Abstract
Ethylene signaling in Arabidopsis begins at a family of five ethylene receptors that regulate activity of a downstream mitogen-activated protein kinase kinase kinase, CTR1. Triple and quadruple loss-of-function ethylene receptor mutants display a constitutive ethylene response phenotype, indicating they function as negative regulators in this pathway. No ethylene-related phenotype has been described for single loss-of-function receptor mutants, although it was reported that etr1 loss-of-function mutants display a growth defect limiting plant size. In actuality, this apparent growth defect results from enhanced responsiveness to ethylene; a phenotype manifested in all tissues tested. The phenotype displayed by etr1 loss-of-function mutants was rescued by treatment with an inhibitor of ethylene perception, indicating that it is ethylene dependent. Identification of an ethylene-dependent phenotype for a loss-of-function receptor mutant gave a unique opportunity for genetic and biochemical analysis of upstream events in ethylene signaling, including demonstration that the dominant ethylene-insensitive phenotype of etr2-1 is partially dependent on ETR1. This work demonstrates that mutational loss of the ethylene receptor ETR1 alters responsiveness to ethylene in Arabidopsis and that enhanced ethylene response in Arabidopsis not only results in increased sensitivity but exaggeration of response.
Ethylene is a simple gaseous molecule that is one of five classic plant hormones, being critical for the control of physiological processes at all stages of plant growth and development. Example processes include seed germination, response to pathogen attack, tissue senescence, and fruit ripening (Abeles et al., 1992). Work to understand the molecular mechanisms of ethylene signaling has utilized Arabidopsis as a model system through mutagenesis and screening for seedlings that display an aberrant ethylene phenotype, resulting in the elucidation of a linear signaling pathway (Kieber, 1997; Johnson and Ecker, 1998; Chang and Shockey, 1999; Bleecker and Kende, 2000; Stepanova and Ecker, 2000).
In Arabidopsis, ethylene perception initiates with binding of ethylene to a family of five receptors (ETR1, ERS1, ETR2, EIN4, and ERS2). Ethylene binding is mediated by a copper cofactor (Rodriguez et al., 1999) that is provided to the receptors by the copper transporter RAN1 (Hirayama et al., 1999; Woeste and Kieber, 2000). The ethylene receptors are structurally similar to a family of proteins from bacteria, collectively known as two-component regulators, which are responsible for sensing changes in the growth environment (Chang and Shockey, 1999; Bleecker and Kende, 2000). As with two-component regulators, the ethylene receptors can be divided into multiple functional domains including a sensor domain that consists of a transmembrane region responsible for ethylene binding (Schaller and Bleecker, 1995; Hall et al., 2000); a GAF domain of unknown function (Aravind and Ponting, 1997); a His kinase domain, of which only ETR1 and ERS1 contain all of the requirements for functionality (Chang et al., 1993; Hua et al., 1995); and, in the case of ETR1, ETR2, and EIN4, a receiver domain predicted to modulate the activity of a downstream factor (Chang et al., 1993; Hua et al., 1998; Sakai et al., 1998).
Downstream of the ethylene receptors is CTR1, a mitogen-activated protein kinase kinase kinase (MAPKKK) that is homologous to mammalian Raf. CTR1 activity is required to suppress ethylene responses, indicating that CTR1 functions as a negative regulator of ethylene signaling (Kieber et al., 1993). At least two ethylene receptors (ETR1 and ERS1) interact with CTR1 (Clark et al., 1998), raising the intriguing possibility that the receptors directly control CTR1 activity. Although ctr1 loss-of-function mutants display a severe ethylene phenotype, these mutants remain ethylene responsive (Larsen and Chang, 2001), suggesting that an alternative mechanism bypassing CTR1 in ethylene signaling exists in Arabidopsis.
The intermediate steps of ethylene signaling are less well defined. EIN2 represents a protein with unknown function that acts downstream of the receptors and CTR1. Loss-of-function mutations in EIN2 result in ethylene insensitivity (Guzmán and Ecker, 1990). Although structurally similar to the N-Ramp family of metal transporters, the role of EIN2 in ethylene signaling remains unclear (Alonso et al., 1999). Ethylene signaling terminates in a transcriptional cascade headed by EIN3 and several EILs (Chao et al., 1997). Loss-of-function mutations in these transcriptional activators confer partial ethylene insensitivity. EIN3 controls transcription of a second transcriptional activator, ERF1, which directly binds to an ethylene response element commonly found in ethylene-inducible genes (Solano et al., 1998).
Several other ethylene-related Arabidopsis mutants have also been described but the corresponding genes have not been reported. These include the ethylene-insensitive mutants ein5 and ein6 (Roman et al., 1995), along with eer1, which opposes ethylene signaling in the hypocotyl and stem (Larsen and Chang, 2001). In addition, loss-of-function mutations have been reported for four of the ethylene receptors (ETR1, ETR2, EIN4, and ERS2; Hua and Meyerowitz, 1998). Mutations in the first three receptors were identified as intragenic mutations that suppress the effects of previously described receptor mutations that confer ethylene insensitivity. Loss-of-function ers2 was identified as a T-DNA insertion in the ERS2 gene. Combination of these mutations into triple and quadruple loss-of-function mutants results in a progressively stronger constitutive ethylene response phenotype, indicating the ethylene receptors function as negative regulators of ethylene signaling. It is predicted that the ethylene receptors are required to maintain CTR1 in an active state in the absence of ethylene. Loss of the ethylene receptors presumably creates a situation where CTR1 is inactive, eliminating repression of ethylene responses.
Analysis of single loss-of-function receptor mutants did not reveal ethylene response phenotypes (Hua and Meyerowitz, 1998). Instead, it was noted that all etr1 loss-of-function mutants displayed a general “growth defect” manifested both in dark-grown hypocotyls and leaves. We have found through extensive analysis of a representative etr1 loss-of-function mutant, etr1-7, that this in actuality represents an increase in responsiveness to ethylene, which is characterized by both a global shift in ethylene sensitivity and an exaggeration in the level of response in certain tissues. This indicates that unlike what has previously been proposed, loss of even a single ethylene receptor in Arabidopsis has ramifications for the control of ethylene signaling and suggests that ETR1 may play a more prominent role than the other receptors in this pathway.
RESULTS
Response of etr1-7 Hypocotyls and Roots to Ethylene
Dark-grown hypocotyls and roots of etr1-7 were examined for their responsiveness to ethylene in comparison with Columbia-0 (Col-0) wild type (wt). For hypocotyls, seedlings were grown for 4 d in the presence of 10 μm aminoethoxyvinyl-Gly (AVG; to reduce endogenous ethylene production) and exposed to a broad range of ethylene concentrations. AVG was not used for root growth analysis because it is severely inhibitory to root growth at this concentration (Larsen and Chang, 2001).
As previously described (Hua and Meyerowitz, 1998), etr1-7 hypocotyls displayed reduced hypocotyl elongation in comparison with wt in air and at all concentrations of ethylene tested (Fig. 1A). Addition of 100 μm AgNO3 to the growth medium (used to eliminate ethylene perception) completely reversed the short hypocotyl phenotype of etr1-7, resulting in etr1-7 hypocotyls that were indistinguishable from wt with regard to length. This demonstrates that the etr1-7 hypocotyl growth inhibition phenotype requires ethylene perception for its manifestation. It is likely that AVG treatment did not completely eliminate ethylene production because etr1-7 hypocotyls were still significantly shorter than wt even in the absence of exogenous ethylene. At a saturating concentration of ethylene, a pronounced difference in hypocotyl length was still observed between wt and etr1-7, indicating that etr1-7 hypocotyls have a greater maximal response than wt.
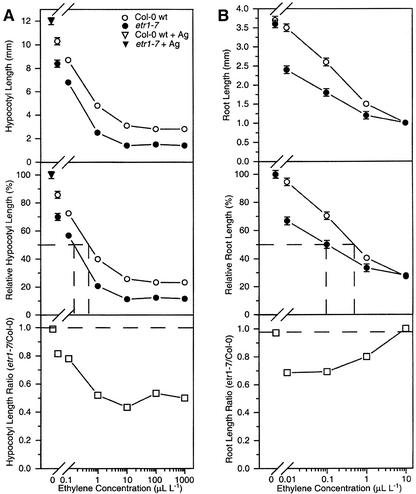
Figure 1.
Dark-grown etr1-7 seedlings have an enhanced response to ethylene. A, Ethylene dose response curves for hypocotyl length of 4-d-old dark-grown wt and etr1-7 seedlings treated with 10 μm AVG. Top, Actual hypocotyl length, including following treatment with 100 μm AgNO3. Middle, Relative inhibition of hypocotyl length (length/length at 100 μm AgNO3), with the concentration of ethylene causing 50% inhibition (−). Bottom, Ratio of etr1-7 hypocotyl length over wt hypocotyl length for each ethylene concentration, with − denoting the predicted ratio if the etr1-7 mutant was not hyperresponsive to ethylene. Mean ± se values were determined from 25 to 30 seedlings. B, Ethylene dose response curves for root length of 4-d-old dark-grown wt and etr1-7 seedlings. Top, Actual root length. Middle, Relative inhibition of root length (length/length at 0 μL L−1 ethylene), with the concentration of ethylene causing 50% inhibition (−). Bottom, Ratio of etr1-7 root length over wt root length for each ethylene concentration, with − denoting the predicted ratio if the etr1-7 mutant were not hyperresponsive to ethylene. Mean ± se values were determined from 25 to 30 seedlings.
Replotting of this data as relative hypocotyl inhibition, with each ethylene-treated sample compared with the respective Ag-treated controls (which represent complete elimination of ethylene response), demonstrates that the etr1-7 hypocotyl phenotype results from a combination of an increase in sensitivity and amplitude of response to ethylene (Fig. 1A). This was seen as a 3- to 4-fold higher concentration of ethylene required to give 50% inhibition of hypocotyl elongation for the wt in comparison with etr1-7. In conjunction with this, etr1-7 hypocotyls displayed exaggeration of response to saturating levels of ethylene, with etr1-7 hypocotyls exhibiting an extreme level of inhibition not achievable by wt. The ratio of hypocotyl length between etr1-7 and wt was not consistent with what would be expected for a general growth defect because this ratio did not remain constant; instead, the differential between the two increased with increasing ethylene concentration, suggesting greater responsiveness by etr1-7 hypocotyls.
Examination of etr1-7 roots revealed that they also have an increase in ethylene sensitivity (Fig. 1B). This was seen as a 4- to 5-fold increase in ethylene concentration required to give 50% root growth inhibition for wt in comparison with etr1-7. Unlike etr1-7 hypocotyls, though, the roots did not exhibit an exaggerated ethylene response because the length of wt and etr1-7 roots was identical at high concentrations of ethylene.
etr1-7 Hypocotyls and Roots Are Hypersensitive to Propylene
Propylene is an ethylene agonist that elicits ethylene responses when applied at concentrations 100-fold higher than ethylene (Abeles et al., 1992; Larsen and Chang, 2001). It was determined whether etr1-7 displayed a similar shift in sensitivity to propylene, which would be consistent with the etr1-7 phenotype being dependent on activity of the ethylene-signaling pathway. Dark-grown hypocotyls and roots of both etr1-7 and wt were tested for propylene responsiveness in the same manner as described for ethylene treatment.
As shown in Figure 2A, etr1-7 hypocotyls demonstrated the same increase in sensitivity to propylene as seen for ethylene treatment. This included a 4-fold increase in propylene concentration required to give 50% growth inhibition for wt in comparison with etr1-7. In addition, etr1-7 hypocotyls in the presence of saturating concentrations of propylene exhibited the same exaggeration of response as seen for ethylene treatment.
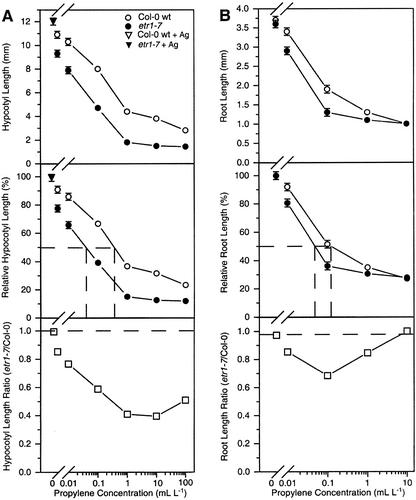
Figure 2.
Dark-grown etr1-7 has enhanced responsiveness to the ethylene agonist propylene in the hypocotyl and root. A, Propylene dose response curves for hypocotyl length of 4-d-old dark-grown wt and etr1-7 seedlings treated with 10 μm AVG. Top, Actual hypocotyl length, including following treatment with 100 μm AgNO3. Middle, Relative inhibition of hypocotyl length (length/length at 100 μm AgNO3), with the concentration of propylene causing 50% inhibition (−). Bottom, Ratio of etr1-7 hypocotyl length over wt hypocotyl length for each propylene concentration, with − denoting the predicted ratio if the etr1-7 mutant was not hyperresponsive to propylene. Mean ± se values were determined from 25 to 30 seedlings. B, Propylene dose response curves for root length of 4-d-old dark-grown wt and etr1-7 seedlings. Top, Actual root length. Middle, Relative inhibition of root length (length/length at 0 μL L−1 propylene), with the concentration of propylene causing 50% inhibition (−). Bottom, Ratio of etr1-7 root length over wt root length for each propylene concentration, with − denoting the predicted ratio if the etr1-7 mutant was not hyperresponsive to propylene. Mean ± se values were determined from 25 to 30 seedlings.
As shown in Figure 2B, treatment of etr1-7 roots with propylene resulted in the same phenotype seen after ethylene treatment. etr1-7 roots displayed increased propylene sensitivity, with wt requiring 2- to 3-fold higher levels of propylene than etr1-7 to cause 50% inhibition of root growth. In addition, as with ethylene treatment, there was no exaggeration of response to propylene in etr1-7 roots.
Effects of Ag and 1-Aminocyclopropane-1-Carboxylic Acid (ACC) on Various Ethylene Response Mutants
Although the reversal of the short hypocotyl phenotype of etr1-7 by Ag treatment suggests that the etr1-7 phenotype is ethylene dependent, it was necessary to demonstrate that Ag treatment did not stimulate an ethylene-independent increase in hypocotyl elongation. Seedlings of the ethylene-insensitive mutant etr1-1, along with wt, etr1-7, and the constitutive ethylene response mutant ctr1-3, were grown in the dark for 4 d either in the presence or absence of Ag and hypocotyl length was subsequently measured (Fig. 3A). It was found that only etr1-7 hypocotyls demonstrated an increase in length with Ag treatment, suggesting that Ag treatment does not stimulate ethylene-independent growth.
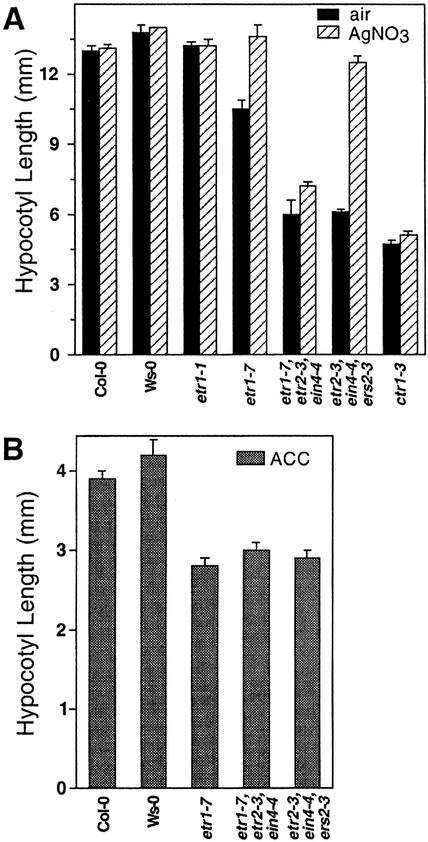
Figure 3.
Effects of Ag and ACC on various ethylene-related mutants. A, Seedlings were grown in the dark either in the presence or absence of 100 μm AgNO3 on vertical plates and hypocotyl lengths were measured after 4 d. Mean ± se values were determined from 25 to 30 seedlings. B, Seedlings were grown in the dark in the presence of 10 μm ACC on vertical plates and hypocotyl lengths were measured after 4 d. Mean ± se values were determined from 25 to 30 seedlings.
In addition, effects of Ag on growth of the triple loss-of-function ethylene receptor mutants were assessed. Both mutants exhibit a constitutive ethylene response independent of exogenous ethylene. Ag treatment had little effect on hypocotyl length of dark-grown etr1-7;etr2-3;ein4-4, with this mutant demonstrating only a slight increase in length after Ag treatment (Fig. 3A). In contrast, treatment of etr2-3;ein4-4;ers2-3 with Ag resulted in almost complete reversal of the mutant phenotype to that of wt, suggesting that hypocotyl shortening in this mutant is not due to a constitutive ethylene response but rather results from hypersensitivity to ethylene. This indicates that this combination of receptors is not required for maintaining CTR1 activity in Arabidopsis.
It was also of interest to determine if exaggeration of ethylene response was unique to etr1 loss-of-function mutants. Dark-grown triple loss-of-function ethylene receptor mutants were treated with 10 μm ACC and compared with both Col-0 and Wassilewskija-0 (Ws-0) wt (Fig. 3B). It was found that both triple loss-of-function mutants displayed the same exaggerated ethylene response as demonstrated by etr1-7.
etr1-7 Leaves Have Increased Expression of Ethylene-Regulated Genes
etr1 loss-of-function mutants were previously described as having reduced leaf expansion (Hua and Meyerowitz, 1998), a phenotype that can be ethylene dependent (Kieber et al., 1993; Hua et al., 1995). To assess whether this reduced leaf expansion may be related to increased ethylene responsiveness, expression of ethylene-regulated genes was tested for both wt and etr1-7 leaves after a 24-h exposure to either subthreshold levels of ethylene or propylene (Chen and Bleecker, 1995; Penninckx et al., 1998; Larsen and Chang, 2001). Northern analysis of 10 μg of total RNA for each sample was performed. Treatment with either 500 nL L−1 ethylene or 500 μL L−1 propylene resulted in substantially higher expression of both basic chitinase and PDF1.2 in leaves of etr1-7 in comparison with wt (Fig. 4A). In addition, there appeared to be higher expression of these genes even in the absence of exogenous ethylene in etr1-7 leaves as compared with wt, suggesting that the etr1-7 leaves may be hypersensitive to even the low level of endogenous ethylene normally produced by the plant. TUA3, an Arabidopsis tubulin, was used as a loading control and showed no obvious difference in expression from sample to sample. These results are consistent with etr1-7 leaves having an increased sensitivity to ethylene and ethylene agonists.
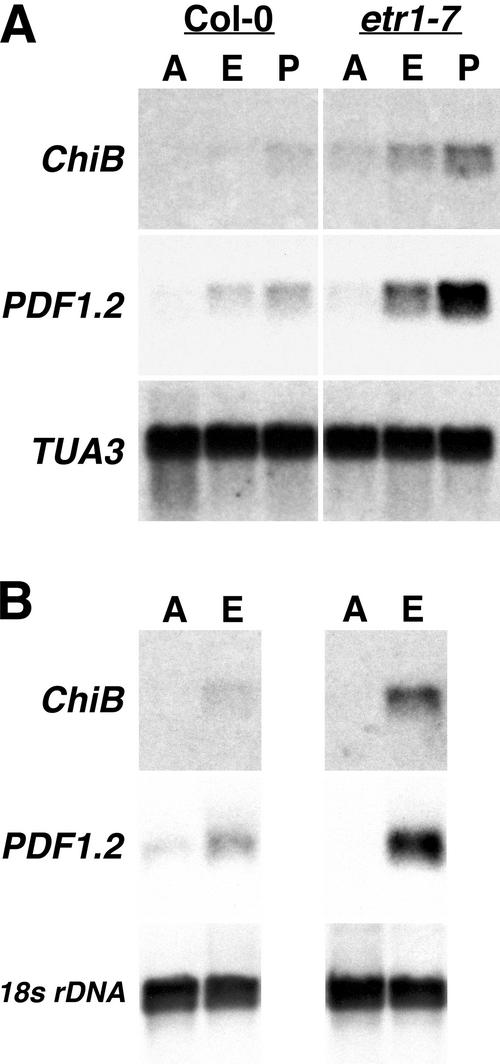
Figure 4.
etr1-7 leaves are hyperresponsive to ethylene and propylene. A, Leaves of 4-week-old Col-0 wt and etr1-7 plants were collected after a 24-h treatment with air (A), 500 nL L−1 ethylene (E), or 500 μL L−1 propylene (P). Ten micrograms of total RNA was electrophoretically separated and northern blotted. The ethylene-responsive genes basic chitinase (ChiB) and PDF1.2 were used as molecular markers for ethylene sensitivity. Arabidopsis tubulin TUA3 was used as a loading control. B, Leaves of 4-week-old Col-0 wt and etr1-7 plants were collected after a 24-h treatment with either air (A) or 100 μL L−1 ethylene (E), which represents a saturating concentration. Five micrograms of total RNA was electrophoretically separated and northern blotted. ChiB and PDF1.2 were used as molecular markers for ethylene responsiveness. Tomato 18S rDNA was used as a loading control.
To determine if the exaggeration of response observed in etr1-7 hypocotyls was also demonstrated in leaves, etr1-7 and wt leaves were exposed to air or a saturating level of ethylene (100 μL L−1) for 24 h and expression patterns of the previously described ethylene inducible genes were assessed (Fig. 4B) by northern analysis of 5 μg of total RNA. Both exposure time and amount of total RNA used were reduced compared with the previous experiment to allow for visualization of differences in level of expression between wt and the mutant at this high level of ethylene. Tomato (Lycopersicon esculentum) 18s rDNA was used as a loading control and showed no discernible difference in expression. In contrast, for both ethylene-regulated genes, treatment with a saturating level of ethylene resulted in a greater maximal level of expression in leaves of etr1-7 compared with wt, demonstrating that etr1-7 leaves also exhibit exaggeration of response to ethylene in conjunction with the observed increase in sensitivity.
The etr1 Loss-of-Function Mutant Phenotype Does Not Result from Ethylene Overproduction
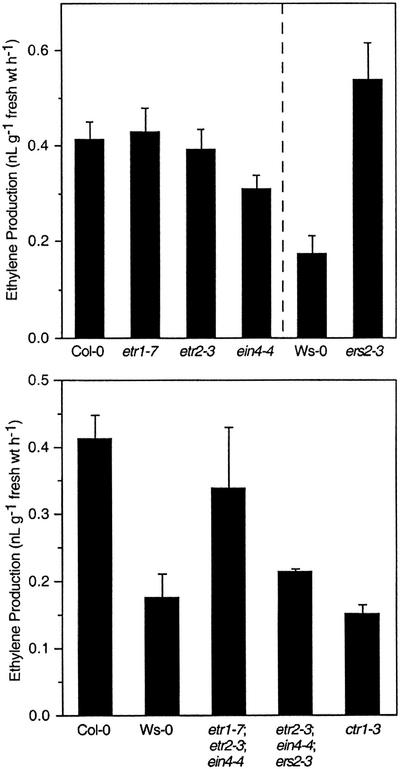
One possible explanation for the apparent change in the ethylene responsiveness of the etr1-7 mutant was that it represents an overproducer of ethylene. This phenotype would not be consistent, though, with observations made in Figure 1, in which etr1-7 hypocotyls give an exaggerated ethylene response in the presence of the ethylene biosynthesis inhibitor AVG. Ethylene generated by dark-grown seedlings of Col-0 wt, Ws-0 wt, ctr1-3, and the published loss-of-function receptor mutants was collected for 12 h and measured using a gas chromatography system. As shown in Figure 5, there was no significant difference in ethylene production for Col-0 wt, etr1-7, etr2-3, or ein4-4, demonstrating that ethylene overproduction cannot be the basis for the observed phenotype for etr1-7. In contrast, ers2-3, a mutation in the Ws-0 background that has no apparent ethylene-related phenotype, produced 3- to 4-fold more ethylene than Ws-0 wt. Analysis of ethylene production by the triple loss-of-function receptor mutants revealed that they produced levels of ethylene at or below what was measured for Col-0 and Ws-0 wt seedlings.
Figure 5.
The etr1-7 phenotype does not result from elevated production of ethylene. A, Ethylene production was measured for dark-grown Col-0 wt, Ws-0 wt, and the single null receptor mutants that correspond to ETR1, ETR2, EIN4, and ERS2 (which is in the Ws-0 ecotype). Ethylene was collected for a period of 12 h and subsequently measured using a gas chromatograph. Ethylene production was calculated based on tissue fresh weight. Mean ± se values were determined for five samples. B, Ethylene production by Col-0 wt, ctr1-3, and the triple loss-of-function receptor mutants was measured as described above. Mean ± se values were determined for five samples.
Receptor Expression in the Loss-of-Function Receptor Mutants
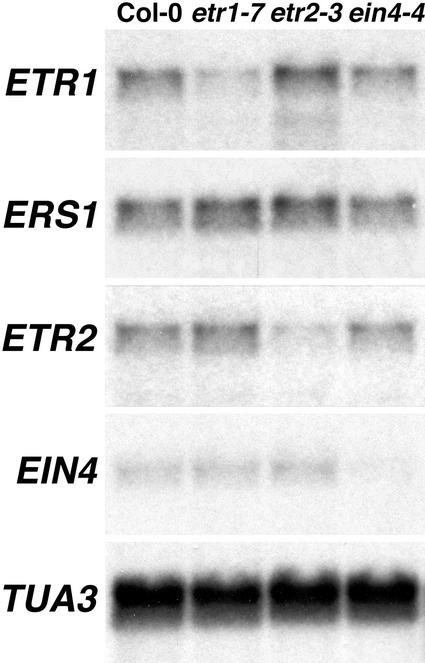
It was not clear why only etr1 loss-of-function mutants have increased ethylene responsiveness. One possibility was that the other loss-of-function receptor mutants had increased expression of one or more of the remaining functional receptors to compensate for the respective genetic lesions. To test this, northern analysis of 10 μg of total RNA isolated from leaves of Col-0 wt, Ws-0 wt, etr1-7, etr2-3, and ein4-4 was performed to assess the expression patterns of four of the ethylene receptors with TUA3 being used as a loading control. As shown in Figure 6, there was little variation in the expression of ETR1, ETR2, EIN4, ERS1, and TUA3 in all samples tested, except for a slight increase in ETR1 expression in etr2-3 and apparent nonsense-mediated decay of receptor mRNA in the corresponding loss-of-function receptor mutants.
Figure 6.
Ethylene receptor expression in the single loss-of-function ethylene receptor mutants. Northern analysis was performed to determine the expression level of the ethylene receptors in receptor loss-of-function mutants. Total RNA from untreated leaves from 4-week-old plants was collected and 10 μg from each sample was electrophoretically separated and analyzed for expression of ETR1, ERS1, ETR2, and EIN4, along with Arabidopsis tubulin TUA3, which was used as a loading control.
ETR2 Weakly Interacts with CTR1's Amino Terminus
It is possible that the apparent importance of ETR1 in ethylene signaling is due to a unique ability of ETR1 to directly regulate CTR1 activity, which can be speculated due to a previously reported association between ETR1 and CTR1 (Clark et al., 1998). Loss of a primary regulator of CTR1 activity may result in the enhanced responsiveness displayed by etr1 loss-of-function mutants. Both a yeast (Saccharomyces cerevisiae) two-hybrid assay and an in vitro binding experiment were performed to determine if a class 2 ethylene receptor such as ETR2 can interact with CTR1.
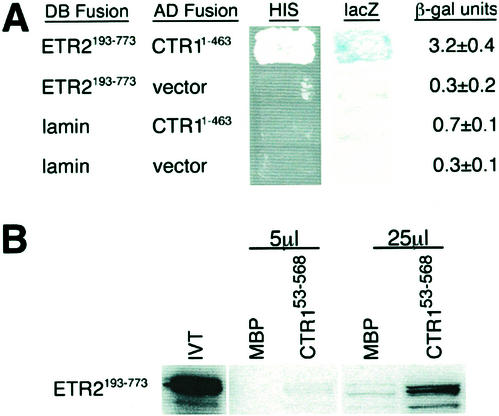
For the yeast two-hybrid assay, a fusion of the Gal4p DNA-binding domain to ETR2193-773 (DB-ETR2193-773), representing the soluble region of ETR2, was tested for its ability to interact with a fusion of the Gal4p activation domain with CTR11-463 (AD-CTR11-463), which represented the amino terminus of CTR1. Although weak, it was consistently found that co-incubation of DB-ETR2193-773 with AD-CTR11-463 resulted in activation of the two reporter genes used in this assay. This included increased capability to grow in the absence of supplied His along with increased β-galactosidase activity in comparison with controls (Fig. 7A).
Figure 7.
ETR2 weakly interacts with CTR1's amino-terminal domain. A, The yeast two-hybrid assay was used to test whether ETR2 can interact with CTR1. A DB-ETR2293-738 fusion protein, representing the cytoplasmic portion of ETR2, was tested for its ability to associate with AD-CTR11-463, which represents the amino-terminal regulatory domain of CTR1. Interaction was assessed by activation of two reporter genes, including restoration of growth in the absence of exogenous His and β-galactosidase activity. For β-galactosidase activity, five transformants of each were measured and the average ± se is presented. B, An in vitro binding assay was used to confirm the association between ETR2 and CTR1. Either 5 or 25 μL of in vitro translated ETR2193-773, radiolabeled with [35S]Met, was associated with maltose-binding protein (MBP) fusions representing either MBP or MBP-CTR153-568. After several washes to remove unbound test protein, samples were separated by SDS-PAGE, fixed, soaked in a fluorographic reagent, and visualized by autoradiography.
To support the findings of the yeast two-hybrid assay, an in vitro binding assay was performed using a fusion of MBP to CTR153-568, which represents the amino-terminal regulatory domain of CTR1—a region previously shown to interact with the ethylene receptors ETR1 and ERS1 in this assay (Clark et al., 1998). Either 5 or 25 μL of 35S-Met-labeled ETR2193-773 were incubated with 5 μg of bacterially expressed MBP or MBP-CTR153-568 bound to amylose resin in vitro. Samples were subsequently washed to remove nonspecific binding and analyzed by SDS-PAGE to determine if the radiolabeled test protein associated with the MBP fusions. Addition of 5 μL of radiolabeled ETR2193-773 resulted in weak binding to MBP-CTR153-568 with no binding to the MBP control (Fig. 7B). A similar pattern of binding occurred when 25 μL of probe was used.
ETR2 Function Is Partially Dependent on ETR1 in Ethylene Signaling
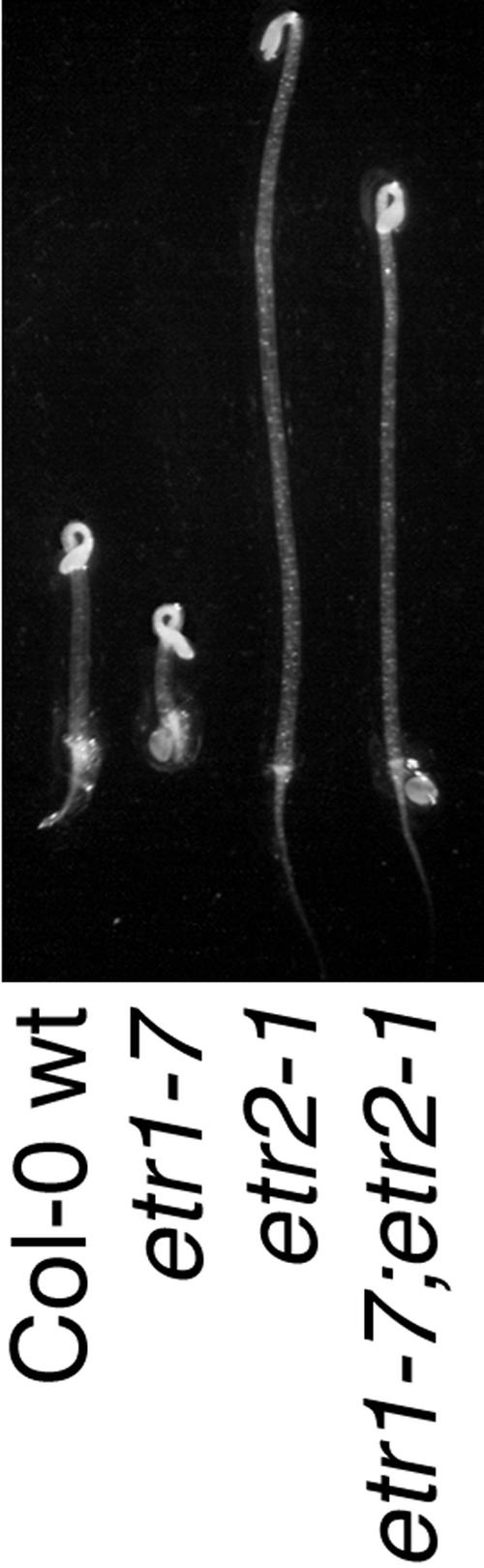
Identification of an ethylene-dependent phenotype for a loss-of-function mutation in a single ethylene receptor gave the means to determine if there is an epistatic relationship between ETR1 and the other receptors in ethylene signaling. A cross between etr1-7 and the dominant ethylene-insensitive mutant etr2-1 (Sakai et al., 1998) was made to assess the relationship between ETR1 and ETR2. F2 progeny were screened for those with long hypocotyls on 10 μm ACC, with these representing lines that carried the etr2-1 mutation. PCR genotyping of seedlings that displayed the etr2-1 phenotype was subsequently performed to identify seedlings that were also homozygous for the etr1-7 mutation. Of these, two independent lines were determined to be etr1-7;etr2-1 double mutants and were subsequently analyzed for the capability of the etr2-1 mutation to reverse the etr1-7 phenotype. Growth of this double mutant in the dark in the absence of ACC resulted in no apparent differences in hypocotyl and root lengths compared with wt and etr2-1 (Tables I and II), which is consistent with the previous observation that the etr1-7 phenotype is reversible by an inhibitor of ethylene perception. In contrast, treatment with 10 μm ACC resulted in a partial ethylene response in the etr1-7;etr2-1 double mutant in comparison with etr2-1 (Tables I and II; Fig. 8). The increased responsiveness was displayed in both the hypocotyl and the root of the double mutant, indicating that ETR2 function may be dependent on ETR1.
Table I.
Hypocotyl lengths of dark-grown ethylene mutants
| Treatment | Col-0 | etr1-7 | etr2-1 | etr1-7;etr2-1 |
|---|---|---|---|---|
| mm | ||||
| Air | 12.0 ± 0.3 | 9.8 ± 0.4 | 12.2 ± 0.3 | 12.2 ± 0.1 |
| 10 μm ACC | 3.8 ± 0.1 | 3.3 ± 0.2 | 12.2 ± 0.2 | 10.7 ± 0.1 |
Each value is the average ± se for >50 seedlings.
Table II.
Root lengths of dark-grown ethylene mutants
| Treatment | Col-0 | etr1-7 | etr2-1 | etr1-7;etr2-1 |
|---|---|---|---|---|
| mm | ||||
| Air | 6.3 ± 0.1 | 4.0 ± 0.1 | 5.7 ± 0.2 | 5.7 ± 0.1 |
| 10 μm ACC | 1.9 ± 0.1 | 1.2 ± 0.1 | 5.8 ± 0.1 | 4.6 ± 0.1 |
Each value is the average ± se for >50 seedlings.
Figure 8.
Double mutant analysis of etr1-7 with etr2-1. A cross between etr1-7 and etr2-1 was made and etr1-7;etr2-1 double mutants were identified. Seedlings of Col-0 wt, etr1-7, etr2-1, and etr1-7;etr2-1 were grown in the dark in the presence of 10 μm ACC for 4 d.
DISCUSSION
Recent work has shown that the ethylene receptors function as negative regulators in ethylene signaling. Mutational loss of multiple receptors results in plants that display a constitutive ethylene response phenotype (Hua and Meyerowitz, 1998), presumably through the loss of activators of CTR1, a downstream MAPKKK that actively suppresses ethylene responses (Kieber et al., 1993). It is probable that the receptors regulate CTR1 activity directly because ETR1, ERS1, and, as we have demonstrated, ETR2, associate with CTR1's amino-terminal regulatory domain in the yeast two-hybrid assay and in vitro (Clark et al., 1998). It was not reported that single or double loss-of-function receptor mutants displayed ethylene response phenotypes (Hua and Meyerowitz, 1998). Through careful analysis of the receptor null mutants, we have found that loss of even one ethylene receptor, specifically ETR1, results in a significant increase in ethylene responsiveness in Arabidopsis. This is consistent with what has previously been shown for tomato, in which loss of LeETR4 results in dramatic morphological changes associated with increased ethylene responsiveness (Tieman et al., 2000), although exaggeration of response to ethylene was not documented as a phenotype associated with this.
etr1-7 was chosen as a representative etr1 loss-of-function allele for our analyses, although all reported alleles exhibit similar phenotypes (Hua and Meyerowitz, 1998). etr1-7 was found to have increased responsiveness to ethylene throughout the plant in both light and dark growth regimens. The enhanced ethylene response was most dramatic in leaves and dark-grown hypocotyls and was manifested as both a shift in sensitivity along with an increase in amplitude of response in these tissues. A shift in sensitivity was also observed for roots, although this tissue did not display exaggerated ethylene response. Similar patterns of responsiveness were seen when etr1-7 was treated with the ethylene agonist propylene, which elicits ethylene responses at a concentration 100-fold higher than ethylene (Abeles et al., 1992). The observation that propylene treatment gave the same phenotypes as ethylene treatment provides additional evidence that the etr1 loss-of-function phenotypes specifically result from increased responsiveness to elicitors of ethylene signaling.
It could be argued that the etr1-7 mutant phenotypes arise from increased ethylene production rather than increased ethylene responsiveness. Even though treatment with inhibitors of both ethylene biosynthesis and perception demonstrate that the phenotypes associated with etr1-7 are ethylene dependent, these inhibitors do not necessarily discriminate between ethylene overproduction and increased ethylene sensitivity. We determined that there was no difference in ethylene production for etr1-7 compared with wt, which supports the hypothesis that the etr1 loss-of-function mutants affect the actual signaling pathway, not ethylene biosynthesis, and that etr1-7 is sensitized to even the low levels of endogenous ethylene produced. Interestingly, our analysis shows that the triple loss-of-function receptor mutant etr2-3;ein4-4;ers2-3 displays a seedling triple response phenotype that is almost completely reversible by the ethylene perception inhibitor Ag. Because this mutant does not overproduce ethylene, we can only conclude that the triple response phenotype displayed by this mutant results from increased sensitivity to the low levels of ethylene normally produced and does not result from loss of capability to activate CTR1. This suggests that the class 2 ethylene receptors are not required for maintaining CTR1 activity in hypocotyls and possibly other tissues and they may function in part to increase the threshold for ethylene signaling.
It is not clear why increased responsiveness to ethylene is displayed only by etr1 loss-of-function mutants and not by the other single loss-of-function receptor mutants (Hua and Meyerowitz, 1998). It is likely that ETR1 plays a greater and/or additional role in ethylene signaling compared with the remaining four receptors. This is possible because ETR1 is unique, being the only Arabidopsis ethylene receptor to possess all predicted requirements associated with two-component regulator activity in other systems (Chang et al., 1993). Alternatively, ethylene-related phenotypes in the other single null mutants may be masked by overexpression of one or more ethylene receptors. Northern analysis of receptor expression argues against the latter because we observed just a slight increase in ETR1 expression only in an etr2 loss-of-function mutant. This contrasts with what was reported for tomato, where in antisense NR tomato plants, LeETR4 was overexpressed at levels 4-fold higher than wt, with this overexpression being predicted to compensate for the loss of NR (Tieman et al., 2000). It should also be noted that it is unlikely that the apparently greater role of ETR1 in ethylene signaling is due to increased expression of ETR1 in relation to the other receptors because our analysis of receptor expression suggests that there is no obvious difference in transcript levels for ETR1, ERS1, and ETR2 in Arabidopsis leaves.
The inflated contribution of ETR1 to ethylene signaling may arise from a greater effectiveness compared with the other receptors with regard to the regulation of CTR1 activity, which presumably occurs through a direct interaction between the receptors and CTR1 (Clark et al., 1998). Our biochemical evidence suggests that an association between ETR2 and CTR1's amino-terminal regulatory domain exists, yet this interaction, along with the association between ERS1 and CTR1, are both significantly weaker than what was previously reported for ETR1 and CTR1 (Clark et al., 1998). It is possible that the severity of the etr1 loss-of-function mutant phenotype arises from the loss of what may be a primary regulator of CTR1 activity. We predict that loss of a primary regulator such as ETR1 would significantly reduce the threshold for response to ethylene because the remaining ethylene receptors would be less effective at maintaining CTR1 in an active state.
Etiolated seedlings of etr1-7;ctr1-1 have a phenotype that is more profound than ctr1 loss-of-function mutants alone (Hua and Meyerowitz, 1998). This additive response is consistent with the report that ctr1 loss-of-function mutants remain ethylene responsive (Larsen and Chang, 2001) and correlates with our finding that hypocotyls of etr1 loss-of-function mutants have an exaggerated response to ethylene in hypocotyls and leaves. These results suggest that a factor additional to CTR1 in ethylene signaling is regulated by the ethylene receptors. One possibility is that there is a second MAPKKK capable of substituting for CTR1 or that works in parallel to CTR1, which may function similarly to CTR1 as a negative regulator of ethylene signaling. We expect that the increased amplitude of response seen in hypocotyls of etr1-7, etr1-7;etr2-3;ein4-4, and etr2-3;ein4-4;ers2-3, along with the adult lethal phenotype demonstrated by etr1-6;etr2-3;ein4-4;ers2-3 (Hua and Meyerowitz, 1998) and possibly ran1-3 (Woeste and Kieber, 2000), result from reduced effectiveness at activating both CTR1 and this predicted second factor in ethylene signaling, thus giving a phenotype more exaggerated than even a ctr1 loss-of-function mutant. The severity of the phenotypes associated with etr1 loss-of-function mutants and the etr1-7;ctr1-3 double mutant indicates that ETR1 may be primarily responsible for activation of this factor. Physiological analysis of the etr1 loss-of-function mutants suggests that this factor does not function in Arabidopsis roots because etr1-7 did not display an exaggerated response in this tissue. Alternatively, there may be other regulators of this factor in roots that can substitute for ETR1.
Dominant mutations causing ethylene insensitivity in Arabidopsis have been found in the ethylene receptors ETR1, ETR2, and EIN4. It is not clear whether the mutant forms of the receptors exert their effects autonomously or through a synergistic relationship with other members of the ethylene receptor family. Because etr1-7 is capable of partially restoring ethylene responsiveness to the dominant ethylene-insensitive mutant etr2-1, this indicates that the ethylene insensitivity conferred by the etr2-1 mutation is partially dependent on functional ETR1 for manifestation of this phenotype. The capability of etr1-7 to suppress the etr2-1 phenotype may arise from a dependence of ETR2 on transphosphorylation as part of a predicted His-to-Asp phosphorelay that is common to two-component regulators in other systems. This would be consistent with the expected order of biochemical events in ethylene signaling because ethylene binding, which the etr2-1 mutation presumably disrupts, should precede changes in the phosphorylation state of the receptors. Dependence of ETR2 activity on transphosphorylation is plausible because only ETR1 possesses all demonstrated requirements for His autophosphorylation (Gamble et al., 1998) and subsequent phosphotransfer (Chang et al., 1993).
In summary, we have found that mutational loss of the Arabidopsis ethylene receptor ETR1 results in a significant increase in sensitivity and, in some tissues, exaggeration of response to ethylene. The increase in sensitivity demonstrated by etr1 loss-of-function mutants is consistent with the model previously proposed by Hua and Meyerowitz (1998), which predicts that the ethylene receptors serve as negative regulators of ethylene signaling. Based on this model, mutational loss of the ethylene receptors results in reduced effectiveness at maintaining CTR1 in an active state, causing either an increase in ethylene sensitivity, or in extreme cases, such as evidenced by loss of multiple receptors, a constitutive ethylene response. The observation that mutational loss of ETR1 results in a measurable shift in ethylene responsiveness, unlike the other receptors in ethylene signaling, argues that ETR1 plays a more prominent role than the other receptors in ethylene signaling, potentially at the level of regulation of CTR1 activity. Further identification and characterization of Arabidopsis mutants that demonstrate these features of enhanced ethylene response, including the previously described eer1 (Larsen and Chang, 2001), should continue to provide valuable insight into the mechanisms that control ethylene signaling.
MATERIALS AND METHODS
Plants and Growth Conditions
For all seedling growth experiments, seeds were surface sterilized and cold stratified at 4°C for 4 d in the dark to synchronize germination. Seeds were then suspended in 0.15% (w/v) agarose and sown on plant nutrient medium plus Suc (Larsen and Chang, 2001). The medium was supplemented with ACC (Sigma, St. Louis), 10 μm AVG (Sigma), or 100 μm AgNO3 (Sigma) as required. For triple response experiments, seedlings were germinated for 4 d in the dark at 20°C. In experiments using ACC, petri dishes were oriented vertically for seedling growth.
All adult plants in this study were grown in soil under a 24-h light cycle at 20°C in a plant growth room supplemented with Gro-Lite fluorescent bulbs (Sylvania, Danvers, MA).
Treatment with Ethylene or Propylene
Ethylene and propylene experiments were done as previously described (Larsen and Chang, 2001).
For treatment of leaves for RNA extraction, adult plants were grown for 4 weeks in air in the previously described plant growth room and then treated with air, ethylene, or propylene in an airtight chamber (Plas Labs, Lansing, MI) for 24 h. Immediately after treatment, leaf tissue was collected and quick frozen for RNA extraction.
Measurement of Ethylene Production
Exactly 100 surface-sterilized seeds were placed in 5-mL glass scintillation vials containing 0.5 mL of plant nutrient medium plus Suc. Uncapped vials were placed into a sterile covered beaker and incubated in the dark for 72 h at 20°C. The vials were then sealed in the dark with a rubber syringe cap for collection of generated ethylene. After a 12-h incubation period, 0.9 mL of headspace was sampled from each vial and the ethylene content was measured using a 6850 series gas chromatography system (Hewlett-Packard, Palo Alto, CA) equipped with a HP Plot alumina-based capillary column (Agilent Technologies, Palo Alto, CA). Tissue fresh weight was measured for each sample.
Northern Analysis
Total RNA was extracted from leaf tissue using the RNeasy Plant Mini Kit (Qiagen, Valencia, CA). Total RNA (10 μg for all experiments except for analysis of gene expression under saturating concentrations of ethylene, where 5 μg of total RNA was used) was separated by electrophoresis in a 1% (w/v) denaturing agarose gel, and the gel was blotted to Zeta-Probe GT Blotting Membrane (Bio-Rad, Hercules, CA). 32P-Labeled probes were generated using the Prime-a-Gene labeling system (Promega, Madison, WI). Prehybridization and hybridization were both carried out at 42°C and washes were done at 42°C and 65°C following the manufacturer's instructions. Results were visualized by autoradiography.
Yeast (Saccharomyces cerevisiae) Two-Hybrid and in Vitro Protein-Binding Assays
For the yeast two-hybrid assay, yeast strain L40 was used as previously described (Clark et al., 1998). Protein fusions were made either to the DNA-binding domain of the bacterial repressor LexA (plexA-NLS) or to the Gal4 transcription activation domain (pACTII). β-Galactosidase activity was quantified using a chlorophenol red-β-d-galactopyranoside-based assay according to manufacturer's instructions (CLONTECH, Palo Alto, CA).
MBP fusion proteins were generated and isolated as previously described (Clark et al., 1998). Radiolabeled ETR2193-773, which represented the soluble portion of ETR2, was synthesized using the TnT T7 Coupled Transcription/Translation System (Promega) using [35S]Met. Assays were performed as previously described (Clark et al., 1998).
Genetic Analysis
Double mutants were generated by crossing etr1-7 (male) to etr2-1 (female). F2 progeny were grown in the dark on 10 μm ACC to isolate ethylene-insensitive individuals (which were either homozygous or heterozygous for etr2-1). Identified individuals were subsequently genotyped by PCR to identify those that were homozygous for the etr1-7 mutation. F3 progeny were grown in the dark on 10 μm ACC to identify lines homozygous for the etr2-1 mutation.
ACKNOWLEDGMENTS
The technical assistance of Dr. Todd Young is sincerely appreciated. We also thank Dr. Daniel Gallie for use of equipment.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.003780.
LITERATURE CITED
- Abeles FB, Morgan PW, Saltveit ME., Jr . Ethylene in Plant Biology. Ed 2. New York: Academic Press; 1992. [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- Aravind L, Ponting CP. The GAF domain: an evolutionary link between diverse phototransducing proteins. Trends Biochem Sci. 1997;22:458–459. doi: 10.1016/s0968-0004(97)01148-1. [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Kende H. Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol. 2000;16:1–18. doi: 10.1146/annurev.cellbio.16.1.1. [DOI] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Chang C, Shockey JA. The ethylene-response pathway: signal perception to gene regulation. Curr Opin Plant Biol. 1999;2:352–358. doi: 10.1016/s1369-5266(99)00004-7. [DOI] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell. 1997;89:1133–1144. doi: 10.1016/s0092-8674(00)80300-1. [DOI] [PubMed] [Google Scholar]
- Chen QG, Bleecker AB. Analysis of ethylene signal-transduction kinetics associated with seedling-growth response and chitinase induction in wild-type and mutant Arabidopsis. Plant Physiol. 1995;108:597–607. doi: 10.1104/pp.108.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KL, Larsen PB, Wang X, Chang C. Association of the Arabidopsis CTR1 Raf-like kinase with the ETR1 and ERS ethylene receptors. Proc Natl Acad Sci USA. 1998;95:5401–5406. doi: 10.1073/pnas.95.9.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble RL, Coonfield ML, Schaller GE. Histidine kinase activity of the ETR1 ethylene receptor from Arabidopsis. Proc Natl Acad Sci USA. 1998;95:7825–7829. doi: 10.1073/pnas.95.13.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AE, Findell JL, Schaller GE, Sisler EC, Bleecker AB. Ethylene perception by the ERS1 protein in Arabidopsis. Plant Physiol. 2000;123:1449–1457. doi: 10.1104/pp.123.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama T, Kieber JJ, Hirayama N, Kogan M, Guzman P, Nourizadeh S, Alonso JM, Dailey WP, Dancis A, Ecker JR. RESPONSIVE-TO-ANTAGONIST1, a Menkes/Wilson disease-related copper transporter, is required for ethylene signaling in Arabidopsis. Cell. 1999;97:383–393. doi: 10.1016/s0092-8674(00)80747-3. [DOI] [PubMed] [Google Scholar]
- Hua J, Chang C, Sun Q, Meyerowitz EM. Ethylene insensitivity conferred by Arabidopsis ERS gene. Science. 1995;269:1712–1714. doi: 10.1126/science.7569898. [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell. 1998;94:261–271. doi: 10.1016/s0092-8674(00)81425-7. [DOI] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell. 1998;10:1321–1332. doi: 10.1105/tpc.10.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PR, Ecker JR. The ethylene gas signal transduction pathway: a molecular perspective. Annu Rev Genet. 1998;32:227–254. doi: 10.1146/annurev.genet.32.1.227. [DOI] [PubMed] [Google Scholar]
- Kieber JJ. The ethylene response pathway in Arabidopsis. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:277–296. doi: 10.1146/annurev.arplant.48.1.277. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Larsen PB, Chang C. The Arabidopsis eer1 mutant has enhanced ethylene responses in the hypocotyl and stem. Plant Physiol. 2001;125:1061–1073. doi: 10.1104/pp.125.2.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx IA, Thomma BP, Buchala A, Metraux JP, Broekaert WF. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell. 1998;10:2103–2113. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez FI, Esch JJ, Hall AE, Binder BM, Schaller GE, Bleecker AB. A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science. 1999;283:996–998. doi: 10.1126/science.283.5404.996. [DOI] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics. 1995;139:1393–1409. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Hua J, Chen QG, Chang C, Medrano LJ, Bleecker AB, Meyerowitz EM. ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc Natl Acad Sci USA. 1998;95:5812–5817. doi: 10.1073/pnas.95.10.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller GE, Bleecker AB. Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science. 1995;270:1809–1811. doi: 10.1126/science.270.5243.1809. [DOI] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Ecker JR. Ethylene signaling: from mutants to molecules. Curr Opin Plant Biol. 2000;3:353–360. doi: 10.1016/s1369-5266(00)00096-0. [DOI] [PubMed] [Google Scholar]
- Tieman DM, Taylor MG, Ciardi JA, Klee HJ. The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proc Natl Acad Sci USA. 2000;97:5663–5668. doi: 10.1073/pnas.090550597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeste KE, Kieber JJ. A strong loss-of-function mutation in RAN1 results in constitutive activation of the ethylene response pathway as well as a rosette-lethal phenotype. Plant Cell. 2000;12:443–455. doi: 10.1105/tpc.12.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]