Abstract
Rice (Oryza sativa) accumulates silicon (Si) in the tops to levels up to 10.0% of shoot dry weight, but the mechanism responsible for high Si uptake by rice roots is not understood. We isolated a rice mutant (GR1) that is defective in active Si uptake by screening M2 seeds (64,000) of rice cv Oochikara that were treated with 10−3 m sodium azide for 6 h at 25oC. There were no phenotypic differences between wild type (WT) and GR1 except that the leaf blade of GR1 remained droopy when Si was supplied. Uptake experiments showed that Si uptake by GR1 was significantly lower than that by WT at both low and high Si concentrations. However, there was no difference in the uptake of other nutrients such as phosphorus and potassium. Si concentration in the xylem sap of WT was 33-fold that of the external solution, but that of GR1 was 3-fold higher than the external solution at 0.15 mm Si. Si uptake by WT was inhibited by metabolic inhibitors including NaCN and 2,4-dinitrophenol and by low temperature, whereas Si uptake by GR1 was not inhibited by these agents. These results suggest that an active transport system for Si uptake is disrupted in GR1. Analysis of F2 populations between GR1 and WT showed that roots with high Si uptake and roots with low Si uptake segregated at a 3:1 ratio, suggesting that GR1 is a recessive mutant of Si uptake.
Silicon (Si) is the second most abundant element, both in terms of weight and number of atoms, in the earth's crust. Si compounds (silicon dioxide or silicates) occupy more than 60% of soil and the concentration of Si in soil solution in the form of silicic acid is between 3.5 and 40 mg Si L−1 (Marschner, 1995). Therefore, all plants rooting in soil contain Si in their tissue. However, the Si content of plant tops greatly varies with species, ranging from 0.1% to 10.0% in dry weight. Based on the Si content of the plant tops and the Si to Ca ratio, plants are classified into Si accumulator, intermediate type, and Si excluder species (for review, see Takahashi et al., 1990). The difference in Si content has been ascribed to the ability of the roots to take up Si. Three uptake modes of Si uptake, active, passive, and rejective uptake, have been suggested for Si accumulator, intermediate type, and Si excluder plants, respectively (Takahashi et al., 1990).
Rice (Oryza sativa) is the most effective Si-accumulating plant known, and accumulates Si to levels up to 10.0% of shoot dry weight. Si deposited in the tissues helps to alleviate water stress by decreasing transpiration, improves light interception characteristics by keeping the leaf blade erect, increases resistance to diseases and pests and lodging, remediates nutrient imbalances, and there are other documented beneficial effects (Epstein, 1994, 1999; Savant et al., 1997; Ma et al., 2001b). Therefore, high Si content of the shoot is considered to be important for the healthy growth of rice and for stability of rice production. Numerous studies have shown that high accumulation of Si in the rice shoot is attributed to the high ability of the roots to take up Si. For example, Okuda and Takahashi (1962a) found that Si uptake by excised roots was much higher than that by excised shoots. Si is taken up in the form of silicic acid, an undissociated molecule (Takahashi and Hino, 1978). Evidence has shown that the uptake of silicic acid is an energy-dependent, active process. Rice roots take up proportionately more Si than water from the culture solution (Okuda and Takahashi, 1962a). Si uptake is not affected by transpiration (Okuda and Takahashi, 1962a), but is inhibited by metabolic inhibitors such as NaCN, 2,4-dichlorophenoxy acetate, and 2,4-dinitrophenol (2,4-DNP; Okuda and Takahashi, 1962b). Si concentration in the rice xylem sap is several hundred-fold higher than that in the external solution after a short exposure time (Okuda and Takahashi, 1962a), indicating that silicic acid is transported across a plasma membrane in the roots against a concentration gradient. All these observations suggest that uptake of Si is mediated through a specific transporter for silicic acid in the rice roots. However, neither genes encoding this transporter nor the transporter protein itself have been isolated. In the present study, we isolated the first known rice mutant that is defective in active Si uptake.
RESULTS
Germanium (Ge) is toxic to plant growth by showing symptoms of brown spots on the leaves, but plant roots cannot discriminate Ge from Si in terms of uptake (Takahashi et al., 1976b, 1976c). This property of Ge helped us to isolate rice mutants defective in Si uptake by using Ge resistance as a selection parameter. In the first screening, 216 mutant candidates (with less or without brown spots on the leaves) were selected from 64,000 M2 seeds. Among these, 117 lines were able to produce M3 seeds. As a result of the second screening using M3 seeds, six lines showed differential resistance to Ge. One of the mutants, GR1 (Ge resistant), showed the most resistance to Ge. In the following studies, only GR1 was characterized in terms of Si uptake.
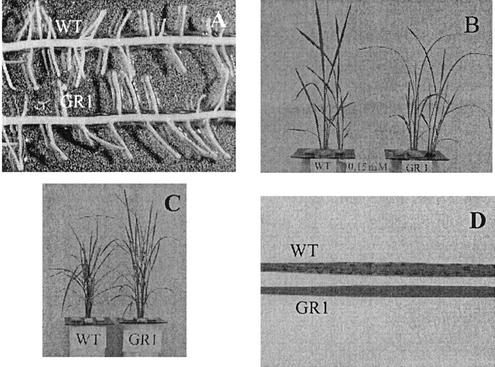
There was no difference in the morphology of the roots between wild-type (WT) and GR1 rice (Fig. 1A). The phenotype of the shoots was also similar except that the leaf blade of GR1 remained droopy when Si was supplied (Fig. 1B).
Figure 1.
Phenotype of WT rice cv Oochikara and a mutant (GR1) defective in Si uptake. A, Roots; B, two lines were cultivated in a nutrient solution containing 0.15 mm Si for 3 weeks; C, two lines were exposed to 20 μm GeO2 for 12 d; D, typical symptoms of Ge toxicity on the leaf.
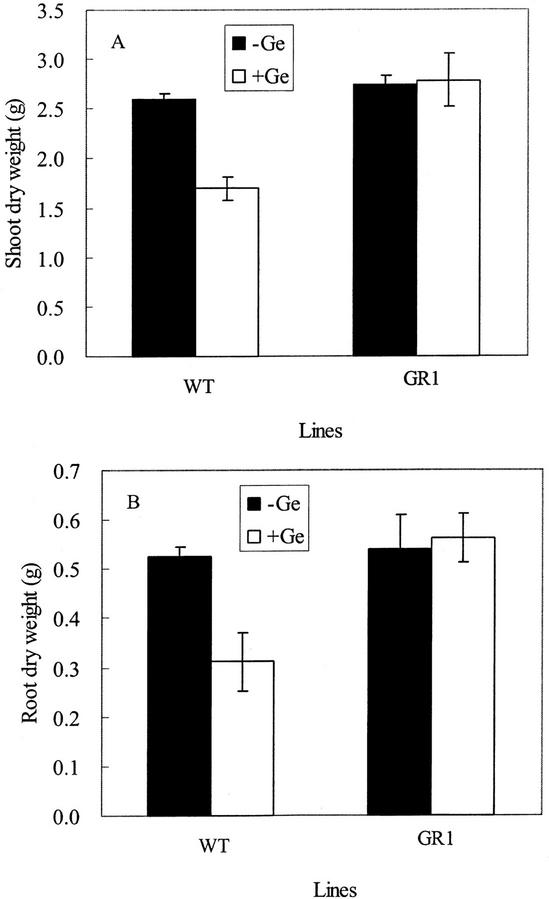
Exposure to Ge caused a 40% reduction in growth of both the roots and the shoots in WT rice, but hardly affected the growth of GR1 (Figs. 1C and 2). Brown spots were observed on the leaf blades on d 3 after Ge treatment in WT, but not in GR1 (Fig. 1, C and D).
Figure 2.
Effect of Ge on the growth of shoot (A) and root (B) of WT rice cv Oochikara and a mutant (GR1) defective in Si uptake. Two lines (26 d old) were exposed to a nutrient solution containing 0 or 20 μm Ge as GeO2 for 12 d. Error bars represent ± sd (n = 3).
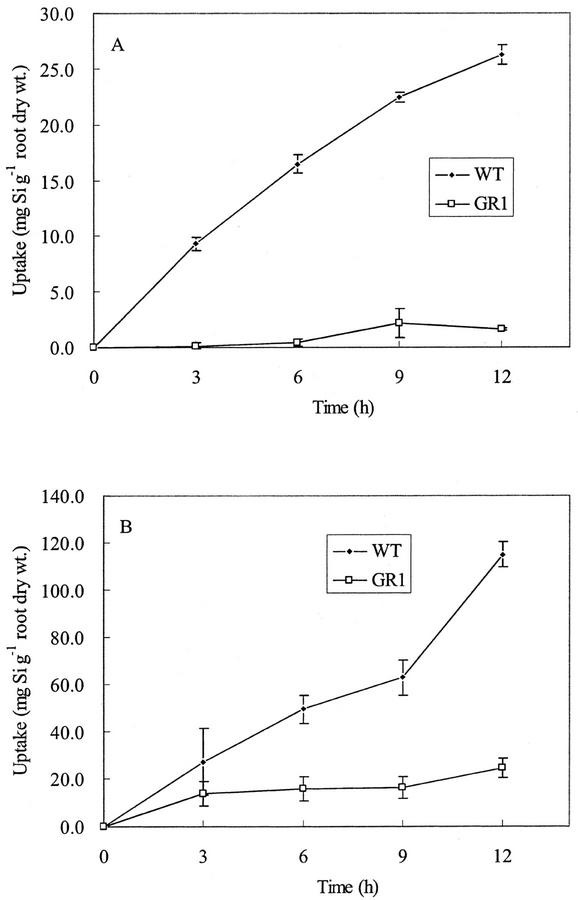
Short-term uptake experiments showed that Si accumulation in WT rice increased linearly with time at both low (0.15 mm) and high (1.5 mm) Si levels (Fig. 3). However, Si uptake by GR1 was much lower than that of WT at either Si concentration. After 12 h, Si taken up by GR1 was 11% and 8% of that taken up by WT at 0.15 and 1.5 mm Si, respectively. Uptake by WT resulted in a rapid and significant decrease in the Si concentration in the external solution, whereas that by GR1 did not change the Si concentration (data not shown). No significant difference was observed in the transpiration rate between WT and GR1 when Si was not supplied.
Figure 3.
Uptake of Si by WT rice cv Oochikara and a mutant (GR1) defective in Si uptake. Twenty-day-old seedlings were placed in a nutrient solution containing 0.15 (A) and 1.5 (B) mm Si as silicic acid. Error bars represent ± sd (n = 3).
In a relatively long-term uptake experiment in solution culture, the Si content of WT shoots was 4.3- and 3.2-fold higher than that of GR1 when Si was supplied at low and high concentration, respectively (Table I). The Si content of the roots was much lower than that of the shoot and there was no difference in the Si content of the roots between WT and GR1. No differences were detected in the content of P and K of both the roots and the shoots between the two lines.
Table I.
Contents of P, K, and Si in the shoot and root of WT rice cv Oochikara and a mutant (GR1) defective in Si uptake
| Mineral Content | Shoot
|
Root
|
||
|---|---|---|---|---|
| 0.15 mm Si | 1.5 mm Si | 0.15 mm Si | 1.5 mm Si | |
| % | ||||
| Phosphorus (P) | ||||
| WT | 0.57 ± 0.01 | 0.54 ± 0.03 | 0.23 ± 0.04 | 0.21 ± 0.05 |
| GR1 | 0.51 ± 0.03 | 0.57 ± 0.04 | 0.20 ± 0.02 | 0.25 ± 0.01 |
| Potassium (K) | ||||
| WT | 3.08 ± 0.34 | 3.18 ± 0.14 | 1.02 ± 0.17 | 1.14 ± 0.31 |
| GR1 | 3.12 ± 0.08 | 3.23 ± 0.16 | 1.01 ± 0.12 | 1.17 ± 0.06 |
| Si | ||||
| WT | 1.46 ± 0.04 | 4.62 ± 0.08 | 0.03 ± 0.01 | 0.12 ± 0.05 |
| GR1 | 0.26 ± 0.06 | 1.43 ± 0.05 | 0.04 ± 0.00 | 0.08 ± 0.04 |
The two lines were grown in a nutrient solution containing 0.15 or 1.5 mm Si as silicic acid for 4 weeks. Values are means ± sd of three replicates.
Soil culture also revealed that the Si content of the shoot of GR1 was lower than that of WT in a soil amended with Si or without added Si (Table II). However, as with solution culture, there were no differences in the content of P and K between WT and GR1.
Table II.
Contents of P, K, and Si in the shoot of WT rice cv Oochikara and a mutant (GR1) defective in Si uptake
| Mineral | Shoot Content
|
|||
|---|---|---|---|---|
| Without Si application
|
With Si application
|
|||
| WT | GR1 | WT | GR1 | |
| % | ||||
| P | 0.44 ± 0.01 | 0.51 ± 0.09 | 0.48 ± 0.04 | 0.53 ± 0.03 |
| K | 4.00 ± 0.43 | 3.96 ± 0.17 | 3.43 ± 0.07 | 3.60 ± 0.41 |
| Si | 1.97 ± 0.11 | 0.92 ± 0.05 | 4.39 ± 0.44 | 1.49 ± 0.19 |
The two lines were grown in a soil amended with Si (2 g Na2SO3 kg−1 soil) or without added Si for 1 month. Values are means ± sd of three replicates.
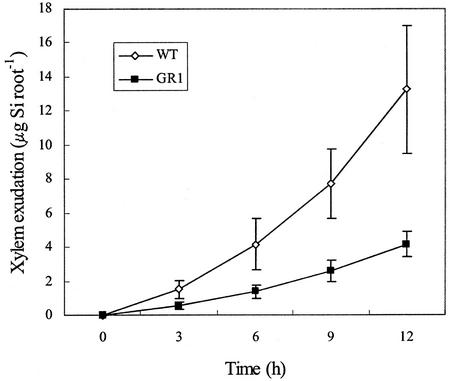
The Si uptake by individual roots was further compared between GR1 and WT using a multicompartment transport box. At 12 h, the Si taken up per excised root was 3 times more in WT than in GR1 (Fig. 4). There was no difference in the number of lateral roots between WT and GR1, with 46.3 ± 9.7 per root for WT, and 42.5 ± 8.7 for GR1 between 2 and 4 cm from the root tip.
Figure 4.
Uptake of Si by individual, excised roots. Ten excised roots (5.5 cm long) from WT rice cv Oochikara and a mutant (GR1) defective in Si uptake were placed in a multicompartment transport box. Twelve milliliters of treatment solution containing 1.5 mm Si as silicic acid was applied to compartment 1 (root apex, 0–3 cm), and 4 mL of treatment solution without Si was added to each of compartments 2 and 3. At times indicated in the figure, the Si exuded from the xylem in compartment 3 was measured. Error bars represent ± sd (n = 3).
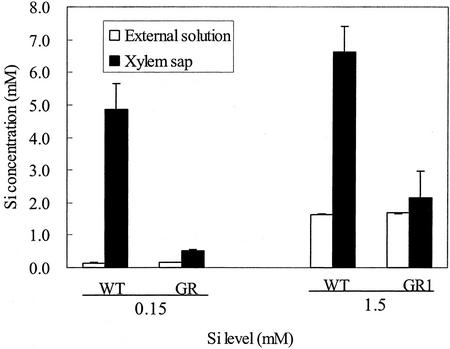
When the external solution contained 0.15 mm Si, the Si concentration in the xylem sap of WT reached about 5 mm after 1 h, a concentration 33-fold greater than that in the external solution (Fig. 5). The xylem sap of GR1 contained only 3 times higher Si concentrations than the external solution. When the external solution contained 1.5 mm Si, the Si concentration in the external solution and in the xylem sap was similar in GR1, but it was 4 times higher in the xylem sap than in the external solution in WT rice.
Figure 5.
Concentration of Si in the external solution and the xylem sap. Xylem sap was collected from WT rice cv Oochikara and a mutant (GR1) defective in Si uptake that were grown in a nutrient solution containing 0.15 and 1.5 mm Si as silicic acid. Xylem sap was collected for 1 h after decapitation. Error bars represent ± sd (n = 3).
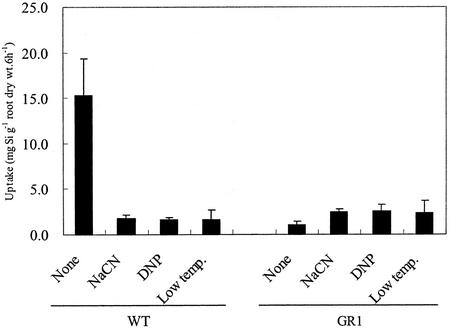
Metabolic inhibitors (NaCN and 2,4-DNP) significantly inhibited Si uptake in WT, but not in GR1 (Fig. 6). Si uptake by WT in the presence of metabolic inhibitors was similar to that by GR1. Low temperature treatment also caused a decrease in Si uptake in WT, but not in GR1.
Figure 6.
Effect of metabolic inhibitors and a low temperature on Si uptake by WT rice cv Oochikara and a mutant (GR1) defective in Si uptake. The uptake experiment was conducted in nutrient solution containing 0.75 mm Si in the presence or absence of inhibitors (1 mm for 2,4-DNP and 10 mm for NaCN) for 6 h. For the low temperature treatment, the plants were exposed to 4°C. Error bars represent ± sd (n = 3).
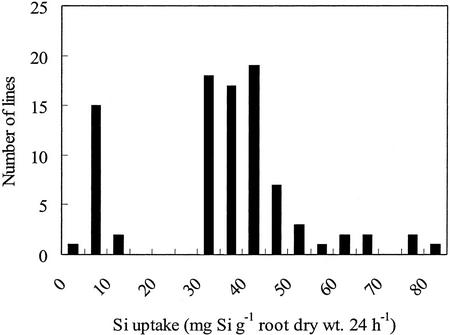
Genetic analysis was performed using F2 populations between WT and GR1 parents. Among 89 seedlings tested, 71 seedlings showed high Si uptake (>30.0 mg Si g−1 root dry weight 24 h−1) and 18 seedlings showed low Si uptake (<12.0 mg Si g−1 root dry weight 24 h−1; Fig. 7; Table III). Because the seedlings showing high Si uptake and low Si uptake segregated at a 3:1 ratio (Table III), it is suggested that low Si uptake in GR1 is controlled by a recessive gene.
Figure 7.
Frequency distributions for Si uptake in a progeny resulting from genetic crosses between WT rice cv Oochikara and a mutant (GR1). Si uptake by each seedling in a nutrient solution containing 1.0 mm Si was determined during 24 h.
Table III.
Segregation ratio of progeny resulting from genetic crosses between WT rice cv Oochikara and a mutant (GR1)
| Phenotype of Progeny
|
Ratio Tested | X2 | P | ||
|---|---|---|---|---|---|
| High Si uptake | Low Si uptake | ||||
| WT × GR1 F2 | 71 | 18 | 3:1 | 1.08 | 0.3–0.5 |
| Si uptake (mg Si g−1 Root dry wt 24 h−1) | >30.0 | <12.0 | – | – | – |
Si uptake by each seedling in a nutrient solution containing 1.0 mm Si was determined during 24 h.
DISCUSSION
Although Si has not been recognized as an essential element for higher plants from a physiological viewpoint, rice requires severalfold more Si than the essential macronutrients, such as N, P, and K, for healthy growth and high production (Savant et al., 1997). Si is taken up in the form of silicic acid. After uptake, Si is translocated to the shoot via the transpiration stream. The distribution of Si among the various parts of a plant, in the leaves in one plant, and along a given leaf, is not uniform. Usually, Si is deposited in greatest quantities in the tissues from which water is lost in greatest quantities, suggesting that Si is transported passively from the root to the shoot in the transpiration stream (Jones and Handreck, 1967). Therefore, the high accumulation of Si in rice is controlled by the ability of the roots to take up Si.
The high content of Si in rice shoots cannot be explained by diffusion of silicic acid across the lipid component of the cell membrane (Raven, 2001). Numerous studies have indicated that a specific active transport system for silicic acid exists in the rice roots (Takahashi et al., 1990; Ma et al., 2001a; Raven, 2001). To understand this transport system, as a first step, we attempted to isolate mutants that are defective in Si uptake.
For isolation of such mutants, Ge resistance was used as a selection parameter. A series of studies revealed that plant roots cannot discriminate Si from Ge in terms of uptake. For example, plants with high Si uptake also take up high Ge (Takahashi et al., 1976b, 1976c). As with Si, the concentration of Ge in the xylem sap of rice was 7 times higher than that in the external solution at 4 h after the application of Ge and increased to 70-fold at the 32nd h (Takahashi et al., 1976a). The uptake of Ge by rice was strongly inhibited by metabolic inhibitors such as 2,4-DNP and 2,4-dichlorophenoxy acetate (Takahashi et al., 1976a). The effect of transpiration on Ge uptake was not observed during a short exposure time in rice (Takahashi et al., 1976a). The uptake of 68Ge by rice decreased with increasing Si concentration in the solution, suggesting that antagonism between Ge and Si occurred. All these facts indicate that the transport system for Si is the same as for Ge. However, unlike Si, Ge is toxic after entering the plant. The typical symptom of Ge toxicity is the occurrence of brown spots on the leaves (Fig. 1, C and D). Therefore, if a line is defective in Si uptake, it reversely means that this line shows high Ge resistance (without brown spots). The visible symptoms of Ge toxicity allow us to rapidly screen mutants showing Ge resistance.
Several mutants resistant to Ge were isolated, and one of them, GR1, which showed the greatest resistance to Ge, was characterized in terms of Si uptake. Uptake experiments of both short and relatively long durations and with both solution and soil culture clearly and consistently indicated that Si uptake by GR1 is much lower than that by WT (Fig. 3; Tables I and II); however, there is no difference in the uptake of P and K (Tables I and II). A recent study reported that lateral roots rather than root hairs play an important role in Si uptake by rice (Ma et al., 2001a). However, no difference in the root morphology, including number of lateral roots, was observed between WT and GR1 (Fig. 1A), suggesting that a specific mutation in a high Si uptake system occurred in GR1. Measures of Si uptake per individual root further support this conclusion (Fig. 4).
Furthermore, the following evidence suggests that GR1 is defective in active Si uptake: First, during uptake by GR1, the Si concentration in the nutrient solution remained unchanged, suggesting that the uptake rates of Si and water by GR1 are similar. In contrast, uptake by WT resulted in a significant and rapid decrease in the Si concentration of the nutrient solution. Second, Si uptake by GR1 was not inhibited by metabolic inhibitors or by a low temperature treatment (Fig. 6). Si uptake in rice is an energy-dependent process and is usually inhibited by metabolic inhibitors and low temperature treatments, as was observed in WT (Fig. 6). Third, the Si concentration in the xylem sap is similar to that in the external solution at 1.5 mm Si in GR1 (Fig. 5), whereas the Si concentration in the xylem sap is much higher than that in the external solution in WT. Taken together, our results suggest that the active uptake system for Si in GR1 was disrupted.
Recently, the role of Si in alleviating biotic and abiotic stresses has generated great interest (for review, see Epstein, 1999; Ma et al., 2001b). Si is the only element that confers the resistance of plants to multiple stresses. Si is also the only element that does not damage plants when accumulated in excess. The beneficial effects of Si on plant growth are largely attributable to the characteristics of a silica gel that is accumulated on the epidermal tissues. However, most important crops are unable to take up Si actively via the roots, even though the concentration in soil solution is high, and, therefore, fail to accumulate high Si in the tops and to benefit from Si. In these crops with low Si uptake, foliar application has been attempted. For example, foliar application of Si is reported to be effective in inhibiting powdery mildew (Uncinula necator) development on cucumber (Cucumis sativus), muskmelon (Cucumis melo), and grape (Vitis vinifera) leaves (Bowen et al., 1992; Menzies et al., 1992). Si applied to leaves may deposit on the surface of leaves and play a similar role to Si taken up from the roots. By improving the Si uptake capacity of these plants genetically, the beneficial effects of Si should also be improved. Understanding the active uptake system for Si in rice roots will be a prerequisite for genetic improvement of Si uptake in other plant species.
A gene family encoding a Si transporter has been identified from a marine diatom (Cylindrotheca fusiformis) that requires Si as an essential element (Hildebrand et al., 1993, 1997). However, similar genes were not found in rice in a sequence homology search. Genetic analysis indicates that the gene encoding the transporter of Si in rice is a single dominant gene (Table III). Recently, the complete genome sequences for the two major subspecies of rice (japonica and indica) have been published (for review, see Kennedy, 2002). This sequence information will help us to isolate the Si transporter gene in rice roots, which is currently being undertaken in our laboratory.
MATERIALS AND METHODS
Screening of Ge-Resistant Mutants
For isolation of rice (Oryza sativa) mutants defective in Si uptake, Ge resistance was used as a selection parameter. Ge is a cognate element of Si and has chemical properties similar to those of Si. Because Ge is toxic to plants, Ge toxicity is a simple way to test for high Si uptake.
M2 seeds of rice (cv Oochikara) that were treated with 10−3 m of sodium azide for 6 h at 25°C were used for screening Ge-resistant mutants. As a first screening, seeds were sterilized in 0.2% (w/v) benomyl solution for 24 h at 30°C and then germinated in water for a further 24 h at 30°C. Germinated seeds (500 each) were placed on a net (30 × 20 cm) that was floated on one-half-strength Kimura B nutrient solution in a 15-L plastic container. The composition of the nutrient solution is reported in our previous paper (Ma et al., 2001a). On d 10, the seedlings were exposed to one-half-strength Kimura B solution containing 50 μm Ge as GeO2 (Wako, Tokyo). At d 5 after treatment, plants without or with less brown spots on the leaves were selected and designated as GR (Ge resistant). These procedures were repeated 16 times and a total of 64,000 seeds were screened.
GR candidates were further grown in nutrient solution without Ge for 1 week and then transferred to a 3-L pot with soil and cultivated to maturity.
A second screening was conducted using M3 seeds harvested from the candidate mutants, as described above for the first screening. Seedlings without or with less brown spots in the leaves were cultivated to maturity and M4 seeds were collected. Screening was performed in a greenhouse.
Ge Resistance
To confirm resistance to Ge, the effect of Ge on growth was compared between the WT and a mutant (GR1), which showed the most tolerance, based on leaf symptoms, to Ge. Seeds of WT rice and GR1 (M5) were surface sterilized in 0.5% (v/v) NaOCl for 15 min, rinsed, and soaked in water overnight at 25°C in the dark. The seeds were then transferred to a net floated on 0.5 mm CaCl2 solution in a plastic container. On d 5, the seedlings (two each per pot) were transferred to a 1.2-L plastic pot containing one-half-strength Kimura B solution (pH 5.6). The solution was renewed every 2 d. After a 26-d preculture, the seedlings were exposed to the same nutrient solution with or without 20 μm GeO2 in a growth chamber with natural light at 25°C. The treatment solution was renewed every 3 d. After 12 d, the roots and shoots were harvested separately, and the fresh and dry weights were recorded.
Si Uptake Experiment
Si uptake by WT rice and GR1 was examined during short-term (up to 12 h) and relatively long-term (both water and soil culture) experiments. For the short-term uptake experiment, two seedlings each (20 d old) were placed in a 180-mL black bottle containing one-half-strength Kimura B solution (pH 5.6) with 0.15 and 1.5 mm Si as silicic acid. Silicic acid was prepared by passing potassium silicate through cation-exchange resin (Amberlite IR-120B, H+ form, Organo, Tokyo). At times indicated in Figure 3, a 1-mL aliquot of uptake solution was taken for determination of Si concentration. Transpiration (water loss) was also recorded at each sampling time. At the conclusion of the experiment, the roots and shoots were harvested separately and their fresh and dry weights were recorded.
For the relatively long uptake experiment, 10-d-old seedlings of each line were transplanted to a 1.2-L plastic pot (two seedlings per pot) containing one-half-strength Kimura B solution with 0.15 or 1.5 mm Si as silicic acid. The solution was renewed every 3 d. The plants were grown at 25°C in a temperature-controlled chamber with natural light. After 4 weeks, the plants were harvested.
Soil culture was also performed for two lines under flooded condition. The soil used was as previously described (Ma et al., 2001a). Two seedlings each (11 d old) were planted in a 1.2-L pot filled with soil previously amended with (NH4)2SO4 (0.47 g kg−1 soil), KCl (0.19 g kg−1 soil), KH2PO4 (0.19 g kg−1 soil), and with or without sodium silicate at 2.0 g kg−1 soil. The plants were cultured at 25°C in a temperature-controlled chamber with natural light. Deionized water was supplied daily. One month later, the shoots were harvested.
Multicompartment Transport Box Experiment
To compare the Si uptake by individual roots of WT rice and GR1, a multicompartment transport box (1.4-cm height × 4.7-cm length × 1.0-cm length) was used (Kawasaki et al., 1984; Ma et al., 2001a). Ten excised roots (5.5 cm long) from 5-d-old seedlings were placed in the box. The box was divided into three compartments. Root apices (0–3 cm) in the first compartment were exposed to one-half-strength Kimura B solution (12 mL) with 0.75 mm Si, whereas the root portions in the second and third compartments were exposed to the same nutrient solution (4 mL for each) without Si. At the times indicated in Figure 4, the solution in the third compartment was replaced with fresh solution, and the Si concentration in this compartment (containing the cut end) as well as the other compartments, was determined. At the conclusion of the experiment, numbers of lateral roots were counted.
Xylem Sap Collection
Seedlings precultured in one-half-strength Kimura B solution with 0.15 or 1.5 mm Si as silicic acid for 4 weeks, were used for collection of xylem sap. Before decapitation, the seedlings were placed in fresh nutrient solution and allowed to take up for 1 h. The top was severed at 3 cm above the roots and xylem sap was then collected for 1 h with a micropipette. The Si concentration in the xylem sap was determined immediately.
Inhibitor and Low Temperature Experiments
To investigate the effect of metabolic inhibitors and low temperature on Si uptake, seedlings (21 d old) were exposed to one-half-strength Kimura B solution containing 0.75 mm silicic acid in the presence or absence of 10−2 m NaCN or 10−3 m 2,4-DNP. Stock solutions of sodium cyanide and 2,4-DNP were dissolved in water and ethanol, respectively. The final concentration of ethanol in the uptake solution was less than 0.4% (v/v) and preliminary experiments showed that this concentration of ethanol did not have any effect on Si uptake. The uptake experiments were performed as described above and the treatment period was 6 h.
For low temperature treatment, seedlings were exposed to one-half-strength Kimura B solution containing 0.75 mm Si that had been precooled at 4°C. After 6 h, the Si concentration in the treatment solution was determined.
Genetic Analysis
F2 populations from WT and GR1 parents were used for Si uptake determination. Si uptake for a total of 89 seedlings (16 d old) was tested in nutrient solution containing 1.0 mm Si as described above. The uptake experiment was conducted at 25°C and the uptake period was 24 h. Chi square analysis was performed.
Determination of Si, P, and K
Plant samples harvested after various treatments were dried at 70°C in an oven for at least 2 d and then ground to a powder. The sample was then microwave digested in a mixture of 3 mL of 62% (w/w) HNO3, 3 mL of 30% (w/w) hydrogen peroxide, and 2 mL of 46% (w/w) HF and the digested sample was diluted to 100 mL with 4% (w/v) boric acid. The Si and P concentration in the digest solution was determined by the colorimetric molybdenum blue method at 600 and 882 nm, respectively. K was determined by flame atomic absorption spectrometry.
ACKNOWLEDGMENTS
We thank Prof. Eiichi Takahashi and Julie Hayes for their critical reading of this manuscript.
Footnotes
This study was supported in part by NSFC (to J.F.M.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010348.
LITERATURE CITED
- Bowen PA, Menzies JG, Ehret D, Samuels L, Glass ADM. Soluble silicon sprays inhibit powdery mildew development on grape leaves. J Am Soc Hortic Sci. 1992;17:906–912. [Google Scholar]
- Epstein E. The anomaly of silicon in plant biology. Proc Natl Acad Sci USA. 1994;91:11–17. doi: 10.1073/pnas.91.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. Silicon. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:641–664. doi: 10.1146/annurev.arplant.50.1.641. [DOI] [PubMed] [Google Scholar]
- Hildebrand M, Higgins DR, Busser K, Volcani BE. Silicon-responsive cDNA clones isolated from the marine diatom Cylindrotheca fusiformis. Gene. 1993;132:213–218. doi: 10.1016/0378-1119(93)90198-c. [DOI] [PubMed] [Google Scholar]
- Hildebrand M, Volcani BE, Gassmann W, Schroeder JI. A gene family of silicon transporters. Nature. 1997;385:688–689. doi: 10.1038/385688b0. [DOI] [PubMed] [Google Scholar]
- Jones LHP, Handreck KA. Silica in soils, plants and animals. Adv Agron. 1967;19:107–149. [Google Scholar]
- Kawasaki T, Moritsugu M, Shimizu G. The absorption and translocation of ions in excised barley roots: a multi-compartment transport box experiment. Soil Sci Plant Nutr. 1984;30:417–425. [Google Scholar]
- Kennedy D. The importance of rice. Science. 2002;296:13. doi: 10.1126/science.296.5565.13. [DOI] [PubMed] [Google Scholar]
- Ma JF, Goto S, Tamai K, Ichii M. Role of root hairs and lateral roots in silicon uptake by rice. Plant Physiol. 2001a;127:1773–1780. [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Miyake Y, Takahashi E. Silicon as a beneficial element for crop plants. In: Datnoff LE, Snyder GH, Korndorfer GH, editors. Silicon in Agriculture. Amsterdam: Elsevier Science Publishing; 2001b. pp. 17–39. [Google Scholar]
- Marschner H. Mineral Nutrition of Higher Plants. San Diego: Academic Press; 1995. Beneficial mineral elements; pp. 405–435. [Google Scholar]
- Menzies J, Bowen P, Ehret D, Glass A. Foliar applications of potassium silicate reduce severity of powdery mildew on cucumber, muskmelon and zucchini squash. J Am Soc Hortic Sci. 1992;117:902–905. [Google Scholar]
- Okuda A, Takahashi E. Studies on the physiological role of silicon in crop plant: VIII. Some examination on the specific behavior of low land rice in silicon uptake. J Soil Sci Manure Jpn. 1962a;33:217–221. [Google Scholar]
- Okuda A, Takahashi E. Studies on the physiological role of silicon in crop plant: IX. Effect of various metabolic inhibitors on the silicon uptake by rice plant. J Soil Sci Manure Jpn. 1962b;33:453–455. [Google Scholar]
- Raven JA. Silicon transport at the cell and tissue level. In: Datnoff LE, Snyder GH, Korndorfer GH, editors. Silicon in Agriculture. Amsterdam: Elsevier Science Publishing; 2001. pp. 41–55. [Google Scholar]
- Savant NK, Snyder GH, Datnoff LE. Silicon management and sustainable rice production. Adv Agron. 1997;58:151–199. [Google Scholar]
- Takahashi E, Hino K. Silica uptake by plant with special reference to the forms of dissolved silica. J Soil Sci Manure Jpn. 1978;49:357–360. [Google Scholar]
- Takahashi E, Ma JF, Miyake Y. The possibility of silicon as an essential element for higher plants. Comments Agric Food Chem. 1990;2:99–122. [Google Scholar]
- Takahashi E, Matsumoto H, Syo S, Miyake Y. Effect of germanium on the growth of plants with special reference to the silicon nutrition (part 3) J Soil Sci Manure Jpn. 1976a;47:217–221. [Google Scholar]
- Takahashi E, Syo S, Miyake Y. Effect of germanium on the growth of plants with special reference to the silicon nutrition (part 1) J Soil Sci Manure Jpn. 1976b;47:183–190. [Google Scholar]
- Takahashi E, Syo S, Miyake Y. Effect of germanium on the growth of plants with special reference to the silicon nutrition (part 2) J Soil Sci Manure Jpn. 1976c;47:191–197. [Google Scholar]