Abstract
The enzyme myrosinase (EC 3.2.3.1) degrades the secondary compounds glucosinolates upon wounding and serves as a defense to generalist pests in Capparales. Certain myrosinases are present in complexes together with other proteins such as myrosinase-binding proteins (MBP) in extracts of oilseed rape (Brassica napus) seeds. Immunhistochemical analysis of wild-type seeds showed that MBPs were present in most cells but not in the myrosin cells, indicating that the complex formation observed in extracts is initiated upon tissue disruption. To study the role of MBP in complex formation and defense, oilseed rape antisense plants lacking the seed MBPs were produced. Western blotting and immunohistochemical staining confirmed depletion of MBP in the transgenic seeds. The exclusive expression of myrosinase in idioblasts (myrosin cells) of the seed was not affected by the down-regulation of MBP. Using size-exclusion chromatography, we have shown that myrosinases with subunit molecular masses of 62 to 70 kD were present as free dimers from the antisense seed extract, whereas in the wild type, they formed complexes. In accordance with this, MBPs are necessary for myrosinase complex formation of the 62- to 70-kD myrosinases. The product formed from sinalbin hydrolysis by myrosinase was the same whether MBP was present or not. The performance of a common beetle generalist (Tenebrio molitor) fed with seeds, herbivory by flea beetles (Phyllotreta undulata) on cotyledons, or growth rate of the Brassica fungal pathogens Alternaria brassicae or Lepthosphaeria maculans in the presence of seed extracts were not affected by the down-regulation of MBP, leaving the physiological function of this protein family open.
Specific β-thioglucosidases known as myrosinases (EC 3.2.3.1) are responsible for the degradation of glucosinolates, secondary metabolites found mainly in the members of the Brassicacea family (Chew, 1988; James and Rossiter, 1991; Louda and Mole, 1991). The myrosinases and glucosinolates are, to a large extent, preformed and most probably require disruption of the plant tissue to become activated. Myrosinase hydrolyses glucosinolates by releasing the Glc moiety. The remaining aglycone spontaneously rearranges into one of several toxic products, such as isothiocyanates, thiocyanates, and nitriles (for review, see Bones and Rossiter, 1996; Rask et al., 2000). The outcome of the degradation depends on the type of glucosinolate being degraded, the local milieu, and additional proteins such as the epithiospecifier protein (Chew, 1988; Louda and Mole, 1991; Lambrix et al., 2001).
Several different myrosinase isoenzymes have been characterized in seeds, seedlings, and vegetative tissues of oilseed rape (Brassica napus; Lenman et al., 1993). More than 20 myrosinase genes seem to be present in oilseed rape (Xue et al., 1992), but few of these genes or cDNAs have been cloned as yet. Myrosinases expressed in seeds can be divided into three different subfamilies, denoted MA, MB, and MC. The three-dimensional structure of one myrosinase, probably an MA isoform, from white mustard (Sinapis alba) has been determined at 1.6 Å resolution and even the structure of some of the carbohydrates present in the sugar trees was resolved (Burmeister et al., 1997). All plant myrosinases characterized to date are glycosylated and are probably transported via the secretory pathway to the myrosin grains present in idioblasts called myrosin cells (Thangstad et al., 1991; Höglund et al., 1992a; Geshi et al., 1998). In seeds of oilseed rape, the myrosin cells are scattered throughout the tissue and constitute 2% to 5% of the total number of embryonic cells. These cells can easily be distinguished from the surrounding cells because they contain less lipid, have a high content of endoplasmic reticulum, and harbor smooth-looking protein bodies, the myrosin grains (Höglund et al., 1991).
Myrosinases that belong to the MA family occur as soluble dimers (140 kD), whereas the MB and MC families of myrosinases are found in complexes with several other proteins (Rask et al., 2000). Some of the proteins interacting with myrosinase have been characterized and are referred to as myrosinase-binding proteins (MBPs) and myrosinase-associated protein (MyAP). MBP and MyAP were first characterized as components of high molecular mass myrosinase complexes (250–1,000 kD) in extracts of oilseed rape seeds that were isolated by size-exclusion chromatography or immunoprecipitation (Lenman et al., 1990; Falk et al., 1995). MBPs have also been demonstrated to occur in 5-d-old etiolated seedlings (Geshi and Brandt, 1998) and mature leaves (Taipalensuu et al., 1997a). The MBPs belong to a family of proteins ranging in molecular mass from approximately 30 to 110 kD, containing seed-specific and vegetative isoforms (Falk et al., 1995; Taipalensuu et al., 1997a; Geshi and Brandt, 1998).
MBP50/52 are major MBPs in seeds and the first proteins to be identified in the complexes (Lenman et al., 1990; Falk et al., 1995). The high-Mr MBPs become more prominent during seedling development, whereas MBP50/52 disappear (Taipalensuu et al., 1997a; Geshi and Brandt, 1998), but the relationship between the low- and high-Mr MBPs is not known and they may have different functions. MBPs are composed of large repeated domains and short repetitive motifs, and it seems likely that alternative splicing and/or proteolytic cleavage of a large precursor generate MBPs of different sizes and structures (Taipalensuu et al., 1997a). The larger repeats show similarities to d-Gal-specific lectins from Artocarpus integer and Maclura pomifera (Taipalensuu et al., 1997a). It has been experimentally demonstrated that certain MBPs do possess lectin properties (Taipalensuu et al., 1997b; Geshi and Brandt, 1998). A possible explanation for the mechanism behind complex formation might be the lectin activity of MBP because the myrosinases and MyAP are glycoproteins and could be bound by lectins. MyAP shows sequence similarities to a lipase from Arabidopsis, but the function of MyAP is not known (Taipalensuu et al., 1997c). MBP and MyAP transcript levels are strongly influenced in vegetative tissue by wounding as well as by treatment with jasmonic acid and salicylic acid (Geshi and Brandt, 1998; Taipalensuu et al., 1997b, 1997c). Considering the inducibility, the lectin property, and the fact that many plant lectins have been shown to be involved in plant defense (Chrispeels and Raikhel, 1991; Peumans and van Damme, 1995) as well as the association of MBP with the myrosinase-glucosinolate system, an involvement of MBP in plant defense seems probable.
The myrosinase complexes from seeds contain several proteins with unknown roles. To begin to understand the function of the MBPs, we have produced oilseed rape seeds lacking MBPs using the antisense technique. We partially purified noncomplex-bound myrosinases using analytical size-exclusion gel chromatography. This approach enabled us to study the effect of MBPs on myrosinase activity, myrosinase localization, and complex formation. To our knowledge, this is the first report describing the necessity of MBP for myrosinase complex formation and the cellular localization of MBP in seed tissue, which indicate that complex formation occurs first upon tissue disruption, e.g. due to wounding. We have also studied the putative defense function of MBP by feeding a generalist insect pest, Tenebrio molitor, with the antisense and untransformed seeds, by testing the preference of flea beetles for cotyledons of wild-type and antisense plants, by testing the effects of seed extracts on growth rate of the Brassica fungal pathogens Alternaria brassicae and Lepthosphaeria maculans, and by studying the degradation product profile of the glucosinolate p-hydroxybenzyl glucosinolate (sinalbin), using complex-bound and free myrosinases.
RESULTS
Myrosinase and MBP Expression in Wild-Type Seeds
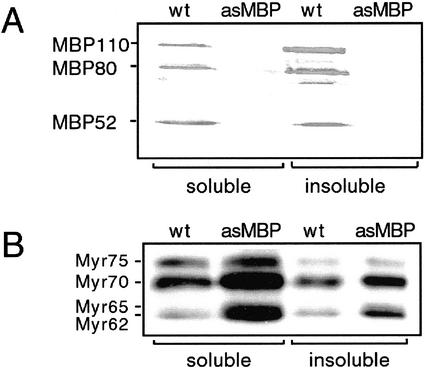
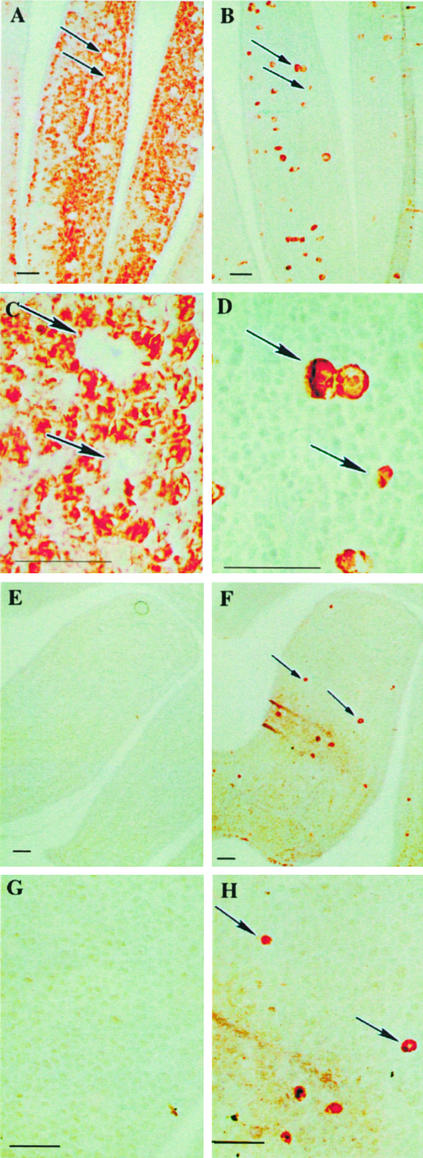
Western-blot analysis of extracts of soluble proteins in the wild-type seeds was carried out using the monoclonal anti-MBP antibody 34:14 recognizing different MBPs (Falk et al., 1995; Geshi et al., 1998). The seeds contained the 50/52-kD MBPs and also the larger MBPs with masses around 80 and 110 kD (Fig. 1A). The major fraction of MBPs was present in the insoluble phase when the total protein content of the fractions was taken into consideration. Blots probed with the monoclonal anti-myrosinase antibody 3D7 showed the presence of four myrosinase bands with approximate molecular masses of 75, 70, 65, and 62 kD showing the presence of all three myrosinase families (Fig. 1B). Immunohistochemistry was used to determine the localization of MBP in wild-type seed tissue using the anti-MBP antibody S4C6 (Fig. 2, A and C). The wild-type seeds contained MBP in virtually all cells in the ground tissue of the mature embryo, except the myrosin cells, the epidermis, and the provascular tissue (Fig. 2, A and C). Myrosinase was exclusively present in the myrosin cells as expected (Fig. 2, B and D).
Figure 1.
Western-blot analysis of MBP and myrosinase in seed extracts from oilseed rape. A, Western-blot analysis using the monoclonal anti-MBP antibody 34:14 of proteins extracted from seeds under nondenaturing conditions (soluble), whereas remaining proteins (insoluble) were solubilized using SDS-containing buffer in control (wt) and antisense MBP (asMBP) samples. B, Western-blot analysis of soluble and insoluble proteins in seed extracts from oilseed rape control (wt) and antisense MBP (asMBP) plants using the myrosinase-specific monoclonal antibody 3D7.
Figure 2.
Immunocytochemistry of myrosinase and MBP in the oilseed rape wild-type (A–D) and antisense (E–H) seeds. In wild-type seeds, MBP was found in all embryonic cells, except the myrosin cells, the epidermis, and the vascular tissue (A and C). Myrosinase was found exclusively in the myrosin cells (B and D). MBP was absent from antisense seeds (E and G), whereas myrosinase expression was confined to the myrosin cells in the seed embryo (F and H), in accordance with the myrosinase localization in the wild type. Scale bars = 10 μm. Arrows in the figure indicate myrosin cells.
Generation and Analysis of Antisense MBP Transgenic Plants
Three-hundred oilseed rape hypocotyls were used for the transformation with a fragment of a seed MBP cDNA in the antisense orientation under control of the napin promoter. The construct was designed based on a common repetitive motif of MBPs to target the whole gene family. Only one of the transformed calli could be generated into a plant and, to analyze if the antisense construct generated an MBP-depleted plant, the transformant was subjected to western-blot analysis of total protein extracts. This analysis showed that the antisense MBP seeds lacked the majority of the MBPs, although a weak band corresponding to an 80-kD MBP was still present (Fig. 1A), which also was confirmed by immunoprecipitation (results not shown). Thus, the antisense transgenics were MBP depleted. In the antisense seeds, all four myrosinases were readily apparent in a standard imidazole buffer extract, indicating their soluble nature (Fig. 1B). Furthermore, the antisense seeds seemed to contain higher levels of the 70-, 65-, and 62-kD myrosinases in the soluble fraction and in the pellet compared with wild-type seeds. The antisense seeds still contained MyAP as revealed by western-blot analysis (data not shown). No obvious morphologic differences between the untransformed and the antisense MBP plants could be observed for the three generations (T3–T5) studied.
Immunohistochemical analysis was used to determine if down-regulation of MBP affected the distribution of myrosinase. MBP could not be detected by immunohistochemical staining of the antisense embryo using the anti-MBP antibody S4C6 (Fig. 2, E and G). However, myrosinase was still only present in the myrosin cells (Fig. 2, F and H). Thus, the exclusive expression of myrosinase in the myrosin cells of the embryo was not influenced by the presence or absence of MBP.
Fractionation of Myrosinases and MBPs by Size-Exclusion Gel Chromatography
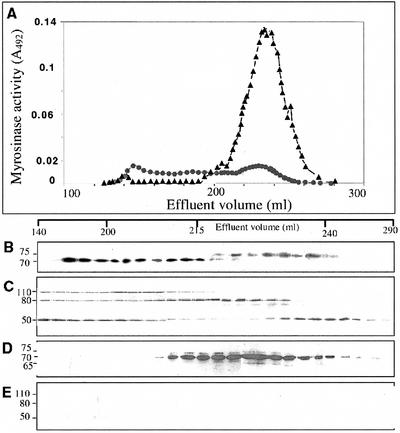
The soluble fraction of seed extracts from the control and antisense MBP plants prepared in imidazole buffer were subjected to size-exclusion gel chromatography on a Sephacryl S-300 column. The relative myrosinase activity and the levels of myrosinase and MBP protein in each fraction were determined (Fig. 3). The elution profile of myrosinase was different in the antisense and the control seed extracts, as judged by western-blot analysis and enzyme activity analysis. Wild-type myrosinases eluted over a large portion of the chromatogram, showing that the 62-, 65-, and 70-kD subunit sizes myrosinases (MB and MC) were constituents of high-Mr complexes of variable sizes (200–1,000 kD), whereas the 75-kD subunit size myrosinase (MA) occurred in a free form in accordance with the size of the dimeric enzyme (Fig. 3B). Also, the MBPs from the nontransformed seed extract eluted over a large part of the chromatogram (Fig. 3C). The distribution of the MBP isoforms showed the presence of different MBPs in complexes of different sizes in extracts of wild-type seeds. The fractions eluting late corresponded to free MBP50/52. In contrast, no myrosinases in the antisense extract were constituents of complexes, but were all present in a free form as judged from the size of dimeric enzymes (Fig. 3D). MA myrosinases are dimeric proteins and, based on the elution profile, all myrosinases in the antisense MBP seeds were found as dimers of similar sizes. MBP could not be detected by western-blot analysis of fractions collected from S-300-fractionated antisense extract (Fig. 3E). The myrosinase activity was higher in the soluble fraction of the antisense seed extract compared with extracts from control seeds (Fig. 3A). Western-blot signals and enzyme activity measurement of complexes seem to correlate, indicating that complex formation does not affect myrosinase activity dramatically. Myrosinase activity in extracts from antisense and nontransformed seeds and in selected gel filtration fractions were all stimulated at low concentrations of ascorbic acid, but showed no obvious differences in the response to different concentrations of ascorbic acid (data not shown).
Figure 3.
Size-exclusion gel chromatography of protein extract from wild-type oilseed rape and antisense MBP seeds separated on an S-300 column. The void (V0) and the total (Vt) volume were 143 and 440 mL, respectively. A, The myrosinase activity, using sinigrin as substrate, was determined on aliquots of the myrosinase-containing fractions from control (●) and antisense (▴) seed extracts. Western-blot analysis of the fractions was performed using the 3D7 antibody, specific for myrosinases, on control (B) and antisense (D) extracts or using the 34:14 anti-MBP antibody on control (C) and the antisense (E) extracts. The elution volumes are shown above the immunoblots.
Degradation Product Profile Analysis
To study if MBP affects the glucosinolate degradation products formed, degradation products from p-hydroxybenzyl glucosinolate were studied after incubation with selected Sephacryl S-300 fractions obtained from the nontransformed and the antisense MBP seed extract. No differences in the pattern of degradation products, in the presence or absence of MBPs, were observed with this substrate (data not shown). The products were p-hydroxybenzyl alcohol and p-hydroxybenzyl isothiocyanate, in addition to an unidentified compound. This experiment was repeated three times with the same result.
Insect Feeding Bioassays and Pathogen Tests
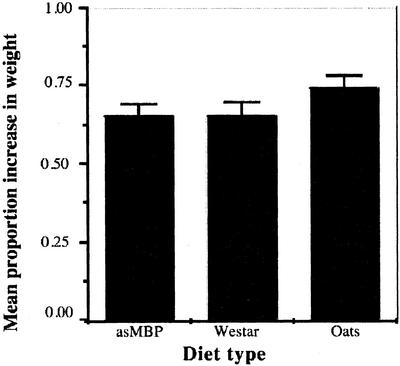
Seeds from antisense plants did not seem to serve as a better source of feed than nontransformed Westar or rolled oats when tested with T. molitor larvae. The absence of MBP did not influence the feeding behavior of the larvae. All larvae increased in weight at the same rate in the different treatments (F = 1.52; df = 2, 123; P = 0.23). The mean percentage of increase in weight and the se for each of the treatments is shown in Figure 4. Because the napin promoter also is active in young green tissue (Girke et al., 2000), we tested if there was any effect on feeding preference of flea beetles on cotyledons from antisense and wild-type plants. The experiments with flea beetles did not show any significant difference (t = 0.26; df = 21; P = 0.80) in damage in three experiments. The potential effect of seed extracts to affect the growth rate of the Brassica fungal pathogens A. brassicae and L. maculans was also tested in vitro. There was no morphological difference in fungi growing on antisense or wild-type seed extracts compared with the negative control (results not shown). No difference in effect between the wild-type and antisense seed extract was observed on the growth rate of A. brassicae or L. maculans (results not shown).
Figure 4.
The mean proportion increase in weight for T. molitor larvae (n = 45 per treatment) fed with different diets after 10 d. Error bars are ses of the mean. Student's t tests of least squared means showed no significant differences between antisense MBP and control Westar seeds (P = 0.92), control Westar seeds and oats (P = 0.12), and antisense MBP seeds and oats (P = 0.15).
DISCUSSION
We have demonstrated that the presence of MBP seems to be a prerequisite for the formation of myrosinase complexes with 62- and 65-kD myrosinases (MB), and 70-kD myrosinase (MC) as judged by size-exclusion chromatography. The absence of MBPs in the antisense seeds enabled us to partially purify all myrosinases found in oilseed rape seeds in a noncomplexed soluble form. It has been suggested earlier that MBPs can change the solubility of myrosinases because of the observed redistribution of myrosinase from the soluble to the insoluble fraction of wounded oilseed rape leaf extracts, which might be due to binding to the strongly induced MBP (Taipalensuu et al., 1997b). Antisense MBP seeds still contained MyAP, but myrosinases were present as free dimers, which indicates that MyAP is not responsible for establishing complexes. This is in agreement with an earlier observation that the major fraction of MyAP is not bound in the myrosinase complexes (Taipalensuu et al., 1996). Our data suggests that MyAP does not bind directly to the myrosinase, but instead binds via MBP.
Myrosinase isoenzymes have been isolated from several different sources (Bones and Rossiter, 1996; Rask et al., 2000), but showed only small, if any, differences in substrate specificity. However, limited information is available concerning the complex-bounded myrosinases, as they give rise to nonhomogeneous myrosinase preparations. To solubilize these myrosinases completely, SDS-containing buffers are needed, which inactivate the enzymes. The insolubility of certain myrosinases has also been a limiting factor when comparing the kinetics of different isoenzymes. For this reason only, soluble myrosinases have been analyzed in previous studies (Björkman and Lönnerdahl, 1973; James and Rossiter, 1991). It is interesting that western-blot analysis indicated that overall levels of different myrosinases are up-regulated in antisense MBP seeds. The myrosinase activity in the soluble fraction from the antisense MBP seeds was also higher compared with that from the control seeds. This could suggest that MBP somehow acts as a repressor of myrosinase expression, but the differences in their cellular localization makes this unlikely. It is evident that seed MB and MC myrosinases have activity also in a free form in accordance with the catalytically active recombinant MB myrosinase produced in Saccharomyces cerevisiae (Chen and Halkier, 1999). This shows that MBPs are not needed for activation of myrosinases. With the antisense seeds in hand, we now have the means to purify in free form some of the myrosinase isoenzymes present within the MB and MC family for further characterization of their properties, and for use in complex reconstitution experiments.
Immunohistochemical staining showed different cellular localization for myrosinases and MBPs in oilseed rape seeds, suggesting that complex formation occurs only after disruption of the seed tissue. This complex formation could be an in vitro artifact, but the presence of only a few proteins in the immunoprecipitates, of which myrosinases are the major fraction, suggests a more specific interaction (Taipalensuu et al., 1996). Furthermore, when oilseed rape seeds germinate, the proposed cellular separation of myrosinase and MBP changes concomitant with a change in MBP isoform profile. In 5-d-old etiolated oilseed rape seedlings, two larger MBPs (97 and 70 kD) were colocalized with myrosinase in the myrosin grains in the myrosin cells (Geshi and Brandt, 1998). This suggested that the complexes can exist in intact seedlings. Another developmental event within the myrosinase-glucosinolate system is the degradation of glucosinolates described to occur during germination (Clossais-Besnard and Larher, 1991). In Indian mustard (Brassica juncea), the glucosinolate sinigrin was shown to be present in aleurone-like cells, but absent from the myrosin cells (Kelly et al., 1998). This shows a spatial separation of myrosinase from glucosinolates, assuming that other glucosinolates have the same localization. Compartmentalization in different cells indicates that to become active, myrosinase or glucosinolates must be transported or the organization of a tissue disrupted. Therefore, colocalization of MBPs and glucosinolates suggests that another function of certain MBPs might be to facilitate the transport or storage of glucosinolates, thus making tissue disruption with subsequent loss of cellular integrity unnecessary in the developmental degradation of glucosinolates. The expression pattern of MBPs in ground tissue showed similarities to the distribution reported for the seed storage proteins napin and cruciferin (Höglund et al., 1992b), suggesting a role for the MBP50/52 as storage proteins. However, the relatively low amounts of MBPs compared with established storage proteins would make this a minor contribution to the amino acid pool and, thus physiologically less significant.
MBP has a highly repetitive structure, containing several jacalin-like repeats suggested to be involved in defense against fungi, insects, and viruses (Peumans and van Damme, 1995). An Arabidopsis resistance gene, RTM1, encoding a protein with similarities to MBP and jacalin-related proteins was recently shown to control long-distance movement of tobacco (Nicotiana tabacum) etch virus (Chisholm et al., 2000). However, in the present study, we found no significant difference between the wild-type and antisense samples in effects on pathogen growth, feeding rate of flea beetles, or growth rate of T. molitor larvae. Lectin-oligosaccharide interactions have also been shown to facilitate glycoprotein folding where the lectin functions as a molecular chaperone associating with folding proteins, but not with fully folded glycoproteins (Saito et al., 1999). Because the MBP-myrosinase complexes are catalytically active toward glucosinolates, it suggests that the myrosinases have an active conformation and, thus have attained a proper fold. Furthermore, the high myrosinase activity in antisense seeds suggests that MBPs are not needed for folding and catalytic activity of myrosinases.
In summary, MBP is necessary for myrosinase complex formation in seed extracts, but does not affect ascorbate dependence, glucosinolate degradation profile, myrosinase localization, or defense against four different pests. Myrosinase-MBP complexes do not seem to exist in intact cells of the seed embryo, but are formed upon disintegration of cellular boundaries e.g. as a result of wounding. It is interesting that the more general secondary product defense system based on degradation of O-glucosides by β-glucosidases has been shown to also harbor a complex-forming protein called β-glucosidase aggregating factor (BGAF) in maize (Zea mays; Esen and Blanchard, 2000; Blanchard et al., 2001). The BGAF is a smaller protein (35 kD) that binds specifically to β-glucosidase and renders it insoluble during extraction. The binding of BGAF to β-glucosidases does not affect enzyme activity or kinetic parameters. The BGAF only binds correctly folded β-glucosidase and forms one type of complex. It is unfortunate that no physiological function has been resolved for BGAF and it can, accordingly, not support functional studies concerned with MBP. Although lectin activity has been experimentally verified for MBP (Taipalensuu et al., 1997b; Geshi and Brandt, 1998), the biochemical or cellular context in which MBP participates and exerts its precise role still remains to be elucidated.
MATERIALS AND METHODS
Protein Extraction
Total protein extracts were prepared from 0.9 g of untransformed control oilseed rape (Brassica napus) seeds or the antisense seeds. The seeds were ground in a chilled mortar on ice, transferred to 4 mL of cold extraction buffer (20 mm imidazole-HCl, pH 6.0, 150 mm NaCl, and 2 mm phenylmethylsulfonyl fluoride), and the test tube was incubated in an “end-over-end” apparatus for 1 h at 4°C. The extract was fractionated by centrifugation at 10,000g for 15 min at 4°C into supernatant (“soluble fraction”) and pellet fractions (“insoluble fraction”). The protein concentration was determined (Bradford, 1976) with bovine serum albumin as a standard.
SDS-PAGE and Western-Blot Analysis
Samples were solubilized in SDS buffer (0.19 m Tris-HCl, pH 6.8, 30% [w/v] glycerol, and 6% [w/v] SDS) containing 25 mm dithiothreitol and were heated prior to separation by SDS-PAGE using 7.5% and 10% (w/v) acrylamide gels (Dobberstein et al., 1979). After completion of the gel electrophoresis, separated proteins were detected by staining with Coomassie Brilliant Blue or by immunoblotting using the mouse monoclonal antibodies 3D7 recognizing the myrosinase isoenzymes, or the MBP-reactive 34:14 antibodies (Lenman et al., 1990).
Immunohistochemical Staining
The seed coat of mature seeds (>40 d after pollination) was removed from control and antisense MBP seeds. The samples were fixed, dehydrated, embedded, and sectioned (7 μm) as described by Höglund et al. (1991). The monoclonal antibodies 3D7 and S4C6 were used for localization of myrosinase and MBPs, respectively (Lenman et al., 1990). In the negative control experiments, the primary antibody was omitted.
Enzyme Assay
Soluble protein extracts from untransformed (control) and antisense MBP seeds, as well as from fractions collected after gel filtration, were assayed for myrosinase activity in the presence of 8.5 mm sinigrin (Sigma Chemical, St. Louis) by measuring the release of Glc using a Glc-oxidase reagent (Merck, Darmstadt, Germany). The myrosinase activity was also measured in the presence of different concentrations, i.e. 0, 0.5, 1, 2, and 5 mm of freshly prepared ascorbic acid. Glucosinolate degradation product profile analysis was performed with radioactive p-hydroxybenzyl glucosinolate (sinalbin) as described (Chen and Halkier, 1999).
Plasmid Construction and Plant Transformation
A DNA fragment was produced by PCR from a pBluescript vector encoding a seed MBP cDNA using the following primers; 5′-ACGTTCCAATGGTAGATCT-3′ (forward) and 5′-GCATGCTATTGATGCCATC-3′ (reverse). The generated fragment was 1,417 bp (nucleotides 1,228–2,660; accession no. BNU59443), which approximately corresponds to the three internal repeats, each consisting of 158 amino acid residues. A modified binary vector, pGA581 (An, 1987), containing the napin promoter construct −1,101 to +45 (Stålberg et al., 1993) was partially digested with BamHI and was completely digested with SacI for removal of the β-glucuronidase gene to replace it with the antisense MBP gene also digested with SacI. Seeds of the oilseed rape cv Westar were obtained from Svalöf-Weibull AB (Svalöv, Sweden). Transformation of oilseed rape hypocotyls was carried out as described (De Block et al., 1989). Successive generations of selfed plants were selected by antibiotics to identify homozygotes of the T3 generation and were further used for the experiments.
Analytical Size-Exclusion Gel Chromatography
Crude protein extracts from control and antisense MBP seeds were separately applied to a Sephacryl S-300 column (85 × 2.6 cm) equilibrated with 20 mm imidazole-HCl, pH 6.0, 150 mm NaCl, and 0.02% (w/v) sodium azide. The same buffer was used for elution of proteins from the column at a flow rate of 1 mL min−1. Fractions of 2 mL were collected and the A280 was monitored. Aliquots were withdrawn for analysis of myrosinase activity, SDS-PAGE, and western-blot analysis.
Insect Feeding Bioassays
Reproductive adults of Tenebrio molitor, a common beetle generalist stored-product pest, obtained from a laboratory culture were isolated for 1 week on a substrate of rolled oats and dried bread. The adults were then removed and larvae were allowed to develop for several weeks to ensure that larvae were of approximately the same age. Larvae were then randomly assigned to a feeding treatment. They were fed with one of three diets: antisense MBP seeds, untransformed Westar seeds, or rolled oats. Larvae were weighed and were then kept individually. Forty-five larvae were assigned to each treatment in two separate experiments. After 10 d, the larvae were weighed again and the percentage of increase in weight was calculated. The data were transformed using an arcsin-square root transformation to achieve a normal distribution. A one-way analysis of variance (factor = larval diet) was then performed on the transformed data. Flea beetles (Phyllotreta undulata) were collected from plots of Brassica plants at the Agricultural University in Uppsala. Twenty insects were offered wild-type and antisense plants at the cotyledon stage in each cage. Cages were kept in growth chambers at 25°C, with a 16-h light/8-h dark regime. Plants were exposed to flea beetles for 8 or 24 h, and the numbers of holes on the cotyledons were counted and pooled for statistical analyses using one-tailed Student's t test.
Pathogen Experiments
The fungal strains Alternaria brassicae 950:31 and Leptosphaeria maculans 950:14 were supplied by Svalöf-Weibull. Fungal strains were grown on potato dextrose agarose (PDA) in the dark at room temperature and were frequently passed through the host to maintain virulence. Seed extracts were prepared from wild-type or antisense seeds essentially as described above. The supernatant was sterile filtrated using a 0.2-μm filter and was used at final concentrations of 0.14%, 1.4%, and 14% (w/v). A disc of mycelium, 1 mm in diameter, was transferred to PDA containing seed extracts. The fungi were grown at room temperature in the dark in 24-well dishes. Growth was determined after 96 h by measuring the area of the mycelium using a digital camera (4742-95; Hamamatsu, Hamamatsu City, Japan) and Image Pro software (version 4; Media Cybernetics, Silver Spring, MD). Triplicate tests were performed with PDA only or PDA with buffer as negative controls. The experiment was repeated and data was pooled for statistical analysis using one-tailed Student's t test.
ACKNOWLEDGMENTS
We thank Ulla Pihlgren and Elfi Öhrén (Department of Plant Biology, Swedish University of Agricultural Sciences) for their expert technical assistance. We also thank Dr. Sixue Chen (Plant Biochemistry Laboratory, Royal Veterinary and Agricultural University, Denmark) for valuable discussions and for providing radioactive sinalbin.
Footnotes
This work was supported by the Swedish University of Agricultural Sciences, by the Nordic Joint Committee for Agricultural Research, by the Swedish Research Council for Agriculture and Forestry, and by the Foundation for Strategic Research.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.003285.
LITERATURE CITED
- An G. Binary Ti vectors for transformation and promoter analysis. Methods Plant Biochem. 1987;153:292–319. [Google Scholar]
- Björkman R, Lönnerdahl B. Studies on myrosinases: enzymatic properties of myrosinases from Sinapis alba and Brassica napusseeds. Biochim Biophys Acta. 1973;327:1221–1231. doi: 10.1016/0005-2744(73)90109-5. [DOI] [PubMed] [Google Scholar]
- Blanchard DJ, Cicek M, Chen J, Esen A. Identification of β-glucosidase aggregating factor (BGAF) and mapping of BGAF binding regions on maize β-glucosidase. J Biol Chem. 2001;276:11895–11901. doi: 10.1074/jbc.M008872200. [DOI] [PubMed] [Google Scholar]
- Bones A, Rossiter JT. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol Plant. 1996;97:194–208. [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burmeister WP, Cottaz S, Driguez H, Iori R, Palmieri S, Henrissat B. The crystal structures of Sinapis alba myrosinase and a covalent glycosyl-enzyme intermediate provide insights into the substrate recognition and active-site machinery of an S-glycosidase. Structure. 1997;5:663–675. doi: 10.1016/s0969-2126(97)00221-9. [DOI] [PubMed] [Google Scholar]
- Chen S, Halkier BA. Functional expression and characterization of the myrosinase MYR1 from Brassica napus in Saccharomyces cerevisiae. Prot Exp Pur. 1999;17:414–420. doi: 10.1006/prep.1999.1158. [DOI] [PubMed] [Google Scholar]
- Chew FS. Biological effects of glucosinolates. In: Cutler HG, editor. Biologically Active Natural Products: Potential Use in Agriculture. Washington, DC: American Chemistry Society; 1988. pp. 155–181. [Google Scholar]
- Chisholm ST, Mahajan SK, Whitman SA, Yamamoto ML, Carrington JC. Cloning of the Arabidopsis RTM1gene, which controls restriction of long-distance movement of tobacco etch virus. Proc Natl Acad Sci USA. 2000;97:489–494. doi: 10.1073/pnas.97.1.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels MJ, Raikhel NV. Lectins, lectin genes, and their role in plant defense. Plant Cell. 1991;3:1–9. doi: 10.1105/tpc.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clossais-Besnard N, Larher F. Physiological role of glucosinolates in Brassica napus: concentration and distribution pattern of glucosinolates among plant organs during a complete life cycle. J Sci Food Agric. 1991;56:25–38. [Google Scholar]
- De Block M, De Brouwer D, Tenning P. Transformation of Brassica napus and Brassica oleracea using Agrobacterium tumefaciens and the expression of the bar and neogenes in the transgenic plants. Plant Physiol. 1989;91:694–701. doi: 10.1104/pp.91.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B, Garoff H, Warren G. Cell free synthesis and membrane insertion of a mouse H-2D histocompatibility antigen and β2-microglobulin. Chem Rev. 1979;17:759–769. doi: 10.1016/0092-8674(79)90316-7. [DOI] [PubMed] [Google Scholar]
- Esen A, Blanchard DJ. A specific beta-glucosidase-aggregating factor is responsible for the beta-glucosidase null phenotype in maize. Plant Physiol. 2000;122:563–572. doi: 10.1104/pp.122.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk A, Taipalensuu J, Ek B, Lenman M, Rask L. Characterization of rapeseed myrosinase-binding protein. Planta. 1995;195:387–395. doi: 10.1007/BF00202596. [DOI] [PubMed] [Google Scholar]
- Geshi N, Andréasson E, Meijer J, Rask L, Brandt A. Myrosinase and myrosinase-binding proteins are co-localized in grains of myrosin cells in cotyledon of Brassica napusL. seedlings. Plant Physiol Biochem. 1998;36:583–590. [Google Scholar]
- Geshi N, Brandt A. Two jasmonate-inducible myrosinase-binding proteins from Brassica napusL. seedlings with homology to jacalin. Planta. 1998;204:295–304. doi: 10.1007/s004250050259. [DOI] [PubMed] [Google Scholar]
- Girke T, Todd J, Ruuska S, White J, Benning C, Ohlrogge J. Microarray analysis of developing Arabidopsis seeds. Plant Physiol. 2000;124:1570–1581. doi: 10.1104/pp.124.4.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund A-S, Lenman M, Falk A, Rask L. Distribution of myrosinase in rapeseed tissues. Plant Physiol. 1991;95:213–221. doi: 10.1104/pp.95.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund A-S, Lenman M, Rask L. Myrosinase is localized to the interior of myrosin grains and is not associated to the surrounding tonoplast membrane. Plant Sci. 1992a;85:165–170. [Google Scholar]
- Höglund A-S, Rödin J, Larsson E, Rask L. Distribution of napin and cruciferin in developing rape seed embryos. Plant Physiol. 1992b;98:509–515. doi: 10.1104/pp.98.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DC, Rossiter JT. Development and characteristics of myrosinase in Brassica napusduring early seedling growth. Physiol Plant. 1991;82:163–170. [Google Scholar]
- Kelly PJ, Bones A, Rossiter JT. Sub-cellular immunolocalization of the glucosinolate sinigrin in seedlings of Brassica juncea. Planta. 1998;206:370–377. doi: 10.1007/s004250050412. [DOI] [PubMed] [Google Scholar]
- Lambrix V, Reichelt M, Mitchell-Olds T, Kliebenstein DJ, Gershenzon J. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia niherbivory. Plant Cell. 2001;13:2793–2807. doi: 10.1105/tpc.010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenman M, Falk A, Rödin J, Höglund AS, Ek B, Rask L. Differential expression of myrosinase gene families. Plant Physiol. 1993;103:703–711. doi: 10.1104/pp.103.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenman M, Rödin J, Josefsson LG, Rask L. Immunological characterization of rapeseed myrosinase. Eur J Biochem. 1990;194:747–753. doi: 10.1111/j.1432-1033.1990.tb19465.x. [DOI] [PubMed] [Google Scholar]
- Louda S, Mole S. Glucosinolates, chemistry and ecology. In: Rosenthal GA, Berenbaum MR, editors. Herbivores: Their Interactions with Secondary Plant Metabolites. San Diego: Academic Press; 1991. pp. 123–164. [Google Scholar]
- Peumans WJ, van Damme EJM. The role of lectins in plant defence. Histochem J. 1995;27:253–271. doi: 10.1007/BF00398968. [DOI] [PubMed] [Google Scholar]
- Rask L, Andréasson E, Ekbom B, Eriksson S, Pontoppidan B, Meijer J. Myrosinase: gene family evolution and herbivory defense in Brassicacea. Plant Mol Biol. 2000;42:93–113. [PubMed] [Google Scholar]
- Saito Y, Ihara Y, Leach MR, Cohen-Doyle MF, Williams DB. Calreticulin functions in vitroas a molecular chaperone for both glycosylated and non-glycosylated proteins. EMBO J. 1999;18:6718–6729. doi: 10.1093/emboj/18.23.6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stålberg K, Ellerström M, Josefsson L-G, Rask L. Deletion analysis of a 2S seed storage protein promoter of Brassica napusin transgenic tobacco. Plant Mol Biol. 1993;23:671–683. doi: 10.1007/BF00021523. [DOI] [PubMed] [Google Scholar]
- Taipalensuu J, Andréasson E, Eriksson S, Rask L. Regulation of the wound-induced myrosinase-associated protein transcript in Brassica napusplants. Eur J Biochem. 1997c;247:963–971. doi: 10.1111/j.1432-1033.1997.00963.x. [DOI] [PubMed] [Google Scholar]
- Taipalensuu J, Eriksson S, Rask L. The myrosinase-binding protein from Brassica napusseeds possesses lectin activity and has a highly similar vegetatively expressed wound-inducible counterpart. Eur J Biochem. 1997b;250:680–688. doi: 10.1111/j.1432-1033.1997.00680.x. [DOI] [PubMed] [Google Scholar]
- Taipalensuu J, Falk A, Ek B, Rask L. Myrosinase-binding proteins are derived from a large wound-inducible and repetitive transcript. Eur J Biochem. 1997a;243:605–611. doi: 10.1111/j.1432-1033.1997.t01-1-00605.x. [DOI] [PubMed] [Google Scholar]
- Taipalensuu J, Falk A, Rask L. A wound- and methyl jasmonate-inducible transcript coding for a myrosinase-associated protein with similarities to an early nodulin. Plant Physiol. 1996;110:483–491. doi: 10.1104/pp.110.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangstad OP, Evjen K, Bones A. Immunogold-EM localization of myrosinase in Brassicacea. Protoplasma. 1991;161:85–93. [Google Scholar]
- Xue J, Lenman M, Falk A, Rask L. The glucosinolate-degrading enzyme myrosinase in Brassicacea is encoded by a gene family. Plant Mol Biol. 1992;18:387–398. doi: 10.1007/BF00034965. [DOI] [PubMed] [Google Scholar]