Abstract
Recent in vitro and in vivo studies have shown that the chemokine fractalkine is widely expressed in the brain and localized principally to neurons. Central nervous system expression of CX3CR1, the only known receptor for fractalkine, has been demonstrated exclusively on microglia and astrocytes. Thus, it has been proposed that fractalkine regulates cellular communication between neurons (that produce fractalkine) and microglia (that express its receptor). Here we show, for the first time, that hippocampal neurons also express CX3CR1. Receptor activation by soluble fractalkine induces activation of the protein kinase Akt, a major component of prosurvival signaling pathways, and nuclear translocation of NF-κB, a downstream effector of Akt. Fractalkine protects hippocampal neurons from the neurotoxicity induced by the HIV-1 envelope protein gp120IIIB, an effect blocked by anti-CX3CR1 antibodies. Experiments with two different inhibitors of the phosphatidylinositol 3-kinase, a key enzyme in the activation of Akt, and with a phospholipid activator of Akt demonstrate that Akt activation is responsible for the neuroprotective effects of fractalkine. These data show that neuronal CX3CR1 receptors mediate the neurotrophic effects of fractalkine, suggesting that fractalkine and its receptor are involved in a complex network of both paracrine and autocrine interactions between neurons and glia.
There is increasing evidence that chemokines play important roles in the central nervous system, ranging from brain development to events underlying the neuropathogenesis of AIDS (1–6). Fractalkine is a novel chemokine that can exist in two forms, either as membrane anchored or as a soluble 95,000 glycoprotein (7, 8). Expression of fractalkine in the brain has been found to be widespread and localized principally to neurons (3, 9–12), whereas expression of its G protein-coupled receptor CX3CR1 (13) is thought to be restricted to microglia and astrocytes (11, 12). Nevertheless, in glia-free cultures of hippocampal neurons, fractalkine can regulate intracellular Ca2+ concentration ([Ca2+]i) and synaptic transmission and activate other signaling pathways, such as extracellular receptor-stimulated kinase (ERK1/2) and cAMP response element binding protein (CREB) phosphorylation (3). The chemokine also produces long-term effects on neuronal survival, which include protection from neurotoxicity induced by the HIV-1 coat protein gp120IIIB (3). These data therefore suggest the presence of fractalkine receptors on neurons.
Here we provide direct evidence for the functional expression of CX3CR1 receptors by hippocampal neurons and demonstrate their involvement in the neuroprotective action of fractalkine. Thus, fractalkine can directly modulate neuronal activity and survival. The effect of fractalkine on hippocampal neurons is mediated by activation of the Ser/Thr kinase Akt, an important component of prosurvival and antiapoptotic mechanisms in neurons and other cell types (14–22). Akt kinase activates several intracellular pathways and transcription factors involved in cell survival (23–29) and also inactivates proapoptotic substrates, such as Bcl-X-associated death inducer (BAD) and caspase-9 (30, 31).
Materials and Methods
Neuronal Cultures.
Hippocampal neurons were obtained as described (3, 32). A feeder layer of secondary astrocytes was used to support their growth and differentiation. Neurons were separated from glia immediately before the experiments.
Neuronal Death.
By using different methods for studying apoptosis, we have shown that gp120IIIB induces apoptosis of hippocampal neurons in cultures (3, 32). Because of its ability to indicate early changes in the nuclear morphology of apoptotic cells, staining with Hoechst 33342 (5 μg/ml; Molecular Probes) was used to quantify apoptotic cell death (32). Treatments were started at the 7th day of culture and in the absence of glia (3). Cell survival was evaluated 24–36 h later. Human soluble fractalkine (R & D Systems) and gp120IIIB (>95% pure; Intracel, Issaquah, WA) were prepared and used as described (3). Dipalmitoyl phosphatidylinositol 3,4-bisphosphate (PI3,4-P2) and 4,5-bisphosphate (PI4,5-P2) were purchased from Matreya (Pleasant Gap, PA) and reconstituted in 1 ml of chloroform/methanol/H2O (1:1:0.3). Aliquots were evaporated under a stream of N2 and stored at −20°C. Before experiments, phospholipids were resuspended and sonicated for 1–2 min.
HEK293 Cells Expressing Rat CX3CR1.
Rat brain cDNA was prepared from 5 μg of total RNA by using SuperScript II reverse transcriptase (Life Technologies). CX3CR1 was amplified by PCR from 6 μl of rat brain cDNA (forward primer: 5′-GCCGCCACCATGCCTACCTCCTTCCCGGAATTGGATC-3′; reverse primer: 5′-GTAGAGTCGGGGTCGGGGAGACCCTTCA-3′). The PCR products were gel purified and cloned into the pGEM-T-Easy vector (Promega) and were sequenced by using automated DNA sequencing and subcloned into pcDNA3.1 (Invitrogen). The resulting clones were verified by restriction analysis and DNA sequencing. HEK293 cells were grown in DMEM containing 10% FBS and 1% penicillin/streptomycin, and transfected in serum-free medium as follows. A total of 10 μg of plasmid DNA was combined with sterile 150 mM NaCl and 20 nmol of polyethyleneimine (25 kDa, Aldrich), and added to the cells. Cells were incubated at 37°C in a 5% CO2-humidified incubator and the medium was replaced with regular culture medium after 1 h. G418 sulfate was used for selection (1,000 mg/liter) and maintenance (500 mg/liter) of positive clones. Colonies were screened for receptor expression by Fura-1 microfluorimetry and Northern blot analysis (data not shown).
Western Blot Analysis.
After removing the glial feeder layer, neurons were washed with balanced salt solution as described (3). Neurons were treated with gp120IIIB and/or fractalkine and the other substances in the absence of glia, unless otherwise specified. An appropriate control (i.e., neurons kept in the absence of glia for the same amount of time of treated neurons) was used for each experiment. Cells were scraped in lysis buffer [25 mM Tris/150 mM NaCl/5 mM NaF/1 mM EDTA/1 mM DTT/1% Nonidet P-40/5 μg/ml each aprotinin, leupeptin, and pepstatin/1 mM 4-(2-aminoethyl)benzeresulfonyl fluoride·HCE (AEBSF)/1 mM vanadate]. Nuclear protein extracts were obtained as described (3). The antibodies to phosphorylated Akt (P-Akt) (Ser-473; 1:4,000), P-IκB-α (Ser-32; 1:1,000), total Akt (1:8,000), and total IκB-α (1:1,000) were obtained from New England Biolabs. The antibody for NF-κB/p65 (1:1,000) was purchased from Santa Cruz Biotechnology and anti-P-ERK1/2 (1:5,000) was obtained from Promega. A purified rabbit anti-rat CX3CR1 antibody (Torrey Pines Biolab, La Jolla, CA) was used for Western blot (0.2 μg/ml), immunostaining, and neutralization experiments (2 μg/ml). When indicated, preadsorption of the anti-CX3CR1 was performed by incubating the antibody (0.2 μg/ml) with the antigen (2 μg/ml) used to rise the antibody itself for 2 h at 4°C in blocker solution. Digitized images of films obtained by enhanced chemiluminescence (SuperSignal West Femto, Pierce) were used for densitometric analysis by using un-scan it software (Silk Scientific, Orem, UT).
Immunofluorescence and Confocal Microscopy.
Cells were fixed in 4% paraformaldehyde, or when also staining for neurofilament, in cold acetone. The anti-CX3CR1 antibody (2 μg/ml) was followed by a biotinylated secondary antibody and streptavidin conjugated to Cy3 or FITC. The mouse antibody against neurofilaments (Chemicon, 1:40) was followed by an FITC-conjugated secondary antibody. Nuclear staining was obtained by using Hoechst 33342 (3 μg/ml). Microphotographs were obtained by using a Leitz Diaplan fluorescence microscope with a ×25 0.60 numerical aperture objective. Cells were imaged by confocal scanning laser microscopy with the Fluoview Confocal System (Olympus, Melville, NY) on an Olympus IX70 inverted microscope with a ×40 oil-immersion objective (Olympus, 1.0 numerical aperture). Digital images were collected by using a step size of 0.3 μm along the z axis at a resolution of 640 × 480 (8 bits per pixel). The three-dimensional renderings were created from z series of 35–45 images.
Fura-2 Microfluorimetry.
Cells were grown on glass cover slips coated with poly-l-lysine (100 μg/ml). Cell loading with 2 μM fura-2/AM, single-cell videoimaging, and calibration of fluorescent signals were performed as described (3).
Statistical Analysis.
One-way ANOVA followed by the Newman-Keuls multiple comparison procedure was used for the statistical analysis of the survival experiments. Paired t tests were used to compare differences in the band densities of immunoblots. Data are reported as means ± SEM with sample sizes for each experiment.
Results
Expression of CX3CR1 on Neurons.
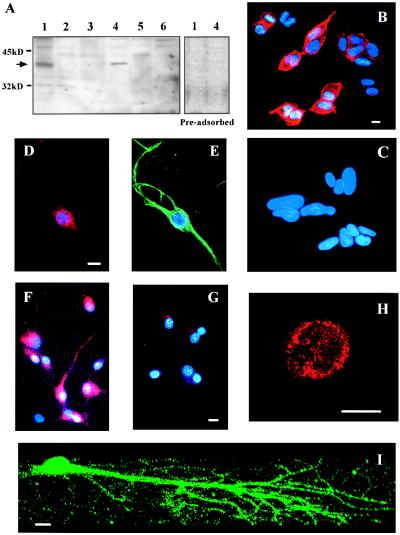
Fig. 1A demonstrates the expression of CX3CR1 receptors by hippocampal neurons (lane 4) and HEK293 cells expressing rat CX3CR1 (lane 1). No bands of the appropriate size were observed in negative controls, including nontransfected HEK293 cells (lane 2) and other cell types of human or rat origin (see figure legend). Similarly, only transfected HEK293 cells and hippocampal neurons showed positive immunostaining for CX3CR1 (Fig. 1 B and D–F, respectively). Preadsorption of the antibody with its antigen blocked immunodetection by Western blot and immunostaining in neurons and HEK293 cells expressing the chemokine receptor (Fig. 1 A and G). The plasma membrane localization of CX3CR1 in hippocampal neurons was demonstrated by confocal microscopy (Fig. 1H). A three-dimensional reconstruction indicated that the receptor was distributed both on the neuronal soma and neurites (Fig. 1I).
Figure 1.
Expression of CX3CR1 on hippocampal neurons. (A Left) Protein extracts (25 μg per lane) from HEK293 cells (lane 1, stably transfected with CX3CR1 and lane 2, wild type); PC12 cells (lane 3); rat hippocampal neurons (lane 4); Ceinge clone 3 oligodendroglial cells (lane 5); and SY5Y neuroblastoma cells (lane 6) were immunoblotted with anti-rat CX3CR1. (A Right) The immunoblotting obtained after preadsorption of the antibody with its antigen. (B–G) Fluorescence microscopy images of stably transfected (B) and wild type (C) HEK293 cells and hippocampal neurons (D, F, and G) immunostained for CX3CR1. Hoechst 33342 (2 μg/ml) was used to stain nuclei of all cells and an antibody against neurofilament protein was used to identify neurons, as shown in E. Preadsorbed anti-CX3CR1 was used in G. (H and I) Confocal microscopy images of two neurons positive for CX3CR1 show the plasma membrane localization of the staining (H) and the distribution of the receptor along neuronal soma and dendritic arbors (I). (Scale bars = 10 μm.)
These findings correlate with previous in vivo studies reporting a relative abundance of neuronal fractalkine expression in the hippocampus and cortex within the adult rat brain (11), and a widespread distribution of glial CX3CR1 (10–12).
Coupling of CX3CR1 to Antiapoptotic Pathways.
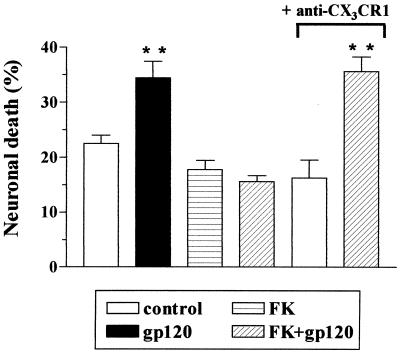
We have previously demonstrated that fractalkine can act as a survival factor for hippocampal neurons and can inhibit neuronal apoptosis induced by the HIV-1 coat protein gp120IIIB (3). We investigated the role of CX3CR1 in this process. As shown in Fig. 2, the ability of fractalkine to prevent gp120-induced neuronal death was blocked by a neutralizing antibody to CX3CR1, whereas fractalkine still prevented gp120IIIB neurotoxicity in the presence of an antibody that did not interact with CX3CR1. When a polyclonal antibody against the chemokine receptor CCR5 was used, for example, neuronal survival was 17 ± 1% in control cells (n = 545), 39 ± 0.8% in gp120-treated cells (n = 369), and 18 ± 2.7% in cells treated with fractalkine + gpIIIB+anti-CCR5 (n = 600). This indicates that the binding of fractalkine to neuronal CX3CR1 activates an antiapoptotic pathway that is able to counteract gp120IIIB neurotoxicity.
Figure 2.
Involvement of CX3CR1 in the neuroprotective effect of fractalkine (FK). Treatment of neurons with a neutralizing anti-rat CX3CR1 antibody (2 μg/ml) prevented fractalkine (100 nM) inhibition of the neurotoxicity induced by gp120IIIB (200 pM). **, P < 0.01 vs. control (500–600 cells per treatment).
Akt kinase has been found to be an important intermediate in the control of apoptosis in many types of cells. Maximal activation of Akt in vitro requires the phosphorylation of Ser-473 as well as Thr-308. However, mutagenesis studies have shown that phosphorylation of Ser-473 alone is able to induce a sevenfold activation of the kinase (33). We therefore studied the effect of fractalkine on Akt activation by measuring the levels of P-Akt in protein extracts from pure hippocampal neurons.
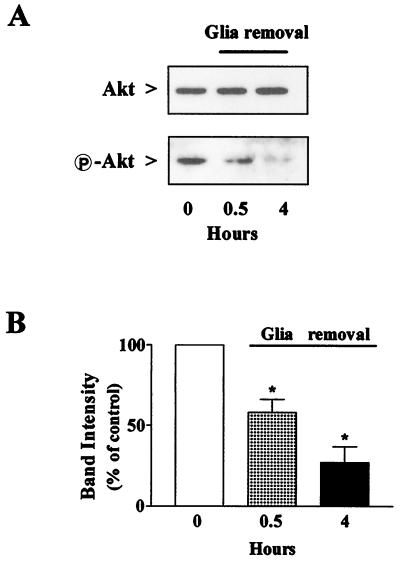
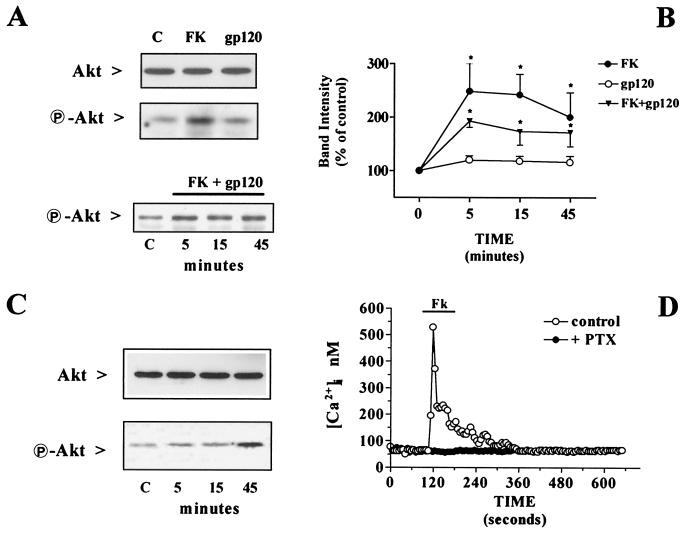
Neurons were treated with fractalkine in the complete absence of their glial feeder layer, which was removed from the cultures 4 h before treatment with the chemokine. Interestingly, when neurons were deprived of glia, a marked reduction of “basal” Akt phosphorylation occurred, whereas total Akt levels did not change (Fig. 3A). The reduction in Akt activation was apparent after only 30 min of glial withdrawal and reached about 25% of control levels after 4 h (Fig. 3B). The addition of fractalkine under the latter experimental conditions produced a significant increase in P-Akt (Fig. 4 A and B). The effect of fractalkine on Akt was not blocked by gp120IIIB (Fig. 4A Lower and B) and the HIV-1 protein alone had no effect on phosphorylation of the kinase (Fig. 4 A and B). Activation of Akt by fractalkine was also observed in HEK293 cells expressing rat CX3CR1 in the absence of other chemokine receptors (Fig. 4C). In these cells, fractalkine also induced a Ca2+ mobilization response (Fig. 4D), similar to that previously observed in hippocampal neurons (3).
Figure 3.
Reduction of Akt phosphorylation in hippocampal neurons after removal of the glial feeder layer. (A) Duplicate samples (40 μg per lane) were immunoblotted with an antibody to total Akt or P-Akt. (B) Densitometric analysis of three different immunoblots revealed >70% reduction of activated Akt after 4 h of glial withdrawal (*, P < 0.05).
Figure 4.
Effect of fractalkine (FK) and gp120IIIB on Akt phosphorylation and neuronal survival. (A) Protein extracts (40 μg per lane) were obtained from neurons treated 15 min with 100 nM fractalkine or 200 pM gp120IIIB (or with fractalkine + gp120 for the indicated time) 4 h after glial deprivation. Immunoblots were conducted with antibodies to total Akt or P-Akt. (B) Densitometric analysis of the immunoblots treated with fractalkine and/or gp120IIIB for 5–45 min showing the increase in P-Akt induced by fractalkine (*, P < 0.05 vs. control, n = 3). (C) Activation of Akt in HEK293 cells expressing CX3CR1 and treated with fractalkine (100 nM) for the indicated time (30 μg per lane). Densitometric analysis of P-Akt bands from two separate experiments showed a twofold increase in the band density after 5- and 15-min treatments with fractalkine, and of more than 400% after 45 min. (D) Calcium responses to fractalkine (100 nM) in CX3CR1-expressing HEK293 cells (n = 87). No response was observed after pertussis toxin (PTX) treatment (200 nM for 16–18 h, n = 56). Representative traces from single cells are shown.
Role of Akt in the Neuroprotective Action of Fractalkine.
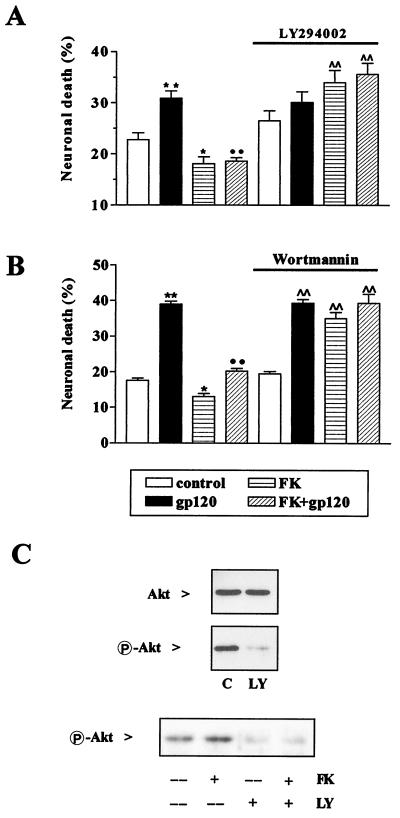
We investigated the involvement of Akt in the neuroprotective action of fractalkine by using a pharmacological approach. LY294002 is a specific inhibitor of phosphatidylinositol 3-kinase (PI 3-kinase) (34), an enzyme that plays an essential role in the activation pathway for Akt (33, 35). We found that relatively short treatments with LY294002 (24–32 h, 50 μM) did not significantly affect hippocampal neuronal survival (Fig. 5A), consistent with previous reports (36), whereas longer treatments (48 h or more) were neurotoxic (data not shown). We tested the effect of LY294002 on neuronal Akt activation and found that LY294002 almost completely abolished basal Akt activation under normal growth conditions (Fig. 5C Upper). Similarly, LY294002 inhibited Akt activation in neurons treated with fractalkine when added 4 h after removal of the glial feeder layer (Fig. 5C Lower).
Figure 5.
Inhibition of PI 3-kinase blocked Akt phosphorylation and neuronal survival. Fractalkine did not prevent gp120-induced neurotoxicity in hippocampal neurons treated with 50 μM LY294002 (A) or 100 nM wortmannin (B). Data are from four different experiments. (A) *, P < 0.05 and **, P < 0.01 vs. control; ••, P < 0.01 vs. gp120; and ∧∧, P < 0.01 vs. LY; (B) *, P < 0.05 and **, P < 0.01 vs. control; ••, P < 0.01 vs. gp120; and ∧∧, P < 0.01 vs. wortmannin. The number of cells analyzed for each data point range from 2,650 to 3,800 in the LY294002 experiments, and 400 to 749 in the wortmannin experiments. (C Upper) Immunodetection of Akt and P-Akt in protein extracts (40 μg per lane) from control neurons and neurons treated with LY294002 for 15 min, immediately after glial removal (the band intensity of P-Akt was reduced to 19 ± 8% by LY294002, n = 3). (C Lower) Neurons were deprived of glia 4 h before treatments with LY294002 ± fractalkine (the band intensity was reduced to 35 ± 8% of control in LY294002 and 32 ± 7% in LY294002 + fractalkine, n = 3, *, P < 0.05).
Next, we evaluated the ability of fractalkine to promote cell survival in neurons treated with LY294002 for 24–27 h. Neurons were separated from glia when treatments with fractalkine and/or gp120IIIB were initiated, both in the absence and presence of LY294002 (Fig. 5A). Under these conditions, neuronal death caused by deprivation of glial trophic factors alone averaged around 20% and additional gp120IIIB neurotoxicity was completely inhibited by fractalkine in the absence of LY294002 (Fig. 5A). In neurons treated with LY294002, the cell death induced by gp120IIIB was comparable to that observed in the absence of the PI 3-kinase inhibitor. Fractalkine was unable to rescue neurons in LY294002-treated cultures (Fig. 5A) and actually potentiated the toxic effect of LY294002 (Fig. 5A). Similar results were obtained with wortmannin, another inhibitor of PI 3-kinase structurally unrelated to LY294002 (Fig. 5B).
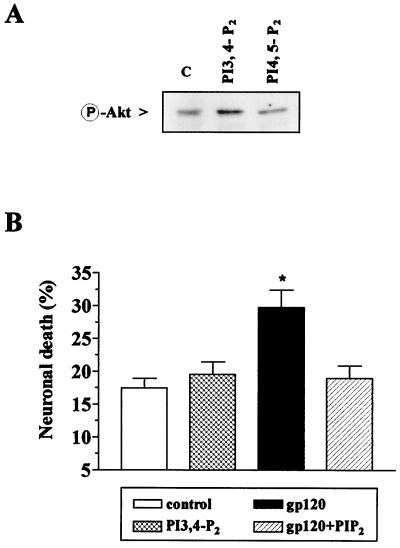
We also tested whether gp120-induced neurotoxicity could be blocked by a phospholipid activator of Akt. PI3,4-P2 is known to induce the activation of Akt in a variety of cell types (33, 35). We therefore tested the effect of PI3,4-P2 and its inactive analogue PI4,5-P2 (35) on Akt activation in hippocampal neurons. Activation of Akt was induced only by PI3,4-P2 (Fig. 6A). Consistent with the activation of Akt by fractalkine, neurons treated with PI3,4-P2 were not killed by gp120IIIB (Fig. 6B).
Figure 6.
Effect of PI3,4-P2 and PI4,5-P2 (5 μM) on Akt phosphorylation and neuronal survival. (A) Protein extracts (40 μg per lane) from neurons treated with PI3,4-P2 or PI4,5-P2 for 15 min 4 h after glia deprivation were immunoblotted for P-Akt. Densitometric analysis of the bands showed a statistically significant increase of 86 ± 3% in the presence of PI3,4-P2 (n = 3, P < 0.05). (B) In survival experiments, PI3,4-P2 ± gp120IIIB was added to the 7th day of culture in the absence of glia. Data are from two different experiments; ≈1,500 cells were analyzed for each treatment (**P < 0.01).
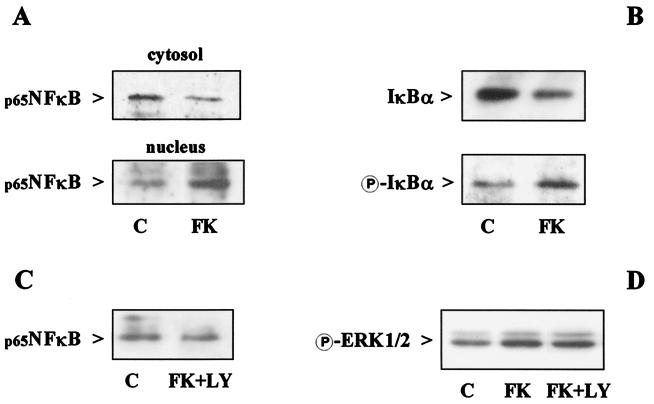
Akt exerts its antiapoptotic action by regulating the activity of several downstream effectors, including transcription factors such as CREB and NF-κB (25–28). NF-κB activity and nuclear translocation are prevented by inhibitory IκB proteins, whose inactivation/degradation are regulated by their phosphorylation. Thus, a reduction in the cytosolic levels of IκB and an increase in its phosphorylated form are generally induced by treatments that cause NF-κB activation. Interestingly, treatment of neurons with fractalkine induced an increase in the nuclear translocation of the p65 subunit of NF-κB (Fig. 7A), associated with a reduction in the cytoplasmic level of its inhibitory protein IκBα and a corresponding augmentation of phosphorylated IκBα (Fig. 7B). NF-κB activation was not observed in the presence of LY294002 (Fig. 7C). LY294002 did not affect other pathways stimulated by fractalkine, such as the activation of ERK1/2 (Fig. 7D). These findings indicate that NF-κB activation by fractalkine is mediated by Akt. However, whether NF-κB is involved in the neuroprotective actions of fractalkine still remains to be determined.
Figure 7.
Effect of fractalkine on Akt downstream pathways. Neurons were treated with 100 nM fractalkine (15 min) in the absence of glia. Nuclear translocation of NF-κB, degradation and phosphorylation of IκBα, and ERK1/2 phosphorylation were evaluated by Western blot analysis of cytoplasmic (40 μg per lane) and/or nuclear (10 μg per lane) protein extracts. (A) Effect of fractalkine on NF-κB cytosolic and nuclear content. Analysis of band density showed a 36 ± 8% reduction in cytosolic NF-κB (P < 0.05, n = 3) and a 73 ± 24% increase in nuclear NF-κB (P < 0.05, n = 4). (B) Effect of fractalkine on IκBα degradation and phosphorylation. Cytosolic IκBα was reduced by 32 ± 7% as determined by densitometric analysis (P < 0.05, n = 4). A 94 ± 3% increase in phosphorylated IκBα was observed (n = 2). (C) LY294002 (50 μM) inhibited the effect of fractalkine (100 nM, 15 min) on NF-κB nuclear translocation by 40 ± 2% (n = 2), but did not significantly affect ERK1/2 phosphorylation (D).
Discussion
The data presented here provide strong evidence that fractalkine can act directly on neurons and activate neuronal survival pathways. Previous studies on fractalkine and its receptor in the central nervous system have suggested a paracrine interaction between neurons that produce fractalkine, and microglia that express CX3CR1 receptors (11). However, the expression of CX3CR1 by hippocampal neurons indicates that these receptors may also be a target for fractalkine produced by microglia and/or astroglia (12) or adjacent neurons. In addition, our findings support the hypothesis of an autocrine interaction as suggested by Pan et al. (8) in the immune system.
In this study, we have shown that the ability of fractalkine to inhibit gp120IIIB-induced neurotoxicity is mediated by the interaction of fractalkine with neuronal CX3CR1 and the subsequent activation of Akt kinase. Activation of Akt kinase by G protein-coupled receptors generally involves a PI 3-kinase-dependent pathway (31, 37, 38). In our experiments, LY294002 completely inhibited fractalkine-induced Akt activation and neuroprotection, suggesting that these effects of fractalkine require PI 3-kinase activation. Surprisingly, fractalkine seemed to potentiate the toxic effect of LY294002. This might be because activation of other pathways by fractalkine (such as activation of ERK1/2 and mobilization of [Ca2+]i) in the absence of Akt activation can result in a death signal, a hypothesis that could also explain the neurotoxicity induced by gp120IIIB, which is unable to activate Akt in hippocampal neurons, although it increases [Ca2+]i (3).
Because gp120IIIB does not affect Akt, the intracellular site of interaction between fractalkine and gp120IIIB seems to be downstream of Akt activation (i.e., gp120 could affect one or more antiapoptotic substrates of Akt). Alternatively, gp120IIIB and fractalkine may activate separate and parallel pathways with opposing results on neuronal survival.
The fact that fractalkine induces NF-κB translocation and that this effect is inhibited by LY294002 suggests a role for this transcription factor in the survival-promoting effect of fractalkine as a downstream effector of Akt. Indeed, a direct correlation between Akt and NF-κB activation has recently been reported (27, 28). NF-κB promotes expression of antiapoptotic genes (39) and increases survival of hippocampal neurons (40). However, additional experiments are needed to clarify the role of NF-κB in the neuroprotective action of fractalkine in general, and in its ability to prevent gp120IIIB neurotoxicity in particular. Although preliminary experiments indicate that gp120IIIB may reduce NF-κB activation in certain circumstances, downstream interactions between chemokines and gp120IIIB could include a variety of additional factors regulated by Akt that are involved in cell survival, such as caspase 9 and/or BAD.
The involvement of fractalkine in the regulation of fundamental neuronal activities, such as synaptic transmission and neuronal survival (3), illustrates some of the potential roles for chemokines in the nervous system. The ability of fractalkine to counteract gp120-induced neurotoxicity, independently of competition at the receptor level, may lead to new insights into the molecular mechanisms of action of gp120 in the brain. However, this also depends on whether two independent and parallel pathways are activated by gp120 and fractalkine. In any case, a complete characterization of the neuronal antiapoptotic pathways activated by chemokines in the central nervous system will facilitate the search for novel therapeutic agents for combating AIDS-associated dementia, as well as other neurodegenerative diseases.
Acknowledgments
We thank Chris P. Mauer for his valuable technical assistance. This work was supported by National Institutes of Health Grants MC40165, DA02121, NS33826, DK44840, and NS21442. A.A.S. was supported by Grant HD07009.
Abbreviations
- P-Akt
phosphorylated Akt
- ERK
extracellular receptor-stimulated kinase
- PI3,4-P2 and PI4,5-P2
dipalmitoyl phosphatidylinositol 3,4-bisphosphate and 4,5-bisphosphate
- PI 3-kinase
phosphatidylinositol 3-kinase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.090017497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.090017497
References
- 1.Zhou Y-R, Kottman A H, Kuroda M, Taniuchi I, Littman D R. Nature (London) 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 2.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, et al. Nature (London) 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 3.Meucci O, Fatatis A, Simen A A, Bushell T J, Gray P W, Miller R J. Proc Natl Acad Sci USA. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaul M, Lipton S A. Proc Natl Acad Sci USA. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein R S, Williams K C, Alvarez-Hernandez X, Westmoreland S, Force T, Lackner A A, Luster A D. J Immunol. 1999;163:1636–1646. [PubMed] [Google Scholar]
- 6.Miller R J, Meucci O. Trends Neurosci. 1999;22:471–479. doi: 10.1016/s0166-2236(99)01408-3. [DOI] [PubMed] [Google Scholar]
- 7.Bazan J F, Bacon K B, Hardiman G, Wang W, Soo K, Rossi D, Greaves D R, Zlotnik A, Schall T J. Nature (London) 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 8.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo J A, Vath J, Gosselin M, Ma J, Dussault B, et al. Nature (London) 1997;387:611–616. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 9.Raport C J, Schweickart V L, Eddy R L, Jr, Shows T B, Gray P W. Gene. 1995;163:295–299. doi: 10.1016/0378-1119(95)00336-5. [DOI] [PubMed] [Google Scholar]
- 10.Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, Kume T, Akaike A, Satoh M. FEBS Lett. 1998;429:167–172. doi: 10.1016/s0014-5793(98)00583-3. [DOI] [PubMed] [Google Scholar]
- 11.Harrison J K, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara R K, Streit W J, Salafranca M N, Adhikari S, Thompson D A, et al. Proc Natl Acad Sci USA. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maciejewski-Lenoir D, Chen S, Feng L, Maki R, Bacon K B. J Immunol. 1999;163:1628–1635. [PubMed] [Google Scholar]
- 13.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall T J, Yoshie O. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 14.Bellacosa A, Testa J R, Staal S P, Tsichlis P N. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 15.Coffer P J, Jin J, Woodgett J R. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 18.Kulik G, Klipper A, Weber M J. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andjelkovic M, Suidan H S, Meier R, Frech M, Alessi D R, Hemmings B A. Eur J Biochem. 1998;251:195–200. doi: 10.1046/j.1432-1327.1998.2510195.x. [DOI] [PubMed] [Google Scholar]
- 20.Blair L A C, Bence-Hanulec K K, Mehta S, Franke T, Kaplan D, Marshall J. J Neurosci. 1999;19:1940–1951. doi: 10.1523/JNEUROSCI.19-06-01940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tilton B, Andjelkovic M, Didichenko S A, Hemmings B A, Thelen M. J Biol Chem. 1997;272:28096–28101. doi: 10.1074/jbc.272.44.28096. [DOI] [PubMed] [Google Scholar]
- 22.Murga C, Laguinge L, Wetzker R, Cuadrado A, Gutkind J S. J Biol Chem. 1998;273:19080–19085. doi: 10.1074/jbc.273.30.19080. [DOI] [PubMed] [Google Scholar]
- 23.Meier R, Alessi D R, Cron P, Andjelkovic M, Hemmings B A. J Biol Chem. 1997;272:30491–30497. doi: 10.1074/jbc.272.48.30491. [DOI] [PubMed] [Google Scholar]
- 24.Berra E, Diaz-Meco M, Mosca J. J Biol Chem. 1998;273:10792–10797. doi: 10.1074/jbc.273.17.10792. [DOI] [PubMed] [Google Scholar]
- 25.Du K, Montminy M. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 26.Kane L P, Smith Shapiro V, Stokoe D, Weiss A. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 27.Nidai Ozes O, Mayo L D, Gustin J A, Pfeffer S R, Pfeffer L M, Donner D B. Nature (London) 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 28.Romashkova J A, Makarov S S. Nature (London) 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 29.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 30.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 31.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Science. 1998;282:1318–1320. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 32.Meucci O, Miller R J. J Neurosci. 1996;16:4080–4088. doi: 10.1523/JNEUROSCI.16-13-04080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 34.Vlahos C J, Matter W F, Hui K Y, Brown R F. J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 35.Franke T F, Kaplan D R, Cantley L C, Tolker A. Science. 1997;275:665–667. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 36.Crowder R J, Freeman R S. J Neurosci. 1998;18:2933–2943. doi: 10.1523/JNEUROSCI.18-08-02933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner S J, Domin J, Waterfield M D, Ward S G, Westwick J. J Biol Chem. 1998;273:25987–25995. doi: 10.1074/jbc.273.40.25987. [DOI] [PubMed] [Google Scholar]
- 38.Ganju R K, Brubaker S A, Meyer J, Dutt P, Yang Y, Qin S, Newman W, Groopman J E. J Biol Chem. 1998;273:23169–23175. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- 39.Wang C-Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S. Nature (London) 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 40.Barger S W, Horster D, Furukawa K, Goodman Y, Krieglstein J, Mattson M. Proc Natl Acad Sci USA. 1995;92:9328–9332. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]