Abstract
Expression patterns of a rice (Oryza sativa) cytochrome c gene OsCc1 and its promoter activity were characterized in transgenic rice plants. OsCc1 transcripts accumulate in most cell types, but to varying levels. Large amounts of OsCc1 transcripts are found in the roots, calli, and suspension cells, but relatively lower in mature leaves, demonstrating its higher levels of expression in non-photosynthetic tissues. Unlike the human cytochrome c gene, which is responsive to cAMP, OsCc1 expression is not enhanced in various rice tissues after dibutyryl cAMP treatments. OsCc1 promoter was linked to the sgfp gene and its activities in different tissues and cell types of transgenic rice plants were analyzed in comparison with the Act1 and RbcS promoters. OsCc1 promoter directs expression in virtually all organs of transgenic plants including roots, leaves, calli, embryos, and suspension cells, showing a particularly high activity in calli and roots. Activity of the OsCc1 promoter was 3-fold higher than Act1 in calli and roots and comparable with RbcS in leaves, representing a useful alternative to the maize (Zea mays) Ubi1 and the rice Act1 promoters for transgene expression in monocots.
Several factors increase the expression of chimeric genes in transgenic plants. The choice of promoters affects transgene transcription, resulting in changes not only in concentration, but also in the stage, tissue, and cell specificity of its expression. Over the years, several well-characterized promoters have been made available for transgene expression in plants. However, most of these promoters are from dicot plants. Presently, the cauliflower mosaic virus (CaMV) 35S promoter and its derivatives are among the most commonly used. The CaMV 35S promoter is active in monocots, but its relative strength is substantially lower in monocot than in dicot cells. In addition, it is inactive in some cell types; for example, pollen (Bruce et al., 1989; Christensen et al., 1992; McElroy and Brettell, 1994). Other dicot promoters have also been used for monocot transformation, but activity tends to be lower than for monocot promoters (Wilmink et al., 1995). For example, Kyozuka et al. (1993) reported that expression of the gusA gene driven by a tomato (Lycopersicon esculentum) RbcS promoter is induced by light. However, the expression of the rice (Oryza sativa) RbcS::gusA gene is significantly higher than expression of the tomato RbcS::gusA gene. Conversely, the use of monocot promoters has resulted in a high degree of gene expression in monocots, including rice (McElroy et al., 1991; Cornejo et al., 1993; Kyozuka et al., 1993). Several promoters have been investigated as useful alternatives to drive a high level of expression in monocot transgenesis; for example, the rice Act1 promoter (McElroy et al., 1991), the rice RbcS promoter (Kyozuka et al., 1993; Jang et al., 1999), and the maize (Zea mays) Ubi1 promoter (Uchimiya et al., 1993). In many cases, introns strongly enhance transgene expression in transgenic plants (Wilmink et al., 1995). In the case of the rice Act1 and maize Ubi1 promoters, the first introns in the 5′-untranslated region of the genes (McElroy et al., 1991; Christensen et al., 1992) were included for their enhanced promoter activities (Christensen and Quail, 1996). The last two are the constitutive promoters that are commonly used in monocot transformation (McElroy and Brettell, 1994). Thus, there is currently a shortage of efficient promoters for ubiquitous gene expression in monocots.
Cytochrome c is a small, peripheral, nuclear-encoded membrane protein located in the intermembrane space of mitochondria. It functions in the catalytic transfer of electrons between respiratory complexes III and IV in eukaryotes. The cytochrome c molecule has been investigated for its functional importance and unique intra-organellar position. Cytochrome c proteins and their nucleotide sequences are highly conserved in organisms as distantly related as yeast (Saccharomyces cerevisiae), mammals, and plants. Therefore, these proteins have been used for many evolutionary comparisons at the molecular level (Syvanen et al., 1989; Kemmerer et al., 1991a, 1991b). In animals, the cytochrome c gene promoter is reported to contain cAMP response element (CRE) and nuclear respiratory factor (NRF)-binding sites (Gopalakrishnan and Scarpulla, 1994). CRE is involved in cAMP-dependent expression, and NRF is involved in the coordination of nuclear and mitochondrial gene activities (Evans and Scarpulla, 1990). Recent focus on the cytochrome c protein has been in relation to its release into the cytosol, which is an indication of apoptotic processes in human cells. In plants, however, little is known about the cytochrome c gene, except that it has been cloned and sequenced in Arabidopsis and rice. This study examined the regulation of a rice cytochrome c gene OsCc1 (accession no. M63704) and its promoter activities in transgenic rice. Our data demonstrate that OsCc1 is expressed in most tissues, and that its expression is particularly high in the non-photosynthetic parts of plants, including the roots, calli, and suspension cells. To evaluate the OsCc1 promoter, it was linked to the sgfp gene and introduced into rice. Using confocal laser scanning microscopy (CLSM) and RNA-blot hybridization, activity of the OsCc1 promoter in various tissues and cell types of transgenic plants was analyzed in comparison with Act1 and RbcS promoters.
RESULTS
Sequence Analysis of the OsCc1 Promoter Region
The physical map of the genomic clone OsCc1 is shown in Figure 1A and the nucleotide sequence of its promoter region was deposited in GenBank under the accession number AF399666. We assigned the transcription start site because the sequence at positions −6 to +4 (CTCGGAATCG) resembles the plant transcription initiation sequence CTCATCA (Joshi, 1987). Several potential regulatory elements were identified in a human cytochrome c promoter (Gopalakrishnan and Scarpulla, 1994). Of particular interest in the OsCc1 promoter are the sequences at positions −218 to −225 (GCACGTGG; I in Fig. 1A) and −180 to −189 (CCGAATGGGC; II in Fig. 1A). These are similar to the sequences of CRE-1 (TGACGTCA) and NRF-1 (CCGCATGCGC), which are binding sites of a human cytochrome c gene promoter (Gopalakrishnan and Scarpulla, 1994). In animal systems, CRE-1 is involved in the cAMP-dependent expression of the cytochrome c gene, and NRF-1 is implicated in a mechanism for coordinating nuclear and mitochondrial genetic systems (Evans and Scarpulla, 1990).
Figure 1.
A, Physical map of the genomic clone (OsCc1) encoding a rice cytochrome c. The solid boxes represent exons. A partial cDNA (cyt) that was used as a probe for RNA-blot experiments is indicated. The transcriptional start site and the translational start codon are marked with +1 and ATG, respectively. Sequences that are similar to those of CRE and NRF1-binding sites are indicated by I and II, respectively. Important restriction enzyme sites are abbreviated as follows: C, ClaI; RI, EcoRI; S, SalI; and X, XbaI. B, The expression of OsCc1 in different tissues of rice. RNA-blot analysis was carried out using total RNA from rice suspension cells (SC), leaves (L), roots (R), and calli (C). C, The response of OsCc1 mRNA expression to the Bt2cAMP treatment. Suspension cells (SC), leaves (L), and roots (R) were treated with 1 mm dibutyryl cAMP (Bt2cAMP) for the time periods indicated, and total RNA was extracted. Total RNA was fractionated on a denaturing agarose gel, blotted to a nylon membrane, and hybridized with a [32P]-labeled cyt cDNA probe, shown in A (OsCc1). The loading of an equal amount of total RNA in each lane was verified by ethidium bromide staining (EtBr).
High-Level Expression of the OsCc1 Gene in Non-Photosynthetic Tissues
Genomic Southern analysis showed that a single copy of OsCc1 is present in the genome of rice cv Nakdong (data not shown). To investigate the expression pattern of the gene, northern-blot hybridization was carried out for different tissues and cells using total RNA prepared with a cDNA fragment (cyt in Fig. 1A). The cyt probe is OsCc1-specific, corresponding to the second and third exons, and the 3′-untranslated region of the gene. The cyt probe detected a single mRNA band of approximately 0.7 kb. As shown in Figure 1B, OsCc1 transcripts accumulated in high concentrations in the roots, calli, and suspension cells, but were present at relatively lower levels in mature leaves, demonstrating higher levels of expression in non-photosynthetic tissues.
The OsCc1 promoter contains a sequence at positions −218 to −225 (GCACGTGG), which is similar to that of CRE-1 (TGACGTCA) in the human cytochrome c gene. This finding prompted us to determine whether OsCc1 is regulated by cAMP in the leaves, roots, and suspension cells. Total RNA was prepared at various time intervals after treatment with Bt2cAMP (Gopalakrishnan and Scarpulla, 1994), and assayed for mRNA levels using RNA-blot hybridization (Fig. 1C). The Bt2cAMP treatment did not result in an increase in OsCc1 mRNA levels at any time interval in any of the tissues, but rather caused a decrease for the 6-h time point for both suspension culture and root cells.
The fact that OsCc1 transcript levels were relatively lower in mature leaves than in non-photosynthetic tissues implies that OsCc1 may be negatively regulated by light during the growth of young seedlings. To determine whether OsCc1 is regulated by light or not, total RNA was prepared from etiolated young seedlings at various times after exposure to light. In contrast to the increased mRNA levels in RbcS, OsCc1 mRNA levels were not changed significantly by light (Fig. 2), suggesting its light-independent expression. The lower level of OsCc1 expression in leaves appears to be due to tissue specificity.
Figure 2.
The response of OsCc1 mRNA expression in etiolated seedlings to light exposure. Etiolated seedlings were treated with light at 150 μmol m2 s−1 for the time periods indicated, and total RNA was extracted. Total RNA was fractionated on a denaturing agarose gel, blotted to a nylon membrane, and hybridized with either a [32P]-labeled cyt cDNA probe, shown in Figure 1A (OsCc1), or the coding region for a small subunit of ribulose-1,5-bisphosphate carboxylase (RbcS). The loading of an equal amount of total RNA in each lane was confirmed by ethidium bromide staining (EtBr). Lane L shows total RNA extracted from mature leaves.
Analysis of OsCc1 Promoter Activity in Different Transgenic Tissues in Comparison with Other Rice Promoters
Figure 3 shows the plasmid components used in rice transformation. The 1.8-kb promoter region of OsCc1 from −1,700 to +100 (Fig. 1A) was linked to sgfp (Chiu et al., 1996), which encodes a modified green fluorescent protein (sGFP). The chimeric OsCc1::sgfp gene was then ligated to the expression cassette that carries the coding region of the phosphinothricin acetyl transferase gene (bar) under the control of the 35S promoter. This procedure generated a pSB-CG (OsCc1::sgfp) plasmid. For comparison, two additional constructs were made: pSB-RG (RbcS::sgfp) and pSBG700 (Act1::sgfp). RbcS::sgfp contains promoter regions of a small ribulose bisphosphate carboxylase/oxygenase subunit (RbcS) from rice (Kyozuka et al., 1993; Jang et al., 1999), whereas Act1::sgfp contains promoter regions from the actin1 (Act1) gene of rice (McElroy et al., 1991). All three of these plasmids contain the bar gene as a selectable marker for transformation under the control of the CaMV 35S promoter. Several independent lines of transgenic rice plants (21 for OsCc1::sgfp, 55 for RbcS::sgfp, and 16 for Act1::sgfp) were obtained by the Agrobacterium tumefaciens-mediated method, and grown in a greenhouse. Phosphinothricin acetyl trans-ferase encoded by the bar gene can detoxify phos-phinothricin-based herbicides (Kim et al., 1999). All the transformants were herbicide resistant, as tested by painting leaves with the commercial herbicide Basta (Jang et al., 1999). For each promoter, three independent lines were randomly chosen for further analysis. Genomic Southern blots were used to determine the number of copies and integration events of transgene in the lines. The strategy for Southern-blot analysis is depicted in Figure 3. The genomic DNAs from OsCc1::sgfp-transformed plants were digested either with NcoI, which excised the intact size of sgfp, or with EcoRV, which cut a unique site of the plasmid. The 0.7-kb fragment corresponding to “NcoI digest” (Fig. 3) appeared in all the transformants, indicating that they contained a full-length sgfp gene (Fig. 4A). The three lines showed distinct band patterns in “EcoRV digest,” suggesting that each line was generated by an independent integration event. The genomic DNAs from Act1::sgfp- or RbcS::sgfp-transformed plants were similarly analyzed, consequently validating that all the lines are independent and copy numbers of corresponding transgene are either one or two (Fig. 4).
Figure 3.
The expression vectors used for rice transformation. pSB-CG (OsCc1::sgfp) consists of the OsCc1 promoter linked to the sgfp coding region, the 3′ region of the potato proteinase inhibitor II gene (3′pinII), and the bar gene expression cassette that contains a 35S promoter/bar coding region/3′ region of the nopaline synthase gene (3′nos). pSBG700 (Act1::sgfp) and pSB-RG (RbcS::sgfp) are identical to pSB-CG except that the rice Act1 and RbcS promoters, respectively, are fused to the sgfp-coding region. Important restriction sites are indicated: EcoRV (E), NcoI (N), XbaI (X), BamHI (B), and SpeI (S). Restriction enzymes followed by the expected fragments and hybridization probe (probe) used for genomic DNA-blot analyses are shown below the map.
Figure 4.
Genomic DNA-blot analysis of transgenic rice plants. A, Genomic DNAs from the leaf tissues of three (1–3) independent lines of OsCc1::sgfp-transformed plants were digested with NcoI (N), EcoRV (E), and hybridized with a 0.7-kb DNA fragment containing the sgfp coding region (see Fig. 3). PC contains NcoI-digested pSB-CG. B, Genomic DNAs from the leaf tissues of three (1–3) independent lines of Act1::sgfp-transformed plants were digested with BamHI (B), XbaI (X), and hybridized with the same probe described in A. PC contains BamHI-digested pSBG700. C, Genomic DNAs from the leaf tissues of three (1–3) independent lines of RbcS::sgfp-transformed plants were digested with XbaI (X), SpeI (S), and hybridized with the same probe described in A. PC contains XbaI-digested pSB-RG. NC, Genomic DNAs from an untransformed control plant; 1X, 3X, and 5X in PC represent one, three, and five genome equivalents of pSB-CG (A), pSBG700 (B), or pSB-RG (C), relative to 5 μg of rice genomic DNA, respectively. The DNA molecular size markers (M) are indicated.
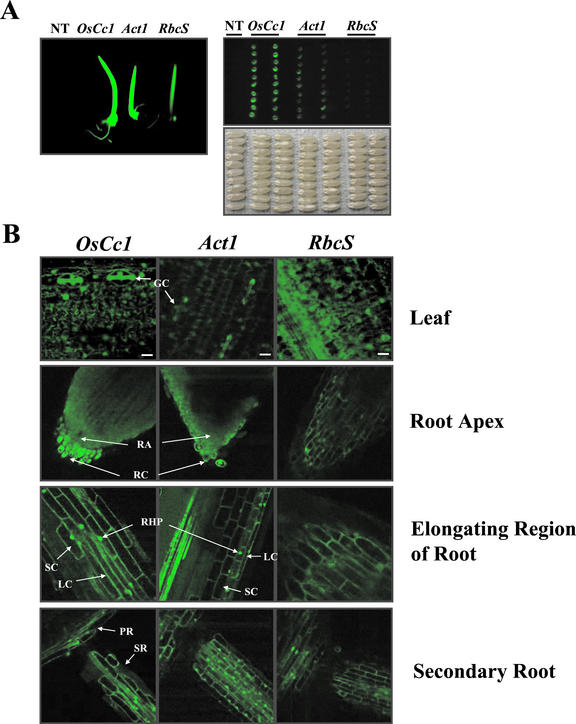
To examine OsCc1 promoter activity at a whole-plant level, sGFP fluorescence in OsCc1::sgfp-transformed plants was examined visually with a digital video imaging system. Non-transformed etiolated seedlings did not show any fluorescence, whereas all tissues of the transgenic etiolated seedlings fluoresced bright green (Fig. 5A, left). The degree and pattern of green fluorescence in the OsCc1::sgfp plant was similar to that of the Act1::sgfp plant. The expression of sGFP was also observed in dry seeds, with the embryos fluorescing strongly (Fig. 5A, right). Intensity of fluorescence in embryos of OsCc1::sgfp plants than in those of Act1::sgfp, or RbcS::sgfp plants. Thus, the OsCc1 promoter is highly active in driving gene expression in most tissues of transgenic rice plants.
Figure 5.
Images of sGFP fluorescence in transgenic rice plants expressing OsCc1::sgfp (OsCc1), Act1::sgfp (Act1), and RbcS::sgfp (RbcS) chimeric genes. A, sGFP fluorescence in transgenic rice seedlings (left) and uncoated dry seeds (right) taken by a digital video imaging system. NT represents an untransformed rice seedling (right) and seeds (left). B, Confocal microscopic image of sGFP fluorescence in a leaf, a root apex, elongating region of a root, and secondary root of transgenic rice plants. GC, Guard cells; RA, root apex; RC, root cap; RHP, root hair protuberances; LC, long cells; SC, short cells; PR, primary root; SR, secondary root. Scale bars = 30 μm.
OsCc1 promoter activity in different tissues was compared with those of the Act1 and rbcS promoters in transgenic rice plants. Using CLSM, we investigated the distribution of sGFP fluorescence in the cells of leaf and root tissues (Fig. 5B). In transformed cells, fluorescence emitted from sGFP was detected in a green channel, whereas fluorescence was not detected in untransformed cells under the same conditions. In leaf cells, OsCc1::sgfp plants had higher levels of sgfp expression than Act1::sgfp plants and comparable levels with RbcS::sgfp plants. In particular, OsCc1::sgfp and Act1::sgfp plants displayed brighter fluorescence in guard and mesophyll cells (Fig. 5B). In contrast, sGFP fluorescence in root cells was much higher in OsCc1::sgfp and Act1::sgfp plants than in RbcS::sgfp plants. Interestingly, OsCc1::sgfp plants showed the greatest GFP fluorescence in the root cap region (RC on Fig. 5B). The OsCc1 and Act1 promoters also directed high levels of sgfp expression in the root hair protuberances of both primary and secondary roots.
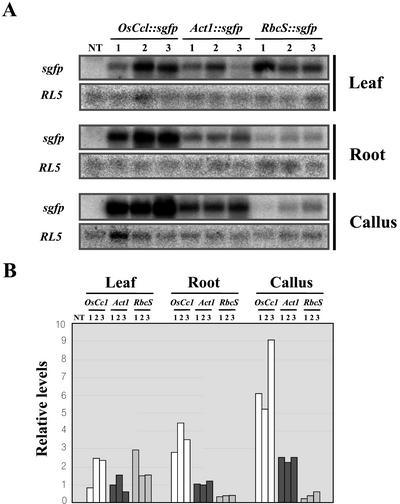
For a quantitative comparison of activities of the promoters, total RNAs were extracted from leaf, root, and callus of three independent lines of OsCc1::sgfp, Act1::sgfp, and RbcS::sgfp plants (shown in Fig. 4) and hybridized with a 0.7-kb DNA fragment containing the sgfp coding region (Fig. 6A). The total RNAs were reprobed with the rice RL5, which encodes the 5S rRNA-binding protein (Kim and Wu, 1993), for equal RNA loading. As shown in Figure 6B, relative levels of sgfp transcripts were calculated and normalized using RL5 as a reference. In leaf tissues, the sgfp transcript levels of OsCc1::sgfp plants were comparable with those of RbcS::sgfp plants, but 2-fold higher than those of Act1::sgfp plants. In roots and calli, sgfp transcript levels of OsCc1::sgfp plants were 3- and 10-fold higher than those of Act1::sgfp and RbcS::sgfp plants, respectively.
Figure 6.
RNA gel-blot analysis and relative levels of sgfp transcripts in transgenic rice plants shown in Figure 4. A, Total RNAs extracted from leaf, root and callus of three (1–3) independent lines of OsCc1::sgfp-, Act1::sgfp-, and RbcS::sgfp-transformed plants and from an untransformed control plant (NT) were hybridized with a 0.7-kb DNA fragment containing the sgfp coding region (see Fig. 3). Hybridizations with the rice RL5 encoding the 5S rRNA-binding protein (Kim and Wu, 1993) were used for equal RNA loading. B, Transcript levels of sgfp shown in A were calculated using those of corresponding RL5 as a reference and the resultant values were then normalized to 1 for that from leaf tissues of Act1::sgfp-transgenic line 1.
Our CLSM and RNA gel-blot data demonstrated that the OsCc1 promoter is similar to the rice Act1 in driving high levels of expression of sgfp in cells that are rapidly dividing or have high metabolic activity. However, the OsCc1 promoter activity was much higher than that of Act1 in leaves, roots, embryos, and calli (Fig. 5C). The OsCc1 promoter is particularly active in guard cells, root cap, apex regions, root hair protuberances, and calli. Thus, OsCc1 is useful as a promoter for ubiquitous expression of transgene in monocots.
DISCUSSION
Cytochrome c is the product of a nuclear gene, and resides in the intermembrane space of mitochondria. It functions in the catalytic transfer of electrons between respiratory complexes III and IV. The amino acid and nucleotide sequence of cytochrome c has been extensively used for studies of molecular evolution because it is present in all eukaryotes, and sequences are strikingly similar among organisms (Syvanen et al., 1989; Kemmerer et al., 1991). With its unique subcellular location, cytochrome c has been a molecule of scientific interest for protein targeting in organelles (von Heijne et al., 1989). In addition, the human cytochrome c gene is regulated by cAMP (Gopalakrishnan and Scarpulla, 1994), and is involved in a mechanism for coordination of nuclear and mitochondrial genetic systems (Evans and Scarpulla, 1990). In contrast to the rapid progress in other organisms, little attention has been given to cytochrome c in plants. It is generally accepted that the main mitochondrial function of generating ATP by oxidative phosphorylation is regulated according to differing requirements during plant development (e.g. the requirements of photosynthetic leaves, roots, or other types of cells). However, gene expression patterns, promoter activity, and the responses of the plant cytochrome c gene to cAMP remain largely unexplored. A rice genomic clone, OsCc1, encoding cytochrome c, was previously sequenced (Kemmerer et al., 1991). Rice contains a single copy of OsCc1 in its genome, as demonstrated by our genomic DNA hybridization. Northern-blot hybridization indicated that high concentrations of OsCc1 transcripts accumulated in the roots, calli, and suspension cells, whereas levels were lower in mature leaves. This suggests that OsCc1 is highly expressed in non-photosynthetic tissues, which is consistent with the fact that chloroplasts function as the main energy supply in photosynthetic tissues, whereas mitochondria are responsible for supplying energy in non-photosynthetic tissues. Undifferentiated plastids are known to cause complex alterations to nuclear gene expression (Hess et al., 1994, 1997; Hedtke et al., 1999). Calli, cultured suspension cells, and roots do not have differentiated chloroplasts, resulting in higher levels of OsCc1 expression than in leaf cells. Because the decrease in OsCc1 mRNA levels in leaves was not due to light exposure (Fig. 4), it is likely that there is specific repression of the gene in leaf tissues.
cAMP is an important signaling molecule in all organisms except plants. In higher plants, cAMP is barely detectable (Trewavas, 1997), so its function has not been elucidated. The OsCc1 promoter contains the sequence GCACGTGG (at positions −218 to −225), which is similar to the TGACGTCA motif. The latter functions as a CRE in a number of cAMP-responsive promoters of animal systems, including a human cytochrome c gene (Gopalakrishnan and Scarpulla, 1994). In our experiments, however, no increase in OsCc1 mRNA levels was detected after Bt2cAMP treatment (Fig. 3B). This result was consistent at all time intervals measured and for all tissues examined, including suspension cells, leaves, and roots. This led us to speculate that OsCc1 expression does not seem to be responsive to cAMP levels in rice cells. However, it remains to be determined whether cAMP levels in the rice cells were actually enhanced by the Bt2cAMP treatment before drawing any conclusion. It is also possible that OsCc1 may be more sensitive to Bt2cAMP treatment in other types of plant cells, or in combination with a plant hormone. This is supported by the observation in animal systems that the cytochrome c gene was not induced in COS-1 cells, but was highly induced in BALB/3T3 fibroblasts after Bt2cAMP treatment (Gopalakrishnan and Scarpulla, 1994).
Despite isolating numerous promoters from a wide variety of plants, only a few of them are commonly used in plant transformation. The CaMV 35S promoter is active in dicot tissues, but has much lower activity in monocots (McElroy et al., 1991; Cornejo et al., 1993; Schledzewski and Mendel, 1994; Wilmink et al., 1995). The rice Act1 (McElroy et al., 1990) and maize Ubi1 (Christensen et al., 1992) promoters are commonly used in monocot transformation because they are significantly more active than the CaMV 35S promoter in these cells (McElroy et al., 1990; Christensen et al., 1992; Cornejo et al., 1993; McElroy and Brettell, 1994; Schledzewski and Mendel, 1994; Wilmink et al., 1995; Christensen and Quail, 1996). Our results demonstrated that the OsCc1 promoter was 2- and 3-fold more active in transgenic rice leaves and roots, respectively, than the Act1 promoter (Figs. 5 and 6). Although OsCc1::sgfp expression varies depending on the plant tissue, it is clear that the OsCc1 promoter is active in most cell types with particular preference in non-photosynthetic tissues, including calli and roots. The feature of the OsCc1 promoter that makes it useful for rice transformation is its ability to drive high levels of expression in calli, which are typically used for the selection and screening of transformed cells. The high activity of the OsCc1 promoter in rapidly dividing cells should allow the clear identification of stable transformants.
The distribution of sGFP fluorescence in transgenic rice plants reveals several other features of the OsCc1 promoter. Young roots showed strong sGFP expression, particularly in the root cap and apex regions. Strong sGFP fluorescence was also observed in the mesophyll and guard cells of leaves. The OsCc1 pattern of expression suggests that this promoter is most active in cells with high metabolic activity, and that it is distinct from other promoters characterized from transgenic rice. CaMV 35S promoter expression is widespread, exhibiting activity in the vascular tissue (Terada and Shimamoto, 1990), and in most root and leaf cell types (Battraw and Hall, 1990). Ubi1 is expressed in many, but not all, cell types. This promoter drives strong expression in young roots, but expression levels decrease drastically as the roots mature. Ubi1 is also active in the vascular system and guard cells of transgenic rice leaves (Cornejo et al., 1993). The Act1 promoter in rice is active in both vegetative and reproductive tissues of transgenic rice plants (McElroy et al., 1990). Thus, the OsCc1 pattern of expression is similar to that of the rice Act1 and CaMV 35S promoters, but is, in fact, stronger than either of these promoters.
In summary, a detailed examination of the expression patterns of the OsCc1::sgfp construct indicates that the gene is expressed in most organs of transgenic rice, consistent with the potential for targeting a wide spectrum of cells. Therefore, the OsCc1 promoter represents a useful alternative to the maize Ubi1 and rice Act1 promoters for the high-level expression of genes in monocots.
MATERIALS AND METHODS
Plant Materials
Transgenic and nontransgenic rice (Oryza sativa) plants were grown in a greenhouse. Embryogenic calli were initiated from mature rice cv Nakdong embryos, and were maintained on solid Murashige and Skoog medium (pH 5.8), containing 1% (w/v) agarose, 30 g L−1 Suc, and 2.5 mg L−1 2,4-dichlorophenoxyacetic acid. Suspension cultures were started from embryogenic calli in liquid AA medium, containing 30 g L−1 Suc, 2.0 mg L−1 2,4-dichlorophenoxyacetic acid, and 0.2 mg L−1 kinetin. The cultures were kept on an incubator that rotated while it shook (120 rpm) in the dark at 26°C.
RNA Isolation and Analysis
Rice plants, cultured cells, and calli were subjected to various treatments. Each sample was soaked in 1 mm Bt2cAMP (Sigma, St. Louis), or was exposed to light at 150 μmol m2 s−1 for the time periods indicated. After treatment, all plant materials were frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted from different tissues of rice by the guanidinium/LiCl method. About 0.1 g of leaves, roots, calli, and etiolated seedlings was homogenized in 1 mL of an extraction buffer, containing 4 m guanidinium isothiocyanate, 25 mm sodium citrate (pH 7.0), 0.5% (w/v) sarkosyl, and 0.1 m β-mercaptoethanol. After homogenization, 0.1 mL of 2 m sodium acetate (pH 4.0), 1 mL of water-saturated phenol (pH 4.5), and 0.2 mL of chloroform:isoamyl alcohol (1:1, v/v) were added. The mixture was vortexed for 30 s, and centrifuged at room temperature for 10 min at 5,000g. The supernatant was removed to a new tube, and an equal volume of isopropanol was added to it. The sample was then incubated at −20°C for 1 h, and centrifuged at 4°C for 10 min at 10,000g. The RNA pellet was dissolved in 0.5 mL of diethyl pyrocarbonate-treated water, and was precipitated with an equal volume of 4 m lithium chloride, followed by incubation at 4°C for at least 1 h. The precipitated RNA was subsequently centrifuged at 4°C for 10 min at 10,000g, washed with 70% (v/v) ethanol, and dried. The RNA pellet was dissolved in 40 mL of diethyl pyrocarbonate-treated water and its concentration was calculated from A260. For the northern-blot analysis, 20 μg of total RNA was electrophoresed on a 1.2% (w/v) agarose gel containing iodoacetamide, and was then blotted onto a nylon membrane (Hybond N+, Amersham, Buckinghamshire, UK) following standard procedures (Sambrook et al., 1989). The membrane was hybridized overnight at 65°C with a [32P]labeled DNA probe, which was prepared according to the manufacturer's instructions using a random primer labeling kit (Takara, Kyoto). Hybridization was carried out overnight in a mixture containing 1 m sodium phosphate buffer and 1 mg of salmon sperm DNA. After hybridization, the membrane was washed once in 2× SSC (0.3 m NaCl and 50 mm sodium citrate, pH 7.0) and 0.1% (w/v) SDS solution at 65°C for 15 min, and then once in 1× SSC and 0.5% (w/v) SDS at 65°C for 15 min, and finally in 0.5× SSC and 0.5% (w/v) SDS at 65°C. The membrane was then exposed on an intensifying plate, and analyzed with a phosphor imager analyzer (FLA 3000, Fuji, Tokyo).
Vector Construction
The OsCc1 promoter sequence (1.8 kb) was PCR amplified using an upstream primer (5′-AACTGGAGGAATTCGGATCTTCGAAGGTAGGC-3′, XhoI site underlined), a downstream primer (5′-AACCATGGCCGC-CGCCGCCGCGAGAACG-3′, NcoI site underlined), and the plasmid pOsCc1 (Kemmerer et al., 1991a) as template. PCR-amplified DNA was digested with XhoI and NcoI, and ligated with XhoI/NcoI-linearized pBluescript KSII containing the sgfp gene (Chiu et al., 1996). This process produced the plasmid pKSCG. Once sequencing verified the regions spanning the junction, the 2.5-kb DNA fragment containing the OsCc1 promoter-sgfp was obtained by digesting pKSCG with XhoI and NotI, and ligating it into XhoI/NotI-linearized pSB105 (Jang et al., 1999). This produced the plasmid pSB-CG. pSB105 contains the potato protease inhibitor II terminator/35S promoter/bar/nopaline synthase terminator between the right and left border sequences of pSB11 (Komari et al., 1996). To construct pSB-RG, the pSK-RG plasmid containing the rbcS promoter linked to the sgfp gene (Chiu et al., 1996) was digested with BamHI and NotI, and was then ligated into BamHI/NotI-linearized pSB105. The rbcS promoter in pSB-RG was replaced by the Act1 promoter of rice (McElroy et al., 1991), producing pSBG700. Finally, the three plasmids were introduced into Agrobacterium tumefaciens LBA4404 by triparental mating (Jang et al., 1999).
A. tumefaciens-Mediated Rice Transformation
For A. tumefaciens-mediated transformation, about 200 mature seeds of rice cv Nakdong were husked and sterilized with 70% (v/v) ethanol for 1 min using gentle shaking. The ethanol was discarded and the seeds were sterilized further with 100 mL of 20% (v/v) commercial bleach for 1 h with gentle shaking. The sterilized seeds were rinsed several times with sterile water. Callus induction, cocultivation with A. tumefaciens, and the selection of transformed calli were carried out as previously described by Jang et al. (1999).
Video Imaging of sGFP Fluorescence
sGFP fluorescence in etiolated seedlings and dry seeds was captured on a digital video imaging system (Chung et al., 2000). The excitation light came from a 250-W halogen lamp. A blue band pass filter with a peak at 480 nm (Edmund, Barrington, NJ) was used with the lamp. The light beam was focused on the sample at an angle of approximately 45° from the vertical, illuminating an area of 4 cm2 with a maximum photon flux density of 2 μmol m−2 s−1. A high-resolution CCD color video camera (model CoolSNAP, Roper Scientific Inc., Trenton, NJ) took images of the sample from a vertical position. Using a zoom lens (Nikon, Tokyo) attached to the CCD, an image was taken of a 20- ×20-mm area of the sample. A green-transmitting band pass filter with a peak at 510 nm (Edmund) was then positioned in front of the lens to transmit only a green fluorescence beam. A personal computer (686-500 MHz; Intel Corp., Seoul, Korea) controlled the CCD camera through an interface board (Roper Scientific Inc.), and collected the image files on the hard drive.
CLSM
The leaves, roots, and calli of the rice plants were observed using a confocal laser scanning microscope (Carl Zeiss LSM510, Zeiss, Jena, Germany) with a standard filter set. The microscope was located at the Korean Basic Science Institute (Daejon). Pseudocolor, similar to the color observed with a fluorescence microscope (Olympus, Tokyo), was added to the images by importing data collected in the green and red channels of the confocal microscope. Sections were taken along the optical axis and were projected into a single image.
ACKNOWLEDGMENT
We are grateful to Dr. Ray Wu (Cornell University, Ithaca, NY) for providing the pOsCc1 plasmid.
Footnotes
This work was supported by the Ministry of Science and Technology through the Crop Functional Genomics Center (grant to J.-K.K.), by the Korea Science and Engineering Foundation through the Plant Metabolism Research Center at Kyung-Hee University (grant to J.-K.K.), and by the Ministry of Education's Brain Korea 21 Project (fellowships to I.-C.J., K.-H.L., and S.I.S.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.002261.
LITERATURE CITED
- Battraw MJ, Hall TC. Histochemical analysis of CaMV 35S promoter-β-glucuronidase gene expression in transgenic rice plants. Plant Mol Biol. 1990;15:527–538. doi: 10.1007/BF00017828. [DOI] [PubMed] [Google Scholar]
- Bruce WB, Christensen AH, Klein T, Fromm M, Quail PH. Photoregulation of a phytochrome gene promoter from oat transferred into rice by particle bombardment. Proc Natl Acad Sci USA. 1989;86:9692–9696. doi: 10.1073/pnas.86.24.9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu W-L, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J. Engineered GFP as a vital reporter in plants. Curr Biol. 1996;6:325–330. doi: 10.1016/s0960-9822(02)00483-9. [DOI] [PubMed] [Google Scholar]
- Christensen AH, Quail PH. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res. 1996;5:213–218. doi: 10.1007/BF01969712. [DOI] [PubMed] [Google Scholar]
- Christensen AH, Sharrock RA, Quail PH. Maize polyubiquitin gene: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol. 1992;18:675–689. doi: 10.1007/BF00020010. [DOI] [PubMed] [Google Scholar]
- Chung BC, Kim J-K, Nahm BH, Lee C-H. In vivo monitoring of green fluorescent protein in transgenic rice. Mol Cell. 2000;10:411–414. [PubMed] [Google Scholar]
- Cornejo M-J, Luth D, Blankenship KM, Anderson OD, Blechl AE. Activity of a maize ubiquitin promoter in transgenic rice. Plant Mol Biol. 1993;23:567–581. doi: 10.1007/BF00019304. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Scarpulla RC. NRF-1: a trans-activator of nuclear-encoded respiratory genes in animal cells. Genes Dev. 1990;4:1023–1034. doi: 10.1101/gad.4.6.1023. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan L, Scarpulla RC. Differential regulation of respiratory chain subunits by a CREB-dependent signal transduction pathway. J Biol Chem. 1994;269:105–113. [PubMed] [Google Scholar]
- Hedtke B, Wagner I, Börner T, Hess WR. Inter-organellar crosstalk in higher plants: Impaired chloroplast development affects mitochondrial gene and transcript levels. Plant J. 1999;19:635–643. doi: 10.1046/j.1365-313x.1999.00554.x. [DOI] [PubMed] [Google Scholar]
- Hess WR, Linke B, Nagy F, Börner T. Effects of plastid differentiation on nuclear gene transcription. In: Schenk HEA, Herrmann RG, Jeon KW, Müllert NE, Schwemmler W, editors. Eukaryotism and Symbiosis. Heidelberg: Springer Verlag; 1997. pp. 233–242. [Google Scholar]
- Hess WR, Müller A, Nagy F, Börner T. Ribosome-deficient plastids affect transcription of light-induced nuclear genes: genetic evidence for a plastid-derived signal. Mol Gen Genet. 1994;242:305–312. doi: 10.1007/BF00280420. [DOI] [PubMed] [Google Scholar]
- Jang I-C, Nahm BH, Kim J-K. Subcellular targeting of green fluorescent protein to plastids in transgenic rice plants provides a high-level expression system. Mol Breed. 1999;5:453–461. [Google Scholar]
- Joshi CP. An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res. 1987;15:6643–6653. doi: 10.1093/nar/15.16.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerer EC, Lei M, Wu R. Isolation and molecular evolutionary analysis of a cytochrome c gene from Oryza sativa (rice) Mol Biol Evol. 1991a;8:212–226. doi: 10.1093/oxfordjournals.molbev.a040639. [DOI] [PubMed] [Google Scholar]
- Kemmerer EC, Lei M, Wu R. Structure and molecular evolutionary analysis of a plant cytochrome c gene: surprising implications for Arabidopsis thaliana. J Mol Evol. 1991b;32:227–237. doi: 10.1007/BF02342745. [DOI] [PubMed] [Google Scholar]
- Kim J-K, Duan X, Wu R, Seok SJ, Boston RS, Jang I-C, Eun MY, Nahm BH. Molecular and genetic analysis of transgenic rice plants expressing the maize ribosome-inactivating protein b-32 gene and the herbicide resistance bar gene. Mol Breed. 1999;5:85–94. [Google Scholar]
- Kim J-K, Wu R. A rice (Oryza sativa L.) cDNA encodes a protein sequence homologous to the eukaryotic ribosomal 5S RNA-binding protein. Plant Mol Biol. 1993;23:409–413. doi: 10.1007/BF00029016. [DOI] [PubMed] [Google Scholar]
- Komari T, Hiei Y, Saito Y, Murai N, Kumashiro T. Vectors carrying two separate T-DNAs for co-transformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J. 1996;19:165–174. doi: 10.1046/j.1365-313x.1996.10010165.x. [DOI] [PubMed] [Google Scholar]
- Kyozuka J, McElroy D, Hayakawa T, Xie Y, Wu R, Shimamoto K. Light-regulated and cell-specific expression of tomato rbcS-gusA and rice rbcS-gusA fusion gene in transgenic rice. Plant Physiol. 1993;102:991–1000. doi: 10.1104/pp.102.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy D, Blowers AD, Jenes B, Wu R. Construction of expression vectors based on the rice actin1 (Act1) 5′ region for use in monocot transformation. Mol Gen Genet. 1991;231:150–160. doi: 10.1007/BF00293832. [DOI] [PubMed] [Google Scholar]
- McElroy D, Brettell RIS. Foreign gene expression in transgenic cereals. Trends Biotechnol. 1994;12:62–68. [Google Scholar]
- McElroy D, Zhang W, Wu R. Isolation of an efficient actin promoter for in rice transformation. Plant Cell. 1990;2:163–171. doi: 10.1105/tpc.2.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schledzewski K, Mendel R. Quantitative transient gene expression: comparison of the promoters for maize polyubiquitin 1, rice actin 1, maize derived Emu and CaMV 35S in cells of barley, maize and tobacco. Transgenic Res. 1994;3:249–255. [Google Scholar]
- Syvanen M, Hartman H, Stevens PF. Classical plant ambiguities extend to the molecular level. J Mol Evol. 1989;28:536–544. doi: 10.1007/BF02602934. [DOI] [PubMed] [Google Scholar]
- Terada R, Shimamoto K. Expression of CaMV35S-GUS gene in transgenic rice plants. Mol Gen Genet. 1990;22:389–392. [Google Scholar]
- Trewavas AJ. Plant cyclic AMP comes in from the cold. Nature. 1997;390:657–658. doi: 10.1038/37720. [DOI] [PubMed] [Google Scholar]
- Uchimiya H, Iwata M, Nojiri C, Smarajeewa PK, Takamatsu S, Ooba S, Anzai H, Christensen AH, Quail PH, Toki S. Biolophos treatment of transgenic rice plants expressing a bar gene prevents infection by the sheath blight pathogen (Rhizoctonia solani) Bio/Technology. 1993;11:835–836. [Google Scholar]
- von Heijne G, Steppuhn J, Herrmann RG. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989;180:535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]
- Wilmink A, van de Ven BCE, Dons JJM. Activity of constitutive promoters in various species from the Liliaceae. Plant Mol Biol. 1995;28:949–955. doi: 10.1007/BF00042079. [DOI] [PubMed] [Google Scholar]