Abstract
Drosophila melanogaster heterochromatin protein 2 (HP2) interacts with heterochromatin protein 1 (HP1). In polytene chromosomes, HP2 and HP1 colocalize at the chromocenter, telomeres, and the small fourth chromosome. We show here that HP2 is present in the arms as well as the centromeric regions of mitotic chromosomes. We also demonstrate that Su(var)2-HP2 exhibits a dosage-dependent modification of variegation of a yellow reporter transgene, indicating a structural role in heterochromatin formation. We have isolated and characterized 14 new mutations in the Su(var)2-HP2 gene. Using wm4h, many (but not all) mutant alleles show dominant Su(var) activity. Su(var)2-HP2 mutant larvae show a wide variety of mitotic abnormalities, but not the telomere fusion seen in larvae deficient for HP1. The Su(var)2-HP2 gene codes for two isoforms: HP2-L (∼365 kDa) and HP2-S (∼175 kDa), lacking exons 5 and 6. In general, mutations that affect only the larger isoform result in more pronounced defects than do mutations common to both isoforms. This suggests that an imbalance between large and small isoforms is particularly deleterious. These results indicate a role for HP2 in the structural organization of chromosomes and in heterochromatin-induced gene silencing and show that the larger isoform plays a critical role in these processes.
THE DNA found inside a eukaryotic nucleus does not exist as such, but is packaged with proteins to form chromatin. By weight, chromatin is approximately one-third DNA, one-third histones, and one-third nonhistone chromosomal proteins, plus a small RNA component. The histones play a major role in packaging and organizing the very long DNA molecules of each chromosome. Analysis of post-transcriptional modifications of histones shows that these basic proteins also play a significant role in determining specific modes of packaging and concomitant gene regulation, interacting with both enzymes and structural proteins that define alternative chromatin states (for reviews, see Richards and Elgin 2002; Khorasanizadeh 2004). One level of packaging, evident by cytological examination of interphase nuclei, is the partitioning of chromatin into euchromatin and heterochromatin. While euchromatin decondenses during interphase, heterochromatin remains relatively more condensed, showing intense staining.
In Drosophila melanogaster, the entire Y chromosome, most of the fourth chromosome, the proximal 40% of the X chromosome, and the pericentric 20% of the major autosomes are heterochromatic. Euchromatic DNA has a high proportion of genes and unique sequences and tends to replicate throughout S phase. Heterochromatin has relatively few genes, is rich in repetitive sequences, and tends to replicate late in S phase; it is often found to be underreplicated (compared to euchromatin) in the polytene chromosomes of insects such as D. melanogaster. Heterochromatin is also characterized by a very low rate of meiotic recombination (Weiler and Wakimoto 1995; Zhimulev et al. 2004).
Euchromatin and heterochromatin appear to differ functionally as well. In a phenomenon termed position-effect variegation (PEV), chromosomal rearrangements that juxtapose euchromatin with heterochromatin at breakpoints often result in the misregulation of the genes found within the regions flanking the breakpoints (Spofford 1976). Misregulation can be seen not only in rearrangements, but also in transgenes, when a gene normally found in euchromatin is placed within a heterochromatic environment. These genes are appropriately expressed (time and place) in some cells but not in others, leading to a mottled or variegated pattern. For example, in the inversion In(1)wm4h, the white gene fails to express in some eye cells, leading to white patches in the eye. Such loss of normal expression, apparently the consequence of heterochromatic packaging, is described as silencing.
Many mutations that dominantly affect PEV have been recovered (Reuter and Wolff 1981; Grigliatti 1991). Suppressors of variegation [Su(var)s] are those mutations that reduce the level of silencing of euchromatic genes when they variegate due to placement in or near heterochromatin. Enhancers of variegation [E(var)s] are mutations that increase the level of silencing of such genes. One of the first Su(var)s to be cloned and characterized was Su(var)205 (James and Elgin 1986; Eissenberg et al. 1990). The Su(var)205 gene codes for heterochromatin protein 1 (HP1). HP1 is a highly conserved 23-kDa protein that contains two identifiable domains, an amino-terminal chromodomain and a carboxy-terminal chromoshadow domain. A variable length amino acid sequence that shows little conservation, the hinge region, separates these two domains. The protein is found primarily in the pericentric heterochromatin, as well as at the telomeres, in a variety of organisms from fission yeast to higher eukaryotes such as mouse and humans (Eissenberg and Elgin 2000). While mutations that reduce the intercellular levels of HP1 have been shown to suppress variegation, mutations that increase the levels of HP1 act as enhancers of variegation. This type of antipodal response to protein dosage has been used to argue that a given protein plays a structural role in heterochromatin formation (Locke et al. 1988). In fact, HP1 has been shown to bind to histone H3 tails that have been methylated at lysine 9, a modification that appears to mark heterochromatin domains (Bannister et al. 2001; Lachner et al. 2001). A mutation in HP1 that disrupts this interaction results in a reduction in the level of silencing (suppression of variegation) (Jacobs et al. 2001). Several proteins have been shown to bind directly to HP1, including the heterochromatic proteins SU(VAR)3-9 (a histone H3-K9 methyltransferase) (Rea et al. 2000; Schotta et al. 2002) and SU(VAR)3-7 (a zinc-finger protein) (Cleard et al. 1997). Changes in the dosage of these proteins again result in an antipodal response from a variegating reporter.
We previously reported the identification of an additional HP1 partner, heterochromatin protein 2 (HP2) (Shaffer et al. 2002). This protein was originally identified through a yeast two-hybrid screen while looking for proteins that interact with HP1. The distributions of HP1 and HP2 show substantial overlap on polytene chromosomes, including prominent association with the chromocenter. In polytene chromosomes where HP1 has been ectopically localized to new euchromatic sites, HP2 is recruited as well, again suggesting an interaction between the two proteins. Lethal mutations in the gene for HP2, Su(var)2-HP2, have been isolated. A total of 17 lethal alleles have now been identified, 3 of which have previously been characterized in detail. Two of these 3 alleles act as suppressors of variegation. In all 3 cases, a molecular lesion was detected in the Su(var)2-HP2 gene (two missense mutations and one truncation mutation) (Shaffer et al. 2002). Taken together, these observations suggest that both HP1 and HP2 are required for proper function of heterochromatin.
The HP2 protein is distinctly different from HP1. In contrast to the small tripartite structure of HP1, HP2 is a very large nuclear protein that exists in two isoforms produced by alternative splicing. The larger isoform, HP2-L, is the product of a 9.7-kb message that has nine exons, coding for a protein with a predicted molecular weight of ∼365 kDa. The smaller isoform, HP2-S, is derived from a transcript in which the fifth and sixth exons are absent, resulting in a protein with a predicted molecular weight of 176 kDa. The only identifiable domains in the protein are a pair of small AT-hook motifs found within the primary sequence of HP2-L. Both isoforms have a skewed amino acid composition rich in serine and the charged amino acids (DERK); for example, in the larger isoform these 5 amino acids make up almost half (43%) of the 3257 amino acids (Shaffer et al. 2002). Recently, the HP1-binding domain in HP2 was mapped to exon 8, common to both the larger and the smaller isoforms of HP2 (Stephens et al. 2005).
To explore the functional role of HP2, identify critical domains, and examine the relationship between the large and small isoforms, we undertook an extensive genetic and phenotypic analysis. Using a matching duplication and deletion containing the Su(var)2-HP2 gene, we show a dose-dependent antipodal effect of HP2 on PEV reminiscent of the dosage sensitivity reported for HP1. We also report the characterization of 14 new alleles in addition to the 3 alleles previously described. Taken together, these 17 alleles fall into three broad classes: 3 missense alleles (one each in exons 6, 8, and 9); 6 nonsense alleles found in exon 6 that are predicted to affect only the larger isoform; and 8 nonsense alleles in exon 8 that should affect both the smaller and the larger isoforms. Most of the exon 6 nonsense alleles and all of the missense alleles are associated with dominant suppression of PEV and high rates of mitotic defects. In contrast to Su(var)205 mutants, which show a high rate of telomere fusions (Fanti et al. 1998), Su(var)2-HP2 mutants show a variety of different aberrant mitotic phenotypes, but not telomere–telomere attachments. These results clearly establish a distinct role for HP2 in both gene silencing and chromosome organization. Moreover, the pattern of mutations and phenotypes demonstrates that HP2-L is essential and suggests that this protein mediates the interaction of HP1 with other critical chromatin components.
MATERIALS AND METHODS
D. melanogaster stocks, crosses, and mutagenesis:
Drosophila stocks were raised on cornmeal–sucrose-based medium (Shaffer et al. 1994). Crosses were carried out at 25° with 70% relative humidity. The methods used for generating mutations with ethyl methanesulfonate (EMS) and for recovery of Su(var)2-HP2 alleles have been described previously (Shaffer et al. 2002). To assess modification of variegation, males carrying the chromosome to be tested were first crossed to In(2L)nocSco/SM6 virgins. Male progeny carrying In(2L)nocSco and the chromosome to be tested were then crossed to virgins carrying either a wm4h chromosome or a chromosome carrying a transgene that variegates for yellow (B079) (Yan et al. 2002). The In(2L)nocSco chromosome was chosen because it contains no major enhancer or suppressor of PEV. Males from these crosses were collected, aged to 3–5 days post-eclosion, and photographed. Eye pigment was extracted and pigment levels were determined (from the optical density at 480 nm) in selected lines as described (Khesin and Leibovitch 1978).
To assess mitotic phenotypes, Su(var)HP2 mutant alleles were balanced over ST. The ST chromosome is a translocation between CyO and TM6B that carries the dominant larval marker Tubby (Tb) (the kind gift of Antonio Garcia Bellido, Universidad Autonoma, Madrid; see also Gatti and Goldberg (1991)). To generate larvae hemizygous for the Su(var)HP2 mutations, we crossed Df(2R)B11/ST flies to flies bearing the Su(var)HP2 mutant allele of interest balanced over ST. Hemizygous mutant larvae were identified on the basis of their non-Tb phenotype.
The Su(var)205 (Sinclair et al. 1983; Eissenberg et al. 1992) and caravaggio (cav) (Cenci et al. 2003) mutant alleles have been described previously.
PCR amplification and sequencing of the Su(var)2-HP2 region:
For sequencing, each putative mutation was placed over a Cy-GFP balancer chromosome and crossed to either the strain used for mutagenesis or a line carrying the Df(2R)B11 deletion over the Cy-GFP chromosome. Progeny carrying the mutation that did not carry the GFP marker were collected. DNA from these individuals was prepared and used as template for PCR amplification (Shaffer et al. 2002). DNA sequencing of each PCR product was carried out using ABI BigDye version 1.1 according to the manufacturer's recommended protocols, except that cycle sequencing was carried out for 80 cycles.
Sequencing of each allele was done using a series of 11 overlapping 1.2-kb PCR products that spanned all of Su(var)2-HP2 (Shaffer et al. 2002). These PCR products covered the entire coding region of the gene as well as ∼900 bases of sequence flanking the coding regions.
Preparation and immunofluorescent staining of mitotic chromosomes:
DAPI-stained chromosome preparations from colchicine-treated larval brains were obtained as described previously and used to score the mutant phenotypes (Pimpinelli et al. 2000).
To obtain metaphase preparations for HP2 immunostaining, noncolchicine-treated brains were fixed for 8 min with 3.7% formaldehyde and 45% acetic acid and squashed in the same fixative. Slides were frozen in liquid nitrogen and, after removing the coverslip, were immersed in cold Tris-buffered saline (TBS). They were then washed in TBS-T (TBS, 0.1% Tween) twice for 5 min, blocked for 30 min with 5% nonfat dry milk in TBS-T, and incubated overnight at 4° with the HP2-specific rabbit antiserum, which recognizes both HP2-L and HP2-S (Shaffer et al. 2002), diluted 1:100 in TBS-T. Slides were then washed twice in TBS-T for 15 min and incubated for 2 hr at room temperature with a CY3-conjugated donkey anti-rabbit secondary antibody (Jackson Laboratories, West Grove, PA), diluted 1:200.
For HP1 immunostaining, noncolchicine-treated brains were fixed in methanol/acetic acid/H2O (10:2:1) and stained with the C1A9 monoclonal antibody (James and Elgin 1986), diluted 1:20 in PBS, according to Pimpinelli et al. (2000).
Immunofluorescent staining of polytene chromosomes:
For Figures 7 and 10, immunofluorescent staining of polytene chromosomes from either Su(var)205 or Su(var)2-HP2 mutant larvae was carried out as described (Stephens et al. 2004). For Figure 9 immunofluorescent staining of polytene chromosomes from either Su(var)205 or cav mutant larvae was carried out as described by Raffa et al (2005). HP2-depleted chromosomes were isolated from nonfluorescent third instar larvae from a cross in which virgin females carrying the Df(2R)B11 deletion over a Cy-GFP balancer chromosome were crossed to males carrying the Su(var)2-HP2 allele of interest over a Cy-GFP chromosome. HP1-depleted polytene chromosomes were generated in a similar manner except that various alleles of Su(var)205/Cy-GFP were crossed to each other and polytene chromosomes were prepared from heteroallelic (nonfluorescent) third instar larvae. Homozygous cav mutant larvae were isolated from a cav/TM6B stock on the basis of their non-Tubby phenotype. The anti-HP1 antibody used in these experiments was the C1A9 monoclonal antibody (James and Elgin 1986). The HP2 antibodies used were either a rabbit antiserum that is specific for the C-terminal exons common to both HP2-L and HP2-S (Shaffer et al. 2002) or a guinea pig (GP) antibody made against a 15-amino-acid peptide found in exon 6 that specifically recognizes HP2-L (Western analysis, data not shown).
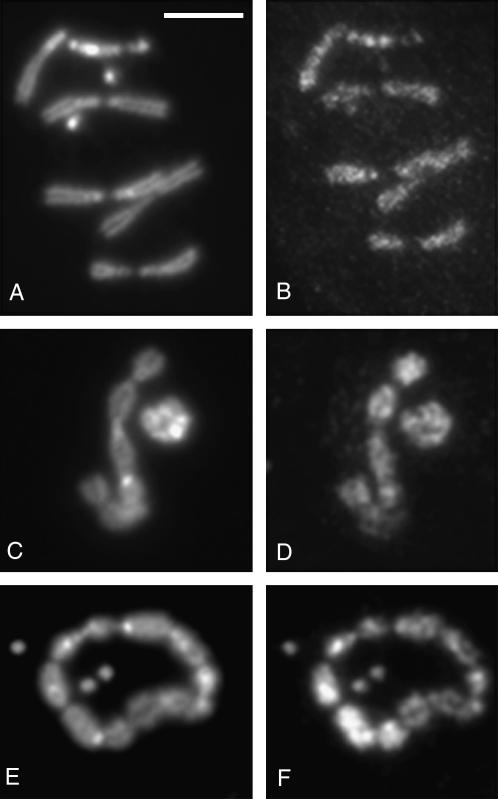
Figure 7.—
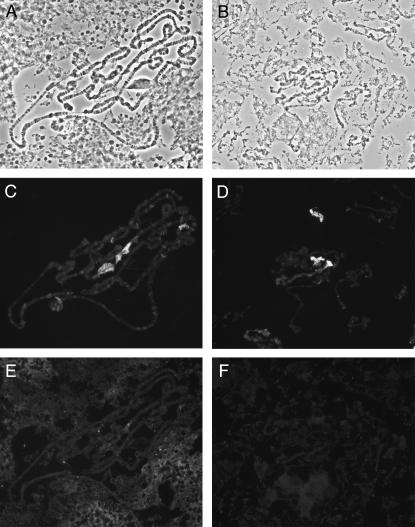
Heterochromatin association of HP1 is not dependent on HP2. Polytene chromosomes from HP2-depleted larvae [carrying HP2 mutant alleles over Df(2R)B11] are shown. (A, C, and E) Polytene chromosomes from a Su(var)2-HP2P2763L/Df(2R)B11 larva; (B, D, and F) Polytene chromosomes from a Su(var)2-HP2Q2259X/Df(2R)B11 larva. (A and B) Phase-contrast photograph of the chromosomes. (C and D) The same chromosomes stained with anti-HP1 antibodies. (E and F) The same chromosomes stained with anti-HP2 rabbit antiserum.
Figure 10.—
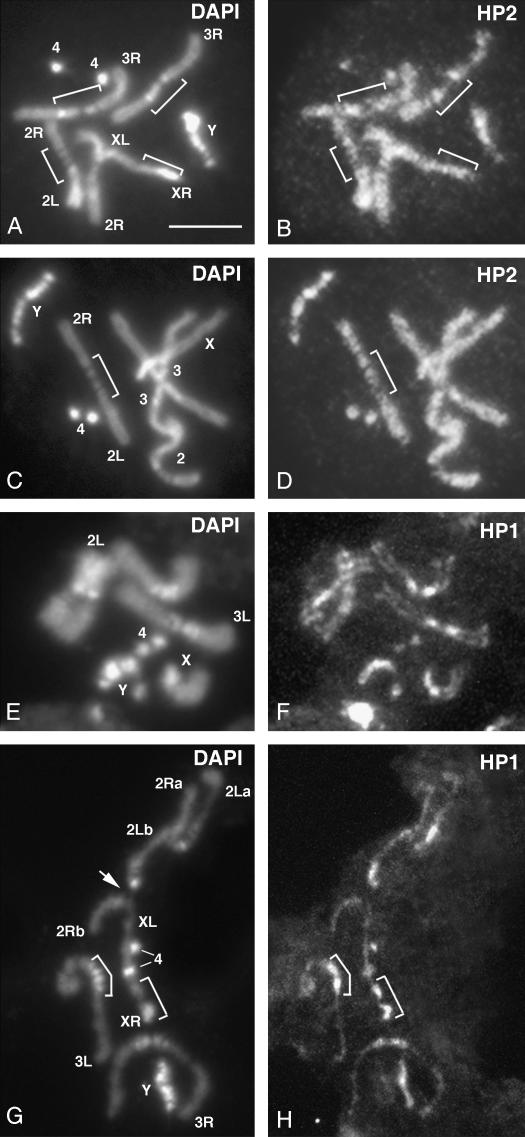
HP2-L, but not HP2-S, requires HP1 for stable binding to the chromocenter of polytene chromosomes. Polytene chromosomes from HP1-depleted larvae [carrying heteroallelic combinations of Su(var)205 alleles] are shown. (A–D) Polytene chromosomes stained with C1A9 mouse anti-HP1 monoclonal antibody. (E and F) Chromosomes stained with anti-HP2 guinea pig antiserum directed against HP2-L;. (G and H) Chromosomes stained with anti-HP2 rabbit antiserum against HP2-L and HP2-S. (A and E) Wild-type chromosomes. (B, C, F, and G) Chromosomes from a Su(var)2055/Su(var)2052 larva. (D and H) Chromosomes from a Su(var)20505/Su(var)20504 larva.
Figure 9.—
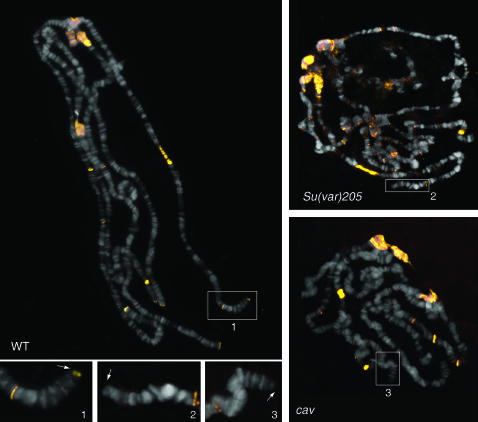
HP2 is lost from telomeres in Su(var)205 and cav mutant polytene chromosomes. Chromosome preparations were immunostained with the anti-HP2 rabbit antiserum that recognizes both isoforms of HP2. Note that HP2 (yellow signals) accumulates at the chromocenter, at several euchromatic bands, and at all telomeres of wild-type chromosomes. In polytene chromosomes from Su(var)205 and cav mutants (right top and right bottom), HP2 is normally concentrated at the chromocenter and at the euchromatic bands but fails to localize at the telomeres. The inserts outline the tips of the 2L polytene chromosome arms and the arrows point to the telomeres.
Western analysis of HP2 mutant lines:
To look for truncated HP2 protein products, nuclei were isolated from 50 females of each mutant line as described (Wallrath et al. 1998). Proteins were extracted from the isolated nuclei by incubation at 4° for 30 min to overnight in a modified nuclear extraction buffer (Nielsen et al. 1999) of 25 mm HEPES, pH 8.0, 400 mm NaCl, 1 mm EDTA, 10% glycerol, and 0.2% NP40. Proteins were size separated by gel electrophoresis and blotted, and the resulting filter was probed using a chicken antibody made against a 15-amino-acid peptide with the sequence of the first 15 N-terminal amino acids of HP2 (Stephens et al. 2005).
RESULTS
HP2 is a structural chromosomal protein:
To help define the role HP2 might be playing in chromosome structure, we immunostained mitotic chromosomes with rabbit antibodies directed against the C-terminal exons common to both HP2-L and HP2-S (Shaffer et al. 2002). Figure 1 shows the staining pattern obtained using mitotic figures from noncolchicine-treated male brains. HP2 is enriched in both euchromatic and heterochromatic regions, which display similar staining intensities. Both euchromatic and heterochromatic regions are longitudinally differentiated into subregions that differ in fluorescence intensity (Figure 1, A and B). This banded appearance is particularly evident in the entirely heterochromatic Y chromosome and in the third-chromosome pericentric heterochromatin. The Y chromosome banding pattern is similar but not identical to the DAPI-staining pattern. The central blocks of the third-chromosome heterochromatin are consistently less fluorescent than the distal blocks (Figure 1, B and D). Extensive staining is seen in the long chromosome arms. Thus, HP2 is neither limited to heterochromatic regions nor uniformly distributed along the mitotic chromosomes, but tends to accumulate in specific chromatin domains. These results suggest that this protein plays a role in chromosome organization.
Figure 1.—
Immunolocalization of HP2 and HP1 along prometaphase chromosomes of wild-type larval brains. Two examples are shown. (A, C, E, and G) DAPI-stained chromosomes. (B, D, F, and H) Chromosomes immunostained for either HP2 (B and D) using a rabbit antiserum (Shaffer et al. 2002) or HP1 (F and H) using the C1A9 monoclonal (James and Elgin 1986). Characteristic heterochromatic regions, designated by brackets, were identified by their DAPI-banding pattern using the cytological map of Drosophila heterochromatin (Gatti and Pimpinelli 1992). In addition, the Y chromosome and the fourth (“dot”) chromosome are largely heterochromatic. In F and H, one of the second chromosomes is bent, so that its 2La and 2Ra arms lie parallel; the other second chromosome displays a prominent secondary constriction (arrow) that corresponds to the histone gene cluster. See text for further explanation. Bar, 5 μm.
Our findings showing HP2 localization along mitotic chromosomes prompted us to ask whether its distribution was comparable to that of HP1. Unfortunately, these two proteins cannot be detected using the same fixation technique. The formaldehyde-based fixation technique required to observe HP2 immunostaining (see materials and methods) does not allow detection of a discernible HP1 signal on mitotic chromosomes (data not shown). Conversely, the methanol/acetic acid fixation routinely used for HP1 immunostaining (Fanti et al. 1998; Pimpinelli et al. 2000) does not result in detection of HP2 (data not shown). Immunostaining of methanol/acetic acid-fixed chromosomes shows that HP1 is specifically enriched in all heterochromatic regions (Figure 1, F and H), consistent with previous results (Fanti et al. 1998). While we cannot rule out the possibility of differential extraction of HP1, the results indicate that in metaphase chromosomes HP2 is equally enriched in euchromatin and heterochromatin, whereas HP1 concentrates only in the heterochromatic domains. This suggests a broad structural role for HP2 but a more specific role for HP1 in metaphase chromosome organization, an inference supported by genetic analysis (see below).
HP2 is an antipodal PEV modifier:
An antipodal modification of PEV in response to HP2 protein dosage, a characteristic of other known heterochromatic structural proteins, would also argue for a structural role of HP2 in chromosome organization. To test for antipodal modification of PEV, we used matching aneuploid rearrangements, Df(2R)B11, a deletion of 42.9 kb, and Dp(2R)214B, a 41.5-kb duplication, which cover a nearly identical section of the second chromosome surrounding the gene for HP2. These two lines were generated in a male recombination scheme in which transposase-induced recombination around a P element inserted just upstream of Su(var)2-HP2 led to the concomitant generation of the deletion and duplication of the proximal 42-kb section, which included the gene for HP2 (Shaffer et al. 2002). Using these two rearrangements, we examined the impact of one, two, or three copies of the Su(var)2-HP2 gene on the expression of a variegating yellow transgene inserted into the pericentric heterochromatin of chromosome 3 (P-element line B079) (Yan et al. 2002). yellow expression from this insert shows an intermediate phenotype in which it is possible to detect both suppression and enhancement of variegation. The two nonrecombinant parental lines used to generate the two aneuploid rearrangements act as controls. The results (Figure 2) show enhancement of variegation in the presence of the duplication (three doses of HP2) and suppression of variegation in the presence of the deletion (one dose of HP2). To quantify the extent of the effect, we counted the number of yellow+ (brown) spots on the abdomens of a number of individuals of each genotype. The parental lines were not significantly different (Figure 2, A and B) with averages (± standard error) of 30.1 ± 2.0 (n = 32) and 26.4 ± 2.4 (n = 26). The duplication line exhibited significantly fewer spots, 17.0 ± 1.2 (n = 39), while the deficiency line exhibited significantly more spots, 84.5 ± 6.7 (n = 11). This antipodal response to HP2 dosage is similar to that seen for HP1 and a few heterochromatin proteins; it suggests a structural role for HP2 in heterochromatin formation.
Figure 2.—
HP2 dosage affects variegated yellow expression. Dissected abdomens from four different D. melanogaster lines carrying different doses of Su(var)2-HP2 were scored for yellow expression from the variegating transgene B079. (A and B) The yellow expression level of flies carrying the two parental chromosomes used to generate the Df(2R)B11 and Dp(2R)214B chromosomes. These two lines each carry two wild-type copies of the gene for HP2. (C) The yellow expression level of a fly carrying one copy of the gene for HP2, generated using Df(2R)B11; note the increased number of pigmented patches. (D) The yellow expression level of a fly carrying three copies of the gene for HP2, generated using Dp(2R)214B; note the decreased number of pigmented patches.
Characterization of lethal alleles of Su(var)2-HP2 shows that HP2-L is essential:
A major step toward understanding the role that HP2 plays in Drosophila can be achieved by identifying mutations in the corresponding gene and examining how the activities of the larger and smaller isoforms compare, i.e., asking whether their activities are redundant or separable. We had previously described the characterization of three Su(var)2-HP2 alleles isolated from a screen designed to recover lethal mutations in Su(var)2-HP2 (Shaffer et al. 2002). To continue this analysis, we sequenced 14 additional lethal Su(var)2-HP2 alleles that had been recovered. We were able to identify 12 of the 14 mutations as double peaks in the sequencing chromatograph [Su(var)2-HP2 alleles found in lines C13, C272, C345, D143, E456, G572, H910, I668, J125, J916, J936, and L606]. However, for two lines (E814 and I308) we were unable to detect a double peak by sequencing DNA from heterozygotes. For these two lines it was necessary to prepare DNA from homozygous individuals as template for PCR amplification prior to sequencing.
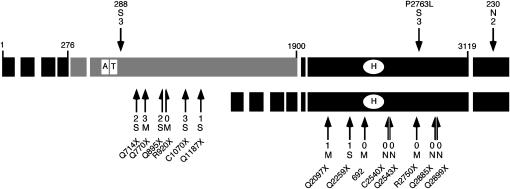
The results of sequencing these 14 alleles are shown in Table 1 and diagrammed in Figure 3. For completeness and comparison, the 3 alleles previously described (Shaffer et al. 2002) are included. The results show a preponderance of truncation alleles generated by nonsense or frameshift mutations, as expected from EMS-induced mutagenesis. The 6 truncation alleles found within exon 6 should affect only the larger isoform [Su(var)2-HP2Q714X, Su(var)2-HP2Q770X, Su(var)2-HP2Q895X, Su(var)2-HP2R920X, Su(var)2-HP2C1070X, and Su(var)2-HP2Q1187X], while the 8 truncation alleles found along the length of exon 8 should affect both the larger and the smaller isoforms. A 47-amino-acid region from exon 8 has recently been identified as a domain sufficient for HP1 binding (Stephens et al. 2005). Three alleles appear to truncate in exon 8 before this domain [Su(var)2-HP2Q2097X, Su(var)2-HP2R2295X, and Su(var)2-HP2692] and 5 after this domain [Su(var)2-HP2C2540X, Su(var)2-HP2Q2543X, Su(var)2-HP2R2750X, Su(var)2-HP2Q2885X, and Su(var)2-HP2Q2889X]. Among the collection of new alleles there is also a new missense allele located in exon 8 [Su(var)2-HP2P2763L]. All of the mutant alleles are lethal over the Df(2R)B11 deletion and in heteroallelic combinations [with the exception of Su(var)2-HP2230, which shows low levels of survival in a few heteroallelic combinations (data not shown)], arguing that the protein is essential.
TABLE 1.
Molecular characterization of Su(var)2-HP2 lethal mutations
| Allele | Line | Location | Exon | Base change | Amino acid change | Su(var) |
|---|---|---|---|---|---|---|
| 288a | 288 | 588 | 6 | C → T | Thr → Iso | 3 |
| Q714X | E456 | 714 | 6 | C → T | Gln → stop | 2 |
| Q770X | J916 | 770 | 6 | C → T | Gln → stop | 3 |
| Q895X | I308 | 895 | 6 | C → T | Gln → stop | 2 |
| R920X | L606 | 920 | 6 | C → T | Arg → stop | 0 |
| C1070X | J125 | 1070 | 6 | C → T | Cys → stop | 3 |
| Q1187X | C272 | 1187 | 6 | T → A | Gln → stop | 1 |
| Q2097X | C13 | 2097 | 8 | C → T | Gln → stop | 1 |
| Q2259X | D143 | 2259 | 8 | C → T | Gln → stop | 1 |
| 692a | 692 | 2370 | 8 | A insertion | 28 aa then stop | 0 |
| C2540X | H910 | 2540 | 8 | T → A | Cys → stop | 0 |
| Q2543X | E814 | 2543 | 8 | C → T | Gln → stop | 0 |
| R2750X | I668 | 2753 | 8 | C → T | Arg → stop | 0 |
| P2763L | G572 | 2763 | 8 | C → T | Pro → Leu | 3 |
| Q2885X | J936 | 2885 | 8 | C → T | Gln → stop | 0 |
| Q2899X | C354 | 2899 | 8 | C → T | Gln → stop | 0 |
| 230a | 230 | 3220 | 9 | A → T | Asp → Iso | 2 |
Alleles are named by indicating the altered amino acid followed by its sequence position and the resulting amino acid, where “X” indicates a stop codon. Location is based on the protein sequence accession NP_610972. See Figure 5 for Su(var) levels.
Alleles from these lines, reported here for completeness, were originally described in Shaffer et al. (2002).
Figure 3.—
Summary of the Su(var)2-HP2 alleles by type and location. Each exon is indicated by a large rectangle; the shaded exons are the HP2-L-specific exons, and the solid exons are common to both HP2-L and HP2-S (HP2-L, top line; HP2-S, bottom line). The small numbers at the ends of some of the exons of the larger isoform indicate the number of the amino acid found at the exon boundary. The open rectangles labeled “A” and “T” indicate the location of the two AT hooks found within HP2-L. The open oval labeled “H” indicates the position of the HP1-binding domain (Stephens et al. 2005). The location of each mutation described is indicated with a solid arrow. Arrows above the larger isoform and pointing downward indicate the position of the three missense alleles. The arrows and half-arrows below the isoforms and pointing upward indicate the locations of the truncation alleles. The number at the end of the arrow indicates the level of suppression of variegation, from near complete (3) to no (0) loss of silencing (see Figure 5). The letter above or below the Su(var) number indicates the extent of mitotic abnormalities: S, very frequent abnormalities; M, medium level of abnormalities; N, no abnormalities (see Table 2). The amino acid change for allele 288 is T588I, the amino acid change for 230 is N3220I, and allele 692 is a single-base insertion of an A, which produces a frameshift at amino acid 2370. The amino acid change for all other alleles is indicated by the allele name.
Mitotic defects associated with Su(var)2-HP2 mutations:
Heteroallelic combinations of Su(var)205 (HP1) alleles show high rates of telomere fusions in mitotic figures (Fanti et al. 1998). To determine whether depletion of HP2 has a similar effect and to assess whether the roles of the two isoforms might be separable, we examined metaphase spreads. Each of the 17 alleles was crossed to a line carrying the Df(2R)B11 deletion; larvae containing both the mutant allele and the deficiency were collected and checked for mitotic phenotype by examining metaphase spreads from brain. Table 2 lists the type and number of observed abnormal mitotic figures. The main abnormality observed in HP2 mutants is an irregular condensation of both euchromatic and heterochromatic regions of the chromosomes (Figure 4). We also observed the frequent appearance of chromosome breaks; these could be either real discontinuities of chromosomal DNA or extreme cases of failure to condense. Other common phenotypes were hypercondensed chromosomes, precocious sister-chromatid separation (PSCS), and the presence of hyperploid/polyploid cells. No telomere fusions were detected in mitotic figures from any of the Su(var)2-HP2 alleles.
TABLE 2.
Mitotic phenotypes associated with Su(var)2-HP2
| Genotype | Total no. of cells scoreda | Abnormal chromosome condensationb | Hypercondensed chromosomesb | Hyperploid/polyploidb | Chromosome breaksb | PSCSb |
|---|---|---|---|---|---|---|
| 288/B11 | 165 | 35.2 | 3.0 | 0 | 3.6 | 10.3 |
| Q714X/B11 | 142 | 18.3 | 11.3 | 7.0 | 5.0 | 17.0 |
| Q770X/B11 | 102 | 10.8 | 7.8 | 11.7 | 4.0 | 8.8 |
| Q895X/B11 | 122 | 39.3 | 6.6 | 0 | 7.3 | 8.1 |
| R920X/B11 | 135 | 13.3 | 3.0 | 1.5 | 5.2 | 3.7 |
| C1070X/B11 | 102 | 28.5 | 14.7 | 5.9 | 5.9 | 7.8 |
| Q1187X/B11 | 111 | 24.3 | 18.9 | 0 | 8.1 | 20.7 |
| Q2097X/B11 | 108 | 7.4 | 12.0 | 8.4 | 7.4 | 3.7 |
| Q2259X/B11 | 129 | 42.6 | 3.1 | 6.2 | 5.4 | 27.9 |
| 692/B11 | 140 | 11.4 | 1.4 | 0.7 | 3.6 | 5.7 |
| C2540X/B11 | 130 | 0.7 | 0 | 0 | 0.7 | 1.4 |
| Q2543X/B11 | 185 | 0.5 | 0 | 1.0 | 0.5 | 1.0 |
| R2750X/B11 | 125 | 16.8 | 13.6 | 1.6 | 8.8 | 12.8 |
| P2763L/B11 | 166 | 24.7 | 0 | 1.2 | 7.8 | 2.4 |
| Q2885X/B11 | 128 | 0 | 0.8 | 0 | 0 | 0 |
| Q2899X/B11 | 230 | 0.9 | 0 | 0 | 0.5 | 0 |
| 230/B11 | 247 | 2.0 | 0 | 1.6 | 0 | 6.0 |
| B11/ST | 157 | 1.3 | 0 | 0 | 0.6 | 0.6 |
| Oregon R | 435 | 0 | 0 | 0 | 0 | 0 |
A minimum of five brains were scored for each genotype.
Each class includes all the cells that exhibit the indicated phenotype. For example, a metaphase figure showing abnormal chromosome condensation, chromosome breakage, and precocious sister-chromatid segregation was included in each of the three corresponding classes.
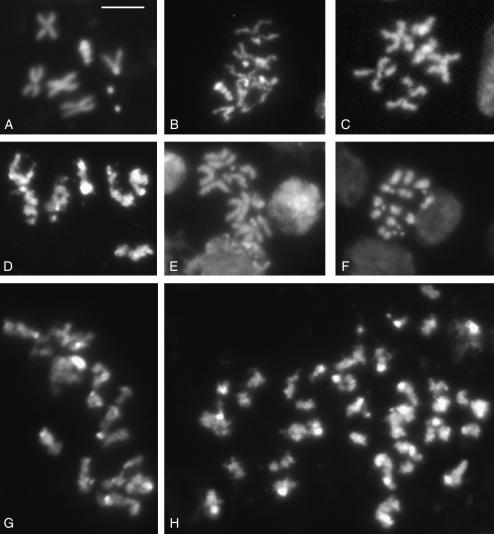
Figure 4.—
Chromosome abnormalities in Su(var)2 -HP2 mutants. (A) DAPI-stained wild-type male metaphase chromosomes. (B–H) Examples of chromosome abnormalities observed in Su(var)2-HP2 mutants. (B–D) Metaphase chromosomes from Su(var)2-HP2Q2259X/Df(2R)B11 (B and C) and Su(var)2-HP2Q895X/Df(2R)B11 (D) mutant brains displaying poorly condensed chromosomes. (E) Metaphase chromosomes from Su(var)2-HP2Q2259X/Df(2R)B11 mutants displaying PSCS. (F) Su(var)2-HP2Q1187X/Df(2R)B11 mutant displaying strongly condensed chromosomes and PSCS. (G and H) Polyploid mutant metaphases from Su(var)2-HP2Q770X/Df(2R)B11 (G) and Su(var)2-HP2Q2097X/Df(2R)B11 (H) mutant brains with poorly condensed chromosomes displaying PSCS. Bar, 5 μm.
The mutations showed a range in expressivity. The results of this analysis, shown in Figure 4 and Table 2, suggest three phenotypic categories, used as descriptors in Figure 3. In Figure 3, the first category (N) includes mutants with a mitotic phenotype that is virtually indistinguishable from that of either wild-type or Su(var)2-HP2+/Df(2R)B11 larval brains [Su(var)2-HP2C2540X, Su(var)2-HP2Q2543X, Su(var)2-HP2Q2885X, Su(var)2-HP2Q2899X, and Su(var)2-HP2230]. All of these are truncations or missense alleles located distal to the HP1-binding domain (see Figure 3). The second category (M) is comprised of mutants with relatively mild mitotic defects [Su(var)2-HP2Q770X, Su(var)2-HP2R920X, Su(var)2-HP2Q2097X, Su(var)2-HP2692, and Su(var)2-HP2R2750X], and the third category (S) includes mutants with a strong mitotic phenotype [Su(var)2-HP2288, Su(var)2-HP2Q714X, Su(var)2-HP2Q895X, Su(var)2-HP2C1070X, Su(var)2-HP2Q1187X, Su(var)2-HP2Q2259X, and Su(var)2-HP2P2763L]. The mutants in the M and S categories do not differ in the type of mitotic defects, only in the frequency. Since all alleles were tested over the Df(2R)B11 deletion chromosome, it is reasonable to assume that any differences in the outcome cannot be attributed to the Df(2R)B11 deletion chromosome but map to the chromosomes carrying the newly generated Su(var)2-HP2 alleles. The results point to the importance of the region including the distal three-quarters of the sixth exon and the seventh and eighth exons up through the HP1-binding domain. All of the alleles located in exon 6 as well as the three alleles located N-terminal to the HP1-binding domain showed abnormalities significantly above background levels. In contrast, four of the five truncations and one of the two missense alleles located C-terminal to the HP1-binding domain show levels of abnormalities insignificantly different from wild-type controls.
Loss of HP2-L results in suppression of variegation:
To further dissect the functional differences between the smaller and larger isoforms, we tested each of the sequenced alleles for suppression of PEV. Three HP2 alleles were previously tested for their effect on PEV: Su(var)2-HP2288, Su(var)2-HP2692, and Su(var)2-HP2230. These lines differed in their ability to suppress white variegation. Of these three alleles, Su(var)2-HP2692, a truncation mutation that affects both the smaller and larger isoforms, showed no Su(var) activity, while the two missense alleles (one in exon 6 and one in exon 9) did suppress PEV (Shaffer et al. 2002). Here we asked whether the newly recovered truncations behave similarly, in particular, how exon 6 truncations compare with the exon 8 truncations in their ability to suppress PEV.
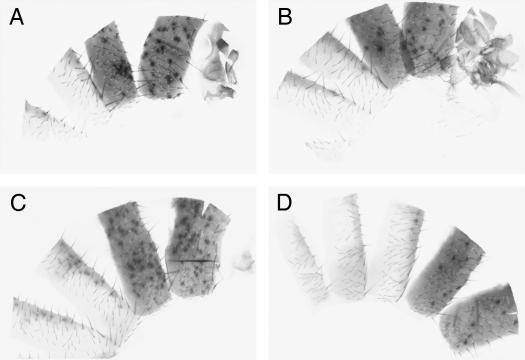
We found significant differences in the average amount of suppression seen with different alleles. To describe the average level of suppression seen from any given allele, we utilized a scoring system from 0 to 3. Figure 5 shows examples of each of the four classes, demonstrating the range of eye pigmentation seen. Five alleles showed no detectable suppression of PEV (level 0; Figure 5A) and usually showed only a few scattered pigmented ommatidia. Four alleles showed only weak suppression (level 1; Figure 5B), in which the eye was still predominantly white but did show larger patches of pigmented cells. Three alleles displayed medium suppression (level 2; Figure 5C) in which the eye was more red than white but still showed some patches without pigment. Finally, five alleles showed strong suppression (level 3; Figure 5D) in which the eye was almost completely red with very small areas of white or lightly pigmented cells, usually found along the posterior edge of the eye.
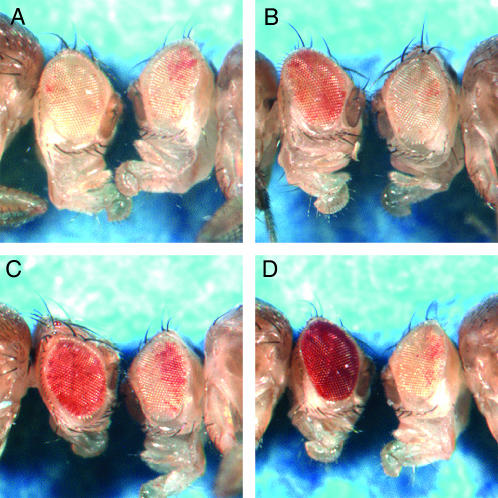
Figure 5.—
Eye phenotypes of Su(var)2-HP2 alleles show a range in suppression of PEV. Males carrying a wm4h X chromosome are shown. In each picture, the left fly carries a Su(var)2-HP2 mutation, whereas the right fly is a sibling carrying a wild-type copy of Su(var)2-HP2. Alleles were separated into four classes as shown: (A) class 0, no suppression, example Su(var)2-HP2Q2540X; (B) class 1, weak suppression, example Su(var)2-HP2Q1187X; (C) class 2, medium suppression, example Su(var)2-HP2Q895X; and (D) class 3, strong suppression, example Su(var)2-HP2Q770X. Quantitative assessment of these example lines showed an effect that ranged from no apparent suppression (OD480 indistinguishable from wm4h homozygotes at 7–9% of wild-type pigment) (classes 0 and 1) to ∼17 and 31% of wild-type pigment (classes 2 and 3) with ∼2% standard error for triplicate samples.
Within each class N, M, and S (indicating mitotic phenotype), the various alleles tended to all show the same class of Su(var) phenotype (see Figure 3). All of the missense alleles showed strong or medium levels of suppression, suggesting that they identify domains important for heterochromatic gene silencing. As a group, truncations in exon 6 show much higher levels of suppression than do truncations in exon 8. Given the dominant phenotype of the Su(var) activity, we cannot formally exclude the possibility that this activity maps outside of the HP2 gene. However, the grouping of the phenotypes of the truncation alleles [strong Su(var) activity for almost all exon 6 truncations and no Su(var) activity for the five truncation alleles downstream of the HP1-binding domain] makes it extremely unlikely that the suppression activity detected is due to second-site mutations found elsewhere on the same chromosome with the HP2 alleles. Collectively, these results indicate the importance of the larger isoform in gene silencing.
While there is a broad correlation, the dominant Su(var) activity and the recessive mitotic abnormalities do not correlate exactly (see Figure 3). Three lines, Su(var)2-HP2692, Su(var)2-HP2R920X, and Su(var)2-HP2R2750X, show no detectable suppression of variegation but do show mild mitotic defects. Conversely, missense mutation Su(var)2-HP2230 shows no mitotic defects, but does show suppression of variegation, indicating that the mitotic function and the silencing activity of HP2 are separable.
Truncation mutations in Su(var)2-HP2 do not produce stable protein:
The results above reveal that truncations in HP2 can have significant dominant effects on variegation and indicate the importance of the larger isoform. Could the lack of a dominant Su(var) phenotype in the mutations with truncations distal to the HP1-binding site be explained by the presence of stable truncated products? Could partial HP2 proteins, truncated in exon 6, have a deleterious impact that causes the variegating phenotype? Or could the balance between HP2-S and HP2-L products be critical? Western analysis of nuclear proteins derived from heterozygous females suggests that most of the truncation mutations do not produce readily detectable stable protein (Figure 6). For this analysis, we used a chicken antibody prepared using a synthetic peptide identical in sequence to the first 15 amino acids of HP2. This epitope is part of the first 276 amino acids common to both the larger and smaller isoform. In only a single case [Su(var)2-HP2C2540X] are we able to detect even small amounts of product at the expected molecular weight. In this case we estimate the total amount of product to be <1% of the total HP2 signal. This suggests that it is unlikely that the dominant Su(var) phenotype is due to a “poison” protein created from the truncation allele. One possible explanation is that an imbalance between amounts of HP2-L andHP2-S, created by truncations in exon 6, has a stronger Su(var) effect than a balanced depletion of both isoforms, presumed to occur as a consequence of truncations in exon 8.
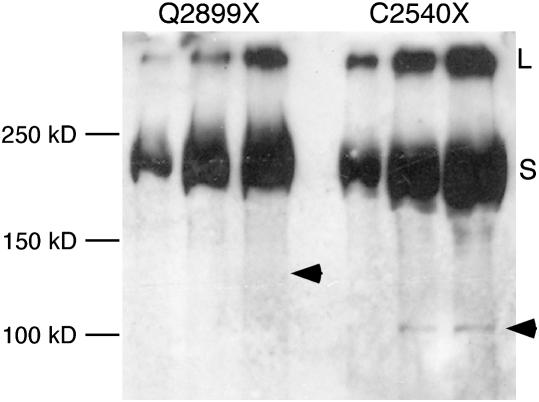
Figure 6.—
Stable truncated products from the HP2 mutations are generally not observed in a Western blot. Proteins extracted from isolated nuclei from females heterozygous for the indicated Su(var)2-HP2 allele (over a wild-type allele) were separated by SDS gel electrophoresis, blotted, and stained for HP2 using the HP2 chicken antiserum (which recognizes the first 15 amino acids of HP2, common to both HP2-L and HP2-S). Three lanes were loaded with increasing amounts of the protein extract in each case. The arrowhead indicates the predicted position of the truncated HP2-S protein. The numbers on the left indicate the position of several molecular weight markers. The letters on the right indicate the position of the full-length HP2-L (L) and HP2-S (S) protein products.
Interdependence of HP1 and HP2 in chromosomal binding:
We had previously shown that recruitment of HP1 protein to ectopic sites in polytene chromosomes results in recruitment of HP2 to these same ectopic sites (Shaffer et al. 2002). This suggests that HP2 might act downstream of HP1. Here we investigate this relationship with respect to the two HP2 isoforms, using flies depleted for HP1, and examine the behavior of HP1 in the presence of the various HP2 mutant alleles. Such experiments should not only help to differentiate the two isoforms, but also might answer important questions regarding HP1 and HP2 binding. Thus, we immunostained polytene and mitotic chromosomes isolated from flies bearing mutations in either Su(var)2-HP2 or Su(var)205 and examined the distributions of HP1 and HP2.
Depletion of HP2 is accomplished by generating larvae that carry one of the Su(var)2-HP2 lethal alleles over the Df(2R)B11 deficiency. We generated such larvae using all three classes of mutations, including five of the lines with truncations in exon 6, four of the lines with truncations in exon 8, and one line with a missense allele. All five alleles with truncations in exon 6 [Su(var)2-HP2Q714X, Su(var)2-HP2Q770X, Su(var)2-HP2Q895X, Su(var)2-HP2C1070X, and Su(var)2-HP2Q1187X; see Figure 3] gave rise to larvae with poorly developed polytene chromosomes that were not suitable for cytological analysis. Cytologically tractable polytene chromosomes were not found even in larvae bearing the Su(var)2-HP2Q2097X mutation, which is the first (most proximal) truncation allele in exon 8. Three alleles with truncations in exon 8 [Su(var)2-HP2Q2259X, Su(var)2-HP2Q2543X, and Su(var)2-HP2C2540X], as well as the exon 8 missense allele Su(var)2-HP2P2750L, did result in larvae that could be collected and used for polytene chromosome preparation and staining. Figure 7 shows the staining pattern obtained using antibodies against HP1 and both isoforms of HP2 in Su(var)2-HP2Q2259X/Df(2R)B11 and Su(var)2-HP2P2750L/Df(2R)B11 larvae. Western analysis of wild-type early stage embryos shows the presence of both the larger and the smaller isoform of HP2 protein well before the start of zygotic transcription (not shown). It is therefore quite likely that there is a maternal supply of HP2 protein provided to the double-mutant embryo as well. This would likely allow for establishment of heterochromatin and survival through embryogenesis. However, as seen in Figure 7, E and F, by the third instar stage little to no HP2 of either isoform is detected by immunostaining of polytene chromosomes. This suggests that little if any maternally loaded protein, or zygotically produced HP2, remains in these surviving larvae. Nonetheless, immunofluorescent staining of these polytene chromosomes with antibodies specific for HP1 showed that in all four cases where HP2 has been mutated, HP1 appears to have a normal level and distribution (Figure 7, C and D). This indicates that HP1 is not dependent on the continuing presence of HP2 to remain stably bound to polytene chromosomes.
Examination of the structure of chromosomes from these same four HP2-depleted lines did illustrate the need for HP2 protein. Polytene chromosomes derived from the Su(var)2-HP2Q2259X/Df(2R)B1 larvae (having an allele with a stop codon early in exon 8, prior to the HP1-binding domain, over the deletion) were clearly abnormal, small, and underreplicated (Figure 7B). In repeated trials carried out in parallel with lines showing normal chromosome morphology, these chromosomes were difficult to spread without breakage. In contrast, the two lines tested with an allele causing truncation after the HP1-binding domain, as well as the exon 8 missense allele, did not show any significant dismorphology of polytene chromosomes (Figure 7A). We note that the missense allele Su(var)2-HP2P2750L shows both a strong dominant Su(var) phenotype and a strong recessive mitotic phenotype but does not show the high levels of chromosomal dismorphology seen with the weaker Su(var)2-HP2Q2259X allele. This suggests that the region(s) of the HP2 protein important for polytene morphology lies distal to the Su(var)2-HP2Q2259X truncation yet outside of the region around the Su(var)2-HP2P2750L allele.
We also investigated what happens to HP2 upon depletion of HP1. We examined both mitotic and polytene chromosomes depleted for HP1 and stained either with the rabbit antiserum that detects both isoforms of HP2 or (in the case of polytene chromosomes) with a GP antiserum specific for an epitope in exon 6, exclusively present in the larger isoform (HP2-L). Figure 8 shows the HP2-staining pattern for mitotic chromosomes depleted for HP1. As previously reported, mitotic chromosomes depleted for HP1 show telomere fusions, often generating rings or chains of chromosomes in mitotic spreads. We do not see any diminishment of HP2 signal in these chromosomes when stained with rabbit antibodies that detect both isoforms of HP2. This indicates that in mitotic chromosomes, the presence of HP2 is not dependent on HP1 for chromosomal binding. Mitotic chromosomes isolated from wild-type larvae do not stain with the GP antibody, presumably due to the masking of the small 15-amino-acid epitope in these highly compacted structures. The lack of the HP2 mutant phenotype (shown in Figure 4), which is readily scored for alleles that should impact only HP2-L, argues that HP2-L as well as HP2-S is present in these chromosomes.
Figure 8.—
HP2 normally localizes on metaphase chromosomes from Su(var)205 mutant larvae. Chromosome preparations were immunostained with the anti-HP2 rabbit antiserum that recognizes both isoforms of HP2. Metaphase chromosomes from Oregon-R (A and B) and Su(var)20505/Su(var)20504 (C–F) larval neuroblasts. (A, C, and E) DAPI staining. (B, D, and F) HP2 localization. Bar, 5 μm.
In polytene chromosomes, however, we are able to detect the HP2-L isoform using the GP antibody, as well as both isoforms using the rabbit antibody. In wild-type chromosomes, the rabbit antibody (directed against the common C-terminal portion of HP2-L and HP2-S) detects signal at telomeres, centric heterochromatin, and a scattering of euchromatic bands (Figure 9). Upon depletion of HP1, we see a loss of detectable signal at telomeres, but not at the chromocenter. Interestingly, we see this same loss of HP2 signal specifically at the telomeres of polytene chromosomes derived from cav mutant larvae (Figure 9); the telomeres of cav polytene chromosomes fail to accumulate both HOAP (the protein encoded by the cav gene) and HP1 (Cenci et al. 2003; G. Cenci and M. Gatti, unpublished results). These results indicate that HP2 localization at chromosome ends is mediated by HP1 and emphasize the special role of HP1 in telomere structure.
While staining with the rabbit antibody indicates a continuous presence of HP2 at the chromocenter, GP antibody, specific to HP2-L, does not show a signal on polytene chromosomes depleted for HP1 (Figure 10). We can clearly detect both HP1 (Figure 10A) and the larger isoform of HP2 (Figure 10E) at the chromocenter of wild-type larvae using the C1A9 monoclonal antibody against HP1 (Figure 10A) and the GP antibody against HP2 (Figure 10E). However, when these same antibodies are used to stain chromosomes derived from a larva depleted for HP1, we find that not only the signal for HP1, but also the signal for HP2-L, is depleted (Figure 10, B and F). As in Figure 9, we do not see a depletion of the HP2 signal in HP1-depleted flies if the rabbit antibody is used (Figure 10, C, D, G, and H). These results indicate the presence of HP2-S but not HP2-L at the chromocenter of HP1-depleted polytene chromosomes. The finding that we can detect the larger isoform in wild-type polytene chromosomes but not in the HP1-depleted chromosomes suggests that the larger isoform is being lost from the polytene chromosomes when HP1 is not present. Note that we cannot rigorously exclude the possibility that the small epitope recognized by the GP antibody is somehow masked in the absence of HP1. Nonetheless, it appears that in the absence of HP1, both isoforms of HP2 are lost from the telomeres but only the larger isoform signal is lost from the centric heterochromatin of polytene chromosomes.
DISCUSSION
HP2 is a structural protein of heterochromatin:
We report here a genetic analysis of HP2, using aneuploid analysis of HP2 dosage as well as a phenotypic analysis of 17 mutant alleles. The aneuploid analysis demonstrates the existence of a dosage-dependent modifier of variegation found within the small 42.9-kb region of 52C. Given that these aneuploid mutations were created by exposure of a P-element insertion to transposase, it is unlikely that the phenotypes observed map outside the small 42-kb region encompassing the gene for HP2. While this region contains several genes, given the interaction between HP1 and HP2 and given that most HP2 lethal alleles also act as dominant Su(var)s, it is reasonable to conclude that the antipodal dosage-dependent Su(var)/E(var) activity found in the region is due to Su(var)2-HP2.
The dosage response seen for HP2 is the same as that for HP1: mutations that reduce the amount of active protein suppress variegation, while mutations that increase the amount of active protein enhance variegation. Similar results have been obtained for a few other heterochromatic proteins that act as suppressors of variegation [e.g., SU(VAR)3-7, a zinc-finger protein (Reuter et al. 1987), and SU(VAR)3-9, a histone H3-K9 methyltransferase (Schotta et al. 2002)]. It has been argued that proteins that display this type of antipodal dosage dependence play a structural role in heterochromatin formation and function (Locke et al. 1988; Schotta et al. 2003). All three of these proteins interact with HP1 and may be involved in a multiprotein complex. From these observations, we infer that HP2 works in concert with other heterochromatin-associated proteins to modulate heterochromatin structure and PEV. In addition, HP2 protein also shows staining along the entire condensed mitotic chromosome. Thus, HP2 is also likely to play a structural role in the maintenance of proper chromosome structure in both the heterochromatic and the euchromatic regions of mitotic chromosomes. It remains possible that HP2 also has some kind of enzymatic activity. As described above for SU(VAR)3-9, chromosomal proteins can have both structural roles and enzymatic activities.
HP2-L is required for gene silencing and heterochromatin spreading and plays a critical role in mitosis:
Seventeen lethal mutations have been described in Su(var)2-HP2. Fourteen mutations are truncation alleles; the remaining 3 are missense alleles. The results reported here demonstrate that truncations that affect only the large isoform, as well as truncations that affect both isoforms of HP2, are homozygous lethal, indicating that the larger isoform is essential for viability. Given that we do not have a mutation that affects the HP2-S exclusively, we cannot investigate the importance of this isoform per se. In addition to the 14 truncation alleles, three amino acid substitution mutations have now been described. These alleles indicate regions of the protein where the primary amino acid sequence appears to be important for activity. Analysis of these regions with programs designed to predict globular domains or regions of ordered structure (Linding et al. 2003a,b) give ambiguous results. This makes it difficult to determine how these mutations disrupt HP2 function, but given the evidence that HP2 is a structural protein (see above), it is likely that these mutations somehow affect protein–protein interactions. Exactly how these mutations disrupt contacts with other proteins cannot be determined as yet; these regions could be directly involved in creating a binding surface or indirectly involved by ensuring folding of the region to create a proper binding domain. The recovery of a missense mutation in exon 6 suggests that the large isoform makes unique contacts.
HP2 alleles, particularly those that are predicted to deplete only the large isoform, result in a suppression of PEV, indicating a defect in the formation and spreading of heterochromatin. The ability of HP1 to interact with histone H3 methylated at lysine 9 through its chromodomain and with the histone H3 K9 methyltransferase [SU(VAR)3-9] through its chromoshadow domain has suggested a model for the spreading and inheritance of this epigenetic structure (Schotta et al. 2002). However, the phenotypes of the HP2 mutant alleles clearly demonstrate that the simple mechanism described is not sufficient; HP2, as well as other proteins, must play a role in generating a stable heterochromatin assembly that is able to spread. Indeed, > 50 suppressors of PEV have been identified in genetic screens (Sinclair et al. 1983; Locke et al. 1988). While many of these appear to be genes that encode the enzymes responsible for shifting the histone modification state from a euchromatic to heterochromatic form [e.g., rpd3, which encodes HDAC1 (Mottus et al. 2000)], others may encode additional structural proteins.
HP2 alleles, when placed over a deletion, show a variety of severe mitotic defects. The data suggest that these mitotic phenotypes reflect a primary problem in chromosome condensation that affects the organization and function of the centromeres. Hypercondensed chromosomes are indeed an expected outcome of a metaphase arrest generated by centromere dysfunction. The PSCS phenomenon can also be attributed to centromere dysfunction preventing proper binding of components of the spindle checkpoint machinery; PSCS has been observed in mutants in spindle checkpoint-related genes such as zw10, zwilch, rod, and bubR1 (Williams et al. 1992, 2003; Basu et al. 1999; Scaerou et al. 1999). Finally, hyperploid and polyploid cells can be the consequence of a complete or partial block in chromosome segregation; this has been observed often in D. melanogaster mutants displaying irregularly condensed mitotic chromosomes (Gatti and Baker 1989). Lagging and improperly condensed chromosomes have also been observed in Schizosaccharomyces pombe as a consequence of the failure to form heterochromatin; in this instance, it has been shown that HP1 is necessary to form a platform for cohesin binding (Nonaka et al. 2002). Remarkably, in none of the HP2 mutants examined do we observe telomeric fusions. This finding is rather surprising, given that the only mitotic phenotype observed in Su(var)205 mutants (defective or diminished HP1) is telomere–telomere attachments. Thus, while HP1 and HP2 form a complex and largely colocalize in polytene chromosomes, they appear to perform different functions in mitotic chromosome maintenance. While HP1 is required for telomere capping, HP2 is essential for proper mitotic chromosome condensation and centromere function.
Interestingly, HP2 function in heterochromatic gene silencing and its function in mitotic chromosomes can be genetically separated in some instances. The exon 9 missense allele Su(var)2-HP2N3220I is the clearest case. Here, an amino acid substitution allele shows a dominant ability to suppress PEV yet does not disrupt mitosis, even when placed over a deletion of the HP2 gene. Also noteworthy are the three alleles with a truncation between the beginning of exon 8 and the HP1-binding domain. As a group, these alleles show weak or no Su(var) activity, yet they show medium-to-high levels of mitotic defects.
These results suggest that HP2-L is important not only for the strong dominant suppressor phenotype but also for the higher rates of recessive mitotic defects. Indeed, most truncations beyond the HP1-binding domain, all of which would affect both the larger and the smaller isoform, tend to have much weaker phenotypes with respect to both PEV and mitotic function, suggesting that reduced but balanced amounts of larger and smaller isoforms may be less deleterious than an imbalance of isoforms. Interestingly, the HP1-binding domain appears to play a role in mitosis, as the three truncations that terminate prior to this domain show higher levels of mitotic defects than do truncations found distal to the HP1-binding domain (see below).
Missense alleles may indicate additional domains of protein–protein interaction:
In addition to the two previously described missense alleles (Shaffer et al. 2002), we identified a third amino acid substitution mutation. These three mutations are not clustered; the Su(var)2-HP2288 allele, a threonine-to-isoleucine substitution, is located at amino acid 588 in the sixth exon; the Su(var)2-HP2P2763L allele, a proline-to-leucine substitution, is located at amino acid 2763 in the eighth exon; and the Su(var)2-HP2230 allele, an asparagine-to-isoleucine substitution, is located very near the C terminus in exon 9 at amino acid 3220 (see Figure 3). Analysis of predicted HP2 protein sequences derived from genomic sequences of HP2 from D. melanogaster, D. pseudoobscura, D. willistoni, and D. virilis show a number of conserved domains that range in size from 11 to 79 amino acids in length (Stephens et al. 2005). The Su(var)2-HP2288 allele maps to a region that shows little to no conservation among the four species. While it changes a threonine to an isoleucine, this threonine does not appear to be within a phosphorylation site (Blom et al. 1999). Intriguingly, the missense allele Su(var)2-HP2P2763L is found within a domain conserved among all four species. This 79-amino-acid conserved domain covers amino acids 2710–2789. Similarly, Su(var)2-HP2230, with an isoleucine substituted for an aspartic acid, is found within a small 11-amino-acid domain, which is seen in D. melanogaster, D. pseudoobscura, and D. virilis but not in D. willistoni. In these three species this small domain is found within 40 amino acids of the C-terminal end of the protein.
It is quite possible that the latter two alleles do indeed disrupt protein–protein interactions. It is notable that while most of the alleles with truncations in exon 8 show weak or no Su(var) activity, these two missense alleles show medium-to-strong Su(var) activity, suggesting the importance of these two conserved domains. Also of note is the lack of HP2 protein on polytene chromosomes isolated from individuals with the missense allele Su(var)2-HP2P2763L placed over Df(2R)B11 (Figure 7E). While it is possible that this proline-to-leucine substitution somehow marks HP2 for degradation, it is more likely that some critical binding domain has been disrupted or that folding has been perturbed, such that the mutated versions of HP2-L and HP2-S can no longer be recruited to or bind to polytene chromosomes.
HP1 is required for HP2 binding at telomeres and required for HP2-L persistence at the centromere of polytene chromosomes:
Protein depletion studies in which the presences of HP1 and HP2 on chromosomes are each tested in the absence of the other show an unexpected level of complexity. All of these experiments are genetic depletion assays in which the only source of the depleted protein is maternal protein provided in the egg. At the point of collecting larvae for staining, the level of the depleted protein has fallen very close to, if not below, our ability to detect it by immunostaining (e.g., Figure 7, E and F; Figure 10, B–D). In the case of depletion of HP2, we do not find a concomitant loss in HP1 signal even when chromosome structure has been impaired (see Figure 7, B and D). This is perhaps not surprising, given the large number of heterochromatic proteins with which HP1 interacts, including H3 modified by K9 methylation.
The binding of HP2 in the absence of HP1 shows a different result. In this case we find domains where HP2 is still detectable and others where it is not. In mitotic and polytene chromosomes, we do not detect a complete loss of both isoforms of HP2 in the absence of HP1; the general association observed continues. However, in polytene chromosomes, we observe a complete loss of HP2 at telomeres and a loss of HP2-L-specific signal from centromeric heterochromatin. The most straightforward interpretation of the latter result would suggest that HP2-L does not remain in polytene heterochromatin the absence of HP1. However, we cannot rule out the possibility that in the absence of HP1, the HP2-L-containing complex has changed in some way that masks the small sixth-exon epitope recognized by the GP antibody.
Nonetheless, the difference observed between the two isoforms in the absence of HP1 suggests a difference in the roles of the larger and smaller isoforms. A plausible explanation for the difference is that there is a domain in the fifth or sixth exon that either destabilizes binding in the polytene chromosomes or increases the likelihood of degradation when HP1 is depleted. For example, a region in exon 6 might interact with other cellular or chromosomal proteins lost from the chromocenter in the absence of HP1. Alternatively, in the absence of HP1, HP2 binding to the heterochromatin may be destabilized to such an extent that HP2-L is depleted by binding to other proteins not found in polytene chromosomes. Given that polytene chromosomes do not go through mitosis, it is tempting to speculate that these “other” proteins might be the proteins that bind HP2 in mitotic chromosomes. Certainly, HP2-L plays an important role in mitotic chromosomes, as shown by the impact of mutations that lead to a stop codon in exon 6, exclusively part of the large isoform. It is also possible that HP2-S has specific partners within the heterochromatin that stabilize its binding even in the absence of HP1; however, given that there are no amino acids in HP2-S that are not found within HP2-L, this seem less likely.
What is the relationship of the larger and smaller isoforms of HP2?
Examination of the truncation alleles by Western analysis suggests that very little if any stable protein product is produced from these alleles. This observation could be explained by the inherent instability of the truncated protein. Alternatively, the absence of the truncation products could be due to the degradation of the mRNA with the nonsense mutation, given the identification of nonsense-mediated decay activity in D. melanogaster (Gatfield and Izaurralde 2004). All exon 6 truncations would thus be expected to act similarly, as they all are predicted to deplete HP2-L yet leave HP2-S intact. Likewise, exon 8 truncations as a group would also be expected to be similar, as they should deplete both the smaller and the larger isoforms. However, some variation in phenotypes is observed. It seems likely that the difference in loss of Su(var) activity in the exon 6 truncation Su(var)2-HP2R920X is due to a second-site mutation not associated with Su(var)2-HP2. However, it is possible that either very small amounts of truncated HP2 protein could cause the shift in phenotypes observed within the exon 8 mutations or that significant amounts of truncated HP2 are produced from these alleles in developmental stages other than that tested.
Clearly the two groups of truncation alleles differ in their Su(var) phenotypes. In general, the truncations in the sixth exon have a more severe phenotype than the downstream truncations, even though exon 6 alleles would be expected to produce normal amounts of wild-type HP2-S isoform. Testing for a dominant Su(var) occurs in the presence of a wild-type chromosome that should produce one dose each of HP2-S and HP2-L. The strong phenotypes associated with exon 6 truncation alleles could be due to the creation of an imbalance between the two isoforms (two doses of HP2-S to one dose of HP2-L). In contrast, each allele was placed over a deletion to examine the mitotic phenotype. In this case, exon 6 nonsense mutations would be expected to produce no HP2-L yet still produce HP2-S, while exon 8 nonsense mutations would be expected to make no wild-type HP2 at all. Yet, here again, the exon 6 truncations as a group give a more severe phenotype. This suggests that the presence of HP2-S in the absence of HP2-L (either depletion or complete loss) strongly interferes with mechanisms for chromatin packaging in both interphase and metaphase.
The presence of a missense mutation in exon 6 suggests the presence of a domain that functions to bind to another chromosomal protein. A high-affinity site for a protein involved in mitosis, which does not occur in polytene cells, might explain the selective loss of HP2-L from the HP1-depleted polytene chromosomes. Indeed, the large size of HP2 suggests that it may interact with multiple heterochromatin proteins, serving to link HP1 with additional components critical for mitotic condensation as well as heterochromatin formation per se.
Acknowledgments
We thank our colleagues for a critical review of this manuscript; Carolyn Craig for technical assistance; and Cory Simpson, Ann Beckert, and Jo Wuller for assistance in the mutant screen. This work was supported by National Institutes of Health grant GM068388 (to S.C.R.E.) and by a grant from the Associazione Italiana per la Ricerca sul Cancro (to M.G.).
References
- Bannister, A. J., P. Zegerman, J. F. Partridge, E. A. Miska, J. O. Thomas et al., 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410: 120–124. [DOI] [PubMed] [Google Scholar]
- Basu, J., H. Bousbaa, E. Logarinho, Z. Li, B. C. Williams et al., 1999. Mutations in the essential spindle checkpoint gene bub1 cause chromosome missegregation and fail to block apoptosis in Drosophila. J. Cell Biol. 146: 13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom, N., S. Gammeltoft and S. Brunak, 1999. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294: 1351–1362. [DOI] [PubMed] [Google Scholar]
- Cenci, G., G. Siriaco, G. D. Raffa, R. Kellum and M. Gatti, 2003. The Drosophila HOAP protein is required for telomere capping. Nat. Cell Biol. 5: 82–84. [DOI] [PubMed] [Google Scholar]
- Cleard, F., M. Delattre and P. Spierer, 1997. SU(VAR)3–7, a Drosophila heterochromatin-associated protein and companion of HP1 in the genomic silencing of position-effect variegation. EMBO J. 16: 5280–5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg, J. C., and S. C. Elgin, 2000. The HP1 protein family: getting a grip on chromatin. Curr. Opin. Genet. Dev. 10: 204–210. [DOI] [PubMed] [Google Scholar]
- Eissenberg, J. C., T. C. James, D. M. Foster-Hartnett, T. Hartnett, V. Ngan et al., 1990. Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 87: 9923–9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg, J. C., G. D. Morris, G. Reuter and T. Hartnett, 1992. The heterochromatin-associated protein HP-1 is an essential protein in Drosophila with dosage-dependent effects on position-effect variegation. Genetics 131: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanti, L., G. Giovinazzo, M. Berloco and S. Pimpinelli, 1998. The heterochromatin protein 1 prevents telomere fusions in Drosophila. Mol. Cell 2: 527–538. [DOI] [PubMed] [Google Scholar]
- Gatfield, D., and E. Izaurralde, 2004. Nonsense-mediated messenger RNA decay is initiated by endonucleolytic cleavage in Drosophila. Nature 429: 575–578. [DOI] [PubMed] [Google Scholar]
- Gatti, M., and B. S. Baker, 1989. Genes controlling essential cell-cycle functions in Drosophila melanogaster. Genes Dev. 3: 438–453. [DOI] [PubMed] [Google Scholar]
- Gatti, M., and M. L. Goldberg, 1991. Mutations affecting cell division in Drosophila. Methods Cell Biol. 35: 543–586. [DOI] [PubMed] [Google Scholar]
- Gatti, M., and S. Pimpinelli, 1992. Functional elements in Drosophila melanogaster heterochromatin. Annu. Rev. Genet. 26: 239–275. [DOI] [PubMed] [Google Scholar]
- Grigliatti, T., 1991. Position-effect variegation—an assay for nonhistone chromosomal proteins and chromatin assembly and modifying factors. Methods Cell Biol. 35: 587–627. [PubMed] [Google Scholar]
- Jacobs, S. A., S. D. Taverna, Y. Zhang, S. D. Briggs, J. Li et al., 2001. Specificity of the HP1 chromo domain for the methylated N-terminus of histone H3. EMBO J. 20: 5232–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, T. C., and S. C. Elgin, 1986. Identification of a nonhistone chromosomal protein associated with heterochromatin in Drosophila melanogaster and its gene. Mol. Cell. Biol. 6: 3862–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khesin, R. B., and B. A. Leibovitch, 1978. Influence of deficiency of the histone gene-containing 38B–40 region on X-chromosome template activity and the white gene position effect variegation in Drosophila melanogaster. Mol. Gen. Genet. 162: 323–328. [DOI] [PubMed] [Google Scholar]
- Khorasanizadeh, S., 2004. The nucleosome: from genomic organization to genomic regulation. Cell 116: 259–272. [DOI] [PubMed] [Google Scholar]
- Lachner, M., D. O'Carroll, S. Rea, K. Mechtler and T. Jenuwein, 2001. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410: 116–120. [DOI] [PubMed] [Google Scholar]
- Linding, R., L. J. Jensen, F. Diella, P. Bork, T. J. Gibson et al., 2003. a Protein disorder prediction: implications for structural proteomics. Structure 11: 1453–1459. [DOI] [PubMed] [Google Scholar]
- Linding, R., R. B. Russell, V. Neduva and T. J. Gibson, 2003. b GlobPlot: exploring protein sequences for globularity and disorder. Nucleic Acids Res. 31: 3701–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke, J., M. A. Kotarski and K. D. Tartof, 1988. Dosage-dependent modifiers of position effect variegation in Drosophila and a mass action model that explains their effect. Genetics 120: 181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottus, R., R. E. Sobel and T. A. Grigliatti, 2000. Mutational analysis of a histone deacetylase in Drosophila melanogaster: missense mutations suppress gene silencing associated with position effect variegation. Genetics 154: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, A. L., J. A. Ortiz, J. You, M. Oulad-Abdelghani, R. Khechumian et al., 1999. Interaction with members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in transcriptional silencing by members of the TIF1 family. EMBO J. 18: 6385–6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka, N., T. Kitajima, S. Yokobayashi, G. Xiao, M. Yamamoto et al., 2002. Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat. Cell Biol. 4: 89–93. [DOI] [PubMed] [Google Scholar]
- Pimpinelli, S., S. Bonaccorsi, L. Fanti and M. Gatti, 2000. Preparation and analysis of Drosophila mitotic chromosomes, pp. 3–23 in Drosophila Protocols, edited by M. Ashburner and R. S. Hawley. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Raffa, G. D., G. Cenci, G. Siriaco, M. L. Goldberg and M. Gatti, 2005. The putative Drosophila transcription factor woc is required to prevent telomeric fusions. Mol. Cell 20: 821–831. [DOI] [PubMed] [Google Scholar]
- Rea, S., F. Eisenhaber, D. O'Carroll, B. D. Strahl, Z. W. Sun et al., 2000. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406: 593–599. [DOI] [PubMed] [Google Scholar]
- Reuter, G., and I. Wolff, 1981. Isolation of dominant suppressor mutations for position-effect variegation in Drosophila melanogaster. Mol. Gen. Genet. 182: 516–519. [DOI] [PubMed] [Google Scholar]
- Reuter, G., J. Gausz, H. Gyurkovics, B. Friede, R. Bang et al., 1987. Modifiers of position-effect variegation in the region from 86C to 88B of the Drosophila melanogaster third chromosome. Mol. Gen. Genet. 210: 429–436. [DOI] [PubMed] [Google Scholar]
- Richards, E. J., and S. C. Elgin, 2002. Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell 108: 489–500. [DOI] [PubMed] [Google Scholar]
- Scaerou, F., I. Aguilera, R. Saunders, N. Kane, L. Blottiere et al., 1999. The rough deal protein is a new kinetochore component required for accurate chromosome segregation in Drosophila. J. Cell Sci. 112(Pt. 21): 3757–3768. [DOI] [PubMed] [Google Scholar]
- Schotta, G., A. Ebert, V. Krauss, A. Fischer, J. Hoffmann et al., 2002. Central role of Drosophila SU(VAR)3–9 in histone H3–K9 methylation and heterochromatic gene silencing. EMBO J. 21: 1121–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotta, G., A. Ebert, R. Dorn and G. Reuter, 2003. Position-effect variegation and the genetic dissection of chromatin regulation in Drosophila. Semin. Cell Dev. Biol. 14: 67–75. [DOI] [PubMed] [Google Scholar]
- Shaffer, C. D., J. M. Wuller and S. C. Elgin, 1994. Raising large quantities of Drosophila for biochemical experiments. Methods Cell Biol. 44: 99–108. [DOI] [PubMed] [Google Scholar]
- Shaffer, C. D., G. E. Stephens, B. A. Thompson, L. Funches, J. A. Bernat et al., 2002. Heterochromatin protein 2 (HP2), a partner of HP1 in Drosophila heterochromatin. Proc. Natl. Acad. Sci. USA 99: 14332–14337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair, D. A. R., R. C. Mottus and T. A. Grigliatti, 1983. Genes which suppress position effect variegation in Drosophila melanogaster are clustered. Mol. Gen. Genet. 191: 326–333. [Google Scholar]
- Spofford, J. B., 1976. Position-effect variegation in Drosophila, pp. 955–1018 in The Genetics and Biology of Drosophila, edited by M. Ashburner and E. Novitski. Academic Press, London.
- Stephens, G. E., C. A. Craig, Y. Li, L. L. Wallrath and S. C. Elgin, 2004. Immunofluorescent staining of polytene chromosomes: exploiting genetic tools. Methods Enzymol. 376: 372–393. [DOI] [PubMed] [Google Scholar]
- Stephens, G. E., E. E. Slawson, C. A. Craig and S. C. Elgin, 2005. Interaction of heterochromatin protein 2 with HP1 defines a novel HP1-binding domain. Biochemistry 44: 13394–13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallrath, L. L., M. J. Swede and S. C. Elgin, 1998. Mapping chromatin structure in Drosophila, pp. 59–77 in Chromatin: A Practical Approach, edited by H. Gould. Oxford University Press, Oxford.
- Weiler, K. S., and B. T. Wakimoto, 1995. Heterochromatin and gene expression in Drosophila. Annu. Rev. Genet. 29: 577–605. [DOI] [PubMed] [Google Scholar]
- Williams, B. C., T. L. Karr, J. M. Montgomery and M. L. Goldberg, 1992. The Drosophila l(1)zw10 gene product, required for accurate mitotic chromosome segregation, is redistributed at anaphase onset. J. Cell Biol. 118: 759–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, B. C., Z. Li, S. Liu, E. V. Williams, G. Leung et al., 2003. Zwilch, a new component of the ZW10/ROD complex required for kinetochore functions. Mol. Biol. Cell 14: 1379–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C. M., K. W. Dobie, H. D. Le, A. Y. Konev and G. H. Karpen, 2002. Efficient recovery of centric heterochromatin P-element insertions in Drosophila melanogaster. Genetics 161: 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhimulev, I. F., E. S. Belyaeva, V. F. Semeshin, D. E. Koryakov, S. A. Demakov et al., 2004. Polytene chromosomes: 70 years of genetic research. Int. Rev. Cytol. 241: 203–275. [DOI] [PubMed] [Google Scholar]