Abstract
Lipid analysis of rosette leaves from Arabidopsis has revealed an accumulation of triacylglycerol (TAG) with advancing leaf senescence coincident with an increase in the abundance and size of plastoglobuli. The terminal step in the biosynthesis of TAG in Arabidopsis is catalyzed by diacylglycerol acyltransferase 1 (DGAT1; EC 2.3.1.20). When gel blots of RNA isolated from rosette leaves at various stages of development were probed with the Arabidopsis expressed sequence tag clone, E6B2T7, which has been annotated as DGAT1, a steep increase in DGAT1 transcript levels was evident in the senescing leaves coincident with the accumulation of TAG. The increase in DGAT1 transcript correlated temporally with enhanced levels of DGAT1 protein detected immunologically. Two lines of evidence indicated that the TAG of senescing leaves is synthesized in chloroplasts and sequesters fatty acids released from the catabolism of thylakoid galactolipids. First, TAG isolated from senescing leaves proved to be enriched in hexadecatrienoic acid (16:3) and linolenic acid (18:3), which are normally present in thylakoid galactolipids. Second, DGAT1 protein in senescing leaves was found to be associated with chloroplast membranes. These findings collectively indicate that diacylglycerol acyltransferase plays a role in senescence by sequestering fatty acids de-esterified from galactolipids into TAG. This would appear to be an intermediate step in the conversion of thylakoid fatty acids to phloem-mobile sucrose during leaf senescence.
Diacylglycerol (DAG) acyltransferase (DGAT; EC 2.3.1.20) mediates the final acylation step in the synthesis of triacylglycerol (TAG). It is present in most plant organs, including leaves, petals, fruits, anthers, and developing seeds (Hobbs et al., 1999). In seeds, TAG is thought to be synthesized within the membranes of the endoplasmic reticulum and subsequently released into the cytosol in the form of oil bodies, which bleb from the cytoplasmic surface of the endoplasmic reticulum (Huang, 1992). The stored TAG is localized in the interior of the oil body, and the surfaces of oil bodies are coated with a monolayer of phospholipid associated with oleosin, the major protein of oil bodies. The acyl chains of the phospholipid monolayer are embedded in the TAG interior of the oil body. Oleosin is a structural protein that is thought to prevent coalescence of oil bodies during seed dehydration (Huang, 1996). That oil bodies originate from the endoplasmic reticulum is consistent with the finding that enzymes of TAG synthesis, including DGAT, are present in microsomal membrane fractions, which are known to contain vesicles of endoplasmic reticulum (Kwanyuen and Wilson, 1986). In addition, TAG can be synthesized in vitro in the presence of microsomes isolated from developing seeds (Lacey et al., 1999).
Although TAG formation in seeds is believed to occur in the ER, there have been several reports indicating that purified chloroplast envelope membranes from leaves are also capable of synthesizing this storage lipid (Siebertz et al., 1979; Martin and Wilson, 1983, 1984). Moreover, TAG is known to be present in plastoglobuli, which are lipid bodies localized in the stroma of chloroplasts (Martin and Wilson, 1984). DGAT is unique to the TAG biosynthetic pathway (Bao and Ohlrogge, 1999), and the finding that different types of membranes are capable of synthesizing TAG suggests that DGAT may have more than one subcellular localization. In fact, three gene families encoding DGAT-like proteins have been identified, specifically the gene family encoding DGAT1, which has high sequence similarity with sterol acyltransferase, the gene family encoding DGAT2, which has no sequence similarity with DGAT1, and the gene family encoding phospholipid:DAG acyltransferase (Lardizabal et al., 2001). DGAT1, DGAT2, and phospholipid:DAG acyltransferase are all capable of catalyzing the final acylation step during TAG synthesis, and this raises the possibility that these separate gene families regulate the synthesis of TAG at different stages of plant development and possibly in different cellular compartments.
DGAT1 has been quite extensively studied in Arabidopsis. The gene is found on chromosome II, approximately 17.5 ± 3 cM from the sti locus and 8 ± 2 cM from the cp2 locus (Zou et al., 1999). It has been established that the Arabidopsis expressed sequence tag (EST) clone E6B2T7 corresponds to the DGAT1 gene, and the full-length cDNA for DGAT1 (approximately 2.0 kb) has been sequenced (Hobbs et al., 1999). Much of the characterization of this gene to date has been focused on determining its effect on seed oil accumulation and the fatty acid composition of TAG in seed oil. To this end, seed-specific mutants have been analyzed (Katavic et al., 1995; Zou et al., 1999; Jako et al., 2001), and among the findings from these studies is the fact that dysfunctional DGAT1 protein results in a decrease in stored TAG and altered TAG fatty acid composition (Katavic et al., 1995). Overexpression of DGAT1 in Arabidopsis seeds engenders an increase in seed size and oil content, suggesting that DGAT catalyzes the rate-limiting step in TAG biosynthesis (Jako et al., 2001). In addition, Routaboul et al. (1999) have demonstrated that when Arabidopsis DGAT1 is inactivated through a frame-shift mutation, seeds are still produced indicating that proteins other than DGAT1 are able to catalyze TAG formation.
In the present study, we report that DGAT1 is up-regulated during senescence of Arabidopsis leaves and that this is temporally correlated with increased levels of TAG-containing fatty acids commonly found in chloroplast galactolipids. The chloroplast is the first organelle of mesophyll cells to be affected by senescence, and the onset of chloroplast senescence appears to be initiated by nuclear-encoded proteins as distinct from chloroplastically encoded proteins (Matile, 1992). Recruitment of membrane carbon from senescing leaves, particularly senescing chloroplasts, to growing parts of the plant is a key feature of leaf senescence, and it involves de-esterification of thylakoid lipids and conversion of the resultant free fatty acids to phloem-mobile Suc (Matile, 1992). De-esterification of thylakoid lipids appears to be mediated by one or more senescence-induced galactolipases (Engelman-Silvestre et al., 1989). The results of the present study indicate that formation of TAG may be an intermediate step in this mobilization of membrane lipid carbon to phloem-mobile Suc during leaf senescence.
RESULTS
Expression of DGAT1 during Rosette Leaf Development and Senescence
Changes in the levels of DGAT1 transcript and its cognate protein during development and senescence of Arabidopsis rosette leaves were examined by northern- and western-blot analysis, respectively. The DGAT1 EST clone E6B2T7 was used as a probe for northern analysis. The alignment of E6B2T7 with the genomic sequence for DGAT1 is illustrated in Figure 1. Western blots were probed with polyclonal antibodies raised against a synthetic peptide corresponding to the last 17 amino acids of the C terminus of DGAT1 (Fig. 1).
Figure 1.
Alignment of the genomic nucleotide sequence of DGAT1 with the EST clone, E6B2T7. Exons are underlined, and the sequence corresponding to E6B2T7 is indicated in bold italics. The deduced amino acid sequence of DGAT1 is also indicated, and the peptide used for antibody generation is highlighted in gray.
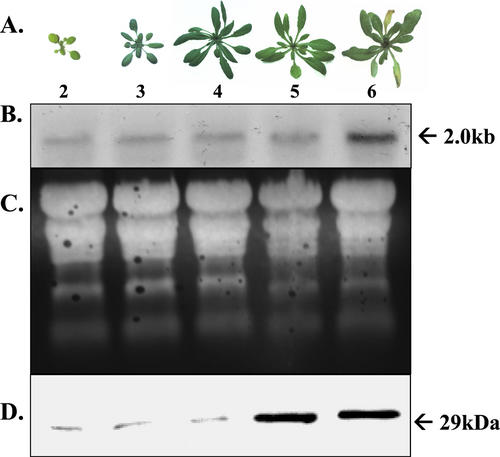
Photographs of Arabidopsis rosettes harvested from 2- through 6-week-old plants are illustrated in Figure 2A. At 2 weeks of age, the rosette leaves are still small, but they enlarge substantially between 2 and 4 weeks of age. By week 5, leaf senescence has engaged, and at week 6 the leaves are visibly yellow reflecting significant depletion of chlorophyll as senescence progresses (Fig. 2A). DGAT1 transcript was detectable in total RNA preparations from all ages of rosette leaves, indicating that there is a basal level of constitutive DGAT1 expression (Fig. 2B). However, the abundance of DGAT1 mRNA changed during leaf development. Specifically, levels were lowest for 2-week-old plants, higher for 3- and 4-week-old plants and then increased again reaching very high levels in the visibly yellow leaves of 6-week-old plants (Fig. 2B). Similar changes in the abundance of DGAT1 protein were evident as well. The protein was present in low amounts in leaves from 2-, 3-, and 4-week-old plants, and then increased sharply through weeks 5 and 6, coincident with the onset of senescence (Fig. 2D). The expected size of the DGAT1 protein is 51 kD, and in some experiments, the native 51-kD protein was detectable. However, the polypeptide routinely detected in western blots probed with DGAT1 antiserum was only 29 kD in size (Fig. 2D) and is presumably a proteolytic catabolite of the native protein.
Figure 2.
Expression analysis of DGAT1 during development and senescence of rosette leaves. A, Photographs of Arabidopsis rosettes at weeks 2 through 6 after planting. B, Northern blot of total RNA isolated from rosette leaves of 2- through 6-week-old Arabidopsis plants. The blot was hybridized with the Arabidopsis EST clone E6B2T7. Each lane contained 10 μg of RNA. C, Ethidium bromide detection of fractionated RNA after transfer to a nylon membrane. Lanes are as in B. D, Western blot of total protein isolated from the rosette leaves of 2- through 6-week-old Arabidopsis plants. Lanes are as in B. Each lane contained 10 μg of protein. The blot was probed with antibody raised against a peptide of DGAT1.
Spatial Expression of DGAT1
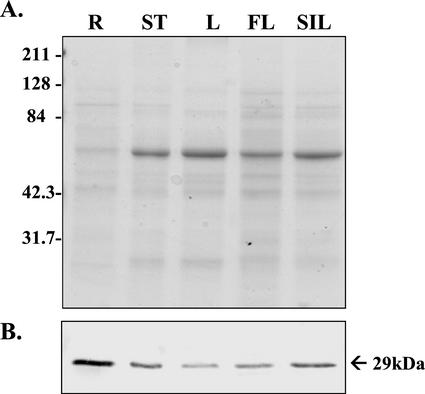
The extent to which DGAT1 is expressed in organs other than rosette leaves was assessed by western-blot analysis. Total protein from roots, stems, non-rosette leaves, flowers, and siliques collected from 6-week-old plants was fractionated by SDS-PAGE and blotted on polyvinylidene difluoride membranes. At this stage of development, the stems and non-rosette leaves had not begun to senesce, the flowers were a mixture of senescing and non-senescing inflorescences, and the siliques were developing but had not yet reached maturity. Probing the blots with DGAT1 peptide antiserum revealed that the protein is present in all of these organs, although it is most abundant in roots and siliques and least abundant in non-rosette leaves (Fig. 3).
Figure 3.
Western-blot analysis of the spatial expression of DGAT1 in 6-week-old Arabidopsis plants. A, SDS-PAGE. Each lane contained 10 μg of protein, and the gel was stained with Coomassie Brilliant Blue. R, Roots; ST, stem; L, non-rosette leaves; FL, flower; and SIL, siliques. B, Corresponding protein blot probed with antibody raised against a peptide of DGAT1. Lanes are as in A. Apparent molecular mass of the immunodetected polypeptide is indicated in kilodaltons.
Lipid Composition of Young and Senescing Rosette Leaves
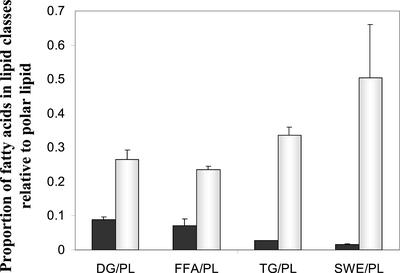
Five distinct classes of lipid, viz., polar lipid, diacylglycerol (DAG), free fatty acids, TAG, and a mixture of steryl and wax esters, were discernible in thin layer chromatograms of total lipid extracts from the rosette leaves of 3- and 6-week-old plants. In both cases, polar lipids consisting of a mixture of phospholipids and galactolipids were dominant, accounting for 84.76% ± 1.34% of the total fatty acid complement in young leaves and 53.03% ± 5.45% of total in senescing leaves. The lower proportion of polar lipids in the senescing leaves reflects higher amounts of neutral lipids, and this was quantified by expressing the fatty acid equivalents of each neutral lipid as a proportion of the polar lipid fatty acid complement (Fig. 4). The concentrations of DAG, free fatty acids, TAG, and steryl/wax esters relative to polar lipids all proved to be much higher for senescing leaves than for young leaves. Steryl/wax esters and TAG proved to be the most abundant neutral lipids in the extract for senescing leaves, whereas DAG and free fatty acids were the most abundant in the extract for young leaves (Fig. 4). Specifically, the ratios of steryl/wax esters and TAG to polar lipid were 30- and 13-fold higher, respectively, in senescing leaves than in young leaves. For DAG and free fatty acids, the corresponding ratios were 3- and 4-fold higher in senescing leaves (Fig. 4). The steryl/wax ester isolate includes lipids from the cuticle, which thickens as the leaves develop and senesce (Post-Beittenmiller, 1996).
Figure 4.
Composition of total lipid extracts from young and senescing rosette leaves. Individual lipid classes were quantified as fatty acid equivalents. The non-polar lipids (DAG [DG], free fatty acids [FFA], TAG [TG], and steryl/wax esters [SWE]) are expressed as a ratio of the polar lipids, phospholipid and galactolipid (PL). The values are means ± se for n = 3. ▪, Young 3-week-old rosette leaves; □, senescing 6-week-old rosette leaves.
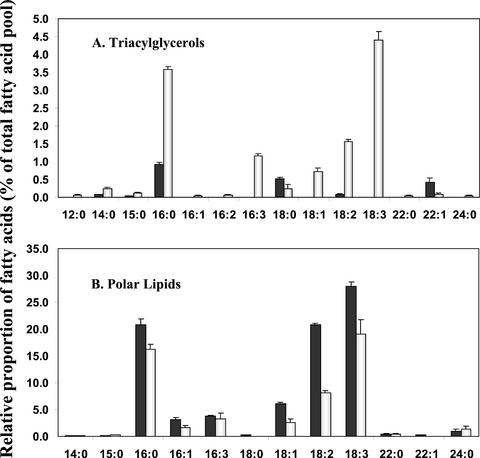
Of particular interest is the finding that there is approximately 13 times more TAG relative to polar lipid in the senescing 6-week-old rosette leaves than in the 3-week-old rosette leaves, for this is consistent with the senescence-related up-regulation of DGAT1. In absolute terms, TAG fatty acid equivalents constitute 12.33% ± 2.20% of the total fatty acid complement in the senescing leaves and only 2.02% ± 0.12% in the young leaves. As well, the fatty acid composition of TAG in senescing leaves is distinguishable from that of young leaves. For young leaves, the major TAG fatty acids proved to be palmitic acid (16:0), stearic acid (18:0), and erucic acid (22:1; Fig. 5A). By contrast, for senescing leaves, the dominant TAG fatty acids included palmitic acid, as for young leaves, as well as linolenic acid (18:3) and hexadecatrienoic acid (16:3; Fig. 5A). Linolenic acid and hexadecatrienoic acid, which are normally associated with the galactolipids of chloroplast membranes (Awai et al., 2001), collectively accounted for 45.03% ± 1.69% of the total TAG fatty acid complement in the 6-week-old senescing leaves (Fig. 5A). Thus, the TAG in the older leaves would appear to be formed at least in part from fatty acids originating from chloroplast membranes.
Figure 5.
Fatty acid composition of TAG and polar lipid from young and senescing rosette leaves. A, Fatty acid composition of TAG from young 3-week-old rosette leaves (▪) and senescing 6-week-old rosette leaves (□). B, Fatty acid composition of polar lipids (phospholipid and galactolipid) from young 3-week-old rosette leaves (▪) and senescing 6-week-old rosette leaves (□). Values are means ± se for n = 3.
Unlike TAG, for polar lipids, the fatty acid composition remained relatively unchanged as senescence was engaged in the rosette leaves. The major fatty acids associated with the polar lipids proved to be palmitic acid (16:0) and the two polyunsaturated fatty acids, linoleic acid (18:2) and linolenic acid (18:3; Fig. 5B). These collectively accounted for 82.22% ± 0.48% of the total polar lipid fatty acid complement for young leaves and 81.59% ± 4.74% of the total for senescing leaves. For young and senescing leaves, linolenic acid was the dominant fatty acid in the polar lipid fraction (Fig. 5B). Inasmuch as linolenic acid is the major fatty acid of galactolipids, this indicates, as expected, that galactolipids are a substantial component of the polar lipid isolate. The presence of hexadecatrienoic acid (16:3) in the polar lipid fraction also signifies the presence of galactolipids, for this fatty acid is uniquely chloroplastic in 16:3 plants such as Arabidopsis (Browse et al., 1986).
DGAT Expression in Chloroplasts
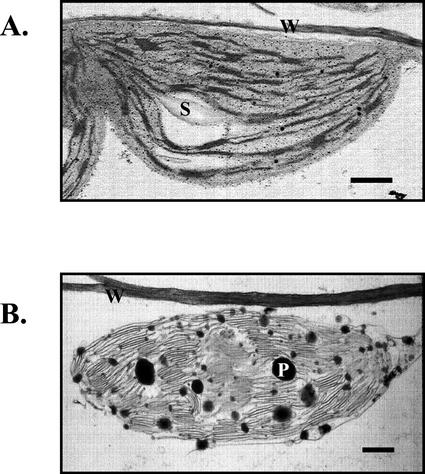
The finding that TAG from senescing rosette leaves of 6-week-old plants contains high levels of fatty acids derived from chloroplastic membranes suggests that the newly synthesized TAG is formed within chloroplasts. This contention is additionally supported by electron microscopic observations indicating greatly increased abundance and size of plastoglobuli in the chloroplasts of 6-week-old senescing rosette leaves in comparison with those of young rosette leaves (Fig. 6). Plastoglobuli are known to contain TAG and are thought to be formed coincident with the dismantling of thylakoid membranes in senescing chloroplasts (Matile, 1992).
Figure 6.
Electron micrographs of chloroplasts in young and senescing rosette leaves. A, Young (3-week-old) rosette leaf chloroplast. B, Senescing (6-week-old) rosette leaf chloroplast. S, Starch grain; W, cell wall; and P, plastoglobule. Bar = 1 μm.
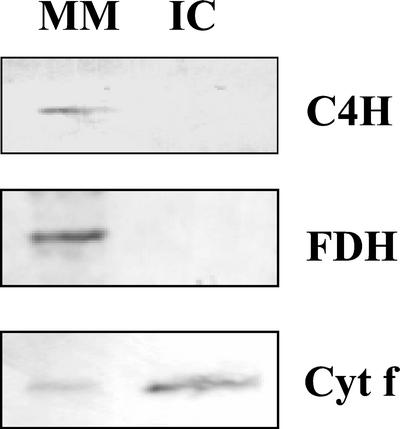
That DGAT1, the protein mediating the terminal step in TAG synthesis, is associated with chloroplasts was confirmed by western-blot analysis. Chloroplasts were isolated from 4.5-week-old rosette leaves and subfractionated into membranes, a composite of thylakoid and envelope membranes, and stroma. Purity of the isolated chloroplasts was confirmed by western-blot analysis using antiserum for formate dehydrogenase, a marker for mitochondria (Colas des Francs-Small et al., 1993), cytochrome P450-cinnamate-4-hydroxylase, a marker for endoplasmic reticulum (Young and Beevers, 1976), and cytochrome f, a marker for thylakoids (Smith et al., 2000). Each of formate dehydrogenase, cytochrome P450-cinnamate-4-hydroxylase and cytochrome f were detectable in immunoblots of microsomal membranes (Fig. 7). The presence of cytochrome P450-cinnamate-4-hydroxylase and cytochrome f in microsomes is in keeping with the fact that this fraction comprises small vesicles formed during tissue homogenization from all cellular membranes, including endoplasmic reticulum and thylakoids. Formate dehydrogenase is a mitochondrial matrix enzyme which would be released into the cytosol during homogenization (Colas des Francs-Small et al., 1993), and its presence in the microsomal fraction reflects the fact that cytosol is occluded within microsomal vesicles as they are formed. However, only cytochrome f was detectable in intact chloroplasts indicating that this fraction is essentially free of intact mitochondria and endoplasmic reticulum (Fig. 7). Because formate dehydrogenase is a mitochondrial matrix enzyme and would not reveal the presence of mitochondrial membrane vesicles in the intact chloroplast fraction, levels of cytochrome c oxidase activity, a marker enzyme for mitochondrial inner membrane (Hodges and Leonard, 1974), were measured in microsomal membranes and intact chloroplasts. The specific activity of cytochrome c oxidase in the intact chloroplast fraction was only 1/25 that of microsomal membranes, indicating that the purified chloroplast fraction is also essentially free of mitochondrial membrane. A further indication of the purity of the intact chloroplast fraction is the fact that cytochrome f, a marker for thylakoid membranes, is highly enriched in intact chloroplasts relative to microsomal membranes in immunoblots of the two fractions loaded with constant protein (Fig. 7).
Figure 7.
Western-blot analysis of purified intact chloroplasts and microsomal membranes isolated from the rosette leaves of 4.5-week-old Arabidopsis plants. Each lane contained 5 μg of protein. MM, Microsomal membranes; IC, intact chloroplasts. The blots were probed with polyclonal antibodies against cytochrome P450-cinnamate-4-hydroxylase (C4H), formate dehydrogenase (FDH), and cytochrome f (Cyt f).
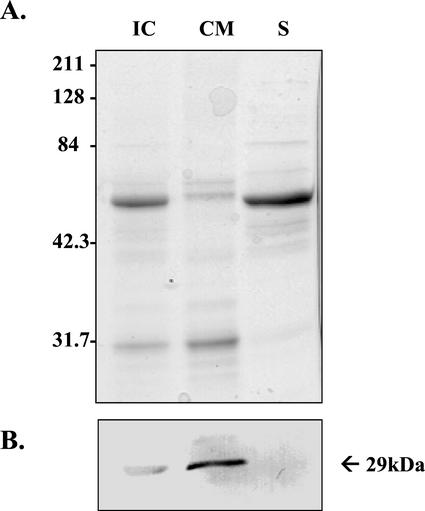
Immunoblots of chloroplasts and their purified subfractions probed with DGAT1 antiserum revealed that the protein is present in intact chloroplasts, enriched in the purified chloroplast membrane fraction, but not detectable in stroma (Fig. 8). Only the large 55-kD subunit of Rubisco is evident in Figure 8A inasmuch as the small 14-kD subunit ran off the end of the gel under the conditions of electrophoresis deployed.
Figure 8.
Western-blot analysis of DGAT1 localization in fractionated chloroplasts isolated from the rosette leaves of 4.5-week-old Arabidopsis plants. A, SDS-PAGE. Each lane contained 5 μg of protein, and the gel was stained with Coomassie Brilliant Blue. B, Corresponding protein blot probed with antibody raised against a peptide of DGAT1. IC, Intact chloroplasts; CM, chloroplast membranes; and S, stroma. Apparent molecular mass of the immunodetected polypeptide is indicated in kilodaltons.
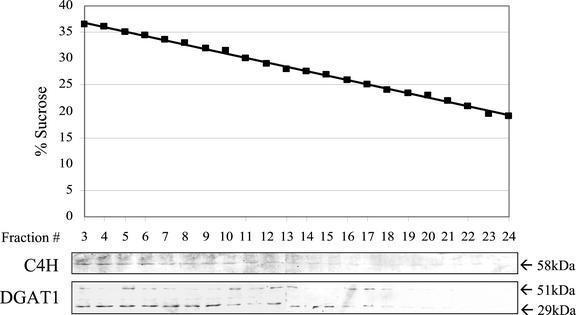
DGAT1 was also detectable in vesicles of endoplasmic reticulum. Microsomal membranes isolated from developing siliques were fractionated by centrifugation through a linear Suc gradient. Western-blot analysis of sequentially removed fractions revealed a parallel distribution of cytochrome P450-cinnamate-4-hydroxylase, a marker for endoplasmic reticulum, and DGAT1 along the gradient (Fig. 9). In these experiments, both the native DGAT1 protein, which has an expected molecular mass of 51 kD, and the 29-kD proteolytic catabolite of the native protein were detectable (Fig. 9). When microsomal membranes from rosette leaves of 4.5-week-old-plants were similarly fractionated and analyzed by western blotting, DGAT1 was not detectable in gradient fractions containing cytochrome P450-cinnamate-4-hydroxylase.
Figure 9.
Western-blot analysis of fractionated microsomes isolated from developing siliques of Arabidopsis plants. The microsomes were fractionated by centrifugation through a continuous Suc density gradient. First panel, Suc concentration in consecutive fractions of the gradient after centrifugation. Second panel, Western blot probed with antibody against cytochrome P450-cinnamate-4-hydroxylase (C4H). Third panel, Western blot probed with antibody against DGAT1. The lanes were loaded with equal volumes of the fractions from the Suc gradient. Lanes 3 through 12 and lanes 13 through 24 are separate gels run concurrently in the same electrophoresis apparatus. Apparent molecular masses of the immunodetected polypeptides are indicated in kilodaltons.
DISCUSSION
Senescence is a highly controlled sequence of events leading ultimately to the death of cells, tissues, or organs (Thompson et al., 1998). It is induced by changes in gene expression and occurs naturally at the end of the life span of an organ or, in the case of monocarpic senescence, the whole plant and can also be engendered prematurely in response to stress (Smart, 1994). Moreover, senescence is not simply a process of programmed cell death invoked when a tissue or organ is no longer needed. It also entails intricately regulated recruitment of nutrients together with their translocation from the senescing tissue to other tissues that are still growing and developing (Smart, 1994). In the case of foliar senescence in plants such as Arabidopsis, for example, there is extensive translocation of nutrients from senescing leaves to the developing seeds (Himelblau and Amasino, 2001).
One of the earliest manifestations of leaf senescence is depletion of chlorophyll reflecting functional decline and dismantling of thylakoid membranes (Thompson et al., 1998). The chloroplast is the first organelle of mesophyll cells to show symptoms of senescence (Matile, 1992). It is noteworthy, however, that although breakdown of thylakoid membranes is initiated early in the leaf senescence cascade, the chloroplast envelope remains relatively intact until the very late stages of senescence (Peoples et al., 1980). Thylakoids are the most abundant membrane in nature (Lee, 2000) and, as such, constitute a rich source of carbon in the form of lipid fatty acids for remobilization during leaf senescence. The galactolipids monogalactosyldiacylglycerol and digalactosyldiacylglycerol collectively comprise approximately 80% of the lipid content of thylakoids (Lee, 2000). As chloroplasts senesce, galactolipid fatty acids are de-esterified and converted to phloem-mobile Suc for translocation out of the senescing leaf. This is achieved through β-oxidation and the glyoxylate cycle, and there is now good evidence for the conversion of leaf peroxisomes to glyoxysomes as senescence is engaged, and for correlative up-regulation of the genes encoding malate synthase and isocitrate lyase, key enzymes of the glyoxylate cycle (De Bellis et al., 1990).
De-esterification of galactolipid fatty acids is thought to be mediated by one or more senescence-induced galactolipases (Kim et al., 2001), and the resultant dismantling of thylakoids is known to be accompanied by a marked increase in the abundance and size of plastoglobuli, lipid bodies within the chloroplast (Matile, 1992). Plastoglobuli are mainly composed of plastoquinone (Bailey and Whyborn, 1963), α-tocopherol (Lichtenthaler, 1969), and TAG (Steinmüller and Tevini, 1985a) but also contain lower concentrations of phytol, which is apparently released from chlorophyll by the action of chlorophyllase, as well as carotenoids and free fatty acids (Steinmüller and Tevini, 1985b). Although the precise role of plastoglobuli has not been elucidated, it is assumed, based on their reduction in size and number during thylakoid biogenesis and their subsequent accumulation and increase in size during thylakoid degradation, that they temporarily store thylakoid lipid metabolites, especially those liberated during leaf senescence (Sprey and Lichtenthaler, 1966; Lichtenthaler, 1969; Lichtenthaler and Weinert, 1970).
In the present study, we have established that enhanced synthesis of TAG and an increase in the abundance and size of plastoglobuli are temporally correlated in senescing rosette leaves of Arabidopsis. TAG levels rose by 13-fold, and plastoglobuli were highly abundant in the chloroplasts of senescing leaves and sparsely present in those of younger non-senescing leaves. This temporal relationship together with the fact that TAG is a major component of plastoglobuli (Steinmüller and Tevini, 1985a) suggests that the incremental TAG in senescing leaves is localized in plastoglobuli. Moreover, the TAG of senescing leaves, unlike that of younger leaves, contains high levels of chloroplastic fatty acids, specifically hexadecatrienoic acid (16:3) and linolenic acid (18:3). These two fatty acids collectively comprise 45.03% ± 1.69% of the total fatty acid complement of TAG from senescing leaves and were not detectable in TAG from the younger leaves. Hexadecatrienoic acid is only found in galactolipids, and although linolenic acid is present to a limited extent in phospholipids, it is the most abundant fatty acid of galactolipids (Miquel et al., 1998). These findings collectively indicate that the TAG in senescing leaves is formed from thylakoid fatty acids released during galactolipid catabolism.
Of particular interest is the finding that the steep increase in TAG levels in senescing rosette leaves was paralleled by strong up-regulation of DGAT1 transcript as well as its cognate protein. This enzyme catalyzes the terminal step in the pathway for TAG synthesis, one in which a fatty acid is added through an ester linkage to the sn-3 carbon of DAG (Hobbs and Hills, 2000). It is the only enzyme in this pathway unique to TAG synthesis (Bao and Ohlrogge, 1999) and has been shown to be rate limiting (Jako et al., 2001). The increase in DGAT1 transcript levels was ascertained by probing northern blots of total RNA with E6B2T7, an EST clone that has been annotated as DGAT1. The transcript depicted by E6B2T7 proved to be 2 kb, the reported size of Arabidopsis DGAT1 mRNA (Hobbs et al., 1999). The increase in DGAT1 protein was discerned by immunoblotting using antibodies raised against a synthetic peptide corresponding to the C terminus of the protein. The expected size of the DGAT1 protein is 51 kD (Hobbs et al., 1999), but the polypeptide routinely immunodetected in western blots was only 29 kD in size. This 29-kD polypeptide appears to be a proteolytic catabolite of the native DGAT1 protein formed during protein extraction and fractionation despite the presence of protease inhibitors. Evidence supporting this contention includes the fact that small amounts of the native 51-kD protein and its catabolite were sometimes detectable on developed blots. In addition, the antibody was raised against a peptide corresponding to the last 17 amino acids of the DGAT1 C terminus, and when this peptide sequence was submitted to BLAST, no significant alignments other than with DGAT1 of Arabidopsis and canola (Brassica napus), close relatives, were returned. Finally, the native 51-kD DGAT1 protein was clearly discernible in immunoblots of protein extracts from leaves of canola probed with the Arabidopsis antibody. Furthermore, the amino acid sequences for DGAT1 of canola and Arabidopsis are 85% identical for the entire protein and 95% identical (16 of 17 amino acids) for the peptide used to generate the antiserum. Thus, DGAT1 in Arabidopsis leaves seems to be more prone to proteolysis during protein extraction and fractionation than its counterpart in canola.
The finding that DGAT1 is present in isolated chloroplasts further supports the contention that TAG is synthesized in the chloroplasts of senescing rosette leaves. Immunoblots of fractionated chloroplasts revealed that DGAT1 is present in intact chloroplasts and purified membranes including thylakoid and envelope but not in the stroma. These findings are consistent with the measurements of DGAT activity in chloroplast subfractions reported by Martin and Wilson (1984), and support their contention that DGAT is associated with chloroplast envelope membranes. This together with the knowledge that chloroplasts possess the enzymes required for the formation of acyl-CoA and DAG (Harwood and Stumpf, 1972; Bertrams and Heinz, 1976; Sanchez and Mancha, 1981; Shimakata and Stumpf, 1982), which serve as substrates for DGAT1, indicates that chloroplasts are capable of synthesizing TAG. Because DGAT1 appears to be associated with the chloroplast envelope, de-esterified galactolipid fatty acids or their corresponding acyl-CoAs destined for conversion to TAG must first be translocated from the thylakoids to the envelope. The mechanism underlying the translocation of TAG formed within the envelope membranes to plastoglobuli is not clear, although recent findings indicate that there is an elaborate system of pathways for lipid transport from the envelope to the interior of the chloroplast (Andersson et al., 2001). In keeping with its perceived association with endoplasmic reticulum in seeds (Huang, 1992), DGAT1 was also immunologically detectable in protein blots of endoplasmic reticulum isolated from developing siliques. However, it was not discernible in western blots of endoplasmic reticulum from rosette leaves, possibly because its abundance in leaf endoplasmic reticulum is too low for detection under the experimental conditions deployed in the present study.
There is growing evidence that plastoglobuli are analogous to oil bodies. Not only do they both store TAG, but plastoglobuli also appear to be coated with a structural protein termed fibrillin or plastid lipid-associated-protein, which is analogous to oil body oleosin (Pozueta-Romero et al., 1997; Kessler et al., 1999; Rey et al., 2000). Fibrillin and oleosin are thought to prevent coalescence of plastoglobuli and oil bodies, respectively (Huang, 1996; Rey et al., 2000). Moreover, recent experiments with transgenic plants in which fibrillin was overexpressed have indicated that the availability of fibrillin regulates the formation of plastoglobuli in much the same way that oleosin regulates the formation of oil bodies (Huang, 1992; Rey et al., 2000). In light of these similarities, it seems likely that plastoglobuli, like oil bodies (Huang, 1992), are formed within a membrane and released from the surface of that membrane into a hydrophilic compartment.
Previous studies have demonstrated activation of TAG synthesis and a concurrent decrease in galactolipids and phospholipid in ozone-fumigated leaves (Sakaki et al., 1990a, 1990b, 1990c). Drought-stressed cotton leaves similarly exhibit a significant decline in polar lipid and a parallel increase in TAG (El-Hafid et al., 1989). Sakaki et al. (1990b) have proposed that the activation of TAG synthesis in ozone-treated leaves serves to sequester fatty acids de-esterified from galactolipids in response to the ozone stress. By analogy, the senescence-related up-regulation of DGAT1 and related synthesis of TAG may serve to sequester galactolipid fatty acids released during chloroplast senescence and, thus, may be an intermediate step in the conversion of thylakoid fatty acids to phloem-mobile Suc. Recent evidence indicates that plastoglobuli of senescing chloroplasts are exuded through the envelope into the cytoplasm (Guiamét et al., 1999). This would enable the fatty acid equivalents of plastoglobuli to gain access to glyoxysomes for β-oxidation and subsequent gluconeogenesis leading to Suc formation.
MATERIALS AND METHODS
Plant Material
Seeds of Arabidopsis, ecotype Columbia, were grown in Promix BX soil (Premier Brands, Brampton, ON, Canada) in 6-inch pots. Freshly seeded pots were maintained at 4°C for 2 d and then transferred to a growth chamber operating at 22°C with 16-h light/8-h dark cycles. Lighting at 150 μmol radiation m−2 s−1 was provided by cool-white fluorescent bulbs. Rosette leaves were harvested after 2, 3, 4, 5, and 6 weeks of growth. Roots, stems, non-rosette leaves, flowers, and siliques were collected from 6-week-old plants. Harvested tissue was either used immediately or frozen in liquid nitrogen and stored at −80°C.
RNA Isolation and Northern Blotting
Total RNA for northern-blot analysis was isolated from Arabidopsis rosette leaves according to Davis et al. (1986). The RNA was fractionated on a 1% (w/v) agarose gel and transferred to nylon membranes (Davis et al., 1986). Immobilized RNA was hybridized overnight at 42°C with radiolabeled DGAT1 EST clone E6B2T7 (Arabidopsis Biological Resource Center, Ohio State University, Columbus). E6B2T7 was labeled with [α-32P]dCTP using a random primer kit (Roche Molecular Biochemicals, Summerville, NJ). The hybridized membranes were washed twice in 2× SSC containing 0.1% (w/v) SDS at 42°C for 15 min and twice in 1× SSC containing 0.1% (w/v) SDS at 42°C for 30 min. Hybridization was visualized by autoradiography after an overnight exposure at −80°C.
Antibody Production and Purification
Antibodies were raised in a rabbit against a DGAT1 peptide (CVLLYYHDLMNRKGSMS), which corresponds to the C terminus of the native protein. The carrier protein, keyhole limpet hemocyanin, was conjugated to the N-terminal Cys of the peptide using m-maleimidobenzoyl-N-hydroxysuccinimide ester according to Drenckhahn et al. (1993) and Collawn and Patterson (1989). The rabbit was injected four times at 2-week intervals with the linked peptide. Two weeks after the final injection, serum was collected. Antibodies were column-purified, and the titer was tested against remaining peptide.
Antibodies were also raised against cytochrome P450-cinnamate-4-hydroxylase fusion protein. For this purpose, the cDNA sequence of mung bean (Vigna radiata) cytochrome P450-cinnamate-4-hydroxylase (accession no. L07634) was amplified by PCR, subcloned into pGEX-5X-3, a glutathione-S-transferase fusion protein expression vector, and expressed in Escherichia coli BL21(DE3). The fusion protein was purified from E. coli extract using glutathione-agarose beads and used as an antigen for generation of polyclonal antibodies in rabbit.
Polyclonal antibodies against formate dehydrogenase were generously provided by Dr. Catherine Colas des Francs-Small (Université Paris-Sud, Orsay cedex, France).
Protein Fractionation and Western Blotting
Rosette leaf, stem, non-rosette leaf, root, flower, and silique tissues were homogenized (0.5 g mL−1) in buffer (50 mm 4–2(2-hydroxyethyl)-1-piperazine propane sulfonic acid [EPPS], pH 7.4, 0.25 m sorbitol, 10 mm EDTA, 2 mm EGTA, 1 mm dithiothreitol [DTT], 10 mm amino-n-caproic acid, 1 mm phenylmethylsulfonyl fluoride [PMSF], 1 mm benzamine, and 1 mm chymostatin) in a mortar and pestle. The homogenates were filtered through Miracloth, and protein was quantified according to Ghosh et al. (1988). SDS-PAGE was performed on Mini protein Dual Slab cells (Bio-Rad, Mississauga, Ontario), and the gels were stained with Coomassie Brilliant Blue R250 (Fairbanks et al., 1971) or transferred to polyvinylidene difluoride membranes using the semidry transfer method (semidry transfer cell, Bio-Rad). The blots were blocked for 30 s in 1 mg mL−1 polyvinyl alcohol (Miranda et al., 1993) and for 1 h in phosphate-buffered saline containing 0.05% (v/v) Tween 20 and 5% (w/v) powdered milk. Primary antibody was diluted 1:500 in phosphate-buffered saline containing 0.05% (v/v) Tween 20 and 1% (w/v) powdered milk. Antigen was visualized using secondary antibody coupled to alkaline phosphatase (Chemicon, Temecula, CA) and the phosphatase substrates p-nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate-p-toluidine salt (Bio-Rad).
Isolation of Chloroplasts and Chloroplast Membranes
Intact chloroplasts were purified from 4.5-week-old Arabidopsis rosettes according to Kunst (1998). In brief, 20 g of leaves from plants dark-treated for 24 h were floated on ice water for 30 min, blotted dry, and homogenized with an Omnimixer (Sorvall Products, Newtown, CT) in 200 mL of homogenization buffer (0.45 m sorbitol, 20 mm Tricine-KOH [pH 8.4], 10 mm EDTA, 10 mm NaHCO3, and 0.05% [w/v] NaN3). The homogenate was filtered through Miracloth and centrifuged at 280g for 90 s, and the pellets were resuspended in 1 mL of ice-cold resuspension buffer (0.3 m sorbitol, 20 mm Tricine-KOH [pH 7.6], 5 mm MgCl2, 2.5 mm EDTA, and 0.025% [w/v] NaN3). This suspension was layered on a Percoll gradient and centrifuged in an HB-4 rotor at 13,300g for 6 min. The lower diffuse band comprising intact chloroplasts was collected with a glass pipette, washed once in resuspension buffer, and resuspended in 1 mL of resuspension buffer.
Chloroplast membranes were obtained as described by Ghosh et al.(1994). Gradient-purified chloroplasts from 20 g of leaf tissue were pelleted and lysed in 1 mL of lysis buffer (10 mm Tricine, pH 7.6, containing 5 mm MgCl2) on ice in the dark for 30 min. At the end of this period, 1 mL of 2× resuspension buffer was added, and the chloroplast membranes (a composite of envelope membranes and thylakoids) were pelleted by centrifugation at 13,000g for 10 min in a microfuge. The membrane pellet was washed three times to remove any contaminating stroma by resuspension in 1.5 mL of resuspension buffer and centrifugation at 13,000g for 10 min in a microfuge. The final membrane pellet was suspended in 0.5 mL of resuspension buffer.
Gradient Fractionation of Microsomes
Microsomal membranes from rosettes and developing siliques were fractionated on a continuous Suc gradient. For isolation of microsomes, rosette leaves (10 g) from 4.5-week-old Arabidopsis plants were homogenized in 100 mL of buffer (3 mm Tris-HCl, pH 7.5, 2 mm EDTA, 250 mm mannitol, 2 mm DTT, 1 mm PMSF, and 5% [w/v] polyvinylpyrrolidone) for 45 s in a Sorvall Omnimixer and for an additional minute in a Polytron homogenizer. Developing siliques (5 g) from 6-week-old plants were homogenized in 50 mL of the same buffer, first in a mortar and pestle with glass beads, and then, as for leaves, for 45 s in a Sorvall Omnimixer and for an additional minute in a Polytron homogenizer. The homogenates were filtered through four layers of cheesecloth and centrifuged at 8,000g for 20 min at 4°C. The supernatant was collected and centrifuged at 100,000g for 1 h at 4°C. The resulting pellets of microsomal membranes were resuspended in 6 mL of storage buffer (6 mm Tris-HCl, pH 7.5, 10% [w/v] glycerol, 250 mm mannitol, 2 mm DTT, and 1 mm PMSF), layered on a continuous Suc density gradient (10%–40%, w/v) and centrifuged in a SW-28 rotor (Beckman Coulter, Inc., Fullerton, CA) at 70,000g for 2 h at 4°C. Fractions (20 drops fraction−1) were collected, and 10 μL from each fraction was used to determine the Suc concentration with a hand refractometer (Bausch & Lomb, Rochester, NY). The fractions were diluted five times with storage buffer and centrifuged at 100,000g for 1 h at 4°C, and the pellets were resuspended in 100 μL of storage buffer.
Lipid Analysis
For lipid analysis, 20 g of rosette leaf tissue from 3- or 6-week-old Arabidopsis plants were homogenized in 60 mL of buffer (50 mm EPPS, pH 7.4, 0.25 m sorbitol, 10 mm EDTA, 2 mm EGTA, 1 mm DTT, 10 mm amino-n-caproic acid, and 4% [w/v] polyvinylpolypyrrolidone) for 45 s in a Sorvall Omnimixer and for an additional minute on a Polytron homogenizer. The homogenate was filtered through four layers of cheesecloth and centrifuged at 3,000g for 10 min. The resulting sediment largely consisted of starch because the tissue homogenization was of sufficient intensity to disrupt cell organelles, which, if intact, might partially sediment under these conditions of centrifugation. The supernatant was collected and used for lipid extraction. Total lipids were extracted according to Bligh and Dyer (1959). Internal standards (diheptadecanoyl l-α-phosphatidylcholine, diarachidin, heptadecanoic acid, triheptadecanoic acid, and cholesteryl arachidate) were added before extraction. Lipid extracts were fractionated by thin layer chromatography (Yao et al., 1991), and the separated lipids were visualized with iodine vapor and identified using authentic standards. Fatty acids of the separated lipid fractions were transmethylated (Morrison and Smith, 1964), and the resultant fatty acid methyl esters were quantified by gas chromatography-mass spectrometry (HP-5890 series II gas chromatograph equipped with a DB Wax column; 30- × 0.25-mm i.d., 0.25-μm film, J&W Scientific, Folsom, CA) and a mass detector (HP-5970) with a scan range of m/z 35–150 operating at 0.16 s/scan. The oven temperature was initially held at 80°C for 5 min and then increased at 10°C per min to 100°C (held for 10 min), 160°C (held for 20 min), and finally 220°C (held for 51 min).
Electron Microscopy
Segments of tissue (approximately 2 mm2) cut from the center of rosette leaves from 3- and 6-week-old Arabidopsis plants were vacuum-infiltrated with 0.02 m sodium phosphate buffer (pH 7.2) and fixed in 4% (w/v) gluteraldehyde in 0.02 m sodium phosphate buffer (pH 7.2) overnight at 4°C. The samples were then washed four times in 0.02 m phosphate buffer (pH 7.2), post-fixed in 1% (w/v) osmium tetroxide in 0.02 m phosphate buffer (pH 7.2) for 2 h at 4°C, and washed four times in water for 30 min. They were then dehydrated in a graded series of acetone, washed four times in 100% (w/v) acetone for 30 min, and embedded in Epon-Araldite. Ultrathin sections (70–90 nm) were stained in lead citrate and uranyl acetate and examined with an electron microscope (CM 10, Philips, Eindhoven, The Netherlands) operating at 60 kV.
Enzyme Assay
Cytochrome c oxidase was assayed as described by Hodges and Leonard (1974).
ACKNOWLEDGMENTS
We thank Dale Weber (University of Waterloo, ON) for assistance with the electron microscopy. Peptide synthesis was conducted in the laboratory of Dr. Giles Lajoie (Department of Chemistry, University of Waterloo).
Footnotes
This work was supported by the Natural Sciences and Engineering Research Council of Canada.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.003087.
LITERATURE CITED
- Andersson MX, Kjellberg JM, Sandelius AS. Chloroplast biogenesis: regulation of lipid transport to the thylakoid in chloroplasts isolated from expanding and fully expanded leaves of pea. Plant Physiol. 2001;127:184–193. doi: 10.1104/pp.127.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awai K, Maréchal E, Block MA, Brun D, Masuda T, Shimada H, Takamiya K-I, Ohta H, Joyard J. Two types of MGDG synthase genes, found widely in both 16:3 and 18:3 plants, differentially mediate galactolipid syntheses in photosynthetic and nonphotosynthetic tissues in Arabidopsis thaliana. Proc Natl Acad Sci. 2001;98:10960–10965. doi: 10.1073/pnas.181331498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JL, Whyborn AG. The osmiophilic globules of chloroplasts: II. Globules of the spinach-beet chloroplast. Biochim Biophys Acta. 1963;78:163–174. [Google Scholar]
- Bao X, Ohlrogge J. Supply of fatty acid is one limiting factor in the accumulation of triacylglycerol in developing embryos. Plant Physiol. 1999;120:1057–1062. doi: 10.1104/pp.120.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrams M, Heinz E. Experiments on enzymatic acylation of sn-glycerol 3-phosphate with enzyme preparations from pea and spinach leaves. Planta. 1976;132:161–168. doi: 10.1007/BF00388898. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Browse J, Warwick N, Sommerville CR, Slack CR. Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the ‘16:3′ plant Arabidopsis thaliana. Biochem J. 1986;235:25–31. doi: 10.1042/bj2350025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas des Frans-Small C, Ambard-Bretteville F, Small ID, Remy R. Identification of a major soluble protein in mitochondria from nonphotosynthetic tissues as NAD-dependent formate dehydrogenase. Plant Physiol. 1993;102:1171–1802. doi: 10.1104/pp.102.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collawn JF, Patterson Y. Production of antipeptide antibodies. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Smith JA, Seidman JG, Struhl K, editors. Current Protocols in Molecular Biology on CD. New York: John Wiley & Sons; 1989. pp. 11.15.1–11.16.1. [DOI] [PubMed] [Google Scholar]
- Davis LG, Dibner MD, Battey JB. Basic Methods in Molecular Biology. New York: Elsevier Science Publishing; 1986. pp. 130–135. [Google Scholar]
- De Bellis L, Picciarelli P, Pistelli L, Alpi A. Localization of glyoxylate-cycle marker enzymes in peroxisomes of senescent leaves and green cotyledons. Planta. 1990;180:435–439. doi: 10.1007/BF00198797. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D, Jons T, Schmitz F. Production of polyclonal antibodies against proteins and peptides. In: Asai DJ, editor. Methods in Cell Biology. Vol. 37. New York: Academic Press; 1993. pp. 7–56. [DOI] [PubMed] [Google Scholar]
- El-Hafid L, Pham AT, Zuily-fodil Y, da Silva JV. Enzymatic breakdown of polar lipids in cotton leaves under water stress: I. Degradation of monogalactosyl-diacylglycerol. Plant Physiol Biochem. 1989;27:495–502. [Google Scholar]
- Engelman-Silvestre I, Bureau J-M, Trémolières A, Paulin A. Changes in membrane phospholipids and galactolipids during the senescence of cut carnations: connection with ethylenic rise. Plant Physiol Biochem. 1989;27:931–937. [Google Scholar]
- Fairbanks G, Steck TL, Wallach DFH. Coomassie blue R250 used in isopropanol-acetic acid. Biochemistry. 1971;10:2606–2618. [Google Scholar]
- Ghosh S, Gepstein S, Heikkila JJ, Dumbroff EB. Use of a scanning densitometer or an ELISA plate reader for measurement of nanogram amounts of protein in crude extracts from biological tissues. Anal Biochem. 1988;169:227–233. doi: 10.1016/0003-2697(88)90278-3. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Hudak KA, Dumbroff EB, Thomspon JE. Release of photosynthetic protein catabolites by blebbing from thylakoids. Plant Physiol. 1994;106:1547–1553. doi: 10.1104/pp.106.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiamét JJ, Pichersky E, Noodén LD. Mass exodus from senescing soybean chloroplasts. Plant Cell Physiol. 1999;40:986–992. [Google Scholar]
- Harwood JL, Stumpf PK. Fat metabolism in higher plants: XLIX. Fatty acid biosynthesis by subcellular fractions of higher plants. Lipids. 1972;7:8–19. [Google Scholar]
- Himelblau E, Amasino RM. Nutrients mobilized from leaves of Arabidopsis thaliana during leaf senescence. J Plant Physiol. 2001;158:1317–1323. [Google Scholar]
- Hobbs DH, Chaofu L, Hills MJ. Cloning of a cDNA encoding diacylglycerol acyltransferase from Arabidopsis thaliana and its functional expression. FEBS Lett. 1999;452:145–149. doi: 10.1016/s0014-5793(99)00646-8. [DOI] [PubMed] [Google Scholar]
- Hobbs DH, Hills MJ. Expression and characterization of diacylglycerol acyltransferase from Arabidopsis thaliana in insect cell cultures. Biochem Soc Trans. 2000;28:687–689. [PubMed] [Google Scholar]
- Hodges TK, Leonard RT. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. In: Fleischer S, Packer L, editors. Methods in Enzymology. Vol. 32. New York: Academic Press; 1974. pp. 392–446. [DOI] [PubMed] [Google Scholar]
- Huang AHC. Oil bodies and oleosins in seeds. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:177–200. [Google Scholar]
- Huang AHC. Oleosin and oil bodies in seeds and other organs. Plant Physiol. 1996;110:1055–1061. doi: 10.1104/pp.110.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jako C, Kumar A, Wei Y, Zou J, Barton DL, Giblin EM, Covello PS, Taylor DC. Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiol. 2001;126:861–874. doi: 10.1104/pp.126.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katavic V, Reed DW, Taylor DC, Giblin M, Barton DL, Zou J, MacKenzie SL, Covello PS, Kunst L. Alteration of seed fatty acid composition by an ethyl methanesulfonate-induced mutation in Arabidopsis thaliana affecting diacylglycerol acyltransferase activity. Plant Physiol. 1995;108:399–409. doi: 10.1104/pp.108.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler F, Schnell D, Blobel G. Identification of proteins associated with plastoglobules isolated from pea (Pisum sativum L.) chloroplasts. Planta. 1999;208:107–113. doi: 10.1007/s004250050540. [DOI] [PubMed] [Google Scholar]
- Kim M-J, Oh J-M, Cheon S-H, Cheong T-K, Lee S-H, Choi E-O, Lee HG, Park CS, Park KH. Thermal inactivation kinetics and application of phospho-and galactolipid-degrading enzyme for evaluation of quality changes in frozen vegetables. J Agric Food Chem. 2001;49:2241–2248. doi: 10.1021/jf001379b. [DOI] [PubMed] [Google Scholar]
- Kunst L. Preparation of physiologically active chloroplasts from Arabidopsis. In: Martínez-Zapater JM, Salinas J, editors. Methods in Molecular Biology: Arabidopsis Protocols. Totowa, NJ: Humana Press; 1998. pp. 43–48. [DOI] [PubMed] [Google Scholar]
- Kwanyuen P, Wilson RF. Isolation and purification of diacylglycerol acyltransferase from germinating soybean cotyledons. Biochim Biophys Acta. 1986;877:238–245. doi: 10.1016/0167-4838(90)90227-7. [DOI] [PubMed] [Google Scholar]
- Lacey DJ, Beaudoin F, Dempsey CE, Shewry PR, Napier JA. The accumulation of TAG within the endoplasmic reticulum of developing seeds of Helianthus annus. Plant J. 1999;17:397–405. [Google Scholar]
- Lardizabal KD, Mai JT, Wagner NW, Wyrick A, Voelker T, Hawkins DJ. DGAT2 is a new diacylglycerol acyltransferase gene family. J Biol Chem. 2001;276:38862–38869. doi: 10.1074/jbc.M106168200. [DOI] [PubMed] [Google Scholar]
- Lee AG. Membrane lipids: it’s only a phase. Curr Biol. 2000;10:R377–R379. doi: 10.1016/s0960-9822(00)00477-2. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. The plastoglobuli of spinach: their size, isolation and lipoquinone composition. Protoplasma. 1969;68:65–66. [Google Scholar]
- Lichtenthaler HK, Weinert H. the correlation between lipoquinone accumulation and plastoglobuli formation in the chloroplasts of Ficus elastica Roxb. Z Naturforsch. 1970;25b:619–623. [Google Scholar]
- Martin BA, Wilson RF. Properties of diacylglycerol acyltransferase from spinach leaves. Lipids. 1983;18:1–6. [Google Scholar]
- Martin BA, Wilson RF. Subcellular localization of TAG synthesis in spinach leaves. Lipids. 1984;19:117–121. doi: 10.1007/BF02534501. [DOI] [PubMed] [Google Scholar]
- Matile P. Chloroplast senescence. In: Baker NR, Thomas H, editors. Crop Photosynthesis: Spatial and Temporal Determinants. New York: Elsevier Science Publishers; 1992. pp. 413–440. [Google Scholar]
- Miquel M, Cassagne C, Browse J. A new class of Arabidopsis mutants with reduced hexadecatrienoic acid fatty acid levels. Plant Physiol. 1998;117:923–930. doi: 10.1104/pp.117.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda PV, Brandelli A, Tezon JG. Instantaneous blocking for immunoblots. Anal Biochem. 1993;209:376–377. doi: 10.1006/abio.1993.1138. [DOI] [PubMed] [Google Scholar]
- Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J Lipid Res. 1964;5:600–608. [PubMed] [Google Scholar]
- Peoples MB, Beilharz VC, Waters SP, Simpson RJ, Dalling MJ. Nitrogen redistribution during grain growth in wheat (Triticum aestivum L.): II. Chloroplast senescence and the degradation of ribulose-1,5-bisphosphate carboxylase. Planta. 1980;149:241–251. doi: 10.1007/BF00384560. [DOI] [PubMed] [Google Scholar]
- Post-Beittenmiller D. Biochemistry and molecular biology of wax production in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:405–430. doi: 10.1146/annurev.arplant.47.1.405. [DOI] [PubMed] [Google Scholar]
- Pozueta-Romero J, Rafia F, Houlne G, Cheniclet C, Carde J-P, Schantz ML, Schantz R. A ubiquitous plant housekeeping gene, PAP, encodes a major protein component of bell pepper chromoplasts. Plant Physiol. 1997;115:1185–1194. doi: 10.1104/pp.115.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey P, Gillet B, Römer S, Eymery F, Massimino J, Peltier G, Kuntz M. Over-expression of a pepper plastid lipid-associated protein in tobacco leads to changes in plastid ultrastructure and plant development upon stress. Plant J. 2000;21:483–494. doi: 10.1046/j.1365-313x.2000.00699.x. [DOI] [PubMed] [Google Scholar]
- Routaboul J-M, Benning C, Bechtold N, Caboche M, Lepiniec L. The TAG1 locus of Arabidopsis encodes for a diacylglycerol acyltransferase. Plant Physiol Biochem. 1999;37:831–840. doi: 10.1016/s0981-9428(99)00115-1. [DOI] [PubMed] [Google Scholar]
- Sakaki T, Kondo N, Yamada M. Free fatty acids regulate two galactosyltransferases in chloroplast envelope membranes isolated from spinach leaves. Plant Physiol. 1990a;94:781–787. doi: 10.1104/pp.94.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki T, Kondo N, Yamada M. Pathway for the synthesis of triacylglycerols from monogalactosyldiacylglycerols in ozone-fumigated spinach leaves. Plant Physiol. 1990b;94:773–780. doi: 10.1104/pp.94.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki T, Saito K, Kawaguchi A, Kondo N, Yamada M. Conversion of monogalactosyldiacylglycerols to triacylglycerols in ozone-fumigated spinach leaves. Plant Physiol. 1990c;94:766–772. doi: 10.1104/pp.94.2.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J, Mancha M. Synthesis of acyl CoAs by isolated spinach chloroplasts in relation to added CoA and ATP. Planta. 1981;153:519–523. doi: 10.1007/BF00385535. [DOI] [PubMed] [Google Scholar]
- Shimakata T, Stumpf PK. Fatty ace synthetase of Spinacia oleracea leaves. Plant Physiol. 1982;69:1257–1262. doi: 10.1104/pp.69.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebertz HP, Heinz E, Linscheid M, Joyard J, Douce R. Characterization of lipids from chloroplast envelopes. Eur J Biochem. 1979;101:429–438. doi: 10.1111/j.1432-1033.1979.tb19736.x. [DOI] [PubMed] [Google Scholar]
- Smart CM. Gene expression during leaf senescence. New Phytol. 1994;126:419–448. doi: 10.1111/j.1469-8137.1994.tb04243.x. [DOI] [PubMed] [Google Scholar]
- Smith MD, Licutalosi DD, Thompson JE. Co-association of cytochrome f catabolites and plastid-lipid-associated protein with chloroplast lipid particles. Plant Physiol. 2000;124:211–221. doi: 10.1104/pp.124.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprey B, Lichtenthaler HK. Zur frage der beziehungen zwischen plastoglobuli und thylakoidgense in gertenkeimlingen. Z Naturforsch. 1966;21b:697–699. [Google Scholar]
- Steinmüller D, Tevini M. Composition and function of plastoglobuli: I. Isolation and purification from chloroplasts and chromoplasts. Planta. 1985a;163:201–207. doi: 10.1007/BF00393507. [DOI] [PubMed] [Google Scholar]
- Steinmüller D, Tevini M. Composition and function of plastoglobuli: II. Lipid composition of leaves and plastoglobuli during beech leaf senescence. Planta. 1985b;163:91–96. doi: 10.1007/BF00395902. [DOI] [PubMed] [Google Scholar]
- Thompson JE, Froese CD, Madey E, Smith MD, Hong Y. Lipid metabolism during plant senescence. Prog Lipid Res. 1998;37:119–141. doi: 10.1016/s0163-7827(98)00006-x. [DOI] [PubMed] [Google Scholar]
- Yao K, Paliyath G, Humphrey RW, Hallet FR, Thompson JE. Identification and characterization of nonsedementable lipid-protein microvesicles. Proc Natl Acad Sci USA. 1991;88:2269–2273. doi: 10.1073/pnas.88.6.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young O, Beevers H. Mixed function oxidase from germinating castor bean endosperm. Phytochemistry. 1976;15:379–385. [Google Scholar]
- Zou J, Wei Y, Jako C, Kumar A, Selvaraj G, Taylor DC. The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J. 1999;19:645–653. doi: 10.1046/j.1365-313x.1999.00555.x. [DOI] [PubMed] [Google Scholar]