Abstract
Myriophyllum spicatum (Haloragaceae) is a highly competitive freshwater macrophyte that produces and releases algicidal and cyanobactericidal polyphenols. Among them, β-1,2,3-tri-O-galloyl-4,6-(S)-hexahydroxydiphenoyl-d-glucose (tellimagrandin II) is the major active substance and is an effective inhibitor of microalgal exoenzymes. However, this mode of action does not fully explain the strong allelopathic activity observed in bioassays. Lipophilic extracts of M. spicatum inhibit photosynthetic oxygen evolution of intact cyanobacteria and other photoautotrophs. Fractionation of the extract provided evidence for tellimagrandin II as the active compound. Separate measurements of photosystem I and II activity with spinach (Spinacia oleracea) thylakoid membranes indicated that the site of inhibition is located at photosystem II (PSII). In thermoluminescence measurements with thylakoid membranes and PSII-enriched membrane fragments M. spicatum extracts shifted the maximum temperature of the B-band (S2QB− recombination) to higher temperatures. Purified tellimagrandin II in concentrations as low as 3 μm caused a comparable shift of the B-band. This demonstrates that the target site of this inhibitor is different from the QB-binding site, a common target of commercial herbicides like 3-(3,4-dichlorophenyl)-1,1-dimethylurea. Measurements with electron paramagnetic resonance spectroscopy suggest a higher redox midpoint potential for the non-heme iron, located between the primary and the secondary quinone electron acceptors, QA and QB. Thus, tellimagrandin II has at least two modes of action, inhibition of exoenzymes and inhibition of PSII. Multiple target sites are a common characteristic of many potent allelochemicals.
Allelopathy (sensu; Molisch, 1937) is considered an effective trait of submerged aquatic angiosperms (Wium-Andersen, 1987; Gross, 1999, and refs. therein) to counteract the usually strong competition for light and carbon with other primary producers, especially phytoplankton and epiphytes (Sand-Jensen and Søndergaard, 1981). Allelopathically active compounds known from terrestrial plants generally act as natural herbicides and often have multiple effects on the metabolism of target organisms (Einhellig, 2001). Some have even been explored as natural substitutes for commercial herbicides (Duke et al., 2000). Few studies have, however, targeted the mode of action of allelochemicals from aquatic angiosperms.
Several allelochemicals isolated from cyanobacteria or higher plants specifically inhibit photosynthesis of target organisms (Einhellig et al., 1993; Gonzalez et al., 1997; Smith and Doan, 1999). This results in a lower primary production and might consequently cause slower growth of competing photoautotrophs. To prove effects on primary production, the radiocarbon assay (fixation of radioactive labeled 14C) has been frequently used, e.g. with extracts from Zostera marina (Harrison and Durance, 1985) and Chara globularis (Wium-Andersen et al., 1983). More detailed studies involved separate measurements of the activity of photosystems I and II (PSI and PSII). Sorgoleone, a p-benzoquinone from sorghum (Sorghum bicolor), inhibits the electron transport between QA and QB (Gonzalez et al., 1997). Many benthic filamentous cyanobacteria produce specific inhibitors of PSII (see Smith and Doan, 1999), like cyanobacterin from Scytonema hofmannii or fischerellin A from Fischerella muscicola and Fischerella ambigua. The former inhibits the photosynthetic electron transport on the acceptor side of PSII (Gleason and Paulson, 1984; Gleason and Case, 1986), whereas the latter interrupts the electron transport at four different sites (Srivastava et al., 1998) and interferes additionally with membrane integrity. These allelopathically active compounds act differently from synthetic herbicides, an apparently common trait of natural herbicides (Duke et al., 2000). Such compounds reveal a great potential for new target sites not yet exploited by commercial herbicides.
Myriophyllum spicatum (also named milfoil hereafter) is a highly competitive submerged macrophyte with strong allelopathic potential (Planas et al., 1981; Agami and Waisel, 1985; Gross et al., 1996). The major active allelochemical has been identified as β-1,2,3-tri-O-galloyl-4,6-(S)-hexahydroxydiphenoyl-d-Glc (tellimagrandin II; Gross et al., 1996). This and other hydrolyzable polyphenols can account for up to 10% of the dry weight of M. spicatum. Part of the inhibitory potential of M. spicatum is attributable to the complexation with and inactivation of cyanobacterial and algal extracellular enzymes, such as alkaline phosphatase (Gross et al., 1996; Gross, 1999). However, this mechanism does not fully explain the strong allelopathic activity. Allelochemicals from M. spicatum interfered with photosynthetic carbon uptake of two common phytoplanktonic diatoms as shown with the radiocarbon method (Gross and Sütfeld, 1994).
Therefore, we investigated the impact of allelopathically active compounds from M. spicatum on photosynthesis of target organisms, especially cyanobacteria. Our objective was to identify the active compound and to characterize its target site and mode of action. We achieved this by measuring PSI and PSII activity, and by application of thermoluminescence (TL) and electron paramagnetic resonance (EPR) spectroscopy.
RESULTS
Inhibition of Photosynthetic Oxygen Evolution
Oxygen evolution by Anabaena sp. PCC 7120 was inhibited in the presence of extracts from M. spicatum. The extract was separated by SPE into a hydrophilic and a lipophilic fraction. The crude extracts used contained approximately 95 μm tellimagrandin II (Table I) and a total of 160 to 170 μg tannic acid equivalents (TAE) mg−1 extracted plant biomass. To test which fraction of the extract exhibited the highest inhibitory effect, the lipophilic and the aqueous fraction were applied separately. The lipophilic fraction contained more than 90% of the phenolic compounds; less than 10% phenolics was found in the hydrophilic fraction. The lipophilic fraction used in a concentration equivalent to 190 μg TAE mL−1 caused an inhibition of approximately 40% (123.1 ± 7.4 μmol O2 mg−1 chlorophyll [Chl] h−1, mean ± se), whereas the corresponding hydrophilic fraction only led to a 10% decrease in oxygen evolution (183.2 ± 7.1 μmol O2 mg−1 Chl h−1) compared with controls (208.3 ± 4.0 μmol O2 mg−1 Chl h−1, n = 3–7 per treatment). Both inhibitory effects were significantly different from solvent controls (Tukey's HSD, P < 0.05). Crude extracts caused a comparable inhibition of other cyanobacteria (Anabaena variabilis ATCC 29413, strain P9; Synechococcus sp. Myr 9801) and the chlorophyte Chlamydomonas sp. Elo-5B (data not shown).
Table I.
Content of tellimagrandin II in the SPE fractions as used in the Clark electrode measurements
| Fractionation | Water | 25% MeOH | 50% MeOH | 75% MeOH | 100% MeOH | Total |
|---|---|---|---|---|---|---|
| μm | ||||||
| 1 | 0.32 | 51.97 | 42.25 | 0.57 | 0.05 | 95.15 |
| 2 | 0.27 | 79.60 | 13.49 | 0.31 | 0.14 | 93.80 |
The tellimagrandin II content was calculated from the peak area of the HPLC analysis. Both fractionations were carried out independently using different plant material in the extraction process.
Identification of the Active Compound
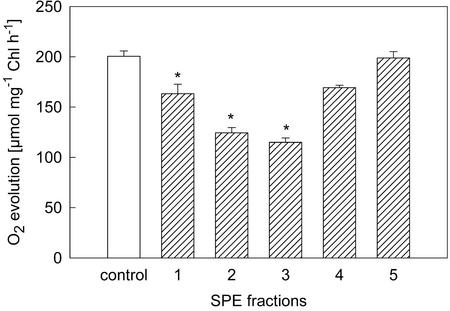
Further fractionation of the crude extract by SPE using procedure II resulted in five fractions that were tested for their inhibitory effect on photosynthetic oxygen evolution. They differed markedly in their effects on Anabaena sp. PCC 7120, as shown in Figure 1. The fractions eluted with 50% and 25% (v/v) methanol, respectively, caused the strongest inhibition (up to 40% reduction of the original activity). HPLC analysis of these fractions revealed a high content of tellimagrandin II in both fractions; the fraction eluted with 50% (v/v) methanol contained 42 μm (final concentration in Clark electrode) and the 25% fraction contained 52 μm tellimagrandin II (Table I). Both fractions contained more than 90% of all phenolic compounds found in the crude extract. These results were repeated with an extract from different M. spicatum material. Because of slight changes in the elution rate in this experiment, we obtained only one active fraction, which contained 85% of the total amount of tellimagrandin II (80 μm; see Table I). This fraction was the only one that caused a statistically significant reduction of photosynthetic oxygen evolution (data not shown). The inhibitory potential of the SPE fractions was apparently correlated with their content of tellimagrandin II.
Figure 1.
Inhibition of photosynthetic O2 evolution of Anabaena sp. PCC 7120 by addition of different fractions of the solid phase extraction (SPE) of M. spicatum extract (1, water; 2, 25% [v/v] MeOH; 3, 50% [v/v] MeOH; 4, 75% [v/v] MeOH; and 5, 100% [v/v] MeOH). The amount of extract used in the experiments was equivalent to 190 μg TAE mL−1. Error bars indicate se; n = 3 to 7 measurements per treatment. Significant differences between treatment and control are shown by an asterisk (Mann Whitney U-test, P < 0.05, experimentwise Bonferroni adjusted).
Because tellimagrandin II was not available in sufficient amount for dose-response measurements, we used tannic acid, a closely related commercially available polyphenol. Tannic acid inhibited oxygen evolution of intact Synechocystis sp. PCC 6803 cells. Concentrations of 5, 50, and 100 μg mL−1 reduced photosynthetic oxygen evolution to 82%, 57%, and 36% of the control (335 ± 35 μmol O2 mg−1 Chl h−1, mean ± se, n = 6 per treatment). Significant differences to the controls were found above concentrations of 50 μg mL−1 (Tukey's HSD, P < 0.05).
Activity Measurements
To determine whether the inhibitor acts directly on PSI or PSII, separate measurements were carried out using spinach (Spinacia oleracea) thylakoid membranes. In controls, PSI activity was approximately 400 μmol O2 uptake mg−1 Chl h−1, and PSII activity about 380 μmol O2-evolution mg−1 Chl h−1. PSI activity of spinach thylakoids measured with methylviologen as electron acceptor was not affected by lipophilic extract fractions of M. spicatum, containing approximately 190 μg TAE mL−1. The experiment was repeated four times (number of replicates per experiment n = 3–7), and did not show a statistically significant difference between treatment and control (Mann Whitney U test, P > 0.05). In contrast, the inhibitory effect of this fraction on oxygen evolution of PSII measured by using the artificial electron acceptor phenyl-p-benzoquinone was pronounced. In three of four experiments, each with at least five individual measurements, a statistically significant reduction of oxygen evolution by 30% was observed (Mann Whitney U-test, P < 0.05). These results indicate that the site of action is located within PSII.
To exclude a putative uncoupler effect of polyphenols in the extract, PSI activity was measured in the absence of NH4Cl. Under these conditions, no change in the rate of O2-uptake was seen in the presence of the lipophilic extract fractions (data not shown).
TL
To further investigate the effect of allelopathically active substances from M. spicatum on electron transport of PSII, TL measurements were performed. In TL, the emitted light originates from charge recombination of trapped charge pairs in PSII. The charge pairs can be identified by their emission temperatures, which strongly depend on the redox potentials of the charge pairs involved. The B- and the Q-band are the most important TL-bands for investigating inhibitory effects of herbicides on the electron transfer in PSII. The recombination of the S2 or S3 states of the manganese cluster of the oxygen-evolving complex at the donor side of PSII with the semiquinone QB− at the acceptor side yields the B-band at approximately 30°C (Rutherford et al., 1982). The recombination of the S2 state with the reduced primary quinone acceptor QA in the presence of herbicides such as 3-(3,4-dichlorpheny)-1,1-dimethylurea (DCMU) results in the Q-band at approximately 5°C (Rutherford et al., 1982).
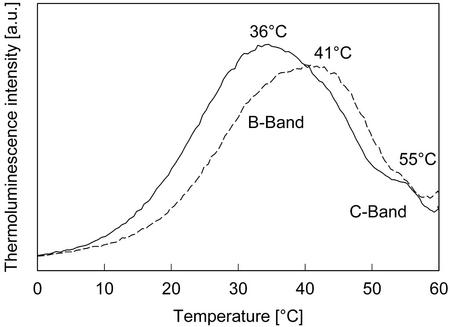
The TL curves obtained with thylakoid membranes are shown in Figure 2. In the control, the B-band had a maximum emission temperature at about 36°C. A small shoulder was seen at about 55°C (C-band), which was assigned to a TyrD+QA− recombination (Demeter et al., 1993; Johnson et al., 1994), where TyrD is a Tyr residue (in PSII) that can be photooxidized. In the presence of the lipophilic fraction, the TL emission was shifted to a higher temperature of about 41°C. It has been described recently that the maximum emission temperature of the B-band is upshifted in the presence of 20 mm formate by approximately 8°C (Bock et al., 2001). Formate competes with HCO3−, one of the physiologically occurring ligands of the non-heme iron between the primary and the secondary quinone acceptors, QA and QB, and so impairs electron transfer (Diner et al., 1991).
Figure 2.
TL signals of thylakoid membranes with no addition (black line) and in the presence of the lipophilic SPE fraction of M. spicatum (dashed line). The amount of extract used in the experiment was equivalent to 190 μg TAE mL−1.
In addition, TL intensities were measured as a function of the number of excitation flashes in the presence and absence of the lipophilic fraction. In the absence of an inhibitor, the B-band oscillates with a period of four representing an active electron transport chain. The period of four oscillation is caused by the turnover of the Mn cluster during the water splitting process. For the production of one molecule oxygen from two water molecules, four light-induced charge separations in the reaction center are required and four positive charges are accumulated. In the presence of the lipophilic fraction, containing approximately 190 μg mL−1 TAE, the oscillation was strongly damped indicating interference with the electron transfer on either the donor or the acceptor side of PSII (data not shown). The same effect was observed using 5 μg mL−1 tannic acid.
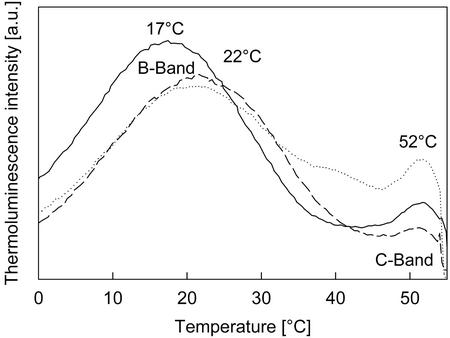
Results of TL measurements with PSII-enriched membrane are shown in Figure 3 and Table II. As can be seen for the control, the maximum emission temperature is positioned at a lower temperature (17°C) than observed with thylakoid membranes (36°C). This effect is because of a partial loss of QB during the isolation procedure; the TL-band reflects a mixture of the Q-band (S2QA− recombination) and the B-band (S2,3QB− recombination). The addition of 20 mm formate shifted the emission temperature to 22°C. The same effect was obtained by the addition of 3 μm tellimagrandin II. Higher formate concentrations (100 mm) led to a larger upshift (ΔT = 12°C). When adding the lipophilic fraction containing about 6 μm tellimagrandin II, a shift of the maximum emission temperature by 12°C was observed similar to the one in the presence of 100 mm formate. Tannic and gallic acid, as well as (+)-catechin also caused a comparable, dose-dependent shift of the maximum temperature of the B-band of PSII-enriched membrane fragments. The emission temperature of the Q-band measured in the presence of the commercial herbicide DCMU, which binds to the QB-binding site, was not changed by the addition of tellimagrandin II or formate. Therefore, we conclude that the redox properties of QA were not altered by tellimagrandin II and that the site of action is localized after QA in the electron transfer chain.
Figure 3.
TL signals of PSII-enriched membrane fragments with no addition (black line) and in the presence of 3 μm tellimagrandin II as pure substance (dashed line) or 20 mm formate (dotted line), respectively.
Table II.
Shift of the B-band of PSII-enriched membrane particles in thermoluminescence after addition of formate, M. spicatum extracts, tellimagrandin II, or pure phenolic compounds
| Experiment Series | Treatment | Concentration | Maximum Temperature | Difference to Control ΔT |
|---|---|---|---|---|
| μm | °C | |||
| I | Control 1 | 0 | 17 | |
| Formate | 20,000 | 22 | 5 | |
| 100,000 | 29 | 12 | ||
| Tellimagrandin II | 3 | 22 | 5 | |
| Extract, with ×μm tellimagrandin II | 6 | 29 | 12 | |
| II | Control 2 | 0 | 22 | |
| Gallic acid | 100 | 22 | 0 | |
| 200 | 32 | 10 | ||
| Tannic acid (μg mL−1)a | (5) | 28 | 6 | |
| (25) | 32 | 10 | ||
| (50) | 35 | 13 | ||
| Catechin | 10 | 28 | 6 | |
| 50 | 35 | 13 | ||
| 100 | 38 | 16 | ||
Two series of experiments with different PSII membrane fragment preparations were used (experiment series I and II), yielding different maximum temperatures for the B band in the controls.
Tannic acid refers to several similar componds (penta- to decagalloyl-Glc), no exact molecular wt; therefore, molarity can be provided for this substance.
EPR Spectroscopy
EPR spectroscopy has played an important role describing many of the key features of the electron transfer pathway in PSII, including the characteristics of the radicals involved. To verify the molecular nature of the interaction between the allelochemical and the non-heme iron, EPR-measurements of PSII-enriched membranes were carried out at low temperature. Dark-adapted material is predominantly characterized by the S1 oxidation state of the water oxidizing complex at the electron donor side, while the primary and secondary electron acceptors, QA and QB are oxidized. Upon continuous illumination at low temperatures (200 K), the Mn-cluster advances by only one oxidation state, yielding S2 and QA− (for reviews, see Diner et al., 1991; Debus, 1992; Carrell et al., 2002).
The donor side of PSII is functional in the presence of M. spicatum lipophilic extract fraction (Table III). The S2 g = 2 multiline EPR signal (Dismukes and Siderer, 1981) is observed unchanged. This indicated that PSII is capable of charge separation, without any damage to the Mn-cluster. In addition, the functionality of the acceptor side was investigated by measuring the iron-semiquinone (Fe2+QA−) EPR signal. Depending on the ligand to the non-heme iron, this signal appears in two forms, the g = 1.9, when HCO3−, the physiological ligand, is present and the g = 1.82 at low pH (Zimmermann and Rutherford, 1986) or when formate is bound (Vermaas and Rutherford, 1984). Both forms were present in the control and also after addition of the lipophilic fraction. Other molecules have also been shown to bind to the non-heme iron, such as cyanide (Koulougliotis et al., 1993) or carboxylate anions (Deligiannakis et al., 1994; Petrouleas et al., 1994). In those cases, alternative signals from the Fe2+QA− complex were reported (g = 1.98, alternate forms of g = 1.82, respectively). Upon addition of 120 mm of cyanide or 100 mm of formate, the g = 1.98 and g = 1.82 forms, respectively, remained unchanged. This lack of competition with other compounds that bind to the non-heme iron indicates that the effect of the active substance on PSII is not attributable to direct binding to the non-heme iron. Addition of 5 mm K3[Fe(CN)6] leads to an oxidation of the non-heme iron in the control. This oxidation was not possible when the lipophilic fraction was present (Table III; Fig. 4). This may indicate that the redox midpoint potential of the iron in PSII is shifted to a more positive value by tellimagrandin II or other phenolic compounds. Fluctuations of the midpoint redox potential of the non-heme iron and their effect on electron transfer from QA to QB are discussed in Deligiannakis et al. (1994).
Table III.
EPR signals of PSII membrane particles in the absence and presence of the lipophilic fraction of M. spicatum extract
| EPR Signal | Control | + Lipophilic Fraction |
|---|---|---|
| S2 multiline (S2 state of the Mn Cluster) | + | + |
| g = 1.9 and g = 1.82 of the QA−-Fe signal | + | + |
| g = 1.82 in the presence of formate | + | + |
| g = 1.98 in the presence of cyanide | + | + |
| Fe3+ signal after addition of 5 mm ferricyanide | + | – |
The amount of extract used in the experiments was equivalent to 190 μg TAE mL−1.
Figure 4.
Difference EPR spectra of the g = 8 and g = 5.6 EPR signal of the oxidized non-heme iron in control (A) and in the presence of lipophilic fraction of M. spicatum (B). EPR conditions: microwave frequency, 9.41 GHz; modulation amplitude, 25 Gauss; microwave power, 20 mW; temperature, 4.3 K.
DISCUSSION
We showed that lipophilic extracts of M. spicatum inhibit photosynthetic electron transport of cyanobacteria and other photoautotrophs. Polyphenols, allelopathically active secondary metabolites in this submerged aquatic angiosperm, were obviously responsible for a reduction in photosynthetic oxygen evolution of various axenic cultures of cyanobacteria as well as unialgal epiphytes isolated from Lake Constance.
SPE fractions containing tellimagrandin II, the major allelopathically active compound (Gross et al., 1996), caused the strongest inhibition of photosynthesis, indicating that this compound is, at least to a great part, responsible for the observed effect (Table I; Fig. 1). During the experiments, tellimagrandin II was not available in sufficient amounts to perform extended dose-response curves, because the substance is not commercially available. Tannic acid (synonym for penta- to decagalloyl-Glc) exhibited a dose-dependent inhibition of photosynthesis in Synechocystis sp. PCC 6803. Although we cannot fully exclude other, yet unknown, secondary metabolites to exert additional inhibitory activity toward photosynthesis, our results with purified extracts and commercially available polyphenols indicate that gallotannins are primarily responsible for the observed inhibition of photosynthesis.
We showed that the lipophilic fraction inhibits photosystem II activity but not PSI. The oxygen consumption of PSI in our assay decreased slightly in the presence of extract, but the difference was never statistically significant. With M. spicatum allelochemicals apparently affecting PSII, we could use TL to refine determination of the target site. TL is a very sensitive and highly specific method suitable to gain information about details of the mode of action of inhibitors within PSII (Vass and Inoue, 1992). The lipophilic extract from M. spicatum caused a shift of the B-band in thylakoid membranes and PSII particles similar to shifts observed with formate applied to purified PSII particles (Figs. 2 and 3). A comparable shift of the B-band was also induced by low concentrations (3 μm) of purified tellimagrandin II. The changes in TL were strictly different from that of commercial phenolic herbicides that bind to the QB-binding site, thereby blocking forward electron transport, resulting in the loss of the B-band and the formation of the Q-band (Vass and Demeter, 1982).
Formate, which causes a similar shift in TL as tellimagrandin II or lipophilic extracts from M. spicatum, binds to the non heme iron between QA and QB as shown by EPR spectroscopy (Vermaas and Rutherford, 1984). However, measurements of the Fe2+QA− signal by EPR in the presence of tellimagrandin II did not reveal a direct interaction of this compound with the non-heme iron (Table II). This is not surprising, because tellimagrandin II is a much larger molecule (Mr 938) than formate (Mr 45), and it is very unlikely that this allelochemical directly binds to the non-heme iron. However, an effect of tellimagrandin II on the redox characteristics of the non-heme iron was shown. In the presence of extract, the oxidation of the non-heme iron by high K3[Fe(CN)6] concentrations was no longer possible, which indicates a rise in the midpoint potential of the non-heme iron (see Deligiannakis et al., 1994). This in turn can interfere with the electron transport between QA and QB and thus explains both the observed decrease in oxygen evolution and the shift of the maximum temperature in the TL measurements. Because ferricyanide interfered with the lipophilic extract as verified by HPLC analysis (data not shown), it was not used in Clark electrode measurements. In the EPR measurements, however, we used it in large surplus to guarantee that it was available in sufficient amount for the desired oxidation.
Most of our studies were performed with isolated spinach thylakoids or cyanobacteria from culture collections. The inhibition of photosynthetic oxygen evolution of an epiphytic cyanobacterium and a chlorophyte from Lake Constance indicates that extracts of M. spicatum act in the same way on naturally occurring primary producers. Further evidence for an ecologically important interference with photosynthesis of epiphytes and phytoplankton was recently demonstrated by others. Allelopathically active exudates from M. spicatum inhibited various cyanobacteria and algae (Körner and Nicklisch, 2002). This study showed, using PAM fluorometry, that allelochemicals from M. spicatum interfere with PSII of competing primary producers. Preliminary results in our laboratory indicate that lipophilic exudates of M. spicatum cause a shift of the B-band in TL comparable with the effect of extract or purified tellimagrandin II. Released polyphenols presumably interfere with photosystem II of algae and cyanobacteria. The release of various polyphenolic compounds by M. spicatum, among them tellimagrandin II, ellagic acid and catechin, was shown in several studies (Gross and Sütfeld, 1994; Gross et al., 1996; Nakai et al., 1999). Exudates from M. spicatum were shown to be growth inhibitory only when a constant exposure to freshly released allelochemicals was provided (Nakai et al., 1999).
In the present study we found that in particular, tellimagrandin II is responsible for the inhibition of PSII activity; a synergistic effect with other polyphenols is likely. At a first glance, a 40% inhibition of PSII activity may be considered weak. Full inhibition of PS by any allelopathically active compound might, however, favor the emergence of resistance in target species (Lopez-Rodas et al., 2001). We suggest that even a moderate reduction of photosynthetic activity may impair normal growth of co-occurring epiphytes and phytoplankton. Our measurements of oxygen evolution with intact cyanobacteria and a chlorophyte reveal that the allelochemicals can pass through cell membranes and reach their target sites. This suggests that such an inhibition is likely to occur in situ.
MATERIALS AND METHODS
Organisms and Cell Fragments Used
Myriophyllum spicatum was collected in September 1999 in Lower Lake Constance near the island Reichenau (Southern Germany) by snorkeling or SCUBA diving and was transported on ice back to the laboratory. There, plants were carefully rinsed with tap water, blotted dry, shock frozen in liquid nitrogen, and stored at −70°C until lyophilization. The freeze-dried material was finely ground and stored in darkness at room temperature.
Anabaena sp. PCC 7120 and Anabaena variabilis sp. ATCC 29413, strain P9, were generally used as axenic indicator organism for photosynthetic inhibition caused by allelopathically active substances from M. spicatum. In addition, we used axenic Synechocystis sp. PCC 6803 and two unialgal epiphytes isolated from submersed macrophytes of Lake Constance (Synechococcus sp. Myr9801 and Chlamydomonas sp. Elo99–5B). Cultures were maintained in cyanobacteria medium (Jüttner et al., 1983) and grown in 300-mL Erlenmeyer flasks at 24°C under permanent light (100 μmol photons m−2 s−1) on a rotary shaker (100 rpm). Chl of cyanobacteria was extracted with methanol and the concentration was determined with an extinction coefficient of ε666 = 6.58 × 104 m−1 cm−1 (Ogawa and Vernon, 1971). The Chl content of Chlamydomonas sp. was determined according to Marker et al. (1980).
To distinguish effects on PSI or PSII, assays were conducted with thylakoid membranes or PSII-enriched membrane fragments from spinach (Spinacia oleracea). The preparation of thylakoid membranes was carried out according to Jensen and Bassham (1966), modified after Laasch (1987). PSII-enriched membrane fragments were prepared from spinach as described by Berthold et al. (1981) with modifications by Johnson et al. (1994). The Chl concentration was determined according to Arnon (1949).
Extraction and Fractionation of Plant Material
We used apical shoot sections for the extracts because they contain the highest amount of bioactive polyphenols (Gross, 2000). Lyophilized material was extracted twice for 2 h on ice under continuous stirring with water:acetone (1:1 [v/v]; 100 mL g−1 dry weight). After centrifugation at 9,000g for 5 min and filtration of the supernatant (Whatman GF/F, Clifton, NJ), the filtrate was evaporated to dryness and resuspended in water:methanol (1:1 [v/v], 1 mL per 125 mg dry weight of initial biomass).
This crude extract was either directly used in measurements of photosynthesis or was fractionated with SPE. Methanol was evaporated from the crude extract, and the remaining water phase was passed over a preconditioned C18-SPE-cartridge (Bond Elut, 500 mg of sorbens, Varian Medical Systems, Palo Alto, CA). Two different SPE fractionations were performed. The first method separated only between the aqueous eluent (named hydrophilic fraction thereafter) and the methanolic eluate containing the compounds adsorbed on the cartridge (named lipophilic fraction). The second procedure used a stepwise elution of the adsorbed compounds. The aqueous eluent was used as one fraction in subsequent assays. Adsorbed lipophilic compounds were then stepwise eluted with 25%, 50%, 75%, and 100% (v/v) methanol (water:methanol mixtures [v/v]). The different fractions were evaporated to dryness, resuspended in 50% (v/v) methanol (1 mL per 125 mg dry weight), and stored at −20°C until use.
Quantification of Hydrolyzable Polyphenols and Tellimagrandin II
Quantification of the total phenolic content of milfoil plant material was performed with the Folin-Ciocalteau assay using tannic acid (T-8406, Sigma-Aldrich, St. Louis) as standard (Gross et al., 1996). Total phenolic content is expressed as TAE. The tellimagrandin II content of extracts and fractions was determined with HPLC analysis. Aliquots of the extracts were chromatographed on an RP-C18 column (250 × 4 mm, Kromasil 100, 5 μm) with solvents A (1% [v/v] acetic acid in ultrapure water) and B (methanol) and the following elution profile: 0 to 20 min 5% to 60% B, 20 to 25 min 60% to 100% B, isocratic with 100% B until end of run (40 min). UV-absorbing compounds were detected at 280 nm, close to the maximum absorbance of most hydrolyzable polyphenols. Tellimagrandin II eluted at 12.9 (± 0.2) min and was quantified using a calibration curve with purified tellimagrandin II.
Pure phenolic compounds related to polyphenols present in M. spicatum, gallic acid, tannic acid, or (+)-catechin (Gross et al., 1996; Nakai et al., 2000), were used in Clark-electrode and TL measurements, all dissolved in 50% (v/v) ethanol. Because tannic acid refers to several similar compounds (penta- to decagalloyl-Glc), no exact Mr can be provided for this substance. According to the Folin-Ciocalteau assay, our M. spicatum extracts contained between 160 and 170 μg TAE mg−1 dry weight. In control experiments, we used the pure phenolic compounds in concentrations comparable with the TAE concentrations detected in M. spicatum extracts.
Measurements of Photosynthetic Activity
Oxygen evolution was measured with a temperature-controlled Clark-type electrode (Hansatech, King's Lynn, UK) at 20°C using saturating white light (I = 2,000 μmol photons m−2 s−1). When adding allelochemicals, the final solvent concentration in the assay was kept below 1%, a concentration that was shown not to affect photosynthetic oxygen evolution in control experiments. The measurement procedure was slightly modified depending on the material used. Cyanobacteria (20 μg Chl mL−1) were pre-incubated together with the inhibitors in cyanobacteria medium plus 1 mm NaHCO3 in dim light for 150 s before starting the measurement. Thylakoid membranes (30–40 μg Chl mL−1) were osmotically shocked in a solution containing 5 mm MgCl2 and 30 mm HEPES (pH 7.5) for 30 s. After this time an equivalent volume of a solution containing 0.6 m sorbitol, 5 mm MgCl2, 100 mm KCl, and 30 mm HEPES (pH 7.9) was added to increase the osmotic potential. We used 10 mm NH4Cl as uncoupler, and 0.5 mm phenyl-p-benzoquinone as electron acceptor for measuring the electron transport activity of PSII. The activity of PSI was measured as the rate of oxygen uptake in the presence of 10 μm DCMU, 5 mm ascorbate, 30 μm 2,6-dichlorophenolindophenol, 0.5 mm methylviologen, 10 mm NH4Cl, and 1 mm NaN3. Data from the photosynthetic oxygen evolution measurements were statistically analyzed with Mann-Whitney U-test or Tukey's HSD test (STATISTICA 99 edition, StatSoft Inc., Tulsa, OK).
TL was measured with an apparatus as described by Krieger et al. (1998). Samples were incubated in the dark at 20°C for 120 s and cooled down to −5°C. At this temperature, a single-turnover flash was given, and the sample was then heated to 60°C with a rate of 0.4°C per s. For measuring the oscillation of TL intensity, saturating single-turnover flashes (1–6; frequency, 1 Hz) were given in subsequent assays. The area under the curve corresponds to the TL intensity and was determined using the curve fitting procedure according to Ducruet and Miranda (1992).
EPR spectroscopy was performed using a spectrometer (ER-300, Bruker, Rheinstetten/Karlsruhe, Germany), equipped with a cryostat (ESR 9, Oxford Biomedical Research, Oxon, UK), a frequency counter (5350B, Hewlett-Packard, Palo Alto, CA), and a Bruker 035 M NMR gaussmeter. Illumination at 200 K was performed in an ethanol/solid CO2 bath in an unsilvered dewar flask, using a 300-W projector lamp. Typical Chl concentrations for the PSII-enriched membranes were 3 to 4 mg Chl mL−1. All control samples were treated with the same amount of methanol. Although the concentrations of methanol used in this study did not interfere with PSII activity, they modify the spectroscopic properties of oxidation states of the Mn-cluster (e.g. see Messinger et al., 1997; Schansker et al., 2002).
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor. The available amount of tellimagrandin II is limited.
ACKNOWLEDGMENTS
We gratefully acknowledge technical assistance from Katharina Kienzler and Claudia Feldbaum, statistical guidance from Daniel Baumgärtner, and linguistic improvements from Raymond Newman. The manuscript further benefited from helpful discussions with Daniela Erhard, Peter Böger, and two anonymous peer reviewers. Christine Postius isolated strain Synechococcus sp. Myr9801 and Enikö Ivanyi isolated strain Chlamydomonas sp. Elo99-5B.
Footnotes
This work was supported by Deutsche Forschungsgemeinschaft (grant nos. SFB 454 to E.M.G. and AL883/3–2 to A.K.L. for construction of the TL apparatus). The European Community granted a Marie Curie individual fellowship to C.G. (no. MCFI–2000–00611).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.011593.
LITERATURE CITED
- Agami M, Waisel Y. Inter-relationship between Najas marina L. and three other species of aquatic macrophytes. Hydrobiologia. 1985;126:169–173. [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold DA, Babcock GT, Yocum CF. A highly resolved, oxygen-evolving photosystem II preparation from spinach thylakoid membranes. FEBS Lett. 1981;134:231–234. [Google Scholar]
- Bock A, Krieger-Liszkay A, Beitia Ortiz de Zarate IBO, Schönknecht G. Cl− channel inhibitors of the arylaminobenzoate type act as photosystem II herbicides: a functional and structural study. Biochemistry. 2001;40:3273–3281. doi: 10.1021/bi002167a. [DOI] [PubMed] [Google Scholar]
- Carrell TG, Tyryshkin AM, Dismukes GC. An evaluation of structural models for the photosynthetic water-oxidizing complex derived from spectroscopic and x-ray diffraction signatures. J Biol Inorg Chem. 2002;7:2–22. doi: 10.1007/s00775-001-0305-3. [DOI] [PubMed] [Google Scholar]
- Debus RJ. The manganese and calcium ions of photosynthetic oxygen evolution. Biochim Biophys Acta. 1992;1102:269–352. doi: 10.1016/0005-2728(92)90133-m. [DOI] [PubMed] [Google Scholar]
- Deligiannakis Y, Petrouleas V, Diner BA. Binding of carboxylate anions at the non-heme Fe(II) of PS II: I. Effects of the QA-Fe2+ and QA-Fe3+ EPR spectra and the redox properties of the iron. Biochim Biophys Acta. 1994;1188:260–270. [Google Scholar]
- Demeter S, Goussias C, Bernat G, Kocacs L, Petrouleas V. Participation of the g=1.9 and g=1.82 EPR forms of the semiquinone-iron complex QA-Fe2+ of photosystem II in the generation of the Q and C thermoluminescence bands, respectively. FEBS Lett. 1993;336:352–356. doi: 10.1016/0014-5793(93)80836-j. [DOI] [PubMed] [Google Scholar]
- Diner BA, Petrouleas V, Wendoloski JJ. The iron-quinone electron-acceptor complex of photosystem II. Physiol Plant. 1991;81:423–436. [Google Scholar]
- Dismukes GC, Siderer Y. Intermediates of a polynuclear manganese centre involved in photosynthetic oxidation of water. Proc Natl Acad Sci USA. 1981;78:274–278. doi: 10.1073/pnas.78.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducruet JM, Miranda T. Graphical and numerical analysis of thermoluminescence and fluorescence F0 emission in photosynthetic material. Photosynth Res. 1992;33:15–27. doi: 10.1007/BF00032979. [DOI] [PubMed] [Google Scholar]
- Duke SO, Dayan FE, Romagni JG, Rimando AM. Natural products as sources of herbicides: current status and future trends. Weed Res. 2000;40:99–111. [Google Scholar]
- Einhellig FA. The physiology of allelochemical action: clues and views. In: Reigosa MJ, Bonjoch NP, editors. First European OECD Allelopathy Symposium: Physiological Aspects of Allelopathy. Spain: Vigo; 2001. pp. 3–25. [Google Scholar]
- Einhellig FA, Rasmussen JA, Hejl AM, Souza IF. Effects of root exudate sorgoleone on photosynthesis. J Chem Ecol. 1993;19:369–375. doi: 10.1007/BF00993702. [DOI] [PubMed] [Google Scholar]
- Gleason FK, Case DE. Activity of the natural algicide cyanobacterin on angiosperms. Plant Physiol. 1986;80:834–838. doi: 10.1104/pp.80.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason FK, Paulson JL. Site of action of the natural algicide cyanobacterin in the blue green alga Synechococcus sp. Arch Microbiol. 1984;138:273–277. [Google Scholar]
- Gonzalez VM, Kazimir J, Nimbal C, Weston LA, Cheniae GM. Inhibition of a photosystem II electron transfer reaction by the natural product sorgoleone. J Agric Food Chem. 1997;45:1415–1421. [Google Scholar]
- Gross EM. Inderjit, KMM Dakshini, CL Foy, eds, Principles and Practices in Plant Ecology: Allelochemical Interactions. Boca Raton, FL: CRC Press; 1999. Allelopathy in benthic and littoral areas: case studies on allelochemicals from benthic cyanobacteria and submersed macrophytes; pp. 179–199. [Google Scholar]
- Gross EM. Seasonal and spatial dynamics of allelochemicals in the submersed macrophyte Myriophyllum spicatum L. Verh Int Ver Limnol. 2000;27:2116–2119. [Google Scholar]
- Gross EM, Meyer H, Schilling G. Release and ecological impact of algicidal hydrolysable polyphenols in Myriophyllum spicatum. Phytochemistry. 1996;41:133–138. [Google Scholar]
- Gross EM, Sütfeld R. Polyphenols with algicidal activity in the submerged macrophyte Myriophyllum spicatum L. Acta Hortic. 1994;381:710–716. [Google Scholar]
- Harrison PG, Durance CD. Reduction in photosynthetic carbon uptake in epiphytic diatoms by water-soluble extracts of leaves of Zostera marina. Mar Biol. 1985;90:117–120. [Google Scholar]
- Jensen RG, Bassham JA. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci USA. 1966;56:1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GN, Boussac A, Rutherford AW. The origin of 40–50°C thermoluminescence bands in photosystem II. Biochim Biophys Acta. 1994;1184:85–92. [Google Scholar]
- Jüttner F, Leonhardt J, Möhren S. Environmental factors affecting the formation of mesityloxide, dimethylallylic alcohol and other volatile compounds excreted by Anabaena cylindrica. J Gen Microbiol. 1983;129:407–412. [Google Scholar]
- Körner S, Nicklisch A. Allelopathic growth inhibition of selected phytoplankton species by submerged macrophytes. J Phycol. 2002;38:862–871. [Google Scholar]
- Koulougliotis D, Kostopoulos T, Petrouleas V, Diner BA. Evidence for cyanide binding at the PS II non-heme iron: effects on the EPR signal for QA iron and on QA/QB electron transfer. Biochim Biophys Acta. 1993;1141:275–282. [Google Scholar]
- Krieger A, Rutherford AW, Jegerschöld C. Thermoluminescence measurements on chloride-depleted and calcium-depleted photosystem II. Biochim Biophys Acta. 1998;1364:46–54. doi: 10.1016/s0005-2728(98)00009-7. [DOI] [PubMed] [Google Scholar]
- Laasch H. Non-photochemical quenching of chlorophyll a-fluorescence in isolated chloroplasts under conditions of stressed photosynthesis. Planta. 1987;171:220–226. doi: 10.1007/BF00391097. [DOI] [PubMed] [Google Scholar]
- Lopez-Rodas V, Agrelo M, Carrillo E, Ferrero LM, Larrauri A, Martin-Otero L, Costas E. Resistance of microalgae to modern water contaminants as the result of rare spontaneous mutations. Eur J Phycol. 2001;36:179–190. [Google Scholar]
- Marker FH, Nusch EA, Rai H, Riemann B. The measurement of photosynthetic pigments in freshwaters and standardization of methods: conclusions and recommendations. Arch Hydrobiol Beih Erg Limnol. 1980;14:91–106. [Google Scholar]
- Messinger J, Robblee JH, Yu WO, Sauer K, Yachandra VK, Klein MP. The S0 state of the oxygen-evolving complex in photosystem II is paramagnetic: detection of an EPR multiline signal. J Am Chem Soc. 1997;119:11349–11350. doi: 10.1021/ja972696a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molisch H. Der Einfluss einer Pflanze auf die andere: Allelopathie. Jena, Germany: Fischer; 1937. [Google Scholar]
- Nakai S, Inoue Y, Hosomi M, Murakami A. Growth inhibition of blue-green algae by allelopathic effects of macrophytes. Water Sci Technol. 1999;39:47–53. [Google Scholar]
- Nakai S, Inoue Y, Hosomi M, Murakami A. Myriophyllum spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa. Water Res. 2000;34:3026–3032. [Google Scholar]
- Ogawa T, Vernon LP. Increased content of cytochromes 554 and 562 in Anabaena variabilis cells grown in the presence of diphenylamine. Biochim Biophys Acta. 1971;226:88–97. doi: 10.1016/0005-2728(71)90180-0. [DOI] [PubMed] [Google Scholar]
- Petrouleas V, Deligiannakis Y, Diner BA. Binding of carboxylate anions at the non-heme Fe(II) of PS II: II. Competition with bicarbonate and effects on the QA/QB electron transfer rate. Biochim Biophys Acta. 1994;1188:271–277. [Google Scholar]
- Planas D, Sarhan F, Dube L, Godmaire H. Ecological significance of phenolic compounds of Myriophyllum spicatum. Verh Int Ver Limnol. 1981;21:1492–1496. [Google Scholar]
- Rutherford AW, Croft AR, Inoue Y. Thermoluminescence as a probe of photosystem II photochemistry: the origin of the flash-induced glow-peaks. Biochim Biophys Acta. 1982;682:457–465. [Google Scholar]
- Sand-Jensen K, Søndergaard M. Phytoplankton and epiphyte development and their shading effect on submerged macrophytes in lakes of different nutrient status. Int Rev Ges Hydrobiol. 1981;66:529–552. [Google Scholar]
- Schansker G, Goussias C, Petrouleas V, Rutherford AW. Reduction of the Mn cluster of the water-oxidizing enzyme by nitric oxide: formation of an S2 state. Biochemistry. 2002;41:3057–3064. doi: 10.1021/bi015903z. [DOI] [PubMed] [Google Scholar]
- Smith GD, Doan NT. Cyanobacterial metabolites with bioactivity against photosynthesis in cyanobacteria, algae and higher plants. J Appl Phycol. 1999;11:337–344. [Google Scholar]
- Srivastava A, Jüttner F, Strasser RJ. Action of the allelochemical, fischerellin A on photosystem II. Biochim Biophys Acta. 1998;1364:326–336. doi: 10.1016/s0005-2728(98)00014-0. [DOI] [PubMed] [Google Scholar]
- Vass I, Demeter S. Classification of photosystem II inhibitors by thermodynamic characterization of the thermoluminescence of inhibitor-treated chloroplasts. Biochim Biophys Acta. 1982;682:496–499. [Google Scholar]
- Vass I, Inoue Y. Thermoluminescence in the study of photosystem II. In: Barber J, editor. The Photosystems: Structure, Function and Molecular Biology. New York: Elsevier Science Publishing; 1992. pp. 259–289. [Google Scholar]
- Vermaas WFJ, Rutherford AW. EPR measurements on the effects of bicarbonate and triazine resistance on the acceptor side of photosystem II. FEBS Lett. 1984;175:243–248. [Google Scholar]
- Wium-Andersen S. Allelopathy among aquatic plants. Arch Hydrobiol Beih Erg Limnol. 1987;27:167–172. [Google Scholar]
- Wium-Andersen S, Anthoni U, Houen G. Elemental sulphur, a possible allelopathic compound from Ceratophyllum demersum. Phytochemistry. 1983;22:2613. [Google Scholar]
- Zimmermann J-L, Rutherford AW. Photoreductant-induced oxidation of Fe2+ in the electron-acceptor complex of photosystem II. Biochim Biophys Acta. 1986;851:416–423. [Google Scholar]