Abstract
1 The action of quinidine sulphate 50 muM has been investigated on frog auricular trabeculae transmembrane currents recorded with a double sucrose gap apparatus. Results were obtained either in current or in voltage clamp conditions. 2 Quinidine modified the time course of repetitive activity elicited by long lasting depolarizing currents and reduced the range of current over which repetitive activity could be triggered, eventually abolishing repetitive responses altogether. 3 Several authors have emphasized the limitations of the voltage clamp method. Taking into account these limitations, the numerical values of the parameters obtained in the present work must not be considered as exact values but may be interpreted as indicators of the variations of the parameters. 4 The results are in agreement with previous findings that the main features of the action of quinidine are to produce (a) a reduced maximum rate of depolarization (MRD), (b) a reduced total amplitude of action potential, (c) a flattening of the plateau of the action potential, (d) a slight prolongation of the tail of the action potential, (e) an increased effective refractory period without greatly prolonging action potential duration, (f) no change of resting potential and of 50% repolarization time. 5 The analysis of ionic conductances has provided explanations for the above effects. 6 Quinidine reduced the reactivation kinetics of the sodium inward current, and decreased sodium conductance and the steady state of activation. These effects account for (a) and (b). 7 Quinidine increased the activation and inactivation time constants of sodium conductances, which account in part for (e). 8 Quinidine delayed reactivation of slow inward current, reduced calcium conductance, and decreased the steady state of activation of calcium conductance. These effects could account for (c). 9 The amplitudes of the two components of the delayed conductances responsible for repolarization were decreased by quinidine, and the time constant of activation for the faster of the two was slowed. These effects could account for (d) and in part for (e).
Full text
PDF
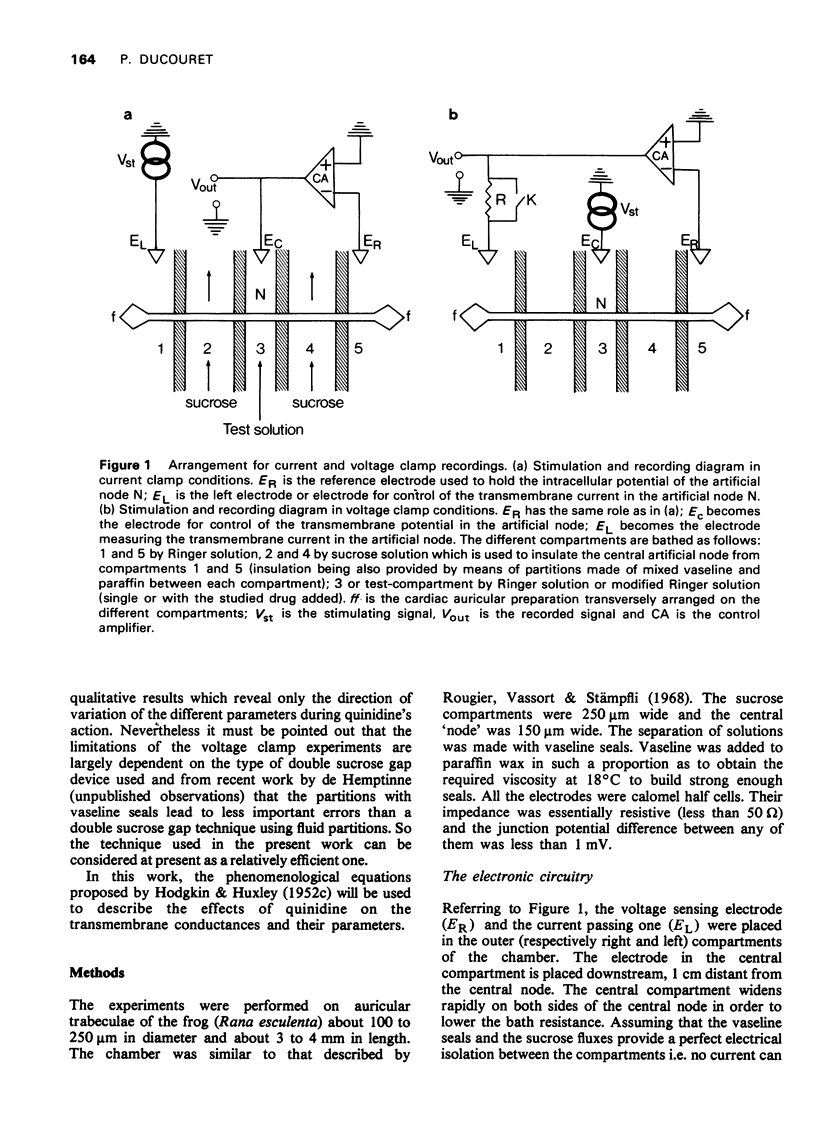


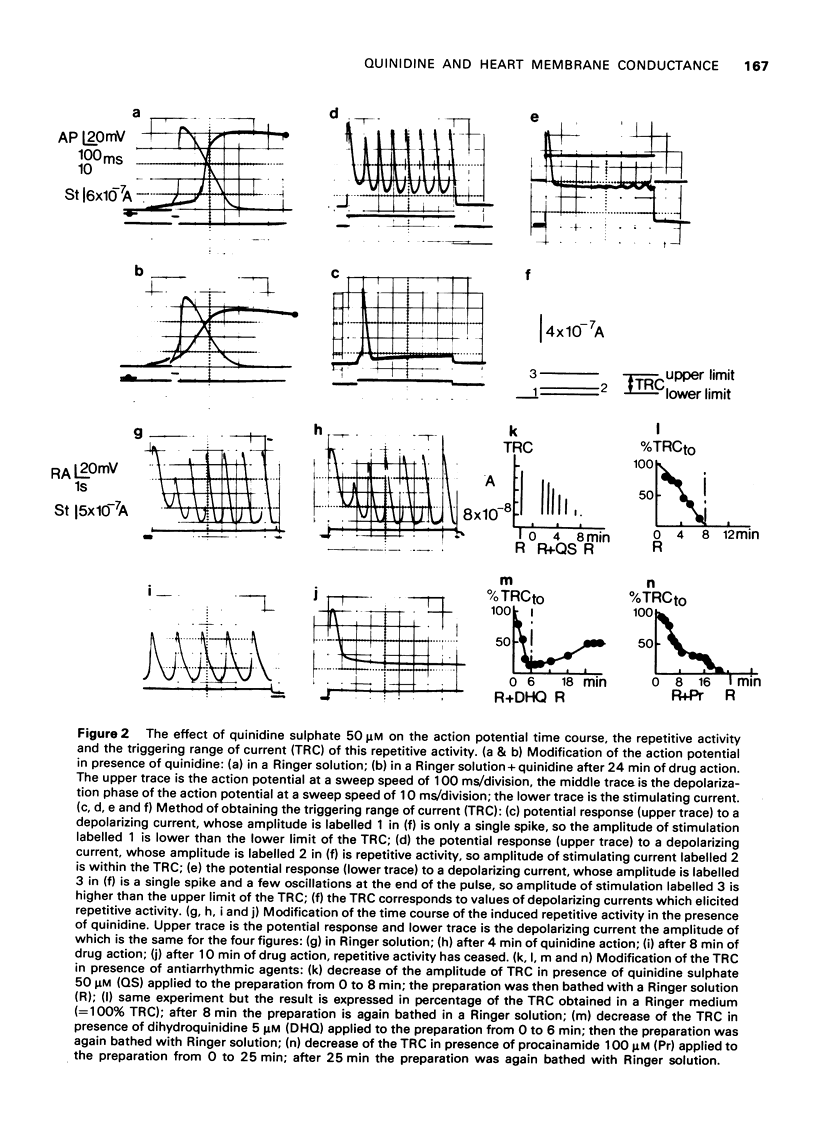
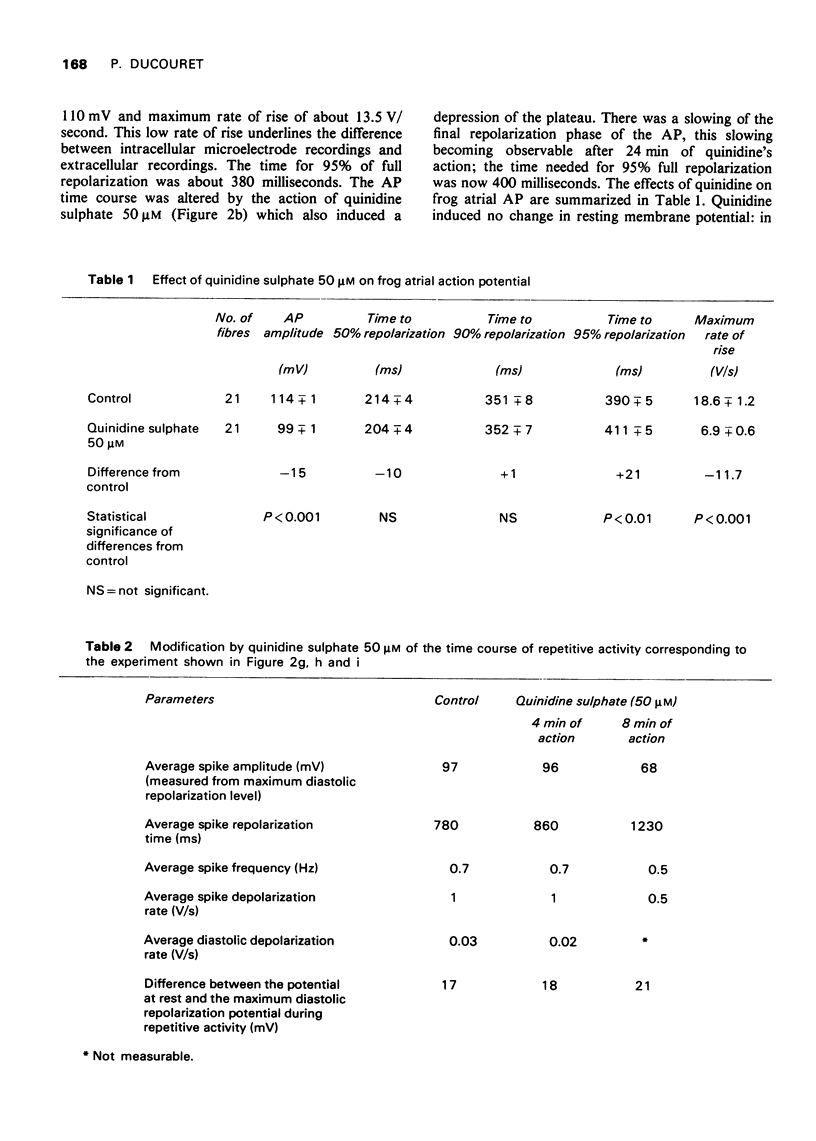
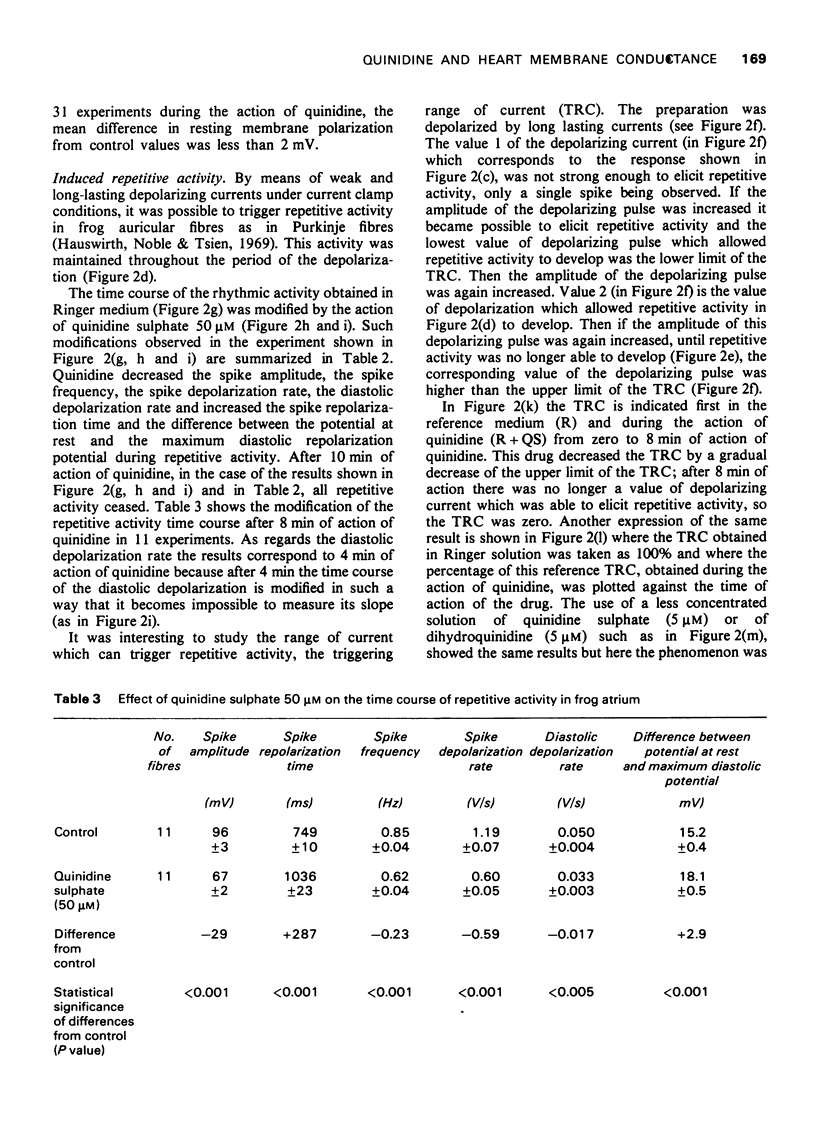
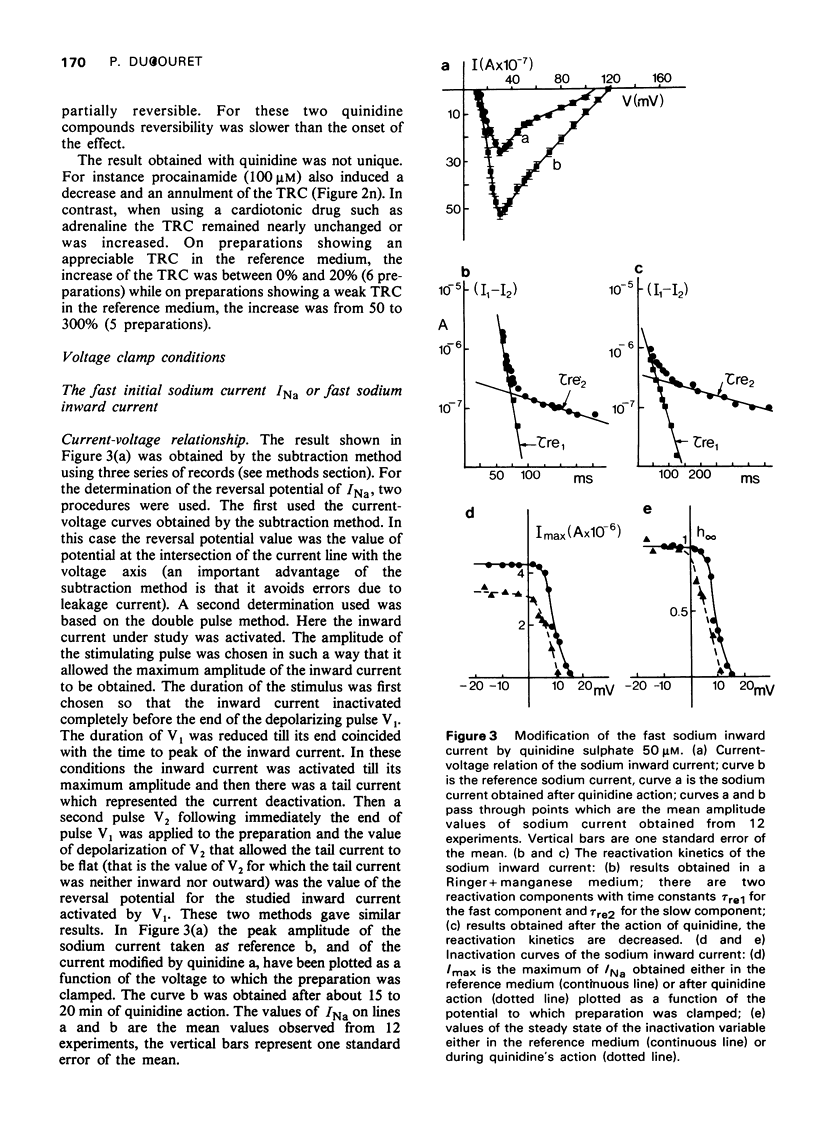








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
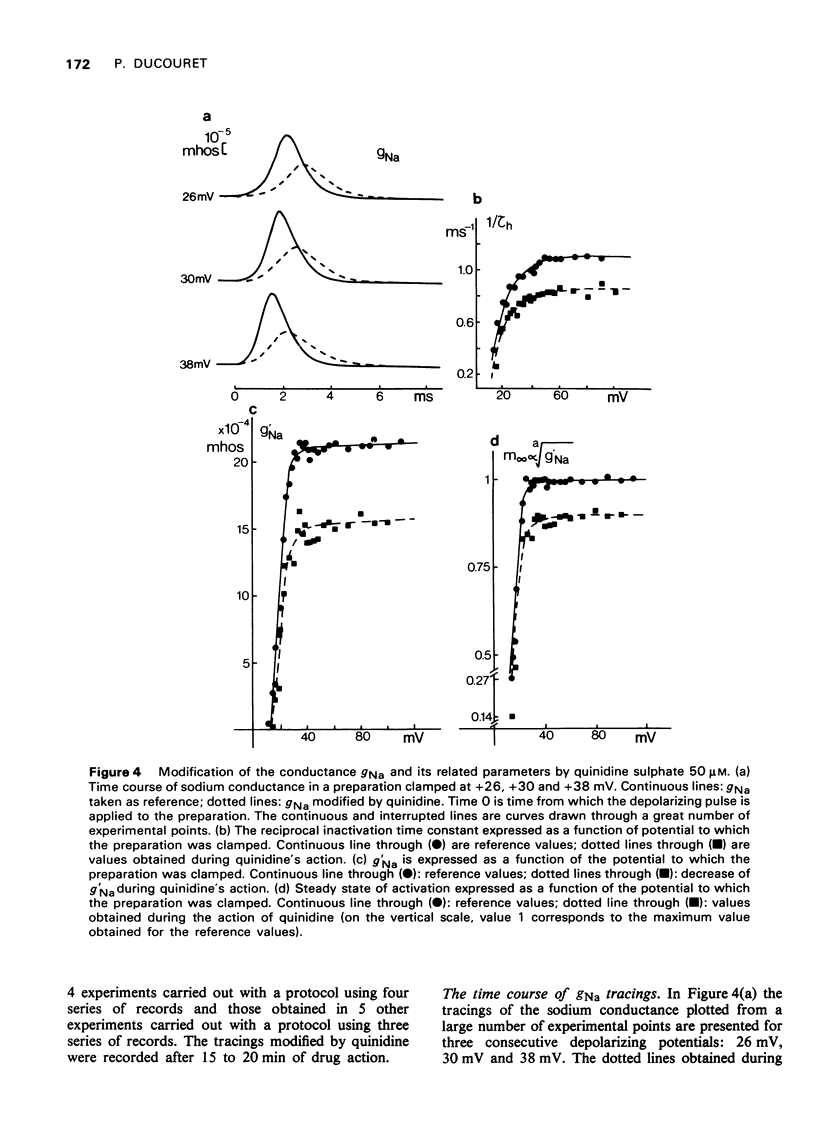
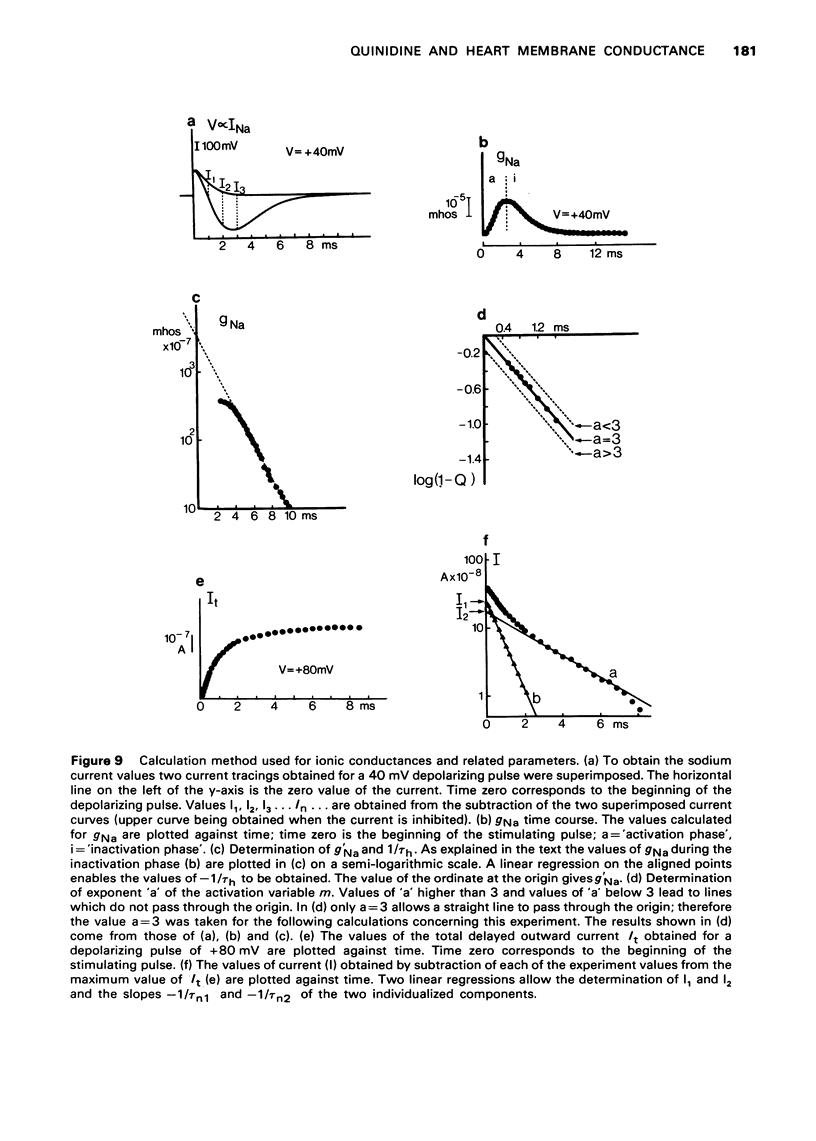
- ANGELAKOS E. T., HASTINGS E. P. The influence of quinidine and procaine amide on myocardial contractility in vivo. Am J Cardiol. 1960 Jun;5:791–798. doi: 10.1016/0002-9149(60)90059-x. [DOI] [PubMed] [Google Scholar]
- Besseau A. Analyse, selon le modèle de Hodgkin-Huxley, des conductances membranaires du myocarde de grenouille (Rana esculenta. J Physiol (Paris) 1972;64(6):647–670. [PubMed] [Google Scholar]
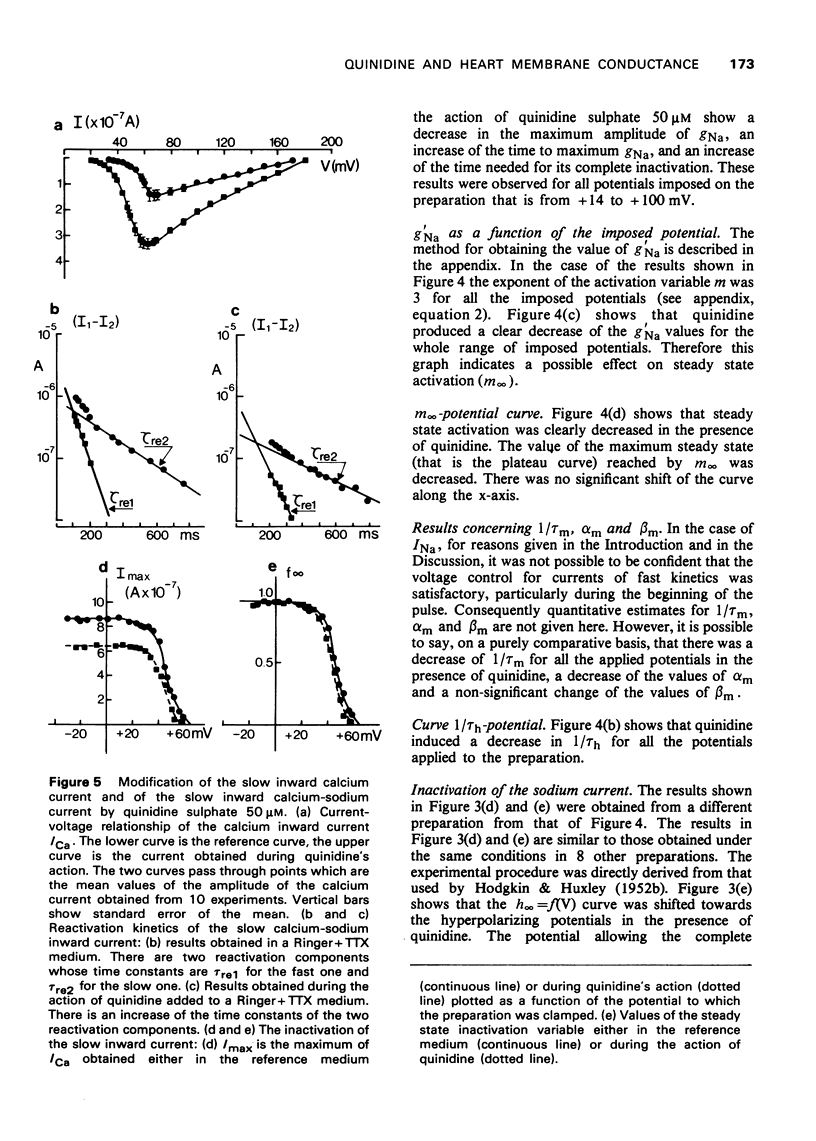
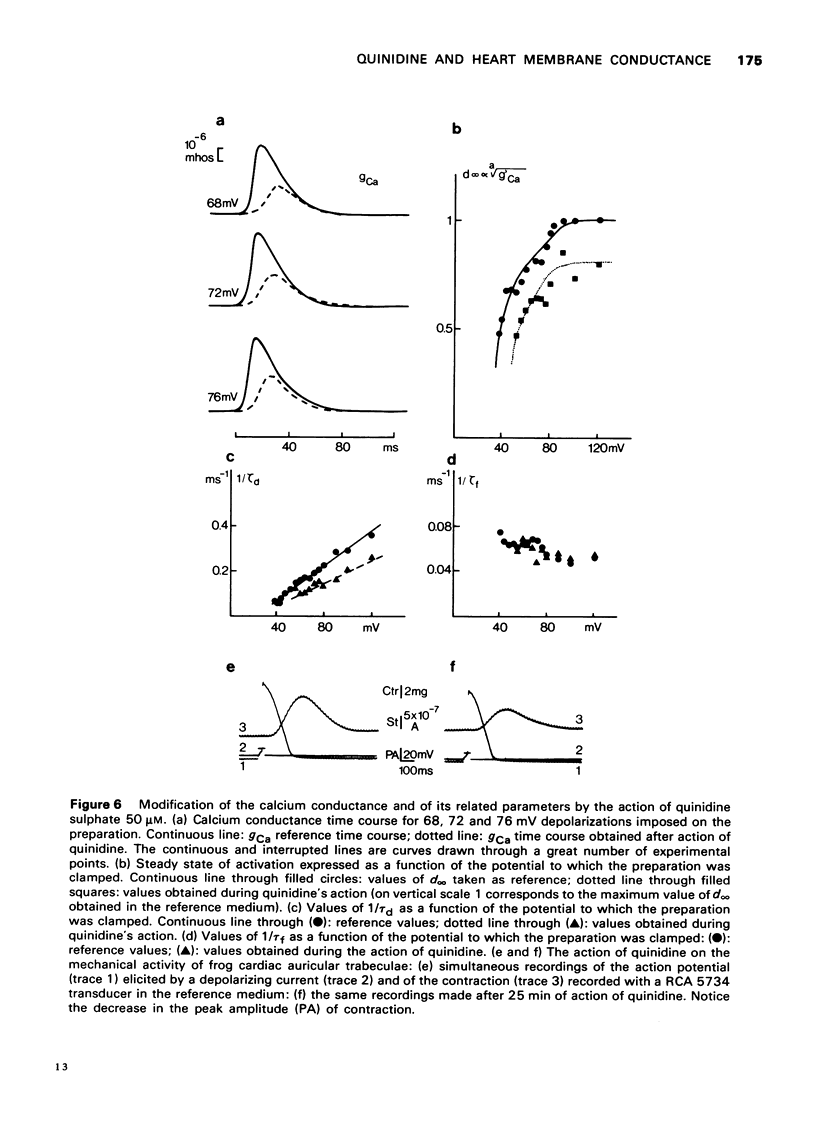
- Brown H. F., Noble S. J. A quantitative analysis of the slow component of delayed rectification in frog atrium. J Physiol. 1969 Oct;204(3):737–747. doi: 10.1113/jphysiol.1969.sp008941. [DOI] [PMC free article] [PubMed] [Google Scholar]
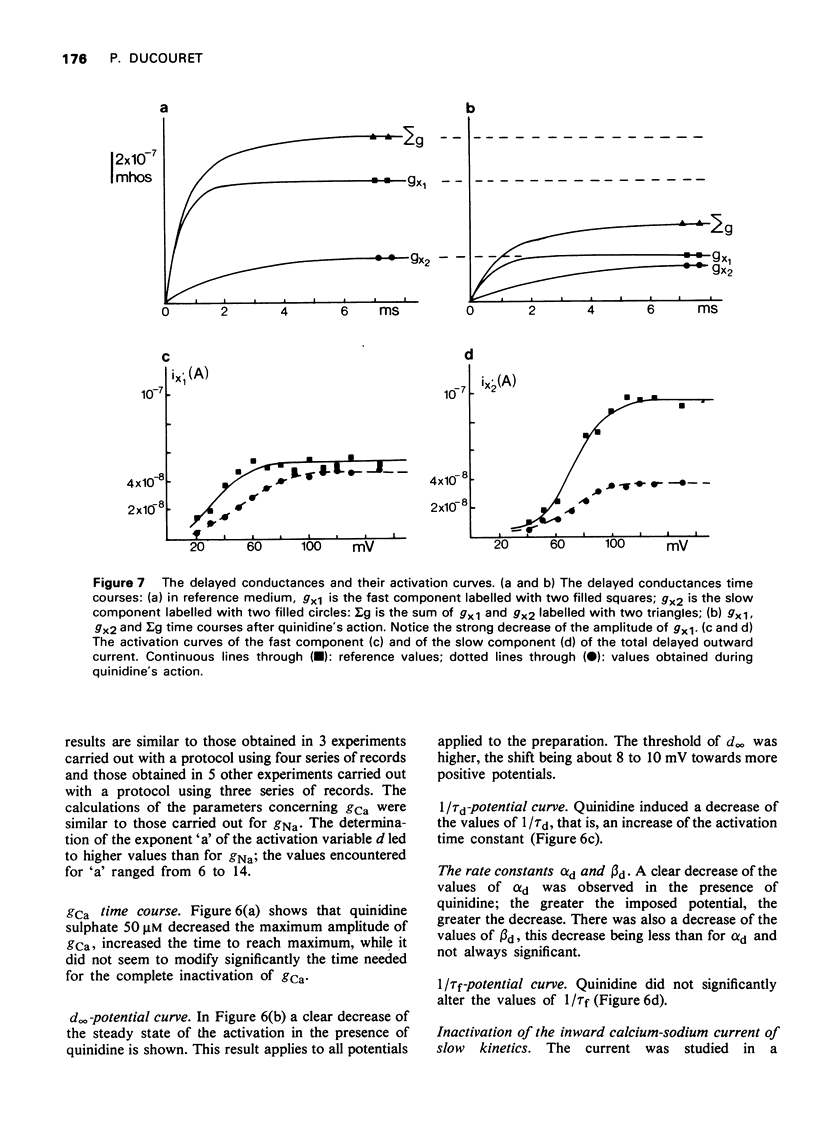
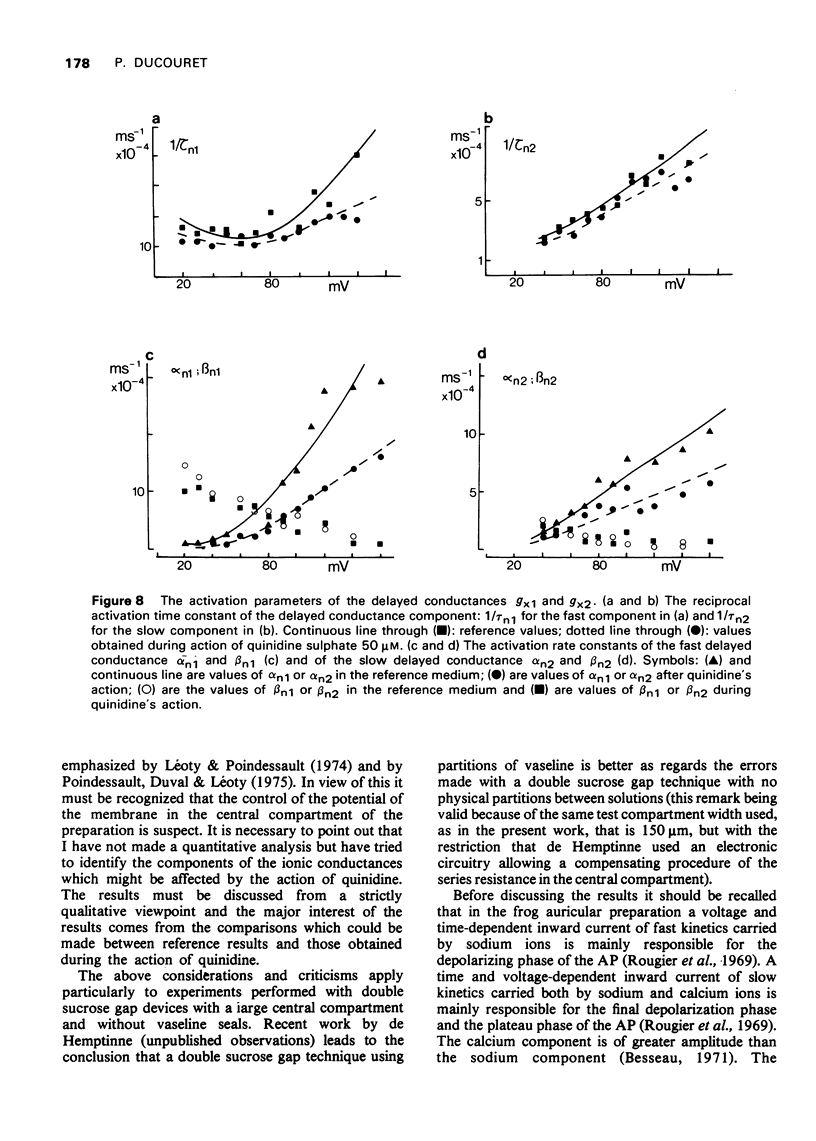
- Brown H. F., Noble S. J. Membrane currents underlying delayed rectification and pace-maker activity in frog atrial muscle. J Physiol. 1969 Oct;204(3):717–736. doi: 10.1113/jphysiol.1969.sp008940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hemptinne A. The frequency dependence of outward current in frog auricular fibres. An experimental and theoretical study. Pflugers Arch. 1971;329(4):332–340. doi: 10.1007/BF00588004. [DOI] [PubMed] [Google Scholar]
- GOODFORD P. J., WILLIAMS E. M. Intracellular Na and K concentrations of rabbit atria, in relation to the action of quinidine. J Physiol. 1962 Mar;160:483–493. doi: 10.1113/jphysiol.1962.sp006861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth O., Noble D., Tsien R. W. The mechanism of oscillatory activity at low membrane potentials in cardiac Purkinje fibres. J Physiol. 1969 Jan;200(1):255–265. doi: 10.1113/jphysiol.1969.sp008691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- Lenfant J., Mironneau J., Aka J. K. Activité répétive de la fibre sino-auriculaire de grenouille: analyse des courants membranaires responsables de l'automatisme cardiaque. J Physiol (Paris) 1972;64(1):5–18. [PubMed] [Google Scholar]
- Léoty C., Poindessault J. P. Proceedings: Effects and compensation of the series resistance in voltage-clamp experiments using double sucrose-gap technique. J Physiol. 1974 Jun;239(2):108P–109P. [PubMed] [Google Scholar]
- Léoty C., Raymond G., Gargouïl Y. M. Tension phasique et tonique de la fibre myocardique de grenouille et les courants ioniques transmembranaires; étude en voltage imposé et par microphotométrie. C R Acad Sci Hebd Seances Acad Sci D. 1970 Oct 28;271(17):1545–1548. [PubMed] [Google Scholar]
- McAllister R. E., Noble D. The effect of subthreshold potentials on the membrane current in cardiac Purkinje fibres. J Physiol. 1967 May;190(2):381–387. doi: 10.1113/jphysiol.1967.sp008216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. E., Noble D. The time and voltage dependence of the slow outward current in cardiac Purkinje fibres. J Physiol. 1966 Oct;186(3):632–662. doi: 10.1113/jphysiol.1966.sp008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOBLE D. A modification of the Hodgkin--Huxley equations applicable to Purkinje fibre action and pace-maker potentials. J Physiol. 1962 Feb;160:317–352. doi: 10.1113/jphysiol.1962.sp006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. Applications of Hodgkin-Huxley equations to excitable tissues. Physiol Rev. 1966 Jan;46(1):1–50. doi: 10.1152/physrev.1966.46.1.1. [DOI] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J Physiol. 1969 Jan;200(1):205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. Reconstruction of the repolarization process in cardiac Purkinje fibres based on voltage clamp measurements of membrane current. J Physiol. 1969 Jan;200(1):233–254. doi: 10.1113/jphysiol.1969.sp008690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmley W. W., Braunwald E. Comparative myocardial depressant and anti-arrhythmic properties of d-propranolol, dl-propranolol and quinidine. J Pharmacol Exp Ther. 1967 Oct;158(1):11–21. [PubMed] [Google Scholar]
- Ramón F., Anderson N., Joyner R. W., Moore J. W. Axon voltage-clamp simulations. A multicellular preparation. Biophys J. 1975 Jan;15(1):55–69. doi: 10.1016/S0006-3495(75)85791-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Localization of beta adrenergic receptors, and effects of noradrenaline and cyclic nucleotides on action potentials, ionic currents and tension in mammalian cardiac muscle. J Physiol. 1974 Oct;242(2):429–451. doi: 10.1113/jphysiol.1974.sp010716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier O., Vassort G., Stämpfli R. Voltage clamp experiments on frog atrial heart muscle fibres with the sucrose gap technique. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(2):91–108. doi: 10.1007/BF00362729. [DOI] [PubMed] [Google Scholar]
- TRAUTWEIN W. Generation and conduction of impulses in the heart as affected by drugs. Pharmacol Rev. 1963 Jun;15:277–332. [PubMed] [Google Scholar]
- Tarr M., Trank J. W. An assessment of the double sucrose-gap voltage clamp technique as applied to frog atrial muscle. Biophys J. 1974 Sep;14(9):627–643. doi: 10.1016/S0006-3495(74)85940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe W. R. Some effects of caffeine and quinidine on sarcoplasmic reticulum of skeletal and cardiac muscle. Can J Physiol Pharmacol. 1973 Jul;51(7):499–503. doi: 10.1139/y73-073. [DOI] [PubMed] [Google Scholar]
- VAUGHAN WILLIAMS E. M. The mode of action of quinidine on isolated rabbit atria interpreted from intracellular potential records. Br J Pharmacol Chemother. 1958 Sep;13(3):276–287. doi: 10.1111/j.1476-5381.1958.tb00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDMANN S. Effects of calcium ions and local anesthetics on electrical properties of Purkinje fibres. J Physiol. 1955 Sep 28;129(3):568–582. doi: 10.1113/jphysiol.1955.sp005379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden M., Kreher P., Aka K. J., Tricoche R. Activité électrique et courants ioniques transmembranaires de la fibre myocardique se singe (famille des cercopithécidés) J Physiol (Paris) 1973;66(4):455–472. [PubMed] [Google Scholar]
- Williams E. M. Progrès dans la connaissance des nouveaux médicaments anti-dysrythmiques. Ann Cardiol Angeiol (Paris) 1973 Jan-Feb;22(1):1–12. [PubMed] [Google Scholar]


