Abstract
In the unicellular green algae Chlamydomonas reinhardtii, high-affinity uptake of iron (Fe) requires an Fe3+-chelate reductase and an Fe transporter. Neither of these proteins nor their corresponding genes have been isolated. We previously identified, by analysis of differentially expressed plasma membrane proteins, an approximately 150-kD protein whose synthesis was induced under conditions of Fe-deficient growth. Based on homology of internal peptide sequences to the multicopper oxidase hephaestin, this protein was proposed to be a ferroxidase. A nucleotide sequence to the full-length cDNA clone for this ferroxidase-like protein has been obtained. Analysis of the primary amino acid sequence revealed a putative transmembrane domain near the amino terminus of the protein and signature sequences for two multicopper oxidase I motifs and one multicopper oxidase II motif. The ferroxidase-like gene was transcribed under conditions of Fe deficiency. Consistent with the role of a copper (Cu)-containing protein in Fe homeostasis, growth of cells in Cu-depleted media eliminated high-affinity Fe uptake, and Cu-deficient cells that were grown in optimal Fe showed greatly reduced Fe accumulation compared with control, Cu-sufficient cells. Reapplication of Cu resulted in the recovery of Fe transport activity. Together, these results were consistent with the participation of a ferroxidase in high-affinity Fe uptake in C. reinhardtii.
Copper (Cu) and iron (Fe) are essential micronutrients and function as catalysts in a variety of oxidation-reduction reactions. Because of their ability to generate free radicals, uptake of Cu and Fe into cells and their assimilation are tightly regulated to prevent both toxicity and deficiency. Carrier-mediated transport systems for Fe and Cu have been identified and characterized in several organisms. The high-affinity Fe uptake system in yeast (Saccharomyces cerevisiae) is composed of three enzymes, whose expression is regulated by the transcription factor AFT1 and is inversely correlated with the cellular Fe content (Yamaguchi-Iwai et al., 1995, 1996). In yeast, Fe is reduced by the Fe3+-chelate reductases, FRE1 and FRE2, before transport by the high-affinity permease, FTR1 (Stearman et al., 1996; Georgatsou et al., 1997). Also participating in uptake is the multicopper oxidase, FET3 (Askwith et al., 1994). A structural and functional interaction between the multicopper oxidase, FET3, and FTR1 has been shown (Stearman et al., 1996) and confirmed by site-directed mutagenesis (Askwith and Kaplan, 1998).
Yeast cells also obtain Fe by means of a second low-affinity uptake system (Dix et al., 1994). The low-affinity Fe uptake activity is catalyzed by the proteins FET4p (Dix et al., 1994) and Smf1p and Smf2p (Liu et al., 1997). In contrast to the high-affinity uptake system, these transporter systems are composed of single proteins with broad metal specificity and preferences for Fe2+ over Fe3+. One further transport system in yeast with homology to FET3 and FTR1 is found in the vacuole and encoded by FET5 and FTH1. This system is presumably involved in sequestration of Fe either in detoxification or as a reservoir for metals to enable the cell to grow under low Fe conditions (Stearman et al., 1996; Spizzo et al., 1997; Urbanowski and Piper, 1999).
The mechanism of Cu uptake in yeast has some similarity to the mechanism for Fe uptake. As for Fe, Cu is reduced by a Cu2+-chelate reductase before uptake (Hassett and Kosman, 1995). Cu permeases are encoded by CTR1 (Dancis et al., 1994a, 1994b) and CTR3 (Knight et al., 1996), and the expression of high-affinity Cu uptake genes is regulated by Cu availability and mediated by the transcription factor MAC1 (Graden and Winge, 1997; Labbe et al., 1997; Yamaguchi-Iwai et al., 1997). The Fe2+ permease, FET4, also functions as a low-affinity Cu transporter and supports normal Cu uptake in yeast (Hassett et al., 2000).
Fe uptake by human cells is somewhat more complex. Transferrin-mediated Fe uptake and a further, not well-understood transferrin-independent uptake system have been described for uptake of Fe from the blood (Aisen et al., 2001). Intestinal Fe acquisition occurs at the brush boarder of the duodenal epithelial cells with subsequent export of the Fe from the epithelial cells into the blood at the basal border. Recently, McKie et al. (2001) identified a mammalian plasma membrane b-type cytochrome with Fe3+-chelate reductase activity in the duodenal mucosa. A divalent cation transporter (DCT1p), also known as NRAMP2p and DMT1p, is responsible for the uptake of Fe2+ from the intestinal lumen (Gruenheid et al., 1995; Gunshin et al., 1997). Fe export from the duodenal epithelial cells requires the Cu-dependent ferroxidase, hephaestin (HEPH; Vulpe et al., 1999), and the permease MTP1p, which is induced under Fe deficiency (Abboud and Haile, 2000).
In Arabidopsis, a reductase activity encoded by FRO2 (Robinson et al., 1999) and an Fe2+ transporter activity, encoded by IRT1 and IRT2 (Eide et al., 1996; Vert et al., 2001), have been shown to be involved in the Fe transport. The expression of IRT2 was localized in external cell layers of the root subapical zone; therefore, it was suggested that this transporter was involved in the Fe uptake into the roots (Vert et al., 2001). In addition, the existence of six genes encoding NRAMP-like proteins was reported in Arabidopsis. It was shown that AtNRAMP1 (Curie et al., 2000) and AtNRAMP3 and 4 (Thomine et al., 2000) complemented the yeast fet3/fet4 mutant and that the AtNRAMP1 accumulated in response to Fe deficiency, whereas AtNRAMP3 and 4 were induced by Fe starvation. Further possible Fe transporters in Arabidopsis are encoded by eight genes homologous to YS1 (yellow stripe), first described in maize (Zea mays) and shown to catalyze the Fe uptake from Fe3+ phytosiderophore complexes (Curie et al., 2001).
Mechanisms analogous to higher plant strategy I and II have been described in algae. Induction of a Fe3+-chelate reductase and Fe uptake activity by Fe deficiency has been best characterized in Chlamydomonas reinhardtii (Eckhardt and Buckhout, 1998; Lynnes et al., 1998; Weger, 1999), although the enzymes catalyzing these reactions and their corresponding genes are unknown. Recently, a distinct increase of a 150-kD protein was observed by SDS-PAGE in the plasma membrane from Fe-deficient C. reinhardtii cells (Herbik et al., 2002). After sequencing of internal peptides of this protein by matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry (MS), homology of two peptides to expressed sequence tag (EST) clones was found (Herbik et al., 2002). Based on physiological experiments and the homology of internal peptides and deduced amino acid sequences to mammalian HEPH and multicopper oxidases in general, it was suggested that this ferroxidase-like protein (FLP) was a component of the Fe uptake complex.
In this study, we have begun a detailed examination of the function of FLP in Fe uptake. Here, we report the full-length cDNA sequence for FLP and the induction of FLP under conditions of Fe deficiency. Furthermore, we have shown that Cu and a multicopper oxidase were required for high-affinity Fe uptake. Thus, the mechanism of high-affinity Fe uptake in C. reinhardtii resembled that found in yeast and not that found in higher plants.
RESULTS
FLP Is a Multicopper Oxidase
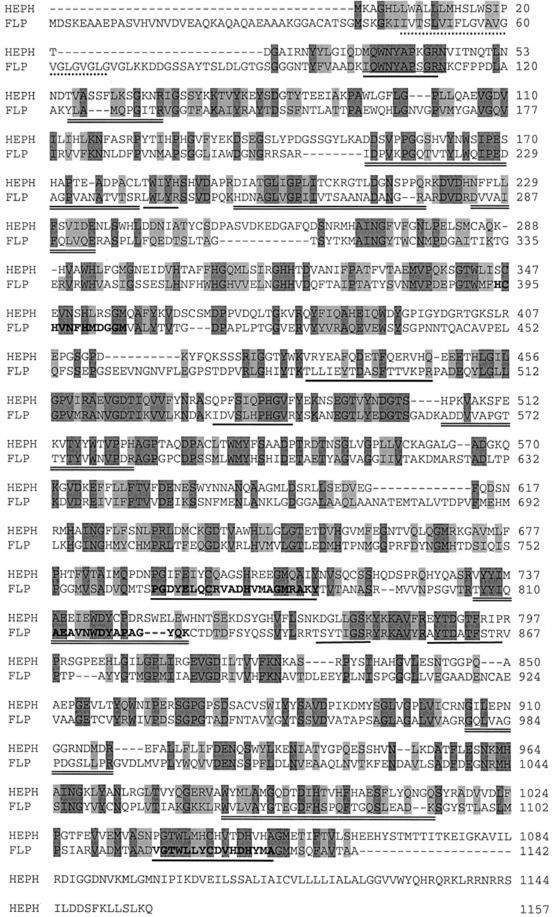
Based on internal partial peptide sequences of FLP (Herbik et al., 2002), two EST clones, AV395796 and AV394010, were obtained and sequenced. Both clones were incomplete. Therefore, 5′-RACE was employed to extend the existing clones and identify a complete cDNA sequence (GenBank accession no. AY074917). Sequence analysis of the cDNA revealed an open reading frame (ORF) of 3,428 nucleotides with a potential ATG start codon at position 250. The cDNA possessed a 3′-untranslated region of about 1,000 bp, which has been found in many nuclear genes of C. reinhardtii (Franzén and Falk, 1992; Dinant et al., 2001), and a polyadenylation signal (TGTAA) located 13 bp upstream of the cDNA poly(A+) sequence. An ORF encoding a protein of 1,142 amino acids with a calculated molecular mass of 131.8 kD and a pI of 4.64 was deduced from the nucleotide sequence. From SDS-PAGE, the molecular mass of the FLP protein was estimated to be 150 kD (Herbik et al., 2002). The predicted sequence contained possible glycosylation sites that may have accounted for the greater molecular mass (PROSITE; Hofmann et al., 1999). Four internal peptides sequenced in the previous study (Herbik et al., 2002) were located in the deduced polypeptide, and peptide maps obtained by MALDI-TOF MS analyses confirmed that the isolated gene coded for FLP (Fig. 1; Herbik et al., 2002). Analysis of the deduced amino acid sequence identified signature sequences for two multicopper oxidase I motifs (767–787 and 1,117–1,137) and one multicopper oxidase II motif (394–405; PROSITE; Hofmann et al., 1999). Hydrophathy analyses predicted a potential transmembrane domain for the FLP protein near to the amino terminus (amino acids 47–68; TMHMM; Moller et al., 2001).
Figure 1.
Comparison of the protein sequence of the FLP from C. reinhardtii with mouse (Mus musculus) HEPH. The alignment was performed using ClustalW from the EMBL database (www.ebi.ac.uk/clustalw). Dark-gray shaded amino acids represent identical residues and light gray indicates conserved substitutions in the alignment. The FLP protein (GenBank accession no. AY074917) and the HEPH protein (GenBank accession no. NP034547) show 31% sequence identity and 46% similarity. Additional features of FLP are also shown. The multicopper oxidase motif I sequences are typed in bold face and underlined, and the multicopper oxidase motif II is typed in bold face. The amino acids 47 to 68 are the putative transmembrane domain and are underlined with a dashed line, the six internal peptide sequences from FLP obtained by MALDI-TOF MS are underlined, and additional peptide maps are underlined twice.
The deduced amino acid sequence was compared with other known multicopper oxidases that have been shown to be involved in Fe transport. The predicted FLP amino acid sequence showed the greatest sequence identity and similarity to mouse HEPH (accession no. NP03447) over its entire length. The FLP and HEPH proteins were identical at 30% of their amino acid residues and displayed 45% similarity (Fig. 1). Homology of FLP was also detected to glycosylphosphatidylinositol-anchored ceruloplasmin (accession no. AAF34175, Rattus norvegicus, 29% identity, 43% similarity) and yeast FET3p (accession no. CAA89768, 26% identity, 45% similarity). Interestingly, a potential transmembrane domain in FLP was predicted near to the amino terminus and not at the carboxy terminus, as was the case for HEPH (Fig. 1).
FLP Expression Was Induced in Response to Fe Deficiency
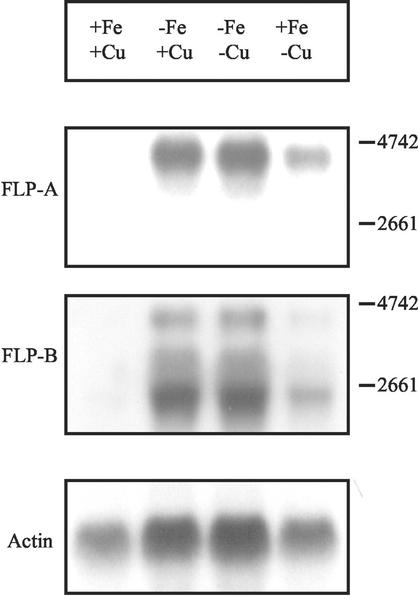
The FLP transcript was examined by probing total RNA with two labeled DNA fragments derived from different regions of the FLP gene (Fig. 2). The FLP-A probe was derived from the 5′ region (1–786) of the FLP gene, and the FLP-B probe was located within the ORF at position 2,228 through 2,902. Compared with the control, FLP was highly expressed after 1 d of Fe deficiency as well as under combined Fe and Cu deficiency. The transcript length of about 4.6 kb agreed with the predicted ORF obtained from the cDNA sequence. After 6 d of Cu deficiency, the expression of FLP was somewhat induced compared with the control. This induction in response to Cu deficiency was distinctly less than after 1 d growth without Fe. Thus, the transcription of the FLP gene was inversely correlated to the supply of Fe and also Cu in the growth media.
Figure 2.
Expression analyses of the FLP gene in Fe- and Cu-deficient C. reinhardtii cells. Northern-blot analysis was performed with total RNA, and cells were grown under sufficient conditions (+), 1 d in Fe-deficient medium (−Fe), 1 d under combined Fe and Cu deficiency (−Fe/−Cu), and 6 d (+Fe/−Cu) in Cu-depleted medium. Two different probes of the FLP gene (FLP-A was derived from the 5′region and FLP-B was located at position 2,228–2,902) were used for hybridization. The 4.5-kb band corresponded to the mature FLP mRNA. A control hybridization of an actin probe using the same blots was also shown.
Repeating the northern analysis using the FLP-B probe, a similar expression pattern was found; however, a signal at 2.5 kb in addition to the 4.6-kb signal was prominent. These two different signals might represent alternative splicing of a single gene, specific degradation or they might represent products from two different genes. However, Southern analyses using the FLP-A probe and FLP-B probe revealed a simple pattern of hybridization (data not shown), suggesting the FLP was unlikely to be coded by multiple genes.
Induction of the Fe3+- and Cu2+-Chelate Reductase Activities Was Time Dependent
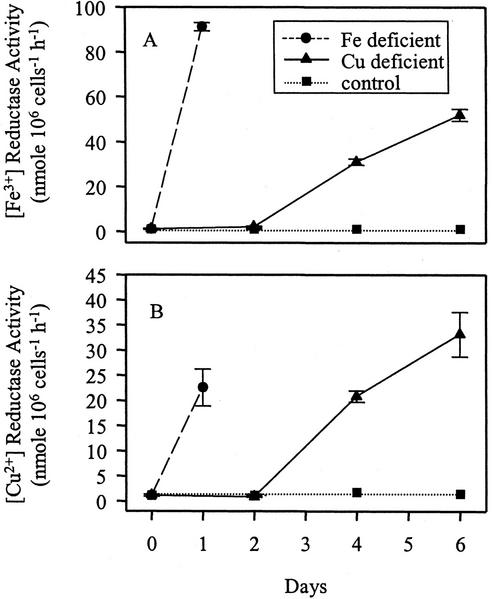
After 1 d of growth in Fe-deficient medium, both the Fe3+- and Cu2+-chelate reductases were induced to the maximum level (Fig. 3, A and B; see also Weger, 1999). However, an increase of both reductase activities was observed after 2 to 3 d of growth on Cu-depleted TAP medium. A maximum induction was reached between 6 and 8 d, although the Fe3+- and the Cu2+-chelate reductase activities were 10-fold lower in Cu-deficient as compared with Fe-deficient cells (Fig. 3). Previous work showed no induction of either reductase after 1 d of growth in Cu-deficient medium (Eckhardt and Buckhout, 1998). From the results shown in Figure 3, it was evident that Cu deficiency increased Cu2+- and Fe3+-chelate reductase activities only after prolonged growth in Cu-deficient medium. Cu deficiency was confirmed by the decrease of the Cu content in Cu-deficient cells (see below).
Figure 3.
Fe3+- and Cu2+-chelate reductase activities of C. reinhardtii, grown in Fe- and Cu-sufficient and deficient media. The cells were grown for 1 d without Fe and up to 6 d without Cu. During the Cu-deficient growth, Tris-acetate-phosphate (TAP) medium was renewed every 2nd d and the cells were diluted to an OD750 of 0.2. Fe3+-chelate (A) and Cu2+-chelate (B) reductase activities were determined. Values represent the mean of three independent assays. ses are shown.
Fe Uptake Was Induced under Fe Deficiency and Inhibited under Cu Deficiency
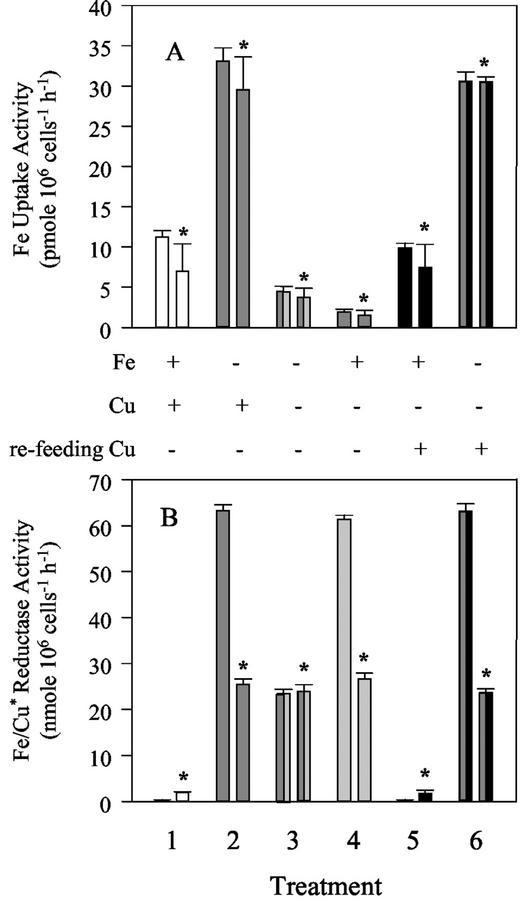
If FLP is a multicopper oxidase, like FET3p or HEPH, its ability to oxidize Fe2+ will depend on Cu ions that activate the enzyme (Stearman et al., 1996). After 1 d of growth in Fe-deficient medium, both the Fe uptake (Fig. 4A) and Fe3+- and Cu2+-chelate reductase activities (Fig. 4B) were increased. Supplying Fe as Fe3+-HEDTA or as Fe2+-HEDTA did not significantly affect the Fe uptake activity (Fig. 4A). Importantly, cells starved of Cu and Fe showed 85% inhibition of the Fe uptake activity compared with uptake under Fe deficiency alone. After reapplication of 6 μm Cu to cells starved of either Cu alone or both Fe and Cu, the Fe uptake and the reductase activities could be restored to the control levels or to the level found under Fe deficiency. These results clearly showed that as in yeast and mammalian cells, a Cu-dependent step was involved in the high-affinity Fe uptake in C. reinhardtii.
Figure 4.
The influence of Cu on Fe uptake (A) and Fe/Cu reductase (B). C. reinhardtii cultures were subjected to different treatments: 1, control; 2, 1 d without Fe; 3, 1 d without Fe and 4 d without Cu; 4, 4 d without Cu; 5, 3 d without Cu, supplemented with Cu for the last day; and 6, 3 d without Cu and Cu resupply by concomitant remove of Fe for the last day. A, Fe was applied as Fe3+-hydroxyethylenediamine triacetic acid (HEDTA), or for Fe2+ uptake 10 mm ascorbic acid was added (bars labeled with an asterisk). B, Fe3+ and Cu2+ (*) reductases were measured. Values represent the mean of three independent assays. ses are shown.
Inhibitory Effect of Tetrathiomolybdate (TTM) on Fe Uptake
If a multicopper ferroxidase were involved in high-affinity Fe transport, inhibition of this ferroxidase should abolish Fe transport. To test this hypothesis, TTM, a well-known inhibitor of multicopper oxidases, was employed (Chidambaram et al., 1984). Fe-deficient and -sufficient C. reinhardtii cells were pre-incubated with TTM and Fe transport activity was determined as described. The Fe uptake was slightly inhibited by the application of 25 μm TTM to Fe-sufficient and -deficient cells (Table I). A greater inhibition of the Fe uptake was obtained by the addition of 250 μm TTM, with the Fe uptake being reduced by 74% in the Fe-sufficient cells and by 89% in the Fe-deficient cells. These results were consistent with the involvement of a ferroxidase in high-affinity Fe transport.
Table I.
Influence of the multicopper oxidase inhibitor TTM on Fe uptake
| Treatment | Nutritional Status
|
|
|---|---|---|
| +Fe | −Fe | |
| Control | 5.66 (100) | 27.23 (100) |
| 25 μm TTM, 0.5 h | 5.99 (106) | 21.53 (79) |
| 25 μm TTM, 4 h | 4.68 (83) | 22.51 (83) |
| 250 μm TTM, 0.5 h | 1.63 (29) | 10.68 (39) |
| 250 μm TTM, 4 h | 1.47 (26) | 2.88 (11) |
Twenty-five and 250 μm TTM were added to a culture containing 4.4 × 108 cells mL−1. TTM was incubated for 0.5 and 4 h and the Fe uptake was measured (pmol 106 cells−1 h−1). These results are averages of two independent assays. Percent inhibition is shown in parentheses.
Micronutrient Content of Cells in Response to Fe and Cu Deficiency
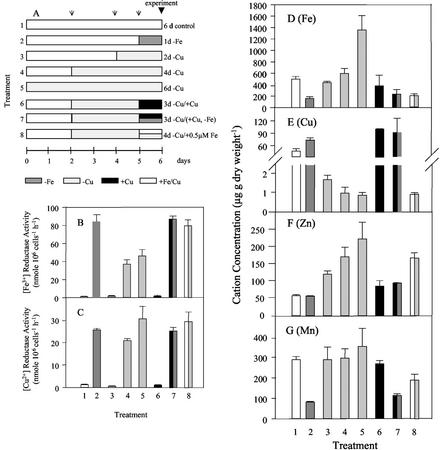
To confirm that the cell were deficient in Fe and Cu, Fe, Cu, Mn, and Zn content were measured in combination with the reductase activities. When grown in full medium, the cells contained 560 μg Fe, 55 μg Cu, 50 μg Zn, and 290 μg Mn g dry weight−1 (Fig. 5, D–G). After 1 d of Fe deficiency, the Fe and Mn content decreased by 70% and 71%, respectively. Surprisingly, Cu content increased by 39%, whereas Zn content remained unchanged. As shown, the Fe3+- and the Cu2+-chelate reductase activities increased under Fe deficiency (Fig. 5, B and C). Removal of Cu for 2, 4, and 6 d led to a corresponding decrease in Cu content and as for Fe deficiency, an increase in both Fe and Cu reductase activities. In contrast to the uptake experiments where the high-affinity Fe uptake was inhibited under Cu deficiency (Fig. 4A), Fe as well as Zn increased with increasing Cu deficiency (Fig. 5, D and F). We speculate that the accumulation of Fe and Zn under Cu deficiency was likely the result of uptake by a low-affinity permease.
Figure 5.
A through C, Total transition metal content of C. reinhardtii cells grown in Fe- and Cu-depleted TAP medium. A, Growth conditions up to 6 d: 1, control; 2, 1 d without Fe; 3, 2 d without Cu; 4, 4 d without Cu; 5, 6 d without Cu, 6, 3 d without Cu, supplemented with Cu for the last day; 7, 3 d without Cu and Cu resupply by concomitant removal of Fe for the last day; and 8, 4 d without Cu and reduction of the Fe supply from 20 to 0.5 μm. Arrows shown in A indicate the medium exchange and simultaneous cell dilution to an A750 of 0.2. B, Fe3+ reductase. C, Cu2+ reductase activities. D, Fe content; E, Cu content; F, Zn content; G, Mn content. These results are averages of two independently grown cultures.
The changes induced by Cu deficiency were reversible. Resupply of Cu to Cu-deficient cells led to a normalization of the content of all four micronutrients to the level of the control. Furthermore, resupply of Cu to Cu- and Fe-deficient cells resulted in micronutrient contents and reductase activities that were similar to those described for Fe-depleted cells. Most importantly, supplying 0.5 μm Fe to Cu depleted cells resulted in maximally induced reductase activity, reduced Fe and Cu content, and accumulation of Zn. Thus, cells grown under Cu deficiency with optimal concentrations of Fe did not accumulate Fe in contrast to Cu-deficient cells that were supplied with supra-optimal Fe, indicating the presence of a low-affinity and a Cu-dependent, high-affinity Fe uptake system in C. reinhardtii.
DISCUSSION
In this report, we present novel evidence linking FLP to high-affinity Fe transport and propose that FLP is a ferroxidase, functioning in the reoxidation of Fe2+ before its uptake into the cell. Initial evidence in support of this hypothesis comes from the deduced FLP amino acid sequence. FLP contains two multicopper oxidase I and one multicopper oxidase signature II motifs. In addition, the amino acid sequence of FLP shows the highest homology to multicopper oxidases in mammals (HEPH and ceruloplasmin) and yeast (FET3). These proteins are ferroxidases that are themselves involved in high-affinity Fe assimilation (Stearman et al., 1996; Askwith and Kaplan, 1998; Mukhopadhyay et al., 1998; Attieh et al., 1999; Vulpe et al., 1999). The involvement of FLP in Fe homeostasis is evident from the regulation of its synthesis. Both the transcription of the FLP gene and synthesis of FLP are greatly increased in Fe-deficient cells and reversed after resupply of Fe. Although we have not demonstrated the ferroxidase activity in FLP directly, we have shown previously that Fe-deficient C. reinhardtii cells have increased p-phenylenediamine oxidase activity compared with Fe-sufficient controls (Herbik et al., 2002). Furthermore, treatment of cells with TTM, an inhibitor of multicopper oxidases, results in increased Fe3+ reductase activity (Herbik et al., 2002) and an inhibition of high-affinity Fe uptake (Table I). These data are at the least consistent with the function of FLP as a ferroxidase.
If FLP is a component of a high-affinity Fe uptake system, one would expect it to be membrane bound. Consistent with this requirement, all data collected thus far indicate a membrane localization of FLP. FLP was initially identified in a membrane fraction highly enriched for the plasma membrane (Herbik et al., 2002), and extraction of this plasma membrane fraction with high salt and/or washing with carbonate at pH 9 does not solubilize FLP (H.I. Reinhardt and T.J. Buckhout, unpublished data). Structural analysis of the primary amino acid sequence of FLP predicts an amino terminal transmembrane domain. The presence of a single transmembrane domain is reminiscent of the predicted structure of mammalian HEPH and yeast FET3; however, in these proteins, the transmembrane domain is located on the carboxy terminus of the protein. Structural analyses also predict that the major portion of the protein is located on the outside surface of the cell (TMHMM; Moller et al., 2001). Thus, with only one transmembrane domain, FLP is unlikely to be a Fe permease. In analogy to HEPH and FET3, we propose that FLP interacts with a presently unidentified Fe transport protein to facilitate the movement of Fe across the membrane.
The proposed involvement of FLP in high-affinity Fe uptake in C. reinhardtii predicted a link between the Cu and Fe nutritional status of the cell and, thus, between Cu availability and Fe acquisition. Results testing this hypothesis confirmed this link and showed that Cu was necessary for high-affinity Fe transport. In Fe-deficient C. reinhardtii, the Fe uptake activity increased with decreasing Fe content (Fig. 4A). Removal of Cu from Fe-deficient cells resulted in an inhibition of Fe uptake activity. Furthermore, Fe uptake activity was also inhibited in Cu-deficient cells (Fig. 4A), and, finally, depletion of cellular Cu was correlated with decreased Fe in cells grown on medium containing sufficient but not supra-optimal Fe and also with the absence of high-affinity Fe transport. The reapplication of Cu to Cu-deficient cells restored the Fe uptake activity to the level of the controls. In general, whenever Cu was removed, the high-affinity Fe uptake activity was inhibited. In yeast, Cu is also required for high-affinity Fe uptake (Askwith et al., 1994; Stearman et al., 1996). Mutations either in the high-affinity Cu transporters (Dancis et al., 1994b; Yuan et al., 1995) or Cu deficiency (Askwith et al., 1994) resulted in impaired Fe uptake. Thus, in C. reinhardtii as in yeast, the involvement of a Cu-containing step and a multicopper oxidase activity in the high-affinity Fe uptake was apparent.
In addition to this high-affinity Fe uptake mechanism, there appeared to be additional low-affinity and low-specificity transport activities that were induced under Fe deficiency. The presence of such an activity would explain the unexpected increase in Cu content in Fe-deficient cells and the accumulation of Fe in Cu-deficient cells fed supra-optimal concentrations of Fe (Fig. 5, D and E). Long-term 59Fe uptake experiments with Cu-depleted cells confirmed the accumulation of Fe when Fe was supplied at high concentrations (data not shown). The presence of low-affinity transporters has been described in yeast. For example, FET4 was shown to catalyze low-affinity Fe transport (Dix et al., 1994, 1997) and low-affinity Cu uptake (Hassett et al., 2000). It was reported that yeast strains lacking high-affinity Fe transport (Δfet3) showed increased expression of FET4 and accumulated transition metals, which resulted in increased metal sensitivity (Li and Kaplan, 1998). The distinct accumulation of Fe and Zn demonstrated an accumulation of transition metals by a Cu-independent system in C. reinhardtii. With reduction of the Fe supply from 20 to 0.5 μm in Cu-depleted cells, the high-affinity, Cu-dependent uptake activity and the low-affinity uptake activity for Fe and Cu were distinguishable. As expected under these conditions, Fe did not accumulate, and the Cu content was reduced to a minimum. However, under these same conditions, the Zn content increased by 200% (Fig. 5F). Although not characterized in this study, these results were consistent with the presence of a low-affinity Fe transport system.
Fe assimilation in C. reinhardtii involves an obligatory reduction of cellular Fe3+-chelates, leading to chelate splitting and subsequent Fe uptake. Both Fe3+-chelate reductase and Fe uptake are induced under Fe deficiency (Eckhardt and Buckhout, 1998; Lynnes et al., 1998; Weger, 1999). What function might a combined reduction of Fe3+-chelates and reoxidation of Fe2+ have in Fe uptake? Several authors have proposed that the ferroxidase imparts specificity and selectivity to high-affinity Fe uptake (Askwith et al., 1996; Askwith and Kaplan, 1998; Eide, 1998) The reductases FRE1 and FRE2 in yeast (Dancis et al., 1992; Hassett and Kosman, 1995) and FRO2 in Arabidopsis are nonspecific and reduce a wide range of Fe3+- as well as Cu2+-chelates (Robinson et al., 1999). Divalent metal carriers from plants (Eide et al., 1996; Korshunova et al., 1999; Curie et al., 2000, Rogers et al., 2000; Eckhardt et al., 2001) as well as low-affinity transporters in yeast (Dix et al., 1994, 1997) are also relatively unspecific. Thus, coupling Fe reduction to a ferroxidase might impart greater substrate specificity to Fe transport. In addition, uptake of Fe2+ into the cell would lead to generation of toxic hydroxyl radicals via the Fenton reaction. Reoxidation of Fe2+ concomitant with uptake of Fe3+ into the cell would avoid the production of oxygen radicals in the cell.
We have demonstrated for the first time, to our knowledge, a Cu-dependent step in high-affinity Fe uptake in C. reinhardtii. Furthermore, we present evidence supporting the idea that this Cu-dependent step involves the multicopper oxidase FLP as a ferroxidase. Thus, the mechanism of Fe uptake in C. reinhardtii resembles that in yeast and not higher plants. Studies are currently under way to test the model with loss-of-function mutants.
MATERIALS AND METHODS
Strains and Culture Conditions
The Chlamydomonas reinhardtii cell wall-deficient mutant strain 83/81 (cw15 mt+) was grown in Fe-sufficient and -deficient TAP medium, as described previously (Eckhardt and Buckhout, 1998). Cell density was estimated at 750 nm (Harris, 1988). Cultures were grown under Cu deficiency essentially as described by Quinn and Merchant (1998). To induce Cu deficiency, cells were collected by centrifugation, washed twice in Cu-free TAP medium, and resuspended in Cu-free TAP medium. Cells were regularly diluted to an A750 of 0.1 to 0.2 every 2nd d and 1 d before the experiment began. All glassware was rinsed overnight with 0.1 n HCl. Chemicals of the highest purity commercially available were used.
Fe3+- and Cu2+-Chelate Reductase Assays and Fe Uptake Measurements
The Fe3+-chelate reductase was measured with 600 μm bathophenanthroline disulfonate as described by Eckhardt and Buckhout (1998). The Cu2+-chelate reductase was measured with 100 μm bathocuproine disulfonate as described by Hill et al. (1996). In general, uptake experiments were conducted as described by Eckhardt and Buckhout (1998) with slight modifications to the uptake buffer (20 mm MES, 20 mm Na-citrate, and 2 mm K-acetate, pH 6.2) and the quench solution (20 mm MES, 20 mm EDTA, and 2 mm CaCl2, pH 6.2). Before each experiment, cells were washed twice and then resuspended in uptake buffer. Five-hundred microliters of this cell suspension (2.3–2.7 × 106 cells mL−1) was combined with 100 μL of substrate solution. When Fe3+ was used as a substrate, the uptake buffer contained 12 μm FeCl3, 14.4 μm HEDTA, and 1,000 cpm μL−1 59FeCl3 (Amersham, Braunschweig, Germany). For Fe2+ uptake, 10 mm ascorbic acid was added. The reaction was stopped after 1, 2, 4.5, 9, 13.5, and 18 min by addition of 10 mL of quench solution. Cells were collected by filtration on glass fiber filters G/FC (Whatman, Kent, UK) and were washed twice with 5 mL of quench solution. The radioactivity was measured by liquid scintillation counting (Liquid Scintillation Analyzer TRI-CARB 2900TR, Hewlett-Packard, Palo Alto, CA).
Micronutrient Analysis
The cells were digested with aqua regia in a microwave oven (2 mL of HNO3 and 6 mL of HCl; Mars 5-XP1500, CEM, Kamp-Lintfort, Germany). To verify the sample preparation, the certified reference material (BCR-CRM 414 Plankton, Institute for Reference Materials and Measurements, Geel, Belgium) was digested by the same method. The total concentrations of Fe, Mn, and Zn were determined by inductively coupled plasma (ICP) atomic emission spectrometry (Optima 3000, Perkin Elmer, Rodgau-Jügesheim, Germany). A quadrupole mass spectrometer with ICP as excitation source (ICP-MS Elan 5000; Perkin Elmer/Sciex, Rodgau-Jügesheim, Germany) was used for Cu determination. Analyses of samples followed external calibration with diluted single element and multielement standards.
Northern Blotting
Total RNA was isolated from C. reinhardtii cells as described by Chomczynski and Sacchi (1987). RNA was separated by agarose gel electrophoresis, blotted onto Hybond N+ (Amersham Biosciences, Freiburg, Germany), and hybridized with the two FLP fragments that were randomly labeled with [32P]dCTP (RediprimeII, Amersham Biosciences). For the reverse transcription (Omniscript, Qiagen, Hilden, Germany), the gene-specific primer GAGCCATGTTGACGGGGAAGTCC (FLP1) was used. For amplification of the 786-bp fragment at the 5′ end of the FLP gene (FLP-A), the primers CCGGGCTATCGGGAACGCCCTTTGGCG (FLP2) and CGCGAATGACCTGACCCACGGCG (FLP3) were used. For the amplification of the second probe, an 874-kb fragment at position 2,028 through 2,902 (FLP-B), the primers ATGCTGTGGATGTACCACTCC (FLP4) and CCCACCTCGGCAATGATCATGG (FLP5) were used. Blots were hybridized and the last stringent washes were at 60°C with 0.1% (w/v) SSC + 0.1% (w/v) SDS for 15 min. The blot was then subjected to autoradiography at −80°C.
5′-RACE and DNA Sequencing
To obtain the 5′ end of the cDNA for the FLP gene, 5′-RACE was performed according to the manufacture's instructions (Roche Molecular Biochemical, Penzberg, Germany). After RNA isolation, contaminating DNA was removed using DNA-free (Ambion, Austin, TX). For cDNA synthesis, Omniscript reverse transcriptase (Qiagen) and AdvantageTM-GC2 polymerase (CLONTECH, Palo Alto, CA) were used to overcome problems with the high GC content of C. reinhardtii sequences. Primer CCAGTCCTGCGTTGTCGTGCTTC (FLP6) was used for first strand synthesis at 42°C, primer GCCACAGGTAGGTGACTGTCTGC (FLP7) was used for the first PCR, and primer GAGCCATGTTGACGGGGAAGTCC (FLP8) was used for the nested PCR. For the cloning of the purified 1.0-kb fragment, a TA cloning kit (Invitrogen, Karlsruhe, Germany) was used. DNA was sequenced by automated ABI 373 sequencer (Applera, Weiterstadt, Germany) using Big Dye Terminator Cycle Sequencing Chemistry.
Note Added in Proof
The C. reinhardtii sequence, FOX1, was deposited in GenBank (accession no. AF450137) by Prof. Sabeebha Merchant (Department of Chemistry and Biochemistry, University of California, Los Angeles), and a report of this work has been recently published (S. La Fontaine, J.M. Quinn, S.S. Nakamoto, M.D. Page, V. Göhre, J.L. Moseley, J. Kropat, S. Merchant [2002] Eykaryot Cell 1: 736–757).
ACKNOWLEDGMENTS
We thank Dr. René Frömmichen for micronutrient analyses, Dr. Anke Koch for the support with the 5′-RACE, Dr. Martin Meixner for DNA sequencing, and Jeane Heyd for excellent technical assistance. The cDNA clone (EST no. AV304010) was generously provided by the Kazusa DNA Research Institute (Kisarazu, Japan).
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (grant to T.J.B.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.013060.
LITERATURE CITED
- Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol. 2001;33:940–959. doi: 10.1016/s1357-2725(01)00063-2. [DOI] [PubMed] [Google Scholar]
- Askwith C, de Silva D, Kaplan J. Molecular biology of iron acquisition in Saccharomyces cerevisiae. Mol Microbiol. 1996;20:27–34. doi: 10.1111/j.1365-2958.1996.tb02485.x. [DOI] [PubMed] [Google Scholar]
- Askwith C, Eide D, Van Ho A, Bernard PS, Li L, Davis-Kaplan S, Sipe DM, Kaplan J. The FET3 gene of S. cerevisiaeencodes a multicopper oxidase required for ferrous iron uptake. Cell. 1994;76:403–410. doi: 10.1016/0092-8674(94)90346-8. [DOI] [PubMed] [Google Scholar]
- Askwith C, Kaplan J. Site-directed mutagenesis of the yeast multicopper oxidase Fet3p. J Biol Chem. 1998;273:22415–22419. doi: 10.1074/jbc.273.35.22415. [DOI] [PubMed] [Google Scholar]
- Attieh ZK, Mukhopadhyay CK, Sesadri V, Tripoulas NA, Fox PL. Ceruloplasmin ferroxidase activity stimulates cellular iron uptake by a trivalent cation-specific transport mechanism. J Biol Chem. 1999;274:1116–1123. doi: 10.1074/jbc.274.2.1116. [DOI] [PubMed] [Google Scholar]
- Chidambaram MV, Barnes G, Frieden E. Inhibition of ceruloplasmin and other copper oxidases by thiomolybdate. J Inorg Biochem. 1984;22:231–239. doi: 10.1016/0162-0134(84)85008-4. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Curie C, Alonso JM, Le Jean M, Ecker JR, Briat JF. Involvement of NRAMP1 from Arabidopsis thalianain iron transport. Biochem J. 2000;347:749–755. [PMC free article] [PubMed] [Google Scholar]
- Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature. 2001;409:346–349. doi: 10.1038/35053080. [DOI] [PubMed] [Google Scholar]
- Dancis A, Roman DG, Anderson GJ, Hinnebusch AG, Klausner RD. Ferric reductase of Saccharomyces cerevisiae: molecular characterization, role in iron uptake, and transcriptional control by iron. Proc Natl Acad Sci USA. 1992;89:3869–3873. doi: 10.1073/pnas.89.9.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancis A, Haile D, Yuan DS, Klausner RD. The Saccharomyces cerevisiaecopper transport protein (CTR1p). Biochemical characterization, regulation by copper, and physiologic role in Cu uptake. J Biol Chem. 1994b;269:25660–25667. [PubMed] [Google Scholar]
- Dancis A, Yuan DS, Haile D, Askwith C, Eide D, Moehle C, Kaplan J, Klausner RD. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell. 1994a;76:393–402. doi: 10.1016/0092-8674(94)90345-x. [DOI] [PubMed] [Google Scholar]
- Dinant M, Baurain D, Coosemans N, Joris B, Matagne RF. Characterization of two genes encoding mitochondrial alternative oxidase in Chlamydomonas reinhardtii. Curr Genet. 2001;39:101–108. doi: 10.1007/s002940000183. [DOI] [PubMed] [Google Scholar]
- Dix DR, Bridgham JT, Broderius MA, Byersdorfer CA, Eide DJ. The FET4 gene encodes the low affinity Fe(II) transport protein of Saccharomyces cerevisiae. J Biol Chem. 1994;269:26092–26099. [PubMed] [Google Scholar]
- Dix DR, Bridgham JT, Broderius MA, Eide DJ. Characterization of FET4 protein of yeast. Evidence for a direct role in the transport of iron. J Biol Chem. 1997;272:11770–11777. doi: 10.1074/jbc.272.18.11770. [DOI] [PubMed] [Google Scholar]
- Eckhardt U, Buckhout TJ. Iron assimilation in Chlamydomonas reinhardtiiinvolves ferric reduction and is similar to strategy I higher plants. J Exp Bot. 1998;49:1219–1226. [Google Scholar]
- Eckhardt U, Mas Marques A, Buckhout TJ. Two iron-regulated cation transporters from tomato complement metal uptake-deficient yeast mutants. Plant Mol Biol. 2001;45:437–448. doi: 10.1023/a:1010620012803. [DOI] [PubMed] [Google Scholar]
- Eide DJ. The molecular biology of metal ion transport in Saccharomyces cerevisiae. Annu Rev Nutr. 1998;18:441–469. doi: 10.1146/annurev.nutr.18.1.441. [DOI] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzén LG, Falk G. Nucleotide sequence of cDNA clones encoding the beta subunit of mitochondrial ATP synthase from the green alga Chlamydomonas reinhardtii: the precursor protein encoded by the cDNA contains both an N-terminal presequence and a C-terminal extension. Plant Mol Biol. 1992;19:771–780. doi: 10.1007/BF00027073. [DOI] [PubMed] [Google Scholar]
- Georgatsou E, Mavrogiannis LA, Fragiadakis GS, Alexandraki D. The yeast Fre1p/Fre2p cupric reductases facilitate copper uptake and are regulated by the copper-modulated Mac1p activator. J Biol Chem. 1997;272:13786–13792. doi: 10.1074/jbc.272.21.13786. [DOI] [PubMed] [Google Scholar]
- Graden JA, Winge DR. Copper-mediated repression of the activation domain in the yeast Mac1p transcription factor. Proc Natl Acad Sci USA. 1997;94:5550–5555. doi: 10.1073/pnas.94.11.5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenheid S, Cellier M, Vidal S, Gros P. Identification and characterization of a second mouse Nramp gene. Genomics. 1995;25:514–525. doi: 10.1016/0888-7543(95)80053-o. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Harris EH. The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. San Diego: Academic Press; 1988. [DOI] [PubMed] [Google Scholar]
- Hassett R, Dix DR, Eide DJ, Kosman DJ. The Fe(II) permease Fet4p functions as a low affinity copper transporter and supports normal copper trafficking in Saccharomyces cerevisiae. Biochem J. 2000;351:477–484. [PMC free article] [PubMed] [Google Scholar]
- Hassett R, Kosman DJ. Evidence for Cu(II) reduction as a component of copper uptake by Saccharomyces cerevisiae. J Biol Chem. 1995;270:128–134. doi: 10.1074/jbc.270.1.128. [DOI] [PubMed] [Google Scholar]
- Herbik A, Haebel S, Buckhout TJ. Is a ferroxidase involved in the high-affinity iron uptake in Chlamydomonas reinhardtii. Plant Soil. 2002;241:1–9. [Google Scholar]
- Hill KL, Hassett R, Kosman D, Merchant S. Regulated copper uptake in Chlamydomonas reinhardtiiin response to copper availability. Plant Physiol. 1996;112:697–704. doi: 10.1104/pp.112.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K, Bucher P, Falquet L, Bairoch A. The PROSITE database, its status in 1999. Nucleic Acids Res. 1999;27:215–219. doi: 10.1093/nar/27.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight SAB, Labbé S, Kwon LF, Kosman DJ, Thiele DJ. A widespread transposable element masks expression of a yeast copper transport gene. Genes Dev. 1996;10:1917–1929. doi: 10.1101/gad.10.15.1917. [DOI] [PubMed] [Google Scholar]
- Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB. The IRT1 protein from Arabidopsis thalianais a metal transporter with a broad substrate range. Plant Mol Biol. 1999;40:37–44. doi: 10.1023/a:1026438615520. [DOI] [PubMed] [Google Scholar]
- Labbe S, Zhu Z, Thiel DJ. Copper-specific transcriptional repression of yeast genes encoding critical components in the copper transport pathway. J Biol Chem. 1997;272:15951–15958. doi: 10.1074/jbc.272.25.15951. [DOI] [PubMed] [Google Scholar]
- Li L, Kaplan J. Defects in the yeast high affinity iron transport system result in increased metal sensitivity because of the increased expression of transporters with broad transition metal specificity. J Biol Chem. 1998;273:22181–22187. doi: 10.1074/jbc.273.35.22181. [DOI] [PubMed] [Google Scholar]
- Liu XF, Supek F, Nelson N, Culotta VC. Negative control of heavy metal uptake by the Saccharomyces cerevisiaeBSD2 gene. J Biol Chem. 1997;272:11763–11769. doi: 10.1074/jbc.272.18.11763. [DOI] [PubMed] [Google Scholar]
- Lynnes JA, Derzaph TLM, Weger HG. Iron limitation results in induction of ferricyanide reductase and ferric chelate reductase activities in Chlamydomonas reinhardtii. Planta. 1998;204:360–365. [Google Scholar]
- McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, Bomford A et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291:1755–1759. doi: 10.1126/science.1057206. [DOI] [PubMed] [Google Scholar]
- Moller S, Croning MD, Apweiler R. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics. 2001;17:646–653. doi: 10.1093/bioinformatics/17.7.646. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay CK, Attieh ZK, Fox PL. Role of ceruloplasmin in cellular iron uptake. Science. 1998;279:714–717. doi: 10.1126/science.279.5351.714. [DOI] [PubMed] [Google Scholar]
- Quinn J, Merchant S. Copper-responsive gene expression during adaptation to copper deficiency. Methods Enzymol. 1998;297:263–279. doi: 10.1016/s0076-6879(98)97020-3. [DOI] [PubMed] [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML. A ferric-chelate reductase for iron uptake from soils. Nature. 1999;397:694–697. doi: 10.1038/17800. [DOI] [PubMed] [Google Scholar]
- Rogers EE, Eide DJ, Guerinot ML. Altered selectivity in an Arabidopsismetal transporter. Proc Natl Acad Sci USA. 2000;97:12356–12360. doi: 10.1073/pnas.210214197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizzo T, Byersdorfer C, Duesterhoeft S, Eide D. The yeast Fet5 gene encodes a Fet3-related multicopper oxidase implicated in iron transport. Mol Gen Genet. 1997;256:547–556. doi: 10.1007/pl00008615. [DOI] [PubMed] [Google Scholar]
- Stearman R, Yuan DS, Yamaguchi-Iwai Y, Klausner RD, Dancis A. A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science. 1996;271:1552–1557. doi: 10.1126/science.271.5255.1552. [DOI] [PubMed] [Google Scholar]
- Thomine S, Wang R, Ward JM, Crawford NM, Schroeder JI. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc Natl Acad Sci USA. 2000;97:4991–4996. doi: 10.1073/pnas.97.9.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowski JL, Piper RC. The iron transporter Fth1p forms a complex with the Fet5 iron oxidase and resides on the vacuolar membrane. J Biol Chem. 1999;274:38061–38070. doi: 10.1074/jbc.274.53.38061. [DOI] [PubMed] [Google Scholar]
- Vert G, Briat JF, Curie C. Arabidopsis IRT2 gene encodes a root-periphery iron transporter. Plant J. 2001;26:181–189. doi: 10.1046/j.1365-313x.2001.01018.x. [DOI] [PubMed] [Google Scholar]
- Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet. 1999;21:195–199. doi: 10.1038/5979. [DOI] [PubMed] [Google Scholar]
- Weger HG. Ferric and cupric reductase activities in the green alga Chlamydomonas reinhardtii: experiments using iron-limited chemostats. Planta. 1999;207:377–384. [Google Scholar]
- Yamaguchi-Iwai Y, Dancis A, Klausner RD. AFT1: a mediator of iron regulated transcriptional control in Saccharomyces cerevisiae. EMBO J. 1995;14:1231–1239. doi: 10.1002/j.1460-2075.1995.tb07106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Iwai Y, Serpe M, Haile D, Yang W, Kosman DJ, Klausner RD, Dancis A. Homeostatic regulation of copper uptake in yeast via direct binding of MAC1 protein to upstream regulatory sequences of FRE1 and CTR1. J Biol Chem. 1997;272:17711–17718. doi: 10.1074/jbc.272.28.17711. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Iwai Y, Stearman R, Dancis A, Klausner RD. Iron-regulated DNA binding by the AFT1 protein controls the iron regulon in yeast. EMBO J. 1996;15:3377–3384. [PMC free article] [PubMed] [Google Scholar]
- Yuan D, Stearman R, Dancis A, Dunn T, Beeler T, Klausner RD. The Menkes/Wilson disease gene homologue in yeast provides copper to a ceruloplasmin-like oxidase required for iron uptake. Proc Natl Acad Sci USA. 1995;92:2632–2636. doi: 10.1073/pnas.92.7.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]