Abstract
Root hairs develop as long extensions from root epidermal cells. After the formation of an initial bulge at the distal end of the epidermal cell, the root hair structure elongates by tip growth. Because root hairs are not surrounded by other cells, root hair formation provides an excellent system for studying the highly complex process of plant cell growth. Pharmacological experiments with actin filament-interfering drugs have provided evidence that the actin cytoskeleton is an important factor in the establishment of cell polarity and in the maintenance of the tip growth machinery at the apex of the growing root hair. However, there has been no genetic evidence to directly support this assumption. We have isolated an Arabidopsis mutant, deformed root hairs 1 (der1), that is impaired in root hair development. The DER1 locus was cloned by map-based cloning and encodes ACTIN2 (ACT2), a major actin of the vegetative tissue. The three der1 alleles develop the mutant phenotype to different degrees and are all missense mutations, thus providing the means to study the effect of partially functional ACT2. The detailed characterization of the der1 phenotypes revealed that ACT2 is not only involved in root hair tip growth, but is also required for correct selection of the bulge site on the epidermal cell. Thus, the der1 mutants are useful tools to better understand the function of the actin cytoskeleton in the process of root hair formation.
Cell division, growth, and differentiation are basic processes underlying plant development. Plant cell growth is a complex process, as the cells are surrounded by a cell wall that limits enlargement of the cell. Therefore, plant cell growth requires a well-coordinated and tightly controlled expansion of the protoplast and the cell wall. Root hairs provide an excellent system for studying the process of cell growth. The root hair structure is not surrounded by other cells that would limit its expansion. As a consequence, aberrations in root hair development are readily observable. Root epidermal cells in Arabidopsis develop into root hair-forming cells (trichoblasts) or non-root hair-forming cells (atrichoblasts), depending on positional cues relative to the underlying cortical cells. Epidermal cells that are in contact with the periclinal wall between two adjacent cortical cells develop into trichoblasts, whereas those that overlie a single cortical cell will become atrichoblasts (Dolan et al., 1993). Once an epidermal cell is determined to become a trichoblast cell, the process of root hair development can be divided into three phases: First, a small bulge forms at the distal end of the epidermal cell; second, a slow-growing root hair develops by tip growth; and third, the tip-growth rate increases and the hair structure fully develops (Dolan et al., 1994). In higher plants, tip growth is found in root hairs and pollen tubes and it constitutes an extreme form of polarized growth in which vesicles containing new cell wall material are fused to the membrane at the very extreme end (tip) of the growing cell (Cai et al., 1997; Yang, 1998; Galway, 2000). In a genetic approach to studying the mechanism of root hair development in Arabidopsis, a large number of root hair mutants has been isolated. Root hairs of these plants show different phenotypes such as reduced tip growth, development of branched root hairs, wavy root hairs, bulbous structures at the root hair basis, or burst root hairs (Schiefelbein and Somerville, 1990; Schiefelbein et al., 1993; Masucci and Schiefelbein, 1994; Grierson et al., 1997; Parker et al., 2000; for review, see Schiefelbein, 2000). Several mutant loci involved in root hair development have been cloned and encode proteins with different functions such as a GTP-binding protein (rhd3), a putative K+ transporter (trh1), a cellulose synthase-like protein (Kojak), and an extracellular, chimeric Leu-rich repeat/extensin protein (lrx1; Wang et al., 1997, 2001; Baumberger et al., 2001; Favery et al., 2001; Rigas et al., 2001).
Biochemical and cytological analyses of root hair and pollen tube development have revealed that a plethora of cellular components are important for tip growth, including the actin and microtubule cytoskeletons, Rac-related proteins, phosphatidylinositol kinases, phosphatidylinositol-4,5-biphosphate, and Ca2+ ions (Bibikova et al., 1997; 1999; Braun et al., 1999; Kost et al., 1999; Miller et al., 1999; Emons and deRuijter, 2000; Esseling et al., 2000; Bao et al., 2001), but also extracellular cell wall-modifying proteins such as xyloglucantransferases and expansins (Baluška et al., 2000; Vissenberg et al., 2001). The actin cytoskeleton is a component of major importance for the cellular architecture and is involved in a variety of cellular activities such as cell polarity, division, elongation, and cytoplasmic streaming (Fowler and Quatrano, 1997; Staiger, 2000). It is a highly dynamic structure that allows a rapid response to changes in development and environmental conditions (Staiger et al., 1997). Actin monomers polymerize to form actin filaments (Holmes et al., 1990) that can aggregate to microfilament bundles. Application of actin filament-interfering compounds lead to aberrant root hair initiation and to a stop of the tip growth process during root hair elongation (Braun et al., 1999; Miller et al., 1999; Baluška et al., 2000).
Arabidopsis contains a family of eight functional actin proteins (ACT) encoded by genes that are predominantly expressed in the vegetative tissues or the reproductive organs (McDowell et al., 1996b; Meagher et al., 1999b). The first group is formed by the three actins ACT2, ACT7, and ACT8, of which ACT2 and ACT8 are most similar, differing in only one amino acid residue (McDowell et al., 1996b; Meagher et al., 1999b). The concomitant expression of several ACT in the same tissue suggests that several isovariants of highly similar but distinct ACT are important for a flexible and dynamic response to changing conditions (Meagher et al., 1999a). Although act2, act4, and act7 T-DNA mutants (McKinney et al., 1995; Gilliland et al., 1998) have not been reported to show any obvious phenotype, the mutations have a deleterious effect that results in a lower frequency of the mutant alleles among the progeny of a heterozygous plant (Gilliland et al., 1998), suggesting that mutations in these actin genes do affect plant development. In addition, the act7 mutant is also affected in callus formation, whereas the act2 mutant shows wild-type development under the same conditions (Kandasamy et al., 2001), suggesting distinct functions of individual actins during plant development.
In this study, we present the isolation and characterization of deformed root hairs 1 (der1) mutant Arabidopsis plants that are affected in root hair development. The mutants were isolated in a screen of an ethylmethanesulfonic acid (EMS)-mutagenized population for seedlings with a phenotype reminisent of lrx1 in which root hairs are frequently swollen and are also shorter than in wild type (Baumberger et al., 2001). Map-based cloning of the DER1 locus revealed that it is mutated in the gene encoding ACT2. The three der1 alleles all harbor missense mutations and show differences in the strength of the phenotype. A detailed characterization of the der1 plants suggests that ACT2 is important throughout the whole process of root hair formation. The site selection of the initial bulge, the positioning of the tip-growth machinery at the bulge, and the process of tip growth per se are strongly affected. These results confirm and extend our understanding of the role of the actin cytoskeleton in root hair development and tip growth.
RESULTS
Isolation of Root Hair Mutants
Arabidopsis seeds of the ecotype C24 were mutagenized with EMS. The seeds were propagated in 12 independent M1 families of 1,000 plants to the M2 generation. Seeds of the M2 generation were grown under sterile conditions on vertical plates for 4 d and were then screened for an aberrant root hair development under the binocular. In total, 70,000 seedlings were analyzed and over 50 der mutants displaying a root hair phenotype were isolated. A number of der mutants display a phenotype with features similar to the lrx1 mutant phenotype. An allelism test revealed that none of them was mutated in the LRX1 locus and that only for der1 were several allelic mutants identified from independent M2 families, which were named der1-1 to der1-3.
Characterization of der1
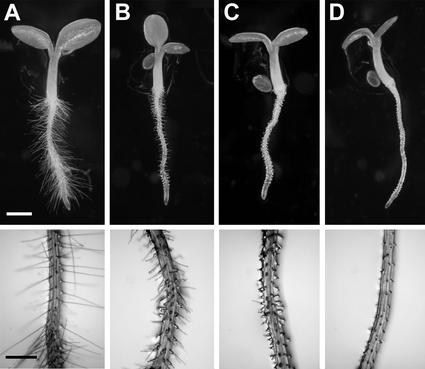
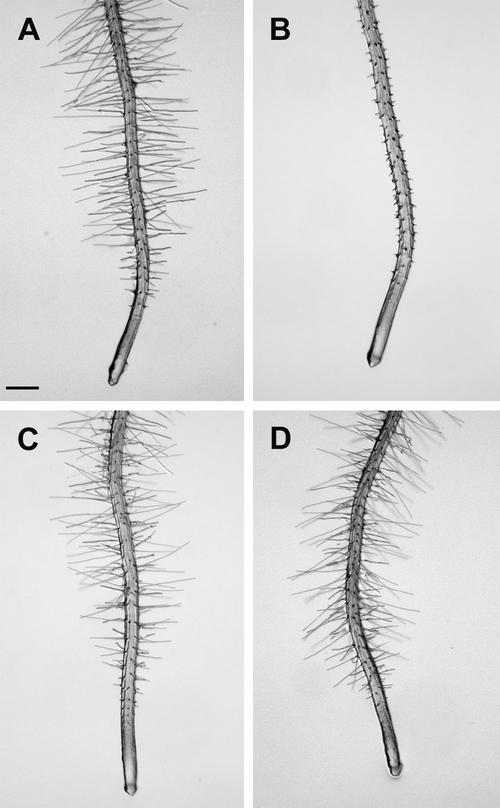
The der1 mutant phenotype could easily be studied under the binocular, as the root hairs are very different from those of C24 wild-type plants (Fig. 1A). A clear difference in strength of the phenotype was observed among the der1 mutants. der1-1 displayed a rather weak phenotype with the lower one-half of the root hair proper being enlarged, whereas the upper one-half of the root hair seemed to develop normally (Fig. 1B). The root hairs were consistently shorter than in wild-type plants. der1-2 and der1-3 showed a comparable phenotype that was much stronger than der1-1. Often, the basis of the root hair was enlarged and the root hair structure was much shorter than in wild type (Fig. 1, C and D).
Figure 1.
der1 plants display a mutant root hair phenotype. Seedlings were grown for 4 d under continuous illumination. Top, Whole seedlings are shown. Bottom, An enlargement of the root hair zone. A, Wild type; B, der1-1; C, der1-2; and D, der1-3. Bars = 1 mm (top) and 0.5 mm (bottom).
Backcrosses of the der1 mutants into wild-type C24 revealed that der1 is a recessive mutation, as the F1 seedlings were not distinguishable from wild type (data not shown). F2 seedlings of this cross segregated in a 1:3 ratio for the mutant phenotype, corroborating that der1 is recessive. Furthermore, the observed segregation suggests that the mutant root hair phenotype is due to a mutation in one genetic locus.
Beside the root hair phenotype, der1 plants did not display any obvious aberrations in development. Trichomes are a second cell type that is not surrounded by other cells. Thus, the effect of the der1 mutation on trichome development was investigated. As the ecotype C24 develops very few trichomes, der1-2 displaying a strong phenotype was crossed with plants of the ecotype Columbia. Young seedlings of the segregating F2 population of this cross were examined, but no aberrant trichome development was observed in plants displaying the der1 phenotype compared with plants with wild-type root hairs. Therefore, we conclude that the expression of the mutant phenotype is limited to root hairs.
Microscopic Investigation of the der1 Phenotype
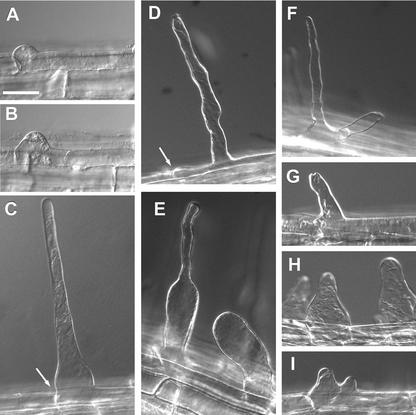
For a more detailed characterization of the der1 mutants, differential interference contrast microscopy (DICM) was used on vertically grown, 4-d-old wild-type and der1 seedlings (Fig. 2). In wild-type seedlings, root hair development was first observed as a bulge at the distal end of the trichoblast cell. After development of the bulge, the root hair proper was formed by tip growth that initiated at the top of the bulge (Fig. 2, A–C). In the der1-1 mutant and also in the strong der1-2 and der1-3 alleles, the initiating bulge formed sometimes rather in the middle than at the distal end of the trichoblast cell (Fig. 2, C and D, arrow). This suggests misplacement of the whole process of bulge initiation. In some cases in the der1-1 mutant, a thin root hair without an enlarged basis developed. However, the structure of these root hairs was irregular with a varying diameter, possibly as a result of asymmetric deposition of cell wall material at the root hair tip or constant slight changes of the growth direction (Fig. 2D). In an alternate manner, the lower one-half of the root hair was strongly enlarged, whereas the upper part was thinner but again very irregular (Fig. 2E). Very often, the enlarged part of the root hair formed, but did not develop further, resulting in a stump-like structure (Fig. 2E). In some cases, two root hairs developed from the initial bulge, suggesting that the initiation of tip growth was disturbed and was not limited to one spot on the initial bulge (Fig. 2F). This is clearly different from branching root hairs as observed in lrx1 mutants, for example (Baumberger et al., 2001). When a short, thin root hair structure was formed, it resembled the root hairs of der1-1 that showed an irregular diameter along the root hair (Fig. 2G). However, the root hairs frequently had a strongly enlarged basis, implying a severely affected bulge-formation and tip-growth process in der1-2 and der1-3 (Fig. 2H). Many times, the initially restricted area of bulge formation grew bigger along the epidermal surface of the trichoblast, and tip growth seemed to initiate at two different positions, although it did not proceed any further (Fig. 2I). A subtle difference between der1-2 and der1-3 was observed in that der1-2 developed the strongly enlarged basis at a higher frequency, whereas root hair structures of der1-3 more often remained thin.
Figure 2.
Root hairs observed by DICM. Roots of 4-d-old seedlings were used for the analysis. A through C, Root hair development in wild-type plants. A and B, An initial bulge is formed at the distal end of the trichoblast. C, The root hair develops by tip growth. D through F, Root hair phenotype of der1-1 mutant. D, Root hairs often show an irregular diameter. E, The lower part of the root hair is enlarged (left) or only an enlarged, short root hair forms (right). F, Two root hairs initiate from the bulge, indicating uncontrolled initiation of tip growth on the bulge. G through I, Root hair phenotype in der1-2 and der1-3 plants. G, Root hairs are very short and have an irregular diameter. H, The root hair basis is frequently enlarged. I, The initial bulge extends over a large part of the epidermal cell and two root hairs appear to develop. Arrows in C and D indicate the distal end of the trichoblast cell. Bar = 40 μm.
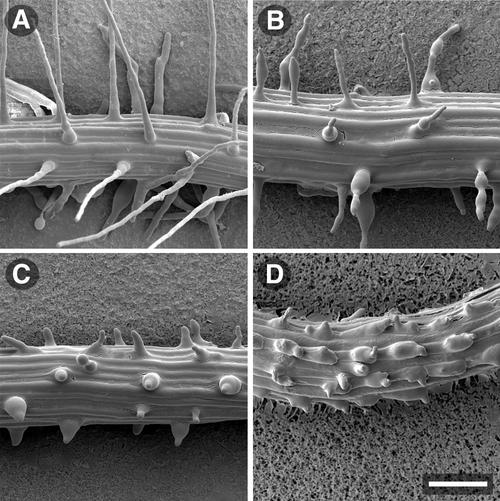
The structure of the wild-type and der1 mutant root hairs was also determined by scanning electron microscopy (SEM; Fig. 3). The SEM analysis confirmed the observations made by DICM. Compared with wild type (Fig. 3A), the lower one-half of root hairs formed by der1-1 seedlings were often enlarged and the stumps were also detectable (Fig. 3B). Again, the root hairs showed an irregular diameter as with DICM, suggesting that this phenotype is not an artifact of sample preparation, but rather is an effect that is genuine to the der1-1 mutation. In plants of the stronger der1-2 and der1-3 alleles, the very short root hair structures with the frequently enlarged basis were readily observable (Fig. 3C). In many trichoblasts, the bulge seemed no longer to be restricted to a small area at the distal end of the trichoblast cell, but rather it extended over a bigger part of the cell surface (Fig. 3D). As a possible consequence of this enlargement, root hair formation was initiated at two points. However, the actual establishment of a root hair proper formed by tip growth and starting from such a structure could never be observed. In some instances, root hair development was observed in normally hairless atrichoblast cell files, which separate the hair-forming trichoblast cell files (Fig. 3D; Dolan et al., 1993). However, this misplacement can also be observed in C24 wild-type plants and thus is not an effect of the der1 mutation (data not shown).
Figure 3.
Root hair-phenotype analysis by SEM. Roots of 4-d-old seedlings were used for the analysis. A, Wild-type C24. B, Representative enlarged root hairs of der1-1 and thin root hairs showing irregular diameters. C and D, der1-2 root hairs. The root hairs are very short and often have an enlarged basis (C). The bulges often are not restricted to the distal end of the trichoblast, but extend over the whole outer cell surface and appear to initiate multiple root hairs (D). Bar = 100 μm.
Map-Based Cloning of the der1 Mutation
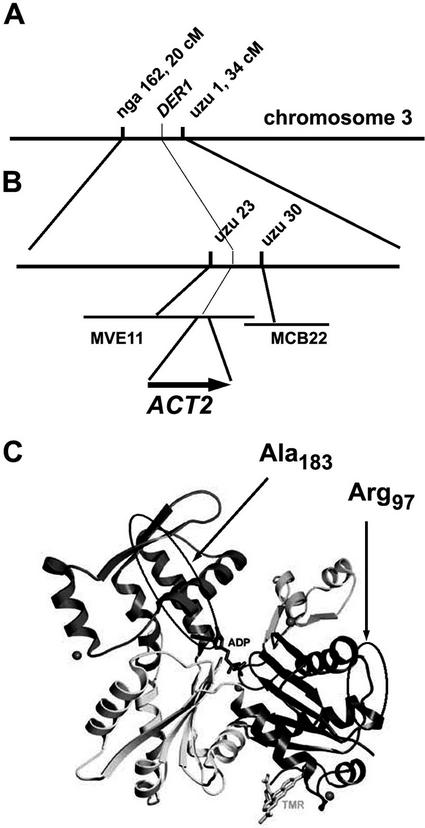
The phenotype of the der1 mutants reflects pleiotropic effects on root hair development and is of considerable interest. Therefore, we decided to clone the DER1 gene by map-based cloning. To this end, the stronger der1-2 allele was used for mapping. As the mutation is in the C24 background and C24 shows DNA polymorphisms with Columbia and Landsberg erecta at a similar frequency, two mapping populations were established. For the initial mapping, genomic DNA of 50 mutant F2 plants of both populations was extracted and analyzed with simple sequence length polymorphism (SSLP; Bell and Ecker, 1994) and cleaved amplified polymorphic sequence (Konieczny and Ausubel, 1993) markers. Several of the markers used for mapping were established based on the polymorphism collection available from Cereon Genetics (http://www.Arabidopsis.org/cereon). This preliminary analysis allowed us to map the der1 locus on chromosome 3 to a region between the SSLP markers nga162 and uzu1, at a distance of 20 and 34 cM from the top of the chromosome, respectively (Fig. 4A). The mapping population of the cross der1-2 × Landsberg erecta was subsequently increased to 1,200 mutant F2 plants. With this population, the DER1 locus was narrowed down to a region between two markers that were established at positions 40,000 on the BAC MVE11 and 16,500 on the adjacent BAC MCB22 (http://www.Arabidopsis.org/chromosomes) with three and one plants, respectively, still showing recombination (Fig. 4B). This interval spans a region of 50 kb and comprises nine annotated genes. Among those, the ACT2 gene, encoded on MVE11 in positions 59,369 through 60,666, seemed a candidate gene as actins are known to be important for cell architecture and specifically to be involved in root hair development. Therefore, the ACT2 gene was sequenced and missense mutations were found in all three der1 alleles compared with the wild-type C24 sequence. In the weaker der1-1 allele, the Ala residue at position 183 (Ala-183) is changed to Val, whereas in the stronger der1-2 and der1-3 the Arg-97 is changed to a His and a Cys, respectively (Fig. 4C).
Figure 4.
Map-based cloning of the DER1 locus. A, In an initial mapping population, the DER1 locus was located on chromosome 3, in an interval between the SSLP markers nga162 and uzu1 at 20 and 34 cM, respectively. B, Using a larger mapping population, DER1 was mapped to a region flanked by the markers uzu23 and uzu30 and spanned by the bacterial artificial chromosome (BAC) clones MVE11 and MCB22. The ACT2 gene is located on the BAC MVE11. C, A model of the actin protein structure in the ADP-bound form. Tetramethylrhodamine-5-maleimide shown in the right lower part was used to facilitate crystallization (Otterbein et al., 2001). The domains that harbor the two amino acids mutated in the der1 alleles are circled.
To examine whether the point mutations have an influence on the expression level of the ubiquitously expressed ACT2, gene expression was tested by northern blotting. Seedlings of wild-type C24 and the weaker der1-1 and stronger der1-3 alleles were grown for 10 d on vertical plates and RNA was extracted from root tissue. Total RNA was separated on an agarose gel, blotted onto a nitrocellulose membrane, and probed with an ACT2-specific probe (An et al., 1996). The result clearly shows that the expression level of act2 is not affected by the point mutations in der1 plants and thus the der1 mutants express the act2 gene at wild-type levels (Fig. 5).
Figure 5.
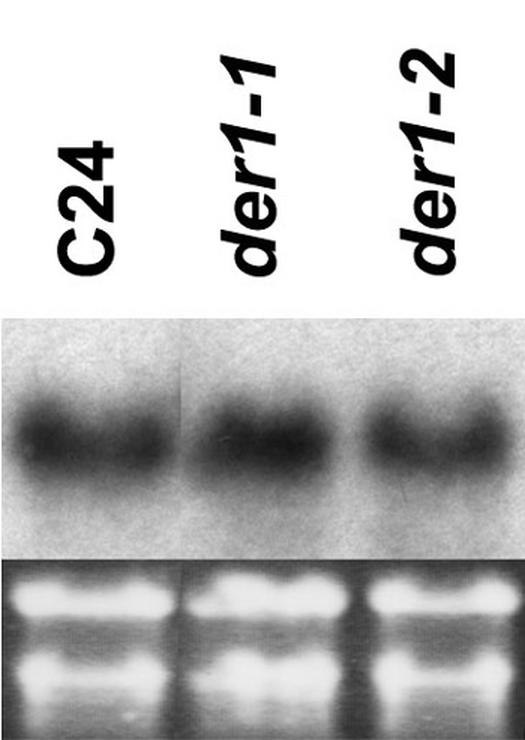
Northern-blot analysis of ACT2 expression in wild-type and der1 mutants. Seedlings were vertically grown for 10 d and root material was used for RNA extraction. Five micrograms of total RNA per lane was blotted and probed with a probe specific for ACT2.
Complementation of the der1 Phenotype with the ACT2 and the Root Hair-Specific LRX1 Promoter
To confirm that the ACT2 gene is responsible for the der1 phenotype, a complementation experiment was performed. To this end, the ACT2 cDNA was isolated from reverse-transcribed and PCR-amplified C24 mRNA and was cloned downstream of a 1.6-kb ACT2 promoter that includes the first intron of the nontranslated region of the ACT2 gene (An et al., 1996). In a second construct, the root hair-specific LRX1 promoter (Baumberger et al., 2001) was used to drive expression of the ACT2 cDNA. The two constructs were transformed into der1-2 plants and for both, segregating T2 populations of six independent transgenic lines were analyzed. The ACT2 cDNA expressed under both promoters complemented the phenotype (Fig. 6). This confirmed that the mutation conferring the root hair phenotype in the der1 plants is in the ACT2 gene. Furthermore, the complementation experiment with the ACT2 cDNA under the control of the LRX1 promoter shows that this root hair-specific promoter is expressed already in early stages of root hair development and confers sufficient ACT2 expression to complement the der1 phenotype. Thus, complementation of the der1 phenotype provides a good system for assessing the promoter activity of a particular gene involved in trichoblast differentiation, as ACT2 is required throughout the whole developmental process.
Figure 6.
Complementation of the der1 mutation. A, Wild-type C24; B, der1-2 used for the complementation experiments. Transgenic der1-2 seedlings expressing the ACT2 cDNA under the control of the ACT2 promoter (C) and the root hair-specific LRX1 promoter (D) show wild-type root hair development. The seedlings were grown for 4 d prior to analysis. Bar = 400 μm.
DISCUSSION
We have identified and characterized three alleles of der1, a mutant that shows severe alterations in root hair development. der1-1 shows an intermediate phenotype with shorter and enlarged root hairs. The stronger der1-2 and der1-3 mutants have a drastically reduced root hair length and often do not develop further than to the initial bulge. The bulge-site selection is often severely affected, resulting in bulges that are sometimes misplaced and often enlarged over a large part of the cell instead of being limited to the distal end of the trichoblast. Map-based cloning of the DER1 locus revealed that it encodes the ACT2 gene, encoding a major actin of the vegetative tissue (An et al., 1996).
der1 plants show a phenotype that suggests that several processes are affected during root hair development. The short root hair phenotype, particularly in the strong der1-2 and der1-3 alleles, indicates that the root hair tip-growth process is affected. This function for actin has been suggested by previous biochemical/cytological studies that showed that interference with the actin cytoskeleton by application of cytochalasin D or latrunculin B stops root hair growth (Bibikova et al., 1999; Braun et al., 1999; Miller et al., 1999). The phenotype of der1-1 seedlings that develop root hairs that are severely disturbed in their structure might reflect loss of directionality of root hair growth and/or unprecise deposition of new cell wall material to the lateral walls instead of the tip region, leading to an enlarged cell. Tip growth includes transport of cell wall material-containing vesicles to the tip, followed by fusion of these vesicles with the plasma membrane. As the actin cytoskeleton is involved in the vesicle transport (Cai et al., 1997; Emons and deRuijter, 2000; Staiger, 2000), interference with this structure by a mutation in an actin protein might be expected to result in uncontrolled trafficking and deposition of vesicles during cell growth. Microtubules have also been shown to be involved in controlling directionality of root hair growth. Treatment of root hairs with microtubule-interfering drugs lead to wavy root hairs that, in contrast to der1-1, exhibit a more regular width (Bibikova et al., 1998). It remains to be shown whether the microtubule structure is affected in der1-1 and thus whether there is a mutual influence between the two cytoskeletons.
In some cases, the bulges in the der1 mutants are not properly positioned to the distal end of the trichoblast cell, and in the stronger der1-2 and der1-3 alleles, they frequently extend over a larger surface of the cell. Thus, ACT2 is important in specifying the area of the cell surface involved in bulge development. This confirms the importance of the actin cytoskeleton as a component defining the cell architecture and polarization (Fowler and Quatrano, 1997). Furthermore, it is in line with previous reports that showed contact of the actin cytoskeleton with the prospective site of bulge formation (Baluška et al., 2000) and rearrangement and focusing of the microfilament bundles in the growing bulge (Braun et al., 1999; Miller et al., 1999), implying a function of the actin cytoskeleton in these processes. In future experiments, it will be interesting to study the rearrangement of the actin cytoskeleton in der1 plants. This will possibly reveal whether ACT2 has a particular function within the cytoskeleton that is required for proper root hair development or whether the cytoskeleton is generally affected in these plants.
It is of interest that the der1 mutant phenotype can only be observed in root hair cells and in no other cell type. A previously reported deleterious effect on the inheritance of a mutation in ACT2 (Gilliland et al., 1998) could not be confirmed, using the inheritance of the strong der1-3 allele in a segregating population as a parameter (data not shown). However, this might also suggest that even this strong allele encodes a partially functional protein and that the deleterious effect can only be detected in an act2-null mutant. Root hairs might be prone to aberrations in cell development as they are not surrounded by other cells that limit their growth. Trichomes represent a cell type that is similar to root hairs in this respect and does also express ACT2 (An et al., 1996). However, no mutant phenotype could be observed in trichomes of der1 plants compared with wild-type C24. Thus, the root hair-specific phenotype suggests that this cell type has distinct characteristics, the composition of the actin cytoskeleton is distinct from other cell types, or the function of ACT2 is particularly important in root hair development. ACT2 is not required for the tip-growth process in pollen as it is not expressed in this tissue (An et al., 1996; Meagher et al., 2000; Kandasamy et al., 2002). The results presented in this work and the lack of requirement of ACT2 for pollen development suggests that in perspective of the actin cytoskeleton, the tip-growth process in these two cell types differs significantly.
Several actin genes of Arabidopsis are expressed concomitantly in the same tissue. At least three actin genes, ACT2, ACT7, and ACT8, are expressed in root hairs (An et al., 1996; McDowell et al., 1996a). The parallel expression of several closely related proteins is thought to provide a higher degree of flexibility to the plant to respond to changes in development (Meagher et al., 1999a). Beside the possible requirement for the expression of several isovariants in the same cell, a possible redundancy in protein function among the different proteins should also prevent an aberrant phenotype upon mutation of a gene encoding one of these proteins. However, our results show that in root hair development, the ACT are not redundant in their function and ACT7 and ACT8 cannot functionally replace ACT2. This is particularly surprising as ACT2 and ACT8 differ in only one amino acid residue (McDowell et al., 1996b). Thus, even small changes in the protein sequence among actins have a tremendous effect on protein function, and the high degree of protein conservation among different actins does not reflect extreme conservation of protein function. In the case of the act7 mutant, a strong effect of the mutation can be observed under tissue culture conditions where act7-derived tissue shows slower growth. However, act2 mutant-derived tissue is not distinguishable from wild type (Kandasamy et al., 2001). Thus, although being expressed together in several tissues, individual ACT have at least partially nonoverlapping functions in different cell types. In an alternate manner, the expression pattern of ACT7 and ACT8 during root hair development might not be sufficiently overlapping with ACT2 to allow complementation, a possibility that cannot be ruled out as the promoter β-glucuronidase fusion constructs used to study the expression pattern of the different actin genes (An et al., 1996; McDowell et al., 1996a) might not fully reflect the expressing profiles of the endogenous genes. To differentiate between the two possibilities, a complementation test of a der1 mutant with constructs expressing ACT7 and ACT8 under the control of the ACT2 promoter will be performed. The fact that an ACT2 cDNA under the control of the root hair-specific LRX1 promoter allows complementation of the der1 phenotype suggests that ACT2 does not require an expression pattern unique to ACT2 to allow proper function of the protein.
The availability of three different act2 mutant alleles exhibiting a mutant phenotype to different degrees while expressing the act2 gene at wild-type levels provides an excellent tool for studying ACT2 function, and it allows us to relate the changes in the protein to its biological function. Both amino acid residues affected in the der1 mutants are conserved throughout the actin family of Arabidopsis (except ACT9, which is encoded by a pseudogene; McDowell et al., 1996b) and in organisms of diverse origin such as ACT1 of Saccharomyces cerevisiae and in rabbit (Oryctolagus cuniculus) skeletal muscle actin isoform (McDowell et al., 1996b and refs. therein). The Ala-183 that is changed to Val in the weaker der1-1 allele is positioned in the α-helix close to the nucleotide binding cleft. Ala-183 is close to an Arg that is important in the ADP conformation of actin where it interacts with the nucleotide through hydrogen bonds (Otterbein et al., 2001). Thus, the mutation might change the structure of the protein and consequently influence the interaction with ADP. der1-2 and der1-3 are mutated in Arg-97, which is changed to His and Cys, respectively. Therefore, the slight difference in the phenotypes of der1-2 and der1-3 must be due to the different amino acids replacing the original Arg. Arg-97 is located in the subdomain1 on the protein surface (Kabsch et al., 1990) and therefore might be important for the establishment of protein-protein interactions. However, it cannot be excluded that these mutations have an influence on the overall protein structure rather than directly interfering with the binding of an interaction partner.
The intermediate phenotype displayed by der1-1 shows that the Ala-183 to Val substitution in ACT2 results in a partially functional protein. Comparison of the root hair phenotypes of der1-2 and der1-3, respectively, with an act2 knockout mutant that was isolated by screening a T-DNA insertion collection (McKinney et al., 1995) should reveal whether the mutations in Arg-97 result in a partially active ACT2.
We have isolated and characterized der1, a genetic locus involved in root hair development. DER1 encodes ACT2, one of two major actin genes expressed in vegetative tissue. The phenotype displayed by the der1 alleles suggests that ACT2 is not only important for root hair tip growth-related processes, but also for the bulge site selection as a first step in root hair development. The der1 lines are an excellent tool for studying redundancy and isovariant dynamics versus complementary but distinct functions among the different members of the actin family of Arabidopsis. The detailed analysis of the actin cytoskeleton in der1 mutants will reveal important information on the function of ACT2 in the establishment of the actin cytoskeleton and will extend our understanding on actin function in plant cell development.
MATERIALS AND METHODS
Plant Material
Arabidopsis seeds of the ecotype C24 were EMS-mutagenized by a standard procedure (Lehle Seeds, Round Rock, TX). Mutagenized M1 seeds were grown in families of 1,000 plants, and M2 seeds of each family were pooled. For isolation of root hair mutants, 70,000 M2 seeds were surface sterilized with 1% (w/v) Na-hypochlorite (Fluka, Buchs, Switzerland) and 0.03% (v/v) Triton X-100, washed three times with sterile water, and stratified for 3 d at 4°C. The seeds were subsequently plated on one-half-strength Murashige and Skoog medium and 0.4% (w/v) Phytoagar (Sigma, Buchs, Switzerland) and were grown for 4 d at 24°C under continuous light. Selected plants were grown to maturity in soil under continuous light at 24°C in growth chambers.
Microscopical Analysis
Prior to the microscopical observations, plants were grown under sterile conditions as described above. The observations were done as described by Baumberger et al. (2001). DICM was performed on an axioplan microscope (Zeiss, Jena, Germany).
Mapping of the der1 Locus
The strong der1-2 allele was used for mapping of the der1 mutation. der1-2 was crossed with Arabidopsis of the ecotypes Columbia and Landsberg erecta. Genomic DNA of 50 mutant F2 plants of each population was extracted following the protocol of Fulton et al. (1995) and was used for initial mapping using SSLP and cleaved amplified polymorphic sequence markers. For the large mapping population, mutant F2 plants were selected and grown for 1 week on soil. After extraction of genomic DNA and testing with the closely linked markers nga162 and uzu1, only F2 plants showing recombination were grown to maturity, and F3 seedlings were tested to confirm the phenotype. Newly generated markers were established based on the Columbia versus Landsberg erecta polymorphism collection of Cereon Genetics (http://www.Arabidopsis.org/cereon).
Cloning Procedures and Plant Transformation
For sequencing, the ACT2 gene was amplified by PCR from genomic DNA of wild-type C24 and der1 mutant plants. To clone the ACT2 cDNA, a cDNA library from C24 (van der Graaff et al., 2000) was used as template for a PCR reaction with the primers Act2-1, AGCGCTGAGGCTGATGATATTCAAC and Act2-2, TCTAGAAACATTTTCTGTGAACGATTC. The resulting fragment contained an Eco 47III site of which the second one-half of the recognition sequence corresponded to the second triplet of the ACT2 coding sequence and an XbaI site at the stop codon. This fragment was cloned into pCR-Blunt II-TOPO (Invitrogen, Basel) and was sequenced. The ACT2 promoter and the LRX1 promoter were amplified by PCR from genomic DNA with the primers ACT2proF1, GGTCATATGTTCAGTTTTTAAGC; ACT2proR1, GCATGCATTTTTTATGAGCTGCAAACAC; and LRX1–1000, AAAAGTGAGGTATTTAGGTCATT; LRX1METR GCATGCATTGCTTATGGGTCAAGAAAC, respectively, cloned into pGEM-T easy (Promega, Wallisellen, Switzerland) and sequenced. For both promoters, a SphI site was introduced such that the G of the ATG start codon corresponded to the first nucleotide of the SphI site. For cloning of the promoter-ACT2 cDNA fusion construct, the promoter was digested with SphI, blunt-ended with T4 DNA-polymerase, cut with XbaI, and ligated with the vector containing the Act2 cDNA, cut with Eco 47III and XbaI. In this way, a perfect promoter-cDNA fusion was obtained without any change in the promoter or the cDNA sequence. Both promoter-ACT2 cDNA fusions were digested with BamHI/XhoI and were cloned into pART7 (Gleave, 1992) digested by the same enzymes. The resulting promoter ACT2-cDNA ocs-termination sequence cassettes were digested with NotI and cloned into pART27 (Gleave, 1992) digested with NotI.
The resulting constructs were transformed into Agrobacterium tumefaciens GV3101 for plant transformation. der1-2 plants were transformed by the floral dip method (Clough and Bent, 1998). The T1 seedlings were germinated on one-half-strength Murashige and Skoog agar plates complemented with 50 μg mL−1 kanamycin for selection of transgenic plants and 150 μg mL−1 Timentin (GlaxoSmithKline, Münchenbuchsee, Switzerland) to inhibit growth of agrobacteria.
RNA Extraction and Northern Blotting
Seedlings were grown for 10 d on vertical plates, root material was collected, and RNA was extracted using Trizol reagent (Invitrogen) following the manufacturer's instructions. Five micrograms of total RNA was separated by gel electrophoresis, blotted on a nylon membrane (GeneScreen Plus; PerkinElmer, Hünenberg, Switzerland), and hybridized with an ACT2-specific probe (An et al., 1996).
ACKNOWLEDGMENTS
We thank Dr. Eric van der Graaff for providing the EMS-mutagenized Arabidopsis population, Dr. Roberto Dominguez for the permission to use the actin structure model, and Cereon Genetics for providing us access to the Arabidopsis polymorphism collection.
Footnotes
This work was supported by the Swiss National Science Foundation (grant nos. 31–51055.97 and 31–61419.00).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.005777.
LITERATURE CITED
- An Y-Q, McDowell JM, Huang SR, McKinney EC, Chambliss S, Meagher RB. Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant J. 1996;10:107–121. doi: 10.1046/j.1365-313x.1996.10010107.x. [DOI] [PubMed] [Google Scholar]
- Baluška F, Salaj J, Mathur J, Braun M, Jasper F, Samaj J, Chua NH, Barlow PW, Volkmann D. Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev Biol. 2000;227:618–632. doi: 10.1006/dbio.2000.9908. [DOI] [PubMed] [Google Scholar]
- Bao YQ, Kost B, Chua NH. Reduced expression of α-tubulin genes in Arabidopsis thaliana specifically affects root growth and morphology, root hair development and root gravitropism. Plant J. 2001;28:145–157. doi: 10.1046/j.1365-313x.2001.01142.x. [DOI] [PubMed] [Google Scholar]
- Baumberger N, Ringli C, Keller B. The chimeric leucine-rich repeat/extensin cell wall protein LRX1 is required for root hair morphogenesis in Arabidopsis thaliana. Genes Dev. 2001;15:1128–1139. doi: 10.1101/gad.200201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CJ, Ecker JR. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics. 1994;19:137–144. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- Bibikova TN, Blancaflor EB, Gilroy S. Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 1999;17:657–665. doi: 10.1046/j.1365-313x.1999.00415.x. [DOI] [PubMed] [Google Scholar]
- Bibikova TN, Jacob T, Dahse I, Gilroy S. Localized changes in apoplastic and cytoplasmic pH are associated with root hair development in Arabidopsis thaliana. Development. 1998;125:2925–2934. doi: 10.1242/dev.125.15.2925. [DOI] [PubMed] [Google Scholar]
- Bibikova TN, Zhigilei A, Gilroy S. Root hair growth in Arabidopsis thaliana is directed by calcium and an endogenous polarity. Planta. 1997;203:495–505. doi: 10.1007/s004250050219. [DOI] [PubMed] [Google Scholar]
- Braun M, Baluška F, von Witsch M, Menzel D. Redistribution of actin, profilin and phosphatidylinositol-4,5-bisphosphate in growing and maturing root hairs. Planta. 1999;209:435–443. doi: 10.1007/s004250050746. [DOI] [PubMed] [Google Scholar]
- Cai G, Moscatelli A, Cresti M. Cytoskeletal organization and pollen tube growth. Trends Plant Sci. 1997;2:86–91. [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Dolan L, Duckett CM, Grierson C, Linstead P, Schneider K, Lawson E, Dean C, Poethig S, Roberts K. Clonal relationships and cell patterning in the root epidermis of Arabidopsis. Development. 1994;120:2465–2474. [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. Cellular organization of the Arabidopsis thaliana root. Development. 1993;119:71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- Emons A, deRuijter N. Actin: a target of signal transduction in root hairs. In: Staiger C, Baluška F, Volkmann D, Barlow P, editors. Actin: A Dynamic Framework for Multiple Plant Cell Functions. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2000. pp. 373–391. [Google Scholar]
- Esseling J, deRuijter N, Emons AMC. The root hair actin cytoskeleton as backbone, highway, morphogenetic instrument and target for signalling. In: Ridge R, Emons A, editors. Root Hairs: Cell and Molecular Biology. Tokyo: Springer-Verlag; 2000. pp. 29–53. [Google Scholar]
- Favery B, Ryan E, Foreman J, Linstead P, Boudonck K, Steer M, Shaw P, Dolan L. KOJAK encodes a cellulose synthase-like protein required for root hair cell morphogenesis in Arabidopsis. Genes Dev. 2001;15:79–89. doi: 10.1101/gad.188801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JE, Quatrano RS. Plant cell morphogenesis: plasma membrane interactions with the cytoskeleton and cell wall. Annu Rev Cell Dev Biol. 1997;13:697–743. doi: 10.1146/annurev.cellbio.13.1.697. [DOI] [PubMed] [Google Scholar]
- Fulton TM, Chunwongse J, Tanksley SD. Microprep protocol for extraction of DNA from tomato and other herbaceous plants. Plant Mol Biol Rep. 1995;13:207–209. [Google Scholar]
- Galway M. Root hair ultrastructure and tip growth. In: Ridge R, Emons A, editors. Root Hairs: Cell and Molecular Biology. Tokyo: Springer-Verlang; 2000. pp. 1–17. [Google Scholar]
- Gilliland LU, McKinney EC, Asmussen MA, Meagher RB. Detection of deleterious genotypes in multigenerational studies: disruptions in individual Arabidopsis actin genes. Genetics. 1998;149:717–725. doi: 10.1093/genetics/149.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleave AP. A versatile binary vector system with a T-DNA organizational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol. 1992;20:1203–1207. doi: 10.1007/BF00028910. [DOI] [PubMed] [Google Scholar]
- Grierson CS, Roberts K, Feldmann KA, Dolan L. The COW1 locus of Arabidopsis acts after RHD2, and in parallel with RHD3 and TIP1, to determine the shape, rate of elongation, and number of root hairs produced from each site of hair formation. Plant Physiol. 1997;115:981–990. doi: 10.1104/pp.115.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes KC, Popp D, Gebhard W, Kabsch W. Atomic model of the actin filament. Nature. 1990;347:44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Kabsch W, Mannherz HG, Suck D, Pai EF, Holmes KC. Atomic structure of the actin: DNAse-I complex. Nature. 1990;347:37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kandasamy MK, Gilliland LU, McKinney EC, Meagher RB. One plant actin isovariant, ACT7, is induced by auxin and required for normal callus formation. Plant Cell. 2001;13:1541–1554. doi: 10.1105/TPC.010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy MK, McKinney EC, Meagher RB. Functional nonequivalency of actin isovariants in Arabidopsis. Mol Biol Cell. 2002;13:251–261. doi: 10.1091/mbc.01-07-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny A, Ausubel FM. A procedure for mapping Arabidopsis mutations using codominant ecotype-specific PCR-based markers. Plant J. 1993;4:403–410. doi: 10.1046/j.1365-313x.1993.04020403.x. [DOI] [PubMed] [Google Scholar]
- Kost B, Lemichez E, Spielhofer P, Hong Y, Tolias K, Carpenter C, Chua NH. Rac homologues and compartmentalized phosphatidylinositol 4, 5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J Cell Biol. 1999;145:317–330. doi: 10.1083/jcb.145.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW. The rhd6 mutation of Arabidopsis thaliana alters root hair initiation through an auxin-associated and ethylene-associated process. Plant Physiol. 1994;106:1335–1346. doi: 10.1104/pp.106.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JM, An YQ, Huang SR, McKinney EC, Meagher RB. The Arabidopsis ACT7 actin gene is expressed in rapidly developing tissues and responds to several external stimuli. Plant Physiol. 1996a;111:699–711. doi: 10.1104/pp.111.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JM, Huang SR, McKinney EC, An YQ, Meagher RB. Structure and evolution of the actin gene family in Arabidopsis thaliana. Genetics. 1996b;142:587–602. doi: 10.1093/genetics/142.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney EC, Ali N, Traut A, Feldmann KA, Belostotsky DA, McDowell JM, Meagher RB. Sequence-based identification of T-DNA insertion mutations in Arabidopsis: actin mutants act2-1 and act4-1. Plant J. 1995;8:613–622. doi: 10.1046/j.1365-313x.1995.8040613.x. [DOI] [PubMed] [Google Scholar]
- Meagher RB, McKinney EC, Kandasamy MK. Isovariant dynamics expand and buffer the responses of complex systems: the diverse plant actin gene family. Plant Cell. 1999a;11:995–1005. doi: 10.1105/tpc.11.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher RB, McKinney EC, Kandasamy MK. The significance of diversity in the plant actin gene family. In: Staiger C, Baluška F, Volkmann D, Barlow P, editors. Actin: A Dynamic Framework for Multiple Plant Cell Functions. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2000. pp. 3–27. [Google Scholar]
- Meagher RB, McKinney EC, Vitale AV. The evolution of new structures: clues from plant cytoskeletal genes. Trends Genet. 1999b;15:278–284. doi: 10.1016/s0168-9525(99)01759-x. [DOI] [PubMed] [Google Scholar]
- Miller DD, de Ruijter NCA, Bisseling T, Emons AMC. The role of actin in root hair morphogenesis: studies with lipochito-oligosaccharide as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J. 1999;17:141–154. [Google Scholar]
- Otterbein LR, Graceffa P, Dominguez R. The crystal structure of uncomplexed actin in the ADP state. Science. 2001;293:708–711. doi: 10.1126/science.1059700. [DOI] [PubMed] [Google Scholar]
- Parker JS, Cavell AC, Dolan L, Roberts K, Grierson CS. Genetic interactions during root hair morphogenesis in Arabidopsis. Plant Cell. 2000;12:1961–1974. doi: 10.1105/tpc.12.10.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas S, Debrosses G, Haralampidis K, Vicente-Agullo F, Feldmann KA, Grabov A, Dolan L, Hatzopoulos P. TRH1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs. Plant Cell. 2001;13:139–151. doi: 10.1105/tpc.13.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein J, Galway M, Masucci J, Ford S. Pollen tube and root hair tip growth is disrupted in a mutant of Arabidopsis thaliana. Plant Physiol. 1993;103:979–985. doi: 10.1104/pp.103.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW. Constructing a plant cell: the genetic control of root hair development. Plant Physiol. 2000;124:1525–1531. doi: 10.1104/pp.124.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW, Somerville C. Genetic control of root hair development in Arabidopsis thaliana. Plant Cell. 1990;2:235–243. doi: 10.1105/tpc.2.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger CJ. Signaling to the actin cytoskeleton in plants. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:257–288. doi: 10.1146/annurev.arplant.51.1.257. [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Gibbon BC, Kovar DR, Zonia LE. Profilin and actin-depolymerizing factor: modulators of actin organization in plants. Trends Plant Sci. 1997;2:275–281. [Google Scholar]
- van der Graaff E, Den Dulk-Ras A, Hooykaas PJJ, Keller B. Activation tagging of the LEAFY PETIOLE gene affects leaf petiole development in Arabidopsis thaliana. Development. 2000;127:4971–4980. doi: 10.1242/dev.127.22.4971. [DOI] [PubMed] [Google Scholar]
- Vissenberg K, Fry SC, Verbelen JP. Root hair initiation is coupled to a highly localized increase of xyloglucan endotransglycosylase action in Arabidopsis roots. Plant Physiol. 2001;127:1125–1135. [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Lockwood SK, Hoeltzel MF, Schiefelbein JW. The ROOT HAIR DEFECTIVE3 gene encodes an evolutionarily conserved protein with GTP-binding motifs and is required for regulated cell enlargement in Arabidopsis. Genes Dev. 1997;11:799–811. doi: 10.1101/gad.11.6.799. [DOI] [PubMed] [Google Scholar]
- Wang X, Cnops G, Vanderhaeghen R, De Block S, Van Montagu M, Van Lijsebettens M. AtCSLD3, a cellulose synthase-like gene important for root hair growth in Arabidopsis. Plant Physiol. 2001;126:575–586. doi: 10.1104/pp.126.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZB. Signaling tip growth in plants. Curr Opin Plant Biol. 1998;1:525–530. doi: 10.1016/s1369-5266(98)80046-0. [DOI] [PubMed] [Google Scholar]