Abstract
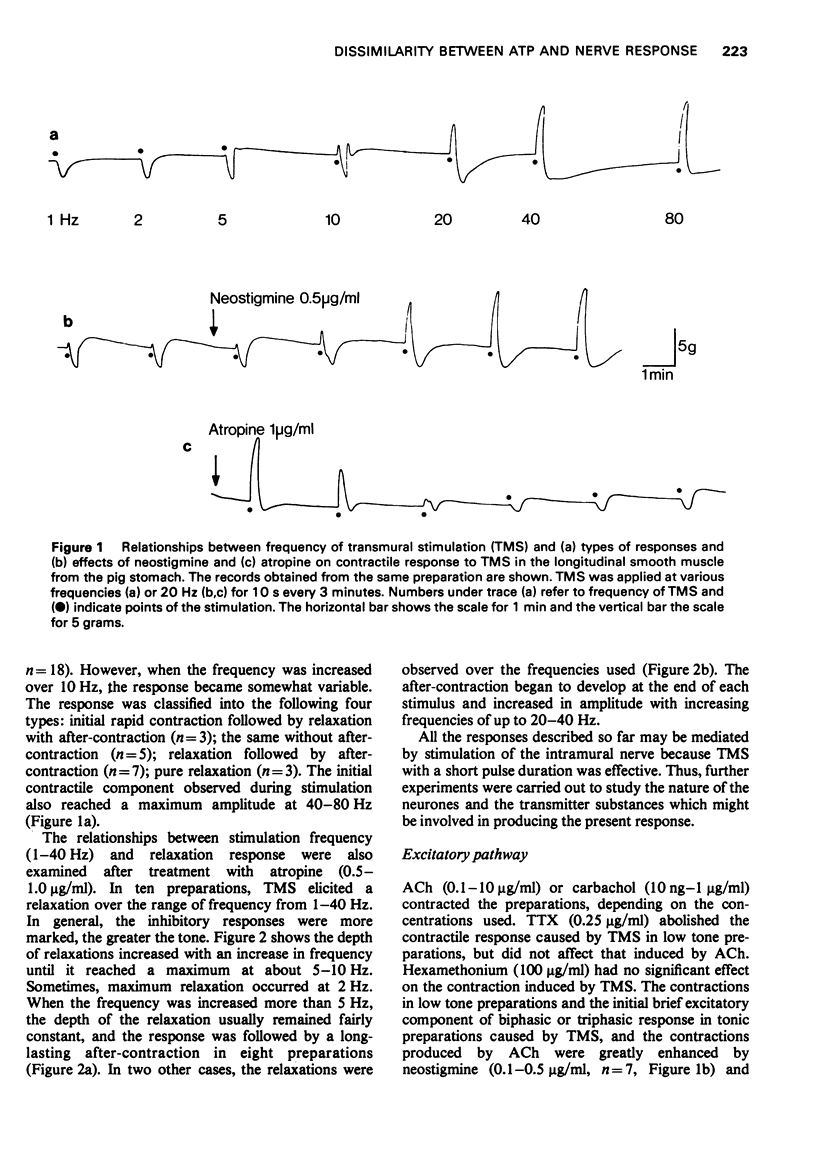
1 Transmural electrical stimulation (TMS) of longitudinal smooth muscle strips taken from the cardiac portion of the pig stomach produced biphasic responses consisting of initial contractions followed by relaxations. The excitatory component was enhanced by neostigmine and abolished by atropine. After atropine treatment, TMS and nicotine or 1,1-dimethyl-4-phenyl-piperazinium, caused a relaxation or a relaxation followed by an after-contraction. All of these responses were abolished or reduced reversibly with tetrodotoxin and cocaine, while hexamethonium only abolished the response to ganglion-stimulating agents.
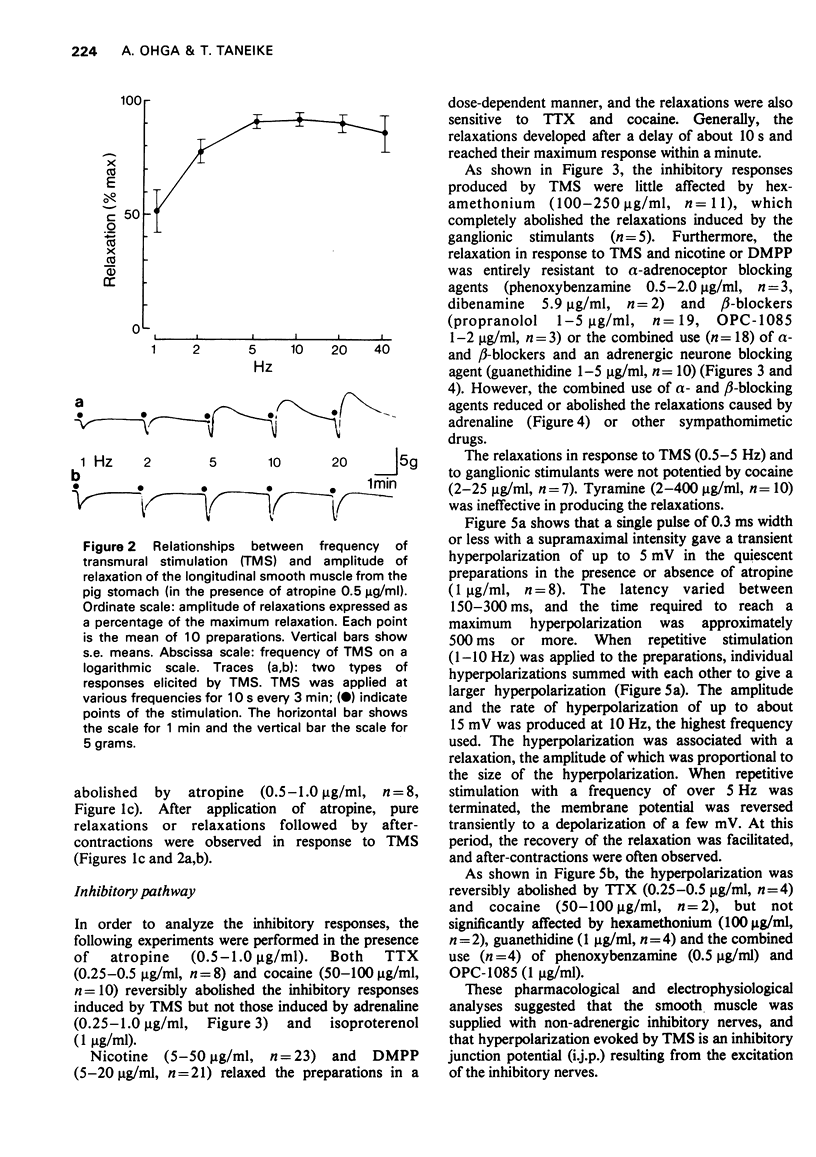
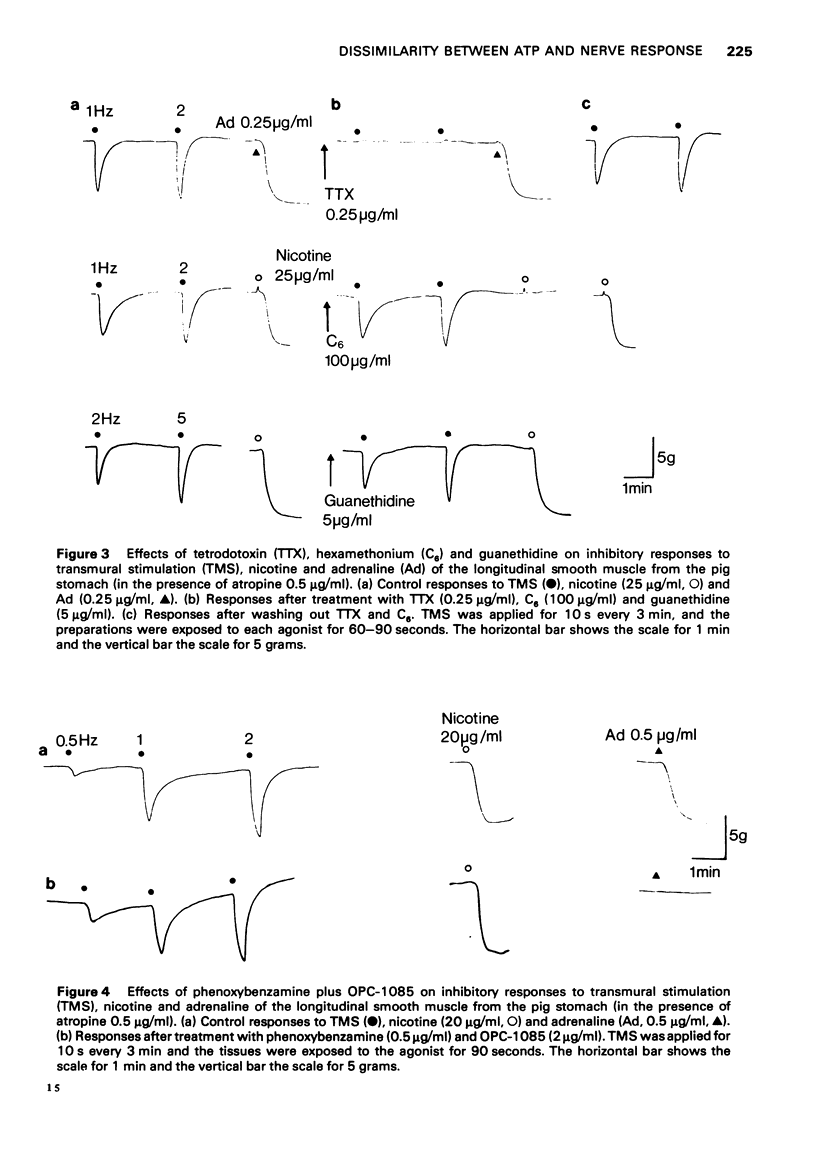
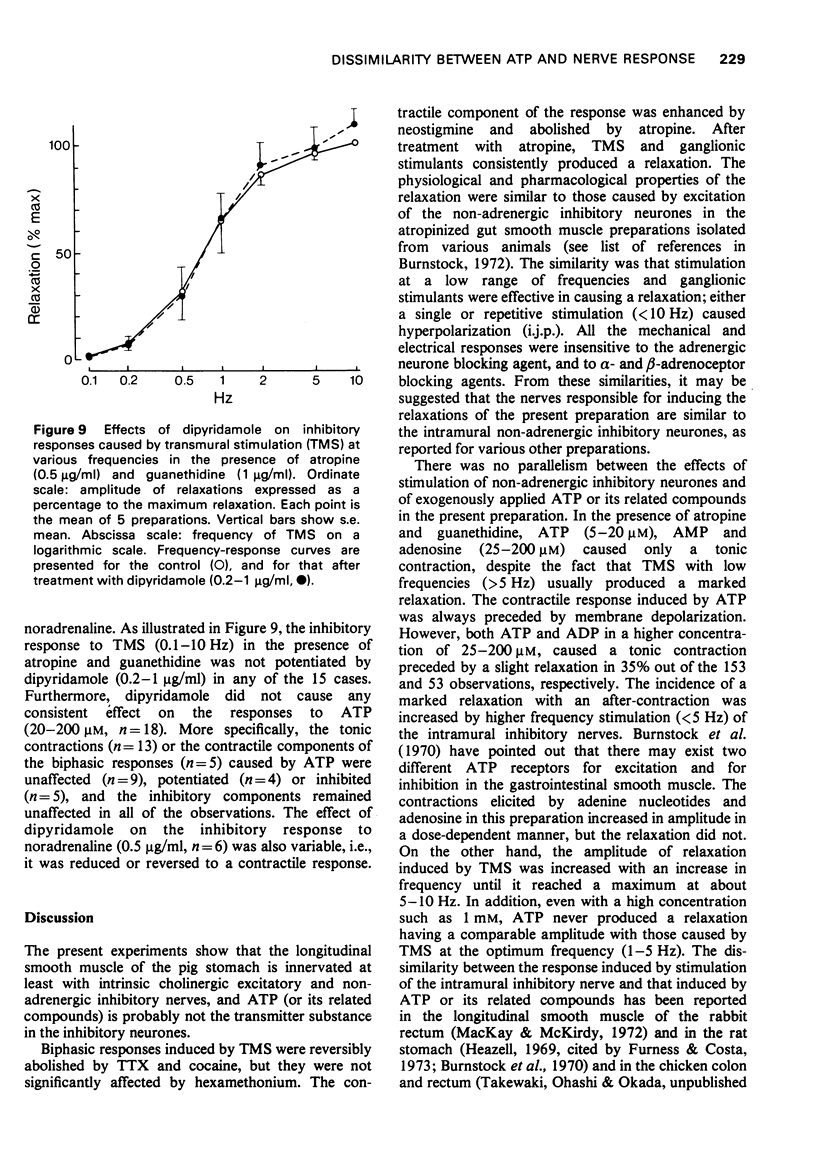
2 The relaxation caused by TMS reached a maximum amplitude at 5-10 Hz, and was entirely resistant to the effects of α- and β-adrenoceptor blocking agents, or a combination of them, and also to guanethidine. These results strongly suggested that the relaxation was elicited by stimulation of intramural non-adrenergic inhibitory neurones.
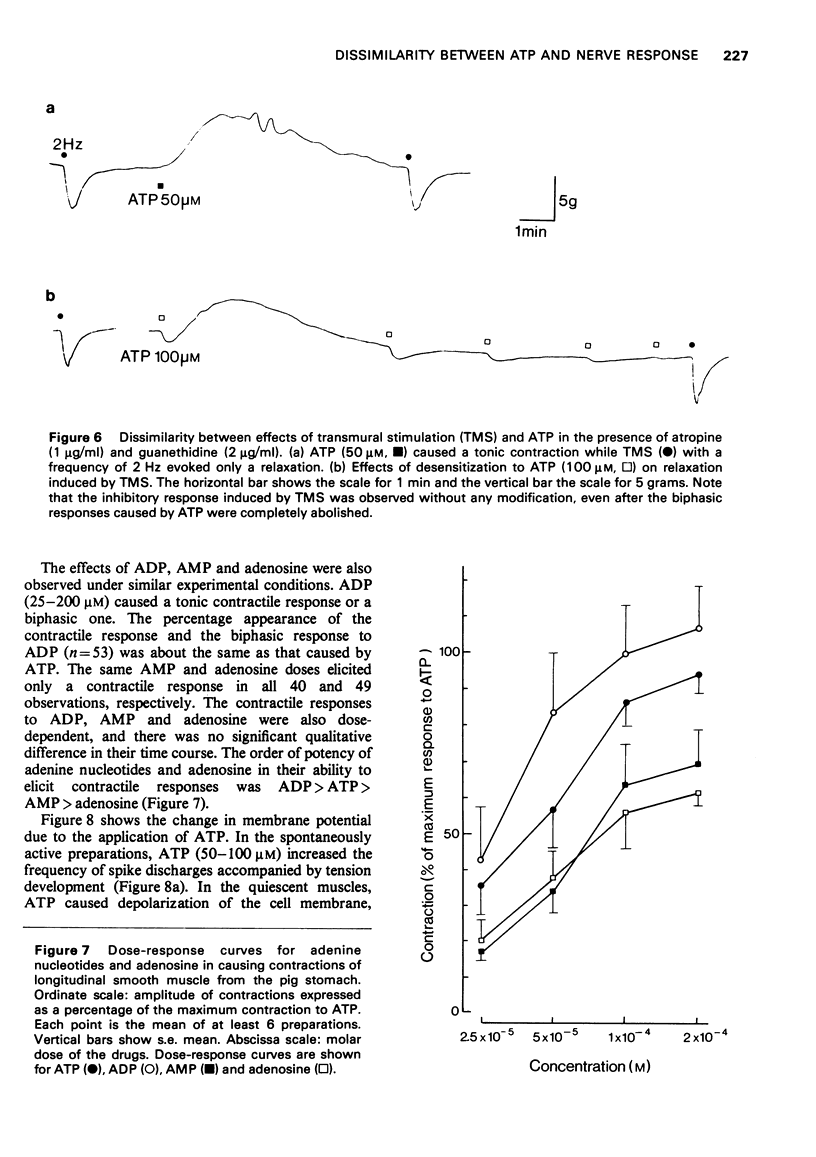
3 In the presence of atropine and guanethidine, adenosine triphosphate (ATP, 5-20 μM) caused only a tonic contraction, and ATP (25-200 μM) or adenosine diphosphate (25-200 μM) produced a contractile response or a biphasic one (tonic contraction preceded by a slight relaxation). Adenosine monophosphate and adenosine caused only the tonic contraction over the range of concentrations (25-200 μM).
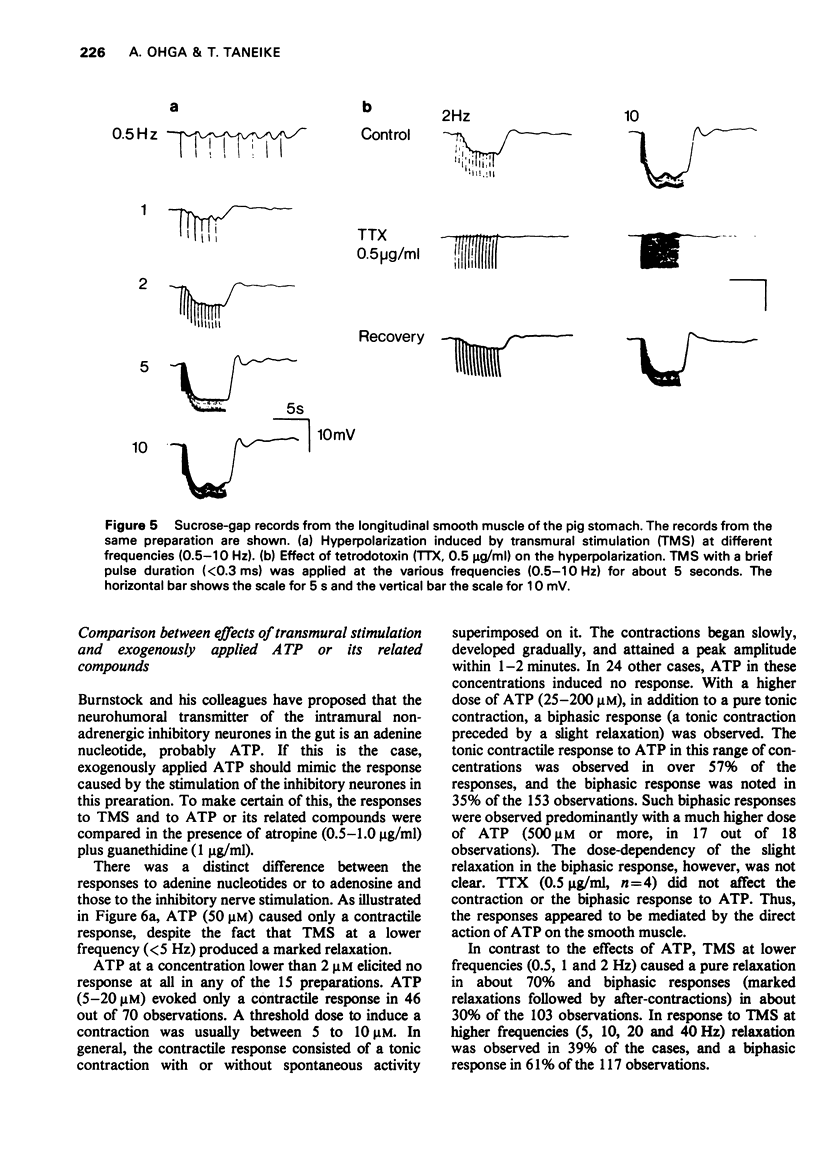
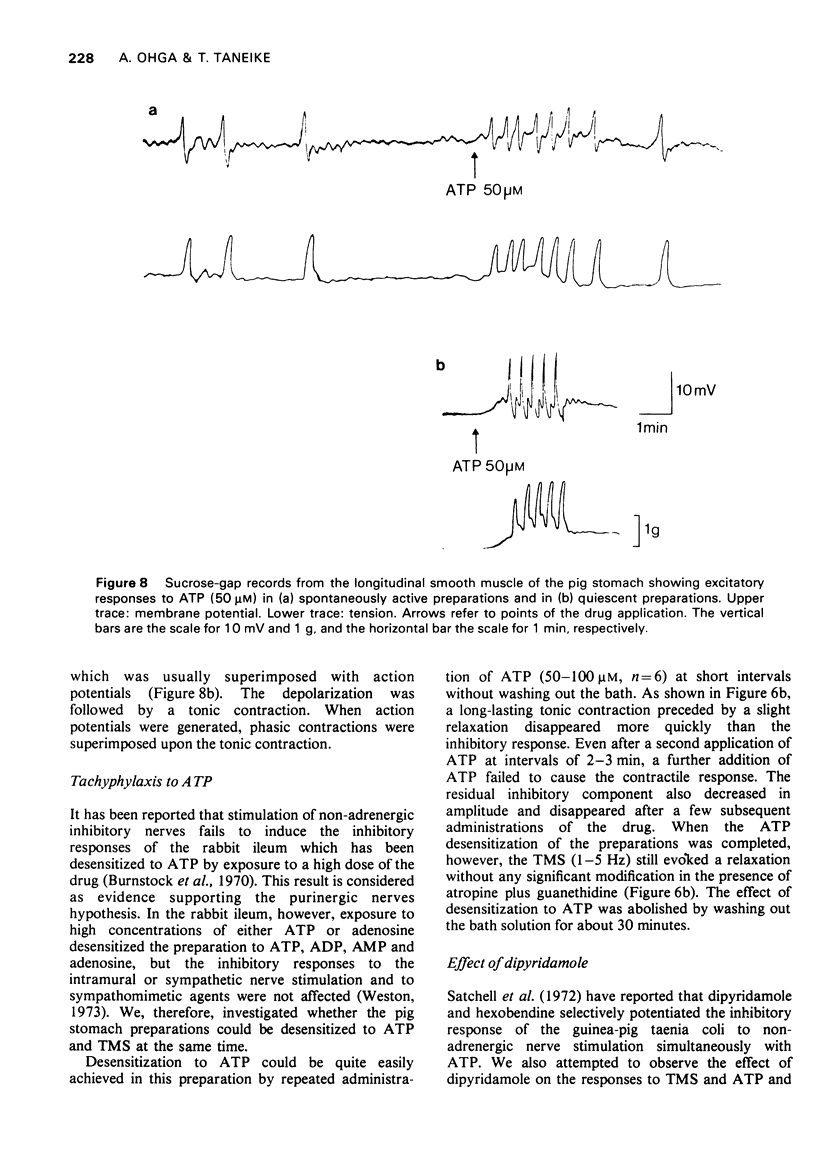
4 Stimulation of the intramural inhibitory neurones of the tissue consistently evoked an inhibitory junction potential, which showed a summation during repetitive stimulation. One the other hand, ATP elicited mainly a small depolarization of a few mV.
5 When the desensitization to ATP of the muscle was achieved in the presence of atropine and guanethidine, the relaxation induced by stimulation of the non-adrenergic inhibitory neurones could be evoked without any modification.
6 Dipyridamole neither potentiated the inhibitory responses due to stimulation of the intramural inhibitory neurones nor showed any consistent effect on the ATP-induced response.
7 From these results, it is unlikely that ATP, or any related compound, is the transmitter substance of the intramural inhibitory neurones in the longitudinal smooth muscle of the pig stomach.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNSTOCK G., STRAUB R. W. A method for studying the effects of ions and drugs on the resting and action potentials in smooth muscle with external electrodes. J Physiol. 1958 Jan 23;140(1):156–167. doi: 10.1113/jphysiol.1958.sp005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Satchell D., Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970 Dec;40(4):668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Burnstock G., Satchell D. G., Smythe A. A comparison of the excitatory and inhibitory effects of non-adrenergic, non-cholinergic nerve stimulation and exogenously applied ATP on a variety of smooth muscle preparations from different vertebrate species. Br J Pharmacol. 1972 Oct;46(2):234–242. doi: 10.1111/j.1476-5381.1972.tb06868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B., Costa M. The nervous release and the action of substances which affect intestinal muscle through neither adrenoreceptors nor cholinoreceptors. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):123–133. doi: 10.1098/rstb.1973.0015. [DOI] [PubMed] [Google Scholar]
- Heazell M. A. Proceedings: Is ATP an inhibitory neurotransmitter in the rat stomach. Br J Pharmacol. 1975 Oct;55(2):285P–286P. [PMC free article] [PubMed] [Google Scholar]
- Hooper M., Spedding M., Sweetman A. J., Weetman D. F. Proceedings: 2-2' pyridylisatogen tosylate: an antagonist of the inhibitory effects of ATP on smooth muscle. Br J Pharmacol. 1974 Mar;50(3):458P–459P. [PMC free article] [PubMed] [Google Scholar]
- Hulme M. E., Weston A. H. Some effects of dipyridamole, hexobendine and lidoflazine on inhibitory processes in rabbit duodenum. Br J Pharmacol. 1974 Apr;50(4):609–611. doi: 10.1111/j.1476-5381.1974.tb08598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay D., McKirdy H. C. Proceedings: Effect of vasopressin and of adenosine triphosphate on the flat preparation of rabbit rectum. Br J Pharmacol. 1972 Feb;44(2):366P–367P. [PMC free article] [PubMed] [Google Scholar]
- Ohashi H., Ohga A. Transmission of excitation from the parasympathetic nerve to the smooth muscle. Nature. 1967 Oct 21;216(5112):291–292. doi: 10.1038/216291a0. [DOI] [PubMed] [Google Scholar]
- Rikimaru A., Fukushi Y., Suzuki T. Effects of imidazole and phentolamine on the relaxant responses of guinea-pig taenia coli to transmural stimulation and to adenosine triphosphate. Tohoku J Exp Med. 1971 Oct;105(2):199–200. doi: 10.1620/tjem.105.199. [DOI] [PubMed] [Google Scholar]
- STAMPFLI R. A new method for measuring membrane potentials with external electrodes. Experientia. 1954 Dec 15;10(12):508–509. doi: 10.1007/BF02166189. [DOI] [PubMed] [Google Scholar]
- Saito K. [Effects of extracellularly applied ATP and related nucleotides on the membrane potential of the guinea-pig taenia coli]. Nihon Heikatsukin Gakkai Zasshi. 1972 Mar;8(1):32–39. doi: 10.1540/jsmr1965.8.32. [DOI] [PubMed] [Google Scholar]
- Satchell D. G., Burnstock G. Comparison of the inhibitory effects on the guinea-pig taenia coli of adenine nucleotides and adenosine in the presence and absence of dipyridamole. Eur J Pharmacol. 1975 Jun-Jul;32(02):324–328. doi: 10.1016/0014-2999(75)90299-x. [DOI] [PubMed] [Google Scholar]
- Satchell D. G., Lynch A., Bourke P. M., Burnstock G. Potentiation of the effects of exogenously applied ATP and purinergic nerve stimulation on the guinea-pig taenia coli by dipyridamole and hexobendine. Eur J Pharmacol. 1972 Sep;19(3):343–350. doi: 10.1016/0014-2999(72)90100-8. [DOI] [PubMed] [Google Scholar]
- Satchell D., Burnstock G., Dann P. Antagonism of the effects of purinergic nerve stimulation and exogenously applied ATP on the guinea-pig taenia coli by 2-substituted imidazolines and related compounds. Eur J Pharmacol. 1973 Sep;23(3):264–269. doi: 10.1016/0014-2999(73)90093-9. [DOI] [PubMed] [Google Scholar]
- Spedding M., Sweetman A. J., Weetman D. F. Antagonism of adenosine 5'-triphosphate-induced relaxation by 2-2'-pyridylisatogen in the taenia of guinea-pig caecum. Br J Pharmacol. 1975 Apr;53(4):575–583. doi: 10.1111/j.1476-5381.1975.tb07397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Fukushi Y., Rikimaru A. [Relaxant effect of adenosine triphosphate and its related nucleotides on the guinea-pig taenia coli]. Nihon Heikatsukin Gakkai Zasshi. 1971 Dec;7(4):207–212. doi: 10.1540/jsmr1965.7.207. [DOI] [PubMed] [Google Scholar]
- Tomita T., Watanabe H. A comparison of the effects of adenosine triphosphate with noradrenaline and with the inhibitory potential of the guinea-pig taenia coli. J Physiol. 1973 May;231(1):167–177. doi: 10.1113/jphysiol.1973.sp010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston A. H. Nerve-mediated inhibition of mechanical activity in rabbit duodenum and the effects of desensitization to adenosine and several of its derivatives. Br J Pharmacol. 1973 Jun;48(2):302–308. doi: 10.1111/j.1476-5381.1973.tb06916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


