Abstract
Tubers of transgenic potato (Solanum tuberosum) plants with decreased activity of the plastidic ATP/ADP transporter AATP1 display reduced levels of starch, modified tuber morphology, and altered concentrations of primary metabolites. Here, we demonstrate that the spontaneous production of hydrogen peroxide, the endogenous content of salicylic acid, and the levels of mRNAs of various defense-related genes are similar in tuber discs of wild-type and AATP1(St) antisense plants. However, upon challenging the tissue with fungal elicitors or culture supernatants of the soft rot-causing pathogen Erwinia carotovora subsp. atroseptica, the AATP1(St) antisense tubers exhibit highly potentiated activation of defense responses when compared with wild-type tissue. The augmented defense responses comprise enhanced accumulation of transcripts of five defense-related genes (β-1,3-GLUCANASE B2 and A1, CHITINASE B3 and A2, and Phe AMMONIA-LYASE) and enhanced elicitation (up to 21-fold) of the early hydrogen peroxide burst. The potentiated activation of cellular defense responses in AATP1(St) antisense tubers is not accompanied by a precedent increase in endogenous salicylic acid levels, but is associated with a strongly enhanced resistance of the tissue to E. carotovora. From these results, we conclude that inhibition of primary metabolic reactions induces a primed state that sensitizes the potato tubers for improved elicitation of various cellular defense responses, which likely contribute to enhanced E. carotovora resistance.
Upon pathogen infection, plants activate a complex set of defense responses that is aimed to reject pathogen attack. However, whether a plant is resistant to a certain pathogen depends on its capacity to effectively induce its various defense mechanisms. This has repeatedly been demonstrated for the so-called gene-for-gene disease resistance (Boch et al., 1998; Yu et al., 1998) and for various induced resistance phenomena (Conrath et al., 2002). Dependent on both the type of pathogen and the plant species attacked, pathogen-induced defense responses include a reinforcement of the cell wall (Bruce and West, 1989; Kauss, 1992; Lamb and Dixon, 1997), the synthesis of antimicrobial phytoalexins (Kombrink et al., 1991), the activation of various defense-related genes including those coding for antimicrobial, so-called pathogenesis-related (PR) proteins (Van Loon and Van Strien, 1999), and the generation of reactive oxygen species such as superoxide anion radicals and hydrogen peroxide (H2O2; Bolwell et al., 1999).
There is evidence for a still poorly understood interaction between primary metabolism and plant defense reactions. For example, the enhanced disease resistance of plant tissue exhibiting increased levels of sugars is called “high-sugar resistance” (Horsfall and Dimond, 1957). This type of pathogen resistance has repeatedly been ascribed to the sugar-mediated activation of various defense-related genes (Johnson and Ryan, 1990; Herbers et al., 1996b). So far, however, detailed analyses of the relationship between increased sugar levels and the activation of PR genes have been done mainly with photosynthetically active plant tissue. For example, exogenous application of sugars to tobacco (Nicotiana tabacum) source leaves was shown to activate various PR genes in this tissue (Herbers et al., 1996b). Interestingly, the sugar-mediated PR gene expression in the leaves appears to be independent on the accumulation of salicylic acid (SA; Herbers et al., 1996b), a signal molecule that often is required for PR gene expression (Dempsey et al., 1999). Sugar-mediated PR gene activation has also been shown in leaves of transgenic tobacco plants ectopically expressing either different types of yeast-derived invertases or reduced activity of a leaf specific H+/Suc symporter (Herbers et al., 1996a). In these experiments, transgenic leaf tissues exhibited significantly increased levels of soluble sugars and constitutively activated PR genes, already without a stimulation by pathogens or elicitors.
In heterotrophic potato (Solanum tuberosum) tubers, however, the situation obviously is of higher complexity. Otazu and Secor (1981) reported that an increase in the level of reducing sugars in potato tubers does not confer resistance rather than enhancing the susceptibility to pathogens. Because the latter observation contrasts with the widely observed high-sugar resistance (Horsfall and Dimond, 1957), it appeared important to study the relationship between altered carbohydrate levels and pathogen resistance also in the heterotrophic potato storage tissue.
We recently reported on transgenic potato plants with altered activity of the plastidic ATP/ADP transport protein AATP1(St) (Tjaden et al., 1998). This transporter catalyzes the import of ATP into heterotrophic plastids, thereby fueling all the anabolic reactions in this cellular compartment (Neuhaus and Emes, 2000). In AATP1(St) overexpressing (sense) plants exhibiting increased transporter activity, the level of tuber starch was enhanced. In tubers of AATP1(St) antisense plants, which display decreased transporter activity, the level of starch was conversely reduced by about 50% compared with the wild type (Tjaden et al., 1998). The metabolic alterations in AATP1(St) sense and antisense tubers were not limited to the level of starch but also influenced soluble metabolites. For example, AATP1(St) antisense tubers accumulated up to 10 times more Glc, exhibited increased ATP to ADP ratios, and increased respiratory activity. In contrast, sense tuber tissues did not exhibit significantly altered Glc levels or ATP to ADP ratios compared with wild-type tubers (Geigenberger et al., 2001).
To investigate whether the observed metabolic alterations might also influence the pathogen response of AATP1(St) antisense plants, we investigated, in the present study, whether AATP1(St) antisense potato tubers display (a) alterations in the induction of the early H2O2 burst, (b) altered accumulation of transcripts encoding defense-related proteins, and/or (c) altered resistance to the soft rot-causing bacterial pathogen Erwinia carotovora subsp. atroseptica.
RESULTS
AATP1(St) Antisense Potato Tubers Exhibit Potentiated H2O2 Elicitation
The release of H2O2 into the apoplast is an early response of plant tissues to pathogen attack (Bradley et al., 1992; Levine et al., 1994; Bolwell et al., 1999). To determine whether there are differences in the capacity to produce extracellular H2O2 between transgenic AATP1(St) and wild-type potato tuber tissue, the release of H2O2 from these two types of tissue was quantified. We first focused on tissues from wild-type and antisense line JT654 plants, because tubers from the latter show strong inhibition of starch biosynthesis and substantially altered levels of primary metabolites (Tjaden et al., 1998; Geigenberger et al., 2001).
As demonstrated in Figure 1, both water-incubated wild-type and AATP1(St) antisense tissue released only low basal levels of H2O2. Within 30 min of incubation, wild-type tissue produced up to 0.28 μm H2O2 and AATP1(St) antisense tissue released up to 0.54 μm H2O2 (Fig. 1). However, as indicated by the sd of the given means, these differences in H2O2 production are not statistically significant. It should be mentioned that all tissues tested have been conditioned in water for 4 h before the respective treatment to induce elicitation competency for H2O2 production (for details, see Fauth et al., 1996).
Figure 1.
Release of H2O2 from wild-type and AATP1(St) antisense potato tuber tissue. Discs from stored (2 month, 8°C) wild-type (white columns) or AATP1(St) antisense tubers (striped columns; line JT 654) were conditioned in sterilized water for 4 h to induce elicitation competency (Fauth et al., 1996). The discs were subsequently transferred to fresh petri dishes and incubated in presence of the given compounds. Pmg elicitor (Pmg) was used at 40 μg mL−1 and Pep-13 at 1 μm. E. carotovora (Eca), and the supernatant of an E. carotovora culture (Eca supernatant) were added as described in “Materials and Methods.” The concentration of H2O2 in the solution was monitored until a maximum at the 30-min time point was reached. Data are means of three independent experiments ± sd.
Interestingly, upon stimulation with a crude cell wall elicitor from the oomycete plant pathogen Phytophthora sojae, the so-called Phytophthora megasperma f. sp. glycinea elicitor (applied at a concentration of 40 μg mL−1), H2O2 release was stimulated by about 21-fold in AATP1(St) antisense tissue (11.4 μm), whereas wild-type tissue showed only an about 1.5-fold increase in H2O2 synthesis (Fig. 1). Pharmacological studies with diphenylene-iodonium chloride and lanthanum chloride suggest that the augmented H2O2 release in AATP1(St) tissue results from enhanced activation of the plasma membrane-localized NAD(P) H oxidase (data not shown). The 13-amino acid elicitor peptide Pep-13, which is present in the crude Pmg elicitor preparation (Nennstiel et al., 1998), also induced a rapid stimulation of H2O2 release in AATP1(St) antisense discs (about 5-fold compared with the water control) but was unable to significantly stimulate H2O2 production in wild-type tissue (Fig. 1).
Cells of E. carotovora, a common soil bacterium causing soft rot of potato tuber tissue, also stimulated H2O2 synthesis in AATP1(St) antisense tuber discs (about 2.9-fold when compared with the water control) but did not induce any significant H2O2 production in wild-type tuber discs (Fig. 1). It seems possible that the lack of detectable H2O2 release by E. carotovora-treated wild-type potato tuber discs might be attributable to destruction of released H2O2 by catalase, which has been reported to be highly present in E. carotovora cells (Miguel et al., 2000). However, the cell-free supernatant of an E. carotovora overnight culture was found to elicit H2O2 release (Fig. 1). As seen before with the Pmg and Pep-13 elicitors, E. carotovora supernatant-stimulated AATP1(St) antisense tuber tissue also synthesized substantially higher amounts of H2O2 than did the corresponding wild-type tissue (Fig. 1).
Potentiated Activation of Defense-Related Genes in AATP1(St) Antisense Potato Tubers
As shown above, AATP1(St) antisense potato tuber tissue does not constitutively produce enhanced amounts of H2O2 (Fig. 1). However, the capacity to synthesize H2O2 upon treatment with elicitors, E. carotovora cells, or bacterial culture supernatant is obviously enhanced in AATP1(St) antisense tuber tissue (Fig. 1). This led us to investigate the activation of another prominent plant response to pathogen attack, namely the induction of different defense-related genes.
Figure 2A demonstrates, that there was essentially no difference between wild-type and AATP1(St) antisense tissue in the steady-state level (0 h, total RNA extracted from freshly prepared wild-type and AATP1(St) antisense tuber tissue) of mRNA encoding Phe-ammonia-lyase (PAL 23), a key enzyme in the phenylpropanoid pathway that leads to a variety of defense-related compounds (Hahlbrock and Scheel, 1989). Incubation of the tuber discs for 4 h in water led to a substantial accumulation of PAL 23 mRNA in both types of tissue (Fig. 2A). This increase in PAL 23 transcript accumulation, however, was much higher in AATP1(St) antisense tissue than it was in tuber tissue from wild-type plants (Fig. 2A). This observation indicates that the wound stimulation during tuber disc conditioning induces some PAL 23 gene activation. The net accumulation of PAL 23 mRNA further increased during another hour of incubation in water, with again higher levels in the AATP1(St) antisense than in the wild-type tuber discs (Fig. 2A). The highest PAL 23 mRNA levels in wild-type tissue were observed upon treatment of conditioned wild-type discs for 1 h with Pmg elicitor (Fig. 2A). Yet, high levels of PAL 23 mRNA also accumulated upon treatment of wild-type discs with the supernatant of an E. carotovora culture for 1 h (Fig. 2A). In case of AATP1(St) antisense tubers, high levels of PAL 23 mRNA accumulated after 1 h of incubation of conditioned tissue in water or after further treatment for 1 h with either Pmg elicitor or the E. carotovora supernatant (Fig. 2A).
Figure 2.
RNA gel-blot analysis of transcripts of defense-related genes in wild-type and AATP1(St) antisense tuber tissue. A, Total RNA was extracted from discs of stored (4 month, 8°C) wild-type or AATP1(St) antisense tubers (line JT 654) directly after punching (0 h). As an alternative, discs were conditioned for 4 h in sterilized water (4, water control) for 1 more (+1, PAL 23 mRNA analysis) or for 9 more h (+9, CHI B3 and A2, BGL B2, and A1 mRNA analysis) in water, Pmg elicitor, or E. carotovora supernatant. Pmg elicitor was used at 40 μg mL−1. The supernatant of an E. carotovora culture (Eca supernatant) was used as described in “Materials and Methods.” B, Total RNA was isolated from discs of the stored wild-type or AATP1(St) antisense tubers directly after punching (0 h). As an alternative, discs were conditioned for 4 h in sterile water (4, water control) and then treated with 40 μg mL−1 Pmg elicitor. At various time points postelicitation, total RNA was extracted from an aliquot of discs and analyzed for the accumulation of BGL B2 transcripts by RNA gel-blot hybridization.
In contrast to PAL 23 mRNA, chitinase B3 (CHI B3) transcripts were not detectable after conditioning wild-type tuber discs for 4 h in water (Fig. 2A). However, after incubation in water for 9 more h, a substantial amount of CHI B3 mRNA was detectable in tuber discs from wild-type plants, indicating that wounding during disc conditioning also causes some CHI B3 activation. The level of Chi B3 mRNA accumulation was not further increased by the presence of Pmg elicitor or E. carotovora supernatant during the 9-h incubation period (Fig. 2A). In contrast, the response of the acidic chitinase A2 (CHI A2) mRNA was less clear (Fig. 2A). However, all of the treatments applied (4 h conditioning, 9 h incubation in water, and challenging with either Pmg elicitor or E. carotovora supernatant for 9 h) resulted in an increase in CHI A2 mRNA (Fig. 2A). As observed for the PAL 23 gene, both the basic CHI B3 and the acidic CHI A2 mRNAs accumulated to much higher levels in AATP1(St) antisense tissue than in the corresponding wild type (Fig. 2A). It is especially the treatment with the fungal Pmg elicitor that substantially increased the level of mRNAs for these two genes (Fig. 2A).
In both wild-type and AATP1(St) antisense tissue, the levels of transcripts encoding β-1,3-glucanases (BGL) B2 and A1 did not rise during conditioning for 4 h in water (Fig. 2A). Nevertheless, the AATP1(St) antisense tissue accumulated substantial levels of BGL B2 mRNA after 4 h of conditioning and subsequent incubation for 9 h in water, probably because of potentiation of the wound stimulus, whereas no BGL B2 transcript accumulation was detectable in the corresponding wild-type tissue (Fig. 2A). The Pmg elicitor caused a pronounced accumulation of both BGL B2 and BGL A1 mRNAs in AATP1(St) antisense tissue but hardly in tissue of wild-type tubers (Fig. 2A). In the case of wild-type tissue, only treatment with the E. carotovora culture supernatant induced the accumulation of remarkable amounts of BGL B2 mRNA, which again is severalfold higher in the AATP1(St) antisense tissue (Fig. 2A).
To ascertain whether the enhanced accumulation of defense gene transcripts seen in the AATP1(St) antisense tuber tissue (Fig. 2A) is associated with more rapid defense gene activation, discs from wild-type and AATP1(St) antisense tubers were conditioned for 4 h and then treated with Pmg elicitor. At various time points posttreatment, discs were harvested and representatively analyzed for the accumulation of BGL B2 mRNA. As is shown in Figure 2B, in AATP1(St) antisense discs, BGL B2 defense gene transcripts accumulated 2 to 3 h earlier than in tuber tissue from wild-type plants. Thus, AATP1(St) antisense potato tuber discs display accelerated and enhanced elicitation of BGL B2 defense gene activation.
Priming for Augmented Activation of Defense Responses in AATP1(St) Antisense Tubers Does Not Correlate with Increases in SA Levels
SA is a well-known endogenous signal that mediates a variety of plant defense responses (Dempsey et al., 1999). For example, treatment with SA or its functional analogs has repeatedly been shown to prime plant tissue for better activation of certain subsequently induced plant defense responses, including activation of the early H2O2 burst (Kauss and Jeblick, 1995) and the expression of various defense-related genes (Katz et al., 1998; Kohler et al., 2002).
To investigate whether the enhanced capacity of the AATP1(St) antisense potato tuber tissue to better activate the above assayed defense responses is correlated with increased levels of SA in this tissue, crude extracts from AATP1(St) antisense and wild-type tubers were analyzed for the accumulation of free and bound SA. Neither extracts from wild-type nor from AATP1(St) antisense tubers contained measurable amounts of free SA (detection limit of SA was 0.6 nmol g−1 fresh weight). In contrast, the level of bound SA was high in both types of tissue. Wild-type tubers contained 10.1 nmol bound SA g−1 fresh weight (sd ± 10%, n = 4), whereas AATP1(St) antisense tubers (line JT 654) even harbored only 2.5 nmol bound SA g−1 fresh weight (sd ± 12%, n = 4). Thus, the primed state for potentiated activation of H2O2 synthesis and augmented defense gene activation in AATP1(St) antisense tubers cannot been ascribed to the enhanced presence of free SA in this tissue.
Enhanced E. carotovora Resistance of AATP1(St) Antisense Potato Tubers
The potentiated activation of both the H2O2 burst and defense gene activation in AATP1(St) antisense tubers suggests that this tissue might possess an enhanced resistance to pathogens. The most prominent potato tuber pathogen is E. carotovora. Upon tuber infection, the bacterium causes soft rot disease (Pérembelon and Kelman, 1980), which is characterized by a rapid maceration of the infected tissue. As a consequence of maceration, the infected tuber tissue turns black (Pérembelon and Kelman, 1980). Therefore, the intensity of black staining can serve as a reliable qualitative marker for potato tuber infection by E. carotovora.
To determine whether there is differences in the E. carotovora resistance of wild-type and AATP1(St) antisense potato tuber tissue, discs were prepared from respective tubers and inoculated with a suspension of E. carotovora cells. After 24 h of incubation under saturated humidity, the black coloration of the infected tissue was analyzed. As is obvious from Figure 3, discs from potato wild-type tuber tissue were heavily infected and turned more or less homogenously black, whereas discs from AATP1(St) antisense tubers hardly exhibited symptoms of successful E. carotovora infection (Fig. 3). In a repeat of the experiment, bacterial population size was determined and found to be 5.0 × 109 ± 4.1 × 108 colony-forming units per tuber disc in the highly macerated wild-type and 2.4 × 109 ± 3.2 × 108 colony-forming units per disc in AATP1(St) antisense tissue. Thus, the bacteria grow more slowly in tuber tissue from the AATP1(St) transgenic line. In this context, it should be mentioned that upon high-titer inoculation or after extended periods of E. carotovora infection, AATP1(St) tuber discs also displayed visible symptoms of disease. However, these symptoms have always been less pronounced than in the corresponding wild-type tuber tissue (data not shown).
Figure 3.
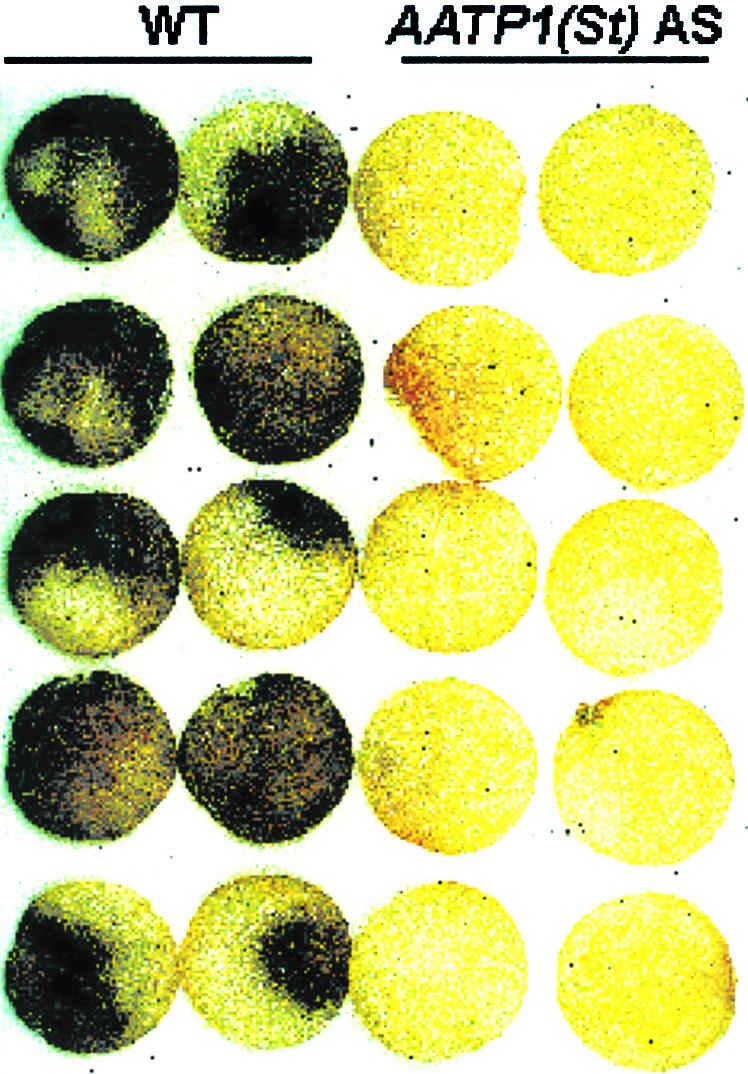
Effect of E. carotovora on discs from wild-type and AATP1(St) antisense tubers. Tuber discs were prepared from stored (2 month, 8°C) wild-type (WT) and AATP1(St) antisense tubers (line JT 654) and incubated with 2,000 E. carotovora cells per disc for 24 h, saturated humidity at 23°C. The extent of infection by E. carotovora is evident by the black coloration of macerated tissue.
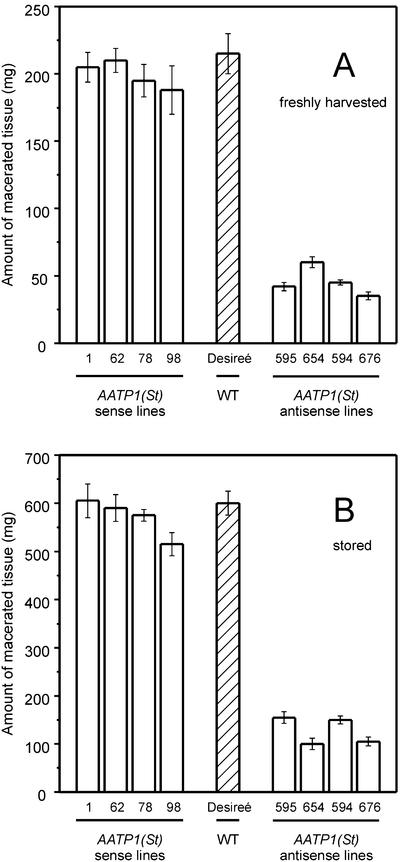
For a quantification of the E. carotovora resistance of AATP1(St) antisense tissue, the loss of macerated tissue was determined (Düring et al., 1993). Figure 4A shows that discs prepared from freshly harvested wild-type tubers lost about 200 mg fresh weight within 24 h of inoculation with E. carotovora. A similar observation has been made with tuber discs prepared from four independent AATP1(St) overexpressing (sense) lines (Tjaden et al., 1998). In contrast, tuber discs from four independent AATP1(St) antisense lines lost only 30 to 50 mg of fresh weight (Fig. 4A), representing up to 6-fold (line 676) increased resistance against E. carotovora.
Figure 4.
Quantification of macerated tissue from E. carotovora treated wild-type and various AATP1(St) sense and antisense tubers. A, Discs were prepared from freshly harvested wild-type (WT), sense (lines JT 1, 62, 78, and 98), and AATP1(St) antisense (lines JT 595, 654, 594, and 675) tubers. Harvested tubers had about 95% of their final size. Infection by E. carotovora was allowed to occur for 24 h under saturated humidity at 23°C. Macerated tissue was washed under a stream of tap water. Weighting of the discs before and after removal of infected tissue allowed quantification of maceration efficiency (Düring et al., 1993). B, Wild-type (WT), sense (lines JT 1, 62, 78, and 98), and AATP1(St) antisense (lines JT 595, 654, 594, and 675) tuber discs were prepared from stored (4 month, 8°C) tubers. Maceration by E. carotovora was allowed to occur for 24 h under high humidity at 23°C. Macerated tissue was removed and determined as given in A. Data given in A and B are means of four independent experiments ± sd.
E. carotovora attacks potato tubers not only during their development in the soil but also during storage (Pérembelon and Kelman, 1980). To analyze whether storage affects the resistance of potato tubers against E. carotovora, infection experiments were performed with discs from tubers that had been stored before at 8°C for 4 months. The amount of tissue lost by wild-type discs from stored tubers is remarkably about three times higher than the one of discs from freshly harvested tubers (about 600 mg; Fig. 4B). Similar to discs from fresh tubers (Fig. 4B), the resistance of all AATP1(St) overexpressing lines was similar to that of wild-type tissue, whereas AATP1(St) antisense tuber tissue exhibited severalfold increased E. carotovora resistance (Fig. 4B). We also demonstrated that potato tubers with reduced activity of ADP-Glc pyrophosphorylase, leading to reduced levels of starch (Müller-Röber et al., 1992), also exhibit increased resistance against E. carotovora (data not shown).
DISCUSSION
AATP1(St) antisense tubers are characterized by a decreased content of starch, increased levels of soluble sugars, and altered concentrations also of other intermediates of primary metabolism (Tjaden et al., 1998; Geigenberger et al., 2001). Here, we have shown that AATP1(St) antisense tuber tissue also displays improved capacity to activate various cellular defense responses but only upon treatment with elicitors, E. carotovora cells, or the E. carotovora culture supernatant (Figs. 1 and 2). The enhanced activation of cellular defense responses in AATP1(St) tubers is associated with an enhanced resistance against E. carotovora attack (Figs. 3 and 4).
Our observation that increased levels of sugars in AATP1(St) antisense potato tubers correlate with an improved ability to resist E. carotovora (Figs. 3 and 4) clearly contrasts with earlier observations made with stored potato tubers (Otazu and Secor, 1981). During storage, tuber sweetening occurs due to starch degradation and the associated accumulation of soluble sugars (ap Rees et al., 1988). This process was reported to correlate with increased susceptibility of the stored tuber tissue to E. carotovora (Otazu and Secor, 1981). However, the data in the present study clearly demonstrate that tuber tissue with high levels of reducing sugars in fact exhibits augmented soft-rot resistance (see the four independent antisense lines in Fig. 4). Therefore, it seems likely that other mechanisms, which differ from the accumulation of reduced sugars, change during tuber storage, thereby conferring enhanced E. carotovora susceptibility.
The synthesis of H2O2 is an early response of plants to pathogen infection (Lamb and Dixon, 1997). Accordingly, the level of apoplastic H2O2 is not constitutively increased in AATP1(St) antisense tubers (Fig. 1). The more or less immediate release of H2O2 upon either elicitor treatment or pathogen infection (Fig. 1), and the immediate suppression of the H2O2 burst by lanthanum chloride and diphenyl iodonium (data not shown) suggest (a) that the plasma membrane localized NADPH oxidase is responsible for the H2O2 release, and (b) that the stimulation of H2O2 production occurred without significant de novo synthesis of proteins rather than via activation of a latent enzyme activity. Therefore, the improved capacity to synthesize H2O2 depends upon at least two criteria: First, an already present plasma membrane-localized enzyme activity has to acquire activity. Second, sufficient substrate supply is a prerequisite for the high H2O2 production rate observed.
It is known that the plant plasma membrane-bound NADPH oxidase is regulated by cytosolic Ca2+ and ATP-dependent protein phosphorylation (Nürnberger and Scheel, 2001). Therefore, it seems possible that the increased ATP to ADP ratio in AATP1(St) antisense tuber tissue (Geigenberger et al., 2001) might induce or facilitate the activation of corresponding protein kinases. In addition, the substrate for the plasma membrane-bound oxidase NADPH is synthesized via the cytosolic oxidative pentose phosphate pathway (Emes and Tobin, 1993). Because inhibition of starch biosynthesis in AATP1(St) tubers correlates with substantially enhanced levels of Glc-6-P (Geigenberger et al., 2001), the key substrate of the oxidative pentose phosphate pathway, more oxidizable carbohydrate substrates are available for an increased metabolic demand.
The improved E. carotovora resistance of AATP1(St) antisense tubers (Figs. 3 and 4, A and B) might, at least in part, be due to the augmented release of antibacterial reactive oxygen species into the apoplast (Fig. 1). This assumption is supported by the finding that heterologous expression of an apoplastic Glc oxidase, which causes constitutively enhanced H2O2 levels, confers strongly enhanced E. carotovora resistance to potato tubers (Wu et al., 1995). However, other factors that might also contribute to the improved E. carotovora resistance should also be kept in mind. As is indicated from Figure 1 and as has been reported by Miguel et al. (2000), E. carotovora contains catalase activity that is able to effectively degrade H2O2. It has recently been shown that an Erwinia chrysanthemi mutant, lacking catalase and, thus, being highly sensitive to H2O2, retained full virulence on potato plants (Miguel et al., 2000). This observation argues against a major role of H2O2 in pathogen rejection, at least in this plant-pathogen combination.
At a first glance, the improved capacity to activate transcription-dependent and -independent defense responses (Figs. 1 and 2) and the increased capacity of the AATP1(St) antisense tuber tissue to better resist E. carotovora infection (Figs. 3 and 4) appears consistent with the frequently observed high-sugar resistance of plant tissues (Horsfall and Dimond, 1957; Vanderplank, 1984). We have previously shown that AATP1(St) antisense tuber tissue is characterized by substantially (up to 10-fold) increased levels of Glc (Geigenberger et al., 2001). Interestingly, the Glc level in a given plant tissue can regulate gene expression (Sheen, 1990, 1994; Jang and Sheen, 1994). There is also ample evidence that increased carbohydrate levels may cause constitutive accumulation of PR gene transcripts (Keil et al., 1986; Johnson and Ryan, 1990; Tsukaya et al., 1991). The latter data originate from promoter characterization of respective genes (Keil et al., 1986; Johnson and Ryan, 1990) but are consistent with results of experiments with intact plant tissues or even whole plants.
To assay possible effects of increased sugar levels on the activation of plant defense responses, experiments have previously been performed in which either fully developed source leaves were fed with Glc, or in which transgenic plants expressing different yeast-derived invertases have been employed. In both cases, the observed increase in soluble sugar levels was associated with constitutively increased PR gene expression (Herbers et al., 1996a, 1996b). Moreover, Glc feeding to heterotrophic cell suspensions from Chenopodium rubrum induced the immediate expression of PAL genes (Ehness et al., 1997). However, the activation mechanism of defense responses in AATP1(St) antisense tuber tissue seems to be unique and much more complex because we did not observe constitutive, preinfectional accumulation of PR gene transcripts (Fig. 2) or permanently increased rates of H2O2 production (Fig. 1). The marked differences in pathogen responses between wild-type and AATP1(St) antisense tuber tissue became obvious only upon stimulation of the tissue with elicitors, E. carotovora cells, or E. carotovora culture supernatant (Figs. 1 and 2).
This phenomenon has striking similarity to the SA-, 2,6-dichloroisonicotinic acid-, benzothiadiazole-, and β-aminobutyric acid-induced, so-called “priming” of plant cells or whole plants for potentiated induction of various cellular defense responses (Conrath et al., 2002). Similar to primed parsley (Petroselinum crispum) cells (Kauss and Jeblick, 1995; Katz et al., 1998; Thulke and Conrath, 1998) and primed cucumber (Cucumis sativus; Fauth et al., 1996) and Arabidopsis (Kohler et al., 2002) plants, AATP1(St) antisense tissue also exhibits an increased capacity for both H2O2 production and the activation of defense-related genes (Figs. 1 and 2). Based on the results in the present study, we therefore conclude (a) that alterations in the primary metabolism may prime potato tuber tissue for stronger activation of various cellular defense responses, thus, possibly contributing to enhanced E. carotovora resistance (Figs. 3 and 4), and (b) that this process is independent on a preceding increase in endogenous SA levels (see above).
Studies on the pathogen resistance of transgenic tobacco leaves, ectopically expressing three different yeast invertase genes, indicated that elevated Glc levels may contribute to a state of enhanced disease resistance. The constitutive activation of defense responses and the enhanced resistance toward potato virus Y in these plants occurred only upon expressing either the cell wall-localized-, or the vacuolar invertase. However, these responses were not seen when the heterologous invertase was present in the cytosol (Herbers et al., 1996a). Yet, all three types of transgenic plants exhibited increased levels of soluble sugars (Herbers et al., 1996a). These observations led to the assumption that the localization of soluble carbohydrates within the cell's endomembrane system, rather than the accumulation of soluble sugars per se, is required for defense response signaling (Herbers et al., 1996a).
We propose that the observed E. carotovora resistance and the augmented ability to activate cellular defense responses in AATP1(St) antisense tubers is not, or at least not exclusively, due to increased Glc levels. This assumption is based on two aspects: First, previous data suggested that Glc needs to accumulate in the plant cell's endomembrane system to induce defense responses (Herbers et al., 1996a). Unfortunately, no protocol exists for assaying the subcellular localization of sugars in potato tuber tissue. However, because a decrease of the amyloplastidic ATP/ADP transporter activity leads to an inhibited access of energy into the storage plastids (Tjaden et al., 1998), it is probable that the sugar accumulation in AATP1(St) tubers occurs in the cytosolic rather than in the endomembrane compartment. Second, the assumed accumulation of Glc in the endomembrane system of transgenic tobacco plants expressing cell wall-localized or vacuolar invertase led to constitutive, preinfectional accumulation of both PR gene transcripts and SA (Herbers et al., 1996a). In marked contrast, the priming of AATP1(St) antisense tissue is associated neither with constitutive accumulation of PR gene transcripts nor with increases in SA levels (Figs. 1 and 2; see “Results”).
Because we accept that increased Glc levels are not the major cause for the augmented ability to activate defense responses, further metabolic alterations need to be considered. AATP1(St) antisense tubers also exhibit an augmented ATP to ADP ratio (Geigenberger et al., 2001). Because ATP-consuming protein phosphorylation is an important cellular reaction in defense response signaling (Nürnberger and Scheel, 2001), it seems possible that an increased cellular energy state is sufficient to improve the cell's response to elicitor treatment or pathogen attack. In addition, we have shown elsewhere that the AATP1(St) antisense tuber tissue exhibits increased respiratory activity (Geigenberger et al., 2001). The increase in respiration might keep the cellular ATP/ADP charge high, even upon induction of energy-consuming pathogen defense responses.
MATERIALS AND METHODS
Cultivation of Potato Plants
Wild-type and transgenic potato (Solanum tuberosum var Désireé) plants were grown in a greenhouse in soil at 22°C during the day and 18°C in the night and were watered daily. The ambient light was extended to 16 h per day with Sont-Agro lights (200 μE, Philips, Eindhoven, The Netherlands) as described before (Tjaden et al., 1998). Potato tissue was analyzed for pathogen resistance either immediately after harvesting fully developed tubers or after storage of the tubers for 2 to 4 months at 8°C.
Qualitative and Quantitative Analysis of Erwinia carotovora subsp. atroseptica Infections on Potato Tuber Discs
E. carotovora subsp. atroseptica cells were supplied by the Deutsche Sammlung für Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany) and were cultivated according to the supplier's advice. To monitor the infection of potato tubers by E. carotovora, discs of 3 mm thickness and 2 cm in diameter were punched out of tubers and incubated in plastic boxes under high humidity (Düring et al., 1993). Bacterial inoculation was done by transferring 2,000 E. carotovora cells in 10 μL of sterilized water into the center of the prepared tuber discs. The discs were subsequently incubated for 24 h at 23°C in the dark.
The increase in bacterial population was estimated in one-half of infected discs from wild-type or AATP1(St) antisense tubers (n = 3). The disc halves were homogenized in 5 mL of sterile water and thoroughly mixed, and serial dilutions of the slurry were plated out on agar plates. After incubation for 2 d at 23°C, colonies were counted, and the original population sizes deducted.
For quantitative analysis of E. carotovora infections, the tuber discs were weighed at the indicated time points postinfection. The macerated tissue was removed by washing off with tap water (Düring et al., 1993). A second determination of weight allowed quantification of the lost (macerated) tissue.
Quantification of Hydrogen Peroxide and Treatment of Tuber Discs for mRNA Measurements
To quantify the amount of released H2O2, four tuber discs (3 mm thickness, 1.5 cm in diameter) from respective plant lines were transferred to a petri dish (5 cm in diameter) containing 8 mL of sterilized water and conditioned for 4 h to acquire elicitation competency (Fauth et al., 1996). To prevent anaerobiosis, the discs were incubated on a laboratory shaker at 30 rpm during conditioning. The elicitor-competent discs were subsequently transferred to fresh petri dishes containing the respective elicitor (see below), the supernatant of an overnight culture of E. carotovora, or E. carotovora cells in 8 mL of sterilized water.
For bacterial infection, an aliquot of E. carotovora overnight culture was transferred to fresh growth medium. Bacteria were grown to A600 nm = 0.6. One milliliter of this suspension was diluted with 7 mL of sterilized water and transferred to petri dishes. Four tuber discs (see above) from wild-type or AATP1(St) antisense plants were subsequently transferred into the diluted growth medium. To assay the effect of the E. carotovora culture supernatant, a corresponding aliquot of culture was centrifuged (10 min, 4,000g, 4°C) to remove bacterial cells. Pmg elicitor (a crude cell wall preparation of Phytophtora sojae) was used at 40 μg mL−1, whereas Pep-13 (an elicitor-active peptide of 13 amino acids that is present in the Pmg elicitor) was used at 1 μm. To prevent anaerobiosis, incubations were done for the given length of time on a shaker at 30 rpm.
H2O2 levels in the supernatant were quantified by ferricyanide-catalyzed oxidation of luminol as described previously (Fauth et al., 1996). Tuber discs to be used for RNA extraction were snap frozen and stored at −70°C.
Extraction of Total RNA and RNA Gel-Blot Hybridization
After punching tuber discs from wild-type or antisense tissue, these were directly snap frozen (0 h). As an alternative, tuber discs were conditioned for 4 h in water to acquire elicitation competency (4 h water-control; for details see Fauth et al., 1996). To check the effect of fungal elicitors on mRNA accumulation, conditioned tuber discs were incubated for 9 more h (1 h for PAL 23 transcript accumulation) in water, 40 μg mL−1 Pmg elicitor, or 1 μm Pep-13. Discs were subsequently transferred into liquid nitrogen until RNA extraction using the Purescript RNA extraction kit (Gentra Systems, Minneapolis) according to the manufacturer's instructions. For RNA gel-blot hybridization analysis, 10 μg of total RNA was denatured and separated on a 1.2% (w/v) agarose-2.5% (v/v) formaldehyde gel essentially as described by Thulke and Conrath (1998). Ethidium bromide was included in the loading buffer to confirm equal sample loading. After blotting to a positively charged nylon membrane (Nytran-Plus, Schleicher & Schuell, Germany) by downstream capillary transfer using 10× SSC (1.5 m sodium chloride and 0.15 m sodium citrate, pH 7.0), RNA was cross-linked to the membrane by UV irradiation. Prehybridization and hybridization were performed at 65°C in 0.25 m NaHPO4, pH 7.2, 1 mm EDTA, 7% (w/v) SDS, and 1% (w/v) bovine serum albumin. Hybridization with alpha-32P-dCTP-labeled cDNA probes was for 16 h. After hybridization the membranes were washed at 65°C for 1 h with two changes of the washing solution (40 mm NaHPO4, pH 7.2, 1 mm EDTA, 5% [w/v] SDS, and 0.5% [w/v] bovine serum albumin). Finally, blots were exposed to Kodak MS x-ray film (Eastman-Kodak, Rochester, NY) at −70°C.
cDNA Clones
The potato PAL 23 clone was kindly provided by Prof. Klaus Hahlbrock and Dr. Günther Strittmatter (Max-Planck-Institut für Züchtungsforschung, Köln, Germany). The cDNA clone encoding potato Chitinase B3 and A2 and Glucanases B2 and A1 from potato were provided by Dr. Erich Kombrink (Max-Planck-Institut für Züchtungsforschung). Plasmid-DNA was harvested from respective clones and digested with restriction enzymes, and the cDNA fragments then were isolated by agarose gel electrophoresis as described (Sambrook et al., 1989). After extraction of the cDNAs from excised gel slices they have been stored at −20°C until random priming labeling and use in the hybridization experiments.
Quantification of SA
The level of both free and bound SA was quantified by HPLC/diode array photometric analysis as described previously (Kauss et al., 1993). Recovery of representative amounts of SA was determined and found to be about 80%.
ACKNOWLEDGMENTS
We thank Prof. Klaus Hahlbrock, Drs. Günther Strittmatter and Erich Kombrink (Max Planck Institut für Züchtungs forschung, Cologne) for providing us various cDNA clones, and Prof. Lothar Willmitzer (Max Planck Institut für Molekulare Pflanzenphysiologie, Golm, Germany) for providing us potato AGPase antisense plants. In the frame of the ad-hoc working group “Novel and Functional Food,” the Bundesforschungsanstalt für Ernährung (Karlsruhe, Germany) provided the greenhouse facilities to T.B.; this is gratefully acknowledged. We thank Corinna Bialek (MPB Cologne GmbH) for skillful technical assistance and Prof. Heinrich Kauss (Universität Kaiserslautern) for critical reading of the manuscript.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. Ne 418/4–1 to the laboratories of U.C. and H.E.N.) and by the Schwerpunkt Biotechnologie of the Federal State Rheinland-Pfalz.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.000802.
LITERATURE CITED
- ap Rees T, Burrell MM, Entwistle TG, Hammond JB, Kirk D, Kruger NJ. Effects of low temperature on the respiratory metabolism of carbohydrates by plants. Symp Soc Exp Biol. 1988;42:377–393. [PubMed] [Google Scholar]
- Boch J, Verbsky ML, Roberston TL, Larkin JC, Kunkel BN. Analysis of resistance gene-mediated defense responses in Arabidopsis thaliana plants carrying a mutation in CPR5. Mol Plant-Microbe Interact. 1998;12:1196–1206. [Google Scholar]
- Bolwell GP, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minobayeva F, Rowntree FG, Wojtaszek P. Recent advances in understanding the origin of the apoplastic oxidative burst in plant cells. Free Radic Res Suppl. 1999;31:137–145. doi: 10.1080/10715769900301431. [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Kjellbom P, Lamb CJ. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Bruce RJ, West CA. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 1989;91:889–897. doi: 10.1104/pp.91.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath U, Pieterse C, Mauch-Mani B. Priming in plant-pathogen interactions. Trends Plant Sci. 2002;7:210–216. doi: 10.1016/s1360-1385(02)02244-6. [DOI] [PubMed] [Google Scholar]
- Dempsey D, Shah J, Klessig DF. Salicylic acid and disease resistance in plants. Crit Rev Plant Sci. 1999;18:547–575. [Google Scholar]
- Düring K, Porsch P, Fladung M, Lörz H. Transgenic potato plants resistant to the phytopathogenic bacterium Erwinia carotovora. Plant J. 1993;3:587–598. [Google Scholar]
- Ehness R, Ecker M, Godt DE, Roitsch T. Glucose and stress independently regulate source and sink metabolism and defense mechanisms via signal transduction pathways involving protein phosphorylation. Plant Cell. 1997;9:1825–1841. doi: 10.1105/tpc.9.10.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emes MJ, Tobin AK. Control of metabolism and development in higher plant plastids. Int Rev Cytol. 1993;145:149–216. [Google Scholar]
- Fauth M, Merten A, Hahn MG, Jeblick W, Kauss H. Competence for elicitation of H2O2 in hypocotyls of cucumber is induced by breaching the cuticle and is enhanced by salicylic acid. Plant Physiol. 1996;110:347–354. doi: 10.1104/pp.110.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Stamme C, Tjaden J, Schulze A, Quick WP, Betsche T, Kersting HJ, Neuhaus HE. Tuber physiology and properties of starch from tubers of transgenic potato plants with altered plastidic adenylate transporter activity. Plant Physiol. 2001;125:1667–1678. doi: 10.1104/pp.125.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahlbrock K, Scheel D. Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:347–369. [Google Scholar]
- Herbers K, Meuwly P, Frommer WB, Métraux J-P, Sonnewald U. Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell. 1996a;8:793–803. doi: 10.1105/tpc.8.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbers K, Meuwly P, Métraux J-P, Sonnewald U. Salicylic acid-independent induction of pathogenesis-related protein transcripts by sugars is dependent on leaf developmental stage. FEBS Lett. 1996b;397:239–244. doi: 10.1016/s0014-5793(96)01183-0. [DOI] [PubMed] [Google Scholar]
- Horsfall JG, Dimond AE. Interactions of tissue sugar, growth substances, and disease susceptibility. Z Pflanzenkr Pflanzenschutz. 1957;27:415–421. [Google Scholar]
- Jang JC, Sheen J. Sugar sensing in higher plants. Plant Cell. 1994;6:1665–1679. doi: 10.1105/tpc.6.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Ryan CA. Wound-inducible potato inhibitor II genes: enhancement of expression by sucrose. Plant Mol Biol. 1990;14:527–536. doi: 10.1007/BF00027498. [DOI] [PubMed] [Google Scholar]
- Katz VA, Thulke OU, Conrath U. A benzothiadiazole primes parsley cells for augmented elicitation of defense responses. Plant Physiol. 1998;117:1333–1339. doi: 10.1104/pp.117.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H. Callose and callose synthase. In: Guro SJ, McPherson MJ, Bowles DJ, editors. Molecular Plant Pathology. Vol. 2. UK: Oxford University Press; 1992. pp. 1–8. [Google Scholar]
- Kauss H, Franke R, Krause K, Conrath U, Jeblick W, Grimmig B, Matern U. Conditioning of parsley (Petroselinum crispum L.) suspension cells increases elicitor-induced incorporation of cell wall phenolics. Plant Physiol. 1993;102:459–466. doi: 10.1104/pp.102.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H, Jeblick W. Pretreatment of parsley suspension cultures with salicylic acid enhances spontaneous and elicited production of H2O2. Plant Physiol. 1995;108:1171–1178. doi: 10.1104/pp.108.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil M, Sanchez-Serrano JJ, Willmitzer L. Primary structure of a proteinase inhibitor II gene from potato (Solanum tuberosum) Nucleic Acids Res. 1986;14:5641–5650. doi: 10.1093/nar/14.14.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler A, Schwindling S, Conrath U. Benzothiadiazole-induced priming for potentiated responses to pathogen infection, wounding and infiltration of water into leaves requires the NPR1/NIM1 gene in Arabidopsis. Plant Physiol. 2002;128:1046–1056. doi: 10.1104/pp.010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E, Hahlbrock K, Hinze K, Schröder M. Molecular responses of potato to infection with Phytophtora infestans. In: Smith CJ, editor. Biochemistry and Molecular Biology of Plant-Pathogen Interaction. Oxford, UK: Clarendon Press; 1991. pp. 312–354. [Google Scholar]
- Lamb C, Dixon R. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Miguel E, Poza-Carrión E, Aguilar I, Llama-Palacios A, García-Olmedo F, Rodríguez-Palenzuela P. Evidence against a direct antimicrobial role of H2O2 in the infection of plants by Erwinia chrysanthemi. Mol Plant-Microbe Interact. 2000;13:421–429. doi: 10.1094/MPMI.2000.13.4.421. [DOI] [PubMed] [Google Scholar]
- Müller-Röber B, Sonnewald U, Willmitzer L. Inhibition of the ADP-glucose pyrophosphorylase in transgenic potatoes leads to sugar-storing tubers and influences tuber formation and expression of tuber storage protein genes. EMBO J. 1992;11:1229–1238. doi: 10.1002/j.1460-2075.1992.tb05167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nennstiel D, Scheel D, Nürnberger T. Characterization and partial purification of an oligopeptide elicitor receptor from parsley (Petroselinum crispum) FEBS Lett. 1998;431:405–410. doi: 10.1016/s0014-5793(98)00800-x. [DOI] [PubMed] [Google Scholar]
- Neuhaus HE, Emes MJ. Development of non-green plastids. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:111–140. doi: 10.1146/annurev.arplant.51.1.111. [DOI] [PubMed] [Google Scholar]
- Nürnberger T, Scheel D. Signal transmission in the plant immune response. Trends Plant Sci. 2001;6:372–379. doi: 10.1016/s1360-1385(01)02019-2. [DOI] [PubMed] [Google Scholar]
- Otazu V, Secor GA. Soft rot susceptibility of potatoes with high reducing sugar content. Phytopathology. 1981;71:290–295. [Google Scholar]
- Pérembelon MCM, Kelman A. Ecology of the soft rot Erwinias. Annu Rev Phytopathol. 1980;18:361–387. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sheen J. Metabolic repression of transcription in higher plants. Plant Cell. 1990;2:1027–1038. doi: 10.1105/tpc.2.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Feedback control of gene expression. Photosynth Res. 1994;39:427–438. doi: 10.1007/BF00014596. [DOI] [PubMed] [Google Scholar]
- Thulke OU, Conrath U. Salicylic acid has a dual role in the activation of defense related genes in parsley. Plant J. 1998;14:35–42. doi: 10.1046/j.1365-313X.1998.00093.x. [DOI] [PubMed] [Google Scholar]
- Tjaden J, Möhlmann T, Kampfenkel K, Henrichs G, Neuhaus HE. Altered plastidic ATP/ADP-transporter activity influences potato (Solanum tuberosum) morphology, amount and composition of tuber starch, and tuber morphology. Plant J. 1998;16:531–540. [Google Scholar]
- Tsukaya H, Ohshima T, Naito S, Chino M, Komeda Y. Sugar-dependent expression of the CHS-A gene for chalcone synthase from petunia in transgenic Arabidopsis. Plant Physiol. 1991;97:1414–1421. doi: 10.1104/pp.97.4.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderplank JE. Sink-induced loss of resistance. In: Vanderplank JE, editor. Disease Resistance in Plants. Ed 2. London: Academic Press; 1984. pp. 107–116. [Google Scholar]
- Van Loon LC, Van Strien EA. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol. 1999;55:85–97. [Google Scholar]
- Wu G, Shortt BJ, Lawrence EB, Levine EB, Fitzsimmons KC, Shah DM. Disease resistance conferred by expression of a gene encoding H2O2-generating glucose oxidase in transgenic potato plants. Plant Cell. 1995;7:1357–1368. doi: 10.1105/tpc.7.9.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I-C, Parker J, Bent AF. Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc Natl Acad Sci USA. 1998;95:7819–7824. doi: 10.1073/pnas.95.13.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]