pharynx abnormalities and cavities within tissue. In comparison, the daf-2(e1370) animal has an enhanced ability to maintain tissue structure and function when exposed to long-term and high-temperature anoxia. One possible reason for the phenotypic difference between the daf-2(e1370) and the wild-type animal is that the daf-2(e1370) animal has the necessary physiological or metabolic state to maintain cell and tissue homeostasis (Hochachka 2000). For example, perhaps the energy-requiring processes involved with tissue/cell viability and function are maintained in the daf-2(e1370) animal during longer periods of anoxia exposure time or when exposed to two stresses (high temperature and anoxia). Second, it is also plausible that the wild-type animals accumulate products detrimental to tissue in anoxia in comparison to the daf-2(e1370) animals. Third, it is possible that reperfusion of oxygen to the animals that did not die during anoxia causes damage and that the daf-2(e1370) animals are better able than wild-type animals to respond to this type of damage.
The mechanism of anoxia-induced tissue damage is not understood at this time but we hypothesize that gpd-2/3 function is involved. This idea is supported by the fact that some of the gpd-2/3(RNAi) animals become uncoordinated and accumulate some tissue damage after 24 hr of anoxia and that the daf-2(e1370);gpd-2/3(RNAi) animal accumulates tissue damage after exposure to long-term or high-temperature anoxia. The disruption of gpd-2/3 function could result in several metabolic changes. The question is, which change(s) in the gpd-2/3(RNAi), daf-2(e1370);gpd-2/3, and daf-2(e1370) animals leads to the anoxia response observed in these studies?
Anoxia-induced motility arrest:
We show that the wild-type animal has the ability to move during the first few hours of anoxia exposure, yet in longer anoxia exposures (≥10 hr) the animal will arrest motility. This response to anoxia may be an important adaptive mechanism because it allows the animal to move, when initially exposed to a stressful environment (anoxia), to a less stressful environment (normoxia or hypoxia); yet if the animal is unable to move from the stressful environment, energy-requiring processes such as motility are arrested. We found that a significant number of the daf-2(e1370) animals in a population did not completely arrest motility even after being exposed to 24 hr of anoxia. This result supports the idea that the daf-2(e1370) animal has the physiological or metabolic state to maintain energy-requiring processes in anoxia.
The daf-2(e1370) prolonged motility in anoxia phenotype was suppressed by daf-16(m26), in that these animals responded to anoxia similarly to wild type. The daf-2(e1370);gpd-2/3(RNAi) animals had a somewhat different phenotype in that they completely arrested motility upon exposure to anoxia. The gpd-2/3(RNAi) animals, but not the daf-16(m26) or daf-16(mu86) animals, also arrested motility when initially exposed to anoxia. Together, these data suggest that the prolonged motility in anoxia phenotype is regulated in a daf-16 manner, but motility during brief exposures to anoxia is dependent on gpd-2 and gpd-3. Thus, it is likely that an additional factor(s) or mechanisms, other than daf-16, regulates gpd-2 and gpd-3 and thus motility in anoxia.
The loss of motility in gpd-2/3(RNAi) animals exposed to brief periods of anoxia is a phenotype that has not been observed previously. This phenotype could be a direct result of decreased GAPDH activity. In wild-type adult C. elegans, there is a correlation between the anoxia-induced arrest of motility with constant levels of protein-bound NADH and an increase in free NADH (Paul et al. 2000). Thus, it would be of interest to determine and compare the levels of free NADH and protein-bound NADH in the daf-2(e1370), wild-type, gpd-2(RNAi), and daf-2(e1370);gpd-2(RNAi) animals exposed to anoxia. Such experiments would allow us to determine if the gpd-2/3(RNAi) animals have reduced levels of NADH due to a reduction of GPD-2/3 activity. It is interesting to note that others have found that the sperm-specific glyceraldehyde-3-phosphate dehydrogenase is required for sperm motility (Miki et al. 2004), suggesting that this isoform may have a role in motility in different cellular types or in different environmental conditions.
Glyceraldehyde-3-phosphate dehydrogenase and anoxia:
In mammals, several glycolytic enzymes, including GAPDH, are induced under hypoxic conditions, which could allow maintenance of ATP levels by increasing anaerobic glycolysis (Duncan and Storey 1992; Graven et al. 1994, 2003). Analyses of the gpd-1(RNAi) and gpd-4(RNAi) animals indicate that gpd-1 and gpd-4 are not required for the adult to respond to and to survive anoxia and others have shown that these genes are required for embryo viability, thus suggesting alternate functional roles between the GPD-2/3 and GPD-1/4 isoforms (Kamath et al. 2003). In this study, we did not identify the requirement of other putative glycolytic genes for the daf-2(e1370) high-temperature anoxia phenotype. Furthermore, unlike gpd-2/3(RNAi) animals, the gpi-1(RNAi), pgk-1(RNAi), or enol-1(RNAi) animals did not exhibit an immediate arrest of motility in response to anoxia. These data suggest either that there are compensatory systems in place for many of the glycolytic enzymes, with the exception of gpd-2 and gpd-3, or that it is not the mere flux through glycolysis that is required for enhanced anoxia survival in the daf-2(e1370). It is also possible that GPD-2 and GPD-3 could have an anoxia-induced cellular function not related to glycolysis and not yet understood. For example, consider that in mice brain-cell lines exposed to hypoxia, there is an increased expression and localization of a specific GAPDH in nuclear fractions suggesting that some of the GAPDH isoforms may have an alternate functional role in addition to glycolysis (Sirover 1999; Yamaji et al. 2003; Sirover 2005).
Our RNAi analysis of gpd-2 and gpd-3 in both wild-type and daf-2(e1370) animals indicates that gpd-2 and gpd-3 are required for anoxia response and survival in the adult nematode. The analysis of daf-2 alleles indicates that the gpd-2/3 mRNA level was highest in daf-2(e1370) animals in comparison to wildtype. These data suggest that decreased levels of gpd-2 and gpd-3 correlate with inadequate anoxia response and survival and that the increased level of gpd-2/3 mRNA correlates with anoxia survival. Together, these data provide additional evidence that gpd-2 and gpd-3 have a role in the daf-2(e1370) animal's ability to survive long-term or high-temperature anoxia.
To date, in C. elegans there is not a genetic mutation that causes a dramatic reduction in anoxia viability in adult hermaphrodites. However, there are RNAi experiments or genetic mutations that reduce the viability of embryos exposed to either anoxia or hypoxia [mdf-2(RNAi) and san-1(RNAi) or (hif-1(ia04), respectively] (Jiang et al. 2001; Nystul et al. 2003). Thus, the response to oxygen deprivation is likely to be dependent on several genetic pathways, the developmental stage of the animal, and oxygen tension. In this report, we found that a reduction in gpd-2/3 function by RNAi will not reduce the survival rate of wild-type adult hermaphrodites exposed to 1 day of anoxia; however, the motility and tissue structure is affected in these animals, suggesting that gpd-2/3 function could be important for the maintenance and recovery of tissues exposed to anoxia. Studies conducted by others indicate that GAPDH has a role in oxygen-deprivation responses in vertebrates and our studies presented here suggest that this is also the case in C. elegans, thus providing further evidence that there are conserved oxygen-deprivation response mechanisms between vertebrates and invertebrates. In conclusion, we suggest that GAPDH expression serves a protective role in animals exposed to oxygen deprivation. The ability to study oxygen deprivation in a genetic model system such as C. elegans will lead to the identification and to a greater understanding of oxygen-deprivation protective mechanisms.
Acknowledgments
We appreciate the valuable input from all members of the Padilla Lab. We especially thank Kent Chapman, Pudur Jagadeeswaran, Vinita Hajeri, and Desh Mohan for valuable comments regarding the manuscript. We also thank the Caenorhabditis elegans Genetics Stock Center, Donald Riddle, and Leon Avery for strains. This work was supported in part by a grant from the National Institutes of Health National Institute of General Medical Sciences (R01GM069419) and University of North Texas internal research support.
References
- Anderson, G. L., 1978. Responses of dauer larvae of Caenorhabditis elegans (Nematoda: Rhabditidae) to thermal stress and oxygen deprivation. Can. J. Zool. 56: 1786–1791. [Google Scholar]
- Blumenthal, T., and K. S. Gleason, 2003. Caenorhabditis elegans operons: form and function. Nat. Rev. Genet. 4: 112–120. [DOI] [PubMed] [Google Scholar]
- Burnell, A. M., K. Houthoofd, K. O'Hanlon and J. R. Vanfleteren, 2005. Alternate metabolism during the dauer stage of the nematode Caenorhabditis elegans. Exp. Gerontol. 40: 850–856. [DOI] [PubMed] [Google Scholar]
- Diaz, R. J., 2001. Overview of hypoxia around the world. J. Environ. Qual. 30: 275–281. [DOI] [PubMed] [Google Scholar]
- Duncan, J. A., and K. B. Storey, 1992. Subcellular enzyme binding and the regulation of glycolysis in anoxic turtle brain. Am. J. Physiol. 262: R517–R523. [DOI] [PubMed] [Google Scholar]
- Epstein, A. C., J. M. Gleadle, L. A. McNeill, K. S. Hewitson, J. O'Rourke et al., 2001. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54. [DOI] [PubMed] [Google Scholar]
- Foe, V. E., and B. M. Alberts, 1985. Reversible chromosome condensation induced in Drosophila embryos by anoxia: visualization of interphase nuclear organization. J. Cell Biol. 100: 1623–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuson, A. L., D. F. Cowan, S. B. Kanatous, L. K. Polasek and R. W. Davis, 2003. Adaptations to diving hypoxia in the heart, kidneys and splanchnic organs of harbor seals (Phoca vitulina). J. Exp. Biol. 206: 4139–4154. [DOI] [PubMed] [Google Scholar]
- Gems, D., A. J. Sutton, M. L. Sundermeyer, P. S. Albert, K. V. King et al., 1998. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics 150: 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghabrial, A., S. Luschnig, M. M. Metzstein and M. A. Krasnow, 2003. Branching morphogenesis of the Drosophila tracheal system. Annu. Rev. Cell Dev. Biol. 19: 623–647. [DOI] [PubMed] [Google Scholar]
- Graven, K. K., R. F. Troxler, H. Kornfeld, M. V. Panchenko and H. W. Farber, 1994. Regulation of endothelial cell glyceraldehyde-3-phosphate dehydrogenase expression by hypoxia. J. Biol. Chem. 269: 24446–24453. [PubMed] [Google Scholar]
- Graven, K. K., D. Bellur, B. D. Klahn, S. L. Lowrey and E. Amberger, 2003. HIF-2alpha regulates glyceraldehyde-3-phosphate dehydrogenase expression in endothelial cells. Biochim. Biophys. Acta 1626: 10–18. [DOI] [PubMed] [Google Scholar]
- Gray, J. M., D. S. Karow, H. Lu, A. J. Chang, J. S. Chang et al., 2004. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature 430: 317–322. [DOI] [PubMed] [Google Scholar]
- Haddad, G. G., 1998. Mechanisms of anoxia tolerance: a novel approach using a Drosophila model system. Adv. Exp. Med. Biol. 454: 273–280. [PubMed] [Google Scholar]
- Haddad, G. G., Y. Sun, R. J. Wyman and T. Xu, 1997. Genetic basis of tolerance to O2 deprivation in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 94: 10809–10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajeri, V. A., J. Trejo and P. A. Padilla, 2005. Characterization of sub-nuclear changes in Caenorhabditis elegans embryos exposed to brief, intermediate and long-term anoxia to analyze anoxia-induced cell cycle arrest. BMC Cell Biol. 6: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand, S. C., 1998. Quiescence in Artemia franciscana embryos: reversible arrest of metabolism and gene expression at low oxygen levels. J. Exp. Biol. 201: 1233–1242. [DOI] [PubMed] [Google Scholar]
- Hermes-Lima, M., and T. Zenteno-Savin, 2002. Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 133: 537–556. [DOI] [PubMed] [Google Scholar]
- Hipkiss, A. R., 2006. On the mechanisms of ageing suppression by dietary restriction: Is persistent glycolysis the problem? Mech. Ageing Dev. 127: 8–15. [DOI] [PubMed] [Google Scholar]
- Hochachka, P. W., 2000. Oxygen, homeostasis, and metabolic regulation. Adv. Exp. Med. Biol. 475: 311–335. [DOI] [PubMed] [Google Scholar]
- Hochachka, P. W., P. L. Lutz, T. Sick, M. Rosenthal and G. E. van den Thillart, 1993. Surviving Hypoxia Mechanisms of Control and Adaptation. CRC Press, Boca Raton, FL.
- Hochachka, P. W., S. C. Land and L. T. Buck, 1997. Oxygen sensing and signal transduction in metabolic defense against hypoxia: lessons from vertebrate facultative anaerobes. Comp. Biochem. Physiol. A Physiol. 118: 23–29. [DOI] [PubMed] [Google Scholar]
- Huang, X. Y., L. A. Barrios, P. Vonkhorporn, S. Honda, D. G. Albertson et al., 1989. Genomic organization of the glyceraldehyde-3-phosphate dehydrogenase gene family of Caenorhabditis elegans. J. Mol. Biol. 206: 411–424. [DOI] [PubMed] [Google Scholar]
- Jiang, H., R. Guo and J. A. Powell-Coffman, 2001. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc. Natl. Acad. Sci. USA 98: 7916–7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath, R. S., A. G. Fraser, Y. Dong, G. Poulin, R. Durbin et al., 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237. [DOI] [PubMed] [Google Scholar]
- Kenyon, C., J. Chang, E. Gensch, A. Rudner and R. Tabtiang, 1993. A C. elegans mutant that lives twice as long as wild type. Nature 366: 461–464. [DOI] [PubMed] [Google Scholar]
- Kimura, K. D., H. A. Tissenbaum, Y. Liu and G. Ruvkun, 1997. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277: 942–946. [DOI] [PubMed] [Google Scholar]
- Lahiri, S., A. Roy, S. M. Baby, T. Hoshi, G. L. Semenza et al., 2005. Oxygen sensing in the body. Prog. Biophys. Mol. Biol. 91: 249–286. [DOI] [PubMed] [Google Scholar]
- Larsen, P. L., P. S. Albert and D. L. Riddle, 1995. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics 139: 1567–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. S., S. Kennedy, A. C. Tolonen and G. Ruvkun, 2003. DAF-16 target genes that control C. elegans life-span and metabolism. Science 300: 644–647. [DOI] [PubMed] [Google Scholar]
- Lee, Y. H., X. Y. Huang, D. Hirsh, G. E. Fox and R. M. Hecht, 1992. Conservation of gene organization and trans-splicing in the glyceraldehyde-3-phosphate dehydrogenase-encoding genes of Caenorhabditis briggsae. Gene 121: 227–235. [DOI] [PubMed] [Google Scholar]
- Link, C. D., A. Taft, V. Kapulkin, K. Duke, S. Kim et al., 2003. Gene expression analysis in a transgenic Caenorhabditis elegans Alzheimer's disease model. Neurobiol. Aging 24: 397–413. [DOI] [PubMed] [Google Scholar]
- Maxwell, P. H., C. W. Pugh and P. J. Ratcliffe, 2001. The pVHL-hIF-1 system. a key mediator of oxygen homeostasis. Adv. Exp. Med. Biol. 502: 365–376. [PubMed] [Google Scholar]
- McElwee, J., K. Bubb and J. H. Thomas, 2003. Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell 2: 111–121. [DOI] [PubMed] [Google Scholar]
- McElwee, J. J., E. Schuster, E. Blanc, J. H. Thomas and D. Gems, 2004. Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J. Biol. Chem. 279: 44533–44543. [DOI] [PubMed] [Google Scholar]
- Miki, K., W. Qu, E. H. Goulding, W. D. Willis, D. O. Bunch et al., 2004. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc. Natl. Acad. Sci. USA 101: 16501–16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton, D. B., 2004. Atypical soluble guanylyl cyclases in Drosophila can function as molecular oxygen sensors. J. Biol. Chem. 279: 50651–50653. [DOI] [PubMed] [Google Scholar]
- Munoz, M. J., 2003. Longevity and heat stress regulation in Caenorhabditis elegans. Mech. Ageing Dev. 124: 43–48. [DOI] [PubMed] [Google Scholar]
- Murphy, C. T., S. A. McCarroll, C. I. Bargmann, A. Fraser, R. S. Kamath et al., 2003. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424: 277–283. [DOI] [PubMed] [Google Scholar]
- Nystul, T. G., J. P. Goldmark, P. A. Padilla and M. B. Roth, 2003. Suspended animation in C. elegans requires the spindle checkpoint. Science 302: 1038–1041. [DOI] [PubMed] [Google Scholar]
- Ogg, S., S. Paradis, S. Gottlieb, G. I. Patterson, L. Lee et al., 1997. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389: 994–999. [DOI] [PubMed] [Google Scholar]
- Padilla, P. A., T. G. Nystul, R. A. Zager, A. C. Johnson and M. B. Roth, 2002. Dephosphorylation of cell cycle-regulated proteins correlates with anoxia-induced suspended animation in Caenorhabditis elegans. Mol. Biol. Cell 13: 1473–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, R. J., J. Gohla, R. Foll and H. Schneckenburger, 2000. Metabolic adaptations to environmental changes in Caenorhabditis elegans. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 127: 469–479. [DOI] [PubMed] [Google Scholar]
- Riddle, D. L., and P. S. Albert, 1997. Genetic and environmental regulation of dauer larva development, pp. 739–768 in C. elegans II, edited by D. L. Riddle, T. Blumenthal, B. J. Meyer and J. R. Priess. Cold Spring Harbor Laboratory Press, Plainview, NY. [PubMed]
- Scott, B. A., M. S. Avidan and C. M. Crowder, 2002. Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science 296: 2388–2391. [DOI] [PubMed] [Google Scholar]
- Semenza, G. L., 2000. HIF-1 and human disease: one highly involved factor. Genes Dev. 14: 1983–1991. [PubMed] [Google Scholar]
- Semenza, G. L., 2001. Hif-1, o(2), and the 3 phds: how animal cells signal hypoxia to the nucleus. Cell 107: 1–3. [DOI] [PubMed] [Google Scholar]
- Shen, C., and J. A. Powell-Coffman, 2003. Genetic analysis of hypoxia signaling and response in C. elegans. Ann. NY Acad. Sci. 995: 191–199. [DOI] [PubMed] [Google Scholar]
- Sirover, M. A., 1999. New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim. Biophys. Acta 1432: 159–184. [DOI] [PubMed] [Google Scholar]
- Sirover, M. A., 2005. New nuclear functions of the glycolytic protein, glyceraldehyde-3-phosphate dehydrogenase, in mammalian cells. J. Cell. Biochem. 95: 45–52. [DOI] [PubMed] [Google Scholar]
- Sulston, J., and J. Hodgkin, 1988. Methods, pp. 587–606 in The Nematode Caenorhabditis elegans, edited by W. Wood. Cold Spring Harbor Laboratory Press, Plainview, NY.
- Timmons, L., D. L. Court and A. Fire, 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263: 103–112. [DOI] [PubMed] [Google Scholar]
- Treinin, M., J. Shliar, H. Jiang, J. A. Powell-Coffman, Z. Bromberg et al., 2003. HIF-1 is required for heat acclimation in the nematode Caenorhabditis elegans. Physiol. Genomics 14: 17–24. [DOI] [PubMed] [Google Scholar]
- Vanfleteren, J. R., and A. De Vreese, 1996. Rate of aerobic metabolism and superoxide production rate potential in the nematode Caenorhabditis elegans. J. Exp. Zool. 274: 93–100. [DOI] [PubMed] [Google Scholar]
- Van Voorhies, W., 2003. The metabolic rate of Caenorhabditis elegans dauer larvae: comments on a recent paper by Houthoofd et al. Exp. Gerontol. 38: 343–344. [DOI] [PubMed] [Google Scholar]
- Van Voorhies, W. A., and S. Ward, 2000. Broad oxygen tolerance in the nematode Caenorhabditis elegans. J. Exp. Biol. 203(Pt. 16): 2467–2478. [DOI] [PubMed] [Google Scholar]
- Wingrove, J. A., and P. H. O'Farrell, 1999. Nitric oxide contributes to behavioral, cellular, and developmental responses to low oxygen in Drosophila. Cell 98: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji, R., K. Fujita, S. Takahashi, H. Yoneda, K. Nagao et al., 2003. Hypoxia up-regulates glyceraldehyde-3-phosphate dehydrogenase in mouse brain capillary endothelial cells: involvement of Na+/Ca2+ exchanger. Biochim. Biophys. Acta 1593: 269–276. [DOI] [PubMed] [Google Scholar]