Abstract
In the big brown bat, Eptesicus fuscus, the response properties of neurons and the cochleotopic (frequency) maps in the auditory cortex (AC) and inferior colliculus can be changed by auditory conditioning, weak focal electric stimulation of the AC, or repetitive delivery of weak, short tone bursts. The corticofugal system plays an important role in information processing and plasticity in the auditory system. Our present findings are as follows. In the AC, best frequency (BF) shifts, i.e., reorganization of a frequency map, slowly develop and reach a plateau ≈180 min after conditioning with tone bursts and electric-leg stimulation. The plateau lasts more than 26 h. In the inferior colliculus, on the other hand, BF shifts rapidly develop and become the largest at the end of a 30-min-long conditioning session. The shifted BFs return (i.e., recover) to normal in ≈180 min. The collicular BF shifts are not a consequence of the cortical BF shifts. Instead, they lead the cortical BF shifts. The collicular BF shifts evoked by conditioning are very similar to the collicular and cortical BF shifts evoked by cortical electrical stimulation. Therefore, our working hypothesis is that, during conditioning, the corticofugal system evokes subcortical BF shifts, which in turn boost cortical BF shifts. The cortical BF shifts otherwise would be very small. However, whether the cortical BF shifts are consequently boosted depends on nonauditory systems, including nonauditory sensory cortices, amygdala, basal forebrain, etc., which determine the behavioral relevance of acoustic stimuli.
Keywords: associative learning, conditioning, hearing, reorganization of tonotopic map, somatosensory cortex
The response properties of neurons and the sensory map in a sensory cortex and subcortical sensory nucleus can be changed by conditioning, learning of a discrimination task, or focal cortical electrical stimulation (see refs. 1–3 for reviews). In the central nucleus of the inferior colliculus (IC) of the big brown bat, Eptesicus fuscus, the best frequency (BF) of a neuron shifts toward the frequency of a conditioned tone that is followed by an unconditioned electric leg stimulus (ESl). The BF shift of ≈1.5 kHz lasts up to 180 min after a 30-min-long conditioning session. Inactivation of the primary auditory cortex (AC) during the conditioning abolishes the collicular BF shift that otherwise would be evoked (4). Focal electrical stimulation of the AC evokes collicular (5, 6) and cortical BF shifts (6, 7) very similar in the amount and duration (retention) to the collicular BF shift evoked by the conditioning. These data indicate that the collicular BF shift evoked by the conditioning is produced by the corticofugal system but do not indicate whether the collicular BF shift is due to the cortical BF shift or vice versa. It has not yet been determined which is the case, because it is very difficult, if not impossible, to inactivate corticofugal fibers selectively without inactivating thalamocortical fibers. One of the aims of the present paper is to present data that strongly suggest that the cortical BF shift is boosted by the collicular BF shift mediated by the corticofugal system.
In the guinea pig, BF shifts evoked by conditioning last for several weeks in the AC (8) but about 1 h in the ventral division of the medial geniculate body (MGB), which receives the input from the central nucleus of the IC (9). Because the BF shifts observed in the AC or the central nucleus of the IC of the big brown bat last up to 3.0 h for cortical electrical stimulation, these BF shifts are somewhat comparable to those obtained from the MGB but quite different from those obtained from the AC of the guinea pig. This difference may be due to the difference in species or the difference in cortical effect between the cortical electrical stimulation and conditioning. The second aim of our paper is to demonstrate that the difference is not due to a species difference but rather due to the difference between cortical electrical stimulation and conditioning. We will demonstrate that long-lasting cortical BF shifts depend on the activity of the auditory corticofugal system and nonauditory systems.
It has been demonstrated that the basal forebrain plays an important role in cortical plasticity in the guinea pig (10, 11) and in the cat (12). The basal forebrain plays the same role in the big brown bat (6). Gao and Suga (4) demonstrated that the somatosensory cortex as well as the AC is necessary for collicular BF shifts evoked by auditory conditioning, i.e., by associative learning; that the corticofugal system plays an essential role in the collicular BF shift; and that the slow return of the collicular BF shift in 180 min does not depend on the corticofugal activity but on the IC itself (4). However, they did not show whether the somatosensory cortex is also necessary for cortical BF shifts. The third aim of the present paper is to report the effect of the bilateral inactivation of the somatosensory cortices during auditory conditioning on cortical BF shifts.
The present paper describes an important aspect of the functional role of the corticofugal system in plasticity (reorganization) of the cochleotopic (i.e., frequency) map in the central auditory system related to associative learning.
Materials and Methods
Preparation.
Experiments were performed with 31 adult big brown bats (E. fuscus). Procedures for animal preparation, acoustic stimulation, ESl and recording of action potentials have been described (4, 13). The protocol for this research was approved by the animal studies committee of Washington University. Under neuroleptanalgesia (Innovar 4.08 mg/kg body weight), a 15-mm-long metal post was glued to the dorsal surface of the bat's skull. Experiments for recording auditory responses from single neurons began 3–4 days after the surgery. The unanesthetized bat was placed in a polyethylene-foam body mold suspended by an elastic band at the center of a soundproof room maintained at a temperature of about 31°C. The head was immobilized by fixing the metal post glued on the skull onto a metal rod with set screws and was adjusted to face directly at a loudspeaker located 74 cm away.
Acoustic and/or Electric Stimuli.
Acoustic stimuli (20-ms-long tone bursts with a 0.5-ms rise–decay time) were delivered to the bat at a rate of five per second. Their frequency and amplitude were varied manually to measure the BF and minimum threshold of a given neuron. Then, the tone bursts were computer controlled. The sharpness of the manually measured tuning curve was used to determine the step size (0.2–1.0 kHz) of a computer-controlled frequency scan: the sharper the tuning curve, the smaller the step. The frequency scan usually consisted of 21 200-ms-long time blocks or of 34 150-ms-long time blocks when a tuning curve was particularly wide. In each scan, a single tone burst was delivered at the beginning of each block, and the frequency of the tone burst was shifted in 20 or 33 steps across the BF of the neuron. The amplitude of the tone bursts in the scan was always set at 10 dB above the minimum threshold of the neuron to detect a shift in BF more easily. An identical frequency scan was delivered 50 or 100 times to obtain an array of poststimulus time histograms as a function of frequency. BFs and frequency-response curves were obtained by counting the total numbers of impulses discharged to 50 or 100 identical tone bursts.
To evoke changes in the BF and frequency-response curve of a cortical or collicular neuron, the bat was conditioned with a 1.0-s-long train of acoustic stimuli (ASt), followed by a 1.0-s gap and then by an ESl (hereafter, ASt + ESl). In ASt, the tone bursts were 50 dB sound pressure level and 10 ms-long and were delivered at a rate of 33 per second. Their frequencies were always 5.0 kHz lower than the BF of a given cortical or collicular neuron to be studied, because ASt evoked the largest BF shift for cortical (7) and collicular neurons (4, 5) with a BF ≈5.0 kHz higher than that of ASt or the BF of cortical neurons electrically stimulated. ESl was a 50-ms-long monophasic electric pulse. The intensity of ESl was just above the threshold (0.15–0.57 mA) for eliciting a just-noticeable leg flexion, which was monitored with a strain gauge. This trace conditioning (ASt + ESl) was 1.0-s-long ASt + 1.0-s gap + 50-ms-long ESl, and backward conditioning (ESl + ASt) was 50-ms-long ESl + 1.0-s gap + 1.0-s-long ASt. A single stimulus or paired stimuli were delivered every 30 s for 30 min (60 times in total). ASt and ESl were the conditioned and unconditioned stimuli, respectively.
Bilateral Inactivation of the Somatosensory Cortex.
In the big brown bat, the corticofugal system is maximally inactivated 20 min after an application of 0.4 μg of muscimol (an agonist of an inhibitory synaptic transmitter, γ-aminobutyric acid) to the AC, and the inactivation stays near the maximum over 220 min and then gradually disappears over 60 min (4). To examine whether the primary somatosensory cortex influences the cortical and collicular BF shifts evoked by conditioning (ASt + ESl), the entire primary somatosensory cortex was inactivated bilaterally during conditioning by 0.4 μg of muscimol applied to each somatosensory cortex 20 min before the conditioning. The method used to apply muscimol to the cortex has been described (4, 14). The primary somatosensory cortex was localized by identifying the somatotopic map (15) and by recording neural responses to touch stimuli before muscimol application.
Acquisition and Processing of Neural Responses.
The responses of cortical and collicular neurons to tone bursts were recorded at 200- to 700-μm depths in the AC or 300- to 2,000-μm depths in the IC with tungsten-wire microelectrodes (≈7-μm tip diameter). A time-amplitude window discriminator was used to select action potentials from a single neuron. In five animals, the electrodes were implanted chronically in the AC, but only two neurons were recorded successfully for more than 10 h.
The BFs and frequency-response curves of cortical and collicular neurons were measured in each bat before and after a 30-min delivery of ASt alone, (ASt + ESl), (ESl + ASt), and/or (ASt + ESl) + (ASt + ESl), which was a “double” conditioning session. In each double conditioning session, the first and second sessions were identical, and the second session was delayed from the end of the first by 10, 90, or 180 min. All of the measurements were based on arrays of poststimulus time histograms obtained as a function of frequency. To minimize a cumulative effect of conditioning, only one neuron was studied in a 1-day experiment, and the same animal was used only after a 1- to 3-day interval. In each 1-day experiment, tone bursts alone were delivered at a rate of five per second over ≈2.0–3.0 h to record single-unit activity and to obtain data in the control condition. This period presumably evoked extinction of BF shifts, if any remained, after a previous conditioning experiment.
To test the significance of a BF shift, a weighted average frequency (i.e., BF) was calculated for the summed response to five consecutive frequency scans. Then, the mean and standard error of these weighted averages were computed, and a two-tailed, paired t test was used to determine whether the weighted-average frequencies obtained before and after conditioning were significantly different at P < 0.01.
Results and Discussion
Shifts in the BFs of Collicular and Cortical Neurons Evoked by Acoustic Stimulation Paired with ESl (Trace Conditioning).
The behavioral response to an ESl was leg flexion and body movement. When an ASt followed by ESl was delivered to the animal for 30 min, the animal showed the conditioned response to ASt in the last 10 min of the 30-min period, and collicular and cortical neurons shifted their BFs toward the frequency of ASt. However, neither a conditioned response nor BF shift was evoked by ASt alone or by backward conditioning (ESl + ASt).
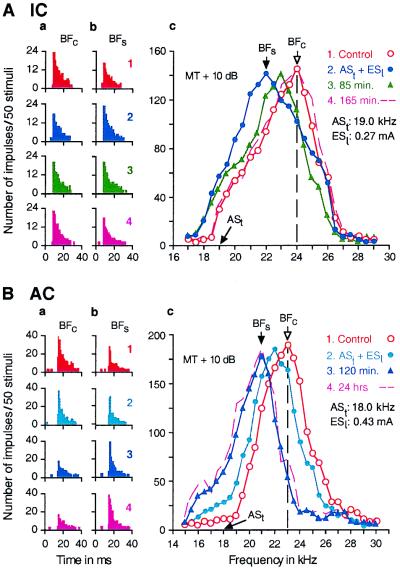
Fig. 1 shows the responses and the frequency-response curves of a single collicular neuron (Fig. 1A) and a single cortical neuron (Fig. 1B). In Fig. 1A, the collicular neuron was tuned to 24 kHz (open circles in Fig. 1Ac). When the animal was conditioned with a 19-kHz ASt paired with ESl, the response of the neuron decreased by 32.3% to a 24-kHz tone burst (Fig. 1A, compare a2 with a1) but increased by 54.5% to a 22-kHz tone burst (Fig. 1A, compare b2 with b1). As a result of these frequency-dependent changes, the frequency-response curve of the neuron shifted to 22 kHz from 24 kHz (Fig. 1A, filled circles in c). Both the response and the frequency-response curve returned (hereafter, recovered) to those in the control condition ≈165 min after the cessation of the 30-min-long conditioning (Fig. 1A, a4, b4, and dashed line in c). All 48 collicular neurons studied showed changes that were basically the same as those described above.
Figure 1.
Changes in the responses (a and b) and frequency-response curves (c) of a single collicular neuron (A) and a single cortical neuron (B) evoked by a 30-min-long conditioning session consisting of 60 stimulus pairs of a short ASt followed by an ESl. All of the data were obtained with tone bursts fixed at 10 dB above the minimum threshold (MT) of a given neuron. In A, ASt was 19.0 kHz, and the BF of the collicular neuron was 24.0 kHz. In B, ASt was 18.0 kHz, and the BF of the cortical neuron was 23.0 kHz. The data in A and B were obtained before (1, control condition), immediately after (2), 85 or 120 min after (3), and 165 min or 24 h after conditioning (4). The poststimulus time histograms in columns a and b, respectively, show the changes in the responses at the BFs in the control (BFc) and “shifted” conditions (BFs), which are indicated by arrows in c. The BF shift of the collicular neuron recovered (i.e., BFs shifted back to BFc) 165 min after the conditioning, but that of the cortical neuron did not recover even 24 h after the conditioning.
In Fig. 1B, the cortical neuron was tuned to 23 kHz (Fig. 1B, open circles in c). When the animal was conditioned with a 18-kHz ASt followed by ESl, the response of the neuron immediately after the conditioning decreased by 13.5% to a 23-kHz tone burst (Fig. 1B, compare a2 with a1) but increased by 10.8% to a 22-kHz tone burst (Fig. 1B, compare b2 with b1). As a result, the BF of the neuron shifted down to 22 kHz from 23 kHz (Fig. 1B, filled circles in c). About 2 h after the conditioning, however, the response decreased further by 72.0% at 23 kHz (Fig. 1B, compare a3 with a1) and increased further by 47.9% at 21 kHz (Fig. 1B, compare b3 with b1). Because of these changes, the frequency-response curve shifted further down to 21 kHz (Fig. 1B, filled triangles in c). The shifted response and frequency-response curve of the neuron showed no sign of recovery as far as we tested (Fig. 1B, e.g., a4, b4, and dashed line in c were obtained 24 h after the conditioning). Of 49 cortical neurons studied, 46 showed changes that were basically the same as those described above. However, the remaining three showed no significant BF shift. In 2 neurons of the 46, responses to tone bursts were recorded up to 26 h after the conditioning. These responses showed no sign of recovery of a shifted BF (open triangles in Fig. 2). The time course of the BF shift and the recovery of BF were dramatically different between collicular and cortical neurons.
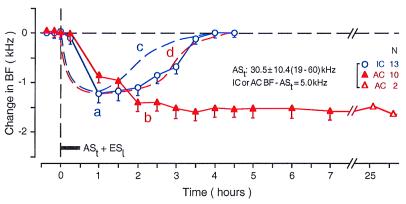
Figure 2.
Time courses of the BF shifts of collicular (curve a, open circles) and cortical neurons (curve b, filled triangles) evoked by a 30-min-long conditioning session consisting of ASt + ESl (horizontal bar). The time course is quite different between the collicular and cortical neurons. Each data point represents the mean and standard error of the values obtained from the number of neurons (N) listed on the right. The mean frequency of ASts ranged from 19 to 60 kHz (30.5 ± 10.4 kHz). The BFs of 13 collicular and 10 cortical neurons studied were 5.0 kHz higher than the frequency of ASt. For comparison, the time courses of the BF shifts of collicular and cortical neurons evoked by a focal electrical stimulation of the AC are also shown in the figure by the dashed curves c and d, respectively (based on refs. 6 and 7). These two dashed curves are similar to curve a.
In Fig. 2, the averaged time courses of the BF shifts of 13 collicular (curve a) and 10 cortical neurons (curve b) evoked by the conditioning show the following five facts. (i) The collicular BF shift was largest (1.12 ± 0.14 kHz) at the end of the conditioning and monotonically recovered ≈180 min after the conditioning. (ii) The cortical BF shift slowly developed and reached a plateau (1.50 ± 0.14 kHz) ≈180 min after the conditioning. The plateau lasted for at least 26 h. (iii) Immediately after the 30-min-long conditioning, the cortical BF shift (0.81 ± 0.13 kHz) was smaller than the collicular BF shift (1.12 ± 0.14 kHz). This difference is significant (P = 0.003). Such a difference is also shown in Fig. 3 A–C. For the 48 collicular and 46 cortical neurons studied, the difference in BF shift at the end of the conditioning was 0.32 ± 0.04 kHz. This difference was highly significant (1.15 ± 0.06 kHz vs. 0.83 ± 0.09 kHz; P < 0.0005). (iv) The cortical BF shift reached a plateau (i.e., largest) when the collicular BF shift had almost recovered, i.e., shifted back to the BF of the control condition. This plateau is also clear in the data shown in Fig. 3. (v) At the peak or plateau, the cortical BF shift is slightly larger than the collicular BF shift (1.50 ± 0.09 kHz vs. 1.12 ± 0.14 kHz; P = 0.03). These data suggest that the collicular change occurs first and evokes the cortical change. The relationship between the collicular and cortical changes was examined further through double conditioning sessions.
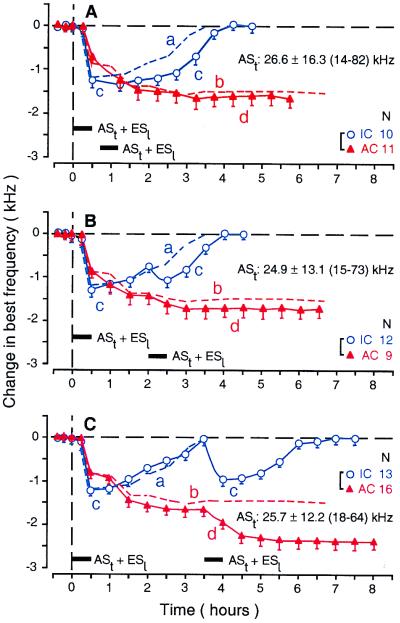
Figure 3.
Time courses of the BF shifts of collicular (curve c, open circles) and cortical neurons (curve d, filled triangles) evoked by two conditioning sessions with an identical 30-min-long conditioning session consisting of ASt + ESl (horizontal bars). The time interval between the first and second conditioning sessions was 10 (A), 90 (B), or 180 min (C). Each data point represents the mean and standard error of the values obtained from the number of neurons (N) listed on the right. The mean frequency and the range of ASts are listed in each figure. The BFs of the neurons studied were 5.0 kHz higher than the frequency of ASt. The dashed curves a and b represent the time courses of collicular (curve a) and cortical BF shifts (curve b) evoked by the first conditioning session alone and are the same as curves a and b in Fig. 2.
Effects of a Second Conditioning Session on BFs of Collicular and Cortical Neurons.
The BF shifts of collicular and cortical neurons were investigated further with a second conditioning session conducted 10, 90, or 180 min after the cessation of the first conditioning session. These time intervals corresponded to the times for the largest, half recovered, and completely recovered collicular BF shift, respectively. When the second conditioning session occurred 10 min after the first, cortical neurons showed no additional BF shift (Fig. 3A, filled triangles), but collicular neurons showed a significant increase in the duration of BF shift (Fig. 3A, open circles). In other words, the collicular change occurred without a cortical change. When the second conditioning session occurred 90 min after the first, cortical neurons tended to show an additional BF shift (increase from 1.50 ± 0.09 kHz to 1.72 ± 0.11 kHz; n = 45), but it was small and not significant (P = 0.24). The plateau of the cortical BF shift evoked by the second conditioning session appeared ≈30 min after that (Fig. 3B, filled triangles). On the other hand, collicular neurons showed an additional BF shift that peaked by the end of the second conditioning session. The BF shift at the peak tended to be smaller than that evoked by the first session, but it was statistically insignificant (1.01 ± 0.12 kHz for the second vs. 1.12 ± 0.14 kHz for the first; n = 12; P = 0.18). The recovery was faster than that evoked by the first session (1.44 ± 0.19 h for the second vs. 2.52 ± 0.22 h for the first; P = 0.0007; Fig. 3B, open circles). When the second conditioning session occurred 180 min after the first, cortical neurons showed an additional prominent BF shift plateau at ≈90 min after the second session (increase from 1.54 ± 0.10 kHz to 2.40 ± 0.11 kHz; n = 16; P < 0.05; Fig. 3C, filled triangles). Collicular neurons, as expected, also showed a BF shift that tended to be slightly smaller than that evoked by the first session (0.92 ± 0.11 kHz for the second vs. 1.15 ± 0.13 kHz for the first; n = 13; P = 0.10). This additional collicular BF shift was largest at the end of the second session (Fig. 3C, open circles). The recovery tended to be faster than that evoked by the first session (2.04 ± 0.17 h for the second vs. 2.61 ± 0.21 h for the first; P = 0.10).
The data shown in Fig. 3 indicate the following three facts. (i) The collicular BF shift is not at all a consequence of the cortical BF shift. (ii) The collicular BF shift always proceeds the cortical BF shift. (iii) The increasing phase of the collicular BF shift is more related to the large cortical BF shift than is its decreasing phase. It seems to boost the cortical BF shift.
A focal electrical stimulation of the AC evokes collicular and cortical BF shifts (5–7). These shifts almost immediately start to develop together, reach the same peak at the same time, and recover in ≈180 min. Although the cortical BF shift tends to last slightly longer than the collicular BF shift (Fig. 2, dashed curves c and d). On the other hand, the collicular BF shift evoked by the conditioning developed with a ≈15-min delay but reached the same peak as the collicular BF shift evoked by the cortical electrical stimulation at nearly the same time and then recovered in ≈180 min (Fig. 2, curve a). Therefore, the collicular BF shift evoked by the conditioning was similar to that evoked by the cortical electrical stimulation. However, the cortical BF shift evoked by the conditioning (Fig. 2, curve b) was quite different from that evoked by the cortical electrical stimulation. It slowly developed with a ≈15-min delay, reached a plateau in ≈180 min, and lasted for many hours after the conditioning.
The cortical electrical stimulation directly stimulates the AC and corticofugal system, whereas the conditioning (ASt + ESl) stimulates the auditory and somatosensory systems and excites brain regions related to associative learning and the cholinergic basal forebrain (see ref. 2 for review). Therefore, the cortical electrical stimulation and the conditioning are quite different from each other but evoke very similar collicular BF shifts. Because an inactivation of the AC during the conditioning abolishes the collicular BF shift that otherwise would be evoked (4), the collicular BF shift evoked by the conditioning must be produced by the auditory corticofugal system.
These findings indicate the following. The cortical electric stimulation does not excite nonauditory systems, such that the cortical BF shift is evoked by the cortical neural net and the corticofugal system, which forms multiple feedback loops via the MGB, IC, and subcollicular auditory nuclei and evokes subcortical (e.g., collicular) BF shifts. On the other hand, in auditory conditioning, the AC and corticofugal system evoke the subcortical (e.g., collicular) BF shifts, which in turn boost the cortical BF shift, depending on the activity of nonauditory systems. Accordingly, the cortical BF shift can be quite different from the subcortical (e.g., collicular) BF shifts in time course and amount.
Effects of Bilateral Inactivation of the Somatosensory Cortex on Collicular and Cortical BF Shifts Evoked by Auditory Conditioning.
The nonauditory systems modulating the cortical BF shift evoked by auditory conditioning include at least the cholinergic basal forebrain (10, 12) and the somatosensory cortex (4). In the big brown bat, the electrical stimulation of the basal forebrain or the somatosensory cortex augments the cortical and collicular BF shifts evoked by electrical stimulation of the AC (6). Bilateral inactivation of the primary somatosensory cortex during auditory conditioning abolishes the collicular BF shift that otherwise would be evoked by the conditioning (4). As described below, it also abolishes the cortical BF shift.
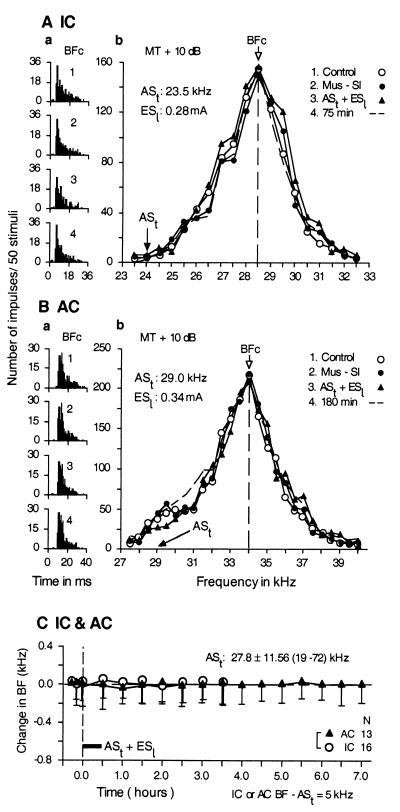
The effect of bilateral inactivation of the primary somatosensory cortex with 0.4 μg of muscimol on the collicular and cortical BF shifts evoked by the conditioning was studied in 16 collicular and 13 cortical neurons. All of the data obtained from 29 neurons were identical. Fig. 4 shows that the bilateral inactivation of the somatosensory cortex during the conditioning abolished the changes in the auditory responses (Fig. 4 Aa and Ba) and frequency-response curves (Fig. 4 Ab and Bb) of a collicular (Fig. 4A) and a cortical neuron (Fig. 4B) that otherwise would be evoked by conditioning. The averaged data of the 16 collicular and 13 cortical neurons shown in Fig. 4C indicate that no BF shifts were evoked by the conditioning when the somatosensory cortex was inactivated bilaterally (0.03 ± 0.06 kHz vs. 0.00 ± 0.11 kHz; P = 0.63).
Figure 4.
Bilateral inactivation of the somatosensory cortex with 0.4 μg of muscimol abolished changes in the responses (a) and frequency-response curves (b) of a single collicular neuron (A) and a single cortical neuron (B) that otherwise would be evoked by auditory conditioning. The poststimulus time histograms in a display the responses to tone bursts at the BF of a given neuron before (1, control condition); during muscimol application to the somatosensory cortices (2); immediately after conditioning under bilateral inactivation (3); and 75 or 180 min after conditioning (4). The frequency-response curves in b were obtained in the above four conditions. The frequencies of conditioned ASt and the current of unconditioned ESl are listed in b. BFc, BF in the control condition. (C) No changes in collicular (filled circles) and cortical BFs (filled triangles) were evoked by the conditioning with ASt + ESl (horizontal bar) because of bilateral inactivation of the somatosensory cortex. Each data point represents the mean and standard error of the values obtained from 16 collicular or 13 cortical neurons (as indicated on the right, N). The mean frequency and the range of ASts used were 27.8 ± 11.56 kHz and 19–72 kHz, respectively. The BFs of 29 neurons studied were 5.0 kHz higher than the frequency of ASt. All of the data were obtained with a tone burst delivered at 10 dB above the minimum threshold (MT) of a given neuron.
One may speculate that muscimol applied to the primary somatosensory cortex during the conditioning might inactivate not only the somatosensory cortex but also the AC and might cause neither collicular nor cortical BF shifts. Therefore, muscimol was applied bilaterally to the dorsoposterior portion to the AC (presumably the visual cortex) during the conditioning, and six collicular and six cortical neurons were studied. All of these 12 auditory neurons showed a normal BF shift for the conditioning. It is thus very unlikely that muscimol applied to the somatosensory cortex inactivated the AC.
Our Working Hypothesis.
By 1990, a number of important findings on learning and memory had been made. (i) Acetylcholine plays an important role in learning and memory (see ref. 16 for review). (ii) The cholinergic basal nucleus of the forebrain projects diffusely and widely to the cerebral cortex (see ref. 17 for review). (iii) The basal forebrain plays an important role in learning and memory (18–20). (iv) The basal forebrain receives an input from the amygdala, which is necessary for the acquisition of conditioned response (21–23). (v) The amygdala receives an input from thalamic nuclei (24). Adapting these findings to auditory conditioning and learning, Weinberger (2, 25) hypothesized that auditory and somatosensory stimuli used for conditioning are associated for associative learning in the medial division of the MGB and the posterior intralaminar complex, both in the thalamus; that the associated signal is then sent to the amygdala, which in turn sends the signal to the basal forebrain; and that the basal forebrain increases cortical acetylcholine levels and amplifies the effect of the medial division of the MGB on the cortical neurons, which are excited by the conditioned stimulus.
Four findings described in the present and previous papers (4), however, do not fit the above hypothesis. (i) The excitation of the AC by an ASt and the excitation of the somatosensory cortex by an ESl both are essential for cortical and collicular BF shifts evoked by conditioning. These sensory cortices indirectly project to the amygdala through the association cortices (26, 27). Therefore, the neural pathway from the sensory cortices to the amygdala is presumably much more important than that from the sensory thalamus to the amygdala for plastic changes in the auditory system related to auditory associative learning. (ii) Collicular changes proceed cortical changes. This fact strongly suggests that collicular changes evoked by the corticofugal system are necessary for boosting cortical changes that otherwise would be very small. (iii) The AC and corticofugal system have an intrinsic mechanism for cortical and subcortical plastic changes, which are highly specific to the parameters characterizing the acoustic stimuli (4, 5, 28, 29). (iv) Cortical changes evoked by conditioning are different from those evoked by focal cortical activation. This fact strongly suggests that the cortical changes that would be evoked by the corticofugal system (i.e., by subcortical changes) are modulated by nonauditory systems (4). Therefore, we want to propose the following working hypothesis.
According to cortical activation evoked by acoustic stimuli, the corticofugal system adjusts and improves subcortical signal processing. Whether the AC changes according to the subcortical change depends on nonauditory systems that suppress or augment cortical changes. The system for suppression hypothesized herein may be related to a mechanism for the extinction of conditioned response, which has been speculated to be evoked by inhibition mediated by the corticofugal system (30). One of the systems for augmentation must consist of nonauditory sensory cortices (the somatosensory cortex in our conditioning experiment), amygdala, and cholinergic basal forebrain. During conditioning with acoustic stimuli followed by an ESl, the auditory and somatosensory cortices indirectly send signals to the amygdala where the sensory signals are associated with each other. When the acoustic stimuli become behaviorally relevant to the animal, the associated signals are sent from the amygdala to the cholinergic basal forebrain, which in turn increases the cortical acetylcholine level. Then, cortical changes based on the corticofugal system (i.e., egocentric selection) are augmented. Our working hypothesis uses part of the hypothesis proposed by Weinberger (2, 25).
Armony et al. (31) found that conditioning with a conditioned acoustic stimulus followed by an unconditioned electric stimulus augmented a short latency response and evoked a long latency response of cortical auditory neurons and that the long latency response was abolished by amygdala lesion, but the short latency response was not. Conditioning changes neural activity in several brain regions, including the auditory and somatosensory cortices, the hippocampus, and the amygdala. The amygdala is necessary for the acquisition of conditioned behavioral response, whereas the hippocampus and sensory cortices are necessary for the memory of the relation between the conditioned and unconditioned stimuli (32–34). Therefore, cortical plasticity evoked by conditioning remains to be evaluated in relation to conditioned response and memory, i.e., in relation not only to the amygdala but also to the hippocampus.
Acknowledgments
We thank Dr. L. S. Green, Dr. J. H. Kass, Dr. M. Konishi, and Mr. N. Laleman for their comments on the manuscript. This work has been supported by National Institute on Deafness and Other Communicative Disorders Grant DC 00175.
Footnotes
Abbreviations: AC, auditory cortex in the cerebrum; ASt, train of acoustic stimuli; BF, best frequency; ESl, electric stimulation of a leg; IC, inferior colliculus in the midbrain; MGB, medial geniculate body in the thalamus.
References
- 1.Irvine D R, Rajan R. Clin Exp Pharmacol Physiol. 1996;23:939–947. doi: 10.1111/j.1440-1681.1996.tb01146.x. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger N M. Neurobiol Learn Mem. 1998;70:226–251. doi: 10.1006/nlme.1998.3850. [DOI] [PubMed] [Google Scholar]
- 3.Buonomano D V, Merzenich M M. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 4.Gao E, Suga N. Proc Natl Acad Sci USA. 1998;95:12663–12670. doi: 10.1073/pnas.95.21.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan W, Suga N. Nat Neurosci. 1998;1:54–58. doi: 10.1038/255. [DOI] [PubMed] [Google Scholar]
- 6.Ma X, Suga N. Assoc. Res. Otolaryngol. Abstr. 2000. , 259. [Google Scholar]
- 7.Chowdhury A S, Suga N. J Neurophysiol. 2000;83:1856–1863. doi: 10.1152/jn.2000.83.4.1856. [DOI] [PubMed] [Google Scholar]
- 8.Weinberger N M, Javid R, Lepan B. Proc Natl Acad Sci USA. 1993;90:2394–2398. doi: 10.1073/pnas.90.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edeline J M, Weinberger N M. Behav Neurosci. 1991;105:618–639. doi: 10.1037//0735-7044.105.5.618. [DOI] [PubMed] [Google Scholar]
- 10.Bakin J S, Weinberger N M. Proc Natl Acad Sci USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjordahl T S, Dimyan M A, Weinberger N M. Behav Neurosci. 1998;112:467–479. doi: 10.1037//0735-7044.112.3.467. [DOI] [PubMed] [Google Scholar]
- 12.Kilgard M P, Merzenich M M. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 13.Suga N, O'Neill W E, Kujirai K, Manabe T. J Neurophysiol. 1983;49:1573–1626. doi: 10.1152/jn.1983.49.6.1573. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Suga N. J Neurophysiol. 1997;78:3489–3492. doi: 10.1152/jn.1997.78.6.3489. [DOI] [PubMed] [Google Scholar]
- 15.Krubitzer L A, Calford M B. J Comp Neurol. 1992;317:1–30. doi: 10.1002/cne.903170102. [DOI] [PubMed] [Google Scholar]
- 16.Bartus R T, Dean R L, III, Beer B, Lippa A S. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 17.Johnston M V, McKinney M, Coyle J T. Proc Natl Acad Sci USA. 1979;76:5392–5396. doi: 10.1073/pnas.76.10.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartus R T, Flicker C, Dean R L, Pontecorvo M, Figueiredo J C, Fisher S K. Pharmacol Biochem Behav. 1985;23:125–135. doi: 10.1016/0091-3057(85)90139-x. [DOI] [PubMed] [Google Scholar]
- 19.Rigdon G C, Pirch J H. J Neurosci. 1986;6:2535–2542. doi: 10.1523/JNEUROSCI.06-09-02535.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wozniak D F, Stewart G R, Finger S, Olney J W, Cozzari C. Neurobiol Aging. 1989;10:173–179. doi: 10.1016/0197-4580(89)90027-4. [DOI] [PubMed] [Google Scholar]
- 21.Krettek J E, Price J L. J Comp Neurol. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- 22.Price J L, Amaral D G. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peinado-Manzano A. Behav Brain Res. 1988;29:61–71. doi: 10.1016/0166-4328(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 24.Ottersen O P, Ben-Ari Y. J Comp Neurol. 1979;187:401–424. doi: 10.1002/cne.901870209. [DOI] [PubMed] [Google Scholar]
- 25.Weinberger N M. Concepts Neurosci. 1990;1:91–123. [Google Scholar]
- 26.Amaral D G, Price J L, Pitkanen A, Carmichael S T. In: The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. Aggleton J P, editor. New York: Wiley–Liss; 1992. pp. 1–66. [Google Scholar]
- 27.Romanski L M, LeDoux J E. Cereb Cortex. 1993;3:515–532. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- 28.Yan J, Suga N. Science. 1996;273:1100–1103. doi: 10.1126/science.273.5278.1100. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Suga N, Yan J. Nature (London) 1997;387:900–903. doi: 10.1038/43180. [DOI] [PubMed] [Google Scholar]
- 30.Teich A H, McCabe P M, Gentile C C, Schneiderman L S, Winters R W, Liskowsky D R, Schneoderman N. Brain Res. 1989;480:210–218. doi: 10.1016/0006-8993(89)91584-9. [DOI] [PubMed] [Google Scholar]
- 31.Armony J L, Quirk G J, Ledoux J E. J Neurosci. 1998;18:2592–2601. doi: 10.1523/JNEUROSCI.18-07-02592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio A R. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- 33.LaBar K S, LeDoux J E, Spencer D D, Phelps E A. J Neurosci. 1995;15:6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips R G, LeDoux J E. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]