Abstract
A β-primeverosidase from tea (Camellia sinensis) plants is a unique disaccharide-specific glycosidase, which hydrolyzes aroma precursors of β-primeverosides (6-O-β-d-xylopyranosyl-β-d-glucopyranosides) to liberate various aroma compounds, and the enzyme is deeply concerned with the floral aroma formation in oolong tea and black tea during the manufacturing process. The β-primeverosidase was purified from fresh leaves of a cultivar for green tea (C. sinensis var sinensis cv Yabukita), and its partial amino acid sequences were determined. The β-primeverosidase cDNA has been isolated from a cDNA library of cv Yabukita using degenerate oligonucleotide primers. The cDNA insert encodes a polypeptide consisting of an N-terminal signal peptide of 28 amino acid residues and a 479-amino acid mature protein. The β-primeverosidase protein sequence was 50% to 60% identical to β-glucosidases from various plants and was classified in a family 1 glycosyl hydrolase. The mature form of the β-primeverosidase expressed in Escherichia coli was able to hydrolyze β-primeverosides to liberate a primeverose unit and aglycons, but did not act on 2-phenylethyl β-d-glucopyranoside. These results indicate that the β-primeverosidase selectively recognizes the β-primeverosides as substrates and specifically hydrolyzes the β-glycosidic bond between the disaccharide and the aglycons. The stereochemistry for enzymatic hydrolysis of 2-phenylethyl β-primeveroside by the β-primeverosidase was followed by 1H-nuclear magnetic resonance spectroscopy, revealing that the enzyme hydrolyzes the β-primeveroside by a retaining mechanism. The roles of the β-primeverosidase in the defense mechanism in tea plants and the floral aroma formation during tea manufacturing process are also discussed.
Floral tea aroma is one of the most important factors to determine the character and quality of each made tea, especially oolong tea and black tea. Fresh tea leaves are virtually odorless or slightly smell of green note, and most of floral aroma compounds are produced by endogenous enzymes during tea manufacturing process of withering, rolling, and fermentation. Monoterpene alcohols such as linalool and geraniol, and aromatic alcohols such as benzyl alcohol and 2-phenylethanol, are known to largely contribute the floral aroma of oolong tea and black tea, and these aroma compounds are present as glycosidic precursors in fresh leaves of tea plants. For the first time, to our knowledge, benzyl and (Z)-3-hexenyl β-d-glucopyranosides were isolated as aroma precursors from a cultivar for green tea (Camellia sinensis var sinensis cv Yabukita; Yano et al., 1991; Kobayashi et al., 1994). We have isolated glycosidic precursors of various aroma compounds as disaccharide glycosides from tea leaves, and most of them were β-primeverosides (6-O-β-d-xylopyranosyl-β-d-glucopyranosides; Guo et al., 1993, 1994; Moon et al., 1994, 1996; Ogawa et al., 1995). Some of tea aroma precursors were isolated as acuminosides (6-O-β-d-apiofuranosyl-β-d-glucopyranosides; Moon et al., 1996; Ma et al., 2001b), and also as a vicianoside (6-O-α-l-arabinopyranosyl-β-d-glucopyranoside; Nishikitani et al., 1996). The quantitative analysis of glycosidic aroma precursors in tea leaves revealed that disaccharide glycosides, especially β-primeverosides, were more abundant (about 3 times) than glucosides in each tea cultivar (Wang et al., 2000). These results indicated that disaccharide glycosides, especially β-primeverosides, are thought to be the main precursors for the tea aroma formation.
A β-primeverosidase (EC 3.2.1.149) capable of hydrolyzing β-primeverosides into a primeverose unit and aglycons was first reported in Primula officinalis (Bridel, 1925). Then β-primeverosidases have been reported to be possibly present in most of higher plants containing β-primeverosides (Plouvier, 1980). Because most of the aroma precursors isolated from tea leaves were found as β-primeverosides, it was likely that there must be a β-primeverosidase for hydrolysis of these aroma precursors in fresh tea leaves. Recently, we found the enzyme, which hydrolyzes these diglycosidic aroma precursors to liberate the floral tea aroma from fresh leaves of a cultivar for green tea (cv Yabukita) (Guo et al., 1995, 1996). This enzyme exhibits the molecular mass of 61 kD on SDS-PAGE and is also present in fresh tea leaves of a cultivar for oolong tea (C. sinensis var sinensis cv Shuixian) (Ogawa et al., 1997) and that for black tea (C. sinensis var assamica) (Ijima et al., 1998).
In this paper, we describe the biochemical and molecular biological characterization of a “diglycosidase,” which is a disaccharide-specific glycosidase to liberate a disaccharide unit and an aglycon. We have succeeded in the purification and cloning of a β-primeverosidase from cv Yabukita. The β-primeverosidase was classified in a family 1 glycosyl hydrolase. The β-primeverosidase exhibited the selective hydrolysis of the disaccharide-aglycon bond of β-primeverosides to liberate a primeverose unit and aglycons. The stereochemistry for hydrolysis by the enzyme revealed that the β-primeverosidase is a retaining glycosyl hydrolase. Thus, this is the first molecular biological characterization of a diglycosidase from higher plants.
RESULTS
Purification of β-Primeverosidase from Fresh Tea Leaves
We reported previously the purification of the β-primeverosidase from juvenile leaves of a cultivar for green tea (cv Yabukita) (Guo et al., 1996), from that for oolong tea (cv Shuixian) (Ogawa et al., 1997), and from that for black tea (C. sinensis var assamica) (Ijima et al., 1998). Because the β-primeverosidase was nearly co-eluted with β-glucosidases from each column chromatography, the final preparations still contained a significant β-glucosidase activity (3%–10% of β-primeverosidase activity). To obtain a pure β-primeverosidase, we improved the purification procedure by application of an additional hydrophobic chromatography to the previously reported procedures, and β-glucosidases were thoroughly eliminated from the β-primeverosidase fractions by tracing both the activities using p-nitrophenyl (pNP) β-glucopyranoside and β-primeveroside as substrates during the whole purification process. The purification procedure is summarized in Table I.
Table I.
Summary of purification of the β-primeverosidase from green tea cv Yabukita
| Purification Step | Total Protein | Total Activity | Yield | Specific Activity | Purification |
|---|---|---|---|---|---|
| mg | unit | % | unit mg protein−1 | fold | |
| Buffer extract | 1,000 | 40 | 100 | 0.04 | 1.0 |
| Acetone precipitate | 220 | 38 | 95 | 0.17 | 4.3 |
| 40% (NH4)2SO4 supernatant | 110 | 32 | 80 | 0.29 | 7.3 |
| Butyl-toyopearl | 7.7 | 3.8 | 9.3 | 0.49 | 12 |
| CM-toyopearl | 0.54 | 0.70 | 1.8 | 1.3 | 33 |
| Mono S | 0.30 | 0.51 | 1.3 | 1.7 | 43 |
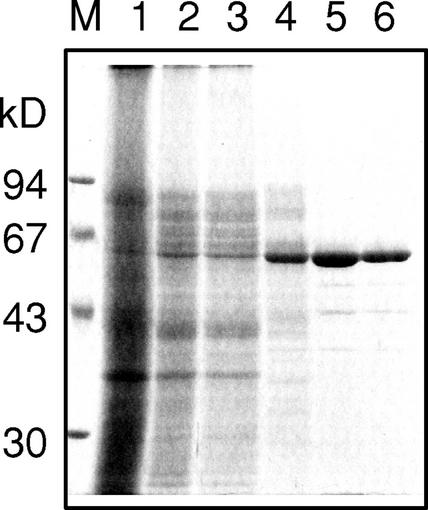
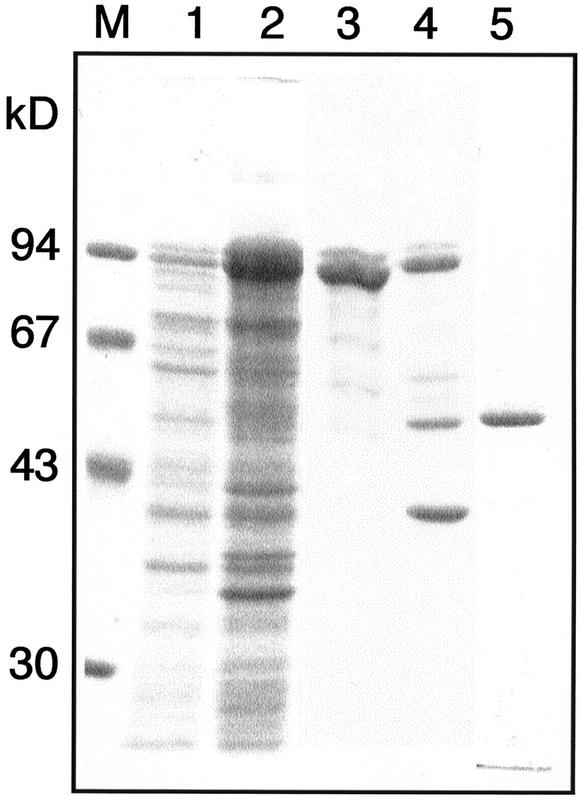
The final preparation (1.7 unit mg−1 of the specific activity for the β-primeverosidase) contained less than 1% of the β-glucosidase activity (0.012 unit mg−1) compared with the β-primeverosidase activity and gave a homogenous 61-kD band on SDS-PAGE (Fig. 1). This preparation was further applied to a reverse-phase HPLC, and a single peak from the HPLC was applied to the amino acid sequence analysis. The amino acid sequences of the N-terminal portion (20 residues) as well as three peptides from a lysyl endopeptidase digest or from a trypsin digest were determined (Fig. 2, underlined).
Figure 1.
SDS-PAGE of fractions from the chromatographic steps of the purification of the β-primeverosidase from fresh green tea leaves. SDS-PAGE was performed using 10% (w/v) polyacrylamide slab gel, and the protein was visualized with silver staining. The migration of size markers is shown to the left of the gel. Lane M, Mr marker; lane 1, the buffer extract; lane 2, acetone precipitate; lane 3, 40% (NH4)2SO4 supernatant; lane 4, Butyl-Toyopearl elute; lane 5, CM-Toyopearl elute; lane 6, Mono S elute.
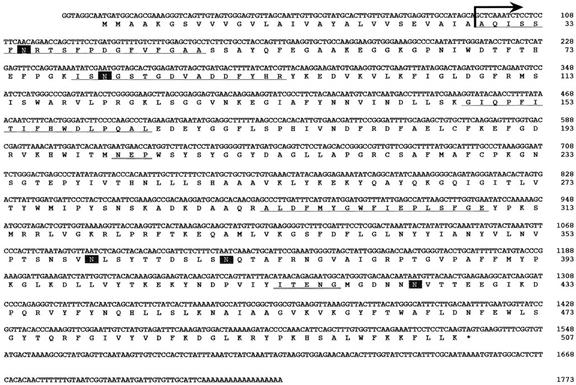
Figure 2.
Nucleotide and predicted amino acid sequences of the β-primeverosidase cDNA from a cultivar for green tea (C. sinensis var sinensis cv Yabukita). Peptide sequences determined from the purified protein are underlined. Arrow indicates the position of the N-terminal amino acid sequence of the purified protein and also the possible cleavage site predicted by PSORT analysis. Possible N-glycosylation sites are boxed. The catalytic residues in the sequence motifs conserved in family 1 glycosyl hydrolases are double underlined.
Isolation of the β-Primeverosidase cDNA
Based on the partial amino acid sequences thus determined, degenerate oligonucleotide primers were synthesized, and the partial cDNA fragment was amplified by PCR. The PCR fragment was used as a probe to screen a cDNA library from tea leaves of a cultivar for green tea (cv Yabukita). The isolated cDNA consists of a 1,524-bp open reading frame encoding a polypeptide of 507 amino acid residues, an 8-bp 5′-untranslated region, a 179-bp 3′-non-coding region, and a poly(A+) tail (Fig. 2). The N-terminal amino acid sequence determined from the purified protein corresponded to the deduced amino acid sequence at the region from 29th to 48th residues, and the three peptide sequences determined from the purified protein were also found in the predicted protein with a perfect agreement (Fig. 2, underlined). It was predicted by PSORT analysis (http://psort.ims.u-tokyo.ac.jp/) that a possible cleavage site for a signal peptidase is present between the amino acid residues Ala-28 and Ala-29 (von Heijne, 1986) and also that the mature protein of 478 amino acid residues will be targeted outside the cells. The pI value of the mature protein was calculated to be 9.21, consistent with that of the purified protein, 9.4 (Guo et al., 1996). The calculated Mr of the mature protein was 54,234, whereas the apparent Mr of the purified β-primeverosidase is estimated by time of flight-mass spectrometry to be 60,480 (Ijima et al., 1998). The result suggests that the posttranslational modification of the β-primeverosidase occurs in plant cells.
The N-Glycosylation of β-Primeverosidase
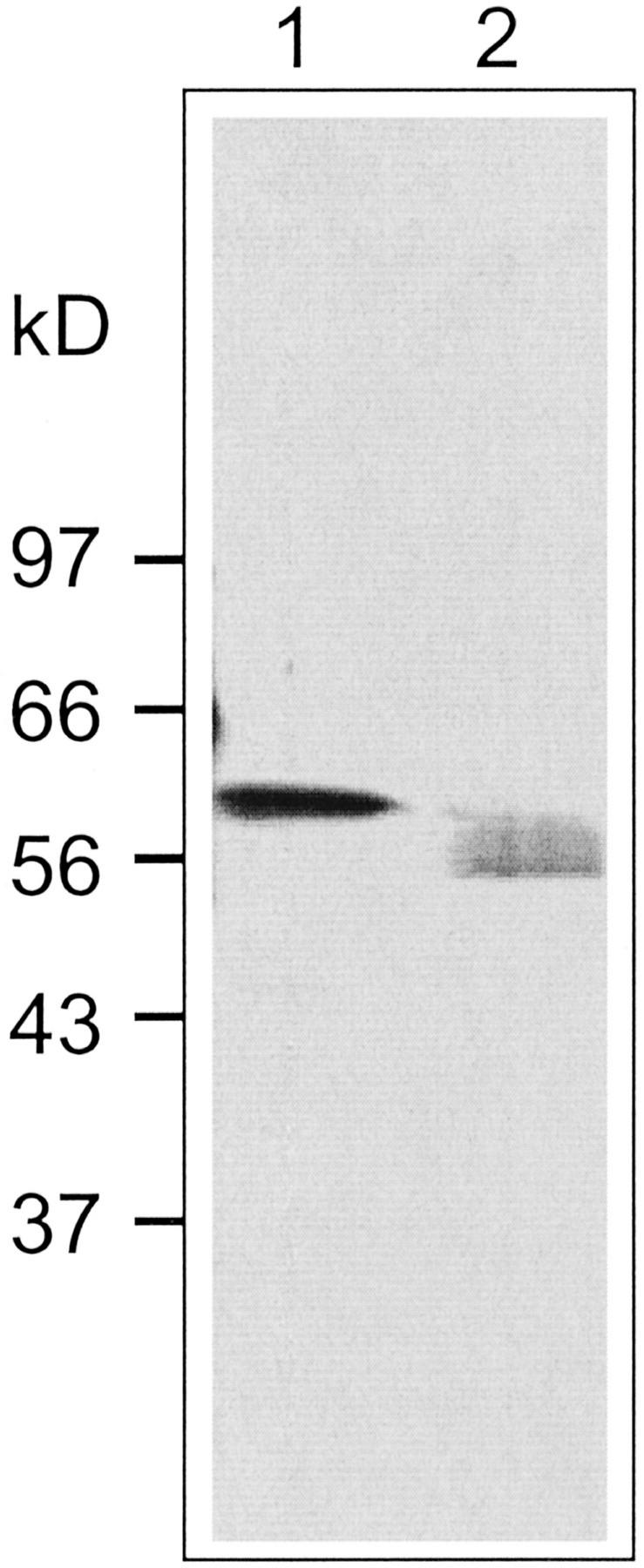
The deduced amino acid sequence of the β-primeverosidase contains the five potential N-Asn glycosylation sites (MOTIF analysis: http://motif.genome.ad.jp/; Fig. 2, boxed). Two Asn residues, Asn-35 and Asn-81, among them are found in the peptide sequences determined from the purified protein, and it is likely that these Asn residues may be glycosylated because of the fact that these Asn residues were not detected by the direct amino acid sequencing. To confirm the glycosylation of the β-primeverosidase, the purified β-primeverosidase was treated with glycopeptidase A and analyzed by SDS-PAGE (Fig. 3). The protein band at 61 kD on SDS-PAGE shifted to the smaller molecular mass around 54 kD, which is good agreement with the calculated molecular mass of the mature form. The glycosylation nature of the β-primeverosidase was also demonstrated by its positive periodic acid-Schiff staining (data not shown). Thus, tea leaf β-primeverosidase is N-glycosylated, and has an N-terminal signal sequence that might target it to the cell wall via the Golgi apparatus.
Figure 3.
Deglycosylation of the purified β-primeverosidase with glycopeptidase A. The β-primeverosidase purified from tea leaves was mixed in 1% (w/v) Triton X-100 and 50 mm 2-mercaptoethanol, and heated at 100°C for 15 min. The sample was treated with glycopeptidase A in the buffer for 24 h, and was analyzed by 10% (w/v) SDS-PAGE. The protein was visualized by silver staining. The migration of size makers is shown to the left of the gel. Lane 1, β-Primeverosidase purified from tea leaves; lane 2, β-primeverosidase treated with glycopeptidase A.
Characterization of the Amino Acid Sequence of β-Primeverosidase
The deduced amino acid sequence of the β-primeverosidase cDNA showed the highest identity to amygdalin hydrolase (58%) from black cherry (Prunus serotina) seeds (Zheng and Poulton, 1995). Interestingly, amygdalin hydrolase catalyzes the hydrolysis of the cyanogenic diglucoside amygdalin [β-gentiobioside (6-O-β-d-glucopyranosyl-β-d-glucopyranoside) of (R)-mandelonitrile], indicating that both the β-primeverosidase and amygdalin hydrolase recognizes the disaccharide glycoside as a substrate. On the other hand, amygdalin hydrolase hydrolyzes the inter-glycosidic bond of β-gentiobioside to release one Glc unit and a monoglucoside prunasin (Zheng and Poulton, 1995). The β-primeverosidase also showed high identities to other plant β-glucosidases such as linamarase from white clover (Trifolium repens; 56%; Tolley et al., 1993), β-glucosidase for indoxyl β-d-glucoside from the indigo plant (Polygonum tinctorium; 55%; Minami et al., 1999), raucaffricine β-glucosidase from Rauvolfia serpentina (53%; Warzecha et al., 2000), strictosidine β-glucosidase from Catharanthus roseus (49%; Geerlings et al., 2000), dhurrinase from Sorghum bicolor (46%; Cicek and Esen, 1998), and myrosinase from Arabidopsis (46%; Xue et al., 1995).
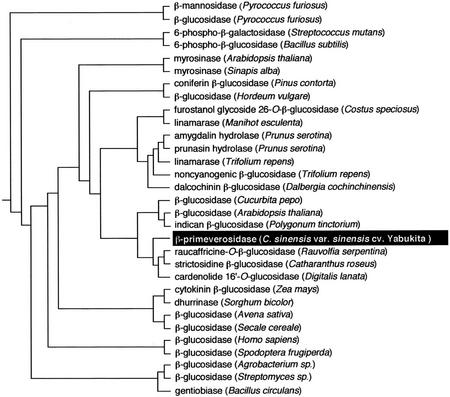
Many glycoside hydrolases have been isolated from bacteria to mammals and have been classified into 83 families according to the amino acid sequence similarity (Henrissat and Bairoch, 1996; http://afmb.cnrs-mrs.fr/CAZY/index.html). Because the tea leaf β-primeverosidase showed high similarities to family 1 glycosyl hydrolases from various kinds of plants, the β-primeverosidase was classified in a family 1 glycosyl hydrolase. This is the first example in this family of the hydrolyzing β-glycosidic bond between the disaccharide and aglycons. Phylogenetic analysis of the β-primeverosidase with various glycosyl hydrolases in this family is shown in Figure 4. The β-primeverosidase was grouped into the cluster of plant β-glucosidases, which act on various glycosides such as alkaloidal glucoside (strictosidine and raucaffricine; Geerlings et al., 2000; Warzecha et al., 2000), cardenolide glycoside (Framm et al., 2000), and indoxyl glucoside (Minami et al., 1999). The β-primeverosidase was only loosely clustered with amygdalin hydrolase despite the high similarity between them because amygdalin hydrolase showed higher identities to prunasin hydrolase and linamarases and was grouped into the cluster of cyanogenic β-glucosidases. On the other hand, the β-primeverosidase was clearly distant from microbial β-glucosidases. Thus, it was suggested that the disaccharide-specific β-primeverosidase is evolved from a monosaccharide-specific plant β-glucosidase.
Figure 4.
A phylogenetic tree of the tea leaf β-primeverosidase with family 1 glycosyl hydrolases from various microorganisms and higher plants. The tree was constructed by alignment of the amino acid sequences using the ClustalW program at the GenomeNet (http://www.genome.ad.jp/) and drawn by the rooted neighbor-joining method. Relative branch lengths are approximately proportional to phylogenetic distance. β-Mannosidase from Pyrococcus furiosus (AAC44387), β-glucosidase from P. furiosus (AAC25555), 6-phospho-β-galactosidase from Streptococcus mutans (AAA16450), 6-phospho-β-glucosidase from Bacillus subtilis (AAA22660), myrosinase from Arabidopsis (AAC18869), myrosinase from Sinapis alba (CAA42534), coniferin β-glucosidase from Pinus contorta (AAC69619), β-glucosidase from Hordeum vulgare (AAA87339), furostanol glycoside 26-O-β-glucosidase from Costus speciosus (BAA11831), linamarase from Manihot esculenta (AAB71381), amygdalin hydrolase from Prunus serotina (PSU26025), prunasin hydrolase from Prunus serotina (AAA93032), linamarase from Trifolium repens (CAA40058), non-cyanogenic β-glucosidase from Trifolium repens (CAA40058), dalcochinin β-glucosidase from Dalbergia cochinchinensis (AAF04007), β-glucosidase from Cucurbita pepo (AAG25897), β-glucosidase from Arabidopsis (AAC16094), indican β-glucosidase from Polygonum tinctorium (BAA78708), β-primeverosidase from C. sinensis var sinensis cv Yabukita (AB088027), raucaffricine-O-β-glucosidase from R. serpentina (AAF03675), strictosidine β-glucosidase from C. roseus (AAF28800), cardenolide 16′-O-glucosidase from Digitalis lanata (CAB38854), cytokinin β-glucosidase from Zea mays (CAA52293), dhurrinase from S. bicolor (AAC49177), β-glucosidase from Avena sativa (AAD02839), β-glucosidase from Secale cereale (AAG00614), cytosolic β-glucosidase from Homo sapiens (CAC08178), β-glucosidase from Spodoptera frugiperda (AAC06038), β-glucosidase from Agrobacterium sp. (AAA22085), β-glucosidase from Streptomyces sp. (CAA82733), and gentiobiase from Bacillus circulans (AAA22266).
β-Glucosidases in this family have been known to hydrolyze the β-glycosydic linkage with net retaining mechanism via a double displacement, and the catalytic residues have been identified (Keresztessy et al., 1994; Zechel and Withers, 2000). The conserved sequence motif of NEP, of which the Glu residue is an acid-base catalyst, is found at the region (202–204 residues) in the β-primeverosidase protein sequence. The β-primeverosidase also contains an ITENG motif at Ile-414 to Gly-418, of which the Glu residue is known to be a catalytic nucleophile of β-glucosidases. The residues involved in the substrate Glc ring recognition (Barrett et al., 1995) are also conserved at Arg-111, His-157, Asn-202, Asn-343, Tyr-345, and Trp-463 in the β-primeverosidase sequence.
Heterologous Expression in Escherichia coli
The cDNA encoding the mature form of the β-primeverosidase was amplified by PCR, and the expression vector, pMALc2-ΔPri, was constructed to express the recombinant fusion protein between a maltose-binding protein and the mature primeverosidase. When expression of the recombinant protein was induced by addition of 1 μm isopropyl-β-d-thiogalactoside (IPTG) at 37°C, all the recombinant proteins were found as inclusion bodies, and the β-primeverosidase activity was not detected in cell lysate (data not shown). The transformed cells were grown at 22°C in the presence of 0.1 mm IPTG, and a part of the recombinant proteins was detected in the soluble fractions (Fig. 5). The recombinant protein was partially purified from the cell lysate by an amylose resin affinity chromatography, and the mature form of β-primeverosidase was released from the fusion protein by digesting with a protease factor Xa and purified by CM-Toyopearl chromatography (Fig. 5).
Figure 5.
SDS-PAGE of the recombinant β-primeverosidase expressed in E. coli. The migration of size marker is shown to the left of the gel. Lane M, Mr marker; lane 1, the supernatant of the crude extracts from the E. coli cells with the expression vector pMALc2-ΔPri; lane 2, the precipitate; lane 3, the recombinant MBP-β-primeverosidase fusion protein purified by an amylose-resin column; lane 4, the MBP-β-primeverosidase fusion protein digested with factor Xa; lane 5, the purified recombinant mature β-primeverosidase.
The substrate specificity of the recombinant protein was determined (Table II). The best substrate for the recombinant β-primeverosidase was a natural substrate, 2-phenylethyl β-primeveroside, and pNP β-primeveroside was also hydrolyzed well. On the other hand, pNP β-d-glucopyranoside was a poor substrate, and neither pNP β-xyloside nor 2-phenylethyl β-d-glucopyranoside was hydrolyzed at all. The crude enzyme from E. coli cells transformed with the vacant pMALc2 vector did not hydrolyze these β-primeverosides at all (data not shown). These results indicate the high specificity of the β-primeverosidase toward β-primeverosides. This pattern of the substrate specificity of the recombinant enzyme was almost identical with that of the native β-primeverosidase purified from tea leaves (Table II; Ma et al., 2001a), indicating that the cDNA actually encodes the tea leaf β-primeverosidase.
Table II.
Substrate specificity of the purified and recombinant β-primeverosidases
| Substrate | Relative
Activity
|
|
|---|---|---|
| Tea β-primeverosidase | Recombinant β-primeverosidase | |
| % | ||
| 2-Phenylethyl β-primeveroside | 100 | 100 |
| 2-Phenylethyl β-glucoside | 0 | 0 |
| p-Nitrophenyl β-primeveroside | 48 | 62 |
| p-Nitrophenyl β-glucoside | 0.5 | 0.3 |
| p-Nitrophenyl β-xyloside | 0 | 0 |
Activity was determined by measuring the release of 2-phenyl ethanol by HPLC or p-nitrophenol by absorbance at 405 nm. Substrates were used at 10 mm concentrations. Relative activity shows the percentage activity detected using 2-phenylethyl β-primeveroside as a substrate.
Mode of Hydrolysis by the β-Primeverosidase
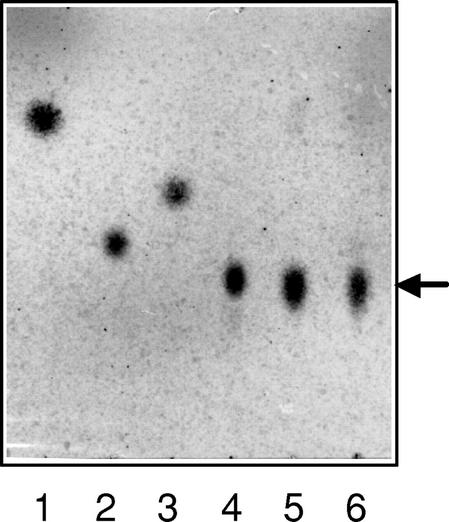
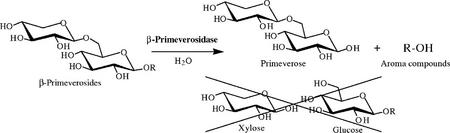
pNP β-primeveroside was incubated with either the β-primeverosidase purified from tea leaves or the recombinant protein produced by E. coli, and the hydrolysate was analyzed by thin-layer chromatography (TLC; Fig. 6). The spot corresponding to primeverose was clearly observed in each of the hydrolysates, but no spots for Glc and Xyl were detected. This confirms that the tea leaf β-primeverosidase is a diglycosidase specifically hydrolyzing the β-glycosidic bond between primeverose and aglycons without cleaving the inter-glycosidic bond of a Xyl-Glc unit.
Figure 6.
TLC of the hydrolysis products of pNP β-primeveroside by the recombinant β-primeverosidase. pNP β-primeveroside was incubated with either the β-primeverosidase purified from tea leaves or the recombinant protein produced by E. coli, and the hydrolysates were analyzed by TLC. TLC was carried out on silica gel 60 F254 plates using a solvent system of butanol:pyridine:water:acetic acid (6:4:3:1 [v/v]). Glycosides and sugars were detected by heating the plate at 120°C after spraying with 0.2% (w/v) naphthoresorcinol in H2SO4:ethanol (1:19 [v/v]). Lanes 1 through 4 were standard. Lane 1, pNP β-primeveroside; lane 2, Glc; lane 3, Xyl; lane 4, primeverose; lane 5, the reaction products by the β-primeverosidase purified from tea leaves; lane 6, the reaction products by the recombinant mature β-primeverosidase. The arrow indicates the position of primeverose.
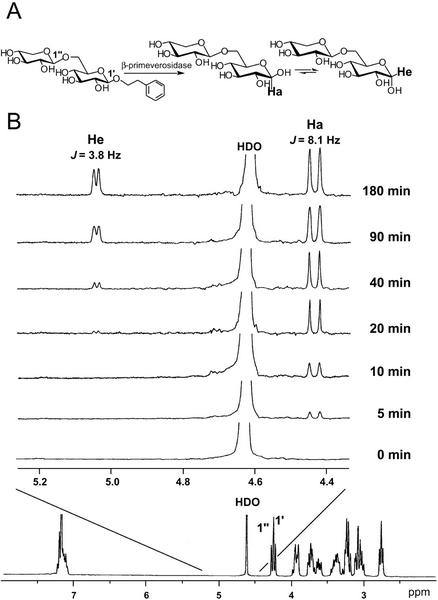
The β-primeverosidase was classified in a family 1 glycosyl hydrolase. Glycosyl hydrolases in family 1 are known to be retaining glycosidases, which catalyze the hydrolysis of the substrates with retaining the anomeric configuration by a double-displacement mechanism through a glucosyl-enzyme covalent intermediate (Zechel and Withers, 2000). The stereochemistry of enzymatic hydrolysis by the β-primeverosidase was analyzed by 1H-NMR spectroscopy (Fig. 7). The 1H-NMR spectra of the reaction mixture containing 2-phenylethyl β-primeveroside and the β-primeverosidase revealed that the β-anomer (Ha, δ 4.43, J = 8.1 Hz) of primeverose was formed first. The α-anomer (He, δ 5.04, J = 3.8 Hz) of primeverose appeared later as a consequence of mutarotation. Thus, the tea leaf β-primeverosidase is a retaining glycosidase, as has been observed for other family 1 β-glucosidases.
Figure 7.
Time course of hydrolysis of 2-phenylethyl β-primeveroside catalyzed by the tea leaf β-primeverosidase, followed by 1H-NMR spectroscopy. A, Postulated retaining hydrolysis of 2-phenylethyl β-primeveroside by the tea enzyme. B, Spectra recorded 0, 5, 10, 20, 40, 90, and 180 min after addition of the enzyme. Ha and He indicate the resonances of H-1 of α- and β-d-Glc, respectively. The full-scan 1H NMR spectrum of the substrate (2-phenylethyl β-primeveroside) is shown at the bottom.
Distribution of the β-Primeverosidase in Tea Shoots
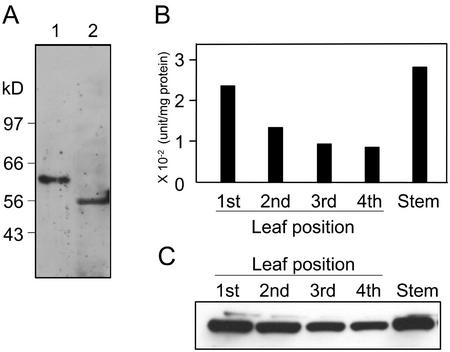
The mature β-primeverosidase lacking the first 28 amino acid residues was expressed as a His-tagged fusion protein in E. coli. The recombinant protein was inactive and formed inclusion bodies (data not shown). The predominant protein with an apparent molecular mass of 54 kD was purified from the insoluble fraction and used to raise polyclonal antibodies in rabbits. The antibodies recognized a single strong band of 61 kD in the crude extract of the tea acetone power and did not show cross-reactivity to other β-glucosidases in the extract (Fig. 8A).
Figure 8.
Distribution of the β-primeverosidase in tea shoots. The acetone powders were prepared from first (including bud), second, third, and fourth leaves, and stem of tea shoots, respectively. The crude extract of each acetone powder was prepared with 10 mm citrate buffer (pH 6.0) by the same procedures as that for the purification. A, Immunoblot analysis was performed by anti-β-primeverosidase antibody. The migration of molecular marker is shown in the left of the gel. Lane 1, Crude extract from the acetone power of tea leaves; lane 2, recombinant His-tagged β-primeverosidase expressed in E. coli. B, β-Primeverosidase activity for each sample was measured with pNP β-primeveroside. C, Immunoblot analysis with the antiprimeverosidase antibody was performed. One microgram of protein was loaded on each lane and separated by 10% (w/v) SDS-PAGE.
To investigate the distribution of β-primeverosidase in tea shoots, we measured the primeverosidase activity and analyzed the amount of the protein by using the anti-β-primeverosidase antibodies in tea shoots (Fig. 8, B and C). Tea shoots were separated to each part (buds; first, second, third, and fourth leaves; and stem), and the crude extract was prepared from the acetone powder of each part of tea shoots. Both the activity and amount of the β-primeverosidase was high in younger leaves, and decreased as the leaf aged. The stem also contained a high amount of the β-primeverosidase. This distribution pattern of the β-primeverosidase in tea shoots is quite similar to that obtained by indirect measurement of the glycosidase activity responsible for the tea aroma formation (Ogawa et al., 1995) as well as to that of the glycosidic precursors of various aroma compounds [2-phenylethanol, benzyl alcohol, geraniol, nerol, methyl salicylate, cis-linalool 3,7-oxide, linalool, cis-linalool 3,6-oxide, trans-linalool 3,6-oxide, and (Z)-3-hexenol; Ogawa et al., 1995].
DISCUSSION
β-Primeverosidase: A Unique Diglycosidase Specific to β-Primeverosides
In addition to β-primeverosides from tea leaves, many kinds of disaccharide glycosides with various kinds of aglycons have been isolated from a wide range of plant species (Williams et al., 1982; Rockenbach et al., 1992; Chassagne et al., 1996; Jaroszewski et al., 1996; Derksen et al., 1998; Lu et al., 1998; Yamamura et al., 1998; van der Plas et al., 1998; Crouzet and Chassagne, 1999; Petrovic et al., 1999; Tamaki et al., 1999; Ma, 2002). Diglycosides are thought to be hydrolyzed by two distinct mechanisms, namely either sequential or simultaneous mechanism. Günata et al. (1988, 1993) suggested that diglycosides are hydrolyzed by endogenous and/or exogenous enzymes in stepwise and sequential reactions, which are catalyzed by a first monosaccharide glycosidase such as α-rhamunosidase, α-arabinosidase, and β-glucosidase to cleave the inter-glycosidic linkage and a second β-glucosidase to release various aglycons from the resultant β-glucosides (the sequential mechanism). This mechanism is supported by the fact that a cyanogenic diglucoside amygdalin [β-gentiobioside (6-O-β-d-glucopyranosyl-β-d-glucopyranoside) of (R)-mandelonitrile found in black cherry seeds] is known to be sequentially hydrolyzed by two distinct β-glucosidases (amygdalin hydrolase and prunasin hydrolase) to release two Glc units and (R)-mandelonitrile (Li et al., 1992). Alternatively, some plants such as Rhamnus dahurica (rhamnodiastase; Suzuki, 1962), Viburnum furcatum (furcatin hydrolase; Imaseki and Yamamoto, 1961), Fagopyrum esculentum (heteroglycosidase; Bourbouze et al., 1975), Vicia angustifolia var segetalis (vicianase; Kasai et al., 1981), Davallia trichomanoides Blume (vicianin hydrolase; Lizotte and Poulton, 1988), and Fagopyrum tataricum (rutinase; Yasuda and Nakagawa, 1994) have been found to contain disaccharide-specific glycosidases (diglycosidases) that are capable of hydrolyzing each disaccharide glycoside to liberate a disaccharide unit and an aglycon (the simultaneous mechanism). Günata et al. (1998) have also detected and identified an enzyme having β-primeverosidase- and/or rutinase-like activity from grape (Vitis vinifera) berry peels. Although some of diglycosidases have been purified from plants (Imaseki and Yamamoto, 1961; Lizotte and Poulton, 1988; Yasuda and Nakagawa, 1994) and also from microorganisms (Narikawa et al., 2000; Yamamoto et al., 2002), they have never been characterized in molecular levels. In this paper, we confirmed the tea leaf β-primeverosidase as a unique diglycosidase by purification from tea leaves and cloning of the cDNA encoding the enzyme. It was found that the β-primeverosidase was able to catalyze the hydrolysis of β-primeverosides to liberate a primeverose unit and aglycons and also that the hydrolysis was specific to the primeverose-aglycon β-glycosidic linkage without cleaving the inter-glycosidic bond between Xyl and Glc (Figs. 6 and 9). It was also revealed that the β-primeverosidase catalyzed the hydrolysis of the glycosyl bond with retention of the anomeric configuration, which is consistent with the classification of the β-primeverosidase in a family 1 glycosyl hydrolase. Thus, this report is the first characterization, to our knowledge, of a disaccharide-specific glycosidase in biochemical and molecular biological levels.
Figure 9.
Proposed reaction scheme of the tea leaf β-primeverosidase. The tea leaf β-primeverosidase catalyzes the hydrolysis of various kinds of disaccharide β-primeverosides to release a primeverose unit and various aroma compounds by a retaining mechanism but does not sequentially hydrolyze β-primeverosides to monosaccharide units.
Ijima et al. (1998) demonstrated that the β-primeverosidase purified from tea leaves hydrolyzed not only β-primeverosides but also β-vicianoside and 6-O-α-l-arabinofranosyl-β-d-glucopyranoside to liberate the corresponding disaccharide units. Günata et al. (1998) also reported that a diglycosidase from grape berry peels acted on various kinds of disaccharide glycosides. These results suggested that a diglycosidase might show broad substrate specificity with respect to the disaccharide glycon moiety. Ma et al. (2001a) recently reported the detail analysis of substrate specificity of the tea leaf β-primeverosidase. The β-primeverosidase was able to hydrolyze not only β-primeveroside but also the other naturally occurring disaccharide glycosides such as β-vicianoside, β-acuminoside, and β-gentiobioside. However, its relative activity to β-primeveroside was much higher (30–100 times) than those to the other natural disaccharide glycosides. This result clearly indicates that the tea leaf β-primeverosidase shows pronounced specificity for β-primeverosides in terms of the glycon moiety. Because many kinds of disaccharide glycosides have been isolated from various species of plants, a number of diglycosidases specific to each kind of disaccharide glycoside may be present in the plants containing the corresponding glycosides. Plouvier (1980) has reported the distribution of diglycosidases in plants and fungi, and suggested that β-primeverosidases and β-gentiobiosidases might be present in most of higher plants containing the corresponding glycosides. We have succeeded in cDNA cloning of a β-acuminosidase from Viburnum furcatum Blume, which contains a large amount of furcatin (p-allylphenyl β-acuminoside) in leaves (M. Mizutani, unpublished data). This is the second example of cDNA cloning of a disaccharide-specific glycosidase from plants. Thus, disaccharide-specific glycosidases such as β-primeverosidase, β-acuminosidase, β-vicianase, and β-rutinase will be distributed throughout the plant kingdom and will form a new group of diglycosidases among the large glycosyl hydrolase family.
Role of β-Primeverosidase in Tea Aroma Formation during the Oolong Tea and Black Tea Manufacturing Process
Fresh tea leaves are virtually odorless or slightly smell of green note. Most floral aroma compounds of oolong tea and black tea are produced by endogenous enzymes during the tea manufacturing process of withering, rolling, and fermentation. This suggests two possibilities for the nature of the enzymes involved in this process. First, the enzymes do not exist in fresh leaves and are induced during tea manufacturing process. Second, the enzymes are present in fresh leaves but are localized separately from the aroma precursors in leaf tissues. The case for the tea leaf β-primeverosidase corresponds to the second case because the β-primeverosidase is constitutively present in fresh tea leaves (Fig. 8). The β-primeverosidase has a signal peptide of 28 amino acid residues, which is predicted to act as a signal to secret the enzyme outside the cells. Perhaps the β-primeverosidase is localized in cell walls, whereas aroma precursor primeverosides are separately present in vacuoles. When leaf tissues are stressed, wounded, or destroyed, the enzyme is able to contact with various aroma precursors and to release the aroma alcohols. It is most likely that the manufacturing processes for oolong tea and black tea induce such interactions between the enzyme and the precursors, and thereby the floral aroma is formed during the manufacturing processes. It was demonstrated previously that β-primeverosidase is a main glycosidase in tea leaves (Guo et al., 1995; Matsumura et al., 1997, Ma et al., 2001a). Most of the aroma precursors have been found as β-primeverosides (Guo et al., 1993, 1994; Moon et al., 1994, 1996), and the quantitative analysis of aroma precursor glycosides revealed that disaccharide glycosides, especially β-primeverosides, are more abundant (about 3 times) than β-glucopyranosides in each tea cultivar (Wang et al., 2000). Furthermore, during the manufacturing process of black tea, β-primeverosides had almost disappeared, whereas the amounts of glucosides were unchanged (Wang et al., 2001), suggesting that the hydrolysis of β-primeverosides by the β-primeverosidase mainly occurs during the fermentation process of black tea. Thus, these results indicate that both β-primeverosides and the β-primeverosidase are the major components for the floral tea aroma formation during the tea fermentation process. The amounts of the β-primeverosidase as well as aroma precursor primeverosides in tea shoots are high in younger leaves and decreased as the leaf aged (Fig. 8; Ogawa et al., 1995). Each made tea, especially a high-quality product of oolong tea and black tea, is traditionally made from young tea leaves (buds to third leaf), and this is quite reasonable in the mechanistic points of view for the tea aroma formation. The quality and quantity of aroma compounds in each made tea are dependent on not only a variety of tea plants but also a producing area and cultivated conditions. The content of the β-primeverosidase in tea shoots may be influenced by these factors. Manipulation of the amounts of aroma precursor β-primeverosides as well as the β-primeverosidase may improve the quality of each made tea.
Physiological Functions in Tea Plants
The β-primeverosidase was classified in a family 1 glycosyl hydrolase. Most plant β-glucosidases in this family are involved in defense mechanisms, in which various toxic aglycons are released by the action of the specific β-glucosidases such as cyanogenic glucosidases (Poulton, 1990; Vetter, 2000) and glucosinolate myrosinases (Rask et al., 2000). Aglycons of β-primeverosides found in tea leaves are (S)-linalool, geraniol, methyl salicylate, linalool oxide, benzyl alcohol, 2-phenylethanol, and 3-hexenol (Guo et al., 1993, 1994; Moon et al., 1994, 1996; Ogawa et al., 1995; Nishikitani et al., 1999). Among them, methyl salicylate is known to be a plant signal compound that induces various defense responses (Ryals et al., 1996), and terpene alcohols such as geraniol and linalool has been shown to have antimicrobial and antifungal activities (Pattnaik et al., 1997). Aromatic alcohol and green leaf volatiles such as 3-hexenol emitted from herbivore-damaged leaves have also been found to act as both direct and indirect defenses, which involve their direct toxicity to insects and the attraction of herbivore enemies, respectively (Mattiacci et al., 1995; Pare and Tumlinson, 1999; Pichersky and Gershenzon, 2002). Thus, the tea leaf β-primeverosidase probably acts as a key enzyme in a defense mechanism by which the β-primeverosidase hydrolyzes β-primeverosides to release these aglycons in response to fungal infection and herbivore feeding. Some of the β-glucosidases involved in defense mechanisms have been found to be induced by methyl jasmonate (Geerlings et al., 2000) or insect feeding (van de Ven et al., 2000). It is also known that most of the β-glucosides are accumulated as nontoxic storage forms in the vacuoles (Klein et al., 1996; Frangne et al., 2002), and on the other hand, plant β-glucosidases are separately localized to cell walls (Kakes, 1985; Mkpong et al., 1990; Hughes et al., 1992), protein bodies (Swain et al., 1992), plastid (Minami et al., 1997; Cicek and Esen, 1998), or the endoplasmic reticulum membrane (Geerlings et al., 2000). The investigation of the tea leaf β-primeverosidase in terms of the regulation of gene expression as well as the intracellular localization will be achieved by using the cDNA and the antibodies prepared in this study.
MATERIALS AND METHODS
Chemicals
pNP β-d-glucopyranoside and pNP β-d-xylopyranoside were purchased from Sigma Chemical (St. Louis). pNP β-primeveroside was enzymatically synthesized by transglycosylation between xylobiose and pNP β-d-glucopyranoside with a β-xylosidase from Aspergillus pulverulentus (Murata et al., 1999). 2-Phenylethyl β-d-glucopyranoside and 2-phenylethyl β-d-primeveroside were chemically synthesized as described (Ma et al., 2001a).
Plant Material
For purification of the β-primeverosidase, the fresh leaves (leaf shoots with up to third or fourth leaf) of a cultivar for green tea manufacturing (Camellia sinensis var sinensis cv Yabukita) were plucked at the National Institute of Vegetables, Ornamental Plants, and Tea (Kanaya, Shizuoka, Japan). For the investigation of the distribution of the β-primeverosidase in tea shoots, the fresh green tea shoots were plucked at Kyoto Prefectural Tea Research Institute (Uji, Kyoto).
Purification of Tea β-Primeverosidase
Fresh juvenile tea leaves of cv Yabukita were finely chopped, crushed in dry ice-acetone by a homogenizer, and filtered in vacuo. The residue was washed with chilled acetone (−20°C) until the filtrate became nearly colorless. The residue was spread on filter paper and then placed in vacuo to remove acetone. The residual acetone powder was stored at −20°C before use. The acetone powder (100 g, equivalent to 600 g of fresh leaves) was suspended in 0.1 m citrate buffer (pH 6.0, 2 L), stirred for 4 h at 4°C, and centrifuged at 14,000g for 20 min. To the supernatant, an equal amount of chilled acetone (−20°C) was gradually added with stirring, and the mixture was left overnight at 4°C. The precipitate obtained by centrifugation at 14,000g for 20 min was dissolved in 0.1 m citrate buffer (pH 6.0, 500 mL). Ammonium sulfate was added up to 40% saturation, and the mixture was centrifuged at 14,000g for 20 min. The supernatant was subjected to a butyl-Toyopearl 650M column (124 × 32 mm, Tosoh, Tokyo) equilibrated with 20 mm citrate buffer (pH 6.0) containing 40% ammonium sulfate. The enzyme fractions were eluted by a linear gradient of ammonium sulfate from 40% to 0% saturation in 1,000 mL of 20 mm citrate buffer (pH 6.0) with a flow rate of 5 mL min−1. The β-primeverosidase fractions were combined and concentrated by ultrafiltration (Amicon PM-10, Grace Japan, Tokyo), and dialyzed against 20 mm citrate buffer (pH 6.0) containing 50 mm NaCl. The fraction was applied to a CM-Toyopearl column (300 × 22 mm, Tosoh), and the β-primeverosidase fractions were eluted by a linear gradient of NaCl from 50 to 150 mm in 500 mL of 20 mm citrate buffer (pH 6.0). The β-primeverosidase fraction was placed on a column (50 × 5 mm) of Mono S HR (Amersham-Pharmacia Biotech, Tokyo) equilibrated with 20 mm citrate buffer (pH 6.0). The β-primeverosidase was eluted with a linear gradient of NaCl from 0.1 to 0.25 m at a flow rate of 1 mL min−1. Because the β-primeverosidase was nearly co-eluted with β-glucosidases from each column chromatography, the fractions containing the β-glucosidase activities were thoroughly eliminated from the β-primeverosidase fractions by tracing both the activities using pNP β-d-glucopyranoside and β-primeveroside as substrates during the whole purification process.
Amino Acid Sequencing
The β-primeverosidase purified as described above was applied to reverse-phase HPLC using a CAPCELL PAK C18 SG300 column (150 × 4.6 mm, SHISEIDO, Tokyo), and a single peak fraction was collected and directly analyzed to determine the N-terminal amino acid sequence. The fraction was further digested with a lysyl endopeptidase (Wako Pure Chemical Industries, Osaka) or trypsin (Wako), and the resultant peptide fragments were separated by reverse-phase HPLC using a CAPCELL PAK C18 SG120 column (250 × 4.6 mm, SHISEIDO). Amino acid sequences were analyzed by automated Edman degradation using the ABI protein sequencer 492 (Applied Biosystems, Norwalk, CT).
Cloning of Tea Leaf β-Primeverosidase cDNA
The total RNA was isolated from juvenile tea leaves by the method of Verwoerd et al. (1989). The mRNA was purified with an oligo(dT) cellulose column type 7 (Amersham-Pharmacia Biotech). The cDNA library was constructed from the mRNA with a double-stranded Uni-ZAP XR vector (Stratagene, La Jolla, CA) after the manufacturer's instruction. The mass excision was performed to make the phagemid library (Stratagene).
The oligonucleotide primer, βPRI1: 5′-GGIGA(T/C) GTIGCIGA(T/C) GA(T/C) TT(T/C) TA(T/C) CA-3′, was degenerated from the internal amino acid sequence, GDVADDFYH, determined from the purified β-primeverosidase. By using a set of βPRI1 and an oligo(dT)16 primer, PCR was performed through 30 cycles of 60 s at 94°C, 90 s at 45°C, and 60 s at 72°C with the mass-excised phagemid library as templates. PCR products were separated by 2% (w/v) agarose gel electrophoresis, and the major band was cloned into a pGEM-T vector (Promega, Madison, WI). The cDNA fragment was labeled with [32P]dCTP by random priming method, and about 500,000 plaques from the tea leaf cDNA library were screened with the labeled fragment as a probe. The partial nucleotide sequences of the positive clones were determined using forward and reverse primers for the pBluescript SK(−) phagemid, and the clone with the longest insert was completely sequenced. The dideoxy chain termination method using an ABI prism Dye termination Cycle Sequencing Reaction Kit (Applied Biosystems) and an ABI Prism 377 Sequencer (Applied Biosystems) carried out DNA sequencing.
Analysis of N-Glycosylation of β-Primeverosidase
For carbohydrate analysis of the purified β-primeverosidase, periodic acid-Schiff staining was performed (Jay et al., 1990). For the deglycosylation of the β-primeverosidase, the β-primeverosidase purified from tea leaves was mixed in 1% (w/v) Triton X-100 and 50 mm 2-mercaptoethanol, and heated at 100°C for 15 min. The sample was treated with glycopeptidase A from Almond (Seikagaku, Tokyo) in the buffer, incubated for 2 d at 37°C, and was analyzed by 10% (w/v) SDS-PAGE.
Expression of the β-Primeverosidase cDNA in Escherichia coli
To obtain the mature form of a recombinant β-primeverosidase, the cDNA coding for the mature β-primeverosidase was amplified by PCR using a set of the two primers: N-terminal primer starting at amino acid 29 residue, 5′-GGATCCGCTCAAATCTCCTCCTTCAAC-3′, containing a BamHI site (underlined); and C-terminal primer, 5′-GTCGACCTACTTGAGGAGGAATTTCTT-3′, containing an SalI site (underlined). The 1.4-kb PCR fragments were cloned into the pGEM-T vector (Promega), and the DNA sequence of the insert was confirmed by sequencing. The insert cDNA coding for the mature β-primeverosidase was isolated by digestion with restriction enzymes, BamHI and SalI. The digested insert was ligated into a pMALc2 vector (New England Biolabs, Beverly, MA) to generate pMALc2-ΔPri. The E. coli (JM109) was transformed with the pMALc2-ΔPri plasmid and the cells were subsequently grown at 37°C and 250 rpm in Luria-Bertani (LB) medium supplemented with 50 μg mL−1 ampicillin overnight. The 10-mL overnight culture was used to inoculate each 1-L aliquot of LB medium supplemented with 100 μg mL−1 ampicillin and 0.2% (w/v) Glc, and the cells were grown at 37°C and 250 rpm until the A600 reached about 0.6. The expression of the mature β-primeverosidase fused with a maltose-binding protein was induced by addition with 0.1 mm IPTG. The cells were further grown at 22°C, 150 rpm for 24 h. The expressed cells were collected by centrifugation at 5,000g for 10 min and were resuspended in 50 mm citrate buffer (pH 6.0), 1 mm EDTA, and 1 mm phenylmethylsulfonyl fluoride. The suspension was sonicated 15 times for 30 s with 30-s intervals, and was centrifuged at 28,000g for 30 min. The obtained supernatant was applied to an amylose resin column (New England Biolabs) equilibrated with the suspension buffer. The fusion protein was eluted with the suspension buffer supplemented with 10 mm maltose. The mature form of the β-primeverosidase was separated from the maltose binding protein by digesting with a protease factor Xa (New England Biolabs), and the protein sample was subjected to further purification by a CM-Toyopearl column chromatography (Tosoh).
Preparation of Anti-β-Primeverosidase Polyclonal Antibodies
The cDNA coding for the mature form prepared above was inserted into the pQE30 vector (Qiagen, Tokyo). The E. coli cells (JM109) were transformed with the expression vector and the cells was subsequently grown at 37°C and 250 rpm in LB medium supplemented with 50 μg mL−1 ampicillin overnight. The 10-mL overnight culture was used to inoculate each 1-L aliquot of LB medium supplemented with 100 μg mL−1 ampicillin and 0.2% (w/v) Glc, and the cells were grown at 37°C and 250 rpm until OD600 reached about 0.6. The expression of β-primeverosidase fused with 6× His tag was induced by the addition with 1 mm IPTG at 37°C for 18 h. The cells were collected by centrifugation at 5,000g for 10 min, resuspended in 100 mm sodium phosphate buffer (pH 7.8) containing 500 mm NaCl, disrupted 15 times for 30 s with 30-s intervals, and centrifuged at 28,000g for 30 min. Because the expressed protein was insoluble and produced as inclusion bodies, the precipitates were dissolved with 100 mm sodium phosphate buffer (pH 7.8) containing 6.0 m guanidium chloride, 500 mm NaCl, and 20 mm imidazole. The His tag fusion protein was purified according to the manufacture's instructions (Qiagen). The fusion protein was further purified by a preparative SDS-PAGE. After staining with 0.3 m CuCl2, the recombinant protein band was excised from the gel and was dialyzed against 250 mm Tris-HCl (pH 9.0) containing 250 mm EDTA for 3 h and then against 20 mm Tris-HCl containing 0.1% (w/v) SDS overnight.
Polyclonal antibodies against the purified recombinant protein were prepared by Hokkaido System Science Co., LTD (Hokkaido, Japan) in New Zealand white rabbits (Oryctolagus cuniculus) by standard methods. IgGs in blood serum were purified on Protein A Sepharose column (Amersham-Pharmacia Biotech) according to the manufacturer's instructions.
Immunoblot Analysis
Tea shoots were separated to each part (buds; first, second, third, and fourth leaves; and stem), and the crude extract was prepared from the acetone powder of each part of tea shoots. One microgram of protein of the crude extract was loaded on 10% (w/v) SDS-PAGE, and transferred to a nylon membrane Hybond N+-ECL (Amersham-Pharmacia Biotech). Bound anti-β-primeverosidase antibody was detected using a goat anti-rabbit IgG conjugated to alkaline phosphatase (Bio-Rad Laboratories, Hercules, CA) and ECL Western Blotting Kit (Amersham-Pharmacia Biotech).
Enzyme Assays
The β-primeverosidase activity was determined using pNP β-primeveroside as an artificial substrate. The incubation mixture (100 μL) was composed of 20 mm citrate buffer (pH 6.0), 5 μL of an enzyme sample solution, and 5 μL of 10 mm substrate solution. Reaction was started by adding an enzyme sample at 37°C and stopped by addition of 50 μL of 1 m Na2CO3. The liberated p-nitrophenol was determined spectrophotometrically at 405 nm. One unit was defined as the amount of enzyme liberating 1 μmol p-nitrophenol min−1 under the assay conditions. When 2-phenylethyl β-primeveroside was used as a natural substrate, the reaction conditions were essentially the same as described above, except for addition of 50 μL of 1 m NaOH containing 15 μg of benzyl alcohol to stop the reaction. The reaction mixture was injected, and HPLC analysis was performed for detection of liberated 2-phenylethanol under the following conditions: column, YMC-pack ODS-AQ (250 × 4.6 mm, YMC, Kyoto); detection, 210 nm with a 996 Photodiode Array Detector (Waters, Milford, MA); column temperature, 40°C; mobile phase, 23% (v/v) MeCN; and flow rate, 1.0 mL min−1. The protein content was determined using the Coomassie Blue protein assay reagent (Pierce, Rockford, IL).
TLC Analysis
TLC was carried out on silica gel 60 F254 plates using a solvent system of butanol:pyridine:water:acetic acid (6:4:3:1 [v/v]). Glycosides and sugars were detected by heating at 120°C after spraying with 0.2% (w/v) naphthoresorcinol in H2SO4:ethanol (1:19 [v/v]).
1H-NMR Analysis for Determination of the Stereochemical Outcome for Hydrolysis by the β-Primeverosidase
The method was essentially described by Wang et al. (1998). 2-Phenylethyl β-primeveroside (10 mg) was dissolved in 0.7 mL of D2O. The β-primeverosidase purified from tea leaves was lyophilized and redissolved in 40 μL of D2O. After the 1H-NMR spectrum of the substrate was recorded, the enzyme (20 μL) was added to an NMR tube containing the substrate, and the tube was incubated at 37°C. The spectra were recorded (eight scans) at the time intervals on a JNM-AL 300 spectrometer (JEOL, Tokyo) at 25°C because the axial proton of β-anomer of the released primeverose was overlapped with the HDO signal at 37°C.
ACKNOWLEDGMENTS
The authors thank the National Institute of Vegetables, Ornamental Plants, and Tea (Kanaya, Shizuoka, Japan) and Kyoto Prefectural Tea Research Institute (Uji, Kyoto) for providing them with green tea leaves. The authors also thank Amano Enzyme Co. (Nagoya, Japan) for pNP β-primeveroside.
Footnotes
This work was partly supported by the Ministry of Education, Science, Sports, and Culture of Japan (grant-in-aid nos. (B)(2) 07456060 and (B)(2)13460049 to K.S.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.011023.
LITERATURE CITED
- Barrett T, Suresh CG, Tolley SP, Dodson EJ, Hughes MA. The crystal structure of a cyanogenic β-glucosidase from white clover, a family 1 glycosyl hydrolase. Structure. 1995;3:951–960. doi: 10.1016/s0969-2126(01)00229-5. [DOI] [PubMed] [Google Scholar]
- Bourbouze R, Pratviel-Sosa F, Percheron F. Rhamnodiastase and α-l-rhamnosidase de Fagopyrum esculentum. Phytochemistry. 1975;14:1279–1282. [Google Scholar]
- Bridel M. Primeverose, primeverosides and primeverosidase. C R Acad Sci Paris. 1925;180:1421–1425. [Google Scholar]
- Chassagne D, Crouzet JC, Bayonove CL, Brillouet JM, Baumes RL. 6-O-α-Arabinopyranosyl-β-d-glucopyranosides as aroma precursors from passion fruit. Phytochemistry. 1996;41:1497–1500. doi: 10.1016/0031-9422(95)00814-4. [DOI] [PubMed] [Google Scholar]
- Cicek M, Esen A. Structure and expression of a dhurrinase (β-glucosidase) from sorghum. Plant Physiol. 1998;116:1469–1478. doi: 10.1104/pp.116.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzet J, Chassagne D. Glycosidically bound volatiles in plants. In: Ikan R, editor. Naturally Occurring Glycosides. New York: John Wiley & Sons; 1999. pp. 225–274. [Google Scholar]
- Derksen GCH, van Beek TA, Groot A, Capelle A. High-performance liquid chromatographic method for the analysis of anthraquinone glycosides and aglycones in madder root (Rubia tinctorumL.) J Chromatogr A. 1998;816:277–281. [Google Scholar]
- Framm JJ, Peterson A, Thoeringer C, Pangert A, Hornung E, Feussner I, Luckner M, Lindemann P. Cloning and functional expression in Escherichia coli of a cDNA encoding cardenolide 16′-O-glucohydrolase from Digitalis lanataEhrh. Plant Cell Physiol. 2000;41:1293–1298. doi: 10.1093/pcp/pcd060. [DOI] [PubMed] [Google Scholar]
- Frangne N, Eggmann T, Koblischke C, Weissenbock G, Martinoia E, Klein M. Flavone glucoside uptake into barley mesophyll and Arabidopsis cell culture vacuoles. Energization occurs by H+-antiport and ATP-binding cassette-type mechanisms. Plant Physiol. 2002;128:726–733. doi: 10.1104/pp.010590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerlings A, Ibanez MM, Memelink J, van Der Heijden R, Verpoorte R. Molecular cloning and analysis of strictosidine β-d-glucosidase, an enzyme in terpenoid indole alkaloid biosynthesis in Catharanthus roseus. J Biol Chem. 2000;275:3051–3056. doi: 10.1074/jbc.275.5.3051. [DOI] [PubMed] [Google Scholar]
- Günata Z, Bitter S, Brilouel JM, Bayonove C, Cordonnier R. Sequential enzymatic hydrolysis of potentially aromatic glycosides from grape. Carbohydr Res. 1988;184:139–149. [Google Scholar]
- Günata Z, Blondeel C, Vallier MJ, Lepoutre JC, Sapis JC, Watanabe N. An endoglycosidase from grape berry skin of cv. M. Alexandria hydrolyzing potentially aromatic disaccharide glycosidase. J Agric Food Chem. 1998;46:2748–2753. [Google Scholar]
- Günata Z, Dugelay I, Sapis JC, Baumes R, Bayonove C. Role of the enzymes in the use of the flavour potential from grape glycosides in winemaking. In: Schreier P, Winterhalter P, editors. Progress in Flavour Precursor Studies. Wheaton, IL: Allured; 1993. pp. 219–234. [Google Scholar]
- Guo W, Hosoi R, Sakata K, Watanabe N, Yagi A, Ina K, Luo S. (S)-Linalyl, 2-phenylethyl, and benzyl disaccharide glycosides isolated as aroma precursors from oolong tea leaves. Biosci Biotechnol Biochem. 1994;58:1532–1534. doi: 10.1271/bbb.58.1532. [DOI] [PubMed] [Google Scholar]
- Guo W, Ogawa K, Yamauchi K, Watanabe N, Usui T, Luo S, Sakata K. Isolation and characterization of a β-primeverosidase concerned with alcoholic aroma formation in tea leaves. Biosci Biotechnol Biochem. 1996;60:1810–1814. [Google Scholar]
- Guo W, Sakata K, Watanabe N, Nakajima R, Yagi A, Ina K, Luo S. Geranyl 6-O-β-d-xylopyranosyl-β-d-glucopyranoside isolated as an aroma precursor from tea leaves for oolong tea. Phytochemistry. 1993;33:1373–1375. doi: 10.1016/0031-9422(93)85093-7. [DOI] [PubMed] [Google Scholar]
- Guo W, Yamauchi K, Watanabe N, Usui T, Luo S, Sakata K. A primeverosidase as a main glycosidase concerned with the alcoholic aroma formation in tea leaves. Biosci Biotechnol Biochem. 1995;59:962–964. [Google Scholar]
- Henrissat B, Bairoch A. Updating the sequence-based classification of glycosyl hydrolases. Biochem J. 1996;316:695–696. doi: 10.1042/bj3160695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MA, Brown K, Pancoro A, Murray BS, Oxtoby E, Hughes J. A molecular and biochemical analysis of the structure of the cyanogenic β-glucosidase (linamarase) from cassava (Manihot esculentaCranz) Arch Biochem Biophys. 1992;295:273–279. doi: 10.1016/0003-9861(92)90518-2. [DOI] [PubMed] [Google Scholar]
- Ijima Y, Ogawa K, Watanabe N, Usui T, Ohnishi-Kameyama M, Nagata T, Sakata K. Characterization of β-primevrosidase, being concerned with alcoholic aroma formation in tea leaves to be processed into black tea, and preliminary observation on its substrate specificity. J Agric Food Chem. 1998;46:1712–1718. [Google Scholar]
- Imaseki H, Yamamoto T. A furcatin hydrolyzing glycosidase of Viburnum furcatumblume. Arch Biochem Biophys. 1961;92:467–474. doi: 10.1016/0003-9861(61)90386-1. [DOI] [PubMed] [Google Scholar]
- Jaroszewski JW, Rasmussen AB, Rasmussen HB, Olsen CE, Jorgensen LB. Biosynthesis of cyanohydrin glycosides from unnatural nitriles in intact tissue of Passiflora morifolia and Turnera angustifolia. Phytochemistry. 1996;42:649–654. doi: 10.1016/0031-9422(96)00065-9. [DOI] [PubMed] [Google Scholar]
- Jay GD, Culp DJ, Jahnke MR. Silver staining of extensively glycosylated proteins on sodium dodecyl sulfate-polyacrylamide gels: enhancement by carbohydrate-binding dyes. Anal Biochem. 1990;185:324–330. doi: 10.1016/0003-2697(90)90302-p. [DOI] [PubMed] [Google Scholar]
- Kakes P. Linamarase and other glucosidases are present in the cell walls of Trifolium repensL. leaves. Planta. 1985;166:156–160. doi: 10.1007/BF00397342. [DOI] [PubMed] [Google Scholar]
- Kasai T, Kishimoto M, Kawamura S. On the free sugars and cyanogenic glycoside in the seed of Vicia angustifolia var. segetalis. Kagawa Daigaku Nougakubu Gakujutsu Houkoku. 1981;32:111–119. [Google Scholar]
- Klein M, Weissenbock G, Dufaud A, Gaillard C, Kreuz K, Martinoia E. Different energization mechanisms drive the vacuolar uptake of a flavonoid glucoside and a herbicide glucoside. J Biol Chem. 1996;271:29666–29671. doi: 10.1074/jbc.271.47.29666. [DOI] [PubMed] [Google Scholar]
- Keresztessy Z, Kiss L, Hughes MA. Investigation of the active site of the cyanogenic β-d-glucosidase (linamarase) from Manihot esculentaCrantz (cassava): II. Identification of Glu-198 as an active site carboxylate group with acid catalytic function. Arch Biochem Biophys. 1994;315:323–330. doi: 10.1006/abbi.1994.1507. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Kubota K, Joki Y, Wada E, Wakabayashi M. (Z)-3-Hexenyl β-d-glucopyranoside in fresh tea leaves as a precursor of green odor. Biosci Biotechnol Biochem. 1994;58:592–593. [Google Scholar]
- Li CP, Swain E, Poulton JE. Prunus serotinaamygdalin hydrolase and prunasin hydrolase. Plant Physiol. 1992;100:282–290. doi: 10.1104/pp.100.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizotte PA, Poulton JE. Catabolism of cyanogenic glycosides by purified vicianin hydrolase from squirre's foot fern (Davallia trichomanoidesBlume) Plant Physiol. 1988;86:322–324. doi: 10.1104/pp.86.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Xu PJ, Chen ZN, Liu GM. Anthraquinone glycosides from Rhynchtechum vestitum. Phytochemistry. 1998;49:1135–1137. [Google Scholar]
- Ma SJ. Enzymatic and chemical studies on β-primeverosidase, a key glycosidase in tea aroma formation. PhD thesis. Japan: Kyoto University; 2002. [Google Scholar]
- Ma SJ, Mizutani M, Hiratake J, Hayashi K, Yagi K, Watanabe N, Sakata K. Substrate specificity of β-primeverosidase, a key enzyme in aroma formation during oolong tea and black tea manufacturing. Biosci Biotechnol Biochem. 2001a;65:2719–2729. doi: 10.1271/bbb.65.2719. [DOI] [PubMed] [Google Scholar]
- Ma SJ, Watanabe N, Yagi A, Sakata K. The (3R,9R)-3-hydroxy-β-ionol disaccharide glycoside is an aroma precursor in tea leaves. Phytochemistry. 2001b;56:819–825. doi: 10.1016/s0031-9422(00)00361-7. [DOI] [PubMed] [Google Scholar]
- Matsumura S, Takahashi S, Nishikitani M, Kubota K, Kobayashi A. The role of diglycosides a tea aroma precursors: synthesis of tea diglycosides and specificity of glycosidases in tea leaves. J Agric Food Chem. 1997;45:2674–2678. [Google Scholar]
- Mattiacci L, Dicke M, Posthumus MA. β-Glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci USA. 1995;92:2036–2040. doi: 10.1073/pnas.92.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y, Shigeta Y, Tokumoto U, Tanaka Y, Yonekura-Sakakibara K, Oh-oka H, Matsubara H. Cloning, sequencing, characterization, and expression of a β-glucosidase cDNA from the indigo plant. Plant Sci. 1999;142:219–226. [Google Scholar]
- Minami Y, Takao H, Kanafuji T, Miura K, Kondo M, Hara-Nishimura I, Nishimura M, Matsubara H. β-Glucosidase in the indigo plant: intracellular localization and tissue specific expression in leaves. Plant Cell Physiol. 1997;38:1069–1074. doi: 10.1093/oxfordjournals.pcp.a029273. [DOI] [PubMed] [Google Scholar]
- Mkpong OE, Yan H, Chism G, Sayre RT. Purification, characterization, and localization of linamarase in cassava. Plant Physiol. 1990;93:176–181. doi: 10.1104/pp.93.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JH, Watanabe N, Ijima Y, Yagi A, Ina K, Sakata K. cis- and trans-Linalool 3,7-oxides and methyl salicylate glycosides and (Z)-3-hexenyl β-d-glucopyranoside as aroma precursors from tea leaves for oolong tea. Biosci Biotechnol Biochem. 1996;60:1815–1819. doi: 10.1271/bbb.60.1815. [DOI] [PubMed] [Google Scholar]
- Moon JH, Watanabe N, Sakata K, Yagi A, Ina K, Luo S. trans- and cis-Linalool 3,6-oxides 6-O-β-d-xylopyranosyl-β-d-glucopyranosides isolated as aroma precursors from leaves for oolong tea. Biosci Biotechnol Biochem. 1994;58:1742–1744. doi: 10.1271/bbb.58.1742. [DOI] [PubMed] [Google Scholar]
- Murata T, Shimada M, Watanabe N, Sakata K, Usui T. Practical enzymatic synthesis of primeverose and its glycoside. J Appl Glycosci. 1999;46:431–437. [Google Scholar]
- Narikawa T, Shinoyama H, Fujii T. A β-rutinosidase from Penicillium rugulosumIFO 7242 that is a peculiar flavonoid glycosidase. Biosci Biotechnol Biochem. 2000;64:1317–1319. doi: 10.1271/bbb.64.1317. [DOI] [PubMed] [Google Scholar]
- Nishikitani M, Kubota K, Kobayashi A, Sugawara F. Geranyl 6-O-β-l-arabinopyranosyl-β-d-glucopyranoside isolated as an aroma precursor from leaves of a green tea cultivar. Biosci Biotechnol Biochem. 1996;60:929–931. doi: 10.1271/bbb.60.929. [DOI] [PubMed] [Google Scholar]
- Nishikitani M, Wang D, Kubota K, Kobayashi A, Sugawara F. (Z)-3-Hexenyl and trans-linalool 3,7-oxide β-primeverosides as aroma precursors from leaves of a green tea cultivar. Biosci Biotechnol Biochem. 1999;63:1631–1633. doi: 10.1271/bbb.63.1631. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Ijima Y, Guo W, Watanabe N, Usui T, Dong S, Tong Q, Sakata K. Purification of a β-primeverosidase concerned with alcoholic aroma formation in tea leaves (cv. Shuixian) to be processed to oolong tea. J Agric Food Chem. 1997;45:877–882. [Google Scholar]
- Ogawa K, Moon JH, Guo W, Yagi A, Watanabe N, Sakata K. A study on tea aroma formation mechanism: alcoholic aroma precursor amounts and glycosidase and activity in parts of the tea plant. Z Naturforsch. 1995;50:493–498. doi: 10.1515/znc-1995-7-805. [DOI] [PubMed] [Google Scholar]
- Pare PW, Tumlinson JH. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999;121:325–332. [PMC free article] [PubMed] [Google Scholar]
- Pattnaik S, Subramanyam VR, Bapaji M, Kole CR. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios. 1997;89:39–46. [PubMed] [Google Scholar]
- Petrovic SD, Gorunovic MS, Wray V, Merfort I. A traxasterol derivative and phenolic compounds from Hieracium gymnocephalum. Phytochemistry. 1999;50:293–296. [Google Scholar]
- Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol. 2002;5:237–243. doi: 10.1016/s1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- Plouvier V. Study and distribution of primeverosidase and gentiobiosidase. C R Ser D. 1980;290:1071–1074. [Google Scholar]
- Poulton JE. Cyanogenesis in plants. Plant Physiol. 1990;94:401–405. doi: 10.1104/pp.94.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask L, Andreasson E, Ekbom B, Eriksson S, Pontoppidan B, Meijer J. Myrosinase: gene family evolution and herbivore defense in Brassicaceae. Plant Mol Biol. 2000;42:93–113. [PubMed] [Google Scholar]
- Rockenbach J, Nahrstedt A, Wray V. Cyanogenic glycosides from Psydrax and Oxyanthusspecies. Phytochemistry. 1992;31:567–570. [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD. Systemic acquired resistance. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H. Hydrolysis of flavonoid glycosides by enzymes (Rhamnodiastase) from Rhamnusand other sources. Arch Biochem Biophys. 1962;99:476–483. doi: 10.1016/0003-9861(62)90296-5. [DOI] [PubMed] [Google Scholar]
- Swain E, Li CP, Poulton JE. Tissue and subcellular localization of enzymes catabolizing (R)-amygdalin in mature Prunus serotinaseeds. Plant Physiol. 1992;100:291–300. doi: 10.1104/pp.100.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki A, Otsuka H, Ide T. Platanionosides A-C, megastigmane diglycosides from the leaves of Alangium platanifolium. J Nat Prod. 1999;62:1074–1076. doi: 10.1021/np990050l. [DOI] [PubMed] [Google Scholar]
- Tolley SP, Barrett TE, Suresh CG, Hughes MA. Crystallization and preliminary crystallographic analysis of the cyanogenic β-glucosidase from the white clover Trifolium repensL. J Mol Biol. 1993;229:791–793. doi: 10.1006/jmbi.1993.1082. [DOI] [PubMed] [Google Scholar]
- van der Plas LHW, Hargendoorn MJM, Jamar DC. Anthraquinoe glycosylation and hydrolysis in Morinda citrifoliacell suspensions: regulation and function. J Plant Physiol. 1998;152:235–241a. [Google Scholar]
- van de Ven WT, LeVesque CS, Perring TM, Walling LL. Local and systemic changes in squash gene expression in response to silverleaf whitefly feeding. Plant Cell. 2000;12:1409–1423. doi: 10.1105/tpc.12.8.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd TC, Dekker BMM, Hoekema A. A smallscale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989;17:2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter J. Plant cyanogenic glycosides. Toxicon. 2000;38:11–36. doi: 10.1016/s0041-0101(99)00128-2. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AW, He S, Grubb JH, Sly WS, Withers SG. Identification of Glu-540 as the catalytic nucleophile of human β-glucuronidase using electrospray mass spectrometry. J Biol Chem. 1998;273:34057–34062. doi: 10.1074/jbc.273.51.34057. [DOI] [PubMed] [Google Scholar]
- Wang D, Kurasawa E, Yamaguchi Y, Kubota K, Kobayashi A. Analysis of glycosidically bound aroma precursors in tea leaves: II. Changes in glycoside contents and glycosidase activities in tea leaves during the black tea manufacturing process. J Agric Food Chem. 2001;49:1900–1903. doi: 10.1021/jf001077+. [DOI] [PubMed] [Google Scholar]
- Wang D, Yoshimura T, Kubota K, Kobayashi A. Analysis of glycosidically bound aroma precursors in tea leaves: I. Qualitative and quantitative analyses of glycosides with aglycons as aroma compounds. J Agric Food Chem. 2000;48:5411–5418. doi: 10.1021/jf000443m. [DOI] [PubMed] [Google Scholar]
- Warzecha H, Gerasimenko I, Kutchan TM, Stockigt J. Molecular cloning and functional bacterial expression of a plant glucosidase specifically involved in alkaloid biosynthesis. Phytochemistry. 2000;54:657–666. doi: 10.1016/s0031-9422(00)00175-8. [DOI] [PubMed] [Google Scholar]
- Williams PJ, Strauss CR, Wilson B, Massywestropp RA. Novel monoterpene disaccharide glycosides of Vitis viniferagrapes and wine. Phytochemistry. 1982;21:2013–2030. [Google Scholar]
- Xue J, Jorgensen M, Pihlgren U, Rask L. The myrosinase gene family in Arabidopsis thaliana: gene organization, expression and evolution. Plant Mol Biol. 1995;27:911–922. doi: 10.1007/BF00037019. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Okada M, Usui T, Sakata K. Isolation and Characterization of a β-primeverosidase-like endo-manner β-glycosidase from Aspergillus fumigatusAP-20. Biosci Biotechnol Biochem. 2002;66:801–807. doi: 10.1271/bbb.66.801. [DOI] [PubMed] [Google Scholar]
- Yamamura S, Osawa K, Ohtani K, Kasai R, Yamasaki K. Antihistaminic flavones and aliphatic glycosides from Mentha spicata. Phytochemistry. 1998;48:131–136. [Google Scholar]
- Yano M, Joki Y, Mutoh M, Kubota K, Kobayashi A. Benzyl glucoside from tea leaves. Agric Biol Chem. 1991;55:1205–1206. [Google Scholar]
- Yasuda T, Nakagawa H. Purification and characterization of the rutin-degrading enzymes in tartary buckwheat seeds. Phytochemistry. 1994;37:133–136. [Google Scholar]
- Zechel DL, Withers SG. Glycosidase mechanism: anatomy of a finely tuned catalyst. Acc Chem Res. 2000;33:11–18. doi: 10.1021/ar970172+. [DOI] [PubMed] [Google Scholar]
- Zheng L, Poulton JE. Temporal and spatial expression of amygdalin hydrolase and (R)-(+)-mandelonitrile lyase in black cherry seeds. Plant Physiol. 1995;109:31–39. doi: 10.1104/pp.109.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]